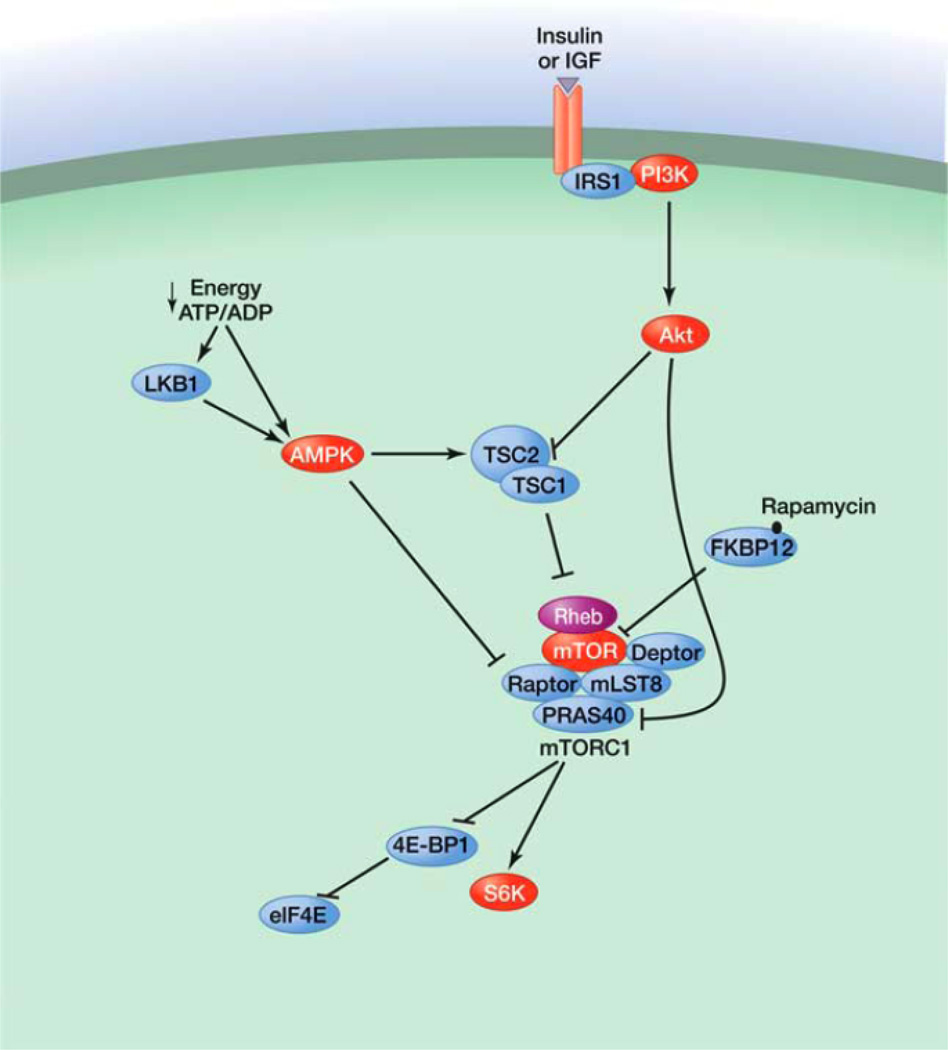
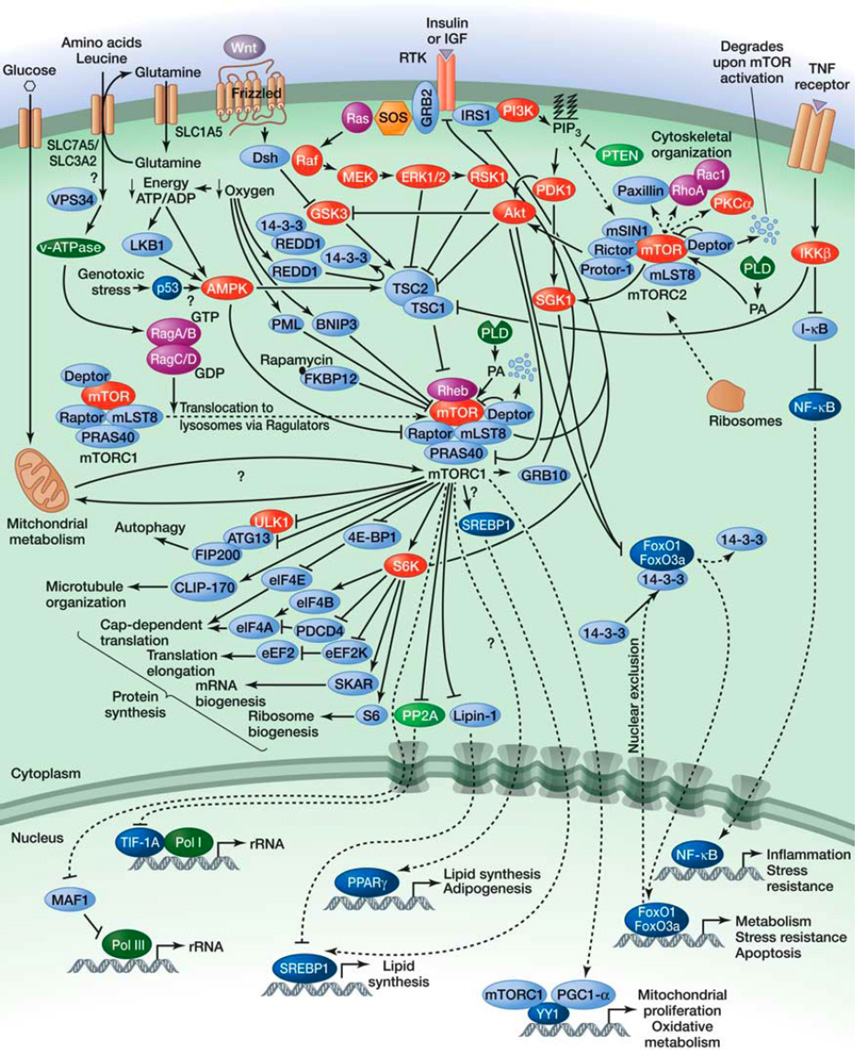
The scarcity of nutrients/energy interspersed with sporadic periods of abundance means that cells must transition between anabolic and catabolic states. An important protein that has evolved to respond to this need is the target of rapamycin (TOR). TOR is a well-conserved serine/threonine kinase that plays an important role in the signaling network that controls growth and metabolism in response to environmental cues. As its name indicates, TOR is the target of amolecule named rapamycin, an anti-fungal macrolide produced by the bacterial species Streptomyces hygroscopicus, which was isolated from a soil sample from the Eastern Islands in the 1970s (Vezina et al. 1975). In addition to its anti-fungal properties, rapamycin strongly inhibits cell growth and proliferation, making this molecule a valuable tool to study cell growth control. In the early 1990s, yeast genetic screens led to the identification of TOR as one mediator of the toxic effect of rapamycin in yeast (Heitman et al. 1991; Kunz et al. 1993). Shortly after, the mammalian TOR (mTOR), now officially known as the mechanistic TOR, was identified as the physical target of rapamycin (Fig. 1) (Brown et al. 1994; Sabatini et al. 1994; Sabers et al. 1995). mTOR interacts with many proteins to form at least two distinct multiprotein complexes: mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2) (Zoncu et al. 2011). In addition to their different protein compositions, the mTOR complexes have important differences in their sensitivities to rapamycin, in the upstream signals they integrate, in the substrates they regulate, and in the biological processes they control (Laplante and Sabatini 2009b). mTORC1 is sensitive to rapamycin and integrates inputs from at least five major signals—growth factors, genotoxic stress, energy status, oxygen, and amino acids— to regulate many processes involved in the promotion of cell growth and proliferation (Fig. 2). With the exception of amino acids, all of these inputs regulate mTORC1 activity by modulating the activity of TSC1–TSC2. TSC1– TSC2 negatively regulates mTORC1 activity by converting Rheb into its inactive GDP-bound state. Growth factors block TSC1–TSC2 activity and activate mTORC1, whereas genotoxic stress, energy deficit, and oxygen deprivation promote TSC1–TSC2 action and inhibit mTORC1. Conversely, amino acids activate mTORC1 through the action of the Rag GTPases. In the presence of amino acids, the Rag GTPases interact with mTORC1, which promotes its translocation from the cytoplasm to lysosomal membranes, where Rheb is thought to reside.
Fig. 1.
A simplified view of the mTOR pathway. (Adapted from Laplante and Sabatini 2009b.)
Fig. 2.
The mTOR pathway.
When activated, mTORC1 promotes protein synthesis mainly by phosphorylating the kinase S6K and the translation regulator 4E-BP1 (Ma and Blenis 2009). This complex also induces lipid biogenesis by activating SREBP1 and PPARg transcription factors (Laplante and Sabatini 2009a). In addition to promoting anabolism, mTORC1 also inhibits catabolism by blocking autophagy through the phosphorylation of the ULK1–Atg13–FIP200 complex. Importantly, sustained mTORC1 activation blocks growth factor signaling by activating many negative feedback loops that inhibit PI3-kinase (PI3K) signaling.
Compared with mTORC1, our knowledge of mTORC2 biology is still very limited. mTORC2 is activated by growth factors through a mechanism that is not well understood. It is insensitive to acute treatment with rapamycin and regulates cell survival, metabolism, and cytoskeletal organization by phosphorylating many AGC kinases, including Akt, SGK1, and PKCa (Laplante and Sabatini 2009b; Zoncu et al. 2011).
References
- Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369:756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- Kunz J, Henriquez R, Schneider U, Deuter-Reinhard M, Movva NR, Hall MN. Target of rapamycin in yeast, TOR2, is an essential phosphatidylinositol kinase homolog required for G1 progression. Cell. 1993;73:585–596. doi: 10.1016/0092-8674(93)90144-f. [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. An emerging role of mTOR in lipid biosynthesis. Curr Biol. 2009a;19:R1046–R1052. doi: 10.1016/j.cub.2009.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009b;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: A mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994;78:35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- Sabers CJ, Martin MM, Brunn GJ, Williams JM, Dumont FJ, Wiederrecht G, Abraham RT. Isolation of a protein target of the FKBP12–rapamycin complex in mammalian cells. J Biol Chem. 1995;270:815–822. doi: 10.1074/jbc.270.2.815. [DOI] [PubMed] [Google Scholar]
- Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY-22, 989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo) 1975;28:721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM. mTOR: From growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]