Abstract
Introduction
The zone of calcified cartilage (ZCC) anchors articular cartilage (AC) to subchondral bone through a layer of intermediate stiffness. The regulation and functional consequences of cartilage calcification may vary with depth from the articular surface. The hypothesis of this study was that the in vitro calcification of immature AC occurs selectively in the deep region and is associated with a local increase in stiffness.
Methods
AC and growth plate cartilage (GPC) from calves were incubated in DMEM, 1% FBS, 100μg/mL ascorbate, and ±10mM ß-glycerophosphate (ßGP) for up to 3 weeks. To assess the time course and effects of cell viability and ßGP, full-depth strips of AC and GPC were analyzed by histology, indentation, and 45Ca++ uptake. To assess the effect of tissue zone, disks harvested from surface and deep zone AC and from reserve and hypertrophic zone of GPC were incubated independently and analyzed by compression and for 45Ca++ uptake and biochemical components.
Results
The deep ~20% of immature AC calcified within 3 weeks, with calcification dependent on cell viability and ßGP. Mineral was deposited continuously around cells in AC but only between cell columns in GPC. The deep zone of AC exhibited a compressive modulus of 0.53 MPa after ßGP-induced calcification, ~4-fold stiffer than AC incubated without ßGP.
Conclusions
Cartilage explants exhibit inherent zone-specific calcification processes, resulting in an increase in stiffness associated with cartilage calcification. Such properties may be useful for engineering a biomimetic ZCC tissue to integrate cartilaginous tissue to bone, thereby forming a mechanically functional osteochondral unit.
Keywords: articular cartilage, growth plate, calcification, calcified cartilage, biomechanics
Introduction
The zone of calcified cartilage (ZCC) is a specialized structure at the native osteochondral junction that functions as a biomechanical connection between articular cartilage and underlying subchondral bone.1,2 The ZCC is 100–300 μm thick and is bound on one side by the tidemark, the gently undulating interface with uncalcified cartilage, and on the other side by the cement line, the highly interdigitated interface with subchondral bone. The intermediate stiffness of the ZCC can facilitate load transfer at the interface between cartilage and bone by reducing stress concentrations that would otherwise occur from the discontinuity in material stiffness of cartilage and bone.2,3 The ZCC is comprised of chondrocytes expressing the hypertrophic phenotype, which are enveloped in a calcified hyaline matrix.4,5
The native ZCC is formed through the calcification of immature articular cartilage matrix during postnatal development. During postnatal growth, chondrocytes near the articular surface proliferate gradually while forming new cartilage.6,7 Concomitantly, chondrocytes in the deep region near the subchondral bone also proliferate and form new cartilage, some of which becomes calcified and is resorbed and replaced by ingrowing bone advancing toward the articular surface.1,6 This process of new cartilage formation and resorption slows after puberty and achieves a steady-state with skeletal maturity.8 In the mature skeleton, deep zone AC is joined to the underlying bone through the ZCC, with the ZCC normally persisting throughout adulthood. Studying calcification of cartilage in immature AC that does not yet have a fully formed ZCC may provide insight into the factors involved in balancing bone ingrowth with the maturation of the calcified cartilage matrix into the ZCC.
Calcification of articular cartilage may occur through a mechanism similar to that in the terminal stages of endochondral ossification of growth plate cartilage (GPC) during long bone growth. Growth plate chondrocytes progressively differentiate while transitioning from the resting zone to the proliferative, and hypertrophic zones, eventually undergoing apoptosis with matrix calcification, vascular invasion, and bone ingrowth and remodeling.9,10 In the lower hypertrophic zone, calcification initiates in the territorial matrix close to chondrocytes and spreads throughout the matrix of the longitudinal septa between columns of lower hypertrophic chondrocytes.11
The coordinated expression of inhibitory and stimulatory factors by cells in different tissue zones12 may be involved in the regulation of cartilage calcification to form the ZCC. Chondrocytes isolated from the deep zone of immature AC readily calcify during in vitro culture, stimulated by factors such as BMPs, thyroid hormone, and organic phosphate.13–16 Chondrocytes from the superficial zone secrete soluble factors that can inhibit mineralization by deep zone chondrocytes during in vitro co-culture,15 but it remains unclear how readily such factors would be transported from the superficial zone to the deep zone through the cartilage matrix in vivo. Using cartilage explants sectioned vertically to preserve full-thickness zonal structure or sectioned horizontally to separate zones and disrupt zonal cross-talk, the effects of the superficial zone on matrix calcification of deep zone AC can be assessed within the transport limitations of the normal tissue environment. Cartilage explant cultures can expand upon chondrocyte cultures by analyzing how calcification is coordinated with remodeling of the existing extracellular matrix. Cartilage explants have been used to study matrix remodeling for integrative repair between cartilage surfaces17 and may also be useful for studying the relationship between matrix calcification and mechanical function.
The mechanical properties of cartilage may be affected by the extent of its mineralization. For compact bone, the Young’s modulus in tension has a strong positive relationship with mineral content even for bones with different porosities and structures,18 and Young’s modulus may increase substantially with even a relatively small increase in mineral content.19 For calcified cartilage, the nanoindentation modulus is positively related to the local mineral content, although lower for calcified cartilage than for subchondral bone for the same mineral content.20,21 The culture of cartilage explants in vitro has been useful to elucidate the mechanisms and consequences of cartilage cell and matrix metabolism as related to proteoglycan depletion in osteoarthritis and the balance between proteoglycan and collagen metabolism during growth.22,23 Previous cultures of explants have focused on the regions near the articular surface; those extending to the deep layers may elucidate the composition-metabolism-function relationships between progressive mineralization of cartilage, mineral content, and mechanical properties of a calcified cartilage matrix.
The hypothesis of this study was that the in vitro calcification of immature articular cartilage (AC) occurs only in the deep zone and is associated with a local increase in mechanical stiffness. The objectives of this study were to determine the effects of [1] culture duration, [2] cell viability and medium supplementation with ß-glycerophosphate, and [3] separation of surface and deep zone cartilage on the in vitro calcification of immature bovine AC.
Materials and Methods
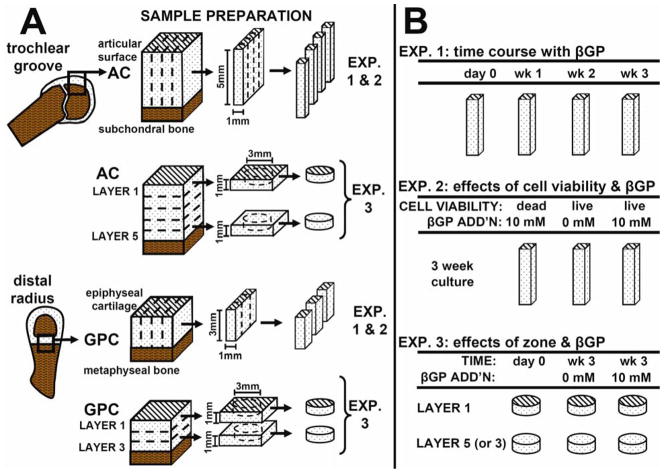
Experimental Design (Fig. 1)
Figure 1.
Sample harvest for AC and GPC explants (A) and experimental design (B).
Experiment 1
The zonal variation and time course of in vitro calcification were assessed by comparing groups analyzed fresh and after 1, 2, or 3 weeks of incubation in medium with addition of ß-glycerophosphate (ßGP). Cartilage strips encompassing the full zonal structure (n = 6–8) were assessed by histology, indentation, and 45Ca++ uptake.
Experiment. 2
The effects of cell viability and ßGP on in vitro calcification were analyzed by killing cells prior to incubation with ßGP, along with live cartilage strips incubated with or without ßGP for 3 weeks. Samples (n = 6–8) were analyzed by histology, indentation and 45Ca++ uptake.
Experiment 3
The effect of zone on in vitro calcification was assessed by separate incubation of surface and deep zone cartilage for 3 weeks with or without ßGP. Samples (n = 6–10) were analyzed fresh and after culture by unconfined compression, 45Ca++ uptake, and assays for sulfated glycosaminoglycan (GAG), collagen, and DNA content. Immature bovine growth plate cartilage (GPC) was used as a positive control tissue for calcification in all experiments.
Cartilage Explant Preparation and Culture (Fig. 1A)
For AC, full-thickness cartilage blocks were harvested from the patellofemoral groove of 1–3 week-old calf (8 animals). For GPC, cartilage blocks were harvested from the distal radius of third-trimester fetal calf from the region between the epiphyseal bone and the metaphyseal bone24 (7 animals). GPC was harvested from fetal calf because at this age the distal radius growth plate was still sufficiently thick (~3 mm) to enable parallel harvest procedures and experiments with AC samples. For Experiments 1 and 2, AC and GPC blocks were sectioned and cut into strips preserving full zonal structure (5×1×1 mm3 for AC, 3×1×1.7 mm3 for GPC). For Experiment 2, some strips were frozen at -80°C for 4h and thawed prior to culture. Loss of cell viability was confirmed using the Live/Dead Viability/Cytotoxicity Kit (Invitrogen, Carlsbad, CA). For Experiment 3, full-thickness cartilage blocks were cut into 1-mm-thick sequential transverse slices (parallel to the cartilage-bone interface for AC or to the metaphyseal bone for GPC), resulting in 5 slices for AC and 3 slices for GPC. From the top and bottom slices, disks (3 mm diameter) were punched. Samples were incubated in medium consisting of DMEM with 1% fetal bovine serum, ascorbic acid (100 μg/mL), 0.5 μCi/mL 45Ca++, antibiotics, amino acids, and HEPES buffer solution at 37°C and 5% CO2. After 3 days, some cultures were supplemented with the addition of 10 mM ßGP. Medium was changed every 3 days until sample termination.
Histology
Upon termination, samples were fixed in 4% paraformaldehyde in phosphate buffered saline (PBS) (pH 7.0) for 18h, embedded in OCT, and sectioned at 8 μm-thickness undecalcified using a cryostat. To assess cell and tissue morphology, sections were stained with hematoxylin and eosin. To localize calcification, sections were stained with 2% Alizarin Red S (pH 4.2) for 2 minutes.25
Biomechanics
Indentation
Each sample strip was subjected to short-duration indentation testing. Using a benchtop mechanical tester (Mach-1TM V500, BioSyntech Canada, Montreal) fitted with a flat-ended indenter tip (0.4 mm diameter) as described previously,26 indentation was performed to a depth of 0.2 mm at a rate of 0.1 mm/s at sites spaced 0.25 mm apart down the centerline of each sample. Indentation load-displacement curves were approximately linear (R2 = 0.96), and the indentation stiffness at each site was obtained by dividing the peak load by the indentation depth. To normalize for strip length, samples were binned into 5 segments or layers, each encompassing 20% of the total strip length, and stiffness values within each layer were averaged.
Compression
Sample disks were subjected to radially-unconfined compression between an impermeable stainless steel post and test chamber bottom surface attached to a mechanical spectrometer (Dynastat, IMASS, Accord, MA), using a loading configuration and setup similar to that described previously.27 Briefly, sample disks were subjected to static compression at amplitudes of 15%, 30% and 45% of the uncompressed thickness, and equilibrium stress-strain data were used to estimate, using least-squares analysis, the equilibrium modulus, E.
Biochemistry
Sample strips in Experiments 1 and 2 were cut into segments of equal length (5 segments for AC or 3 segments for GPC) to allow separate biochemical analysis of different tissue layers. The number of layers was chosen to correspond to the initial length of the strips (5 mm for AC, 3 mm for GPC), and layer 1 was defined to start from the articular surface for AC and from the epiphysis for GPC. Sample strip layers and whole disks from Experiment 3 were solubilized by digestion with proteinase K (Roche Diagnostics, Indianapolis, IN) in 5% EDTA at 60°C for 18 h. Portions of the sample digests were mixed with EcoLume scintillation fluid and assessed for calcium uptake by scintillation counting for incorporated 45Ca++ radioactivity using a LKB/Wallac RackBeta 1214 Liquid Scintillation Counter, which has been correlated with total mineral accumulation.28,29 A portion of radio-labeled medium (with known calcium concentration) was analyzed for 45Ca++ radioactivity in order to estimate a conversion factor between counts and absolute calcium content. For Experiment 3, portions of the sample tissue digest were analyzed to quantify content of sulfated GAG 30, hydroxyproline31, and DNA32. DNA was converted to cell number using a conversion constant of 7.7 pg DNA per cell33 and hydroxyproline was converted to collagen by assuming a mass ratio of collagen:hydroxyproline equal to 7.25:1.34
Statistics
Data are presented as mean ± SEM and were log-10 transformed for statistical analysis. In Experiments 1 & 2, ANOVA was used to assess effects with a fixed factor of tissue layer (1–5 for AC, 1–3 for GPC) and a repeated factor of culture duration (0, 1, 2, or 3 weeks) or culture condition (dead tissue with ßGP, live tissue with no ßGP, live tissue with ßGP). In Experiment 3, ANOVA was used to assess effects with a fixed factor of tissue layer (1 or 5 for AC, 1 or 3 for GPC) and repeated factor of culture condition (freshly isolated, culture with no ßGP, culture with ßGP). The relationship between equilibrium modulus and calcium, water, GAG, and collagen content in Experiment 3 was analyzed using linear regression. Tukey’s post-hoc comparisons were used, with P values less than 0.05 considered significant.
Results
Experiment 1: Time course with ßGP
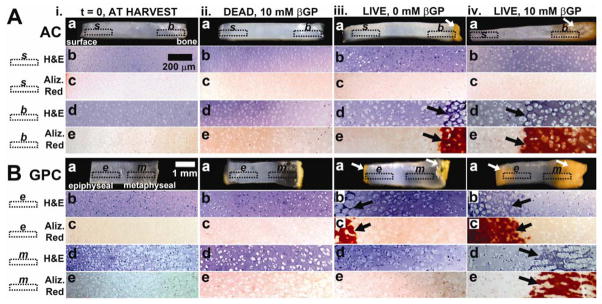
During the 3-week culture period, AC and GPC samples developed an orange-brown pigmentation which appeared during the culture period in regions of calcification (Fig. 2A.iv.a, B.iv.a). In AC, the orange-brown coloration was evident in the deep region of samples by week 2 and remained in the deep ~25% of tissue. In GPC, the orange-brown pigmentation appeared on both the metaphyseal and epiphyseal ends during week 1 of culture and advanced towards the middle to cover about ~50% of the sample area by week 3. The source of the pigmentation is unclear but is not associated with tissue fixation.
Figure 2.
Macroscopic and microscopic views of AC (A) and GPC (B), fresh (i) and after 3wk incubation of dead tissue in medium supplemented with 10 mM β-glycerophosphate (ii), live tissue in medium with 0mM β-glycerophosphate (iii), and live tissue with 10 mM β-glycerophosphate (iv). Micrographs are of regions indicated: s, b, e, m. Arrows indicate areas of calcification.
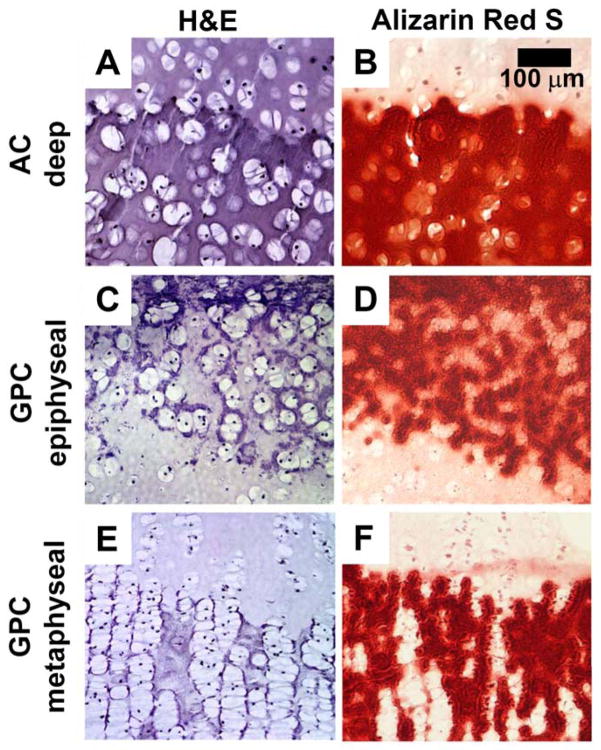
Histological staining with H&E and Alizarin Red S showed different calcification patterns for AC and GPC (Fig. 2, 3, S1). In AC, enlarged chondrocytes and calcified matrix were localized to the deep ~25% of AC. Chondrocytes appeared to be randomly organized (Fig. 2A.iv.d, 3A) with Alizarin Red-stained matrix completely surrounding the cells (Fig. 2A.iv.e, 3B). For GPC, there was a different pattern of calcification for the epiphyseal and metaphyseal ends. At the epiphyseal side, the organization of cells and mineral was similar to deep AC (Fig. 2B.iv.b, 2B.iv.c, 3CD), while at the metaphyseal side, the chondrocytes were stacked in columns (Fig. 2B.iv.d, 3E) with Alizarin Red-stained matrix between cell columns (Fig. 2B.iv.e, 3F).
Figure 3.
Deep region of AC (A, B), epiphyseal end of GPC (C, D), and metaphyseal end of GPC (E, F) after incubation in medium supplemented with 10 mM β-glycerophosphate for 3 weeks. Sections are stained with Hematoxylin & Eosin (A, C, E) or Alizarin Red S (B, D, F).
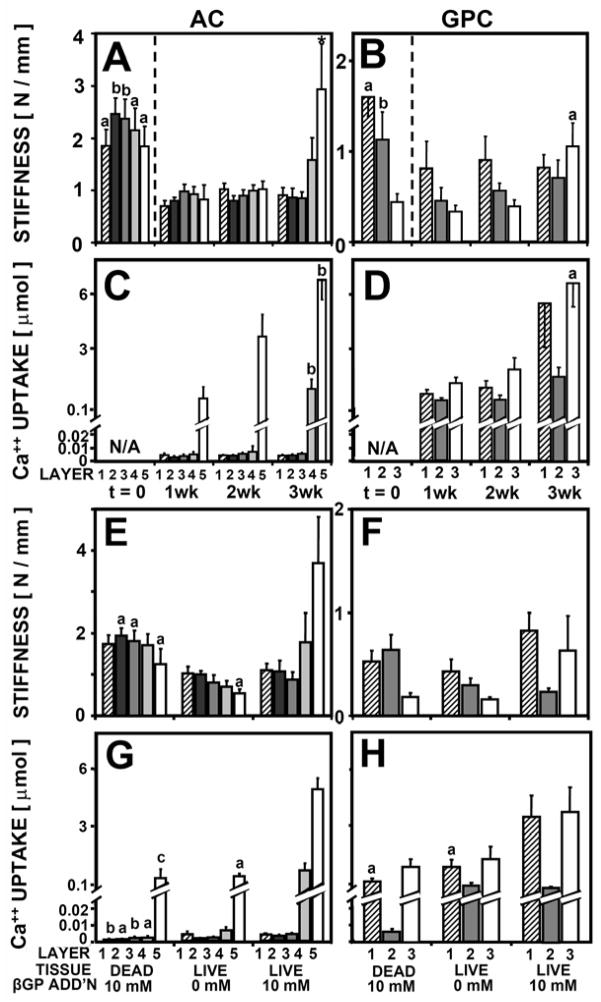
Indentation stiffness was dependent on culture duration for both AC and GPC (P < 0.01), with a significant interaction with tissue layer for AC (P < 0.005) but not for GPC (P = 0.07) (Fig 4AB) (Table 1). For AC, the indentation stiffness of layer 5 (deep 20%) at week 3 was 2.9 N/mm, 4-fold higher than its stiffness at week 1 (P < 0.05). For GPC, the indentation stiffness of layer 3 at week 3 was 1.1 N/mm, 3-fold higher than at week 1 (P < 0.05).
Figure 4.
Indentation stiffness (A, B, E, F) and 45Ca++ uptake (C, D, G, H) by AC (A, C, E, G) and GPC (B, D, F, H) as a function of tissue layer and culture duration up to 3 weeks, n = 6–8 (A-D) and as a function of tissue layer, cell viability, and media supplementation with βGP after incubation for 3 weeks, n = 6–8 (E-H). Significant differences from t=1wk (A-D) or from live tissue with 10 mM βGP addition (E-H) are indicated by a (p<0.05), b (p<0.01), c (p<0.001).
Table 1.
Indentation stiffness and equilibrium modulus of AC and GPC, fresh and after 3 weeks of culture with either no βGP or 10 mM βGP.
| tissue | layer | indentation stiffness [ N / mm ] | equilibrium modulus [ MPa ] | ||||
|---|---|---|---|---|---|---|---|
| fresh | 0 mM βGP |
10 mM βGP |
fresh | 0 mM βGP |
10 mM βGP |
||
| AC | 1 | 1.85 | 1.03 | 0.91 | 0.24 | 0.12 | 0.13 |
| 2 | 2.46 | 1.00 | 0.86 | --- | --- | --- | |
| 3 | 2.37 | 0.80 | 0.87 | --- | --- | --- | |
| 4 | 2.15 | 0.71 | 1.58 | --- | --- | --- | |
| 5 | 1.84 | 0.55 | 2.93 | 0.33 | 0.057 | 0.53 | |
| GPC | 1 | 1.60 | 0.44 | 0.82 | 0.23 | 0.064 | 0.64 |
| 2 | 1.13 | 0.29 | 0.71 | --- | --- | --- | |
| 3 | 0.44 | 0.16 | 1.05 | 0.035 | 0.006 | 0.16 | |
Ca++ uptake was dependent on culture duration for both AC and GPC (P < 0.001), with a significant interaction with tissue layer for AC (P < 0.001) (Fig 4CD). For AC, Ca++ uptake by layer 5 increased 6-fold from 1.2 μmol to 6.6 μmol Ca++ between week 1 and 3 (P < 0.01). Similarly, Ca++ uptake by layer 3 of GPC increased from 1.2 μmol to 6.7 μmol Ca++ between week 1 and 3 (P < 0.05).
Experiment 2: Effects of cell viability and βGP
The effects of cell viability and βGP were evident in the macroscopic appearance of AC and GPC samples. Dead AC samples did not change in appearance from time of harvest (Fig. 2A.ii.a), whereas AC with no βGP had some orange pigmentation at the deep edge that did not extend into the tissue (Fig. 2A.iii.a). For GPC, both dead samples and samples with no βGP developed orange pigmentation on the epiphyseal and metaphyseal edges that did not extend into the tissue (Fig. 2B.ii.a, 2B.iii.a). Histological staining revealed similar cell and mineral organization as described in Experiment 1.
Indentation stiffness was dependent on cell viability and βGP for AC (P < 0.01) with a significant interaction with tissue layer (P <0.01) (Fig. 4EF) (Table 1). The stiffness of layer 5 of AC was ~3-fold lower for dead samples and samples without βGP than for samples with βGP (P <0.05) (Fig. 4E). Indentation stiffness of GPC layers 1 and 3 followed a similar trend (Fig 4F). Ca++ uptake was dependent on cell viability and βGP for AC (P < 0.001) and for GPC (P <0.001), with a significant interaction with tissue layer for AC (P < 0.001) (Fig. 4GH).
Ca++ uptake by layer 5 of AC was 10-fold lower for dead samples and samples with no βGP than for samples with βGP (P < 0.05) (Fig. 4G). Ca++ uptake by GPC layers 1 and 3 followed a similar trend (Fig. 4H).
Experiment 3: Effects of zone and βGP
The effects of tissue zone and βGP were evident in the macroscopic appearance of AC and GPC disks. Disks from AC layer 5 and from GPC incubated with βGP became orange-brown in color, while disks from AC layer 1 remained white and glossy.Samples incubated with no βGP did not change in appearance from time of harvest.
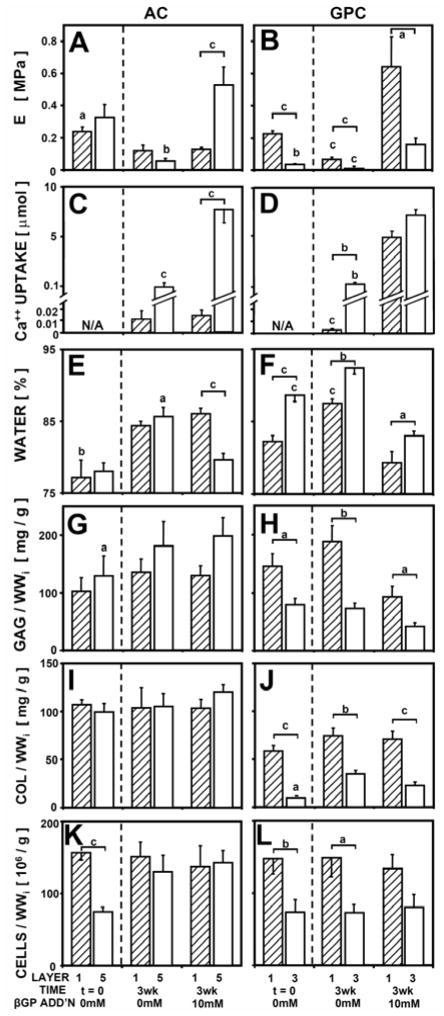
The equilibrium unconfined compressive modulus was dependent on βGP for both AC (P < 0.01) and GPC (P <0.001), with an interaction with tissue zone for AC (P < 0.01) (Fig. 5AB) (Table 1). For layer 1 of AC, βGP did not affect the modulus, which was ~0.1 MPa either with or without βGP. For layer 5 of AC, samples with βGP had a modulus of 0.53 MPa, 9-fold higher than samples without βGP (P < 0.001). For GPC, both layer 1 and 3 had a higher modulus with β GP.
Figure 5.
Unconfined compressive equilibrium modulus (A, B), 45Ca++ uptake (C, D), water content (E, F), and sulfated glycosaminoglycan content (G, H), collagen contenct (I, J), and cell number (K, L) for AC (A, C, E, G, I, K) and GPC (B, D, F, H, J, L) , as a function of tissue layer and media supplementation with βGP, n = 6–10. Significant differences from tissue with βGP or between tissue layers 1 and 5 (or 3) are indicated by a (p<0.05), b (p<0.01), c (p<0.001).
Ca++ uptake was dependent on βGP for both AC (P < 0.001) and GPC (P <0.001), with an interaction with tissue zone for AC (P < 0.01) (Fig. 5CD). Ca++ uptake results followed similar trends as described in Experiments 1 and 2.
Water content was dependent on βGP for AC (P < 0.001) and GPC (P <0.001), with an interaction with tissue zone for AC (P < 0.05) (Fig. 5EF). For AC samples, layer 5 AC with βGP had 5% lower water content than samples without βGP (P < 0.05), but there was no difference for layer 1. Water content of both layers 1 and 3 GPC decreased with βGP (P < 0.001). Biochemical content, including GAG, collagen, and cell number, did not change with βGP for AC or GPC (Fig, 5GHIJKL). GAG, collagen, and cell content were higher in layer 1 of GPC than in layer 3 (P < 0.05).
The equilibrium modulus correlated positively with Ca++ content for AC (P < 0.001, R2 = 0.70) and for GPC (P < 0.01, R2 = 0.30) and correlated negatively with water content for AC (P < 0.01, R2 = 0.18) and for GPC (P < 0.001, R2 = 0.44). For fresh samples and samples incubated without βGP, equilibrium modulus correlated positively with collagen content for AC (P < 0.001, R2 = 0.49) and with both GAG and collagen content for GPC (P < 0.001, R2 = 0.71).
Discussion
The results presented here indicate that the deep ~20% of immature AC can undergo calcification within 3 weeks of in vitro explant culture, with functional biomechanical consequences related to the extent of calcification as governed by cell viability and medium supplementation with βGP as a phosphate source. Compared to fresh tissue, incubation of cartilage in medium without βGP resulted in lower tissue stiffness and compressive modulus for all tissue zones, whereas incubation in media with βGP resulted in calcification and markedly higher tissue stiffness and modulus (2-fold higher than fresh tissue and 4-fold higher than tissue incubated without βGP) in specific regions of AC and GPC. The regions of cartilage that calcified in vitro were the 1 mm of AC adjacent to the subchondral bone (layer 5) and 1 mm from each end of GPC adjacent to the epiphyseal and metaphyseal bone (layers 1 and 3). Zonal variations in calcification were maintained whether zones were incubated together as intact tissue (Experiments 1 and 2) or incubated separately (Experiment 3). Calcification patterns in AC and GPC had a distinct structure of mineral organization around cells, consistent with their expected in vivo phenotypes. In the deep immature AC, mineral was deposited around cells, similar to the organization seen in epiphyseal GPC but different from metaphyseal GPC, in which mineral was deposited primarily between cell columns. Such structure-metabolism-function relationships have implications for the mechanisms and consequences of native cartilage maturation, as well as strategies for tissue engineering to establish the ZCC.
The results of the study reflect, in part, various parameters chosen for study in explant culture, including the use of β-GP as a phosphate source in the calcifying medium to modulate mineral deposition in the cartilage matrix. Medium supplementation with 10 mM βGP has been commonly used in studies of osteoblast cultures to promote calcium phosphate deposition in vitro,35 with βGP acting as a source of phosphate due to its efficient hydrolysis by the alkaline phosphatase secreted by cells and present in serum.35,36 In cultures of cells such as osteoblasts, the use of βGP exceeding 2 mM may result in supraphysiological levels of medium phosphate (compared to physiological levels of 1.0–1.5 mM phosphate and ~3 mM organic phosphate),35 leading to non-specific precipitation of mineral in vitro. In the present study, the low serum concentration (1%) and a relatively low rate of alkaline phosphatase secretion by chondrocytes within cartilage explants, along with the hindered transport of macromolecules within cartilage, may have maintained a physiological process of mineral precipitation in the cartilage matrix.
The calcified cartilage formed in vitro from deep immature AC had an equilibrium modulus of 0.53 MPa in unconfined compression, 4-fold higher than the modulus for cartilaginous calcified tissue formed in vitro by AC deep zone chondrocytes in a previous study.37 The higher stiffness of calcified explants may be due to higher Ca++ content as well as higher collagen content from using the native matrix rather than building a new cartilaginous matrix. The equilibrium modulus of calcified metaphyseal GPC was 0.16 MPa, 3-fold lower than calcified deep zone AC despite having similar Ca++ uptake. This can be attributed to the lower collagen and GAG content in the GPC matrix compared to the AC. The stiffness of in vitro mineralized explants in the present study was still lower than that of native ZCC in bovine femur, which has an elastic modulus of 320 MPa estimated in three-point bending.38
The selective in vitro calcification of immature deep zone AC over 3 weeks is consistent with previous studies and extends those findings by providing a tissue-level view of in vitro calcification. The calcification of the deep 20% of AC explants in vitro in the presence of βGP supports previous findings that deep zone articular chondrocytes cultured in monolayer or at high density mineralize under stimulatory conditions, while superficial zone chondrocytes can resist mineralization.14–16 Unlike monolayer chondrocyte cultures, which are subject to dedifferentiation39 and may have effects on cell hypertrophy and mineralization, explant cultures maintain the chondrocyte phenotype since the cells remain within their native extracellular matrix.
In the present study, the surface region (layer 1) of AC resisted mineralization in the presence of βGP, but did not inhibit mineralization of the deep zone when incubated together as intact full-thickness samples. Further investigation is needed to determine whether any inhibitory factors were secreted by the superficial zone chondrocytes and whether such factors were unable to inhibit mineralization in the deep zone due to hindered transport through the cartilage matrix. In the present study, incubation of deep zone AC for 3 weeks in medium with βGP resulted in a Ca++ content of 7.1 μmol per disk, or 8.4 wt% Ca++. This is similar to the Ca++ content of 9.1 wt% found for the ZCC of 9-month-old bovine,40 but lower than the 21 wt% in the ZCC of a 2-year-old bovine5 and the 24 wt% in ZCC of mature human patella.41 In mineralizing chondrocyte cultures, deep zone chondrocytes can form mineral deposits within 1 day after addition of calcification medium, with progressive mineralization and accumulation of up to 6.5 wt% Ca++ content after 8 weeks of culture.15,16 Incubation of AC explants for longer times in vitro may result in mineral content approaching levels found in native mature ZCC.
The form of mineral deposited in the deep layer of immature AC explants and in GPC explants remains to be established. The calcification endpoint measures of 45Ca++ uptake and Alizarin Red S staining were chosen to provide complementary information; however, they do not differentiate between different mineral phases, since they are both indices of calcium bound in the cartilage matrix. It remains to be determined whether the deposited mineral was comprised of amorphous calcium deposits or a more mature phase such as hydroxyapatite.
The use of young animals in the present study may have affected the type of calcified cartilage matrix that was formed. In immature AC where the ZCC has not yet developed, full-thickness AC explants include the articular epiphyseal complex cartilage, which normally becomes replaced by bone to expand the secondary ossification center and provide radial growth.42 Thus, the calcification of deep immature AC in the present study is not necessarily equivalent to the formation of mature ZCC in vivo, which is likely a late event in the terminal differentiation of chondrocytes deriving from the superficial zone.7 The use of immature AC may serve as a model to study transplantation of engineered cartilaginous tissues, which would likely resemble immature cartilage in terms of cellularity and matrix content and may respond similarly to in vitro stimuli as immature AC. In addition, the calcification of immature cartilage matrix in vitro could be applicable to form a ZCC-like tissue that can be functionally equivalent to the native ZCC in an engineered osteochondral graft.
The modulated increase in stiffness associated with tissue calcification may be useful for tissue engineering methods to create a ZCC-like tissue to facilitate the biomimetic attachment of a cartilaginous tissue to a bony substrate. Anchoring cartilaginous tissue to bone is an important step toward the ultimate goal of fabricating a large osteochondral graft capable of withstanding physiological joint articulation soon after implantation. The in vitro culture of AC explants is useful not only for providing insight into critical biological questions in the development of the native osteochondral interface, but also for designing methods to integrate the cartilage-bone interface in chondral and osteochondral graft treatments for damaged cartilage or osteoarthritis.
Supplementary Material
Acknowledgments
Funding: This work was supported by NIH and an HHMI Professor’s Award to UCSD in support of RLS.
Footnotes
Conflict of Interest: All authors have no conflict of interest.
Contributor Information
Jennifer Hwang, Email: jenhwang@ucsd.edu.
Espoir M Kyubwa, Email: ekyubwa@ucsd.edu.
Won C Bae, Email: wbae@ucsd.edu.
William D Bugbee, Email: bugbee.william@scrippshealth.org.
Koichi Masuda, Email: koichimasuda@ucsd.edu.
References
- 1.Oegema T, Jr, Carpenter R, Hofmeister F, Thompson RC., Jr The interaction of the zone of calcified cartilage and subchondral bone in osteoarthritis. Microsc Res Tech. 1997;37:324–32. doi: 10.1002/(SICI)1097-0029(19970515)37:4<324::AID-JEMT7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 2.Muller-Gerbl M, Schulte E, Putz R. The thickness of the calcified layer of articular cartilage: a function of the load supported? J Anat. 1987;154:103–11. [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson DD, Brown TD, Radin EL. The influence of basal cartilage calcification on dynamic juxtaarticular stress transmission. Clin Orthop Rel Res. 1993;286:298–307. [PubMed] [Google Scholar]
- 4.Hunziker EB, Quinn TM, Hauselmann HJ. Quantitative structural organization of normal adult human articular cartilage. Osteoarthritis Cartilage. 2002;10:564–72. doi: 10.1053/joca.2002.0814. [DOI] [PubMed] [Google Scholar]
- 5.Lovell T, Eyre D. Unique biochemical characteristics of the calcified cartilage zone of articular cartilage. Trans Orthop Res Soc. 1988;13:511. [Google Scholar]
- 6.Mankin HJ. Localization of tritiated thymidine in articular cartilage of rabbits I. growth in immature cartilage. J Bone Joint Surg Am. 1962;44-A:682–98. [Google Scholar]
- 7.Hunziker EB, Kapfinger E, Geiss J. The structural architecture of adult mammalian articular cartilage evolves by a synchronized process of tissue resorption and neoformation during postnatal development. Osteoarthritis Cartilage. 2007;15:403–13. doi: 10.1016/j.joca.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Lane LB, Bullough PG. Age-related changes in the thickness of the calcified zone and the number of tidemarks in adult human articular cartilage. J Bone Joint Surg Br. 1980;62:372–5. doi: 10.1302/0301-620X.62B3.7410471. [DOI] [PubMed] [Google Scholar]
- 9.Ballock RT, O'Keefe RJ. The biology of the growth plate. J Bone Joint Surg Am. 2003;85-A:715–26. [PubMed] [Google Scholar]
- 10.Farnum CE, Wilsman NJ. Determination of proliferative characteristics of growth plate chondrocytes by labeling with bromodeoxyuridine. Calcif Tissue Int. 1993;52:110–9. doi: 10.1007/BF00308319. [DOI] [PubMed] [Google Scholar]
- 11.Hall BK, Newman S. Cartilage: Molecular Aspects. CRC Press; 1991. p. 268. [Google Scholar]
- 12.Huitema LF, Vaandrager AB. What triggers cell-mediated mineralization? Front Biosci. 2007;12:2631–45. doi: 10.2741/2260. [DOI] [PubMed] [Google Scholar]
- 13.Oyajobi BO, Frazer A, Hollander AP, Graveley RM, Xu C, Houghton A, Hatton PV, Russell RG, Stringer BM. Expression of type X collagen and matrix calcification in three-dimensional cultures of immortalized temperature-sensitive chondrocytes derived from adult human articular cartilage. J Bone Miner Res. 1998;13:432–42. doi: 10.1359/jbmr.1998.13.3.432. [DOI] [PubMed] [Google Scholar]
- 14.Wu LN, Ishikawa Y, Genge BR, Wuthier RE. Chondrocytes isolated from tibial dyschondroplasia lesions and articular cartilage revert to a growth plate-like phenotype when cultured in vitro. J Cell Physiol. 2005;202:167–77. doi: 10.1002/jcp.20105. [DOI] [PubMed] [Google Scholar]
- 15.Jiang J, Leong NL, Mung JC, Hidaka C, Lu HH. Interaction between zonal populations of articular chondrocytes suppresses chondrocyte mineralization and this process is mediated by PTHrP. Osteoarthritis Cartilage. 2008;16:70–82. doi: 10.1016/j.joca.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Kandel RA, Boyle J, Gibson G, Cruz T, Speagle M. In vitro formation of mineralized cartilagenous tissue by articular chondrocytes. In Vitro Cell Dev Biol Anim. 1997;33:174–81. doi: 10.1007/s11626-997-0138-7. [DOI] [PubMed] [Google Scholar]
- 17.Ahsan T, Lottman LM, Harwood FL, Amiel D, Sah RL. Integrative cartilage repair: inhibition by beta-aminoproprionitrile. J Orthop Res. 1999;17:850–7. doi: 10.1002/jor.1100170610. [DOI] [PubMed] [Google Scholar]
- 18.Currey JD. The effect of porosity and mineral content on the Young's modulus of elasticity of compact bone. J Biomech. 1988;21:131–9. doi: 10.1016/0021-9290(88)90006-1. [DOI] [PubMed] [Google Scholar]
- 19.Currey J. Collagen and the Mechanical Properties of Bone and Calcified Cartilage. 1. Vol. 1. New York, NY: Springer; 2008. pp. 397–420. [Google Scholar]
- 20.Gupta HS, Schratter S, Tesch W, Roschger P, Berzlanovich A, Schoeberl T, Klaushofer K, Fratzl P. Two different correlations between nanoindentation modulus and mineral content in the bone-cartilage interface. J Struct Biol. 2005;149:138–48. doi: 10.1016/j.jsb.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Ferguson VL, Bushby AJ, Boyde A. Nanomechanical properties and mineral concentration in articular calcified cartilage and subchondral bone. J Anat. 2003;203:191–202. doi: 10.1046/j.1469-7580.2003.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kempson GE, Tuke MA, Dingle JT, Barrett AJ, Horsfield PH. The effects of proteolytic enzymes on the mechanical properties of adult human articular cartilage. Biochim Biophys Acta. 1976;428:741–60. doi: 10.1016/0304-4165(76)90205-1. [DOI] [PubMed] [Google Scholar]
- 23.Asanbaeva A, Masuda K, Thonar EJ, Klisch SM, Sah RL. Mechanisms of cartilage growth: modulation of balance between proteoglycan and collagen in vitro using chondroitinase ABC. Arthritis Rheum. 2007;56:188–98. doi: 10.1002/art.22298. [DOI] [PubMed] [Google Scholar]
- 24.Boustany NN, Gray ML, Black AC, Hunziker EB. Correlation between synthetic activity and glycosaminoglycan concentration in epiphyseal cartilage raises questions about the regulatory role of interstitial pH. J Orthop Res. 1995;13:733–9. doi: 10.1002/jor.1100130513. [DOI] [PubMed] [Google Scholar]
- 25.McGee-Russell S. Histochemical methods for calcium. J Histochem Cytochem. 1958;6:22–42. doi: 10.1177/6.1.22. [DOI] [PubMed] [Google Scholar]
- 26.Bae WC, Schumacher BL, Sah RL. Indentation probing of human articular cartilage: effect on chondrocyte viability. Osteoarthritis Cartilage. 2007;15:9–18. doi: 10.1016/j.joca.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Chen AC, Bae WC, Schinagl RM, Sah RL. Depth- and strain-dependent mechanical and electromechanical properties of full-thickness bovine articular cartilage in confined compression. J Biomech. 2001;34:1–12. doi: 10.1016/s0021-9290(00)00170-6. [DOI] [PubMed] [Google Scholar]
- 28.Pourmand EP, Binderman I, Doty SB, Kudryashov V, Boskey AL. Chondrocyte apoptosis is not essential for cartilage calcification: evidence from an in vitro avian model. J Cell Biochem. 2007;100:43–57. doi: 10.1002/jcb.20977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boskey AL, Stiner D, Doty SB, Binderman I, Leboy P. Studies of mineralization in tissue culture: optimal conditions for cartilage calcification. Bone Miner. 1992;16:11–36. doi: 10.1016/0169-6009(92)90819-y. [DOI] [PubMed] [Google Scholar]
- 30.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–7. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 31.Woessner JF. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–7. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 32.McGowan KB, Kurtis MS, Lottman LM, Watson D, Sah RL. Biochemical quantification of DNA in human articular and septal cartilage using PicoGreen and Hoechst 33258. Osteoarthritis Cartilage. 2002;10:580–7. doi: 10.1053/joca.2002.0794. [DOI] [PubMed] [Google Scholar]
- 33.Kim YJ, Sah RLY, Doong JYH, Grodzinsky AJ. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem. 1988;174:168–76. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- 34.Pal S, Tang L-H, Choi H, Habermann E, Rosenberg L, Roughley P, Poole AR. Structural changes during development in bovine fetal epiphyseal cartilage. Collagen Rel Res. 1981;1:151–76. doi: 10.1016/s0174-173x(81)80017-9. [DOI] [PubMed] [Google Scholar]
- 35.Chung CH, Golub EE, Forbes E, Tokuoka T, Shapiro IM. Mechanism of action of beta-glycerophosphate on bone cell mineralization. Calcif Tissue Int. 1992;51:305–11. doi: 10.1007/BF00334492. [DOI] [PubMed] [Google Scholar]
- 36.Khouja HI, Bevington A, Kemp GJ, Russell RG. Calcium and orthophosphate deposits in vitro do not imply osteoblast-mediated mineralization: mineralization by betaglycerophosphate in the absence of osteoblasts. Bone. 1990;11:385–91. doi: 10.1016/8756-3282(90)90131-h. [DOI] [PubMed] [Google Scholar]
- 37.Allan KS, Pilliar RM, Wang J, Grynpas MD, Kandel RA. Formation of biphasic constructs containing cartilage with a calcified zone interface. Tissue Eng. 2007;13:167–77. doi: 10.1089/ten.2006.0081. [DOI] [PubMed] [Google Scholar]
- 38.Mente PL, Lewis JL. Elastic modulus of calcified cartilage is an order of magnitude less than that of subchondral bone. J Orthop Res. 1994;12:637–47. doi: 10.1002/jor.1100120506. [DOI] [PubMed] [Google Scholar]
- 39.Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–24. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 40.Kandel R, Hurtig M, Grynpas M. Characterization of the mineral in calcified articular cartilagenous tissue formed in vitro. Tissue Eng. 1999;5:25–34. doi: 10.1089/ten.1999.5.25. [DOI] [PubMed] [Google Scholar]
- 41.Zizak I, Roschger P, Paris O, Misof BM, Berzlanovich A, Bernstorff S, Amenitsch H, Klaushofer K, Fratzl P. Characteristics of mineral particles in the human bone/cartilage interface. J Struct Biol. 2003;141:208–17. doi: 10.1016/s1047-8477(02)00635-4. [DOI] [PubMed] [Google Scholar]
- 42.Carlson CS, Hilley HD, Henrikson CK. Ultrastructure of normal epiphyseal cartilage of the articular-epiphyseal cartilage complex in growing swine. Am J Vet Res. 1985;46:306–13. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.