Abstract
Background
Near-infrared (NIR) fluorescence imaging using indocyanine green (ICG) has the potential to improve sentinel lymph node (SLN) mapping of breast cancer. In the current randomized clinical trial, the value of blue dyes when used in combination with NIR fluorescence was assessed. Furthermore, the possibility to perform SLN mapping without radiotracers was preliminarily examined.
Methods
Clinical trial subjects were 24 consecutive breast cancer patients scheduled to undergo SLN biopsy. All patients received standard of care using 99mTechnetium-nanocolloid and received 1.6 mL of 500-µM ICG injected periareolarly. Patients were randomly assigned to undergo SLN biopsy with or without patent blue. To assess the need for radiocolloids to localize the SLN(s), the surgeon did not use the handheld gamma probe during the first 15 min after the axillary skin incision.
Results
SLN mapping was successful in 23 of the 24 patients. No significant difference was found in signal-to-background ratio between the patent blue group and no patent blue group (8.3 ± 3.8 vs. 10.3 ± 5.7, respectively, P = 0.32). In both groups, 100% of SLNs were radioactive and fluorescent, and in the patent blue group, only 84% of SLNs were stained blue. In 25% of patients, the use of the gamma probe was necessary to localize the SLN within the first 15 min.
Conclusion
This study shows that there is no benefit of using patent blue for SLN mapping in breast cancer patients when using NIR fluorescence and 99mTechnetium-nanocolloid. NIR fluorescence imaging outperformed patent blue in all patients.
INTRODUCTION
Sentinel lymph node (SLN) mapping is regarded as standard of care in staging of the axilla in breast cancer patients with clinically negative axillary lymph nodes [1]. To locate the SLN, different techniques can be used. Combining a radiotracer and blue dye staining achieves the highest identification rates (95%–97%) and is therefore preferred [2–5]; however, both methods have several disadvantages. Radioactive colloids require involvement of a nuclear physician, do not provide visual information intraoperatively, and the time window in which they can be used is limited due to the half-life of 99mTechnetium. Moreover, a substantial portion of patients undergo SLN mapping using only a blue dye, because radioactive isotopes are not widely available in every medical center. The percentage of patients in whom only blue dye staining is used for SLN mapping varies from 4% to 50% in developed countries [6–9]. Blue dyes cannot be seen through skin and fatty tissue, permit only limited visualization of afferent lymphatic vessels, and in case a lumpectomy is performed, tattooing of the breast can be seen up until several months after blue dye injection.
Due to these disadvantages, optical imaging using the near-infrared (NIR) fluorescence lymphatic tracer indocyanine green (ICG) has been put forward as an alternative for, or an addition to conventional SLN mapping. Feasibility of this technique has been extensively reported in breast cancer patients [10–16] and other cancer types [17]. A previous dose-finding study performed by our group recently demonstrated that NIR fluorescence using a dose of 500-µM ICG adsorbed to human serum albumin was most convenient [18]. A follow-up double-blind randomized clinical trial demonstrated no advantages of premixing ICG with human serum albumin in comparison to ICG alone at a dose of 500 µM for NIR fluorescence SLN mapping [19].
In the clinical trials discussed above, the NIR fluorescence signal of ICG was consistently visualized earlier than the blue dye staining. In the total of 60 SLNs that were detected in the 2 studies (N = 42 patients), only 48 (80%) were stained blue, while 60 (100%) SLNs could be detected with NIR fluorescence [18,19]. Therefore, NIR fluorescence imaging has the potential to replace blue dyes in SLN mapping in breast cancer patients. If the use of blue dye staining could be omitted, disadvantages like tattooing of the breast and blue staining of the surgical field in case of a lumpectomy could be prevented. Furthermore, the use of blue dyes may interfere with NIR fluorescence imaging by absorbing the fluorescent light and thereby decreasing the NIR fluorescent signal. Finally, anaphylactic reactions to blue dyes, although rare, can be life threatening. Because NIR fluorescent light penetrates relatively deep into tissue (up to 0.5–1 cm), it also has the potential to replace the use of radiotracers. However, omitting the use of radiotracers may only be reserved for selected patients, for example those with a sufficiently low body mass index (BMI).
In the current randomized clinical trial, the added value of using blue dye staining when used in combination with NIR fluorescence and radiotracer was assessed. Furthermore, the concordance between NIR fluorescence, radiocolloids, and patent blue was assessed, and the possibility of performing SLN mapping without radiotracers was preliminarily explored.
METHODS
Preparation of Indocyanine Green
ICG (25-mg vials) was purchased from Pulsion Medical Systems (Munich, Germany) and was resuspended in 10 cc of sterile water for injection to yield a 2.5-mg/ml (3.2 mM) stock solution. To obtain a 500-µM dilution of ICG, 7.8 mL of the 3.2-mM ICG solution was diluted in 42.8 mL of sterile water. In a previous study, we determined that the optimal dose of ICG lies between 400 µM and 800 µM [18], therefore a dose of 500 µM was chosen.
Intraoperative Near-Infrared Imaging System (Mini-FLARE™)
SLN mapping was performed using the Mini-Fluorescence-Assisted Resection and Exploration (Mini-FLARE™) image-guided surgery system, as described earlier [18]. Briefly, the system consists of 2 wavelength isolated light sources: a “white” light source, generating 26,600 lx of 400 to 650 nm light, and a “near-infrared” light source, generating 7.7 mW/cm2 of 760-nm light. Color video and NIR fluorescence images are simultaneously acquired and displayed in real time using custom optics and software that separate the color video and NIR fluorescence images. A pseudo-colored (lime green) merged image of the color video and NIR fluorescence images is also displayed. The imaging head is attached to a flexible gooseneck arm, which permits positioning of the imaging head at extreme angles virtually anywhere over the surgical field. For intraoperative use, the imaging head and imaging system pole stand are wrapped in a sterile shield and drape (Medical Technique Inc., Tucson, AZ).
Clinical Trial
This randomized, single-institution trial comparing NIR fluorescence SLN mapping with or without patent blue was approved by the Medical Ethics Committee of the Leiden University Medical Center and was performed in accordance with the ethical standards of the Helsinki Declaration of 1975. All patients planning to undergo a SLN procedure for invasive breast cancer or high-risk carcinoma in situ were eligible for participation in the trial. Patients had clinically negative axillary nodes as assessed by palpation and ultrasonography. Exclusion criteria were pregnancy, lactation or an allergy to iodine, shellfish, or indocyanine green. All patients gave informed consent and were anonymized.
As part of the SLN procedure, patients were injected periareolarly with approximately 100 MBq 99mTechnetium-nanocolloid the day before surgery. Before the start of the operation, patients were randomly assigned to receive or not receive an injection with patent blue. Patients were randomized by the Department of Surgery and treatment allocation was performed by block randomization. For patients randomized to be injected with patent blue, 1 mL total of patent blue (Bleu Patenté V, Guerbet, Brussels, Belgium) was injected intradermally and periareolarly at 4 sites before the start of the operation. All patients were intradermally and periareolarly injected with 1.6 mL total of 500-µM ICG at 4 sites before the start of the operation. Patent blue and ICG injections were performed by the surgeon. Subsequently, gentle pumping pressure was applied to the injection site for 1 min. After surgical scrub and sterile covering of the operation field, NIR fluorescence imaging was performed with the imaging head of the Mini-FLARE™ at approximately 30-cm distance to the surgical field. The lights in the operating room were turned off, and the surgical field was illuminated at a high fluence rate using the white light surgical luminary of the Mini-FLARE™ imaging system. Camera exposure times were between 5 to 250 ms. A SLN exhibiting a signal-to-background ratio (SBR) ≥ 1.1 in situ was considered positive by NIR fluorescence.
To assess the need for radiocolloids to localize the SLN(s), the surgeon did not use the handheld gamma probe during the first 15 min of the operation starting from the axillary skin incision. In case the SLN(s) were not localized using only ICG or ICG in combination with patent blue within the first 15 min, the surgeon was allowed to use the handheld gamma probe for SLN localization.
Routine histopathological frozen analysis of SLNs was performed during surgery. After frozen section analysis, SLNs were fixed in formalin and embedded in paraffin for routine hematoxylin and eosin staining and immunohistopathological staining for AE1/AE3 at 3 levels, with an interval of 150 to 250 µm, according to the Dutch guidelines for SLN analysis. Patients underwent an axillary lymph node dissection if the SLN was found to contain metastases. If micrometastases (< 0.2 mm) or isolated tumor cells were found, no axillary lymph node dissection was performed.
Power Calculation and Statistical Analysis
To show non-inferiority, a power calculation based on data from our previous studies [18,19] revealed that 24 patients are needed to achieve 91% power to detect a difference of 5.0 in SBR between the 2 groups with the null hypothesis that the mean SBR of each group is 10.0 ± the standard deviation of 3.5; and the alternative hypothesis that the mean of the no blue dye group is 15.0 with a significance level of 0.05 using a 2-sided 2-sample t test. For statistical analysis, SPSS statistical software package (Version 16.0, Chicago, IL) was used. To compare patient characteristics, SBR and the number of SLNs identified between the patent blue group and no patent blue group, the independent-sample t test and chi-square test were used. P < 0.05 was considered significant.
RESULTS
Patient and Tumor Characteristics
Twenty-four consecutive breast cancer patients undergoing SLN mapping using 99mTechnetium-nanocolloid and ICG were randomized to be injected with or without patent blue. The median age of the included patients was 59 years (range: 39–75) and the median BMI was 24 kg/m2 (range: 19–47). BMI was significantly higher in the no patent blue group (P = 0.042). Other patient, tumor, and treatment characteristics were equally distributed over the treatment groups (Table 1). The average time between injection of ICG and the skin incision was not significantly different between treatment groups and was 15.2 ± 3.0 min and 13.2 ± 3.0 min for the patent blue and no patent blue patient groups, respectively. No adverse reactions associated with the use of ICG or the Mini-FLARE imaging system occurred. No postoperative complications of the sentinel lymph node procedure were observed.
Table 1.
Patient and Tumor Characteristics
| Patent Blue (N = 12) |
No Patent Blue (N = 12) |
||||
|---|---|---|---|---|---|
| Characteristic | N | % | N | % | P |
| Age in years (Median, Range) |
54 (39–75) | 67 (48–71) | 0.15 | ||
| Body Mass Index (median, range) |
23.5 (19–34) | 28 (20–47) | 0.042* | ||
| Skin Type | 0.62 | ||||
| - II* | 2 | 17 | 2 | 17 | |
| - III+ | 10 | 83 | 10 | 83 | |
| Previous Procedure of Breast |
0.54 | ||||
| - Excision fibroadenoma |
1 | 8 | 0 | 0 | |
| - Neoadjuvant Chemotherapy |
2 | 17 | 1 | 8 | |
| - Neoadjuvant Hormonal Therapy |
0 | 0 | 1 | 8 | |
| Multifocality | 0 | 0 | 1 | 8 | 0.31 |
| Tumor side | 0.41 | ||||
| - Left | 6 | 50 | 7 | 59 | |
| - Right | 6 | 50 | 5 | 42 | |
| Tumor localization | 0.69 | ||||
| - Upper Outer | 7 | 59 | 7 | 59 | |
| - Lower Outer | 0 | 0 | 0 | 0 | |
| - Lower Medial | 0 | 0 | 0 | 0 | |
| - Upper Medial | 3 | 25 | 3 | 25 | |
| - Central | 2 | 17 | 2 | 17 | |
| Type of Operation | 0.36 | ||||
| - Mastectomy | 2 | 17 | 3 | 25 | |
| - Wide Local Excision | 9 | 75 | 9 | 75 | |
| - SNB Only | 1 | 8 | 0 | 0 | |
| Pathological Tumor Size in mm (Median, Range) |
15 (5–35) | 16 (5–50) | 0.12 | ||
| Histological Type | 1 | ||||
| - Infiltrating Ductal type Adenocarcinoma |
10 | 83 | 10 | 83 | |
| - Infiltrating Lobular type Adenocarcinoma |
1 | 8 | 1 | 8 | |
| - Ductal Carcinoma In Situ |
1 | 8 | 1 | 8 | |
| Histological Grade | 0.56 | ||||
| - I | 4 | 33 | 3 | 25 | |
| - II | 4 | 34 | 3 | 25 | |
| - III | 3 | 2 | 5 | 42 | |
| - No grading possible (DCIS) |
1 | 8 | 1 | 8 | |
Skin type II: White: usually burns easily; tans minimally (Northern European),
Skin type III: III. White (average): sometimes burns; tans gradually to light brown (Central European)
Intraoperative NIR Fluorescence Imaging
In 23 of 24 patients, at least 1 SLN (Figure 1) was identified (Table 2). In 1 patient included in the no patent blue group, no SLN could be detected, even when using the gamma probe A total of 19 and 18 SLNs were resected in the patent blue and no patent blue groups, respectively. In the patent blue group, 19 of 19 (100%) of SLNs were NIR fluorescent, 17 of 19 (89%) SLNs were radioactive and 16 of 19 (84%) SLNs were blue. In the no patent blue group, 18 of 18 (100%) SLNs were NIR fluorescent and 18 of 18 (100%) of SLNs were radioactive. The afferent lymphatics were visualized percutaneously in 83% and 75% of patients in the patent blue and no patent blue patient groups, respectively. No significant difference was observed. In all patients that were administered with patent blue, the NIR fluorescence signal in the SLN was detected before patent blue was visualized.
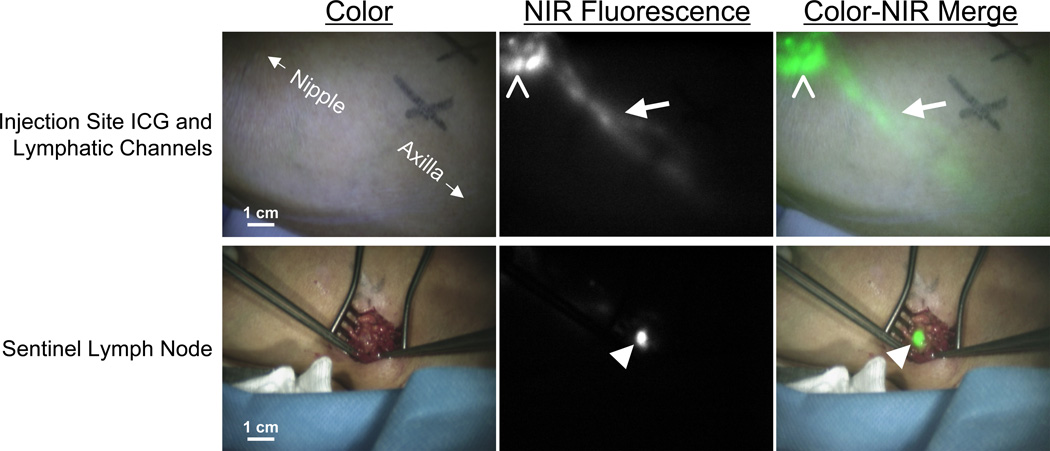
Figure 1. NIR fluorescence imaging during sentinel lymph node mapping in a breast cancer patient.
In the upper row, the periareolar injection site (open arrowhead) and an afferent lymphatic channel (arrow) are clearly visualized. In the lower row, identification of the SLN (arrowhead) with NIR fluorescence imaging is demonstrated 10 min after incision. Camera exposure times were 30 ms (top row) and 100 ms (bottom row). Scale bars represent 1 cm. Patent blue was omitted in this patient.
Table 2.
SLN Identification Results
| Characteristic | Total (N = 24) |
Patent Blue (N = 12) |
No Patent Blue (N = 12) |
P | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Number of SLNs Identified | 37 | 19 | 18 | ||||
| Number of SLNs Identified per Patient |
0.54 | ||||||
| - No SLN | 1 | 4 | 0 | 0 | 1 | 8 | |
| - One SLN | 12 | 50 | 7 | 58 | 5 | 42 | |
| - Two SLNs | 9 | 38 | 4 | 33 | 5 | 42 | |
| - Three SLNs | 1 | 4 | 1 | 8 | 0 | 0 | |
| - Four SLNs | 1 | 4 | 0 | 0 | 1 | 8 | |
| Average Number of SLNs Identified (SD) |
1.5 ± 0.8 | 1.6 ± 0.7 | 1.5 ± 1.0 | 0.81 | |||
| Method of Detection | |||||||
| - Radioactive | 35 | 95 | 17 | 89 | 18 | 100 | |
| - Blue | 16 | 84 | 16 | 84 | 0 | 0 | |
| - Fluorescent | 37 | 100 | 19 | 100 | 18 | 100 | |
| Signal-to-Background Ratio | 9.2 ± 4.8 | 8.3 ± 3.8 | 10.3 ± 5.7 | 0.32 | |||
| Percutaneous Lymph Drainage Visualization |
0.84 | ||||||
| - Yes | 12 | 50 | 6 | 50 | 6 | 50 | |
| - Partially | 7 | 29 | 4 | 33 | 3 | 25 | |
| - No | 5 | 21 | 2 | 17 | 3 | 25 | |
| Average Time between Injection and Skin Incision (min, SD) |
14.8 ± 3.3 | 15.2 ± 3.0 | 13.8 ± 3.9 | 0.17 | |||
| Average Time between Skin Incision and SLN Resection (min, SD) |
12.2 ± 7.9 | 12.4 ± 7.7 | 18.1 ± 18.9 | 0.35 | |||
| Histology sentinel lymph node | 0.10 | ||||||
| - Negative | 16 | 67 | 6 | 50 | 10 | 83 | |
| - Isolated Tumor Cells | 4 | 17 | 4 | 33 | 0 | 0 | |
| - Micrometastases | 2 | 8 | 0 | 0 | 2 | 17 | |
| - Macrometastases | 2 | 8 | 2 | 17 | 0 | 0 | |
| Axillary Lymph Node Dissection | 0.14 | ||||||
| - No | 22 | 92 | 10 | 83 | 12 | 100 | |
| - Yes | 2 | 8 | 2 | 17 | 0 | 0 | |
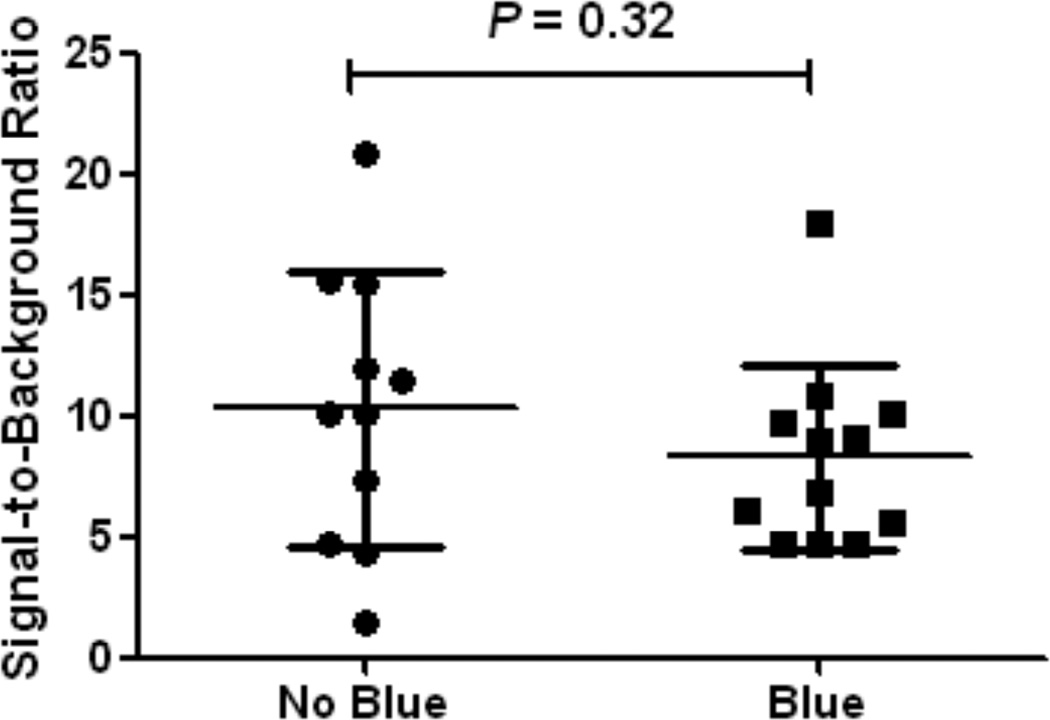
Average brightness of the SLN, expressed in signal-to-background ratio (SBR), was 8.3 ± 3.8 and 10.3 ± 5.7 for the patent blue and the no patent blue group, respectively (Figure 2; Table 2). No significant difference in SBR was observed between the treatment groups (P = 0.32). Average time between skin incision and SLN identification was 12.4 ± 7.7 min and 18.1 ± 18.9 min for the blue dye and no blue dye patient groups, respectively. No significant difference was observed (P = 0.35). In one patient in the no patent blue group, time between skin incision and SLN detection was 66.91 min. This patient received neoadjuvant chemotherapy, and the SLN was located in the area next to the latissimus dorsi muscle. Excluding this outlier, average time between skin incision and SLN identification was 12.4 ± 7.7 min and 13.2 ± 10.3 for the patent blue and no patent blue treatment groups, respectively (P = 0.83).
Figure 2. Difference in brightness of SLNs between treatment groups.
Signal-to-background ratios (mean ± S.D.) of breast SLNs are plotted. The SBRs of blue and no blue patient groups were not significantly different.
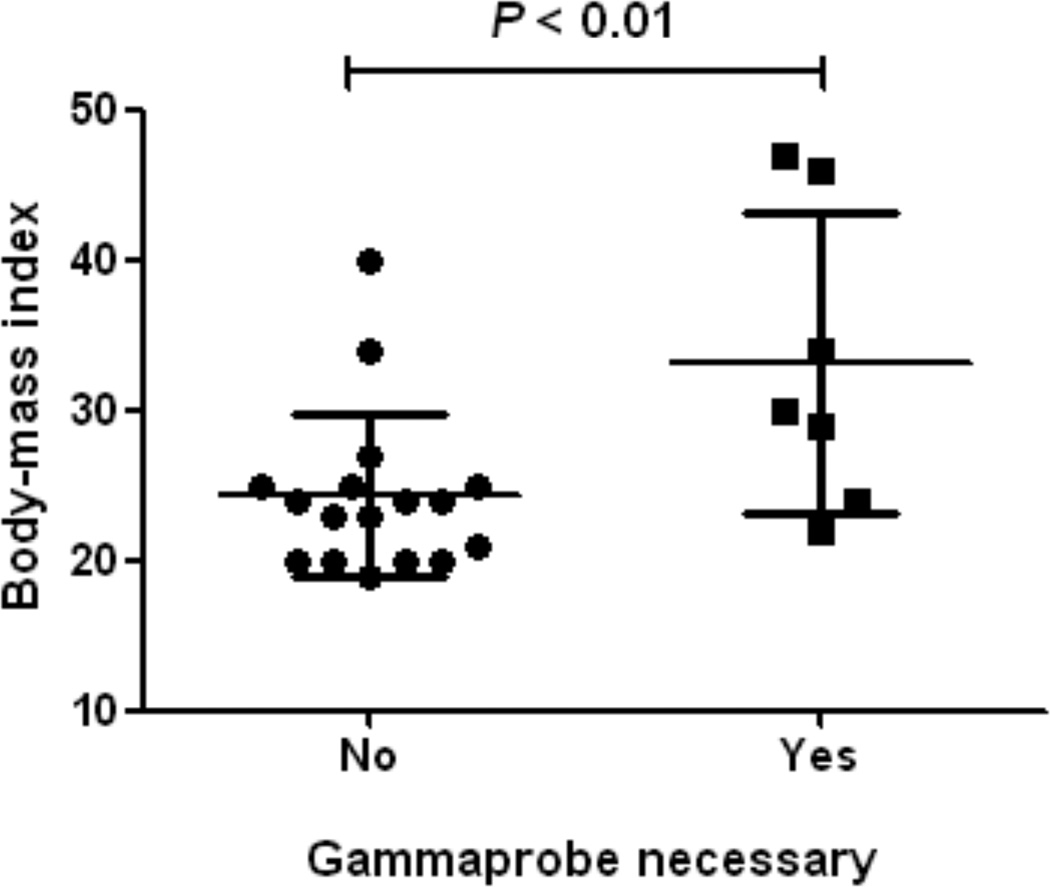
To assess the need for radiotracers to localize the SLN(s), the surgeon did not use the handheld gamma probe during the first 15 min starting from skin incision. After the first 15 min, the use of the handheld gamma probe was necessary to locate the SLN(s) in 2 patients in the patent blue group and 4 patients in the no patent blue group. In all other patients, the handheld gamma probe was only used to verify the SLN for radioactivity ex vivo and to verify if any SLNs were missed. The average BMI of patients in whom the gamma probe was needed for SLN identification (33.1 ± 9.9) was significantly higher than in patients in whom the gamma probe could be omitted (24.4 ± 5.4; P < 0.01; Figure 3).
Figure 3. Influence of body-mass index on the necessity to use the gamma probe.
The BMI of patients was plotted. The average BMI of patients in whom the gamma probe was needed for SLN identification (33.1 ± 9.9) was significantly higher than in patients in whom the gamma probe could be omitted (24.4 ± 5.4) (P < 0.01).
DISCUSSION
NIR fluorescence imaging has been extensively described as a tool for the SLN procedure in various types of cancer. In previous work, we demonstrated that a dose of 500 µM of ICG, without premixing with human serum albumin, was optimal to perform NIR fluorescence SLN mapping in breast cancer [18,19]. In the current randomized clinical trial, the added value of patent blue staining, when used in combination with NIR fluorescence, was assessed in breast cancer patients undergoing the SLN procedure. Regarding time to identify the SLN, no significant difference between the 2 treatment groups was observed. In one patient in the no patent blue group, the SLN mapping lasted 67 min. A possible explanation for this relatively long time to locate the SLN could be that this patient was treated with neoadjuvant chemotherapy (NAC). It has been proposed that NAC alters the lymphatic drainage network, thereby possibly hampering the accuracy of the SLN procedure.[20] This could possibly be an explanation for the difficult identification of the SLN in 1 of our patients. However, in the current study, 2 more patients were treated with NAC and in those patients time between skin incision and SLN identification was in concordance with the average time between skin incision and SLN identification that was observed in all other patients (approximately 13 min). A meta-analysis of studies that examined the SLN procedure in patients treated with NAC included 1273 patients and reported an identification rate of 90% and a false-negative rate of 12% [21]; additionally, a more recent meta-analysis reported similar results [22]. These results are not significantly different from meta-analyses of SLN biopsy in patients who are naïve to chemotherapy [23]. Nonetheless, results of SLN biopsy after NAC remain conflicting. The prospective multicenter German SENTINA trial is currently accruing patients to evaluate the accuracy of the SLN procedure after NAC.
Comparing the patent blue and no patent blue patient groups, brightness of the SLNs was higher in the no patent blue group (difference in SBR is 2.0); however, this difference was not significant. Furthermore, in the patent blue group, only 84% of NIR fluorescent SLNs were stained blue, which is in concordance with previous studies [18,19]. Patent blue and ICG were injected at the same time prior to surgery, at the same location, and by the same person. Furthermore, ICG and patent blue have comparable hydrodynamic diameters [24,25]. A potential reason for SLNs that are not stained blue but are NIR fluorescent is the sensitivity of NIR fluorescence imaging systems, which can detect significantly lower doses of tracer compared to blue dye staining [11,26,27]. These results indicate that NIR fluorescence using ICG can replace blue dye staining, which would have several advantages. First, the use of blue dyes stains the surgical field in an unnatural color that persists over the course of several months after surgery. Second, blue dyes cannot be visualized when covered by overlying tissue, while ICG can be detected through millimeters to a centimeter of overlying tissue. Third, in many cases, ICG can be seen percutaneously, which allows lymphatic mapping before surgery, which possibly decreases time to identify the SLN.
An obvious next step in the optimization of the SLN procedure would be to evaluate the need for radiotracers. Although radiotracers have superior tissue penetration, they expose caregivers and patients to ionizing radiation, and they can only be detected using a gamma probe, which does not provide the surgeon with visual information. Furthermore, the time window for SLN identification is limited due to the short half-life (6 hours) of 99mTechnetium. To explore the necessity of intraoperative radiotracers in addition to NIR fluorescence, the surgeon was not allowed to use the gamma probe during the first 15 min of the surgery. In 6 of 24 patients (25%), the surgeon could not identify the SLN within the first 15 min and in one patient, no SLN could be detected at all. In the current study, average BMI of patients in whom the gamma probe was used for SLN identification was significantly higher than in patients in whom the gamma probe was not necessary. These results are in concordance with previous studies that showed a significant correlation between time to identify the SLN and BMI [28,29]. Furthermore, when only patent blue is used for SLN mapping, the SLN detection rate is significantly higher in patients with a BMI < 30 compared to patients with a BMI > 30 [30]. This suggests that BMI plays an important role in selecting those patients whom are eligible for NIR fluorescence SLN mapping without the use of radiotracers. However, the current study was not powered to compare the sensitivity of NIR fluorescence and radiocolloids, and this has to be addressed in a future sufficiently powered clinical trial. Furthermore, because larger series will be required to determine the safety and SLN identification rate when radiotracers are omitted, the use of NIR fluorescence imaging using ICG as a lymphatic tracer is at present particularly attractive to hospitals unable to work with radioactive isotopes,.
Another approach within the field of SLN mapping is combining fluorescence and radioactivity in one lymphatic tracer by simply premixing ICG and 99mTc-NanoColl (complex: ICG-99mTc-NanoColl), which has been performed in prostate cancer patients [17]. Using this multimodal tracer injected by the nuclear physician before surgery, time of surgery will probably be shortened because no dye injection and massage is needed in the surgical theatre. A clinical trial in breast cancer patients using this hybrid multimodal radiocolloid is currently ongoing in our center.
In conclusion, this randomized trial showed no advantage of using patent blue for the SLN procedure in breast cancer when NIR fluorescence and radiotracers are used. Combining these results with previous work, a dose of 500-µM ICG injected in a total of 1.6 ml is recommended for NIR fluorescence SLN mapping in breast cancer patients and patent blue can be omitted.
ACKNOWLEDGMENTS
We thank the following individuals for the contribution to this study: Gemma Ranke, Elly Krol-Warmerdam, Annemarie Voet-van den Brink, Gerlinda van Gent-de Bruijn (Breast Cancer Unit) and Linda van der Hulst (Central Pharmacy). We thank Lindsey Gendall for editing. This work was supported in part by NIH grants R01-CA-115296 and R21-CA-130297 and the Dutch Cancer Society grant UL2010-4732. This research was performed within the framework of CTMM, the Center for Translational Molecular Medicine, project MUSIS (grant 03O-202). Joost van der Vorst is an MD-medical research trainee funded by The Netherlands Organisation for Health Research and Development (grant 92003593).
Footnotes
CONFLICT OF INTEREST
Joost R. van der Vorst, M.D.: None.
Boudewijn E. Schaafsma, M.D.: None
Floris Verbeek: None
Merlijn Hutteman, M.Sc., Ph.D.: None.
J. Sven D. Mieog, M.D, Ph.D.: None.
Clemens W.G.M. Lowik, Ph.D.: None
Gerrit-Jan Liefers, M.D., Ph.D.: None.
John V. Frangioni, M.D., Ph.D.: FLARE™ technology is owned by Beth Israel Deaconess Medical Center, a teaching hospital of Harvard Medical School. It has been licensed to the FLARE™ Foundation, a nonprofit organization focused on promoting the dissemination of medical imaging technology for research and clinical use. Dr. Frangioni is the founder and chairman of the FLARE™ Foundation. The Beth Israel Deaconess Medical Center will receive royalties for sale of FLARE™ Technology. Dr. Frangioni has elected to surrender post-market royalties to which he would otherwise be entitled as inventor, and has elected to donate pre-market proceeds to the FLARE™ Foundation.
Cornelis J.H. van de Velde, M.D., Ph.D.: None.
Alexander L. Vahrmeijer, M.D., Ph.D.: None.
REFERENCES
- 1.Cox CE, Pendas S, Cox JM, et al. Guidelines for sentinel node biopsy and lymphatic mapping of patients with breast cancer. Ann Surg. 1998;227:645–651. doi: 10.1097/00000658-199805000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goyal A, Newcombe RG, Chhabra A, Mansel RE. Factors affecting failed localisation and false-negative rates of sentinel node biopsy in breast cancer--results of the ALMANAC validation phase. Breast Cancer Res Treat. 2006;99:203–208. doi: 10.1007/s10549-006-9192-1. [DOI] [PubMed] [Google Scholar]
- 3.Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8:881–888. doi: 10.1016/S1470-2045(07)70278-4. [DOI] [PubMed] [Google Scholar]
- 4.Zavagno G, De Salvo GL, Scalco G, et al. A Randomized clinical trial on sentinel lymph node biopsy versus axillary lymph node dissection in breast cancer: results of the Sentinella/GIVOM trial. Ann Surg. 2008;247:207–213. doi: 10.1097/SLA.0b013e31812e6a73. [DOI] [PubMed] [Google Scholar]
- 5.Straver ME, Meijnen P, van Tienhoven G, et al. Sentinel Node Identification Rate and Nodal Involvement in the EORTC 10981-22023 AMAROS Trial. Ann Surg Oncol. 2010;17:1854–1861. doi: 10.1245/s10434-010-0945-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonoo H, Noguchi S. Results of questionnaire survey on breast cancer surgery in Japan 2004–2006. Breast Cancer. 2008;15:3–4. doi: 10.1007/s12282-007-0017-9. [DOI] [PubMed] [Google Scholar]
- 7.Davis KG, Schriver JP. Prevalence of teaching sentinel lymph node biopsy for breast cancer in general surgery residency programs. Curr Surg. 2002;59:420–422. doi: 10.1016/s0149-7944(02)00618-9. [DOI] [PubMed] [Google Scholar]
- 8.Keshtgar M, Aresti N, Macneil F. Establishing axillary Sentinel Lymph Node Biopsy (SLNB) for early breast cancer in the United Kingdom: a survey of the national training program. Eur J Surg Oncol. 2010;36:393–398. doi: 10.1016/j.ejso.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Quan ML, Hodgson N, Lovrics P, et al. National adoption of sentinel node biopsy for breast cancer: lessons learned from the Canadian experience. Breast J. 2008;14:421–427. doi: 10.1111/j.1524-4741.2008.00617.x. [DOI] [PubMed] [Google Scholar]
- 10.Kitai T, Inomoto T, Miwa M, Shikayama T. Fluorescence navigation with indocyanine green for detecting sentinel lymph nodes in breast cancer. Breast Cancer. 2005;12:211–215. doi: 10.2325/jbcs.12.211. [DOI] [PubMed] [Google Scholar]
- 11.Murawa D, Hirche C, Dresel S, Hunerbein M. Sentinel lymph node biopsy in breast cancer guided by indocyanine green fluorescence. Br J Surg. 2009;96:1289–1294. doi: 10.1002/bjs.6721. [DOI] [PubMed] [Google Scholar]
- 12.Tagaya N, Yamazaki R, Nakagawa A, et al. Intraoperative identification of sentinel lymph nodes by near-infrared fluorescence imaging in patients with breast cancer. Am J Surg. 2008;195:850–853. doi: 10.1016/j.amjsurg.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 13.Hirche C, Murawa D, Mohr Z, Kneif S, Hunerbein M. ICG fluorescence-guided sentinel node biopsy for axillary nodal staging in breast cancer. Breast Cancer Res Treat. 2010;121:373–378. doi: 10.1007/s10549-010-0760-z. [DOI] [PubMed] [Google Scholar]
- 14.Hojo T, Nagao T, Kikuyama M, Akashi S, Kinoshita T. Evaluation of sentinel node biopsy by combined fluorescent and dye method and lymph flow for breast cancer. Breast. 2010;19:210–213. doi: 10.1016/j.breast.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Troyan SL, Kianzad V, Gibbs-Strauss SL, et al. The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann Surg Oncol. 2009;16:2943–2952. doi: 10.1245/s10434-009-0594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sevick-Muraca EM, Sharma R, Rasmussen JC, et al. Imaging of lymph flow in breast cancer patients after microdose administration of a near-infrared fluorophore: feasibility study. Radiology. 2008;246:734–741. doi: 10.1148/radiol.2463070962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Poel HG, Buckle T, Brouwer OR, Valdes Olmos RA, van Leeuwen FW. Intraoperative Laparoscopic Fluorescence Guidance to the Sentinel Lymph Node in Prostate Cancer Patients: Clinical Proof of Concept of an Integrated Functional Imaging Approach Using a Multimodal Tracer. Eur Urol. 2011 doi: 10.1016/j.eururo.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 18.Mieog JS, Troyan SL, Hutteman M, et al. Toward Optimization of Imaging System and Lymphatic Tracer for Near-Infrared Fluorescent Sentinel Lymph Node Mapping in Breast Cancer. Ann Surg Oncol. 2011 doi: 10.1245/s10434-011-1566-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hutteman M, Mieog JS, van der Vorst JR, et al. Randomized, double-blind comparison of indocyanine green with or without albumin premixing for near-infrared fluorescence imaging of sentinel lymph nodes in breast cancer patients. Breast Cancer Res Treat. 2011;127:163–170. doi: 10.1007/s10549-011-1419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuerer HM, Hunt KK. The rationale for integration of lymphatic mapping and sentinel node biopsy in the management of breast cancer patients receiving neoadjuvant chemotherapy. Semin Breast Dis. 2002;5:80–87. [Google Scholar]
- 21.Xing Y, Foy M, Cox DD, et al. Meta-analysis of sentinel lymph node biopsy after preoperative chemotherapy in patients with breast cancer. Br J Surg. 2006;93:539–546. doi: 10.1002/bjs.5209. [DOI] [PubMed] [Google Scholar]
- 22.van Deurzen CH, Vriens BE, Tjan-Heijnen VC, et al. Accuracy of sentinel node biopsy after neoadjuvant chemotherapy in breast cancer patients: a systematic review. Eur J Cancer. 2009;45:3124–3130. doi: 10.1016/j.ejca.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Fraile M, Rull M, Julian FJ, et al. Sentinel node biopsy as a practical alternative to axillary lymph node dissection in breast cancer patients: an approach to its validity. Ann Oncol. 2000;11:701–705. doi: 10.1023/a:1008377910967. [DOI] [PubMed] [Google Scholar]
- 24.Michel CC, Levick JR. Variations in permeability along individually perfused capillaries of the frog mesentery. Q J Exp Physiol Cogn Med Sci. 1977;62:1–10. doi: 10.1113/expphysiol.1977.sp002369. [DOI] [PubMed] [Google Scholar]
- 25.Ohnishi S, Lomnes SJ, Laurence RG, et al. Organic alternatives to quantum dots for intraoperative near-infrared fluorescent sentinel lymph node mapping. Mol Imaging. 2005;4:172–181. doi: 10.1162/15353500200505127. [DOI] [PubMed] [Google Scholar]
- 26.Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003;7:626–634. doi: 10.1016/j.cbpa.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Fujisawa Y, Nakamura Y, Kawachi Y, Otsuka F. Indocyanine green fluorescence-navigated sentinel node biopsy showed higher sensitivity than the radioisotope or blue dye method, which may help to reduce false-negative cases in skin cancer. J Surg Oncol. 2012 doi: 10.1002/jso.23045. [DOI] [PubMed] [Google Scholar]
- 28.Aliakbarian M, Memar B, Jangjoo A, et al. Factors influencing the time of sentinel node visualization in breast cancer patients using intradermal injection of the radiotracer. Am J Surg. 2011;202:199–202. doi: 10.1016/j.amjsurg.2010.06.035. [DOI] [PubMed] [Google Scholar]
- 29.Pritsivelis C, Garcia Mendonca CA, Pinheiro Pessoa MC, et al. Failure predictors of the sentinel lymph node in patients with breast cancer using Tc-99m sulfur colloid and periareolar injection. Q J Nucl Med Mol Imaging. 2007;51:189–193. [PubMed] [Google Scholar]
- 30.Nos C, Freneaux P, Guilbert S, et al. Sentinel lymph node detection for breast cancer: which patients are best suited for the patent blue dye only method of identification? Ann Surg Oncol. 2001;8:438–443. doi: 10.1007/s10434-001-0438-1. [DOI] [PubMed] [Google Scholar]