Abstract
The accumulation of acetylcholine receptors (AChRs) at nerve terminals is critical for signal transmission at the neuromuscular junction, and rapsyn is essential for this process. Previous studies suggest that AChRs might direct rapsyn self-clusters to the synapse. In vivo experiments with fluorescently tagged AChR or rapsyn in zebrafish larvae revealed that rapsyn self-clusters separate from AChRs did not exist before synapse formation. Examination of rapsyn in the AChR-less mutant sofa potato revealed that rapsyn in the absence of AChR was localized in the Golgi complex. Expression of muscle-type AChR in sofa potato restored synaptic clustering of rapsyn, while neuronal type AChR had no effect. To determine if this requirement of protein interaction is reciprocal, we examined the mutant twitch once, which has a missense mutation in rapsyn. While the AChRs distributed non-synaptically on the plasma membrane in twitch once, mutant rapsyn was retained in the Golgi complex. We conclude that AChRs enable the transport of rapsyn from the Golgi complex to the plasma membrane through a molecule-specific interaction.
Introduction
Neuromuscular junctions (NMJs) have historically provided a framework for studying synapse formation in the nervous system (Sanes and Lichtman, 2001). Molecules involved in NMJ formation include MuSK (DeChiara et al., 1996), rapsyn (Burden, 1985), agrin (Reist et al., 1992), dok-7 (Okada et al., 2006), and lrp4 (Kim et al., 2008). The mechanism of their interaction, however, still remains unresolved. Zebrafish larvae provide an excellent system to observe synapse formation in vivo (Lefebvre et al., 2007), due to their transparent body and rapid, ex-utero development. At 1 day post fertilization (dpf), focal synapses (FS) form in the center of individual muscle cells. As the embryo matures, non-focal synapses (NFS) develop, which are distributed in multiple locations of a single cell, including the myotendinous edge. Studies using MuSK mutants and morpholinos against Wnt signaling molecules show that FSs depend on Wnt – MuSK signaling (Jing et al., 2009). Thus the molecular signaling cascade in FSs is similar to that of mammalian NMJs.
Among factors involved in the formation of NMJs, AChRs were initially considered passive players, receiving directions from upstream molecules in the signaling cascade. However, studies with zebrafish suggest that AChRs localize rapsyn to the synapse (Ono et al., 2001; 2004). In a zebrafish mutant sofa potato, which harbors a mutation in the AChR δ subunit, AChR pentamers fail to reach the plasma membrane of myocytes. In these mutants, rapsyn clusters were absent at the synapse. When wild type δ subunit was expressed in mutant muscle cells, the synaptic localization of rapsyn was restored. Active roles of AChRs in synapse formation have also been proposed for other systems including cultured myotubes (Marangi et al., 2001; Bruneau et al., 2008), C.elegans (Gally et al., 2004), and AChR α1 subunit knockout mouse (An et al., 2010). Initial self-clustering of rapsyn, on the other hand, is supposed to occur independent from AChRs. When expressed in fibroblasts without co-transfection of AChRs, rapsyn self-clusters were observed (Phillips et al., 1991a; Apel et al., 1995). In sofa potato zebrafish, clusters of exogenously-introduced rapsyn-GFP were observed extra-synaptically (Ono et al., 2001). We therefore hypothesized that in the process of synapse formation, rapsyn self-clusters initially form separate from AChRs, and AChRs localize them to the synapse. To examine this hypothesis, we used stable zebrafish transgenic lines that express AChR or rapsyn, each tagged with fluorescent molecules, and performed time-lapse analysis of synapse formation in wild type or NMJ mutant backgrounds.
Methods
Zebrafish
All adult fish were maintained in stand-alone, self-circulating AHAB systems (Aquatic Ecosystems, Apopka, FL) and Tecniplast systems (Tecniplast USA, West Chester, PA) following the guidelines of the IACUC at NIH/NIAAA. Embryos obtained from crosses of male and female adults were reared and maintained at 28°C. The mutant lines of sofa potato and twitch once, soptj19d and twoth26e, respectively, were described previously (Granato et al., 1996; Ono et al., 2002; 2004). Larvae were used for experiments at stages before their sex was determined.
To observe synapse development in vivo, we used three lines of transgenic zebrafish (Fig. 1A). Muscle cells of a stable fish line tg(α-actin: δ2YFP) (Epley et al., 2008) expressed AChR δ subunit tagged with YFP. The chimera subunit forms pentamers with other AChR subunits. Pentamers containing the AChRδ-YFP subunit were functional, with regard to the protein interaction and the channel gating. Two lines were newly established for this study. Tg(α-actin: rapsynCFP) expressed rapsyn conjugated with CFP in muscle cells, and tg(HuC:mCherry) expressed mCherry in all neurons. Tg(α-actin: δ2YFP), tg(α-actin:rapsynCFP) and tg(HuC:mCherry) will be referred to as AChRδ–YFP, rapsyn-CFP and HuC-mCherry hereafter. In addition, tg(α-actin:α7-YFP) was also established (Fig. 5B). The zebrafishα-actin promoter drove the expression of α7-YFP, which was based on the rat α7 gene (Séguéla et al., 1993) and had an insertion of YFP in the III–IV cytoplasmic loop.
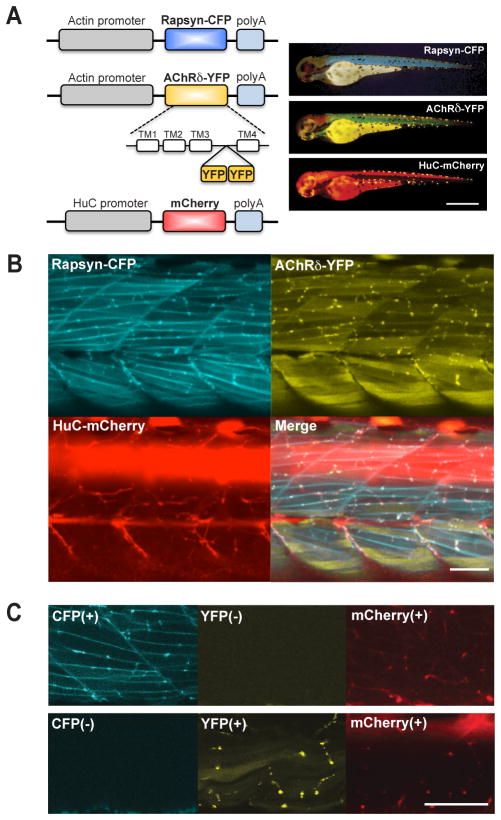
Fig 1.
Fluorescence visualized synaptic proteins. A. A schema of constructs to express CFP, YFP and mCherry (left). Established stable lines were crossed so that a single larva expresses the three transgenes (right). The displayed larva (3 dpf) expressed rapsyn-CFP, AChRδ-YFP and mCherry, and the fluorescence was visualized with corresponding filter cubes. Scale: 500 μm. B. Using confocal microscopy, three signals overlapped in 3 dpf larvae. C. The larva shown in top panels lacked AChRδ-YFP, and the larva in lower panels lacked rapsyn-CFP. Signals corresponding to the missing transgenes were blank, showing the lack of signal bleed-through. Scale: 50 μm in B and C.
Fig 5.
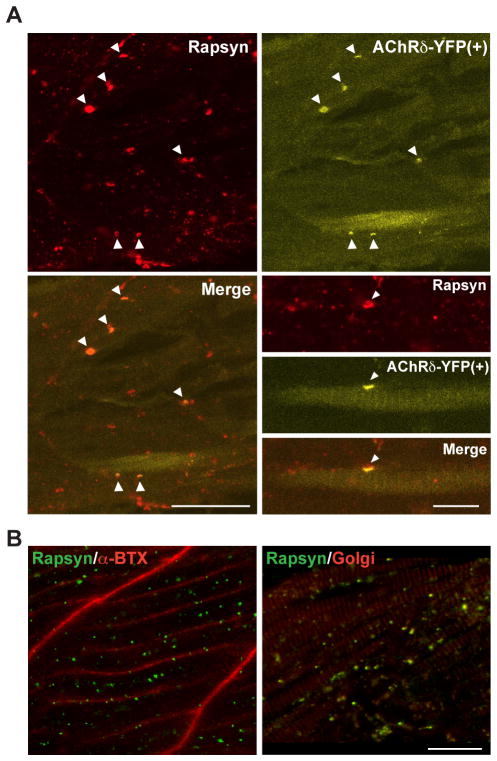
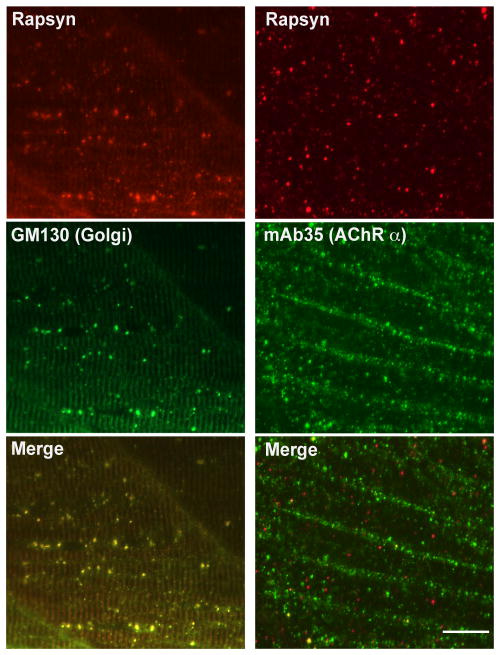
Rescue of rapsyn transport by muscle-type AChRs. A. Expression of AChRδ-YFP in sofa potato mutants restored the membrane distribution of rapsyn. In muscle cells expressing YFP, the distribution of rapsyn visualized by antibody (upper left) and that of AChR visualized by YFP (upper right) co-localized, as indicated by arrowheads. The merged image is shown at the lower left. Note only a subpopulation of muscle cells expressed AChRδ-YFP and formed co-clusters of AChR/rapsyn. Scale: 50 μm. Higher magnification pictures of a single muscle cell expressing YFP are shown at the lower right. Rapsyn and AChR (arrowheads) are co-localized on the plasma membrane. Scale: 20 μm. B. A sofa potato larva expressing α7-YFP in all muscle cells. While α7-YFP is detected on the plasma membrane (α-Btx shown in red; left), rapsyn (green) does not co-localize with α7-YFP but co-localizes with the Golgi marker (GM-130 shown in red; right). Scale: 20 μm
Transgenes were placed in plasmids containing megalinker recognition sequences or Tol2 sequences, and clones were verified by sequencing. Injection of DNA constructs with Meganuclease or transposase into fertilized zebrafish eggs at 1 cell stage was performed as previously described (Ikenaga et al., 2011). Injected embryos were raised to adult and out-crossed to search for germ-line transmission using an Olympus MVX fluorescent stereomicroscope. Embryos used for imaging were heterozygous for a particular transgene. Multiple stable lines were established for each clone. Synapses observed by fluorescence were not qualitatively different between lines. As discussed in the results section, the amount of transgene expression affected the distribution of its product. Even in a single batch of larvae the expression level of a transgene was variable. Therefore we generally selected embryos with the lowest expression level. Stable lines were crossed to generate embryos expressing transgenes in the background of wild type, sofa potato or twitch once.
For stochastic expression of rapsyn, two genes were inserted in a plasmid (Fig. 6). The first gene was EGFP driven by the CMV promoter, and the second was the zebrafish rapsyn gene driven by the zebrafish α-actin promoter.
Fig 6.
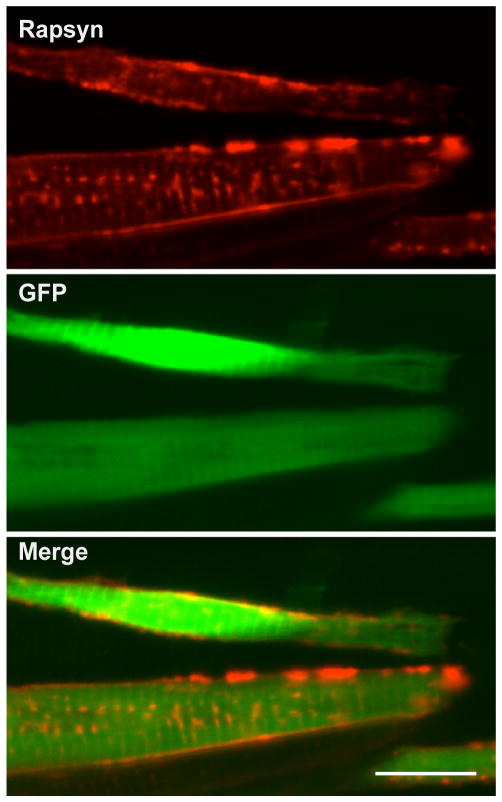
Overexpression of rapsyn enables its transport to the plasma membrane. A sofa potato larva injected with a plasmid expressed rapsyn and cytoplasmic GFP at 3 dpf. Anti-rapsyn antibody staining (red) and GFP signal (green) are shown. Note the distribution of rapsyn on the plasma membrane in GFP (+) cells. Scale: 20μm
Confocal imaging
All images were taken on the Zeiss LSM510 Meta Confocal microscope with a 40X C-Apo objective (N.A.1.2) and analyzed in Photoshop (Adobe Systems, San Jose, CA). For time-lapse imaging, an embryo was anesthetized in egg water containing 0.1g/L MS-222 (Tricaine methanesulfonate, Sigma, St. Louis, MO) for 10 mins before being transferred to a glass-bottom dish. The embryo was observed for 30 mins with a Zeiss LSM510Meta Confocal microscope (Carl Zeiss Microimaging, Thornwood, NY). After observation, the embryo was transferred to MS-222-free egg water. After 1 hr, the voluntary movement of embryos was confirmed before subjecting the embryo to the next round of confocal observation. In order to observe the same area of trunk throughout the time-lapse analysis, we imaged the ventral region of an identical body segment. By scanning through the whole depth of the ventral segment, we were able to identify corresponding areas between rounds of observations depending on the pattern of axon arbors and the muscle cell orientation. For confocal microscopy, rapsyn-CFP, AChRδ– YFP and HuC-mCherry were excited with 458, 514 and 561nm laser lines, respectively. Bandpass filters of 465–510 nm and 520–555 nm were used for the emission of CFP and YFP respectively, and a longpass filter > 576 nm for mCherry. Bleed-through of signals between CFP and YFP signals with the employed optical conditions was not observed (Fig. 1C). Labeling of AChR with α-bungarotoxin was performed as previously described (Ono et al., 2001). Numbers of examined optical regions from multiple larvae are shown as n in the text, and representative images are shown in figures.
Immunostaining
A rabbit anti-rapsyn antibody (Abcam, San Francisco, CA) raised against a synthetic peptide corresponding to the C terminal residues of human rapsyn was used. Antibody specificity for zebrafish rapsyn was confirmed in wild type embryos, which displayed rapsyn clusters in middle regions of muscle cells (Fig. 3A–D). Mouse anti-GM130 antibody (BD Biosciences, Franklin Lakes, NJ) and anti-58K Golgi protein antibody (Abcam) were used to visualize the Golgi complex. GM130 is a 130kDa cis-Golgi matrix protein homologous to the Golgi autoantigen golgin 95 (Fritzler et al., 1993; Nakamura et al., 1995). 58K Golgi protein is located on the microtubule-binding outer surface of the Golgi complex (Bloom and Brashear, 1989). mAb35 (Sigma-Aldrich) is a monoclonal anti-nicotinic acetylcholine receptor antibody which recognizes α subunits including α1 (Tzartos et al., 1981). PDI is a protein disulfide isomerase, primarily located in the ER lumen (Ellgaard and Ruddock, 2005). Mouse anti-PDI antibody was used as an ER marker (BD Biosciences, Franklin Lakes, NJ).
Fig 3.
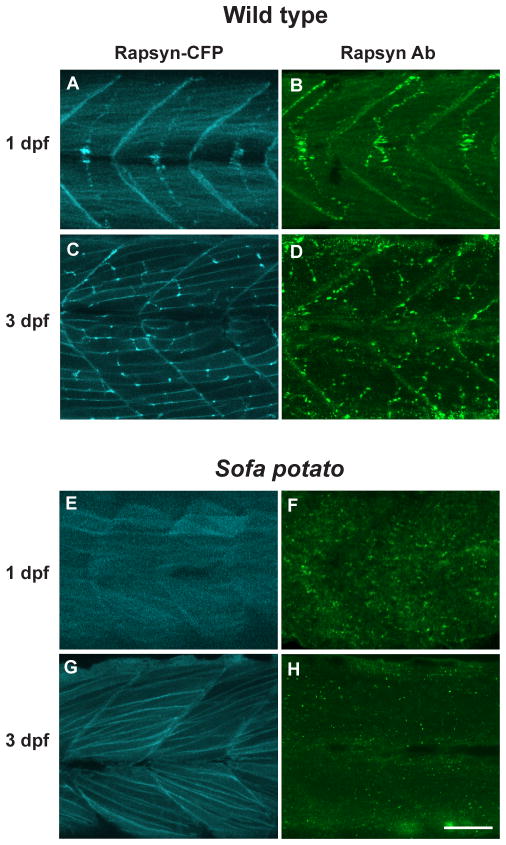
Rapsyn was visualized in a rapsyn-CFP (+) larva with CFP (A, C and E, G) and in native larva stained with rapsyn-antibody (B, D and F, H). The distribution of CFP and anti-rapsyn antibody staining were similar at 1 dpf (A, B) or 3 dpf (C, D). The distribution of rapsyn was also examined in the sofa potato background, with (E, G) or without (F, H) the rapsyn-CFP transgene. Scale: 50μm.
Immunohistochemistry was performed as previously described, with slight modifications (Ono et al., 2001). Briefly, embryos were anesthetized in 0.1g/L MS-222, and incubated in 4% PFA for 4 hrs at 4°C, and were stored in 100% methanol overnight. Heads were removed with a razor blade and embryos were washed with water for 2 min. For blocking, embryos were gently shaken in PBS containing 1% normal goat serum (NGS), 3% bovine serum albumin (BSA) and 0.1% triton X-100 for 30 min, and then incubated in 1:200 rabbit anti-rapsyn antibody, mouse anti-GM130 antibody or anti 58K Golgi protein antibody in PBS containing 1% NGS, 3% BSA and 0.01% triton X-100 overnight at 4°C. Embryos were washed in PBS containing 0.01% triton X-100 for 2 hrs, followed by incubation in 1:200 goat anti-rabbit or anti-mouse secondary antibody (Invitrogen, Carlsbad, CA). After washing in PBS-t for 2 hrs, samples were mounted on glass-covered dishes with Fluoromount-G (Southern Biotech, Birmingham, AL) for imaging.
Results
Time lapse imaging of fluorescence-tagged synaptic molecules at the NMJ
In order to observe synapse development in vivo, we used three lines of transgenic zebrafish, AChRδ-YFP, rapsyn-CFP and HuC-mCherry. YFP, CFP and mCherry signals visualize AChRs, rapsyns and motor neuron axons, respectively. When three transgenes were expressed in a single embryo, fluorescent molecules overlapped in puncta in mature NMJs (n= 10; Fig. 1B). A wide-field observation of these transgenic fish over several days of development displayed an overall pattern of synaptic type transition in agreement with previous studies (Flanagan-Steet et al., 2005; Panzer, 2006; Lefebvre et al., 2007). Briefly, at 1 day post fertilization (dpf) FSs predominate. As the development progresses, muscle cells in shallower layers start to develop NSFs. The distribution of AChRδ-YFP and rapsyn-CFP corroborated previous reports of NMJ formation and therefore validated our method of using fluorescence-tagged molecules. When larvae lacking the expression of YFP or CFP were observed, the channel corresponding to the missing transgene displayed a blank image, suggesting that signal bleed-through between channels did not occur with the employed optical conditions (n= 3; Fig. 1C).
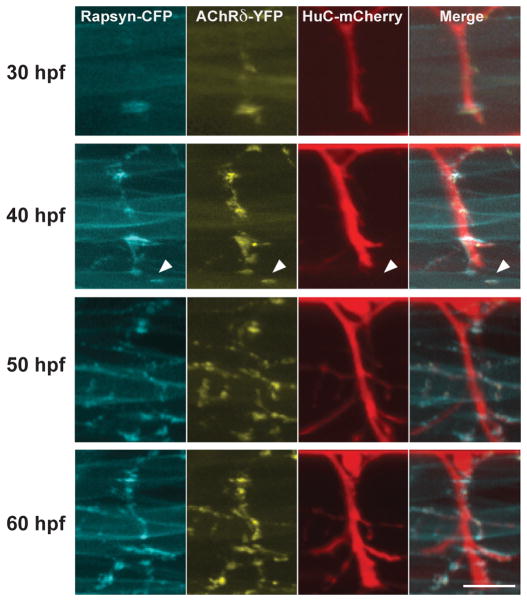
When FSs initially formed, AChR and rapsyn formed clusters before the arrival of neural terminals, as was reported previously in mammalian as well as zebrafish NMJs (Fig. 2). Extending motor neuron axons later reached these pre-clusters. Therefore, the clustering of AChR and rapsyn visualized by tagged fluorescent molecules indeed represented initial stages of synapse formation. In this time frame, we examined whether we could observe rapsyn clusters before their association with AChRs. Contrary to our expectation, the timing of rapsyn-CFP cluster formation always coincided with that of AChRδ-YFP, and we did not observe rapsyn-CFP self-clusters without AChRδ-YFP in developing myocytes (n= 8).
Fig 2.
Time-lapse imaging of NMJ formation. 3D reconstructed images of a single larva at 30, 40, 50 and 60 hpf. Arrowheads indicate a pre-cluster of rapsyn-CFP and AChRδ-YFP. Scale: 20 μm.
Rapsyn without AChRs were retained in the Golgi complex
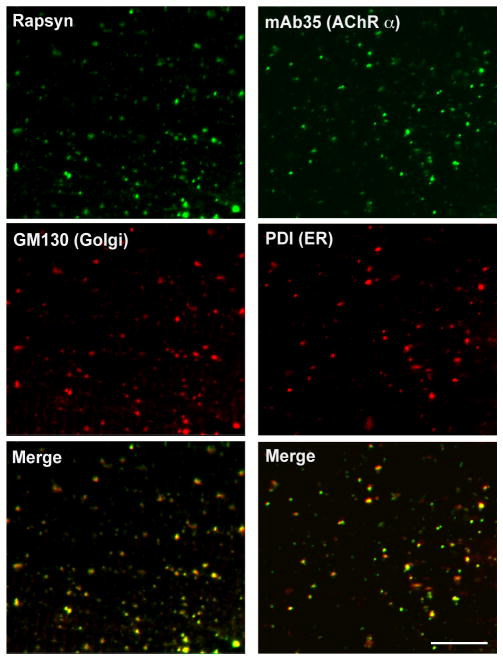
Because we did not observe AChR-less rapsyn clusters in normal development, we examined whether we could observe rapsyn-CFP self-clusters in the AChR-less mutant, sofa potato. Sofa potato lacks expression of AChR on the muscle cell surface due to a missense mutation in the δ subunit (Ono et al., 2004). Therefore we examined rapsyn-CFP distribution in the sofa potato background. Unexpectedly, its distribution was diffuse on the plasma membrane and clustering was minimal at both 1 dpf and 3 dpf (n= 4; Fig. 3E&G). In contrast, rapsyn displayed a FS-like distribution at 1 dpf (Fig. 3A) and NFS-like distribution at 3 dpf (n= 4; Fig. 3C) in wild type larvae, as expected. Suspecting that over-expression or addition of CFP to rapsyn may have disrupted the native distribution of rapsyn in sofa potato mutants, we visualized endogenous rapsyn with a rapsyn-specific antibody. In these larvae, small accumulations of rapsyn were observed (n= 4; Fig. 3F&H). Based on previous reports that rapsyn localized partially to the Golgi complex (Marchand et al., 2002; Gervásio and Phillips, 2005), we examined whether these accumulations represent the Golgi complex by double staining with the Golgi specific antibody GM130. The staining of rapsyn in sofa potato larvae overlapped with that of the GM130 (n= 5; Fig. 4, left panels). This result was further confirmed by using another Golgi marker, 58K Golgi (data not shown).
Fig 4.
Rapsyn and AChR subunit in sofa potato. A 3 dpf sofa potato larva was stained with anti-rapsyn antibody and anti-GM130 antibody (left panels). Signals overlapped, as shown in the merged image. A 3 dpf sofa potato larva was stained with the mAb35 and anti-PDI antibody (right panels). Signals overlapped, as shown in the merged image. Scale: 20μm.
In sofa potato, AChR subunits other than the δ subunit; α1, β1b, γ and ε, (Mongeon et al., 2011) are expected to remain at stages where their assembly is obstructed (Green and Millar, 1995). We examined the localization of AChR subunits using mAb35, which binds to the α1 subunit of the muscle nicotinic acetylcholine receptor. Most of mAb35 staining colocalized with ER specific anti PDI staining in sofa potato (n= 3; Fig. 4, right panels). Thus the failure of assembly in sofa potato leads to the retention of AChR subunits in the ER, and rapsyn retained in the Golgi complex lacks interaction with AChR (Fig. 9).
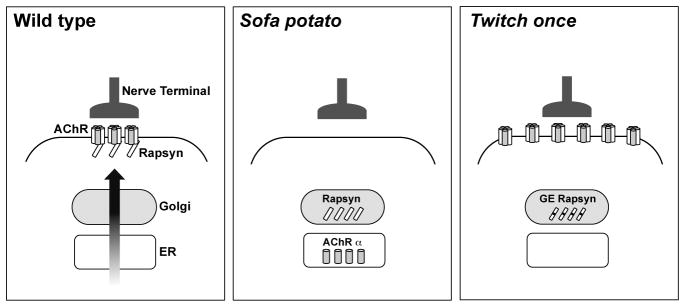
Fig 9.
A schema of the neuromuscular junction in wild type, sofa potato and twitch once backgrounds. Note that over-expression changes the distribution, and over-expressed proteins are not shown in this schema. Only AChR α subunits are shown in sofa potato, which mAb35 visualized. GE rapsyn designates rapsyn with G130E mutation. The distribution of AChR and rapsyn displayed distinct patterns, revealing the role of their interactions in the proper trafficking.
Recovery of rapsyn transport by expression of muscle-type AChR
Sofa potato mutants harbor a point mutation (L28P) near the N-terminus of the AChR δ subunit, and muscle-specific expression of wild type δ subunit tagged with YFP, δ2YFP, led to improved escape behavior (Epley et al., 2008). In order to examine whether this process involves the recovery of rapsyn transport to the synapse, wild type AChRδ-YFP was transiently introduced in sofa potato mutant larvae. In larvae displaying partial recovery of behavior, immunostaining was performed and rapsyn was localized on the plasma membrane in association with the AChR (n= 3; Fig. 5A).
In order to examine the specificity of AChR in the rapsyn transport, we expressed an AChR subunit not natively expressed in the muscle cell. α7 subunits are expressed widely in the central nervous system, and they form homopentamers without the need of additional subunits (Dani and Bertrand, 2007). In sofa potato larvae expressing α7YFP, rapsyn failed to reach the plasma membrane even though α7YFP was found on the plasma membrane (n= 3; Fig. 5B). This result is in contrast to the heterologous expression of δ2YFP in sofa potato (Fig. 5A) and suggests that the effect of AChR on the transport of rapsyn is highly specific and muscle–type AChRs are required for the transport of rapsyn from the Golgi complex to the plasma membrane.
The unexpected membrane distribution of rapsyn-CFP in sofa potato (Fig. 3E&G) may be caused by a different mechanism because AChR subunits in these larvae are retained in the ER and do not reach the plasma membrane. The change of rapsyn molecular structure by the addition of CFP may have affected its transport, or the membrane localization of rapsyn-CFP may result from the increased expression of rapsyn driven by the strong α-actin promoter. In order to test these two possibilities, we over- expressed rapsyn without a fused fluorescent moiety in the sofa potato background. The injected plasmid contained two genes: GFP, driven by the CMV promoter, and rapsyn, driven by the α-actin promoter. Muscle cells that over-expressed rapsyn, as evidenced by the co-expression of cytosolic GFP, displayed rapsyn protein on the plasma membrane (n= 4; Fig. 6). This result shows that rapsyn, when over-expressed, can reach the plasma membrane without AChRs.
AChRs do not require interaction with rapsyn for their transport
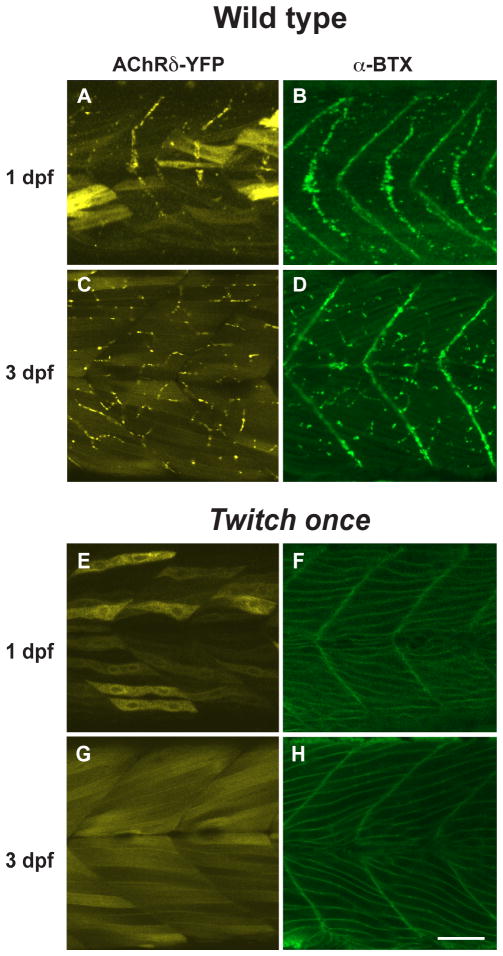
The interaction between AChRs and rapsyn molecules may also be necessary for the AChRs to exit the Golgi complex or ER. To test this potential reciprocity, we examined the rapsyn mutant, twitch once. Rapsyn is non-functional in twitch once larvae due to a G130E mutation in the 4th tetratricopeptide repeat (TPR) domain (Ono et al., 2002). While the high expression level of AChRδ-YFP in the cytoplasm obscured the distribution of YFP on the plasma membrane, staining with α-BTX of non-permeabilized muscle cells clearly visualized AChRs on the plasma membrane of twitch once larvae (n= 4; Fig 7E–H). Though rapsyn protein in twitch once harbors a missense mutation G130E, the rapsyn antibody still recognizes the mutant rapsyn because its epitope is near the C-terminus. Staining with the rapsyn antibody revealed that the G130E rapsyn was localized in the Golgi complex (n= 3; Fig. 8, left panels). Separate distributions of AChR and rapsyn, AChR on the plasma membrane and rapsyn in the Golgi complex, suggest that the interaction between them is lost due to the G130E mutation. The separate distribution was further confirmed with mAb35 antibody staining (n= 3; Fig. 8, right panels). Therefore, AChRs can reach the plasma membrane independent of interaction with rapsyn. Once on the plasma membrane, however, AChRs do require rapsyn for their synaptic localization because the synaptic localization of AChR was absent in the twitch once mutant (Fig. 7E–H). Rapsyn therefore plays a role in synapse formation only after AChRs reach the plasma membrane (Fig. 9).
Fig 7.
The distribution of AChR was observed in AChRδ-YFP (+) larva with YFP (A, C and E, G) and in native larvae stained with α-Btx (B, D and F, H) in the wild type (A–D) or the twitch once background (E–H). While the YFP molecule filling the cytoplasm obscures the AChRδ-YFP distribution in twitch once (E, G), the membranous distribution of AChRs is obvious with α-Btx (F, H), which stains only assembled pentamers. Scale: 50μm.
Fig 8.
Immunostaining with the anti-GM130 antibody and the anti-rapsyn antibody showed that G130E rapsyn localized to the Golgi complex in a twitch once larva (left panels). G130E rapsyn and AChR were visualized by anti-rapsyn antibody and the mAb35 (right panels). Overlap of signals was minimal. Scale: 20μm
Discussion
In this study, we used transgenic / mutant zebrafish and revealed the mechanism of AChR / rapsyn transport from the Golgi complex to the plasma membrane. Rapsyn has been proposed to interact with various proteins at the NMJ (Apel et al., 1995; Antolik et al., 2006; 2007; Chen et al., 2007; Zhang et al., 2007; Borges et al., 2008; Luo et al., 2008), and it is well established that rapsyn is critical for the high-density accumulation of AChRs at nerve terminals. Biochemical analyses have shown that AChRs and rapsyn are co-localized within distal exocytic routes and sorting/targeting are mediated by the lipid raft microdomain (Marchand et al., 2000; 2002). In torpedo electrocytes and COS-7 cells, rapsyn and AChRs were found in the same post-Golgi vesicles and co-transported to the plasma membrane. This implies that the interaction of AChR and rapsyn may start early in the transport process and their interaction may play an essential role. We show here that rapsyn molecules require AChRs to exit the Golgi complex, demonstrating for the first time that this molecular interaction is required for proper trafficking to the plasma membrane. We further show that the requirement is not reciprocal, i.e. rapsyn exerts its effect on AChR only after the post-Golgi vesicle fuses with the plasma membrane.
It has been generally considered that rapsyn first forms self clusters and receptor clustering happens subsequently through its binding to rapsyn (Ramarao and Cohen, 1998). This hypothesis was mainly based on comparison of fibroblasts transfected with AChR, rapsyn, or both (Phillips et al., 1991a). In spite of some studies suggesting earlier interaction of rapsyn and AChR in the transport pathway (Marchand et al., 2000; 2002), this schema has been widely accepted. In zebrafish we also reported previously that the expression of rapsyn-GFP in sofa potato, which was the only method to visualize rapsyn in zebrafish due to the lack of a good rapsyn-specific antibody, led to extra-synaptic clusters, some of which were on the plasma membrane (Ono et al., 2001; 2004). Based on this finding and the conventional model of rapsyn self-clusters on the plasma membrane, we proposed that AChRs direct rapsyn clusters to the synapse. We now showed that the membranous distribution of rapsyn in the absence of AChRs occurs through its over-expression (Fig. 6). In light of current findings, we still propose that AChRs localize rapsyn to the synapse. However, they do so by allowing rapsyn to exit the Golgi complex, rather than by directing rapsyn self-clusters.
Rapsyn is a cytoplasmic protein whose linkage to the plasma membrane is mediated by myristoylation at the N-terminus (Phillips et al., 1991b). It contains several protein motifs: TPR domains, a coiled-coil domain and a RING-H2 domain (Ramarao and Cohen, 1998). Mutagenesis studies suggested that the TPR domains mediate self-clustering while the coiled-coil domain causes the clustering of AChR by binding to its cytoplasmic loop (Ramarao, 2000; Borges et al., 2008). This view, based mainly on heterologous cell systems, may require re-evaluation in view of our current data and other studies. In the absence of AChRs, we did not obtain evidence that rapsyn formed self-clusters except that they localize to the Golgi complex and are represented as intracellular puncta (Fig. 3&4). When rapsyn molecules reach the plasma membrane by over-expression, on the other hand, they can form self-clusters. The distribution of rapsyn-GFP in sofa potato exhibited clusters (Ono et al., 2001) though rapsyn-CFP seemed more diffuse on the plasma membrane (Fig. 3E&G). This difference may result from the expression levels of the transgene, or the protein structure by addition of different fluorescent molecules. In either case, it is questionable whether these self-clusters represent the intrinsic nature of rapsyn. It is also possible that some of the extra-synaptic rapsyn clusters observed in previous studies, from our group as well as others, may have been in the Golgi complex rather than on the plasma membrane. Also, the distinct distributions of AChR and G130E rapsyn suggest that the interaction between rapsyn and AChRs was lost due to a mutation in the 4th TPR. The loss of interaction between rapsyn and AChR was also reported in rapsyn mutations of human population, where rapsyn missense mutations identified in congenital myasthenic families in TPR1, 3 and 5, led to reduced co-clustering of rapsyn and AChR in cell culture systems (Ohno et al., 2002). The global structure of rapsyn may be changed due to these mutations, and rapsyn functions may not be as modular as initially considered.
It is intriguing that the distribution of rapsyn is strongly affected by its expression level. In sofa potato, wild type rapsyn exits the Golgi complex only when it is over-expressed (Fig. 6). Conversely, G130E rapsyn in twitch once does not exit the Golgi complex (Fig. 8), but with over-expression it can reach the plasma membrane and make small non-synaptic clusters (Ono et al., 2002). When proteins are over-expressed in general, interactions with other molecules can be affected, complicating the interpretation of results. Rapsyn changes its distribution dramatically depending on its expression level. We are not aware of another protein whose over-expression alone enables its transport from the Golgi complex to the plasma membrane. Unless its expression level is strictly controlled, such as in a knock-in system, the interpretation of rapsyn mutagenesis studies will be difficult. Different levels of rapsyn expression may partially explain inconsistent findings in the literature. Certain molecules are known to localize to the Golgi complex, such as enzymes whose functions are related to the glycosylation, which takes place in the Golgi complex. Golgi retention signals of the enzymes, GlcNAcTI, GalT or ST, have been studied extensively and assigned to transmembrane regions (Colley, 1997; Opat et al., 2001). However the transmembrane regions do not share high homologies, and the retention mechanism is not clear. Two hypotheses were based on studies of such glycosyltransferases. The first is that transmembrane sequences of certain membrane proteins are too short to enter cholesterol-rich transport vesicles, and a second proposes that oligomerization of proteins make them incompatible with transport vesicles. Results in this paper may be compatible with the latter hypothesis, since rapsyn does not have a transmembrane region. When rapsyn exits the Golgi complex, factor(s) rendering rapsyn oligomers incompatible with the transport vesicle may either be competitively inhibited by AChRs, or titrated out by the increased amount of rapsyn.
The process of transport from the Golgi complex to the plasma membrane revealed in this study begs further questions, in particular with regard to other synaptic molecules. MuSK is located at the prospective synapse of a myocyte, interacts with Dok-7 and plays an essential role in the formation of AChR/rapsyn pre-clusters (Lin et al., 2001; Yang et al., 2001). Without MuSK, clusters of rapsyn and/or AChR do not form at the synapse (DeChiara et al., 1996). Hence, MuSK or dok-7 may affect the transport of rapsyn/AChRs from the Golgi complex to the plasma membrane. Alternatively MuSK/dok-7 may function in the processing only after AChR/rapsyn reaches the plasma membrane. Several elegant in vivo studies examined the dynamics of rapsyn/AChRs on the plasma membrane (Bruneau and Akaaboune, 2007; 2010). However, it will be very useful to examine their interactions before they reach the plasma membrane. Further studies on the rapsyn/AChR transport will lead to a better understanding of synapse formation.
Acknowledgments
We thank Dr. Michael Linhoff for his technical help in the immunohistochemistry of rapsyn. We thank Drs. Yujin Won and Meghan Mott for their help in maintaining the fish facility. We thank Drs. Henry Puhl and Meghan Mott for critical reading of the manuscript. This study was supported by the intramural program of NIH/NIAAA.
Footnotes
No conflict of interest
References
- An MC, Lin W, Yang J, Dominguez B, Padgett D, Sugiura Y, Aryal P, Gould TW, Oppenheim RW, Hester ME, Kaspar BK, Ko C-P, Lee K-F. Acetylcholine negatively regulates development of the neuromuscular junction through distinct cellular mechanisms. Proc Natl Acad Sci USA. 2010;107:10702–10707. doi: 10.1073/pnas.1004956107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antolik C, Catino DH, O’Neill AM, Resneck WG, Ursitti JA, Bloch RJ. The actin binding domain of ACF7 binds directly to the tetratricopeptide repeat domains of rapsyn. Neuroscience. 2007;145:56–65. doi: 10.1016/j.neuroscience.2006.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antolik C, Catino DH, Resneck WG, Bloch RJ. The tetratricopeptide repeat domains of rapsyn bind directly to cytoplasmic sequences of the muscle-specific kinase. NSC. 2006;141:87–100. doi: 10.1016/j.neuroscience.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Apel ED, Roberds SL, Campbell KP, Merlie JP. Rapsyn may function as a link between the acetylcholine receptor and the agrin-binding dystrophin-associated glycoprotein complex. Neuron. 1995;15:115–126. doi: 10.1016/0896-6273(95)90069-1. [DOI] [PubMed] [Google Scholar]
- Bloom GS, Brashear TA. A novel 58-kDa protein associates with the Golgi apparatus and microtubules. J Biol Chem. 1989;264:16083–16092. [PubMed] [Google Scholar]
- Borges LS, Yechikhov S, Lee YI, Rudell JB, Friese MB, Burden SJ, Ferns MJ. Identification of a motif in the acetylcholine receptor beta subunit whose phosphorylation regulates rapsyn association and postsynaptic receptor localization. J Neurosci. 2008;28:11468–11476. doi: 10.1523/JNEUROSCI.2508-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneau E, Akaaboune M. The dynamics of the rapsyn scaffolding protein at individual acetylcholine receptor clusters. J Biol Chem. 2007;282:9932–9940. doi: 10.1074/jbc.M608714200. [DOI] [PubMed] [Google Scholar]
- Bruneau EG, Akaaboune M. Dynamics of the rapsyn scaffolding protein at the neuromuscular junction of live mice. J Neurosci. 2010;30:614–619. doi: 10.1523/JNEUROSCI.4595-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneau EG, Brenner DS, Kuwada JY, Akaaboune M. Acetylcholine receptor clustering is required for the accumulation and maintenance of scaffolding proteins. Curr Biol. 2008;18:109–115. doi: 10.1016/j.cub.2007.12.029. [DOI] [PubMed] [Google Scholar]
- Burden SJ. The subsynaptic 43-kDa protein is concentrated at developing nerve-muscle synapses in vitro. Proc Natl Acad Sci USA. 1985;82:8270–8273. doi: 10.1073/pnas.82.23.8270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Qian L, Yang Z-H, Huang Y, Ngo ST, Ruan N-J, Wang J, Schneider C, Noakes PG, Ding Y-Q, Mei L, Luo Z-G. Rapsyn Interaction with Calpain Stabilizes AChR Clusters at the Neuromuscular Junction. Neuron. 2007;55:247–260. doi: 10.1016/j.neuron.2007.06.031. [DOI] [PubMed] [Google Scholar]
- Colley KJ. Golgi localization of glycosyltransferases: more questions than answers. Glycobiology. 1997;7:1–13. doi: 10.1093/glycob/7.1.1-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- DeChiara TM, Bowen DC, Valenzuela DM, Simmons MV, Poueymirou WT, Thomas S, Kinetz E, Compton DL, Rojas E, Park JS, Smith C, DiStefano PS, Glass DJ, Burden SJ, Yancopoulos GD. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell. 1996;85:501–512. doi: 10.1016/s0092-8674(00)81251-9. [DOI] [PubMed] [Google Scholar]
- Ellgaard L, Ruddock LW. The human protein disulphide isomerase family: substrate interactions and functional properties. EMBO Rep. 2005;6:28–32. doi: 10.1038/sj.embor.7400311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epley KE, Urban JM, Ikenaga T, Ono F. A modified acetylcholine receptor delta-subunit enables a null mutant to survive beyond sexual maturation. J Neurosci. 2008;28:13223–13231. doi: 10.1523/JNEUROSCI.2814-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan-Steet H, Fox MA, Meyer D, Sanes JR. Neuromuscular synapses can form in vivo by incorporation of initially aneural postsynaptic specializations. Development. 2005;132:4471–4481. doi: 10.1242/dev.02044. [DOI] [PubMed] [Google Scholar]
- Fritzler MJ, Hamel JC, Ochs RL, Chan EK. Molecular characterization of two human autoantigens: unique cDNAs encoding 95- and 160-kD proteins of a putative family in the Golgi complex. J Exp Med. 1993;178:49–62. doi: 10.1084/jem.178.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gally C, Eimer S, Richmond JE, Bessereau J-L. A transmembrane protein required for acetylcholine receptor clustering in Caenorhabditis elegans. Nature. 2004;431:578–582. doi: 10.1038/nature02893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervásio OL, Phillips WD. Increased ratio of rapsyn to ACh receptor stabilizes postsynaptic receptors at the mouse neuromuscular synapse. J Physiol (Lond) 2005;562:673–685. doi: 10.1113/jphysiol.2004.077685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granato M, van Eeden FJ, Schach U, Trowe T, Brand M, Furutani-Seiki M, Haffter P, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Odenthal J, Nüsslein-Volhard C. Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development. 1996;123:399–413. doi: 10.1242/dev.123.1.399. [DOI] [PubMed] [Google Scholar]
- Green WN, Millar NS. Ion-channel assembly. Trends Neurosci. 1995;18:280–287. [PubMed] [Google Scholar]
- Ikenaga T, Urban JM, Gebhart N, Hatta K, Kawakami K, Ono F. Formation of the spinal network in zebrafish determined by domain-specific pax genes. J Comp Neurol. 2011;519:1562–1579. doi: 10.1002/cne.22585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing L, Lefebvre JL, Gordon LR, Granato M. Wnt signals organize synaptic prepattern and axon guidance through the zebrafish unplugged/MuSK receptor. Neuron. 2009;61:721–733. doi: 10.1016/j.neuron.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N, Stiegler AL, Cameron TO, Hallock PT, Gomez AM, Huang JH, Hubbard SR, Dustin ML, Burden SJ. Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell. 2008;135:334–342. doi: 10.1016/j.cell.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre JL, Jing L, Becaficco S, Franzini-Armstrong C, Granato M. Differential requirement for MuSK and dystroglycan in generating patterns of neuromuscular innervation. Proc Natl Acad Sci USA. 2007;104:2483–2488. doi: 10.1073/pnas.0610822104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Burgess RW, Dominguez B, Pfaff SL, Sanes JR, Lee KF. Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature. 2001;410:1057–1064. doi: 10.1038/35074025. [DOI] [PubMed] [Google Scholar]
- Luo S, Zhang B, Dong X-P, Tao Y, Ting A, Zhou Z, Meixiong J, Luo J, Chiu FCA, Xiong WC, Mei L. HSP90 beta regulates rapsyn turnover and subsequent AChR cluster formation and maintenance. Neuron. 2008;60:97–110. doi: 10.1016/j.neuron.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangi PA, Forsayeth JR, Mittaud P, Erb-Vögtli S, Blake DJ, Moransard M, Sander A, Fuhrer C. Acetylcholine receptors are required for agrin-induced clustering of postsynaptic proteins. EMBO J. 2001;20:7060–7073. doi: 10.1093/emboj/20.24.7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand S, Bignami F, Stetzkowski-Marden F, Cartaud J. The myristoylated protein rapsyn is cotargeted with the nicotinic acetylcholine receptor to the postsynaptic membrane via the exocytic pathway. J Neurosci. 2000;20:521–528. doi: 10.1523/JNEUROSCI.20-02-00521.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand S, Devillers-Thiéry A, Pons S, Changeux J-P, Cartaud J. Rapsyn escorts the nicotinic acetylcholine receptor along the exocytic pathway via association with lipid rafts. J Neurosci. 2002;22:8891–8901. doi: 10.1523/JNEUROSCI.22-20-08891.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongeon R, Walogorsky M, Urban J, Mandel G, Ono F, Brehm P. An acetylcholine receptor lacking both and subunits mediates transmission in zebrafish slow muscle synapses. J Gen Physiol. 2011;138:353–366. doi: 10.1085/jgp.201110649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N, Rabouille C, Watson R, Nilsson T, Hui N, Slusarewicz P, Kreis TE, Warren G. Characterization of a cis-Golgi matrix protein, GM130. J Cell Biol. 1995;131:1715–1726. doi: 10.1083/jcb.131.6.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno K, Engel AG, Shen X-M, Selcen D, Brengman J, Harper CM, Tsujino A, Milone M. Rapsyn mutations in humans cause endplate acetylcholine-receptor deficiency and myasthenic syndrome. Am J Hum Genet. 2002;70:875–885. doi: 10.1086/339465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Inoue A, Okada M, Murata Y, Kakuta S, Jigami T, Kubo S, Shiraishi H, Eguchi K, Motomura M, Akiyama T, Iwakura Y, Higuchi O, Yamanashi Y. The muscle protein Dok-7 is essential for neuromuscular synaptogenesis. Science. 2006;312:1802–1805. doi: 10.1126/science.1127142. [DOI] [PubMed] [Google Scholar]
- Ono F, Higashijima S, Shcherbatko A, Fetcho JR, Brehm P. Paralytic zebrafish lacking acetylcholine receptors fail to localize rapsyn clusters to the synapse. J Neurosci. 2001;21:5439–5448. doi: 10.1523/JNEUROSCI.21-15-05439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono F, Mandel G, Brehm P. Acetylcholine receptors direct rapsyn clusters to the neuromuscular synapse in zebrafish. J Neurosci. 2004;24:5475–5481. doi: 10.1523/JNEUROSCI.0851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono F, Shcherbatko A, Higashijima S-I, Mandel G, Brehm P. The Zebrafish motility mutant twitch once reveals new roles for rapsyn in synaptic function. J Neurosci. 2002;22:6491–6498. doi: 10.1523/JNEUROSCI.22-15-06491.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opat AS, van Vliet C, Gleeson PA. Trafficking and localisation of resident Golgi glycosylation enzymes. Biochimie. 2001;83:763–773. doi: 10.1016/s0300-9084(01)01312-8. [DOI] [PubMed] [Google Scholar]
- Panzer JA. In Vivo Imaging of Preferential Motor Axon Outgrowth to and Synaptogenesis at Prepatterned Acetylcholine Receptor Clusters in Embryonic Zebrafish Skeletal Muscle. J Neurosci. 2006;26:934–947. doi: 10.1523/JNEUROSCI.3656-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips WD, Kopta C, Blount P, Gardner PD, Steinbach JH, Merlie JP. ACh receptor-rich membrane domains organized in fibroblasts by recombinant 43-kildalton protein. Science. 1991a;251:568–570. doi: 10.1126/science.1703661. [DOI] [PubMed] [Google Scholar]
- Phillips WD, Maimone MM, Merlie JP. Mutagenesis of the 43-kD postsynaptic protein defines domains involved in plasma membrane targeting and AChR clustering. J Cell Biol. 1991b;115:1713–1723. doi: 10.1083/jcb.115.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramarao MK. Role of Rapsyn Tetratricopeptide Repeat and Coiled-coil Domains in Self-association and Nicotinic Acetylcholine Receptor Clustering. Journal of Biological Chemistry. 2000;276:7475–7483. doi: 10.1074/jbc.M009888200. [DOI] [PubMed] [Google Scholar]
- Ramarao MK, Cohen JB. Mechanism of nicotinic acetylcholine receptor cluster formation by rapsyn. Proc Natl Acad Sci USA. 1998;95:4007–4012. doi: 10.1073/pnas.95.7.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reist NE, Werle MJ, McMahan UJ. Agrin released by motor neurons induces the aggregation of acetylcholine receptors at neuromuscular junctions. Neuron. 1992;8:865–868. doi: 10.1016/0896-6273(92)90200-w. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2:791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- Séguéla P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzartos SJ, Rand DE, Einarson BL, Lindstrom JM. Mapping of surface structures of electrophorus acetylcholine receptor using monoclonal antibodies. J Biol Chem. 1981;256:8635–8645. [PubMed] [Google Scholar]
- Yang X, Arber S, William C, Li L, Tanabe Y, Jessell TM, Birchmeier C, Burden SJ. Patterning of muscle acetylcholine receptor gene expression in the absence of motor innervation. Neuron. 2001;30:399–410. doi: 10.1016/s0896-6273(01)00287-2. [DOI] [PubMed] [Google Scholar]
- Zhang B, Luo S, Dong X-P, Zhang X, Liu C, Luo Z, Xiong W-C, Mei L. Beta-catenin regulates acetylcholine receptor clustering in muscle cells through interaction with rapsyn. J Neurosci. 2007;27:3968–3973. doi: 10.1523/JNEUROSCI.4691-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]