In a constantly changing environment, we are surrounded by cues that enable us to predict future events. These cues elicit motivational states that drive us to adaptively shift our behaviors. For example, aversive motivational states, induced by cues paired with electric shock, can result in escape or freezing behavior (Orsini and Maren, 2012), as well as suppressed feeding in food-deprived animals (Petrovich et al., 2009). The ability of these cues to direct many of these behaviors depends on the integrity of the central nucleus of the amygdala (CeA; Petrovich et al., 2009; Orsini and Maren, 2012).
Learning theorists have also noted that, like more canonical aversive conditioned stimuli, cues associated with the omission of an expected reward can themselves evoke an aversive motivational state. Amsel (1958) refers to this as a “frustrative event” and suggests that constant omission of expected reward is associated with fear and anxiety, as evidenced by avoidance behavior. Similar to fear-evoking conditioned stimuli, the omission of a reward appears to recruit the CeA; for example, Calu et al. (2010) found that CeA neurons selectively increased their firing during a period of reward omission.
Insight into what role the CeA may have in contributing to aversive behavior comes from a report by Gozzi and colleagues (2010), who suggested that neurons within the CeA are responsible for directing emotionally salient information into separate neuronal circuits subserving distinct behaviors. They revealed the existence of two distinct populations of GABAergic neurons within the lateral division of the CeA (CeL). One neuronal population routes information to brainstem structures via the medial division of the CeA (CeM) and is involved in passive fear responses, such as freezing. The other activates cholinergic forebrain targets, which are engaged during active fear responses such as avoidance. Importantly, the study demonstrates that distinct CeA projections can gain access to circuits that underlie both reflexive and instrumental behaviors.
Based on extant literature, cues that elicit similar aversive states can drive divergent behavioral strategies, and the CeA is important across these situations. It is unclear, however, whether the representation of the aversive cue within the CeA codes for motivational value or behavioral strategy. We would predict that if the CeA encodes value, the same population of neurons would be activated by presentation of an aversive cue, regardless of the behavioral strategy the animal engages in. Conversely, if the CeA is preferentially involved in selecting the appropriate form of behavioral expression, we would expect the neuronal representation of aversive cues in the CeA to vary with the types of responses the cues drive. To address this important question, Purgert and colleagues (2012) assessed CeA neuronal involvement during the presentation of shock and reward-omission cues.
Purgert et al. (2012) first trained rats to pull a chain in order to receive food rewards, and then split the animals into three groups: food omission and shock training (O-S), food omission and shock control training (O-SC), or food omission control and shock training (OC-S). Separate cues were paired with each of the aversive outcomes, and all animals were presented with both cues. Control training for each condition consisted of unpaired cue-aversive stimulus presentations. In their first experiment, the authors took advantage of the relative timing of expression of the immediate early genes (IEGs) Arc (active immediately following a behavioral experience) and Homer1a (active 30 minutes following a behavioral experience). After presenting the reward-omission and shock cues 30 minutes apart, they stained CeA neurons for these IEGs. They observed increased expression of Homer1a and Arc expression in all subregions of the CeA following conditioned cue presentation relative to control cue presentations. However, most cells were single labeled, indicating that reward omission and shock cues recruited separate populations of neurons.
The CeA has a well documented role in the expression of freezing to a shock-paired cue (for review, see Orsini and Maren 2012); in order to determine whether the CeA is similarly necessary for producing the behavioral responses to reward omission, Purgert and colleagues (2012) performed neurotoxic lesions of the CeA during presentation of the reward-omission cue. Whereas control animals showed diminished chain pulling in response to the omission cue, indicative of behavioral aversion, CeA lesions abolished this response. Together with the first experiment, these results suggest that while the CeA is generally necessary for the behavioral expression of aversion, distinct microcircuits are activated in different behavioral situations. This is consistent with a role for CeA neural representations of aversive cues in coding behavioral expression rather than motivational value.
Although the amygdala as a whole is broadly regarded as the primary neural substrate for aversion and fear, these central states can arise from different situations and direct disparate, often mutually exclusive, behavioral responses. While both the shock-cue and omission cue appear to induce aversive states in the animal, they differ both in terms of 1) the qualitative nature of the aversive state: a central ‘fear’ state vs. Amsel’s ‘frustrative’ state, and 2) how these states direct behavior: shock cues here provoke freezing while reward omission cues diminished instrumental responding. Thus, it is possible that the different activated neural populations could be representing either of these differences. However, previous literature suggests a preferential role for the CeA in behavioral expression rather than encoding motivational value. Indeed, Swanson (2003) has proposed that the CeA is in fact an extension of the striatum, both in terms of its structural organization as well as its role in using motivational information to direct behavior via projections to autonomic and motor nuclei. Moreover, several behavioral manipulations have revealed a preferential role for the CeA in behavioral expression rather than coding for an aversive state. For example, Choi et al. (2010) have shown that post-training lesions of the CeA do not abolish fear, but rather shift expression of fear from freezing to avoidance responses. However, lesions to other parts of the amygdala, such as the lateral nucleus, abolish all forms of fear expression. These findings are consistent with a role for CeA neurons in directing different forms of behavior in response to an aversive cue, while other components of the circuit may encode motivational value.
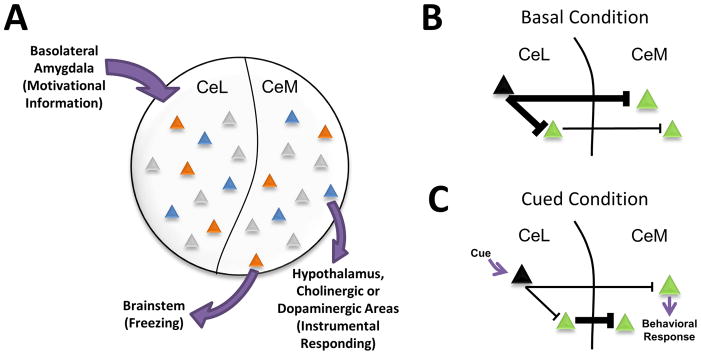
The CeA is well positioned for this task. GABAergic efferents project predominantly from the CeM, their activity sculpted both by local inhibitory connections and by inhibitory inputs from the CeL. CeM targets include sympathetic and motor centers in the brainstem and hypothalamus as well as neuromodulatory nuclei in the midbrain and forebrain. This anatomical connectivity allows the CeA to direct typical fear responses, such as freezing and startle, while also allowing access to instrumental responding via its hypothalamic, dopaminergic, and cholinergic projections (LeDoux, 2000). Indeed, Gozzi and colleagues (2010) have recently demonstrated that modulation of specific subpopulations of CeL neurons can support either passive fear responses, such as freezing, or active fear responses. Together with the findings presented by Purgert et al. (2012), this suggests that non-overlapping populations of neurons in the CeA route motivationally relevant information into circuits that support different types of behavioral responses (Fig 1A).
Figure 1. A Model of Behavioral Selection by Neurons in the Central Amygdala.
A. Cues associated with different types of aversive stimuli are represented in distinct patterns of neural activity (indicated by different colored triangles) within the central nucleus of the amygdala (CeA). Salient information about the cues is relayed to the CeA via afferents from the basolateral amygdala. Cues paired with different forms of aversive stimuli, such as shock (orange) or omission of expected reward (blue), recruit non-overlapping populations of neurons in both the lateral (CeL) and medial (CeM) subdivisions of the CeA (Purgert et al., 2012). We hypothesize that these networks of coactive neurons not only represent distinct aversive cues, but also target different downstream structures in order to select appropriate behavioral responses. B, C. A general model by which both subregions of the CeA may be recruited by aversive cues. Under basal conditions (B), cue-inhibited neurons (black) provide tonic inhibition throughout CeL/CeM microcircuits involved in behavioral expression. Upon cue presentation (C), inhibition of these neurons results in the disinhibition of projection cells (green) in both the CeL and CeM, allowing for activity across both subnuclei and the selection of an appropriate behavioral response. (Orange triangles – shock cue activated neurons; Blue triangles – omission cue activated neurons, Black triangles – cue inhibited neurons, Green triangles – projection neurons)
It is important to note that Purgert and colleagues (2012) found that reward-omission cues and shock cues evoked activity in non-overlapping populations of neurons across both medial and lateral subregions of the CeA. As the authors note, this is in contrast to work that reported differences in activity between the CeM and CeL during aversive stimulus presentations. Purgert et al. (2012) discuss recent work by Haubensak et al. (2010) that shows that the CeL is preferentially recruited during fear acquisition, whereas the CeM is recruited more heavily during fear expression. However, these recent accounts of CeA microcircuitry suggest that a primary role of CeL GABAergic neurons is to shape the firing patterns of CeM output neurons. For example, neural activity in the CeL, originally induced by fear conditioning, does not change during fear recall, while CeM neural activity diminishes with changes in fear expression (Duvarci et al., 2011). Together with anatomical and functional data that demonstrate CeL neurons predominantly project onto CeM neurons, this suggests more of an organizational role for CeL in CeM output than direct control over behavior. As further evidence for this, Haubensak et al. (2010) used a combination of genetic and electrophysiological techniques to selectively target a specific population of CeL neurons that inhibit both a population of downstream neurons in CeM and a distinct population of CeL neurons. This particular population of cells is thought to correspond with so-called CeLOFF cells and control fear expression to a shock- paired tone by providing tonic inhibition throughout the expression circuit. When a fearful cue is presented, CeLOFF cells are inhibited, releasing its downstream targets from inhibition and allowing fear expression. Thus, after presentation of the shock-associated stimulus, we would expect to see activation in both the lateral and medial divisions of the CeA, as a result of the disinhibition of the CeLoff targets in both CeL and CeM (Fig 1B,C). This hypothesis is consistent with the data presented by Purgert and colleagues (2012). Given that the CeM is the primary output structure of the CeA, and that the CeA has a critical role in behavioral expression in response to aversive stimuli, it is not surprising that distinct neurons were activated in the CeM for the reward omission cue. However, it is intriguing that non-overlapping populations were also observed in the CeL, as this suggests completely dissociated neural representations of aversive cues prior to the output step. One technical limitation of assessing IEG activation, however, is that it does not allow analysis of suppression of neural activity. Given the largely inhibitory nature of processing in the CeA, measures of increased activity alone do not provide information about synaptically connected neural networks. In other words, simultaneously active cells observed by Purgert and colleagues (2012) are unlikely to represent monosynaptically connected neurons in a network because their activity would likely inhibit downstream targets; rather, their simultaneous recruitment may reflect complex coordinating activity by interneurons in the circuit. Further work is needed to characterize the specific cellular interactions that allow these neural representations to emerge.
Together with previous literature, these new results by Purgert et al. (2012) allow us to propose a model by which the CeA may be involved in the selection of appropriate actions in response to discrete aversive stimuli (Fig. 1). By activating distinct neural representations that then project to downstream targets involved in different types of behavioral outputs, CeA microcircuitry may lie at the crux of aversive behavioral selection. This hypothesized pattern of activity is consistent with recent work characterizing CeA circuits in freezing expression (Haubensak et al., 2010; Duvarci et al., 2011), although its role in directing other types of behaviors is unknown. Further characterization is needed regarding how these coactive networks arise and are organized, their specific anatomical targets, and whether neural representations of different cues interact to suppress other, competing behaviors.
Acknowledgments
The authors would like to acknowledge their funding sources: NIMH (CAO; F31MH091822); University of Michigan (AB; Rackham Regents Fellowship). KEP was supported by R01MH065961. The authors would also like to thank Dr. Joshua Berke for his comments during the preparation of this manuscript.
References
- Amsel A. The role of frustrative nonreward in noncontinuous reward situations. Psychol Bull. 1958;55:102–119. doi: 10.1037/h0043125. [DOI] [PubMed] [Google Scholar]
- Calu DJ, Roesch MR, Haney RZ, Holland PC, Schoenbaum G. Neural correlates of variations in event processing during learning in central nucleus of amygdala. Neuron. 2010;68:991–1001. doi: 10.1016/j.neuron.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JS, Cain CK, LeDoux JE. The role of amygdala nuclei in the expression of auditory signaled two- way active avoidance in rats. Learn Mem. 2010;17:139–147. doi: 10.1101/lm.1676610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Popa D, Pare D. Central amygdala activity during fear conditioning. J Neurosci. 2011 doi: 10.1523/JNEUROSCI.4985-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozzi A, Jain A, Giovannelli A, Bertollini C, Crestan V, Schwartz AJ, Tsetsenis T, Ragozzino D, Gross CT, Bifone A. A neural switch for active and passive fear. Neuron. 2010;67:656–666. doi: 10.1016/j.neuron.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Kunway PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong HW, Deisseroth K, Callaway EM, Fanselow MS, Luthi A, Anderson DJ. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev. 2012 doi: 10.1016/j.neubiorev.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Ross CA, Mody P, Holland PC, Gallagher M. Central, but not basolateral, amygdala is critical for control of feeding by aversive learned cues. J Neurosci. 2009;29:15205–15212. doi: 10.1523/JNEUROSCI.3656-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purgert RJ, Wheeler DS, McDannald MA, Holland PC. Role of Amygdala Central Nucleus in Aversive Learning Produced by Shock or by Unexpected Omission of Food. J Neurosci. 2012;32:2461–2472. doi: 10.1523/JNEUROSCI.5090-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. The amygdala and its place in the cerebral hemisphere. Ann N Y Acad Sci. 2003;985:174–184. doi: 10.1111/j.1749-6632.2003.tb07081.x. [DOI] [PubMed] [Google Scholar]