Abstract
Visual experience plays a critical role in the development of direction selective responses in ferret visual cortex. In visually naïve animals, presentation of bidirectional `training' stimulus induces rapid increases in the direction selective responses of single neurons that can be predicted by small but significant direction biases that are present in vivo 2-photon imaging of calcium signals to further explore the contribution of visual experience to the emergence of direction selective responses in ferret visual cortex. The first set of experiments was designed to determine whether visual experience is required for the development of the initial neighborhood bias. In animals that were dark-reared until the time of eye-opening, we found that individual neurons exhibited weak direction selective responses accompanied by a reduced but statistically significant neighborhood bias, indicating that both features arise without the need for visual experience. The second set of experiments employed a unidirectional training stimulus to assess the relative roles of the neighborhood bias and visual experience in determining the direction preference that cortical neurons acquire during direction training. We found that neurons became more responsive to the trained direction even when they were located in regions of the cortex with an initial neighborhood bias for the direction opposite the training stimulus. Taken together, these results suggest an adaptive developmental strategy for the elaboration of direction selective responses, one in which experience-independent mechanisms provide a symmetry-breaking seed for the instructive effects of visual experience.
Introduction
The role of experience in the maturation of the response properties of neurons in visual cortex has been the subject of intense investigation since the original description of orientation and direction selective responses in visual cortex (Hubel and Wiesel, 1959). It is generally agreed that the basic properties that characterize mature cortical circuits are evident at the onset of visual experience (Hubel and Wiesel, 1963); but the number of neurons displaying these features and their response selectivity is far less than that found after several weeks of experience (Blakemore and Van Sluyters, 1975, Fregnac and Imbert, 1984). While proper maturation of cortical function depends on visual experience, the exact contribution of experience-dependent and -independent mechanisms to circuit construction remains unclear.
The robust columnar representation of direction-selective responses in ferret visual cortex provides a unique model system in which to assess the contribution of experience-dependent and -independent mechanisms to cortical development. At eye opening, direction selectivity is poorly developed. Direction selectivity and its columnar structure reach mature levels over several weeks via a process that requires visual experience (Li et al., 2006). In visually naïve animals, these changes can be induced in hours through repeated presentation of bidirectional drifting stimuli. Using 2-photon imaging, individual cortical neurons were found to exhibit significant increases in direction selectivity. Moreover, the direction preference that a neuron acquired could be predicted by small but significant direction biases that were present in neighboring neurons at the onset of training (Li et al., 2008).
While these observations imply a strong impact of visual experience on the development of direction selectivity, fundamental questions remain. First, is the initial neighborhood bias that lays the foundation for the map of direction preference derived independent of visual experience? Second, how do the initial neighborhood biases and the properties of visual stimulation interact to influence the direction preference that a neuron acquires? Is the initial bias an immutable “fate map” that dictates the direction preference that emerges with experience, or does the motion stimulus exert a significant influence over the direction preference that a neuron acquires?
We first imaged the visual cortex of animals dark-reared until eye-opening and found a reduced but significant neighborhood bias in direction preference, consistent with the idea that the initial seed for the direction map arises without visual experience. Second, we challenged the capacity of the initial neighborhood bias to predict a neuron's direction preference by limiting the motion training to a single direction. Neurons became more selective to the trained direction, even when they were located in regions of the cortex with an initial neighborhood bias for the direction opposite the training stimulus. Moreover, unidirectional training was found to shift neighborhood biases towards the direction of the training stimulus in regions where the initial neighborhood bias would have been strengthened by bidirectional training. Taken together, these findings suggest an adaptive developmental strategy for the construction of the map of direction preference; one in which experience-independent mechanisms serve as a catalyst for the instructive effects of visual experience.
Materials and Methods
All experimental procedures were approved by the Institutional Animal Care and Use Committee at Duke University Medical Center. Light-reared (that is, typically-reared) ferrets were reared in a 12 hour light/dark cycle environment, whereas dark-reared ferrets were raised in complete darkness starting at age postnatal day 14–17 until the time of the experiment as described in (Li et al., 2006). To verify that the enclosure of the dark-reared animals remained dark at all times, a roll of unexposed photographic film was kept in enclosure, and a new frame was exposed to the environment each day. At the conclusion of dark rearing, the film was developed and verified to be “blank”.
Ferrets of both sexes were prepared for 2-photon calcium imaging (Stosiek et al., 2003, Ohki et al., 2005) as described in detail in Li et al., (2008). Briefly, ferrets were anaesthetized with ketamine (50 mg/kg) and isoflurane (2% for surgery, 0.8–1% during imaging). Oregon Green 488 BAPTA-1 acetoxymethyl ester (Invitrogen) was pressure injected into cortex, and changes in calcium fluorescence were monitored with an Ultima IV 2-photon microscope (Prairie Technologies) driven by a mode-locked Chameleon laser (810 nm, Coherent).
Cells were identified manually by the experimenter through visual inspection. This identification process was performed blind to knowledge of physiological responses. Circular regions of interests 12 pixels (5.5μm) in diameter were manually drawn in the centers of cell somas. These regions of interests were smaller than the cells to reduce possible intrusion of the neuropil signal into somatic responses. As in our previous study, image sequences taken at different times were aligned with correlation (Li et al., 2008). Each cell was examined to ensure it was still clearly present in the second recording, and small manual corrections were made as necessary. The quality of the identification and alignment was checked offline by a second investigator, and corrections were made if necessary by removing objects that could not be unambiguously identified as cells by both investigators or by adding any cells that were missed. Cells that appeared to be astrocytes on the basis of characteristic morphology were excluded.
The training protocol consisted of a 5s drifting grating stimulation followed by a 10s interstimulus interval. The protocol continued for 20 min and was followed by 10 min of no stimulation. This entire procedure was repeated for several hours. Visual stimulation and analysis were performed using custom software for Matlab (The MathWorks) and the Psychophysics Toolbox (Brainard, 1997, Pelli, 1997).
Analysis
The response to each stimulus was calculated as where FSTIM is the average response during each frame when the stimulus was on, and F0 is the average response during the final 3 seconds of the interstimulus period before stimulus onset.
Cells were considered to be significantly visually responsive if an ANOVA test indicated that the mean responses to all stimulation conditions (including blank) were statistically different. Cells with significant orientation responses were identified by plotting each trial's response as a point in orientation space: , where S(θi) is raw at direction θi. Cells were considered to exhibit significant orientation selectivity if the mean of the points was different from 7 (0, 0) by Hotelling's t2-test with P < 0.05.
To examine orientation responses for all neurons, including those that did not exhibit significant orientation responses, we computed the circular variance (Ringach et al., 2002):
The circular variance varies between 0 (the total response is at 1 orientation) and 1 (the response is indifferent to orientation), and has the quality that it is a relatively reliable (low noise) index for both very selective and poorly selective cells. Here, we report 1-CV so that values near 1 are highly orientation selective, while values near 0 are unselective.
Responses to cells that exhibited significant orientation selectivity (Hotelling's test) where fit with a 2-peak Gaussian function (Carandini and Ferster, 2000):
where ROFFSET is a constant offset, θPREF is the preferred direction angle, RPREF is the above-offset response to the preferred direction, Ropp is the above-offset response to the opposite direction, is the tuning width (half-width at half height) and ang(x)=min(x,x−180,x + 180) wraps angular difference values onto the interval 0° to 180°.
Direction index values were calculated as
where PREFERRED = R(θPREF) and OPPOSITE = RθPREF + 180). This direction index varies from 0 (no direction selectivity) to 1 (no positive response to the opposite direction). The minimum function restricts DI to be between 0 and 1 in some cells where the OPPOSITE response was below BLANK.
To compare the magnitude of direction selectivity for each neuron relative to the trained direction in unidirectional training experiments, we used the direction ratio (DR) measurement, which we calculated as
The direction index and direction ratio above are based on mean responses and do not consider variation across trials. To statistically examine the direction preference for each tuning curve, we used the bootstrap algorithm (Press et al., 1992) and simulated 100 recordings of 12 trials by drawing from the actual recorded trials randomly with replacement. We then calculated direction angle preferences and direction index values for these 100 simulated recordings, and used these 100 simulations to derive measures of direction preference uncertainty and local coherence. We defined the direction preference uncertainty index for each cell as the percentage of these 100 bootstrap simulations that exhibited direction preferences that were 90° or more away from the dominant direction preference obtained in the 100 simulations. The dominant direction preference was determined by examining the mean orientation preference for all 100 simulations, and identifying which of the 2 opposite directions orthogonal to that orientation were preferred in most simulations (ties were broken by randomly choosing a direction). Thus, the uncertainty index varied from 0 (all 100 simulations agreed) to 50% (50 simulations exhibited a direction preference that differed by 90° or more from the dominant direction preference).
The local coherence index LCI(θ)for a cell was defined to be the percentage of nearby neurons that had a direction angle preference that matched a reference angle θ (within 45°) minus the percentage of nearby neurons that had the opposite direction angle preference (within 45°). Distance was measured in the horizontal plane: if multiple depths were recorded at the same point on the cortical surface, only horizontal distance between cells was considered. For each cell, we computed 100 values of the local coherence index by drawing the direction angle preference of every neighboring cell from the likelihood distribution estimated by the bootstrap procedure above. We took the median of these 100 values to be the local coherency index for each cell. We used this Monte Carlo approach because we felt it provided a better estimate of the true local coherence index rather than simply using the raw data. These simulations take into account the biological and measurement uncertainty of each cell's direction angle preference.
Results
Impact of early experience on the emergence of map structure
In our previous 2-photon imaging study (Li et al., 2008), we found evidence for a weak but significant neighborhood bias in direction preference that was present at the onset of motion or flash training. Some animals in our study had not yet opened their eyes, but a majority of animals were examined 1–2 days after natural eye opening. Therefore, it is unclear whether visual experience, either through the closed lids before eye opening (Krug et al., 2001, Akerman et al., 2002(Krug et al., 2001, Akerman et al., 2002), or after eye opening, contributed to the weak direction selectivity and the neighborhood bias that we observed.
To examine the contribution of visual experience to direction selective responses prior to training, we compared the results from light-reared animals to animals that were raised in complete darkness from P14–P17 until the time of the experiment, which occurred between P31–P34. The light-reared animals consisted of 5 animals from our previous study (Li et al., 2008) as well as 6 animals from the unidirectional training portion of this paper. As in our previous study, we assessed orientation and direction selectivity with presentations of 4 separate orientations of sine wave gratings (spatial frequency 0.06–0.08cpd, temporal frequency 4 Hz) that drifted along an axis of motion orthogonal to the grating orientation in 1 of 2 opposite directions, totaling 8 different directions 45 degree apart (Figure 1ab).
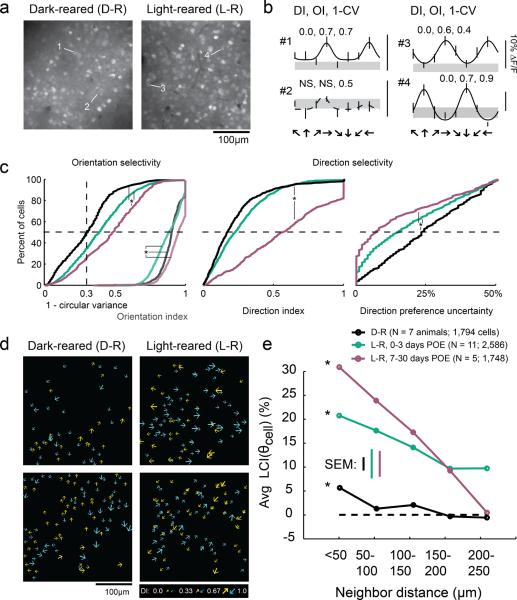
Figure 1.
Weak local direction biases are present in dark-reared animals. a) Fluorescent imaging fields of neurons loaded with the calcium indicator OGB-1 in dark-reared and light-reared animals. b) Tuning curves of cells indicated in a); gray region indicates the response to a blank stimulus (+/− SEM), error bars indicate SEM; solid curves indicate tuning curve fits to cells exhibiting significant orientation selectivity, dashed curves indicate fits to insignificant cells. Numbers indicate direction index (DI), orientation index (OI), and 1-circular variance. DI and OI could only be reliably calculated for cells that exhibited significant orientation selectivity (see Materials and Methods). c) Left: 1-circular variance for all responsive cells (left set of curves), and OI for cells that were significantly orientation-selective (right set of curves); note that OI and 1-CV are plotted on the same axes. Middle: DI for cells exhibiting significant orientation selectivity. Right: Direction preference uncertainty index; * indicates significant differences (KW-test, K-W post-hoc, p<0.05). PEO is days post-eye-opening. d) Plots of direction selectivity of individual cells in dark-reared and light-reared imaging fields. Arrows indicate preferred direction; for visualization, half of direction space is represented by yellow arrows, and the other half by blue arrows; size of arrow indicates strength of DI as indicated in key. e) Local coherence index as a function of neighborhood distance. Both light-reared groups and dark-reared animals exhibited a significant LCI relationship with distance (ANOVA, see text), although LCI was weaker in dark-reared animals than in light-reared animals that had less than 3 days of visual experience.
Layer 2/3 neurons in animals that had been dark-reared exhibited levels of orientation selectivity (as quantified by the orientation index and 1-circular variance) and direction selectivity (direction index) that were slightly less than those of the naïve light-reared group and far from that seen in light-reared animals with more than a week of visual experience (Figure 1c). We also quantified the certainty with which we could assign a preferred direction to these cells, using bootstrap analysis to account for variation in responses from trial to trial (see Materials and Methods). Layer 2/3 neurons in dark-reared animals exhibited slightly higher median direction preference uncertainty values than light-reared animals (23% dark-reared vs. 15% light-reared, Kruskal-Wallis p<0.001), indicating that direction responses in dark-reared animals were modestly more variable than those in light-reared animals. Finally, dark-reared animals also exhibited a modestly lower percentage of visually responsive cells as compared to light-reared animals (dark-reared: mean 39%, range 10–98%; light-reared: mean 70%, range 37–94%, t-test p<0.017).
Despite the slight reductions of orientation and direction selectivity and visual responsiveness in dark-reared animals, summary schematics from individual animals (Figure 1d) indicate that weak local direction biases develop in the absence of visual experience. To quantify these local neighborhood biases we used a local coherence index that was evaluated with respect to each cell's direction preference, LCI(θcell). For a single cell, LCI(θcell) is defined to be the fraction of neighboring cells that match the reference cell's direction preference (within 45°) minus the fraction of neighboring cells that differ from the reference cell's direction preference (within 45°). Analysis of the spatial structure of the direction preferences in the dark-reared animals revealed a statistically significant neighborhood bias that varied with distance (Figure 1e). As shown previously (Li et al. 2008), light-reared animals exhibit a significant average LCI(θ)cell that falls off with distance (both ANOVA, p< 0.001), consistent with a coherent map structure; older light-reared animals exhibit a much stronger LCI(θ)cell relationship with distance than younger light reared animals. The average LCI(θ)cell value value for dark-reared animals was weaker than the light-reared animals; nevertheless, this value varied significantly with distance (ANOVA p< 0.001). Thus, the weak neighborhood biases in the spatial arrangement of direction preference that are present at the onset of motion training emerge without the need for visual experience.
Previously, we demonstrated that the neighborhood bias at the onset of motion training could be used to predict the direction preference that emerged from training with a bidirectional motion stimulus. This observation was taken as evidence that the weak neighborhood bias serves as a seed for the experience-dependent development of the direction map. To determine whether the neighborhood bias derived without visual experience could serve a similar role, we sought to examine the impact of direction training in the animals that had been dark-reared up to the onset of training.
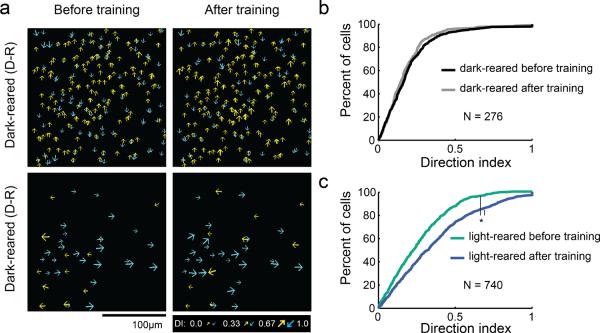
We were surprised to find that 3–6 hours of bidirectional or unidirectional training, a duration that was highly effective in light-reared animals, was ineffective in the dark-reared animals (Figure 2). Dark-reared animals did not exhibit training- induced increases in direction index values (K-W test, p= 0.2). This result, by itself, suggests that dark rearing prior to the time of eye opening either slows or prohibits the maturation of direction-selective responses. Because the duration of these training experiments was limited by the lifetime of the calcium indicator, we were unable to test whether direction training effects would be evident with longer periods of training. However, we have shown previously that animals dark-reared until eye opening and then reared for several weeks under normal conditions do develop strong columnar maps of direction preference (Li et al., 2006). Taken together, these results imply that dark rearing prior to the time of eye opening slows, but does not prohibit, the experience-dependent maturation of direction selective responses.
Figure 2.
Motion training was ineffective in dark-reared animals. a) Imaging fields of direction selectivity before and after bidirectional or unidirectional motion training for dark-reared animals. Cumulative density histograms of direction index values before and after training for dark-reared animals (b) and light-reared animals (c). Differences in DI were not significant for dark-reared animals (K-W, p=0.2), but were significant for light-reared animals (K-W, p<0.001).
Unidirectional training
Having established that the neighborhood bias emerges in the absence of visual experience, we next sought to understand how the neighborhood bias and the properties of the visual stimulus contribute to the direction preference that a neuron acquires. At one extreme, it is possible that the spatiotemporal properties of the visual stimulus are largely irrelevant to the direction preference that a neuron acquires: the neighborhood bias established prior to motion training may be the equivalent of a `fate map', predetermining the outcome of motion training. At the other extreme, the spatiotemporal properties of the visual stimulus may play an instructive role in determining the direction preference that a neuron acquires: the direction preferences associated with early neighborhood biases may be provisional, and overwritten if they are not reinforced with corresponding motion stimuli.
In order to distinguish between these 2 alternatives, we examined the impact of unidirectional motion training on the emergence of direction preferences in typically-reared ferrets that had fewer than 2 days of visual experience after natural eye opening (n = 6; postnatal day 32–35). Before training, we observed strong orientation selectivity and weak direction selectivity that was slightly biased (Figure 3ab) for 1 direction of motion, as in our previous study (Li et al., 2008). In general, each imaging field contained more cells that were biased in 1 particular direction, which we termed the majority direction, as compared to the opposite direction, the minority direction. We selected 1 of these directions for 3–6 hours of unidirectional training (5 animals in the minority direction, 1 animal in the majority direction), followed by a reassessment of direction preferences.
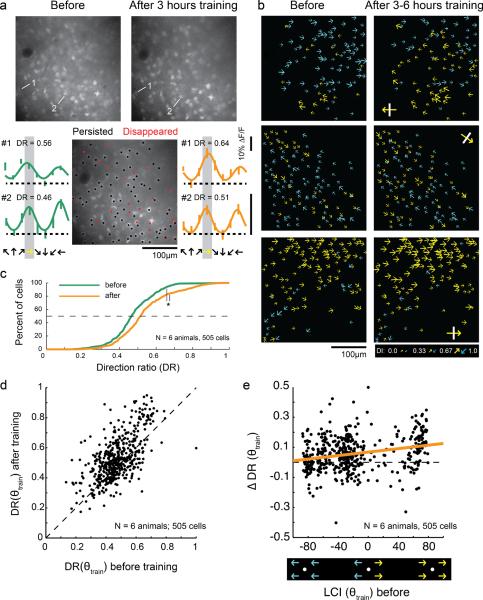
Figure 3.
Motion training in a single direction increases responses to the trained direction. a) Top: Representative 2-photon images before and after unidirectional motion training. Bottom: Cell history (persisted/disappeared—see methods) over the course of training is depicted in the middle field. Individual tuning curves for 2 cells are shown before (left) and after (right) unidirectional training. Yellow arrows indicates trained direction in all panels. b) Plots of direction selectivity in individual cells before and after training. Yellow arrows indicate cells that preferred the training direction (within 180°); blue arrows indicate cells that preferred the opposite direction (within 180°). Yellow arrows with white bars indicate actual training direction. c) Direction ratio (DR) cumulative histogram before and after training (N=number of animals, number of cells) * indicates significant difference (t-test, p<0.001). d) DR before and after unidirectional training for individual cells. Dashed line indicates unity line. On average, cells with initial DR values greater than or less than 0.5 exhibited an increase in DR with training (t-test, p<0.001). e) Change in DR with unidirectional training as a function of initial local coherence index with respect to the trained direction (neighborhood size: 150μm). Cells with both positive and negative initial LCI(θtrain) values exhibited increases in selectivity for the trained direction (F-tests of regression line slope, p<0.001). Thick line is regression line.
We restricted our analysis to cells that were both significantly responsive and could be unambiguously identified before and after training (see Materials and Methods). Direction preferences and index values for neurons in 3 animals are shown graphically in Figure 3b. In all these cases, yellow arrows indicate cells whose direction preferences matched the direction that was used for training, and blue arrows indicate cells whose direction preferences matched the opposite direction. After training, many cells appear to have been recruited to prefer the trained direction; that is, there are more yellow cells on the right panels of Figure 3b than on the left panels.
To examine this effect quantitatively, we developed an index, called the “direction ratio” (DR), which was defined to be the response to the trained direction relative to sum of the responses to the trained and opposite directions (Figure 3c). After unidirectional training, there was a significant increase in the direction ratio (t-test, p<0.001), indicating that cells responded more strongly to the trained direction after training than before.
The increase in the distribution of DR after unidirectional training indicates that the median cell became more responsive to the trained direction, but it does not tell us if all cells, or a subset of cells, exhibited this behavior. For example, it is possible that only the cells that initially exhibited biases for the trained direction become more strongly biased as a result of the training, while cells biased towards the opposite direction remain unchanged. To address this question, we plotted DR for individual cells before and after training (Figure 3d). Mean DR increased significantly for those cells that were initially biased for the trained direction (initial DR greater than 0.5) and for those cells that were biased against the trained direction (initial DR less than 0.5), indicating that, on average, both groups increased their responses to the stimulated direction as a result of training (t-test, p<0.001 for both).
These results indicate that the spatiotemporal properties of the motion training stimulus have a significant impact on the direction selective responses that cortical neurons acquire. But they do not allow us to assess the degree to which these training effects are constrained by the neighborhood biases that exist at the onset of training. Individual cells biased for and against the trained direction are found throughout the initial weak direction map, both in regions where the majority of the neighboring cells are biased for the trained direction, and in regions where the majority are biased against the trained direction. In principle, increases in DR could be restricted to regions where the majority of the neighboring cells were already biased for the training direction at the onset of training. Alternatively, the effects of the training stimulus may be strong enough to impact the selectivity of neurons in regions that exhibit neighborhood biases for the direction opposite the training stimulus.
To determine whether the influence of the training stimulus was limited to regions of cortex that exhibited an initial neighborhood bias consistent with the training stimulus, we examined the change in direction ratio induced by training as a function of each cell's initial local coherence index LCI(θtrain) (Figure 3e). Cells on the right side of the graph were initially surrounded by cells that exhibited biases for the trained direction (positive LCI(θtrain)values), while cells on the left side of the graph were initially surrounded by cells that exhibited biases for the opposite direction (negative LCI(θtrain) values). On average, DR increased for both positive and negative values of LCI(θtrain) (both F-tests of regression slope, p<0.001) indicating that the direction of the motion training stimulus can influence the direction selectivity of cortical neurons even in regions that start with a neighborhood bias for the direction opposite the training stimulus. Moreover, Figure 4a shows that the effectiveness of the motion training stimulus in altering the DR of cortical neurons varied as a function of the initial neighborhood bias. A regression line fit to the data shows that the greatest changes in DR were found in cortical regions that exhibited the strongest initial neighborhood bias towards the training stimulus, and the weakest effects were found in the regions that exhibited the strongest initial bias towards the direction opposite the training stimulus.
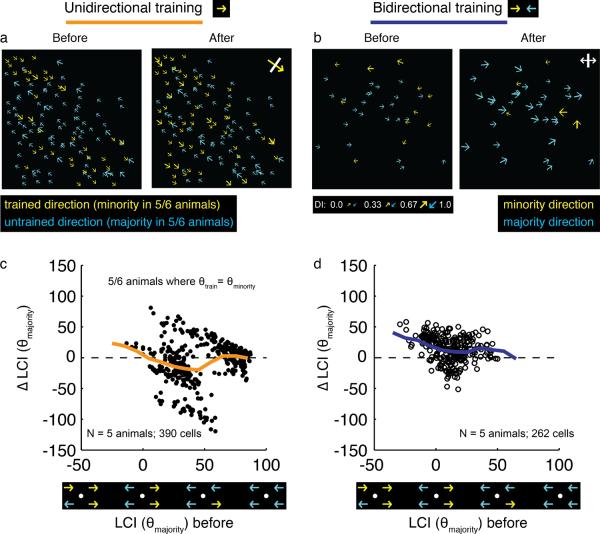
Figure 4.
Single direction training alters the local neighborhood bias so that it becomes more biased for the stimulated direction. a) Imaging field before and after unidirectional training; yellow color indicates training direction, while blue indicates opposite direction; for 5/6 animals, the yellow corresponds to the minority direction, while blue corresponds to majority direction. b) Imaging field before and after bidirectional training; yellow indicates the field's minority direction, blue indicates the field's majority direction. c, d) Changes in local coherence with respect to the majority direction with unidirectional training (c) bidirectional training (d). Thick lines are sliding means. In (c), only the 5 animals that were trained with the minority direction are plotted. In unidirectional training, the local neighborhood of most cells shifted away from the bias present at the onset of training and towards the direction that was used for training (sign test, p<0.001). By contrast, in bidirectional training, the local neighborhood of most cells shifted towards the bias that was present at the onset of training (sign test, p<0.001).
These results suggest that both the initial neighborhood bias and the direction of the training stimulus play instructive roles in determining the direction preference that neurons acquire. But is experience with a single direction of motion capable of eroding an initial neighborhood bias for the direction opposite the training stimulus and replacing it with a neighborhood bias that matches the training stimulus? To address this question, we calculated the change in local coherence with respect to the prevailing majority direction (that is, the direction to which the imaging field as a whole was initially biased), for the 5 of 6 animals that were stimulated with the minority direction. Training with a unidirectional motion stimulus resulted in changes in local coherence values that were, on average, negative (Figure 4c; sign test, p<0.001), indicating that the neighborhood biases were moving away from the initial values and towards the direction specified by the training stimulus. This result is in contrast to results obtained with bidirectional training in our previous study (Li et al. 2008). Training with a bidirectional motion stimulus resulted in changes in local coherence values that were positive, indicating that, on average, initial neighborhood biases were strengthened as a result of the training (Figure 4d; sign test, p<0.001). Thus, at least in cortical areas with a modest initial bias, experience with a single direction of motion for 3–6 hours appears to be sufficient to override the initial neighborhood bias and increase the number of neighboring neurons that prefer the direction of the training stimulus.
Discussion
These results show that experience-independent mechanisms have the capacity to build weak direction-selective responses and the neighborhood biases that predict the future structure of direction columns in ferret visual cortex. But the impact of these experience-independent mechanisms in determining the direction preference that a neuron will acquire is limited. A small amount of experience with a single direction of motion is sufficient to induce a selective increase in the response of cortical neurons to the direction of the training stimulus, regardless of their initial direction bias. Moreover, single direction training can override weak neighborhood biases for the direction opposite the training stimulus, suggesting that columnar identity is not fixed by events that precede eye opening.
An experience-independent seed
The fact that weak directional biases emerge in cortical neurons without the need for visual experience, and that these biases are shared by nearby neurons is consistent with a number of studies showing that there are orientation- and direction-selective responses at the onset of visual experience (Hubel and Wiesel, 1963, Blakemore and Van Sluyters, 1975, Daw and Wyatt, 1976, Fregnac and Imbert, 1978, Krug et al., 2001), but the number of neurons displaying these features and the selectivity of the responses are far less than that found following several weeks of visual experience. The degree of direction selectivity, and the strength of the neighborhood relations are far less in dark-reared animals compared to animals that were normally reared. Given the rapid impact of visual experience on the maturation of direction selectivity, it is likely that this difference reflects the absence of some facilitating effect of light through closed (Krug et al., 2001, Akerman et al., 2002) or open lids. Whether light at this early stage serves a permissive role (perhaps by altering levels of activity or gene expression) or an instructive role (providing spatiotemporal cues relevant for motion direction) remains unclear. Nevertheless, both the property of direction selectivity and the seed for its arrangement in a columnar fashion have their origin in developmental processes that do not require visual experience.
The mechanisms that are responsible for guiding the experience-independent emergence of direction selectivity in cortical circuits remain to be determined. Endogenous patterns of neuronal activity that impinge on, or originate in, cortical circuits could share some of the instructive properties of visually driven activity. Activity patterns that have the characteristics of waves (Huberman et al., 2008) could serve an instructive role in building weak directional biases, as has been suggested in computational models with spike-timing dependent plasticity (STDP) (Wenisch et al., 2005).
Alternatively, the weak direction selectivity that is apparent without visual experience could arise in retina and be relayed to cortex via the lateral geniculate nucleus (LGN). Although cortical direction selectivity is generally regarded as an emergent property of cortical circuits, recent studies in mouse have shown that a class of direction-selective retinal ganglion cells (DSRGCs) sends its axons to the LGN in this species (Huberman et al., 2009), and strongly direction-selective neurons have been described in rabbit and mouse LGN (Swadlow and Weyand, 1985, Kaye et al., 2011). Moreover, direction-selective responses in retinal ganglion cells emerge without the need for visual experience (Daw and Wyatt, 1974, Chan and Chiao, 2008, Elstrott et al., 2008). Whether there are comparable classes of DSRGCs in carnivores, and whether these neurons project to LGN, remain unclear. But even if DSRGCs project to LGN in ferret, the fact that cortical direction selectivity is so immature at the onset of experience, and fails to mature without visual experience suggests that DRGCs cells can provide, at most, the initial seed upon which cortical circuits elaborate direction selectivity under the influence of visual experience. In mouse visual cortex, however, direction-selective responses are present at eye opening, and are unaffected by dark rearing, leaving open the possibility of a more significant contribution of DRGCs to cortical direction selectivity in this species (Rochefort et al., 2011).
Although we have argued that the initial neighborhood biases that are present at eye opening (and survive dark rearing) are the initial seed for the emergence of mature direction-selective responses, we were unable to show that the spatial pattern of direction selectivity in dark-reared animals could predict the effects induced by motion training. The failure to see training effects is likely due to a difference in the amount of time required to reach detectable changes in direction selectivity in dark-reared animals, since animals dark-reared until eye opening go on to acquire mature levels of direction selectivity if they are exposed to light-rearing conditions in the next 2 weeks of development (Li et al., 2006). The levels of local coherence at the start of motion training were significantly lower in the dark-reared animals than in the light-reared animals, and this difference alone might impact the time it takes for training effects to reach detectable levels. A more intriguing possibility is that dark rearing could have substantial effects on the maturation of the circuit elements that are critical for activity-dependent plasticity. Similar effects of dark rearing have been described for ocular dominance plasticity (Cynader and Mitchell, 1980, Mower et al., 1981, Morales et al., 2002, Pizzorusso et al., 2002, Sugiyama et al., 2008 Maffei, 2008, 4377–84).
Unidirectional training
The results of the unidirectional training experiments described here are consistent with previous experiments in tadpole tectum (Engert et al., 2002) and cat visual cortex (Daw and Wyatt, 1976). The vast majority of neurons recorded from the cat following unidirectional rearing responded preferentially to the experienced direction of motion. These experiments were conducted over a greater length of time (several weeks) and were initiated after direction-selective responses had benefited from early visual experience. Importantly, these experiments could not distinguish whether the effects were due to the loss of responses from neurons that originally responded preferentially to the direction opposite the experienced direction (selection) or a shift in the direction preference of neurons so that they now responded preferentially to the training stimulus (instruction). Indeed, what has limited all previous efforts to understand how the immature response properties present at the onset of experience are guided to maturity by visual experience is the inability to track the properties of individual neurons before and after training. The present results provide compelling evidence that short periods of unidirectional training early in development selectively increase the response of neurons to the training stimulus. Moreover, the effects of motion training are qualitatively similar for the neurons that initially preferred the training direction as well as those that initially preferred the opposite direction, indicating that exposure to a single motion training stimulus is capable of shifting the direction preference of cortical neurons towards the direction of the training stimulus.
While increased selectivity for the training stimulus was found for cells that initially preferred the training stimulus and those that preferred the opposite direction, the impact of single direction training was correlated with the strength and sign of the cell's initial neighborhood bias. Furthermore, the strongest effects were found in regions where neighboring neurons preferred the training direction, and the weakest effects were found in regions where neighboring neurons preferred the direction opposite the training stimulus. Neurons that reside in neighborhoods that are rich in neurons that respond preferentially to the training stimulus may have more correlated spike trains, and large numbers of stimulus driven spikes could tend to reinforce the preference for the training direction of motion. Conversely, neurons in neighborhoods that strongly prefer the opposite direction will have weaker responses to the training stimulus and may have fewer opportunities for correlated activity that would promote synaptic strengthening.
While the initial neighborhood bias appears to influence the impact that unidirectional training has on direction selectivity, unidirectional training also impacts neighborhood bias. With bidirectional training, the initial neighborhood bias is most likely to prevail; but with unidirectional training, neighborhoods that are weakly biased against the training stimulus shift toward the direction of the training stimulus. Thus, with a few hours of experience, asymmetrical training is capable of overriding the initial neighborhood bias leading to an expansion in the representation of the training stimulus. What remains unclear is whether more of cortex would be dominated by neurons that prefer the trained direction, if training were continued for a longer period of time. Other studies that have examined the impact of rearing animals with lid suture or with exposure to a single orientation have documented the increase in area or number of cells that respond preferentially to the “experienced” condition; but they also show that even long periods of deprivation do not erase the representation of the deprived stimulus (Blakemore and Van Sluyters, 1975, Daw and Wyatt, 1976, Fregnac and Imbert, 1978, Mrsic-Flogel et al., 2007). This may depend on the maturational state of cortical circuits at the onset of deprivation, and/or the tuning bandwidth of the “deprived” neurons (i.e., the degree to which they are responsive to the rearing stimulus). Further studies employing genetically-encoded calcium sensors will be necessary to establish the full potential of visually driven activity to determine direction preferences of cortical neurons.
Acknowledgements
We thank Julie Heiner for technical assistance and Rebekah Corlew and the other members of the Fitzpatrick lab for helpful discussions. This work was supported by grants from the Whitehall Foundation to LEW and the US National Institutes of Health to DF and SDV.
Footnotes
Contributions: SDV, YL, MC, LEW, and DF designed the experiments; YL and MC performed the experiments; SDV, YL, MC, and GS analyzed the data. DF, SDV, YL, and MC wrote the paper with comments from all authors.
References Cited
- Akerman CJ, Smyth D, Thompson ID. Visual experience before eye-opening and the development of the retinogeniculate pathway. Neuron. 2002;36:869–879. doi: 10.1016/s0896-6273(02)01010-3. [DOI] [PubMed] [Google Scholar]
- Blakemore C, Van Sluyters RC. Innate and environmental factors in the development of the kitten's visual cortex. J Physiol. 1975;248:663–716. doi: 10.1113/jphysiol.1975.sp010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Carandini M, Ferster D. Membrane potential and firing rate in cat primary visual cortex. J Neurosci. 2000;20:470–484. doi: 10.1523/JNEUROSCI.20-01-00470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YC, Chiao CC. Effect of visual experience on the maturation of ON-OFF direction selective ganglion cells in the rabbit retina. Vision Research. 2008;48:2466–2475. doi: 10.1016/j.visres.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Cynader M, Mitchell DE. Prolonged sensitivity to monocular deprivation in dark-reared cats. J Neurophysiol. 1980;43:1026–1040. doi: 10.1152/jn.1980.43.4.1026. [DOI] [PubMed] [Google Scholar]
- Daw NW, Wyatt HJ. Raising rabbits in a moving visual environment: an attempt to modify directional sensitivity in the retina. The Journal of physiology. 1974;240:309–330. doi: 10.1113/jphysiol.1974.sp010612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw NW, Wyatt HJ. Kittens reared in a unidirectional environment: evidence for a critical period. The Journal of physiology. 1976;257:155–170. doi: 10.1113/jphysiol.1976.sp011361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstrott J, Anishchenko A, Greschner M, Sher A, Litke AM, Chichilnisky EJ, Feller MB. Direction selectivity in the retina is established independent of visual experience and cholinergic retinal waves. Neuron. 2008;58:499–506. doi: 10.1016/j.neuron.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engert F, Tao HW, Zhang LI, Poo MM. Moving visual stimuli rapidly induce direction sensitivity of developing tectal neurons. Nature. 2002;419:470–475. doi: 10.1038/nature00988. [DOI] [PubMed] [Google Scholar]
- Fregnac Y, Imbert M. Early development of visual cortical cells in normal and dark-reared kittens: Relationship between orientation selectivity and ocular dominance. J Physiol. 1978;278:27–44. doi: 10.1113/jphysiol.1978.sp012290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregnac Y, Imbert M. Development of neuronal selectivity in primary visual cortex of cat. Physiol Rev. 1984;64:325–434. doi: 10.1152/physrev.1984.64.1.325. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields of single neurones in the cat's striate cortex. J Physiol. 1959;148:574–591. doi: 10.1113/jphysiol.1959.sp006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive Fields Of Cells In Striate Cortex Of Very Young, Visually Inexperienced Kittens. J Neurophysiol. 1963;26:994–1002. doi: 10.1152/jn.1963.26.6.994. [DOI] [PubMed] [Google Scholar]
- Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Wei W, Elstrott J, Stafford BK, Feller MB, Barres BA. Genetic identification of an On-Off direction-selective retinal ganglion cell subtype reveals a layer-specific subcortical map of posterior motion. Neuron. 2009;62:327–334. doi: 10.1016/j.neuron.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye AP, Marshel JH, Callaway EM. Society for Neuroscience. Washington, D.C.: 2011. Direction selectivity in the mouse LGN revealed by in vivo two-photon calcium imaging; p. 269.205. [Google Scholar]
- Krug K, Akerman CJ, Thompson ID. Responses of neurons in neonatal cortex and thalamus to patterned visual stimulation through the naturally closed ids. J Neurophysiol. 2001;85:1436–1443. doi: 10.1152/jn.2001.85.4.1436. [DOI] [PubMed] [Google Scholar]
- Li Y, Fitzpatrick D, White LE. The development of direction selectivity in ferret visual cortex requires early visual experience. Nat Neurosci. 2006;9:676–681. doi: 10.1038/nn1684. [DOI] [PubMed] [Google Scholar]
- Li Y, Van Hooser SD, Mazurek M, White LE, Fitzpatrick D. Experience with moving visual stimuli drives the early development of cortical direction selectivity. Nature. 2008;456:952–956. doi: 10.1038/nature07417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales B, Choi SY, Kirkwood A. Dark rearing alters the development of GABAergic transmission in visual cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:8084–8090. doi: 10.1523/JNEUROSCI.22-18-08084.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mower GD, Berry D, Burchfiel JL, Duffy FH. Comparison of the effects of dark rearing and binocular suture on development and plasticity of cat visual cortex. Brain Research. 1981;220:255–267. doi: 10.1016/0006-8993(81)91216-6. [DOI] [PubMed] [Google Scholar]
- Mrsic-Flogel TD, Hofer SB, Ohki K, Reid RC, Bonhoeffer T, Hübener M. Homeostatic regulation of eye-specific responses in visual cortex during ocular dominance plasticity. Neuron. 2007;54:961–972. doi: 10.1016/j.neuron.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Ohki K, Chung S, Ch'ng YH, Kara P, Reid RC. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature. 2005;433:597–603. doi: 10.1038/nature03274. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision. 1997;10:437–442. [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- Press WH, Flannery BP, Teukolsky SA, Vetterling WT. Numerical recipes in C. Cambridge University Press; Cambridge: 1992. [Google Scholar]
- Ringach DL, Shapley RM, Hawken MJ. Orientation selectivity in macaque V1: diversity and laminar dependence. J Neurosci. 2002;22:5639–5651. doi: 10.1523/JNEUROSCI.22-13-05639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort NL, Narushima M, Grienberger C, Marandi N, Hill DN, Konnerth A. Development of direction selectivity in mouse cortical neurons. Neuron. 2011;71:425–432. doi: 10.1016/j.neuron.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Stosiek C, Garaschuk O, Holthoff K, Konnerth A. In vivo two-photon calcium imaging of neuronal networks. Proc Natl Acad Sci U S A. 2003;100:7319–7324. doi: 10.1073/pnas.1232232100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama S, Di Nardo AA, Aizawa S, Matsuo I, Volovitch M, Prochiantz A, Hensch TK. Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell. 2008;134:508–520. doi: 10.1016/j.cell.2008.05.054. [DOI] [PubMed] [Google Scholar]
- Swadlow HA, Weyand TG. Receptive-field and axonal properties of neurons in the dorsal lateral geniculate nucleus of awake unparalyzed rabbits. J Neurophysiol. 1985;54:168–183. doi: 10.1152/jn.1985.54.1.168. [DOI] [PubMed] [Google Scholar]
- Wenisch OG, Noll J, Hemmen JL. Spontaneously emerging direction selectivity maps in visual cortex through STDP. Biol Cybern. 2005;93:239–247. doi: 10.1007/s00422-005-0006-z. [DOI] [PubMed] [Google Scholar]