Summary
Gene therapy has been shown to be a powerful new approach to the treatment of brain diseases. Brain neurodegenerations, brain tumors, inherited brain diseases, and autoimmune disorders are currently recognized as proper targets for gene therapeutics. Advances in the development of viral vectors (especially improvements in their immune profiles), the capacity to regulate transgene expression, and identification of appropriate therapeutic constructs have made the transition into clinical trials for gene therapy possible. One particular remaining challenge is the immune response that could be raised against either the viral vectors themselves or any regulatory or therapeutic transgenes. Because of the structure of brain immune responses, viral gene transfer into the brain can, under certain circumstances, be invisible to the systemic immune response and thus not generate a deleterious immune attack. If, however, the systemic immune system is primed against any vector antigen, the systemic immune response eliminates transgene expression and thus curtails the therapeutic efficacy of gene therapy. Mechanistic studies of brain immune responses indicate that the adaptive arm of the immune system may indeed be able to kill transduced cells. To move neurological gene therapy into the clinic in an effective and safe manner, these are the developments needed: novel viral vectors that either display a reduced capacity to stimulate an adaptive immune response or become invisible to the immune system after the delivery of the vector genome to the nucleus of transduced cells, and ways either to steer the immune response away from cytotoxic responses or to induce tolerance to gene therapy products.
Keywords: Gene therapy, immune responses, neuroimmunology, immunological synapse, viral vectors
INTRODUCTION: GENE THERAPY FOR NEUROLOGICAL DISORDERS
The natural lifecycle of all viruses consists of 1) infecting target host cells, 2) transferring their own genetic material into the host cells, 3) commandeering the host's cellular machinery to express viral proteins, 4) virion assembly, and finally 5) release of new viral particles to continue the cycle. The exquisite ability of viruses to carry their DNA into target cells and express their virally encoded genes has made them a choice tool as vectors to transfer therapeutic genes into diseased organs. Thus, over the past 20 years researchers have developed the molecular techniques to transform pathogenic viruses into viral vectors, a genetically re-engineered replication-competent virus containing foreign therapeutic genes yet incapable of causing viral disease. In most cases, viral genomes are genetically modified to remove genomic sequences imparting replicative and pathogenic functions; however, some sequences from the wild-type viral genome are maintained. Such vectors are usually referred to as first-generation vectors. In oncolytic vectors, however, viral replication is redirected to tumor cells to selectively kill them.
More recently researchers have developed viral vectors that have been deleted of all viral protein coding sequences, retaining only those needed for genome packaging and replication. Such vectors are grown in tissue culture and packaged into virions, using helper viruses. These helper-dependent vectors have a larger capacity for transgenes and regulatory sequences and for altered immune reactivity. The majority of these high-capacity helper-dependent or amplicon vectors are derived from adenoviruses (HC-Ad) or herpes simplex viruses (HSV-1/a). The viral vectors most frequently used are derived from adenoviruses, herpes simplex viruses, and retroviruses. A comprehensive summary of most viral vector systems proposed or currently used in gene therapy clinical trials is presented in TABLE 1.
TABLE 1.
Gene Transfer Vehicles Used in Gene Therapy Applications
| Ad | HC-Ad | Oncolytic Ad | HSV-1/r | HSV-1/a | AAV | Retrovirus | Lentivirus | Vaccinia virus | Measles virus | Microinjection | Transfection | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Size, kb | 36 | 30−36 | 36 | 152 | 152 | 4.68 | 3.5−9.2 | 35−9.2 | 186 | 16 | Unlimited | Unlimited |
| Cloning capacity | 7.5 | ∼30 | <7 | 30 | 30−130 | 2−4.5 | ∼8 | ∼10 | 30 | 4 | Unlimited | Unlimited |
| Transduction of postmitotic cells? | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | No | No | No | No |
| In vivo? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No |
| In vitro? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No |
| Long-term expression? | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | ? | No |
| Vaccination? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | — | — |
| Vector titers, i.u./mL | 1012 | 1011 | 108 | 108 | 108 | 109 | 107 | 109 | 106−108 | 107 | — | — |
Abbreviations: AAV = adeno-associated virus; Ad = adenovirus; HC-Ad = high-capacity, helper-dependent adenovirus; HSV = herpes simplex virus; i.u. = infectious units.
Viral vector-based treatments of neurodegenerative diseases
Because treatment for chronic neurodegenerations such as Alzheimer's or Parkinson's disease usually requires long-term delivery of a therapeutic agent, viral vectors are attractive candidates for delivery of therapeutic genes to the diseased brain. Their long-term persistence, high levels of expression, and ability to infect tissues of the central nervous system (CNS) make viral vectors ideal for the treatment of neurodegenerative diseases. The following is a brief summary of various gene therapy strategies to treat neurodegenerative diseases, including results from human clinical trials using gene therapy as a therapeutic agent.
Alzheimer's disease
Alzheimer's disease (AD) is characterized by deposition of extracellular β-amyloid plaques, intracellular neurofibrillary tangles, synaptic loss, and neurodegeneration with symptomatic presentation including progressive cognitive decline and memory loss. No early diagnosis is currently possible, and the only approved treatments use cholinesterase inhibitors to delay the onset of memory loss without substantially altering disease progression. Most proposed gene therapy approaches aim to reduce amyloid plaques and tangles or introduce neurotrophic factors to reduce the death of brain cells.
Immunization against β-amyloid to target amyloid deposits in the brain has also been attempted. Initial clinical trials of this approach encountered serious side effects, such as brain inflammation (including increased microglia activation, macrophage recruitment, and T cell infiltration), which forced the cessation of this trial. Brain inflammation was most likely the result of a T-cell inflammatory response caused by T cells specific for β-amyloid, the intraparenchymal epitope target of the T cells. As a consequence, the immunogen utilized is being engineered to avoid the activation of T cells while maintaining a strong antibody response that could help clear the amyloid load of the brain.
The recent discovery of mutations in the SORL1 gene that may underlie pathology in so-called sporadic cases of AD could offer further direct avenues to manipulating the genetic causes of the most common, nonfamilial form of AD.1 The neurotrophic activity of nerve growth factor (NGF)2 has led to rescue-degenerating basal forebrain cholinergic neurons.3 However, injections of NGF into the ventricles of patients with AD not only had no striking therapeutic effects, but also had serious toxic effects, including pain and weight loss.4 In consequence, an ex vivo gene therapy approach was developed that implants autologous fibroblasts transduced ex vivo with retroviral vectors expressing NGF into the nucleus basalis of Meynert.5 A phase I/II dose-escalating, randomized study of an adeno-associated virus (AAV) vector encoding NGF is currently ongoing.
Parkinson's disease
The second most common neurodegenerative disorder is Parkinson's disease (PD), which occurs both in sporadic form and, far less commonly, in familial form. In patients suffering from PD, there is a progressive loss of dopaminergic neurons in the substantia nigra and other brain stem nuclei. There are ∼400,000 dopaminergic neuron cells in the human midbrain.6 Patients with PD suffer from various motor impairments, including resting tremor, bradykinesia, and rigidity, as well as balance problems, autonomic nervous dysfunction, and (at late stages) cognitive and psychiatric symptoms. Currently, there are 11 gene loci linked to the familial form of PD, named PARK1 through PARK11, and genes have been identified for six of them: SNCA, Parkin, UCH-LA, Pink1, PARK7, and LRRK2.7
In the past 10 years, gene therapy approaches for PD have developed in three main directions: 1) transduction of multiple genes essential for the synthesis of dopamine, to restore dopamine levels; 2) transduction of genes encoding growth factors, differentiation factors, transcription factors, and antiapoptotic proteins, to prevent ongoing neurodegeneration of nigrostriatal dopamine neurons; and 3) improvements and further developments of vector and promoter systems to reduce toxicity, modulate immune responses, increase longevity of expression, and regulate transgene expression.
A clinical trial for PD, ongoing in 2007, uses an AAV vector encoding the therapeutic glutamic acid decarboxylase gene (GAD) to manage the tremors associated with late-stage PD. This therapeutic approach aims to stimulate an inhibitory GABA-ergic pathway after gene transfer of GAD into the subthalamic nucleus.8,9
An AAV vector carrying the therapeutic gene aromatic-l-amino-acid decarboxylase (AADC) is being evaluated in clinical trials combined with the administration of l-dopa, the current standard of care for the treatment of the dyskinesia associated with PD. Outstanding efficacy was observed in nonhuman primates when AAV-mediated gene transfer of AADC was combined with gene transfer of tyrosine hydroxylase (TH) and GTP cyclohydrolase I (CH1) encoded on two other AAV vectors.10 To circumvent the small cloning capacity of AAV vectors, a lentiviral vector system encoding a tricistronic expression cassette containing all three therapeutic genes is currently under development for human clinical trials.11
Neuroprotective gene therapy should be especially useful in early PD stages, when a significant number of nigral neurons could still be protected from further degeneration. It has been shown that gene transfer of glial cell-derived neurotrophic factor (GDNF),12 brain cell-derived neurotrophic factor (BDNF),13 the neurodifferentiation factor sonic hedgehog,14 the transcription factor Gli,15 and neurturin16 protect nigrostriatal neurons from neurotoxic insults in rat and primate models of PD. For potential clinical application, uncertain consequences of long-term growth factor expression, such as downregulation of TH17 and questions regarding timing and regulation of therapy need to be addressed. A double-blind, phase II, open-label study of an AAV vector encoding neurturin sponsored by Ceregene (San Diego, CA) is currently in process and recruiting patients.18
Other paradigms of gene therapy for PD that are currently being tested in animals models include the transduction of dopaminergic neurons with JNK-interacting protein 1 (JIP-1), sonic hedgehog, a secreted neurodifferentiation factor,19 apoptosis protease activating factor 1 (Apaf-1)20 dominant negative inhibitor, neuronal apoptosis inhibitor protein (NAIP),21 Hsp70,22 and Parkin.23
IMMUNE RESPONSES AGAINST VIRAL VECTORS USED IN GENE THERAPY
Innate immune responses and gene therapy as pharmacology
Although gene therapy uses disabled viruses, the virions themselves can still cause acute dose-dependent inflammation, including microglial activation, macrophage recruitment, and antigen-nonspecific T-cell infiltration. The reason is that viral vectors are packaged into identical capsids as wild-type viruses. Initial inflammatory responses against viruses are usually caused by the interaction of viral capsid proteins with specific innate immune receptors, such as Toll receptors. The structure of the immune system of the brain is such that injection of viral vectors into the brain stimulates innate inflammatory responses without necessarily inducing a linked systemic adaptive immune response.
Detailed dose–response studies have shown that inflammatory responses to adenovirus are strictly dose-dependent. In one study, Thomas et al.24 studied the short-term (3 days) and long-term (30 days) inflammatory consequences of administering increasing doses of adenoviral vectors delivered in small volumes, injected directly into the striatum of mice. A limited (small volume), and small dose of an infectious particulate antigen, such as the viral vectors, delivered directly into the brain parenchyma will not stimulate the systemic adaptive immune response. Such systemic immune ignorance is thought to be due to the very limited, if any, availability of vector antigens to the general systemic circulation and lymphoid organs, and thus, the lack of priming of an adaptive antivector immune response.
In the studies performed by Thomas et al.,24 vector doses from 106 to 109 infectious units (i.u.) were injected directly into the striatum. Cellular inflammation (i.e., microglia activation, macrophage infiltration, antigen-nonspecific T cell recruitment) and transgene expression were monitored over time. At a short time after vector injection (3 days after), β-galactosidase expression was detected in brains injected with 106i.u. Expression levels increased with escalating doses until reaching a plateau at 108 i.u.. Cytotoxicity increased in parallel with increasing doses of vectors injected. Local cytotoxicity was minimal at doses less than 108 i.u. (the plateau dose for expression), but after the injection of 109 i.u. (the highest dose) there was a substantial loss of immunore-activity for the astrocyte marker GFAP and the neuronal marker NeuN, suggesting a loss of both astrocytes and neurons. Notably, 1 × 109 i.u. of heat-inactivated adenoviral vectors failed to cause any significant frank brain inflammation or leukocyte infiltration. This demonstrates that acute toxicity is indeed caused by intact viral particles, but not the virion proteins or DNA itself.
With longer periods of time (in this case, 30 days after the injection of vector into the striatum), doses of vectors that at 3 days showed increased inflammation in the form consisting of increased markers for monocytes and lymphocytes had a corresponding decrease in transgene expression at 30 days, in the form of reduced transgene expression at 108 i.u. and no expression after injection of 109 i.u.. At doses of 106 and 107 i.u., however, there was no decrease in transgene activity at 30 days.
The cytotoxicity at the dose of 109 i.u. was caused by acute inflammatory induced cell death, as evidenced by the increase of apoptotic cells detected by TUNEL staining. Concomitant to the brain cell loss, Thomas et al. 24 detected a corresponding increase in persistent brain inflammation and activation of microglia, and continued presence of monocytes and leukocytes. The long-term persistence of macrophages and CD8+ T cells and the increased expression of major histocompatibility complex class 1 (MHC-1) expression correlated directly with acute cytotoxicity. There was also a positive correlation between short-term and long-term (>30 days) brain cellular inflammation and long-term loss of transgene expression from adenoviral vectors at the 109 i.u. dose.
Conversely, when brains injected with noncytotoxic doses (i.e., <1 × 108) were examined 30 days later, any initial inflammatory mononuclear cell infiltration and microglia activation had completely resolved. This indicates that the acute innate inflammatory response caused by adenoviral vectors in naïve animals is dose-dependent, transient, and self-limiting and that it provides therapeutically acceptable levels of transgene expression without any long-term inflammation, monocyte recruitment, or cytotoxicity at doses less than 1 × 108.
In follow-up work, Zirger et al.25 demonstrated that interferon-regulated and chemokine mRNAs did not increase at the noncytotoxic doses of Thomas et al.24 This work also used increasing doses of adenovirus, from 105 to 108, and observed an increase in αβ-interferon-regulated genes such as OAS, IRF1, and PKR (EIF2AK2); and chemokines, such as RANTES (CCL5), MCP-1 (CCL2), and interferon-γ (IFNγ)-inducible protein 10, were only significantly increased at the dose of 108 i.u., thus, above the threshold established for activation of local microglia, and recruitment of circulating mononuclear cells, established by Thomas et al.24 Moreover production of mRNAs for αβ-interferon-regulated genes and chemokines was transient with expression of most mRNAs returning to baseline by 7 days after injection into the brain. This indicates that innate inflammatory responses to adenovirus (i.e., increase in expression of interferon-inducible genes, and chemokine genes) are dose-dependent; above a particular threshold the injection of adenoviral vectors increases expression of chemokines and induces local cytotoxicity. Once this inflammatory threshold is crossed, long-term, potentially chronic (i.e., 6 months) brain inflammation ensues.26
The inflammatory threshold to adenoviral vectors injected into the brain is 1 × 108 i.u. Once this threshold is crossed, increased expression of interferon-inducible and chemokine-encoding genes, activation of local microglia, and recruitment of circulating monocytes and lymphocytes occur. Injections of vector concentrations below this threshold do not cause any innate increased recruitment of inflammatory cells to the brain, do not cause glial or neuronal toxicity, do not cause an increase in expression of interferon-regulated genes or chemokine genes, and achieve long-term transgene expression for up to at least 1 year. The significance of this work for the use of adenoviruses as vectors in gene therapy for neurological disorders is the demonstration that the dose-dependent increased inflammatory gene expression or recruitment of inflammatory cells caused by adenovirus must be taken into account when planning experimental or clinical trials.
This work also highlights the crucial importance of preparing high-quality viral vectors. Errors in the titration of these vectors (i.e., mistakes in the calculation of the amount of vectors injected into the brain) will have serious, deleterious consequences for long-term therapeutic transgene expression in the brain. At the right dose, adenoviruses are very effective therapeutics vehicles for gene therapy in the brain, as seen in experimental animal models for Parkinson's disease and Alzheimer's disease, as well as other neurodegenerations.
Structure of the brain immune system
Brain immune reactivity is contingent on antigen reaching one or both of two fundamentally different immune compartments: 1) the brain ventricles, choroid plexus, and meninges and 2) the brain parenchyma. Like the immune system of other organs, the brain ventricles, meninges, and choroid plexus contain all the cellular, vascular and lymphatic components necessary for immune reactivity. Dendritic cells (DC), which are the main cell type capable of inducing primary T-cell responses, are found within the meninges, choroid plexus, and CSF under noninflammatory conditions.27,28 Antigens within the meninges, choroid plexus, and CSF can be captured by DCs, triggering their migration to the cervical lymph nodes (CLN), which are the primary lymph nodes draining the brain and CSF.29 The brain parenchyma, however, whose endothelial cells form a tight diffusion barrier—the blood–brain barrier—is devoid of DC in its naïve state and lacks classical lymphatic drainage.30 In addition to these structural differences between the two compartments, there are a number of molecular mechanisms by which the brain parenchyma dampens local intraparenchymal immune reactivity.
Administration of antigens into either immune compartment of the brain illustrates the profound differences in their immunoreactivity. Injection of a particulate antigen or infectious agent (e.g., live influenza virus, bacille Calmette-Guérin [BCG], or nonreplicative adenoviral vectors) exclusively and selectively into the brain parenchyma causes innate inflammatory responses, but fails to stimulate systemic adaptive immune responses.31-33 By contrast, injection of the same type of antigen into the ventricular system induces both an innate inflammatory and a systemic adaptive immune response.32-34 Injection of a diffusible antigen, however, one that can easily diffuse between compartments (e.g., ovalbumin [OVA]), does induce a systemic B-cell response.35 An explanation for differential priming of lymphocytes in the distinct CNS compartments may reside in the inability of particulate antigen to drain from the brain parenchyma, either through a cellular or diffusible route. Thus, particulate antigens injected into the brain parenchyma cause local microglial activation, but are never transported to the lymph nodes to prime a systemic immune response. Soluble antigen can diffuse from the brain to the ventricles and thus eventually reach the lymph nodes, where it will stimulate a systemic immune response.
This differential immune reactivity is thought to reside, at least in part, in the distribution of DC. The DC localize predominantly to lymphoid tissue, where they take up antigen, and mature into potent antigen-presenting cells (APC). Alternatively, they acquire antigen at inflamed sites and traffic back to lymphoid tissue to activate T cells.36 Recent data have also suggested that monocytes recruited to sites of acute inflammation can acquire the phenotype of DC and present antigen to primed T cells, thus propagating T-cell responses.37 DC uptake of foreign antigen in the ventricular system is likely to trigger migration of DC to the CLN. Alternatively, antigens could drain from the brain directly into deep CLN, although this theoretical possibility remains to be formally proven.
Irrespective of the antigen transport and delivery mode, naïve T cells are primed in the CLN, expand, and traffic to the site of insult, where they exert effector function upon antigen re-encounter. Thus, although DC can enter the CNS parenchyma to activate the function of infiltrating T cells during infectious, toxic, or tumor-induced inflammation, the initial activation of naïve T cells preceding disease onset likely occurs in the CLN. A potential role has been proposed for DC in presenting antigenic epitopes to naïve T cell clones during chronic inflammation in diseases such as MS or in viral demyelinating disease models.38 Local lymphoid-like structures that could serve to sustain such signals and could provide the necessary anatomical organization to do so are currently being examined in greater depth.39
Adaptive immune responses against adenoviruses injected into the brain
Because of the particular structure of the brain immune system, careful injection of noninflammatory doses of nonreplicating viral vectors into the brain provides long-term therapeutic transgene expression in the brain and achieves therapeutically effective transgene expression in experimental models of animal disease. If, however, the viral vectors reach the peripheral organs or the systemic circulation, then a systemic, adaptive, anti-adenoviral immune response ensues that can almost completely eliminate transgene expression from the brain. In this case, T cells are the main cells responsible for eliminating transgene expression from the brain. T cells are highly efficient at eliminating transgene expression from the brain, being able to recognize an injection of as little as 1 × 103 i.u. of vector (equivalent to 1000 transduced cells).40
If animals have been injected with adenovirus into the brain and subsequently immunized against adenovirus within 30−60 days, transgene expression in the brain will have been reduced to 50−100%. Barcia et al.40 demonstrated that an adaptive immune response was able to eliminate transgene expression from as little as 1000 i.u. injected into the brain. For these experiments, increasing concentrations of adenovirus were injected into the brain, followed by a systemic immunization against adenovirus. The systemic immune response induced was able to eliminate transgene expression from doses that ranged from 103 to 107 i.u. injected into the brain. That the immune system could eliminate expression from as little as 1000 infected cells to the brain indicates the high efficiency by which the adaptive immune system can eliminate transgene expression from the brain.
Further experiments by Barcia et al.41 and Zirger et al. (personal communication) have shown that CD8+ T cells and CD4+ T cells are necessary for immune-mediated elimination of transgene expression from the brain. Studies by Barcia el al.40 have also shown that the elimination of transgene expression is independent of the promoter used to drive transgene expression: whether viral, housekeeping, or cell type specific promoters are used to drive transgene expression, the immune system can eliminate transgene expression from all of these promoters.
These issues would appear to provide a fatal blow to the use of adenoviral vectors in gene therapy; however, only first-generation adenoviral vectors were used in these experiments. New generations of viral vectors have been produced recently, high-capacity, helper-dependent adenoviruses (HC-Ad) that allow the insertion of up to 30−34 kb of transgenic sequences. Most important, the genomes of these vectors do not encode any viral proteins. The genomes of these high-capacity, helper-dependent adenoviral vectors thus do not produce any adenoviral protein that could be recognized as antigenic epitopes by the immune system.
In a series of articles, Thomas et al.,24,42 Xiong et al.,43 O'Neal et al.,44 Mian et al.,45 Parks et al.,46 Maione et al.,47 and others48 have now demonstrated that, even in the presence of immunization against adenovirus prior to injection of HC-Ad into the brain, transgene expression from these viruses remained stable, and was shown to persist for up to 1 year. These vectors could therefore be used even in human patients that have been pre-exposed to adenovirus before being subjected to gene therapy.
Are HC-Ad vectors completely immune to the adaptive arm of the immune system? Once the genome of these viruses has reached the nucleus, they are. Before they infect the cells, however, the viral capsid of these vectors could of course be neutralized by anti-adenovirus antibodies. Nonetheless, the adaptive arm of the immune system, the T cells, would have a very short period of time during which they can recognize cells infected with HC-Ad, if capsid proteins were transiently presented on MHC Class 1 molecules. Such proteins would be provided only by the capsid, however, and because the genome of these vectors does not encode for any of these viral proteins, once these proteins from the viral capsid have been metabolized, this vector effectively becomes immune to the antiviral T cells. Further engineering of these vectors shows that adenoviruses are effective vehicles for long-term therapeutic transgene expression in the brain.
What causes loss of transgene expression?
Although it is clear that the adaptive immune response can clear transgene expression from the brain, how it does so, and what the consequences are to the transfused cells, has not yet been determined in sufficient detail. In theory, two main possibilities could account for the loss of transgene expression. On the one hand, T cells could selectively turn off transgene expression from transduced cells, through the secretion of effector molecules such as IFNγ. Alternatively, T cells could eliminate transgene expression by killing transduced cells.
The consequences of the particular mechanism of clearance of virally transfused cells are important because if T cells merely block expression from the viral genome, no anatomical damage is being done. If, however, T cells eliminate transgene expression by killing transduced cells, the consequences to gene therapy would be more serious. To date, work done in various laboratories remains inconclusive on this issue.
The issue may be more complicated than is initially apparent. If we take the literature on immune response against brain viral infections as an example, we soon realize that most groups hold the idea that the clearing of viral brain infections proceeds mainly through noncytolytic mechanisms. Upon detailed analysis of the published data, however, it becomes apparent that this conclusion is based mainly on an inability to detect cell death in the nervous system. Because the number of infected or transduced cells is slightly at or below 5% for the total amount of brain cells in any particular brain region, it is likely that the detection of a loss of such a small percentage of brain cells would be difficult to detect experimentally. In addition, the brain repairs itself rather effectively through glyosis. In this case, astrocytes are able to divide and fill the space left by dead brain cells. Furthermore, any dead brain cells are quickly phagocytosed by local microglial cells and possibly by incoming macrophages, thereby keeping brain inflammation at a reasonable minimum.
The ultimate consequences of the mechanisms of elimination of transgene expression are important. If the immune system turns off transgene expression, no permanent anatomical damage occurs. However, if the immune system kills transduced cells, the underlying disease could worsen. Therefore, the mechanisms by which the immune system abolishes transgene expression from transduced brain cells need to be firmly established.
Cell biology of brain immune responses
Until now, intercellular interactions between immune cells and target brain cells have been mostly extrapolated from studies of each cell type at the population level. Thus, there is relatively little information on the in vivo cell biology of T cell interactions with individual infected brain cells studied at the single-cell level. Over the last 10 years, immunological synapses have been characterized as the cellular substrate of intercellular communication in the immune system. Immunological synapses that form at the junction between T cells and antigen presenting cells consist of a rearrangement of membrane proteins (intercellular adhesion molecules such as ICAM-1, and T cell antigen receptor [TCR]) and intracellular TCR downstream signaling tyrosine kinases, as well as cytoskeletal structures and intracellular organelles of the secretory pathway of the T cells.49-56 Although various types of arrangements of T cell proteins have been found at these intercellular junctions, a canonical structure, known as the mature (or Kupfer-type) immunological synapse, has been described as consisting of the following arrangement: a peripheral supramolecular activation cluster (p-SMAC) comprising a ring of adhesion molecules that anchor the membrane of the T cell to the membrane of the APC and a central SMAC (c-SMAC) with a higher concentration of TCR and signaling molecules. Immunological synapses have been described for both CD4 and CD8-T cells and natural killer (NK) cells in contact with various types of APCs (e.g., dendritic cells, B cells, or target cells).
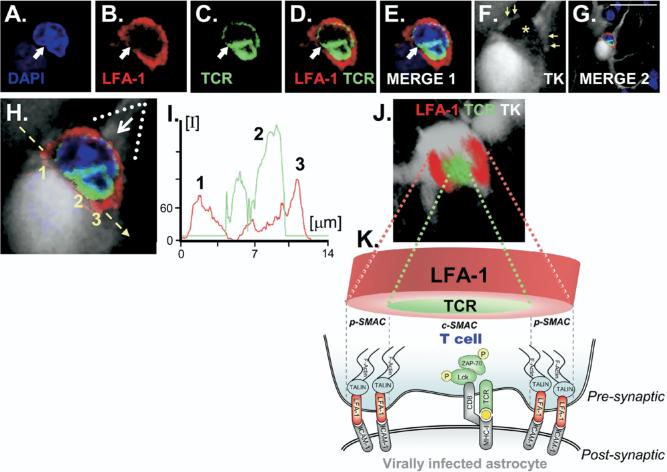
In a contribution toward understanding the cellular basis of neuroimmune interactions in the brain in vivo, it was recently shown that anti-adenoviral CD8 T cells infiltrate the brain and form Kupfer-type mature immunological synapses with MHC-1 expressing astrocytes.40,57 These immunological synapses were characterized through the formation of the classical supramolecular activation clusters (SMACs), which constitute the hallmark of immunological synapses (FIG. 1). In this model, a nonreplicating adenoviral vector was used to target predominantly astrocytes in the rat brain, resulting in a fixed number of astrocytes harboring viral genomes. This virus is replication-defective and thus cannot directly kill infected cells. Because the parenchymal CNS infection itself does not induce significant mononuclear cell infiltration, nor upregulation of inflammatory mediators, nor a systemic anti-adenoviral immune response, the systemic anti-adenoviral immunization was induced with a different Ad vector injected systemically. Systemic anti-adenoviral immunization triggered a systemic anti-adenoviral immune response, which led to overt full-blown brain inflammation. This inflammation consisted mainly of an infiltration into the brain parenchyma of CD8 T cells and macrophages and a perivascular infiltration of CD4 T cells.
FIG. 1.
Supramolecular activation cluster (SMAC) formation at immunological synapses in vivo, between T cells and infected astrocytes in the brain. (A–F) Confocal images of nuclei stained with 4′, 6-diamidino-2-phenylindole (DAPI; blue), leukocyte function-associated antigen 1 (LFA-1 immunoreactivity; red), T cell antigen receptor (TCR; green), and the virally infected cell (thymidine kinase [TK]; white). Scale bars = 15 μm. In (F), the yellow asterisk indicates the location of the T cell in close apposition to the infected astrocyte and yellow arrows indicate structures apparently surrounding the T cell. (G,H) Low (G) and high (H) magnification of the immunological synapse.. (I) Graphic representation of the intensity of fluorescence measured at the interface—indicated by the yellow arrow in (H)—of the immunological synapse. The relative intensity of fluorescence of LFA-1 (red) and TCR (green) shows the expected distribution, with more intense LFA-1 staining toward the outer p-SMAC and stronger TCR in the central c-SMAC. (J) A three-dimensional (3D) reconstructed image illustrates the characteristic structure of the peripheral p-SMAC (outer LFA-1 ring) and central c-SMAC (inner TCR cluster) of the mature Kupfer-type immunological synapse. The image shown in (J) was rotated so that the plane of the interface of the immunological synapse, indicated by the broken yellow arrow in (H), could be observed from above; the white arrow in (H) shows the angle of vision of the 3D reconstruction in (J). (K) A diagrammatic view of a T cell contacting an infected astrocyte illustrates the localization of molecules involved in the immunological synapse, as well as polarized phosphorylated tyrosine kinases, a consequence of TCR engagement of cognate antigen being presented on major histocompatibility complex MHC-I on the surface of astrocytes. (Modified from Barcia et al.41 [J Exp Med 2006;203:2095−2107].)
The systemic anti-adenoviral immune response resulted in a significant reduction in the number of brain astrocytes that express adenoviral proteins, and a concomitant reduction in the number of viral genome copy numbers present in the CNS. Loss of infected cells was dependent on both CD4 and CD8 T cells. The presence of CD8 T cells within the brain parenchyma suggests the operation of direct cytolytic mechanisms in the elimination of infected cells. Although no direct evidence for apoptotic astrocytes was obtained, macrophages containing remains of infected astrocytes were found throughout the area of the brain that had been cleared of infected cells. This suggests that the formation of immunological synapses may represent the microanatomical substrate underlying CD8 T cell effector functions in the CNS, and mediate the antiviral clearing of CD8 T cells.
The importance of these studies is the demonstration that immunological synapse do indeed form in vivo in the brain during the clearing of virally infected astrocytes by the adaptive immune response. Their in vivo description in the context of an antiviral immune response highlights their physiological role as the structure underlying neuroimmune interactions in vivo. Also, the existence of immunological synapses in the brain during the clearing of virally infected brain cells opens up the examination of neuroimmune interactions and pathways at the single-cell level.
Implications of the experimental study of immune responses against adenoviral vectors for gene therapy for neurodegeneration, brain tumors, viral infections, and autoimmune disease in the brain
For the last 10 years, various research groups have shown that immune responses against adenoviral vectors can be deleterious for brain structure in function, eventually leading to the loss of transgene expression and brain cell death. The evidence suggests that the T-cell response can identify infected cells in the brain and either eliminate them physically or functionally. Further evidence has accumulated in at least two species that T cells can actually eliminate vector-transduced brain cells. Should these data be correct, the logical conclusion would be to avoid using such vectors in clinical gene therapy trials. However, it is difficult to compare immune responses across species. Further complications arise from trying to gauge the strength of the immune response in humans who may have been exposed to wild-type adenovirus decades before being exposed to the gene therapy. Although the threat of a deleterious immune response remains, it is almost impossible to model such responses in experimental animal species in a way that establishes credible expectations for translating these experiments into humans.
Two options remain. Either experiments are performed in humans with no prior exposure to adenovirus, or in those in whom no such a response can be detected, or novel vectors must be developed specifically for use in clinical trials. The first option retains the threat of an immune response that, at a minimum, may eliminate therapeutic transgene expression and, at a maximum, may compromise normal brain tissue, thereby worsening the underlying disease. The second option is more complicated, but a number of novel viral vectors exist with a much more favorable immune profile. Within the area of adenoviral vectors, the vector structure of HC-Ad is such that, after established gene transfer, no antigenic viral epitopes remain within infected cells; barring the development of an immune response against the therapeutic transgene, these vectors are effectively invisible to the immune system.
The discovery of immunological synapses has opened up experimental exploration of intercellular interactions during brain immune responses, both during autoimmune or infectious immunopathology, as during antivector immune responses. Work in this area indicates that the immune cells may well be capable of eliminating viral vector–transduced cells. Although this work permits a much more detailed understanding of the cellular mechanisms underlying immune clearing of infected cells and tumor cells from the brain, it also suggests the use of alternative vectors in gene therapy, vectors that (at least to the best of our current understanding) would remain invisible to a cytotoxic immune response. This may delay the implementation of clinical trials, but it will speed achieving clinical safety and efficacy of gene therapies for the treatment of brain diseases.
Acknowledgments
The gene therapy projects for neurological diseases are funded by grants from the National Institutes of Health (NIH) National Institute of Neurological Disorders and Stroke (1 R01 NS44556.01, to M.G.C.), NIH National Institute of Diabetes and Digestive and Kidney Diseases (1 R03 TW006273-01, M.G.C.), NIH National Institute of Neurological Disorders and Stroke (1 R01 NS 42893.01, U54 NS045309-01, and 1R21 NS047298-01, to P.R.L.), and the Bram and Elaine Goldsmith Chair in Gene Therapeutics (P.R.L.), as well as The Linda Tallen and David Paul Kane Annual Fellowship to M.G.C. and P.R.L. We also thank the generous funding our Institute receives from the Board of Governors at Cedars–Sinai Medical Center. We thank S. Melmed, R. Katzman, and D. Meyer for their superb administrative and organizational support, and for their academic leadership.
REFERENCES
- 1.Rogaeva E, Meng Y, Lee JH, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39:168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg MB, Friedmann T, Robertson RC, et al. Grafting genetically modified cells to the damaged brain: restorative effects of NGF expression. Science. 1988;242:1575–1578. doi: 10.1126/science.3201248. [DOI] [PubMed] [Google Scholar]
- 4.Eriksdotter Jonhagen M, Nordberg A, Amberla K, et al. Intracerebroventricular infusion of nerve growth factor in three patients with Alzheimer's disease. Dement Geriatr Cogn Disord. 1998;9:246–257. doi: 10.1159/000017069. [DOI] [PubMed] [Google Scholar]
- 5.Tuszynski MH, Thal L, Pay M, et al. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med. 2005;11:551–555. doi: 10.1038/nm1239. [DOI] [PubMed] [Google Scholar]
- 6.Hardman CD, Henderson JM, Finkelstein DI, Horne MK, Paxinos G, Halliday GM. Comparison of the basal ganglia in rats, marmosets, macaques, baboons, and humans: volume and neuronal number for the output, internal relay, and striatal modulating nuclei. J Comp Neurol. 2002;445:238–255. doi: 10.1002/cne.10165. [DOI] [PubMed] [Google Scholar]
- 7.Hodaie M, Neimat JS, Lozano AM. The dopaminergic nigrostriatal system and Parkinson's disease: molecular events in development, disease, and cell death, and new therapeutic strategies. Neurosurgery. 2007;60:17–28. doi: 10.1227/01.NEU.0000249209.11967.CB. discussion 28–30. [DOI] [PubMed] [Google Scholar]
- 8.During MJ, Kaplitt MG, Stern MB, Eidelberg D. Subthalamic GAD gene transfer in Parkinson disease patients who are candidates for deep brain stimulation. Hum Gene Ther. 2001;12:1589–1591. [PubMed] [Google Scholar]
- 9.Kaplitt MG, Feigin A, Tang C, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 10.Muramatsu S, Fujimoto K, Ikeguchi K, et al. Behavioral recovery in a primate model of Parkinson's disease by triple transduction of striatal cells with adeno-associated viral vectors expressing dopamine-synthesizing enzymes. Hum Gene Ther. 2002;13:345–354. doi: 10.1089/10430340252792486. [DOI] [PubMed] [Google Scholar]
- 11.Wong LF, Goodhead L, Prat C, Mitrophanous KA, Kingsman SM, Mazarakis ND. Lentivirus-mediated gene transfer to the central nervous system: therapeutic and research applications. Hum Gene Ther. 2006;17:1–9. doi: 10.1089/hum.2006.17.1. [DOI] [PubMed] [Google Scholar]
- 12.Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 13.Hyman C, Hofer M, Barde YA, et al. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350:230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- 14.Torres EM, Monville C, Lowenstein PR, Castro MG, Dunnett SB. Delivery of sonic hedgehog or glial derived neurotrophic factor to dopamine-rich grafts in a rat model of Parkinson's disease using adenoviral vectors Increased yield of dopamine cells is dependent on embryonic donor age. Brain Res Bull. 2005;68:31–41. doi: 10.1016/j.brainresbull.2005.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suwelack D, Hurtado-Lorenzo A, Millan E, et al. Neuronal expression of the transcription factor Gli1 using the Tα1 alpha-tubulin promoter is neuroprotective in an experimental model of Parkinson's disease. Gene Ther. 2004;11:1742–1752. doi: 10.1038/sj.gt.3302377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dass B, Kladis T, Chu Y, Kordower JH. RET expression does not change with age in the substantia nigra pars compacta of rhesus monkeys. Neurobiol Aging. 2006;27:857–861. doi: 10.1016/j.neurobiolaging.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Georgievska B, Kirik D, Bjorklund A. Overexpression of glial cell line-derived neurotrophic factor using a lentiviral vector induces time- and dose-dependent downregulation of tyrosine hydroxylase in the intact nigrostriatal dopamine system. J Neurosci. 2004;24:6437–6445. doi: 10.1523/JNEUROSCI.1122-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. National Institutes of Health [June 15, 2007];Double-Blind, Multicenter, Sham Surgery Controlled Study of CERE-120 For Efficacy and Safety in Subjects With Idiopathic Parkinson's Disease [Internet] ClinicalTrials.gov Available at: http://www.clinicaltrials.gov/ct/show/NCT00400634?order=1.
- 19.Suwelack D, Hurtado-Lorenzo A, Millan E, et al. Neuronal expression of the transcription factor Gli1 using the Tubulin a-1 promoter is neuroprotective in an experimental model of Parkinson's disease. Gene Ther. 2004;11:1742–1752. doi: 10.1038/sj.gt.3302377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mochizuki H, Hayakawa H, Migita M, et al. An AAV-derived Apaf-1 dominant negative inhibitor prevents MPTP toxicity as antiapoptotic gene therapy for Parkinson's disease. Proc Natl Acad Sci U S A. 2001;98:10918–10923. doi: 10.1073/pnas.191107398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crocker SJ, Wigle N, Liston P, et al. NAIP protects the nigrostriatal dopamine pathway in an intrastriatal 6-OHDA rat model of Parkinson's disease. Eur J Neurosci. 2001;14:391–400. doi: 10.1046/j.0953-816x.2001.01653.x. [DOI] [PubMed] [Google Scholar]
- 22.Dong Z, Wolfer DP, Lipp HP, Bueler H. Hsp70 gene transfer by adeno-associated virus inhibits MPTP-induced nigrostriatal degeneration in the mouse model of Parkinson disease. Mol Ther. 2005;11:80–88. doi: 10.1016/j.ymthe.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Lo Bianco C, Schneider BL, Bauer M, et al. Lentiviral vector delivery of parkin prevents dopaminergic degeneration in an alpha-synuclein rat model of Parkinson's disease. Proc Natl Acad Sci U S A. 2004;101:17510–17515. doi: 10.1073/pnas.0405313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas CE, Birkett D, Anozie I, Castro MG, Lowenstein PR. Acute direct adenoviral vector cytotoxicity and chronic, but not acute, inflammatory responses correlate with decreased vector-mediated transgene expression in the brain. Mol Ther. 2001;3:36–46. doi: 10.1006/mthe.2000.0224. [DOI] [PubMed] [Google Scholar]
- 25.Zirger JM, Barcia C, Liu C, et al. Rapid upregulation of interferon-regulated and chemokine mRNAs upon injection of 108 international units, but not lower doses, of adenoviral vectors into the brain. J Virol. 2006;80:5655–5659. doi: 10.1128/JVI.00166-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dewey RA, Morrissey G, Cowsill CM, et al. Chronic brain inflammation and persistent herpes simplex virus 1 thymidine kinase expression in survivors of syngeneic glioma treated by adenovirus-mediated gene therapy: implications for clinical trials. Nat Med. 1999;5:1256–1263. doi: 10.1038/15207. [DOI] [PubMed] [Google Scholar]
- 27.McMenamin PG. Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. J Comp Neurol. 1999;405:553–562. [PubMed] [Google Scholar]
- 28.Pashenkov M, Teleshova N, Link H. Inflammation in the central nervous system: the role for dendritic cells. Brain Pathol. 2003;13:23–33. doi: 10.1111/j.1750-3639.2003.tb00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cserr HF, Knopf PM. Cervical lymphatics, the blood-brain barrier and the immunoreactivity of the brain: a new view. Immunol Today. 1992;13:507–512. doi: 10.1016/0167-5699(92)90027-5. [DOI] [PubMed] [Google Scholar]
- 30.Bechmann I, Galea I, Perry VH. What is the blood-brain barrier (not)? Trends Immunol. 2007;28:5–11. doi: 10.1016/j.it.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Cartmell T, Southgate T, Rees GS, Castro MG, Lowenstein PR, Luheshi GN. Interleukin-1 mediates a rapid inflammatory response after injection of adenoviral vectors into the brain. J Neurosci. 1999;19:1517–1523. doi: 10.1523/JNEUROSCI.19-04-01517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevenson PG, Hawke S, Sloan DJ, Bangham CR. The immunogenicity of intracerebral virus infection depends on anatomical site. J Virol. 1997;71:145–151. doi: 10.1128/jvi.71.1.145-151.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matyszak MK, Perry VH. The potential role of dendritic cells in immune-mediated inflammatory diseases in the central nervous system. Neuroscience. 1996;74:599–608. doi: 10.1016/0306-4522(96)00160-1. [DOI] [PubMed] [Google Scholar]
- 34.Matyszak MK. Inflammation in the CNS: balance between immunological privilege and immune responses. Prog Neurobiol. 1998;56:19–35. doi: 10.1016/s0301-0082(98)00014-8. [DOI] [PubMed] [Google Scholar]
- 35.Knopf PM, Harling-Berg CJ, Cserr HF, et al. Antigen-dependent intrathecal antibody synthesis in the normal rat brain: tissue entry and local retention of antigen-specific B cells. J Immunol. 1998;161:692–701. [PubMed] [Google Scholar]
- 36.Caux C, Ait-Yahia S, Chemin K, et al. Dendritic cell biology and regulation of dendritic cell trafficking by chemokines. Springer Semin Immunopathol. 2000;22:345–369. doi: 10.1007/s002810000053. [DOI] [PubMed] [Google Scholar]
- 37.Leon B, Lopez-Bravo M, Ardavin C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity. 2007;26:519–531. doi: 10.1016/j.immuni.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 38.McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med. 2005;11:335–339. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- 39.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6:205–217. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 40.Gerdes C, Xiong C, Chemin W, et al. Immunological thresholds in neurological gene therapy: highly efficient elimination of transduced cells may be related to the specific formation of immunological synapses between T cells and virus-infected brain cells. Neuron Glia Biol. 2007;2:309–327. doi: 10.1017/S1740925X07000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barcia C, Thomas CE, Curtin JF, et al. In vivo mature immunological synapses forming SMACs mediate clearance of virally infected astrocytes from the brain. J Exp Med. 2006;203:2095–2107. doi: 10.1084/jem.20060420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas CE, Schiedner G, Kochanek S, Castro MG, Lowenstein PR. Peripheral infection with adenovirus causes unexpected long-term brain inflammation in animals injected intracranially with first-generation, but not with high-capacity, adenovirus vectors: toward realistic long-term neurological gene therapy for chronic diseases. Proc Natl Acad Sci U S A. 2000;97:7482–7487. doi: 10.1073/pnas.120474397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiong W, Goverdhana S, Sciascia SA, et al. Regulatable gutless adenovirus vectors sustain inducible transgene expression in the brain in the presence of an immune response against adenoviruses. J Virol. 2006;80:27–37. doi: 10.1128/JVI.80.1.27-37.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Neal WK, Zhou H, Morral N, et al. Toxicity associated with repeated administration of first-generation adenovirus vectors does not occur with a helper-dependent vector. Mol Med. 2000;6:179–195. [PMC free article] [PubMed] [Google Scholar]
- 45.Mian A, Guenther M, Finegold M, Ng P, Rodgers J, Lee B. Toxicity and adaptive immune response to intracellular transgenes delivered by helper-dependent vs. first generation adenoviral vectors. Mol Genet Metab. 2005;84:278–288. doi: 10.1016/j.ymgme.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 46.Parks R, Evelegh C, Graham F. Use of helper-dependent adenoviral vectors of alternative serotypes permits repeat vector administration. Gene Ther. 1999;6:1565–1573. doi: 10.1038/sj.gt.3300995. [DOI] [PubMed] [Google Scholar]
- 47.Maione D, Della Rocca C, Giannetti P, et al. An improved helper-dependent adenoviral vector allows persistent gene expression after intramuscular delivery and overcomes preexisting immunity to adenovirus. Proc Natl Acad Sci U S A. 2001;98:5986–5991. doi: 10.1073/pnas.101122498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barcia C, Jimenez-Dalmaroni M, Kroeger KM, et al. Sustained, one year expression from high-capacity helper-dependent adenoviral vectors delivered to the brain of animals with a pre-existing systemic anti-adenoviral immune response: implications for clinical trials. Mol Ther. 2007 doi: 10.1038/sj.mt.6300305. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cemerski S, Das J, Locasale J, et al. The stimulatory potency of T cell antigens is influenced by the formation of the immunological synapse. Immunity. 2007;26:345–355. doi: 10.1016/j.immuni.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dustin ML, Cooper JA. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat Immunol. 2000;1:23–29. doi: 10.1038/76877. [DOI] [PubMed] [Google Scholar]
- 51.Friedl P, den Boer AT, Gunzer M. Tuning immune responses: diversity and adaptation of the immunological synapse. Nat Rev Immunol. 2005;5:532–545. doi: 10.1038/nri1647. [DOI] [PubMed] [Google Scholar]
- 52.Grakoui A, Bromley SK, Sumen C, et al. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. [PubMed] [Google Scholar]
- 53.Huppa JB, Davis MM. T-cell-antigen recognition and the immunological synapse. Nat Rev Immunol. 2003;3:973–983. doi: 10.1038/nri1245. [DOI] [PubMed] [Google Scholar]
- 54.Huse M, Lillemeier BF, Kuhns MS, Chen DS, Davis MM. T cells use two directionally distinct pathways for cytokine secretion. Nat Immunol. 2006;7:247–255. doi: 10.1038/ni1304. [DOI] [PubMed] [Google Scholar]
- 55.Lee KH, Holdorf AD, Dustin ML, Chan AC, Allen PM, Shaw AS. T cell receptor signaling precedes immunological synapse formation. Science. 2002;295:1539–1542. doi: 10.1126/science.1067710. [DOI] [PubMed] [Google Scholar]
- 56.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 57.Barcia C, Thomas C, Zirger J, et al. Immune system regulation of transgene expression in the brain 1: systemically activated immune responses silence adenoviral vector-encoded transgene expression in the brain through both non-cytolytic and cytotoxic mechanisms. Mol Ther. 2004;9(Suppl 1):174–175. [Google Scholar]