Abstract
Melanomas from different patients have been shown to express shared tumor antigens, which can be recognized in the context of the appropriate MHC class I molecules by cytolytic T cells. To determine if T-cell-defined melanoma antigens are expressed on other tumors of neuroectodermal origin, four melanoma-specific cytotoxic T lymphocyte (CTL) cultures derived from tumor-infiltrating lymphocytes (TIL) were tested for lysis of a panel of 23 HLA-A2+ neuroectodermal tumor cell lines of various histologies, including retinoblastoma (1), neuroblastoma (8), neuroepithelioma (6), astrocytoma (2), neuroglioma (1), and Ewing's sarcoma (5). Low expression of MHC class I and/or ICAM-1 molecules was found on 22 of 23 neuroectodermal tumor lines, and could be enhanced by treatment with interferon γ (IFNγ). Following IFNγ treatment, three Ewing's sarcoma lines were lysed by at least one melanoma TIL culture, and levels of lysis were comparable to melanoma lysis by these TIL. Lysis could be inhibited by monoclonal antibodies directed against MHC class I molecules and against CD3, indicating specific immune recognition of tumor-associated antigens. None of the other neuroectodermal tumors tested were lysed by TIL, but they could be lysed by non-MHC-restricted lymphokine-activated killer cells. This demonstration of immunological cross-reactivity between melanomas and Ewing's sarcomas, two tumors of distinct histological types with a common embryonic origin, has implications for the developmental nature of these CTL-defined tumor antigens. It also raises the possibility that specific antitumor immunotherapies, such as vaccines, may be reactive against more than one form of cancer.
Keywords: Melanoma, Neural crest, Ewing's sarcoma, Tumor-infiltrating lymphocyte
Introduction
Clinical studies have shown that adoptive immunotherapy with melanoma-specific cytotoxic T lymphocytes (CTL) derived from some melanoma patients, in combination with interleukin-2 (IL-2), can induce objective regressions of metastatic disease [19, 25]. In vitro studies with many of these melanoma-specific CTL have demonstrated recognition of autologous and allogeneic HLA-matched melanomas, but not HLA-unmatched melanomas nor nonmelanoma tumors, as demonstrated by cytolysis and secretion of cytokines [9, 10, 17, 20]. The hypothesis that melanoma-specific CTL can recognize commonly expressed melanoma-associated antigens presented on allogeneic tumors in the context of appropriate MHC class I molecules [6] is now supported by the direct demonstration of shared antigens through gene cloning techniques [2, 28] and peptide isolation [21, 22]. The CTL-defined melanoma antigen MZ2-E, encoded by the MAGE-1 gene and presented in the context of HLA-A1, is expressed by a variety of cultured and fresh tumors including other derivatives of the neuroectoderm such as small-cell lung cancer, medullary carcinoma of the thyroid, and neuroblastoma [28, 31] (J. Weber et al., personal communication). Another melanoma-associated antigen defined by T cell reactivity, SK29-Ab, is HLA-A2-restricted and commonly expressed by melanomas as well as normal melanocytes [2]. SK29-Ab is the product of the normal tyrosinase gene, and is thus considered a differentiation antigen. Recently, melanoma-specific HLA-A2-restricted CTL clones have been shown to recognize cultured normal melanocytes, supporting the notion that melanoma-associated antigens may be lineage-specific [1]. In addition, monoclonal antibodies (mAb) directed against melanoma-associated antigens may bind a variety of nonmelanoma tumors or normal tissues that have in common their embryonic origin from the neuroectoderm [8, 26].
Taken together, these findings suggest that melanoma-associated antigens defined by T cell recognition may be presented by tumors of other histologies that are also derived from the neuroectoderm. To our knowledge, T cell recognition of HLA-compatible tumors of different histologies has not yet been documented, although this might be expected from the distribution of common melanoma antigens detected at the mRNA level [2, 28] (J. Weber et al., personal communication). To address this question, we used melanoma-specific HLA-A2-restricted CTL derived from tumor-infiltrating lymphocytes (TIL) to screen a panel of HLA-A2+ neuroectodermal tumors for recognition. Screened tumors included derivatives of the neural crest (neuroblastoma, neuroepithelioma, Ewing's sarcoma) and the neural tube (astrocytoma, retinoblastoma, neuroglioma). We found that Ewing's sarcomas, but not tumors from other neuroectodermal tumor histologies, were lysed by melanoma-specific CTL. Lysis was MHC-class-I restricted and T-cell-receptor(TCR)-mediated, indicating specific antigen recognition.
Materials and methods
TIL cultures
TIL cultures were established from metastatic melanoma lesions as previously described [24]. Briefly, resected specimens were minced and enzymatically digested overnight. Viable mononuclear and tumor cells were retrieved by separation over Ficoll/Hypaque gradients (Organon Teknika Corp., Durham, N. C.). TIL cultures were maintained in medium that consisted of RPMI-1640 medium containing antibiotics, 10 mM HEPES buffer (Biofluids Inc., Rockville, Md.) and 2 mM l-glutamine (MA Whittaker Bioproducts, Walkersville, Md.). This medium was supplemented with 10% heat-inactivated human AB serum. Conditioned medium from 3- to 4-day allogeneic lymphokine-activated killer cell (LAK) cultures was added to TIL cultures at a final concentration of 20% (v/v), and IL-2 was added to the cultures at a final concentration of 6000 IU/ml (a gift of the Chiron Corporation, Emeryville, Calif.). Under these conditions, a selective outgrowth of lymphocytes was observed. Cultures were maintained at 37° C in 5% CO2 and passaged weekly for 3–8 weeks. Cultures were then cryo-preserved, and subsequently thawed and recultured for use in assays.
Lymphokine-activated killer cell cultures
Peripheral blood mononuclear cells (PBMC) obtained from normal donors were cultured in RPMI-1640 medium supplemented with 2% heat-inactivated human AB serum, antibiotics, and IL-2 6000 IU/ml as previously described [24]. LAK cells were used as effectors in lysis assays on culture days 3–7. Superuatants from 3- to 4-day cultures were used to supplement TIL medium.
Generation of vaccinia-virus-specific H-2Kd-restricted murine CTL
We used the Vac-Kd system previously described [18] to evaluate antigen processing and presentation by neuroectodermal tumors (Vac-Kd a generous gift from Dr. J. Yewdell and Dr. J. Bennick, NIH, Bethesda, Md.). To generate effector cells, female 6- to 8-week-old BALB/c mice were primed with intravenous injection of 100-plaque forming units (PFU) of vaccinia virus. Spleens were removed at 2 weeks, dispersed to single-cell suspensions, and stimulated in vitro by vaccinia-virus-infected BALB/c splenocytes at a 2:1 ratio. Cells were cultured in Iscove's modified medium with 7.5% heat-inactivated fetal calf serum (FCS) for 7 days.
Generation of influenza-M1-specific HLA-A2-restricted human CTL
We used the influenza M1 peptide system previously described [17] to evaluate antigen presentation by HLA-A2+ neuroectodermal tumors. PBMC from an HLA-A2+ donor were cultured at a density of 3 × 106 cells in 2 ml Iscove's modified medium supplemented with 10% human AB serum, with 1 μM M1 58–66 peptide (GILGFVFTL, more than 98% pure; Multiple Peptide Systems, San Diego, Calif.). IL-2 (30 IU/ml) was added to the culture on day 3. Responding cells were harvested on day 7 and mixed with stimulator cells prepared by incubating irradiated PBMC from the same donor with 1 μM M1 58–66 peptide. On day 8, IL-2 was added to the culture at a concentration of 60 IU/ml. The cultured T cells exhibited HLA-A2-restricted, M1-specific lysis after three or four restimulations.
Tumor cell cultures
A total of 30 neuroectodermal tumor cell lines of various histologies were collected for this study. They included 2 retinoblastoma lines: WERI-Rb-1 and Y-79 (from American Type Culture Collection, ATCC, Rockville, Md.), 10 neuroblastoma lines: SK-N-AS, SK-N-DZ, NLF, SMS-KAN, SK-N-DW, SK-N-F1, LA-N-1 (from Dr. O. M. E1-Badry, NIH, Bethesda, Md.), IMR-32, SK-N-SH, and SK-N-MC (ATCC), 2 neuroepithelioma lines: TC-32 and CHP-100 (Dr. O. M. E1-Badry), 4 clones of CHP-100: A1.2/A, A1.2/C, B3, and C3 (generous gifts of Dr. L. Neckers, NIH, Bethesda, MD), 3 astrocytoma lines: U-87 MG, U-373 MG and CCF-STTG1 (ATCC), 2 glioblastoma lines: U-138 MG and T-98-G (ATCC), 1 glioma line: Hs 683 (ATCC), 1 neuroglioma line: H4 (ATCC), and 5 Ewing's sarcoma lines: RD-ES (ATCC), TC-71, 6647, A4573 and 5838 (Dr. M. Tsokos NIH, Bethesda, Md.). Melanoma cell lines were established from metastatic lesions in our laboratory. All tumor cell cultures were maintained as adherent monolayers in RPMI-1640 medium + 10% FCS. In some experiments, recombinant human interferon γ (IFNγ, a generous gift of Genentech Inc., South San Francisco, Calif.) was added to tumor cultures at a concentration of 500 U/ml for 72 h prior to phenotypic o1 functional assays.
B cell lines
B lymphoblastoid cell lines were generated from PBMC transformed with the Epstein-Barr virus (EBV) using standard techniques. These were maintained as suspension cultures in RPMI-1640 medium + 10% FCS.
Flow-cytometric analysis of tumor cell lines
Adherent cell cultures were harvested with 0.02% EDTA (Versene, Biofluids) and phenotyped using flow cytometry as previously described [24]. Monoclonal antibodies (mAb) directed against HLA determinants included W6/32 (against HLA-A, -B, -C molecules, monomorphic determinant, Sera Labs, Westbury, N. Y.), anti-HLA-DR (L243, purchased from Becton Dickinson, Mountain View, Calif.), and MA 2.2 (against HLA-A2 and HLA-A29, Incstar, Stillwater, Minn.). The anti-ICAM-1 mAb 84H10 was a gift of Dr. S. Shaw (NIH, Bethesda, Md.). Monoclonal antibodies directed against melanoina-associated antigens included 48.7 (anti-high-molecular weight Ag), 96.5 (anti-p97 Ag) (both gifts of Dr. J. Carasquillo, NIH, Bethesda, Md.), and R24 (Mel-1, anti-GD3 ganglioside, Signet Labs, Dedham, Mass.). Fluorescein-conjugated goat anti-(mouse IgG) F(ab)′2 (GAMIg, Boehringer Mannheim Biochemicals, Indianapolis, Ind.) was used to counterstain when necessary. Anti-Thy1.2 (against murine T cells, Becton Dickinson) was used as a negative control.
Cytotoxicity testing
The cytolytic activities of TIL were analyzed with standard 4-h 51Cr-release assays, as previously described [24]. Target cells were harvested with EDTA, washed and labeled with 200 μCi 51Cr for 90 min. Effector cells were coincubated with targets in 96-well plates for 4 h; superuatants were collected and counted with a gamma counter (LKB Instruments Inc., Gaithersburg, Md.). Percentage lysis was calculated by the formula: 100× (experimental release − spontaneous release)/(maximum release − spontaneous release). Inhibition of lysis with mAb was performed by pretreating the chromated targets with either anti-Thy1.2 or W6/32, or the effectors with anti-CD3 (anti-Leu4, Beckton Dickinson) for 30 min prior to coculturing targets and effectors.
Vaccinia virus infection of tumor cell lines
The generation of vaccinia virus constructs containing the HLA-A2.1 gene (Vac-A2), the β2-microglobulin gene (Vac-β2) and the mouse H-2Kd gene (Vac-Kd), and the method of target cell infection, have been described elsewhere [17, 18]. Vac-wt indicates unaltered (wild-type) vaccinia virus. Briefly, target cells were incubated with 10 PFU/cell of the appropriate vaccinia virus construct for 90 rain at 37°C, in RPMI medium supplemented with 0.1% bovine serum albumin. Cells were then diluted to a concentration of 5 × 105 cells/m1 and incubated for an additional 90 min in RPMI-1640 medium + 10% FCS, after which they were labeled with 5lCr for 1 h. Lysis of these targets was then evaluated in standard 51Cr-release assays.
M1 peptide pulsing of cells
Tumor or EBV-B cells were incubated with 0.6 μM influenza M1 58–66 peptide for 60 rain while chromating, as previously described [17]. Cells were then washed and incubated with the appropriate effector cells in a standard 4-h 51Cr-release assay.
HLA typing
HLA serotyping was performed by the NIH HLA laboratory using the Amos modified method [30]. The HLA types of TIL and EBV-B cell lines were determined by analysis of patients' PBMC. The HLA types of melanoma and Ewing's sarcoma cell lines were determined by direct typing of tumor cells.
Results
Characterization of cytolytic HLA-A2-restricted melanoma TIL used to detect shared neuroectodermal tumor antigens
Polyclonal CTL populations derived from four patients' melanomas (TIL 660, 1073, 1074, 1143) were used to screen neuroectodermal tumors for the presence of tumor-associated antigens shared with melanomas. These TIL were generated from individuals expressing the HLA-A2 determinant common to 50% of Caucasians, and have previously been shown to use the HLA-A2 molecule as one restriction element for recognition of tumor antigens shared by a variety of melanomas but not present on normal tissues of nonmelanocytic lineage or nonmelanoma tumors [ 10, 13, 17]. Many bulk-cultured TIL populations express heterogeneous activities, with different CTL clones using different HLA restriction elements for specific recognition of melanomas [9, 10]. However, experiments in which the gene encoding HLA-A2.1, the most common HLA-A2 subtype, has been introduced into melanomas not naturally expressing this molecule, offer direct evidence that HLA-A2.1 is at least one restriction element used by our TIL [13, 17].
Table 1 presents the results of two experiments that demonstrate the ability of TIL 1073, 1074 and 1143 to recognize a common melanoma antigen presented in the context of HLA-A2. The melanoma cell line 624-mel was derived from an HLA-A2+ patient and has been shown by flow cytometry and HLA serotyping to express this molecule. This melanoma was lysed by all three TIL lines tested, while the HLA-A2− cell line 397-mel was not recognized by any of the three TIL lines. After infecting 397-mel with vaccinia virus constructs containing the HLA-A2.1 and β2-microglobulin genes, TIL were able to recognize 397-mel. However, TIL were not able to recognize 397-mel if the target was infected with only the β2-microglobulin gene, in the absence of HLA-A2.1. In contrast, LAK cells lysed 397-mel both prior to and after vaccinia infection, since their target recognition is not MHC-restricted. The results from these representative experiments demonstrate that the polyclonal TIL cultures used in this study contain populations of T cells that recognize a common melanoma antigen presented in the context of HLA-A2. While TIL 1073 and 1074 have not been shown to use restriction elements other than HLA-A2 for melanoma recognition, TIL 1143 also contain a subpopulation of melanoma-specific CTL restricted HLA-Cw7, and alloreactive CTL directed against HLA-A24.
Table 1.
Lysis of Vac-A2-infected cell lines by HLA-A2-restricted melanoma-specific tumor-infiltrating lymphocytes (TIL)
| Targets | Vaccinia infectiona |
E: T | Cytotoxicity (% lysis) with effectors: |
|||
|---|---|---|---|---|---|---|
| TIL 1073 | TIL 1074 | TIL 1143 | LAK cells | |||
| Experiment 1 | ||||||
| 397-melb | None | 40:1 | −3 | −4 | −5 | 58 |
| 10:1 | −3 | −2 | 5 | 29 | ||
| β2 | 40:1 | 0 | −3 | 0 | 76 | |
| 10:1 | −1 | −2 | −1 | 46 | ||
| A2/β2 | 40:1 | 29 | 19 | 23 | 80 | |
| 10:1 | 14 | 15 | 17 | 53 | ||
| 624-melc | None | 40:1 | 28 | 32 | 30 | 53 |
| 10:1 | 18 | 28 | 21 | 29 | ||
| Experiment 2 | ||||||
| 397-mel | None | 40:1 | 6 | −1 | −1 | 54 |
| 10:1 | 2 | 2 | −1 | 20 | ||
| β2 | 40:1 | −15 | −15 | −16 | 60 | |
| 10:1 | −11 | −11 | −27 | 34 | ||
| A2/β2 | 40:1 | 38 | 25 | 35 | 69 | |
| 10:1 | 22 | 13 | 19 | 30 | ||
| 624-mel | None | 40:1 | 29 | 21 | 35 | 51 |
| 10:1 | 21 | 13 | 17 | 34 | ||
Results are from 4-h 51Cr-release assays after infection of targets with appropriate vaccinia virus construct(s). E:T, effector: target ratio
β2, vaccinia β2-microglobulin construct; A2/β2, vaccinia HLA-A2.1 and vaccinia β2-microglobulin in constructs
HLA-A2−
HLA-A2+
The fourth CTL population used in our study, TIL 660, has been extensively characterized. It has been demonstrated to lyse allogeneic HLA-A2+ melanoma cell lines as well as unmatched melanoma cell lines that were engineered to express HLA-A2.1 either through infection with an HLA-A2.1 vaccinia construct or stable transfection with the HLA-A2.1 gene [13, 17]. TIL 660 do not lyse Epstein-Barr-virus-transformed B cells or a variety of colon and breast carcinomas expressing HLA-A2.1, and thus seem to be melanoma-specific. When assayed at an early point in their culture (prior to day 40), polyclonal TIL 660 also lyse an HLA-A2− allogeneic melanoma cell line with which they share only HLA-Cw7. This HLA-Cw7-restricted recognition is inhibited by the anti-(MHC class I) mAb, W6/32 (data not shown).
Thus, the four TIL cultures used in this study share the property of HLA-A2-restricted recognition of commonly expressed melanoma antigens, while TIL 660 and 1143 can utilize additional class I antigens for specific melanoma recognition.
Cell-surface phenotypes of neuroectodermal tumors screened for expression of tumor antigens shared with melanoma
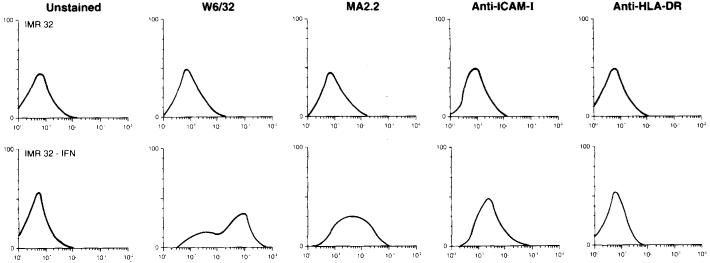
We collected 30 neuroectodermal tumor lines and used flow cytometry to analyze cell-surface expression of molecules important for CTL recognition. Many of these lines were deficient in expression of MHC and adhesion molecules, as expected [7, 14, 16]. For our initial screening for lysis by HLA-A2-restricted TIL, we sought to identify target cell lines expressing HLA-A2 as well as the adhesion molecule ICAM-1, or lines that could express these molecules after a 3-day exposure to IFNγ. A total of 23 such lines were identified, including 8 neuroblastomas, 6 neuroepitheliomas, 2 astrocytomas, 1 retinoblastoma, 1 neuroglioma, and 5 Ewing's sarcomas (Table 2). Although it was rare that a tumor line failed to express any detectable MHC class I or ICAM-1 before IFNγ treatment (example: retinoblastoma Y-79), most lines expressed levels below those expressed on melanoma lines recognized by our TIL (501-mel, 624-mel). HLA-A2 positivity by flow cytometry was enhanced after IFNγ treatment in most of the 23 lines presented in Table 2, and all but two (Y-79 and neuroblastoma SK-N-FI) expressed levels of HLA-A2 similar to those expressed by the control melanomas. However, ICAM-1 was, in general, poorly expressed by these lines and not as readily up-regulated by IFNγ. Of note, only one of 21 lines tested for HLA-DR expression was positive (astrocytoma U-87 MG), and up-regulation with IFNγ was observed infrequently. Figure 1 shows a representative example of a neuroectodermal tumor phenotype before and after IFNγ treatment.
Table 2.
Effect of interferon γ (IFNγ) on MHC and adhesion molecule expression by neuroectodermal tumor cell lines
| Cell lines | IFNγa | Mean channel number |
|||||
|---|---|---|---|---|---|---|---|
| Anti-Thy1.2 | GAMIg | Anti-MHC-I (W6/32) |
Anti-HLA-A2/29 (MA2.2) |
Anti-HLA-DR (L243) |
Anti-ICAM-1 (84H10) |
||
| Neuroblastoma | |||||||
| SK-N-DZ | − | 4 | 5 | 9 | 9 | 5 | 7 |
| + | 5 | 5 | 1008 | 202 | 5 | 40 | |
| NLF | − | 4 | 4 | 42 | 44 | 3 | 5 |
| + | 4 | 4 | 66 | 35 | 3 | 47 | |
| SMS-KAN | − | 24 | 10 | 52 | 18 | 27 | 28 |
| + | 13 | 13 | 984 | 225 | 13 | 14 | |
| SK-N-DW | − | 10 | 7 | 123 | 55 | 10 | 27 |
| + | 13 | 11 | 500 | 84 | 15 | 85 | |
| SK-N-FI | − | 10 | 11 | 65 | 30 | 10 | 16 |
| + | 12 | 14 | 290 | 19 | 19 | 44 | |
| LA-N-1 | − | 24 | 42 | 31 | 62 | 38 | 52 |
| + | 36 | 44 | 238 | 80 | 48 | 130 | |
| IMR-32 | − | 9 | 9 | 15 | 16 | 8 | 13 |
| + | 8 | 10 | 617 | 100 | 8 | 40 | |
| SK-N-MC | − | 6 | 8 | 29 | 24 | 7 | 38 |
| + | 7 | 10 | 97 | 133 | 11 | 205 | |
| Neuroepithelioma | |||||||
| TC-32 | − | 5 | 6 | 92 | 56 | 6 | 8 |
| + | 7 | 8 | 525 | 187 | 8 | 40 | |
| CHP-100 | − | 6 | 6 | 202 | 69 | 6 | 8 |
| + | 9 | 10 | 247 | 146 | 8 | 38 | |
| Clones of CHP-100 | |||||||
| A1.2/A | − | 5 | 5 | 125 | 54 | 5 | 4 |
| + | 5 | 6 | 328 | 167 | 6 | 5 | |
| A1.2/C | − | 6 | 5 | 171 | 89 | 5 | 5 |
| + | 7 | 7 | 353 | 196 | 7 | 6 | |
| B3 | − | 5 | 5 | 190 | 72 | 6 | 9 |
| + | 10 | 12 | 270 | 192 | 12 | 16 | |
| C3 | − | 5 | 7 | 155 | 61 | 5 | 5 |
| + | 7 | 9 | 237 | 78 | 26 | 50 | |
| Astrocytoma | |||||||
| U-87 MG | − | 9 | 8 | 48 | 68 | 43 | 37 |
| + | 9 | 9 | 41 | 74 | 83 | 87 | |
| U-373 MG | − | 7 | 7 | 140 | 55 | 10 | 9 |
| + | 8 | 8 | 147 | 67 | 71 | 43 | |
| Retinoblastoma | |||||||
| Y-79 | − | 7 | 7 | 7 | 8 | 7 | 7 |
| + | 7 | 8 | 13 | 18 | 7 | 8 | |
| Neuroglioma | |||||||
| H4 | − | 6 | 11 | 99 | 9 | 6 | 7 |
| + | 5 | 8 | 458 | 47 | 203 | 10 | |
| Ewing's sarcoma | |||||||
| 6647 | − | 4 | 8 | 143 | 103 | 5 | 12 |
| + | 5 | 10 | 434 | 337 | 8 | 98 | |
| TC-71 | − | 5 | 5 | 311 | 204 | 7 | 16 |
| + | 7 | 11 | 707 | 266 | 33 | 109 | |
| RD-ES | − | 8 | 5 | 83 | 31 | 9 | 31 |
| + | 7 | 13 | 595 | 184 | 11 | 95 | |
| A4573 | − | 5 | 5 | 64 | 6 | ND | 6 |
| + | 6 | 6 | 538 | 83 | ND | 63 | |
| 5838 | − | 6 | 10 | 106 | 45 | ND | 19 |
| + | 6 | 8 | 258 | 24 | ND | 49 | |
| Melanoma | |||||||
| 501-mel | − | 6 | 8 | 151 | 130 | 5 | 103 |
| + | 5 | 7 | 239 | 117 | 102 | 149 | |
| 624-mel | − | 7 | 7 | 133 | 43 | 7 | 54 |
| + | 8 | 9 | 209 | 57 | 8 | 88 | |
| 938-melb | − | 8 | 9 | 264 | 12 | 8 | 63 |
| + | 9 | 9 | 549 | 11 | 250 | 600 | |
Neuroectodermal tumor phenotype by flow-cytometric analysis before and after treatment with IFNγ. Fluorescein-conjugated anti-Thy1.2 is a negative control for staining with W6/32 and L243. Fluorescein-conjugated goat anti-mouse immunoglobulin (GAMIg) was used as a counterstain for MA2.2 and 84H10. ND, not done
IFNγ 500 U/ml for 72 h
938-mel, an HLA-A2-negative melanoma, is not lysed by HLA-A2-restricted TIL but can be lysed by other melanoma-specific TIL sharing the appropriate restriction element [6]
Fig. 1.
Flow-cytometric analysis of MHC and ICAM-1 molecule expression by the neuroblastoma line IMR-32. IMR-32 does not bind the mAb W6/32 (directed against a monomorphic determinant on HLA-A, -B, -C), MA2.2 (against HLA-A2 and -A29), anti-ICAM-I or anti-HLA-DR prior to IFNγ treatment. After IFNγ treatment (500 U/ml for 72 h), expression of MHC class I molecules (W6/32), HLA-A2 (MA2.2), and ICAM-1, but not HLA-DR, is detected
Owing to homology among human HLA molecules, most anti-HLA mAb cross-react with more than one MHC antigen. The mAb MA2.2, used in this study, is known to cross-react with HLA-A2 and HLA-A29. Since HLA-A29 is relatively infrequent in the Caucasian population (approximately 6%), we assumed for screening purposes that cells staining with MA2.2 expressed HLA-A2. In cases where TIL recognition of neuroectodermal tumors was observed, direct HLA serotyping of tumor lines was performed to characterize these interactions further (see below).
Recognition of neuroectodermal tumor cell lines by melanoma-specific TIL
The 23 neuroectodermal tumors identified in Table 2, which expressed HLA-A2/A29 either constitutively or as a consequence of IFNγ exposure, were used as targets for lysis by HLA-A2-restricted melanoma TIL in 4-h 51Cr-release assays. TIL 1073 failed to recognize any neuroectodermal tumor target. However, TIL 660, 1074 and 1143 were reactive with 3 of 5 Ewing's sarcomas tested. Representative data are shown in Table 3. TIL 660 lysed RD-ES, TIL 1074 lysed TC-71, and TIL 1143 lysed these two tumors as well as 6647. Lysis occurred only after targets had been cultured in IFNγ, and was similar in degree to lysis of the HLA-A2+ melanoma line 624-mel. TIL 1074 and 1143 were tested against the entire panel of 23 neuroectodermal tumors and failed to consistently recognize any other lines. Of note, the Ewing's sarcomas were lysed by non-MHC-restricted LAK cells even before IFNγ exposure, and lysis by LAK cells decreased after IFNγ (RD-ES, 6647, 624-mel), as is typically observed, showing that all targets were lysable and that the effect of IFNγ on TIL lysis was not nonspecific.
Table 3.
Lysis of Ewing's sarcoma cell lines by melanoma-specific TIL
| Cytotoxicity (% lysis at E:T = 40:1) with effectors: |
|||||
|---|---|---|---|---|---|
| Targets | IFNγa | TIL 660 | TIL 1074 | TIL 1143 | LAK cells |
| RD-ES | − | 6 | 0 | 0 | 75 |
| + | 25 | 0 | 25 | 48 | |
| TC-71 | − | 1 | 9 | 0 | 48 |
| + | 10 | 26 | 31 | 58 | |
| 6647 | − | 3 | 2 | 2 | 65 |
| + | 0 | 3 | 38 | 26 | |
| 624-mel | − | 55 | 25 | 34 | 55 |
| + | 65 | 35 | 49 | 26 | |
Results are from 4-h 51Cr-release assay. Lysis above 10% is considered significant. LAK, lymphokine-activated killer
IFNγ pretreatment of targets, 500 U/ml for 72 h
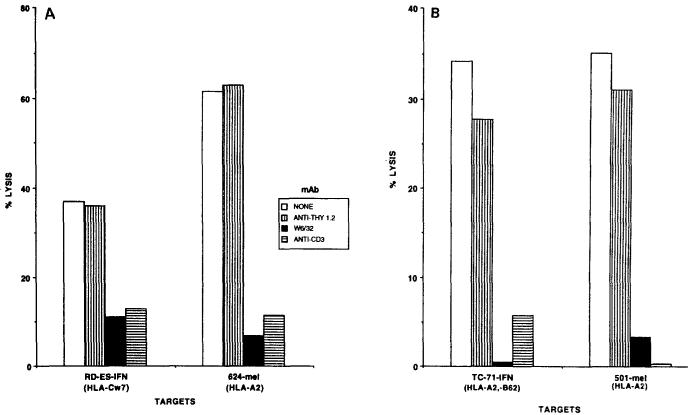
Cytolysis of IFN-treated Ewing's sarcomas by melanoma TIL could be inhibited by mAb directed against MHC class I molecules (W6/32) and against the TCR (anti-Leu4), demonstrating the immunological specificity of these interactions. Figure 2 shows representative experiments for TIL 660 lysis of RD-ES and TIL 1074 lysis of TC-71, and Fig. 3 for TIL 1143 lysis of RD-ES, TC-71, and 6647. In each experiment, TIL lysis of an HLA-A2+ melanoma (501-mel or 624-mel) was also effectively blocked by W6/32 and anti-Leu4. The mAb anti-Thy1.2 was used as a negative control in these experiments, and failed to inhibit TIL lysis significantly. Simultaneous assays using LAK cells to lyse the 3 Ewing's sarcomas failed to demonstrate antibody blocking (data not shown). Thus, melanoma-specific TIL seemed to behave like classical CTL in their recognition of Ewing's sarcomas, lysing these targets in an MHC-class-I-restricted, TCR-dependent manner.
Fig. 2A, B.
Recognition of Ewing's sarcomas by melanoma-specific tumor-infiltrating lymphocytes (TIL). A Lysis of interferon-γ(IFNγ)-treated Ewing's sarcoma cell line RD-ES by TIL 660 is inhibited by the anti-MHC class I mAb W6/32 and by anti-CD3. The irrelevant mAb anti-Thy1.2, directed against murine T cells, is used as a negative control and does not inhibit lysis. TIL 660 and RD-ES-IFN are matched only at one HLA locus, HLA-Cw7. TIL 660 and 624-mel are matched at HLA-A2 and demonstrate the same pattern of MHC-class-I-restricted, T-cell-receptor-dependent lysis. B Lysis of IFNγ-treated Ewing's sarcoma cell line TC-71 by TIL 1074 is inhibited by W6/32 and anti-CD3, but not by anti-Thy1.2. TIL 1074 and TC-71 are matched at HLA-A2 and HLA-B62. TIL 1074 and 501-mel are matched only at HLA-A2 and demonstrate the same MHC-class-I-restricted, TCR-dependent interaction. Inhibition of LAK cell lysis of these targets by these mAb was not observed (data not shown)
Fig. 3.
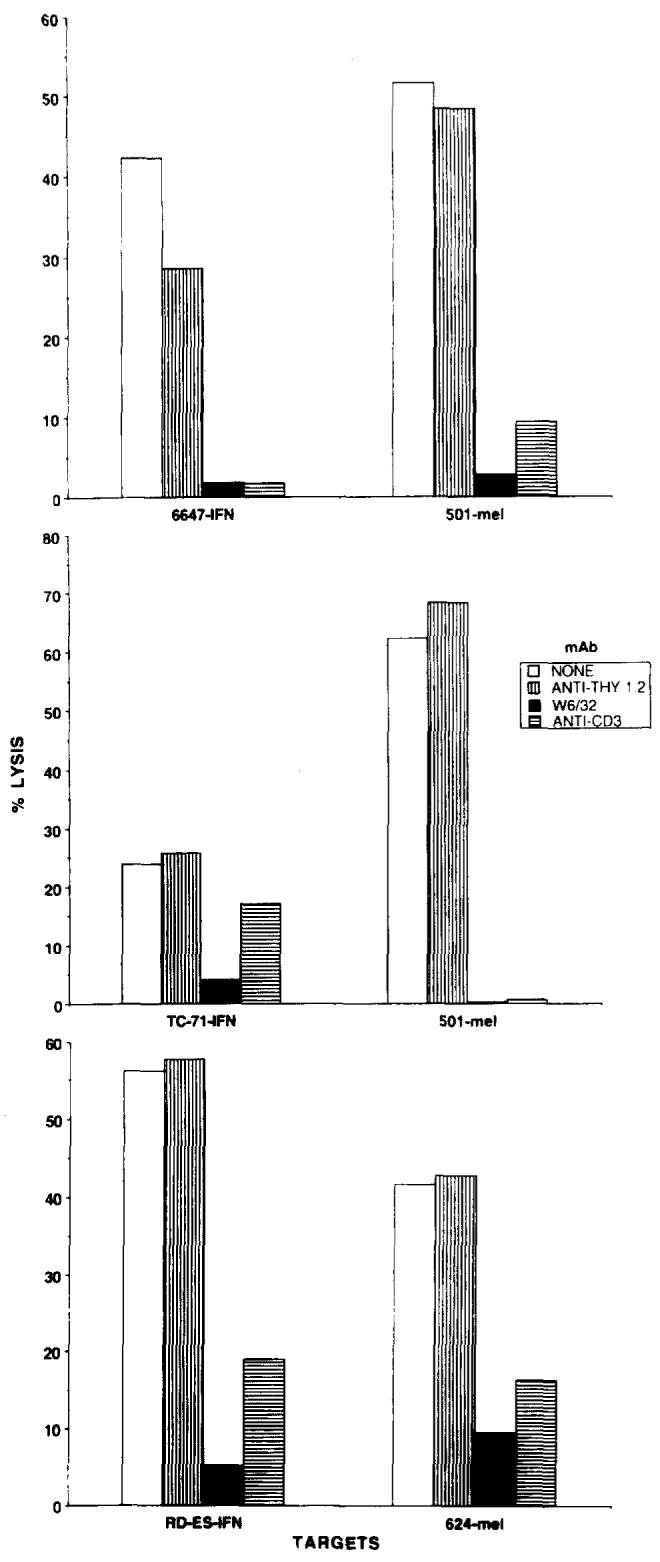
Lysis of the IFNγ-treated Ewing's sarcoma cell lines 6647, TC-71, and RD-ES by TIL 1143 is inhibited by the mAb W6/32 and anti-CD3, but not anti-Thy1.2. mAb failed to block lymphokine-activated killer cell lysis of these targets in the same experiment (data not shown), but did inhibit TIL lysis of the HLA-A2+ melanomas 501-mel and 624-mel
Evaluation of HLA-A2− neuroectodermal tumors
We identified 7 neuroectodermal tumors that expressed MHC class I and ICAM-1 molecules by flow cytometry but did not express HLA-A2. To screen for the presence of antigen sharing with melanomas, four of these tumors were infected with vaccinia vectors encoding HLA-A2.1 and β2-microglobulin, and were then used as targets for lysis by TIL 1073 and 1074. Targets included 2 neuroblastomas (SK-N-SH and SK-N-As), 1 glioma (Hs 683), and 1 astrocytoma (CCF-STTG1). Although infected targets expressed HLA-A2 molecules, as assessed by flow cytometry, they were not lysed by TIL, which nevertheless lysed HLA-A2-infected 397-mel cells (naturally HLA-A2−) in the same experiment (data not shown). As a positive control, all targets were lysed by LAK cells. Thus, we were unable to identify additional tumors that could be recognized by melanoma-specific TIL.
Identification of possible MHC class I restriction elements mediating the TIL/Ewing's sarcoma interactions
Complete HLA serotypes of TIL (patient PBMC) and IFN-treated Ewing's sarcomas were obtained using the standard microcytotoxicity method, in order to evaluate possible class I molecules involved in recognition (Table 4). TIL 660 shared only HLA-Cw7 with RD-ES. Since these TIL were known to contain a melanoma-reactive HLA-Cw7-restricted subpopulation, Ewing's sarcoma recognition was presumed to be HLA-Cw7-restricted as well. It is of note that RD-ES had been presumed to be HLA-A2+ by virtue of reactivity with the mAb MA2.2 (Table 2), but was actually HLA-A2−, HLA-A29+, in accord with the known cross-reactivity of the MA2.2 mAb. The restriction element HLA-Cw7 was not expressed on RD-ES unless the cells had been pretreated with IFNγ, providing one possible explanation for the necessity of IFNγ for TIL 660 recognition.
Table 4.
MHC class I phenotypes of TIL and Ewing's sarcoma cell lines
| Cell linea | HLA locus |
||
|---|---|---|---|
| A | B | Cw | |
| TIL 660 | 2,26 | 45,49 | 4,7 |
| TIL 1074 | 2,3 | 7,62 | 3,–b |
| TIL 1143 | 2,11 | 7,60 | 3,7 |
| RD-ES-IFN | 24,29 | 44,62 | 3,7 |
| TC-71-IFN | 2,33 | 62,– | 7,– |
| 6647-IFNc | 2d | NE | NE |
HLA serotyping using the modified Amos technique. NE, not evaluable
Patient PBMC typed for TIL cultures, IFNγ treated lines typed for Ewing's sarcoma
Not determined
Unable to be typed by conventional techniques
Expression of HLA-A2 demonstrated by flow cytometry and ability of cells to present M1 influenza peptide to HLA-A2-restricted CTL
HLA typing showed that TIL 1074 shared HLA-A2 and HLA-B62 with TC-71. As these TIL have been shown to recognize a variety of HLA-A2+ melanomas, but could not be shown to use HLA-B62 as a restriction element for melanoma recognition, it is likely that HLA-A2 is utilized for Ewing's sarcoma recognition as well.
Finally, TIL 1143 express specific anti-melanoma reactivities mediated by HLA-A2 and -Cw7. Thus, their recognition of RD-ES, an HLA-A2− but HLA-Cw7+ target, could be HLA-Cw7-restricted. Recognition of TC-71 is possible through the shared HLA-A2 and HLA-Cw7 antigens. The tumor 6647 could not be typed with conventional techniques, because of technical difficulties; however, this line can present an HLA-A2-specific influenza peptide to HLA-A2-restricted influenza-specific CTL (see Table 5, below). This strongly suggests that 6647 is HLA-A2+ and provides one possible restriction element for the interaction with TIL 1143. As TIL 1143 contain an allo-A24-reactive subpopulation, alloreactivity could not be excluded as a mechanism for recognition of RD-ES (HLA-A24+) or 6647 (HLA type undetermined).
Table 5.
Recognition of influenza M1-peptide-pulsed Ewing's sarcoma cell lines by HLA-A2-restricted influenza-specific CTL
| Cytotoxicity (% lysis) with effectors: |
|||||
|---|---|---|---|---|---|
| Targets | HLA-A2 | IFNγa | M1 peptideb |
Anti-M1 CTLc |
LAK cellsd |
| 6647 | + | − | − | 1 | 51 |
| + | 69 | 41 | |||
| + | − | 2 | 5 | ||
| + | 72 | 8 | |||
| TC-71 | + | − | − | 5 | 45 |
| + | 44 | 39 | |||
| + | − | 8 | 29 | ||
| + | 58 | 23 | |||
| RD-ES | − | − | − | 4 | 49 |
| + | 4 | 67 | |||
| + | − | 2 | 48 | ||
| + | 6 | 42 | |||
| 501-EBV | + | − | − | 7 | 9 |
| + | 42 | 6 | |||
| 586-EBV | − | − | − | 2 | 14 |
| + | 4 | 8 | |||
Results are from a 4-h 51Cr-release assay
IFNγ 500 U/ml for 72 h
Targets pulsed with 0.6 μM M1 58-66 peptide for 60 min
Effector: target ratio = 10
Effector: target ratio = 40
In summary, HLA phenotyping identified one or more possible restriction elements for each TIL/Ewing's sarcoma interaction observed. In no instance did TIL and the Ewing's sarcoma express completely disparate HLA types.
Evaluation of the requirement for IFNγ in TIL recognition of Ewing's sarcoma: expression of MHC molecules on target cells
HLA serotyping and flow-cytometric analysis identified tumors TC-71 and 6647 as being HLA-A2+ even before exposure to IFNγ. However, the results of repeated experiments confirmed that IFNγ pretreatment was required for TIL lysis of these targets to occur. Exposure to IFNγ did enhance MHC class I molecule expression on Ewing's sarcomas considerably (Table 2). Thus, we asked if pre-IFN levels of class I expression were sufficient for antigen presentation. CTL specific for the HLA-A2.1-restricted influenza M1 58–66 peptide were used to assess antigen presentation by Ewing's sarcoma cells pulsed with this peptide. As shown in Table 5, anti-M1 CTL recognized 6647 and TC-71 pulsed with M1 peptide both before and following a 3-day exposure to IFNγ, and IFNγ did not seem to enhance CTL lysis. Unpulsed 6647 and TC-71 tumor cells were not recognized by M1-specific CTL, nor were HLA-A2− RD-ES cells under any condition. Lysis of peptide-pulsed 6647 and TC-71 targets equalled or exceeded lysis of pulsed HLA-A2+ EBV-transformed B cells, which expressed abundant quantities of the relevant HLA restriction molecule. Target cell lysability was confirmed using LAK cell effectors.
These data indicate that presentation of HLA-A2-restricted epitopes by 6647 and TC-71 is not influenced by exposure of these tumors to IFNγ. However, we have not addressed antigen presentation by other possible restriction elements in these peptide experiments. HLA serotyping of TC-71 and RD-ES before and after IFNγ exposure showed that HLA-B and -C elements were expressed poorly or not at all by untreated cells, and that their expression was enhanced by IFNγ; on the other hand, HLA-A molecules were detectable regardless of IFNγ exposure. These findings are similar to observations of locus-specific MHC class I antigen down-regulation in melanoma cell lines [15]. Thus TIL 660 and 1143, which seem to use HLA-Cw7 to recognize RD-ES, would require IFNγ for expression of this restriction element and presentation of the putative tumor-related epitope.
Evaluation of the effect of IFNγ on antigen-processing functions of Ewing's sarcoma cell lines
Previous studies have identified antigen-processing defects in human tumor cell lines, particularly small-cell lung carcinomas. These are related to deficiencies in proteasome components as well as peptide transporters and can be overcome by exposure of tumor cells to IFNγ [18]. To examine whether Ewing's sarcoma cells are deficient in antigen processing, we used a previously described model system [18] in which target cells are infected by a recombinant vaccinia vector encoding viral proteins as well as the murine MHC class I molecule H-2Kd (Vac-Kd), and then tested for lysis by vaccinia-specific H-2Kd-restricted murine CTL. Table 6 shows an experiment in which 3 Ewing's sarcoma lines were infected with either wild-type vaccinia (Vac-wt) or Vac-Kd and tested for lysis by two murine Vac-specific CTL lines. Human tumor cells infected with Vac-wt were not lysed by the H-2Kd-restricted murine CTL, regardless of whether tumors were pretreated with IFNγ. However, tumors infected with Vac-Kd were recognized both before and after IFNγ treatment. Although there was a suggestion of enhanced lysis of Vac-Kd-infected tumor 6647 following IFNγ treatment, pre-IFN lysis levels were still well above values observed using the processing-defective T2 cell line [4, 12] infected with Vac-Kd as a target. As a negative control, TIL 888, which specifically lyse their autologous melanoma, failed to lyse any Ewing's sarcoma cells whether infected with Vac or treated with IFNγ. Thus, we were unable to demonstrate antigen-processing defects in our Ewing's sarcoma lines that could account for the IFNγ effect observed on TIL lysis.
Table 6.
Recognition of VAC-Kd-infected Ewing's sarcoma cell lines by vaccinia-specific H-2Kd-restricted murine CTL
| Cytotoxicity (% lysis) with effectors: |
||||||
|---|---|---|---|---|---|---|
| Cell line | Vaccinia infectiona | IFNγb | E:T | CTL-1 | CTL-2 | TIL 888 |
| TC-71 | VAC-WT | − | 40:1 | −4 | −2 | −5 |
| 10:1 | −1 | −1 | −3 | |||
| + | 40:1 | −1 | 9 | 7 | ||
| 10:1 | 4 | 14 | 12 | |||
| VAC-Kd | − | 40:1 | 34 | 18 | −3 | |
| 10:1 | 17 | 15 | 2 | |||
| + | 40:1 | 23 | 18 | 3 | ||
| 10:1 | 19 | 17 | 2 | |||
| RD-ES | VAC-WT | − | 40:1 | 2 | 2 | 0 |
| 10:1 | 7 | 1 | 4 | |||
| + | 40:1 | 0 | 2 | 5 | ||
| 10:1 | 1 | 3 | 5 | |||
| VAC-Kd | − | 40:1 | 22 | 5 | −13 | |
| 10:1 | 11 | −3 | −8 | |||
| + | 40:1 | 28 | 21 | −2 | ||
| 10:1 | 20 | 13 | 3 | |||
| 6647 | VAC-WT | − | 40:1 | −1 | 2 | −1 |
| 10:1 | 1 | 4 | 1 | |||
| + | 40:1 | 4 | 4 | 7 | ||
| 10:1 | 3 | 5 | 5 | |||
| VAC-Kd | − | 40:1 | 21 | 16 | −2 | |
| 10:1 | 18 | 11 | 12 | |||
| + | 40:1 | 36 | 30 | −3 | ||
| 10:1 | 29 | 15 | 0 | |||
| T2 | VAC-Kd | − | 40:1 | 1 | −2 | −2 |
| 10:1 | 4 | −1 | 1 | |||
| + | 40:1 | −2 | −3 | −3 | ||
| 10:1 | 1 | 0 | 2 | |||
| 888-mel | None | − | 40:1 | −2 | −1 | 40 |
| 10:1 | 2 | 1 | 29 | |||
Results are from a 4-h 51Cr-release assay after infection of targets with appropriate vaccinia construct
VAC-WT, vaccinia virus wild type; VAC-Kd, vaccinia virus H-2Kd construct
IFNγ 500 U/ml for 72 h
Expression of antibody-defined melanoma-associated antigens by neuroectodermal tumor cell lines
Several studies have demonstrated that a variety of tumors of neuroectodermal origin express antigens defined by mAb that react with melanomas. We examined this phenomenon to reconfirm previously reported results as well as to examine correlations of expression of B-cell-defined antigens (binding of mAb) and T-cell-defined antigens (recognition by CTL). Analysis of 11 neuroectodermal tumor cell lines by flow cytometry with three “melanoma-specific” mAb demonstrated that all cell lines expressed antigens defined by R24 and 48.7. Of 11 cell lines, 6 also bound the mAb 96.5. The antigens defined by these mAb were expressed on the 3 Ewing's sarcoma lines recognized by melanoma-specific TIL as well as other neuroectodermal tumors not recognized by TIL (data not shown). Therefore, there appears to be no correlation between expression of B-cell-defined antigens and T-cell-defined antigens in this study.
Discussion
Cytolytic T cells can recognize commonly expressed melanoma-associated antigens in the context of a variety of HLA-A, -B, and -C, molecules [5, 6, 9, 10, 17, 28, 29]. Such MHC-class-I-restricted T cells have also been shown to recognize cultured normal melanocytes [1], suggesting targeting of a differentiation antigen. Most CTL described as recognizing shared melanoma antigens have been HLA-A2-restricted. It is unclear whether this is a function of relatively diminished expression of HLA-B and -C alleles on melanomas [15], or if there is an immunological dominance of certain HLA alleles at the T cell level, as has been suggested [5]. Certainly, the high frequency of the HLA-A2 gene in the Caucasian population increases the likelihood of finding reactive T cell/melanoma combinations restricted by this allele in any given tissue collection.
The evidence that melanoma-associated CTL epitopes might be differentiation antigens, observed at the T cell recognition level [1] and also at the mRNA level [2, 28], led us to screen other tumors of neuroectodermal origin for the presence of these antigens. We chose to use four different HLA-A2-restricted melanoma-specific CTL derived from melanoma TIL for this purpose. The ease of identifying HLA-A2+ neuroectodermal tumor targets made this project feasible.
Our data show that 3 neuroectodermal tumors, among a panel of 23 candidate lines, could be recognized by 3 melanoma-specific CTL in a TCR-dependent, MHC-class-I-restricted interaction. These 3 tumors share the histological classification of Ewing's sarcoma, a rare malignant tumor of adolescence formerly believed to be of mesenchymal origin (hence “sarcoma”). Ewing's sarcomas are undifferentiated tumors now generally accepted to originate from parasympathetic neuronal tissue, on the basis of chromosomal and biochemical studies [3, 11, 23]. It was necessary to treat the 3 Ewing's sarcomas, RD-ES, TC-71, and 6647, with IFNγ for T cell recognition to occur, possibly because of the need to enhance expression of MHC and/or adhesion molecules (Table 2). Although experiments using the Vac-Kd expression system failed to demonstrate a definite role for IFNγ in enhancing antigen processing in these lines, it is possible that IFNγ increased expression of the T cell epitope(s) through alternative mechanisms, perhaps at the level of gene transcription or translation. Of interest, the Ewing's sarcoma antigens recognized by melanoma-specific CTL did not seem to be shared among the 3 lines (Table 3). Thus, TIL 660, which can recognize shared melanoma antigens via the HLA-A2 and HLA-Cw7 alleles, lysed RD-ES (HLA-Cw7+) but not TC-71 (HLA-A2+, Cw7+ or 6647 (HLA-A2+), even though the latter 2 tumors could be lysed by TIL 1074 and 1143. Likewise, TIL 1074, which appear to be solely HLA-A2-restricted, recognize TC-71 (HLA-A2+) but not 6647 (also HLA-A2+). While TIL 1143 lysed all 3 Ewing's sarcomas, the multiple specificities of this polyclonal culture complicate interpretations of tumor antigen sharing. Individual melanoma lines have been shown to express multiple distinct tumor antigens recognized by specific CTL [21, 22, 27, 29]. Thus, while our polyclonal TIL cultures are all capable of specifically recognizing the HLA-A2+ melanomas 501-mel, 624-mcl, and a variety of others, they do not necessarily react with a single tumor antigen. It is possible that the tumor-associated antigens recognized on the Ewing's sarcoma lines RD-ES, TC-71, and 6647 are shared with melanomas, but are not commonly shared among most Ewing's sarcomas. Clarification of these issues requires definitive identification of the relevant antigens through gene cloning or peptide sequencing, or development of reactive monoclonal T cell populations for study. To date, assays with two HLA-A2-restricted melanoma-specific CTL clones have failed to demonstrate antigen sharing with Ewing's sarcomas (data not presented). Also, the 3 Ewing's sarcoma lines lysed by our TIL failed to express message for three molecularly defined melanoma antigens recognized by T cells: MZ2-E, MART-l, and gpl00 (J. Weber, Y. Kawakami, personal communication).
The results of this study confirm expectations that tumors embryologically related to melanoma might express shared tumor-associated T cell epitopes, and underline the developmental nature of these epitopes. At this time, it is unclear why T cell recognition was restricted to Ewing's sarcomas rather than other types of neuroectodermal tumors. Further clarification awaits ongoing efforts to identify these epitopes directly. Our data suggest that specific clinical immunotherapies targeted against these epitopes, such as vaccines, may be applicable to more than one type of cancer.
Acknowledgements
The authors wish to thank Dr. Francesco Matincola for helpful discussions, Dr. Paul E Robbins for anti-MI CTL, Toni Simonis for HLA typing, Bert O'Neil for assistance with vaccinia experiments, Arnold Mixon and Ellen Fitzgerald for flow cytometric analyses, and Etta Owens for preparation of this manuscript.
We also thank Dr. Drew Pardoll for critical review of the manuscript.
Note added in proof
The authors have become aware of a recent report by D. Rimoldi et al. (Int J Cancer 54: 527, 1993) showing that MAGE 1-specific CTL can recognize melanomas as well as glioblastomas and neuroblastomas expressing this gene.
References
- 1.Anichini A, MacCalli C, Mortarini R, Salvi S, Mazzocchi A, Squarcina P, Herlyn M, Parmiani G. Melanoma cells and normal melanocytes share antigens recognized by HLA-A2-restricted cytotoxic T cell clones from melanoma patients. J Exp Med. 1993;177:989. doi: 10.1084/jem.177.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brichard V, Van Pel A, Wolfel T, Wolfel C, De Plaen E, Lethe B, Coulie P, Boon T. The tyrosinase gene codes for an antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med. 1993;178:489. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavazzana AO, Miser JS, Jefferson J, Triche TJ. Experimental evidence for a neural origin of Ewing's sarcoma of bone. Am J Pathol. 1987;127:507. [PMC free article] [PubMed] [Google Scholar]
- 4.Cerundolo V, Alexander J, Anderson K, Lamb C, Cresswell P, McMichael A, Gotch F, Townsend A. Presentation of viral antigen is controlled by a gene in the major histocompatibility complex. Nature. 1990;345:449. doi: 10.1038/345449a0. [DOI] [PubMed] [Google Scholar]
- 5.Crowley NJ, Darrow TL, Quinn-Aleen MA, Seigler HF. MHC-restricted recognition of autologous melanoma by tumor specific cytotoxic T-cells: evidence for restriction by a dominant HLA-A allele. J Immunol. 1991;146:1692. [PubMed] [Google Scholar]
- 6.Darrow DL, Slingluff CL, Siegler HF. The role of HLA class I antigen in recognition of melanoma cells by tumor-specific cytotoxic T lymphocytes: evidence for shared tumor antigens. J Immunol. 1989;142:3329. [PubMed] [Google Scholar]
- 7.Favrot MC, Combaret V, Goillot E, Tabone E, Bouffet E, Dolbeau D, Bouvier R, Coze C, Michon J, Philip T. Expression of leucocyte adhesion molecules on 66 clinical neuroblastoma specimens. Int J Cancer. 1991;48:502. doi: 10.1002/ijc.2910480405. [DOI] [PubMed] [Google Scholar]
- 8.Hellstrom I, Garrigues HJ, Cabasco L, Mosley GH, Brown JP, Hellstrom KE. Studies of a high molecular weight human melanoma-associated antigen. J Immunol. 1983;130:1467. [PubMed] [Google Scholar]
- 9.Hom SS, Topalian SL, Simonis T, Mancini M, Rosenberg SA. Common expression of melanoma tumor-associated antigens recognized by human tumor infiltrating lymphocytes: analysis by HLA restriction. J Immunother. 1991;10:153. [PubMed] [Google Scholar]
- 10.Hom SS, Schwartzentruber DJ, Rosenberg SA, Topalian SL. Specific release of cytokines by lymphocytes infiltrating human melanomas in response to shared melanoma antigens. J Immunother. 1993;13:18. doi: 10.1097/00002371-199301000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Horowitz ME, Tsokos MG, Delaney TF. Ewing's sarcoma. Cancer Clin. 1992;42:300. doi: 10.3322/canjclin.42.5.300. [DOI] [PubMed] [Google Scholar]
- 12.Hosken NA, Bevan MJ. Defective presentation of endogenous antigen by a cell line expressing class I molecules. Science. 1990;248:367. doi: 10.1126/science.2326647. [DOI] [PubMed] [Google Scholar]
- 13.Kawakami Y, Zakut R, Topalian SL, Stotter H, Rosenberg SA. Shared human melanoma antigens: Recognition by tumor-infiltrating lymphocytes in HLA-A2.1-transfected melanomas. J Immunol. 1992;148:638. [PubMed] [Google Scholar]
- 14.Main EK, Monos DS, Lampson LA. IFN-treated neuroblastoma cell lines remain resistant to T cell-mediated allo-killing, and susceptible to non-MHC-restricted cytotoxicity. J Immunol. 1988;141:2943. [PubMed] [Google Scholar]
- 15.Marincola FM, Shamamian P, Simonis TB, Abati A, Fetsch P, Restifo NP, Mulé JJ, Rosenberg SA. Locus specific down-regulation of HLA class I in melanoma cell lines. Proc Am Assoc Cancer Res. 1993;34:453. [Google Scholar]
- 16.Naganuma H, Kiessling R, Patarroyo M, Hansson M, Handgretinger R, Gronberg A. Increased susceptibility of IFN-γ treated neuroblastoma cells to lysis by lymphokine-activated killer cells: participation of ICAM-1 induction of target cells. Int J Cancer. 1991;47:527. doi: 10.1002/ijc.2910470410. [DOI] [PubMed] [Google Scholar]
- 17.O'Neil BH, Kawakami Y, Restifo NP, Bennink JR, Yewdell JW, Rosenberg SA. Detection of shared MHC restricted human melanoma antigens following vaccinia virus mediated transduction of genes coding for HLA. J Immunol. 1993;151:1410. [PMC free article] [PubMed] [Google Scholar]
- 18.Restifo NP, Esquivel F, Kawakami Y, Yewdell JW, Mulé JJ, Rosenberg SA, Bennink JR. Identification of human cancers deficient in antigen processing. J Exp Med. 1993;177:265. doi: 10.1084/jem.177.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA, Simpson C, Carter C, Bock S, Schwartzentruber D, Wei JP, White DE. Use of tumor infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma: preliminary report. N Engl J Med. 1988;819:1676. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 20.Schwartzentruber DJ, Topalian SL, Mancini M, Rosenberg SA. Specific release of GM-CSF, TNF-α and IFN-γ by human tumor infiltrating lymphocytes following autologous tumor stimulation. J Immunol. 1991;146:3674. [PubMed] [Google Scholar]
- 21.Slingluff CL, Cox AL, Henderson RA, Hunt DF, Engelhard VH. Recognition of human melanoma cells by HLA-A2.1-restricted cytotoxic T lymphocytes is mediated by at least six shared peptide epitopes. J Immunol. 1993;150:2955. [PubMed] [Google Scholar]
- 22.Storkus WJ, Zeh HJ, Maeurer MJ, Salter RD, Lotze MT. Identification of human melanoma peptides recognized by class I restricted tumor infiltrating T lymphocytes. J Immunol. 1990;151:3719. [PubMed] [Google Scholar]
- 23.Thiele CJ. Pediatric peripheral neuroectodermal tumors, oncogenes, and differentiation. Cancer Invest. 1990;8:629. doi: 10.3109/07357909009018932. [DOI] [PubMed] [Google Scholar]
- 24.Topalian SL, Muul LM, Rosenberg SA. Expansion of human tumor infiltrating lymphocytes for use in immunotherapy trials. J Immunol Methods. 1987;102:127. doi: 10.1016/s0022-1759(87)80018-2. [DOI] [PubMed] [Google Scholar]
- 25.Topalian SL, Solomon D, Avis FP, Chang AE, Freerksen DL, Linehan WM, Lotze MT, Robertson CN, Seipp CA, Simon P, Simpson CG, Rosenberg SA. Immunotherapy of patients with advanced cancer using tumor-infiltrating lymphocytes and recombinant interleukin-2: a pilot study. J Clin Oncol. 1988;6:839. doi: 10.1200/JCO.1988.6.5.839. [DOI] [PubMed] [Google Scholar]
- 26.Urmacher C, Cordon-Cardo C, Houghton AN. Tissue distribution of GD3 ganglioside detected by mouse monoclonal antibody R24. Am J Dermatopathol. 1989;11:577. doi: 10.1097/00000372-198912000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Van Den Eynde B, Hainaut P, Herin M, Knuth A, Lemoine C, Weynants P, Van Der Bruggen P, Fauchet R, Boon T. Presence on a human melanoma of multiple antigens recognized by autologous CTL. Int J Cancer. 1989;44:634. doi: 10.1002/ijc.2910440413. [DOI] [PubMed] [Google Scholar]
- 28.Van Der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van Den Eynde B, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 29.Wolfel T, Klehmann E, Muller C, Schutt KH, Meyer Zum Buschenfelde KH, Knuth A. Lysis of human melanoma cells by autologous cytolytic T-cell clones: identification of human histocompatibility leukocyte antigen A2 as a restriction element for three different antigens. J Exp Med. 1989;170:797. doi: 10.1084/jem.170.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zachary AA, Teresi GA, editors. ASHI Laboratory Manual. 2nd edn. American Society for Histocompatibility and Immunogenetics; New York: 1990. p. 195. [Google Scholar]
- 31.Zakut R, Topalian SL, Kawakami Y, Mancini M, Eliyahu S, Rosenberg SA. Differential expression of MAGE-1, -2, -3 mRNA in transformed and normal human cell lines. Cancer Res. 1993;53:5. [PubMed] [Google Scholar]