Abstract
Cannabinoid receptors, CB1R, are present in glomeruli of the main olfactory bulb (MOB). The functions of CB1R and their endogenous activators, endocannabinoids, for glomerular signaling are unknown. Glomeruli contain at least three types of neurons: periglomerular (PG), external tufted (ET), and short-axon (SA) cells. PG cells form inhibitory GABAergic dendrodendritic synapses with ET cells. ET cells form excitatory glutamatergic dendrodendritic synapses with PG and SA cells. In mouse brain slices, we used whole-cell patch-clamp recordings to study the role of CB1R in regulating PG and ET cells. Cannabinoids displayed strong, direct inhibitory effects on PG cells and weak effects on ET cells. Single pulses or a train of pulses of depolarizing current injected into an ET cell evoked suppression of IPSCs. This suggests retrograde endocannabinoid signaling, namely, depolarization-induced suppression of inhibition (DSI) in ET cells. Our results support the hypothesis that burst firing of ET cells triggers the release of endocannabinoids which in turn directly inhibit PG cells and reduce GABA release from PG cells. This, in turn, can result in a transient reduction of PG cell inhibitory input to ET cells.
Keywords: AM251, cannabinoids, DSI, glomerulus, patch-clamp electrophysiology, signal transduction
Introduction
Endocannabinoids (eCBs) were found to act as retrograde signaling molecules in the hippocampus, cerebellum, neocortex, and amygdala (reviewed in Alger, 2002; Kano et al., 2009). eCBs act at CB1R at presynaptic terminals to reduce transmitter release (GABA, glutamate). One type of short-term synaptic plasticity, originally observed in the cerebellum and hippocampus, is mediated by eCBs, namely DSI (depolarization-induced suppression of inhibition). In DSI, eCBs are released from depolarized principal neurons and travel to presynaptic inhibitory interneurons to transiently reduce presynaptic firing and neurotransmitter (GABA) release.
Immunohistochemical and autoradiographic studies indicate that CB1R is present in the MOB glomerular layer (Herkenham et al., 1991; Pettit et al. 1998; Tsou et al., 1998; Moldrich and Wenger 2000). Neurons in the glomerular layer fall into three subpopulations: periglomerular (PG), external tufted (ET), and short-axon (SA) cells (reviewed in Ennis et al., 2007). PG cells are GABAergic, SA cells express both GABA and dopamine, and ET cells are glutamatergic (Hayar et al., 2004b; Kiyokage et al., 2010). PG cells receive input from the olfactory nerve or dendrodendritic glutamatergic input from ET or mitral cells (Hayar et al., 2004b; Ennis et al., 2007). PG cells contain GABA and presynaptically inhibit olfactory receptor neurons through GABAergic transmission (Aroniadou-Anderjaska et al., 2000; Murphy et al., 2005). ET cells receive spontaneous bursts of IPSCs from PG cells at inhibitory GABAergic synapses (Hayar et al., 2005). Neurons in the glomerular layer are immunoreactive for enzymes that synthesize eCBs (Allen Mouse Brain Atlas 2009; Okamoto et al 2007; Piomelli 2003). ET cells can be a potential source of eCBs.
To determine the function of the eCB system in olfactory glomeruli, we used whole-cell patch-clamp recording and tested the effects of agonists/antagonists of CB1R on cellular and network activity.
Materials and Methods
Wildtype mice of either sex (C57BL/6J, Jackson Laboratory, Bar Harbor, ME) were used in agreement with IACUC and NIH guidelines. 16–25 day old mice were decapitated, the MOBs dissected out, and immersed in artificial cerebrospinal fluid (ACSF) at 4°C, as previously described (Heinbockel et al., 2004). Horizontal slices (400 μm-thick) were maintained at 30 ± 0.5°C by superfusion with oxygenated ACSF.
Recording methods have been described previously (Heinbockel et al., 2004). Recording pipettes (5-8 MΩ) were pulled on a Flaming-Brown P-97 puller (Sutter Instrument Co., Novato, CA) from 1.5 mm O.D. borosilicate glass with filament. Data were obtained using a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA) and digitized on computer disc (Clampex 10.1, Molecular Devices). Data were also collected through a Digidata 1440A Interface (Molecular Devices) and digitized at 10 kHz.
The ACSF consisted of (in mM): NaCl 124, KCl 3, CaCl2 2, MgSO4 1.3, glucose 10, sucrose 4.4, NaHCO3 26, NaH2PO4 1.25 (pH 7.4, 300 mOsm), saturated with 95 O2/5% CO2. For intracellular recording of spiking activity, the pipette-filling solution consisted of (mM) K-gluconate 144, MgCl2 2, HEPES 10, Mg2ATP 5, Na3GTP 0.5, NaCl 2, EGTA 0.2. For IPSCs, EPSCs recording and DSI experiments, electrodes were filled with a low-Cl− or high-Cl−-based solution depending on the experiment. Low-Cl−-based pipette solution contained the following composition (in mM): 125 cesium methanesulfonate CsMeSO3, 1 NaCl, 10 phosphocreatine ditris salt, 5 ATP, 0.5 GTP, 0.5 EGTA, 10 HEPES, 10 QX-314 [2(triethylamino)-N-(2,6-dimethylphenyl) bromide], pH 7.3 with 1N CsOH (290 mOsm). High-Cl−-based pipette solution contained the following composition (in mM): 110 cesium cloride, 10 tetraethylammonium-Cl, 2 NaCl, 10 phosphocreatine ditris salt, 5 ATP, 0.5 GTP, 0.5 EGTA, 10 HEPES, 10 QX-314, pH 7.3 with 1N CsOH (290 mOsm).
ET cells were identified by their spontaneous intrinsic burst firing (Fig. 2A) that persisted in synaptic blockers, a pear-shaped cell body located in the bottom half of the glomerular layer, an extensively ramifying apical dendrite in one glomerulus, and absence of lateral dendrites in the external plexiform layer (Hayar et al., 2004b; Liu and Shipley, 2008). PG cells were identified by their location around the glomerular neuropil and electrophysiological characteristics including membrane potential, input resistance and membrane capacity (Shao et al., 2009). SA cells were excluded from PG cells based on the fact that compared to PG cells, SA cells have relative lower input resistance, higher firing frequency and larger cell bodies (Hayar et al., 2004b; Kiyokage et al., 2010; Aungst et al., 2003). We recorded from two different PG cell subtypes that were both located at the periphery of individual glomeruli but exhibited different firing behaviors and input resistances. One subtype displayed sustained spontaneous spiking with relatively low input resistance (sPG cell), whereas the other subtype required depolarizing current to exhibit firing (nPG cells) and had relatively smaller cell bodies with higher input resistance and a more negative membrane potential. A PG cell body was relatively smaller than that of an ET cell. ET cells received spontaneous EPSCs and bursts of IPSCs from PG cells, whereas PG cells received spontaneous bursts of EPSCs (Hayar et al., 2004a, b, 2005).
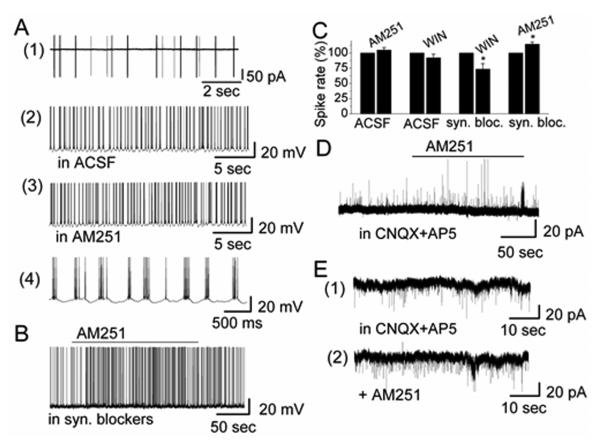
Figure 2.
CB1R modified the activity of and synaptic transmission to ET cells. A(1) Extracellular recording from an ET cell in cell-attached voltage-clamp mode to initially identify ET cells by their burst firing pattern. A(2), A(3) The burst firing pattern of an ET cell recorded in absence and presence of CB1R antagonist AM251 in whole-cell current-clamp mode. The burst firing pattern of A(2) is shown at higher time resolution in A(4). B AM251 increased the burst firing rate of an ET cell in the presence of synaptic blockers (10 μM CNQX + 50 μM D-AP5 and 5 μM gabazine). C Group data illustrating the role of CB1R for burst firing of ET cells in absence and presence of synaptic blockers. Asterisks indicate significance level (* - p < 0.05). D AM251 induced an increase of sIPSCs in an ET cell in the presence of CNQX + D-AP5 with low-Cl−-based pipette solution. Holding potential is at 0 mV. IPSCs appeared as upward deflections. E AM251 induced an increase of sIPSCs in ET cell in the presence of CNQX + D-AP5 with high-Cl−-based pipette solution. Holding potential was at − 60 mV. sIPSCs appeared as downward deflections.
The following drugs were bath applied: L-2-amino-5-phosphonopentanoic acid (AP5, APV), 6-cyano-7-nitroquinoxaline-2-3-dione (CNQX), (2-(3-carboxypropyl)-3-amino-6-(4 methoxyphenyl)-pyridazinium bromide (gabazine, SR-95531), (R)-(+)-[2,3-Dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3,-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate (WIN55,212-2 mesylate), N-(Piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4methyl-1H-pyrazole-3-carboxamide (AM251). Chemicals were supplied by Sigma-Aldrich (St. Louis, MO) and Tocris (Ellisville, MO).
Data were analyzed using Clampfit (Axon Instruments) and Mini Analysis (Synaptosoft, Decatur, GA) software. Detection of events (intracellularly recorded spontaneous (s)EPSCs and spontaneous (s)IPSCs) was performed off-line using Mini Analysis program. We quantified the averaged integral area which is an indication of both frequency and amplitude of sIPSCs. We calculated the integral area of individual sIPSCs within 1.3 sec to determine total synaptic area (A = number of events for 1.3 sec × mean area of sIPSCs). The calculation of DSI was slightly modified as follows: DSI (%) = 100 × [Apost/Apre] (Fortin et al., 2004). Apost is the total area of 1.3 sec sIPSCs after depolarization. Apre, the total area of 1.3 sec sIPSCs before depolarization, was obtained by measuring the total area of three continuous 1.3 sec sIPSCs before depolarization and averaging the value. Membrane potentials were calculated from the steady-state membrane potential that occurred after a single action potential. Minimal membrane potential was measured as membrane potential for burst firing (Liu and Shipley, 2008). Numerical data were expressed as the mean ± SEM. Tests for statistical significance (p < 0.05) were performed using paired Student’s t-test, and non-parametric Wilcoxon signed rank test for paired data of small sample sizes (~5), or one-way ANOVA followed by the Bonferroni test for multiple comparisons.
Results
Regulation of periglomerular cell activity through CB1R
PG cells are likely candidates for direct effects of eCBs since CB1R is robustly expressed in the glomerular layer (Moldrich and Wenger 2000). PG cells are neurochemically and functionally heterogeneous (Ennis et al., 2007; Shao et al., 2009; Kiyokage et al., 2010). Recordings were obtained from 49 sPG cells and 12 nPG cells. sPG cells generated firing patterns that were clearly distinct from those of ET cells (Ennis et al., 2007; Hayar et al., 2004b, 2005). sPG cells had an average Vm of −57.7 ± 0.6 mV (n = 49), an input resistance of 732.3 ± 36.9 MΩ (n = 49), and cell capacitance of 6-11 pF. nPG cells had a more negative membrane potential (−69.9 ± 2.8 mV, n = 12), a higher input resistance (1065.1 ± 192.3 MΩ, n = 12) and cell capacitance of 4-8 pF.
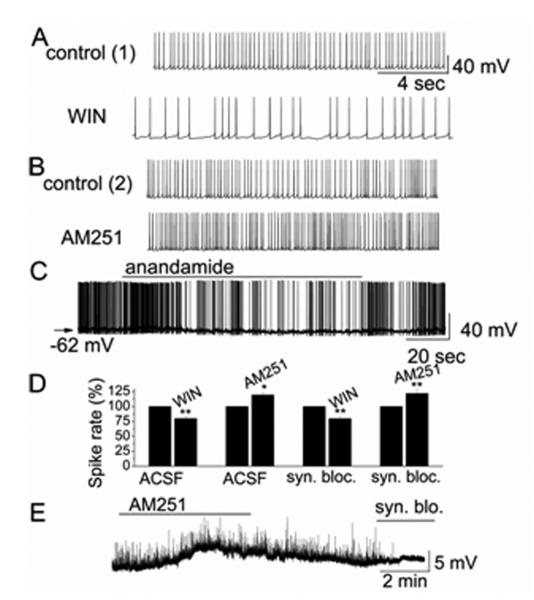
We first tested if a selective CB1R agonist regulated PG cells. sPG cells exhibited spiking with an average firing rate of 3.9 ± 0.3 Hz (n = 31). WIN (10 μM) reversibly decreased the firing rate of sPG cells (control: 4.4 ± 0.4 Hz vs. in WIN: 3.2 ± 0.4 Hz, n = 11, p = 0.0001) and hyperpolarized Vm (ΔVm = −1.4 ± 0.2, n = 11, p = 0.02) (Fig. 1). Similarly, CB1R selective agonist anandamide displayed inhibitory effects on firing rate of sPCs (control: 3.7 ± 0.6 Hz vs. in anandamide: 2.5 ± 0.6 Hz, n = 7, p = 0.008) and hyperpolarization of Vm (ΔVm = 1.8 ± 0.3, n = 7, p = 0.002) (Fig. 1C). In contrast, CB1R antagonist AM251 reversibly increased sPG cell firing (control: 4.0 ± 0.5 Hz vs. in AM251: 5.2 ± 0.6 Hz, n = 7, p = 0.01) and depolarized them (ΔVm = 1.9 ± 0.4 mV, n = 6, p = 0.02) (Fig. 1B). nPG cells also responded with depolarization to CB1R inactivation (Fig. 1E). AM251 (10 μM) depolarized by 5.7 ± 1.6 mV (n = 5; p = 0.037, Wilcoxon) whereas WIN (10 μM) hyperpolarized nPG cells by −2.3 ± 0.6 mV (n = 5; p = 0.043).
Figure 1.
CB1R regulated the activity of periglomerular cells. A, B. Original recordings illustrate that CB1R agonist WIN reduced the firing rate of an sPG cell. CB1R antagonist AM251 increased spiking of an sPG cell. Recordings for WIN and AM251 are from the same sPG cell. C Anandamide reduced firing and hyperpolarized membrane potential in an sPG cell. D Summary of cannabinoid effects on sPG cell activity in absence and presence of synaptic blockers. E. Original recording illustrates depolarization of an nPG cell following bath application of AM251. sEPSPs were abolished following bath application of synaptic blockers (syn. blo.).
To determine if the effects of eCBs on PG cells were mediated by CB1R, we tested if AM251 blocked the WIN effects. In the presence of 10 μM AM251, bath application of 10 μM WIN failed to induce a decrease in firing rate (in AM251: 4.3 ± 0.6 Hz vs. in AM251 plus WIN: 4.01 ± 0.7 Hz, n = 5, p = 0.66) or change in membrane potential (ΔVm = 0.05 ± 0.02 mV, n = 5, p = 0.90). These results implied that CB1R was involved in cannabinoid-mediated modulation of PG activity.
To further determine if the actions of cannabinoids on PG cells were mediated through CB1R expressed by PG cells, we tested the effects of CB1R activation/inactivation on PG cells in the presence of ionotropic glutamate and GABAA receptor blockers (synaptic blockers; CNQX, 10 μM; APV, 50 μM; gabazine, 5 μM). The effects of WIN (10 μM) and AM251 (10 μM) on firing and membrane potential of PG cells persisted in the presence of synaptic blockers (Fig. 1D). In synaptic blockers, AM251 increased reversibly the firing rate of sPG cells from 4.5 ± 0.9 Hz to 5.9 ± 0.9 Hz (n = 5, p = 0.01) and depolarized Vm (ΔVm = 1.6 ± 0.2, n = 5, p = 0.02) (Fig. 1D). In contrast, WIN reduced the firing rate of sPG cells in synaptic blockers from 4.2 ± 0.6 Hz to 3.0 ± 0.5 Hz (n = 6, p = 0.003) and hyperpolarized Vm (ΔVm = −0.9 ± 0.2, n = 6, p = 0.02) (Fig. 1D). The effects of CB1R drugs on sPG cells in synaptic blockers were not significantly different from those without blockers (p > 0.05 determined by ANOVA and Bonferroni post-hoc analysis). For nPG cells, AM251 (10 μM) depolarized cells in synaptic blockers by 5.0 ± 1.0 mV (n = 5; p = 0.043), and WIN (10 μM) hyperpolarized cells by −1.9 ± 0.4 mV (n = 5; p = 0.042). These results indicated that CB1R directly regulated membrane properties of both PG cell subtypes.
Cannabinoids regulate activity of and synaptic transmission to ET cells
CB1R-regulated activity of PG cells may modulate transmitter release and synaptic transmission to ET cells. We tested the effects of CB1R drugs on the activity of ET cells (n = 39). Neither agonist AM251 (10 μM) nor antagonist WIN (10 μM) significantly influenced overall firing frequency and membrane potential of ET cells (spiking: control: 5.36 ± 1.28 Hz vs. in AM251: 5.49 ± 1.31 Hz, n = 5, p = 0.89; Vm: control: −52.7 ± 1.02 mV vs. in AM251: −52.0 ± 1.16 mV, n = 5, p = 0.26; spiking: control: 4.97 ± 1.25 Hz vs. in WIN: 4.71 ± 1.23 Hz, n = 6, p = 0.46; Vm: control: −52.8 ± 1.08 mV vs. in WIN: −53.20 ± 0.93 mV, n = 6, p = 0.60) (Fig. 2A). However, in synaptic blockers, CB1R drugs had a modest effect on ET cells. In this condition, AM251 slightly increased firing of ET cells (control: 5.46 ± 0.73 Hz mV vs. in AM251: 6.10 ± 0.76 Hz, n = 9, p = 0.002) (Fig. 2B). We did not observe a significant membrane depolarization by AM251 (control: −53.5 ± 1.1 mV vs. in AM251: −52.8 ± 1.1 mV, n = 9, p = 0.18). In synaptic blockers, WIN slightly decreased firing of ET cells (control: 4.70 ± 0.73 Hz mV vs. in WIN: 3.52 ± 0.89 Hz, n = 5, p = 0.043) but failed to change Vm (control: −53.1 ± 1.1 mV vs. in WIN: −53.9 ± 1.1 mV, n = 5, p = 0.10). The effects of CB1R drugs in synaptic blockers indicate that CB1R mediated a direct effect on ET cells (Fig. 2C).
CB1R regulated activity of PG cells and might modify GABA release and transmission to ET cells. This hypothesis was supported by our observation that AM251 increased sIPSC frequency and WIN decreased sIPSC frequency in ET cells. The effect of AM251 on sIPSCs was tested with both low-Cl− and high-Cl−-based pipette solution. In whole-cell voltage-clamp recording mode with low-Cl− pipette solution and holding potential at 0 mV, AM251 increased the frequency of sIPSCs in blockers of ionotropic glutamate receptors (CNQX, D-AP5) (Figure 2D). In this condition, sIPSCs were upward and exhibited some rundown. Thus, a high-Cl− pipette solution was used in the subsequent experiments. In blockers of ionotropic glutamate receptors, AM251 evoked an increase in sIPSC frequency from 1.08 ± 0.27 Hz to 1.67 ± 0.33 Hz (n = 5, p = 0.033) associated with an increase of current amplitude from 22.8 ± 4.3 pA (CNQX, D-AP5) to 26.5 ± 4.8 (n = 5, p = 0.042) (Fig. 2E). sIPSCs were completely eliminated by gabazine (sIPSC frequency: <0.01 Hz, n = 5, p = 0.0001), indicating that currents were mediated by GABAA receptors. The results indicated that AM251 directly activated PG cells and enhanced their GABA release.
CB1R Mediated Depolarization-Induced Suppression of Inhibition (DSI)
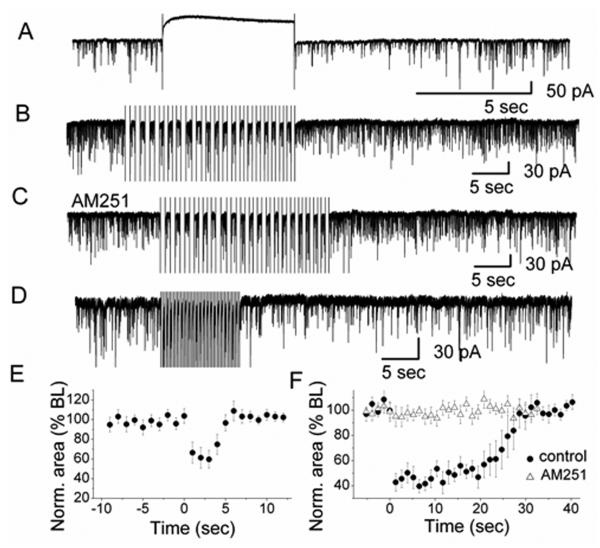
DSI has not been demonstrated in the olfactory system. Based on our above results, we hypothesized that DSI might be present in the glomerular layer of MOB. First, we tested whether DSI can be induced by depolarization of the postsynaptic neuron, ET cells. With a 5-sec depolarizing voltage step from a holding potential of −60 mV to 0 mV, DSI was clearly visible in ET cells as a decrease in the amplitude and frequency of sIPSCs (Fig. 3A). Immediately after a single depolarizing step, the sIPSC area was suppressed by 40.3 ± 9.0 % of control and then gradually recovered (n = 7; p < 0.05) (Fig. 3E). To determine a possible functional role of DSI in glomeruli, we mimicked spontaneous rhythmic bursting of ET cells by applying a train of depolarizing steps. A train of depolarizing steps resulted in a transient reduction in sIPSC area (20 steps, 0.75 Hz) (Fig. 3B). Suppression of sIPSCs reached a maximum of 59.5 ± 5.3% within 7 sec after the train of depolarizing steps with significant suppression lasting 25 sec (n = 12; p < 0.05) (Fig. 3B, F). In the presence of AM251, DSI was completely eliminated (n = 10) suggesting that DSI was mediated by CB1R (Fig. 3C, F). The bursting frequency of ET cells ranged from 0.5 to 6.5 Hz with a mean frequency of 2.7 bursts/sec (Hayar et al., 2004a). In addition, the sustained depolarization during a burst is much lower than 0 mV which is only transiently reached during action potentials. Therefore, we used a train of depolarizing steps to −30 mV at 2 Hz to test if ET cell bursting can evoke DSI. Depolarizing voltage pulses at 2 Hz (20 steps, pulse duration: 250 ms) evoked a reduction of sIPSCs in ET cells that was similar to the result obtained with voltage steps at 0.75 Hz to 0 mV. The maximal suppression at 2 Hz was 56.4 ± 7.9% (n = 7). Trains of depolarizing voltage steps evoked suppression of inhibition (DSI), suggesting that spontaneous rhythmic bursting of ET cells triggers the release of eCBs from ET cells. The data suggests that eCBs function as retrograde messengers to reduce GABA release from PG cells which in turn, regulates the activity of PG cell synaptic targets such as ET cells.
Figure 3.
Depolarization-induced suppression of inhibition (DSI) in olfactory glomeruli. A A depolarizing voltage step evoked DSI in a representative ET cell. High Cl−-based pipette solution was used for recording sIPSCs. Depolarization was achieved by stepping from −60 mV holding potential to 0 mV for 5 sec. B In the presence of CNQX and 5-AP, a train of 20 voltage steps to 0 mV (0.75 Hz; step duration: 667 ms) transiently reduced sIPSCs in an ET cell. Holding potential was −60 mV. C. In the presence of AM251, no sIPSC suppression was observed. D. A train of 20 voltage steps to −30 mV (2 Hz; step duration: 250 ms) transiently reduced sIPSCs in an ET cell (in CNQX and 5-AP). E Normalized sIPSCs area illustrating the magnitude and time course of DSI elicited by a 5-sec depolarizing pulse (n = 7). The averaged values between 0 – 5 sec after the end of the voltage step were significantly different from the baseline (ANOVA and Bonferroni post-hoc analysis, p < 0.05). F Normalized sIPSC area illustrating the magnitude and time course of DSI elicited by a train of depolarizations to 0 mV (n =12) in control and in the presence of AM251 (n = 10). In control conditions, the averaged values between zero to 25 seconds after the end of the train of voltage steps were significantly different from the baseline (ANOVA and Bonferroni post-hoc analysis, p < 0.05).
Discussion
We present electrophysiological evidence that the eCB system plays a functional role in regulating neuronal activity and signaling in MOB glomeruli. We found evidence for the existence of CB1R-mediated retrograde signaling among glomerular neurons in the form of DSI. Our results support the idea that eCBs released from ET cells function as retrograde messengers to control the excitability of presynaptic neurons, i.e., PG cells, and to regulate their transmitter release.
Our electrophysiological evidence is consistent with immunohistochemical studies that show CB1R is present in the glomerular layer (Moldrich and Wenger, 2000). We added new evidence that CB1R is expressed by PG cells since a CB1R agonist/antagonist directly regulated PG cell activity. Bursting is intrinsic to ET cells and mediated by several ET cell intrinsic conductances (Liu and Shipley, 2008). In the presence of synaptic blockers, a CB1R agonist/antagonist regulated the activity of ET cells. The results suggest that CB1R is expressed in ET cells and may participate in modulating ET cell activity. However, we did not observe a change in ET cell activity as a result of activation or blockade of CB1R in the absence of synaptic blockers. We hypothesize that the direct excitatory effect of a CB1R antagonist on ET cells is opposed by increased GABAergic synaptic input from PG cells onto ET cells, i.e., the enhanced GABA release from PG cells triggered by a CB1R antagonist may dominate and mask the CB1R antagonist-evoked direct excitation of ET cells.
In other brain systems, eCBs are synthesized and released from neuronal cell bodies as a result of cellular excitation (Kano et al., 2009). One potential source of eCBs in the MOB is neurons that synapse onto presynaptic cells, i.e., PG cells, and receive feedback synaptic inputs. ET cells possess these characteristics, and could be a potential eCB source in the MOB. This hypothesis was supported by our observation of DSI in ET cells. ET cells displayed DSI depending on voltage step duration and step number. With step duration of 1 sec, we did not observe clear DSI in ET cells (data not shown). However, when the step duration was increased to 5 seconds, transient DSI was evoked. A train of depolarizing voltage steps (>3) strengthened the inhibition of sIPSCs, suggesting that excitation of ET cells in the form of rhythmic bursting triggers the release of eCBs and regulates glomerular activity. Bursting of neurons may modulate eCB release not only in the MOB but also in other brain systems.
Since ET cells receive monosynaptic sensory input, the inhibitory effect of CB1R on PG cells by eCBs reduces inhibitory input to ET cells and could enhance ET cell sensitivity to weak sensory inputs by depolarizing the membrane potential closer to spike threshold. eCBs released by ET cells inhibit PG cells, reduce their GABA release and, in turn, could modify the firing pattern of ET cells and could potentially also reduce inhibition of mitral cells and presynaptic olfactory nerve terminals. Activation of CB1R on PG cells may thus increase the overall sensitivity of the glomerulus to sensory inputs.
Acknowledgements
Supported by Whitehall Foundation, U.S. PHS grants S06GM08016 (MBRS-SCORE, NIGMS/NIH), 8G12MD007597 (RCMI, NIH-NIMHD).
Bibliography
- Alger BE. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 2002;68:247–286. doi: 10.1016/s0301-0082(02)00080-1. [DOI] [PubMed] [Google Scholar]
- Allen Institute for Brain Science . Allen Mouse Brain Atlas [Online] Seattle, WA: 2009. http://mouse.brain-map.org. [Google Scholar]
- Aroniadou-Anderjaska V, Zhou FM, Priest CA, Ennis M, Shipley MT. Tonic and synaptically evoked presynaptic inhibition of sensory input to the rat olfactory bulb via GABA(B) heteroreceptors. J Neurophysiol. 2000;84:1194–1203. doi: 10.1152/jn.2000.84.3.1194. [DOI] [PubMed] [Google Scholar]
- Aungst JL, Heyward PM, Puche AC, Karnup SV, Hayar A, Szabo G, Shipley MT. Centre-surround inhibition among olfactory bulb glomeruli. Nature. 2003;426:623–629. doi: 10.1038/nature02185. [DOI] [PubMed] [Google Scholar]
- Ennis M, Hamilton KA, Hayar A. Neurochemistry of the main olfactory system. In: Lajtha A, Johnson DA, editors. Handbook of Neurochemistry and Molecular Neurobiology. Sensory Neurochemistry. Springer; Heidelberg: 2007. pp. 137–204. [Google Scholar]
- Fortin DA, Trettel J, Levine ES. Brief trains of action potentials enhance pyramidal neuron excitability via endocannabinoid-mediated suppression of inhibition. J Neurophysiol. 2004;92:2105–2112. doi: 10.1152/jn.00351.2004. [DOI] [PubMed] [Google Scholar]
- Hayar A, Ennis M. Endogenous GABA and glutamate finely tune the bursting of olfactory bulb external tufted cells. J Neurophysiol. 2007;98:1052–1056. doi: 10.1152/jn.01214.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Karnup S, Shipley MT, Ennis M. Olfactory bulb glomeruli: external tufted cells intrinsically burst at theta frequency and are entrained by patterned olfactory input. J Neurosci. 2004a;24:1190–1199. doi: 10.1523/JNEUROSCI.4714-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Karnup S, Ennis M, Shipley MT. External tufted cells: a major excitatory element that coordinates glomerular activity. J Neurosci. 2004b;24:6676–6685. doi: 10.1523/JNEUROSCI.1367-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Shipley MT, Ennis M. Olfactory bulb external tufted cells are synchronized by multiple intraglomerular mechanisms. J Neurosci. 2005;25:8197–8208. doi: 10.1523/JNEUROSCI.2374-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinbockel T, Heyward P, Conquet F, Ennis M. Regulation of main olfactory bulb mitral cell excitability by metabotropic glutamate receptor mGluR1. J Neurophysiol. 2004;92:3085–3096. doi: 10.1152/jn.00349.2004. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Kiyokage E, Pan YZ, Shao Z, Kobayashi K, Szabo G, Yanagawa Y, Obata K, Okano H, Toida K, Puche AC, Shipley MT. Molecular identity of periglomerular and short axon cells. J Neurosci. 2010;30:1185–1196. doi: 10.1523/JNEUROSCI.3497-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Shipley MT. Multiple conductances cooperatively regulate spontaneous bursting in mouse olfactory bulb external tufted cells. J Neurosci. 2008;28:1625–1639. doi: 10.1523/JNEUROSCI.3906-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldrich G, Wenger T. Localization of the CB1 cannabinoid receptor in the rat brain. An immunohistochemical study. Peptides. 2000;21:1735–1742. doi: 10.1016/s0196-9781(00)00324-7. [DOI] [PubMed] [Google Scholar]
- Murphy GJ, Darcy DP, Isaacson JS. Intraglomerular inhibition: Signaling mechanisms of an olfactory microcircuit. Nat Neurosci. 2005;8:354–364. doi: 10.1038/nn1403. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Wang J, Morishita J, Ueda N. Biosynthetic pathways of the endocannabinoid anandamide. Chem Biodivers. 2007;4:1842–1857. doi: 10.1002/cbdv.200790155. [DOI] [PubMed] [Google Scholar]
- Pettit DA, Harrison MP, Olson JM, Spencer RF, Cabral GA. Immunohistochemical localization of the neural cannabinoid receptor in rat brain. J Neurosci Res. 1998;51:391–402. doi: 10.1002/(SICI)1097-4547(19980201)51:3<391::AID-JNR12>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signaling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Shao Z, Puche AC, Kiyokage E, Szabo G, Shipley MT. Two GABAergic intraglomerular circuits differentially regulate tonic and phasic presynaptic inhibition of olfactory nerve terminals. J Neurophysiol. 2009;101:1988–2001. doi: 10.1152/jn.91116.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]