Abstract
Objectives
Pregnancy leads to rheumatoid arthritis remission in humans. The objective of this study was to determine if the SKG mouse could serve as a model for pregnancy-associated inflammatory arthritis amelioration. In addition, the maternal peripheral blood mononuclear cell (PBMC) transcriptome was assessed to define a biomarker associated with remission.
Methods
Cohorts of zymosan-treated pregnant SKG mice and controls were monitored for arthritis progression. Microarray analysis evaluated alterations in gene expression in maternal PBMCs at embryonic day 14.5 (E14.5) between arthritic and pregnancy-remitted mice. A selected target, serum amyloid A3 (SAA3), was further investigated using quantitative reverse transcriptase PCR (qRT-PCR) and an enzyme-linked immunosorbent assay (ELISA).
Results
Pregnancy resulted in complete or partial remission in the majority of the zymosan-treated SKG mice. Twenty-seven transcripts were differentially expressed in the PBMCs between arthritic and pregnancy-remitted mice. Expression and plasma SAA3 levels decreased with pregnancy-induced arthritis amelioration and plasma SAA3 levels correlated with arthritis severity.
Conclusions
These results establish the SKG mouse as a model system to study pregnancy-induced amelioration of arthritis. These studies also establish SAA3 as a biomarker of arthritis amelioration in SKG mice. This model can be used to elucidate the molecular and cellular mechanisms underlying the impact of pregnancy on the maternal immune system that results in arthritis amelioration.
Keywords: SAA3, rheumatoid arthritis, microarray, pregnancy, SKG mouse
Introduction
Rheumatoid Arthritis (RA) is a chronic inflammatory autoimmune disease that affects approximately 1–2% of the population [1]. Many RA patients fail to achieve durable remission or relapse due to the loss of efficacy of current treatments [2]. During pregnancy, 75% of women with RA have amelioration of their disease often within the first trimester and 16% have complete remission [1, 3]. Since this observation was first described in 1931 [4], many researchers have investigated the potential mechanism for pregnancy-associated RA remission including cortisol, sex steroid hormones, and other circulating factors. However, the mechanism by which pregnancy ameliorates RA remains unknown [1].
More recently, the degree of maternal and paternal MHC disparity was shown to correlate with the durability of the RA remission during pregnancy [5], leading to the hypothesis that the early immunological events in pregnancy that establish tolerance to the fetal allograft contribute to RA remission. Mechanistic studies to address this hypothesis will benefit from an animal model, as studies in pregnant humans are challenging and rightfully limited by ethical concerns. In these studies the SKG mouse model of inflammatory arthritis is evaluated as a potential model for such mechanistic studies. While others have utilized collagen-induced models of inflammatory arthritis (CIA), the SKG model is different in that it arose from a spontaneous mutation in a BALB/c mouse colony, it requires an environmental trigger as suspected for humans and finally SKG mice develop not only arthritis but also develop the extra-articular manifestations of disease as seen in humans with RA [6–9].
In previously published work, we have demonstrated that SKG mice treated with a single dose of zymosan (SKG+Z) develop inflammatory arthritis [10]. Clinically observable disease begins by 3 weeks and the disease severity plateaus at 6–7 weeks post zymosan treatment [10]. Using this mouse model of inflammatory arthritis, we tested whether pregnancy alters arthritis progression and then determined changes in the peripheral immune system in response to pregnancy by assessing the maternal PBMC transcriptome. Furthermore, we validated one of the identified targets, SAA3, as a biomarker of pregnancy-induced arthritis amelioration.
Materials and methods
Mice
Female SKG mice on the BALB/c background and male C57BL/6 mice (B6) were bred and housed in a pathogen-free animal care facility at National Jewish Health in accordance with institutional guidelines.
Arthritis induction and timed mating
Eight-week-old female littermate SKG mice were injected intraperitoneally with 2 mg zymosan A (Sigma-Aldrich) or saline (controls) to induce arthritis. Mice were monitored weekly for signs of arthritis according to a previously published scoring system [9, 10]. On day 21-post injection SKG mice were bred overnight with an allogeneic C57BL/6 male to provide MHC disparity. Presence of a vaginal plug marked day E0.5. Pregnant and non-pregnant mice were either followed until day 77 post zymosan injection, or sacrificed on E14.5 (day 36 post-zymosan injection). Pregnant mice followed until day 77 post zymosan injection delivered pups on E19.5 and pups were weaned at post-natal day 10. At time of sacrifice, blood and joint tissues were harvested and analyzed as described below.
Blood collection and processing
Mice were bled on day E14.5 or equivalent by cardiac puncture at time of necropsy. Plasma was separated from blood cells by centrifugation at 300 × g for 5 minutes at 4°C. Aliquots were stored at −80°C. Red blood cells were lysed in ACK solution (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA in dH2O). After centrifugation at 300 × g for 10 min at 4°C, the PBMC pellet was lysed in Trizol (Invitrogen) and RNA isolated following manufacturer’s instructions and stored at −80°C for gene expression profiling.
Histology
Mice were euthanized and joints fixed in 10% formalin solution, decalcified in 0.5 M EDTA and embedded in paraffin. Four-micrometer thick tissue sections were stained with toluidine blue and imaged (Premier Laboratories).
Gene expression profiling
100 ng of high quality RNA, as determined by Agilent bioanalyzer Pico chips (RNI > 8.0), was converted to cDNA by amplification methods using Ovation kit (NuGen) with the whole blood reagent to manage the globin content of the samples. Labeled samples were hybridized to Affymetrix Mouse Gene 1.0 ST GeneChips using standard Affymetrix protocols. Analyses of microarray data were performed using the opensource R (version 2.11) statistical software [11]. In addition to our in-house statistical functions, we used R statistical packages from the Bioconductor consortium [12]. Raw data were transformed using the Robust Multi-chip Average normalization method for background correction [13]. Probe sets were filtered by only including those with the significant “Detection Above Back Ground” call in all samples. The statistical power was increased by pruning a priori genes that did not vary over any chips, and with other techniques (e.g. a test of replicability) as appropriate. Student’s t-tests were performed and combined with the False Discovery Rate (FDR) correction for multiple testing [14].
Quantitative RT-PCR analysis
200 ng high quality RNA, isolated as described above, was reverse transcribed using a Promega Improm-II Reverse Transcription System following the manufacturer’s instructions. Next, qRT-PCR was performed using TaqMan Universal PCR Master Mix, No AMPErase UNG (Applied Biosystems) with SAA3 (Applied Biosystems; Mm00441203_m1) as the target gene and ribosomal protein 18S (Applied Biosystems; Mm02601777_g1) as the endogenous reference gene. The samples were amplified on a Bio-Rad CFX96 C1000 Thermal Cycler using the following conditions: 10 minutes at 95°C and 40 cycles of 15 seconds at 95°C and 1 minute at 60°C. A known source of SAA3 RNA (LPS stimulated macrophages, MH-S) was used as a positive control [15, 16]. Results were analyzed using the Bio-Rad CFX96 Software and relative SAA3 expression was calculated using the ΔΔCt method as the efficiency of both probe sets were similar (<10% different).
SAA3 enzyme-linked immunosorbent assay
Plasma samples were analyzed in duplicate for the presence of SAA3 using an ELISA according to the manufacturer’s protocol (Millipore). Immunoreactive complexes were detected with 3,3′,5,5′-Tetramethylbenzidine and were read at 450 nm in a VERSAmax tunable microplate reader (Molecular Devices). Detectable SAA3 concentrations ranged from 0.078 to 5 µg/mL. Intra-assay and inter-assay variation ranged from 3–14% CV and 10–13% CV, respectively. 100% specificity to mouse SAA3 was reported in manufacturer protocol.
Statistical analysis
Prism 5 software was used for statistical analysis. Student’s t-test was used to analyze differences in number of live fetuses between treated and nontreated mice and one-way ANOVA was used for comparison among all treatment groups. Relative Risk was calculated using a two-tailed Fisher’s exact test with a 95% confidence interval. The Kruskal-Wallis one-way analysis of variance by rank was used to analyze differences in median SAA3 expression or plasma concentration between treatment groups. Posttest analyses were performed using Dunn’s multiple comparison tests. Linear correlation was used to determine the association between plasma concentration and PBMC RNA expression of SAA3. Nonlinear regression was calculated to analyze the relationship between plasma SAA3 concentration and arthritis score. Results were considered significant with a p-value <0.05.
Results
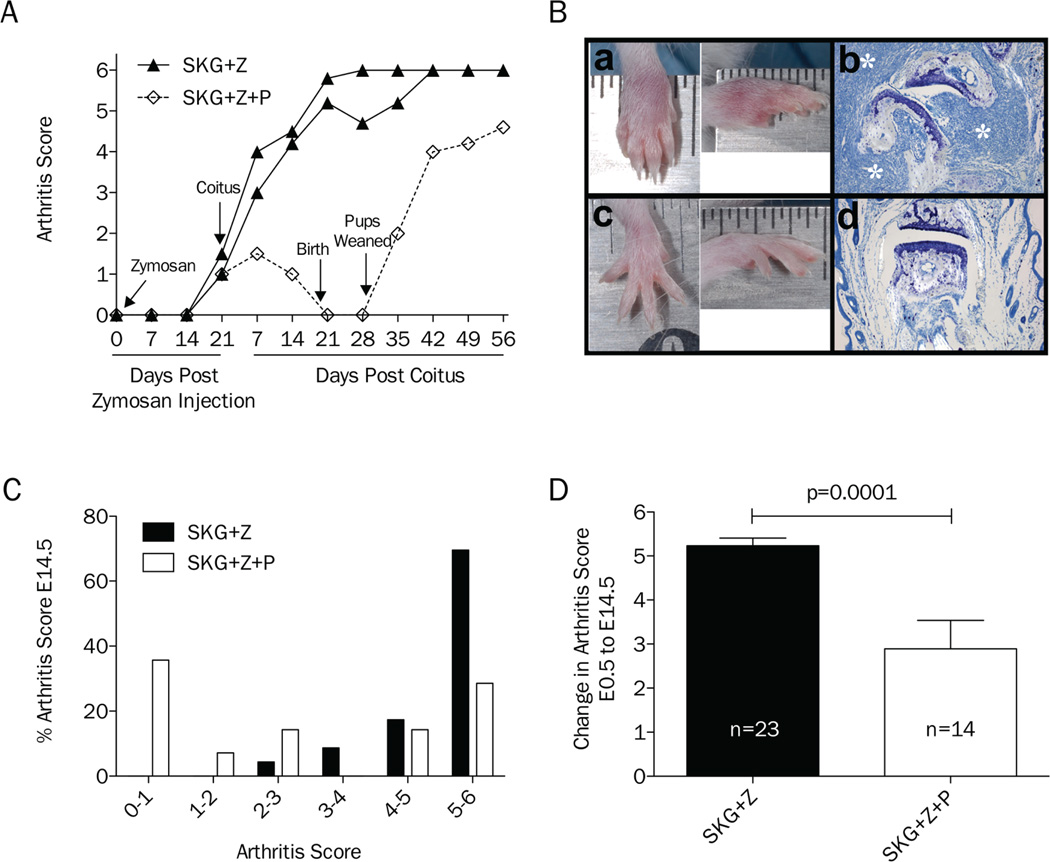
The kinetics of inflammatory arthritis and pregnancy development were monitored to establish if pregnancy could induce remission of arthritis in the SKG mouse model and to determine the gestational age with maximal change in arthritis score to guide timing of necropsies in subsequent cohorts. The arthritis score kinetics of an initial representative cohort of 8-week-old littermate female mice (n=3) injected with zymosan and bred to a C57BL/6 stud 3 weeks post zymosan injection is shown in Figure 1. This cohort was followed through pregnancy and 5 weeks post-delivery. One littermate became pregnant (SKG+Z+P) and experienced complete remission of arthritis. Following delivery, arthritis returned (Figure 1A). In subsequent experiments, mice were euthanized at day E14.5 for histologic analysis and collection of PBMCs. Histologic evaluation of the joints demonstrated regression of the inflammatory infiltrate and pannus in SKG+Z+P mice compared to SKG+Z mice (Figure 1B). Evaluation of multiple cohorts of mice, SKG+Z+P (n=14) had lower arthritis scores as compared to SKG+Z mice (n=23) at E14.5 (Figure 1C). In order to combine data between cohorts of mice, which had slightly different levels of arthritis at time of coitus, we looked at the change in arthritis score from E0.5 to E14.5. The change in arthritis score was significantly lower in SKG+Z+P mice (p≤0.0001) as compared to SKG+Z (Figure 1D). Although the majority of SKG+Z mice developed disease, there was variability in the degree of remission observed in SKG+Z+P mice, not unlike what is seen with pregnancy in women with RA [1, 3]. Specifically, 28.6% of SKG+Z+P mice remitted completely (SKG+Z+P(R)) with an arthritis score <1, and 35.7% partially remitted (SKG+Z+P(PR)) with an arthritis score lower than the corresponding SKG+Z littermates. The remaining 35.7% of mice did not experience improvement in arthritis score (SKG+Z+P(NR)) and disease progressed during pregnancy similar to SKG+Z littermates controls. Comparing SKG+Z+P(R) and SKG+Z+P(PR) to SKG+Z+P(NR), the relative risk of arthritis development is significantly lower for zymosan-treated pregnant mice as compared to non-pregnant zymosan-treated controls (RR 0.41, 95% CI 0.20–0.84, p=0.0028). There were no significant differences in the number of live fetuses between SKG+P and SKG+Z+P mice (p=0.91) or across all pregnancy categories [SKG+P, SKG+Z+P(R), SKG+Z+P(PR), SKG+Z+P(NR)] (p=0.50) (data not shown).
Figure 1.
Pregnancy induces remission of inflammatory arthritis in SKG mice. A, Single representative cohort of SKG mice treated with zymosan and then mated to compare arthritis kinetics in pregnant (n=1) and non-pregnant (n=2) mice through pregnancy and post-delivery. B, Gross morphology of wrist joints of SKG mice treated with zymosan from both non-pregnant (a) and pregnant (c) mice on gestational day E14.5. Representative histology of digit joints stained with toluidine blue from SKG+Z (b) and SKG+Z+P (d) mice at day E14.5. White asterisks identify synovial lymphocytic infiltrate and pannus formation (c). C, Distribution of percentage of arthritis scores for non-pregnant (n=23) and pregnant (n=14) zymosan-treated SKG mice on gestational day E14.5. D, Change in arthritis score for non-pregnant (n=23) and pregnant (n=14) zymosan-treated SKG mice from gestational day E0.5 to E14.5.
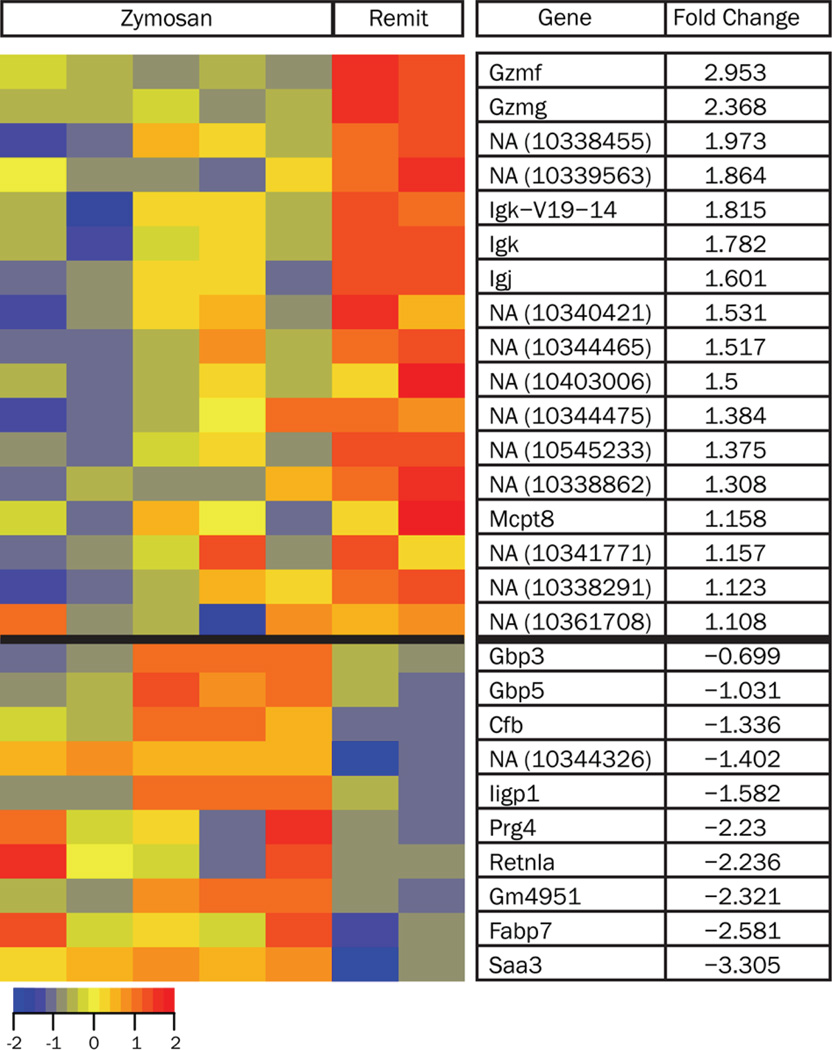
As pregnancy alters the development of inflammatory arthritis in SKG+Z mice, we next sought to determine the impact of pregnancy on the maternal immune system. To accomplish this, we determined the transcriptome of the maternal PBMCs, serving as a window into alterations of the systemic maternal immune response. A cross-sectional approach was used to compare SKG+Z and SKG+Z+P(R) mice at E14.5. Analysis of the gene expression data comparing SKG+Z mice (n=5) with SKG+Z+P(R) mice (n=2) identified 27 differentially expressed genes using a threshold of FDR=0.05 (Figure 2). Of interest, SAA3 was found to be down-regulated 3.3 fold in SKG+Z+P(R) mice compared to SKG+Z.
Figure 2.
Heat map of differentially expressed genes in the PBMCs of zymosan-treated (SKG+Z) compared with pregnant remitted mice (SKG+Z+P(R)). The normalized log intensity values for the 27 differentially expressed probe sets were centered to the median value of each probe set and colored on a range of −2.0 to +2.0. Red denotes up-regulated expression levels and blue denotes down-regulated expression levels as compared to the median value. Columns contain data from a single mouse PBMC sample, and rows correspond to a single probe ID. Rows are ranked by fold change of mean of SKG+Z+P(R) compared to mean of SKG+Z.
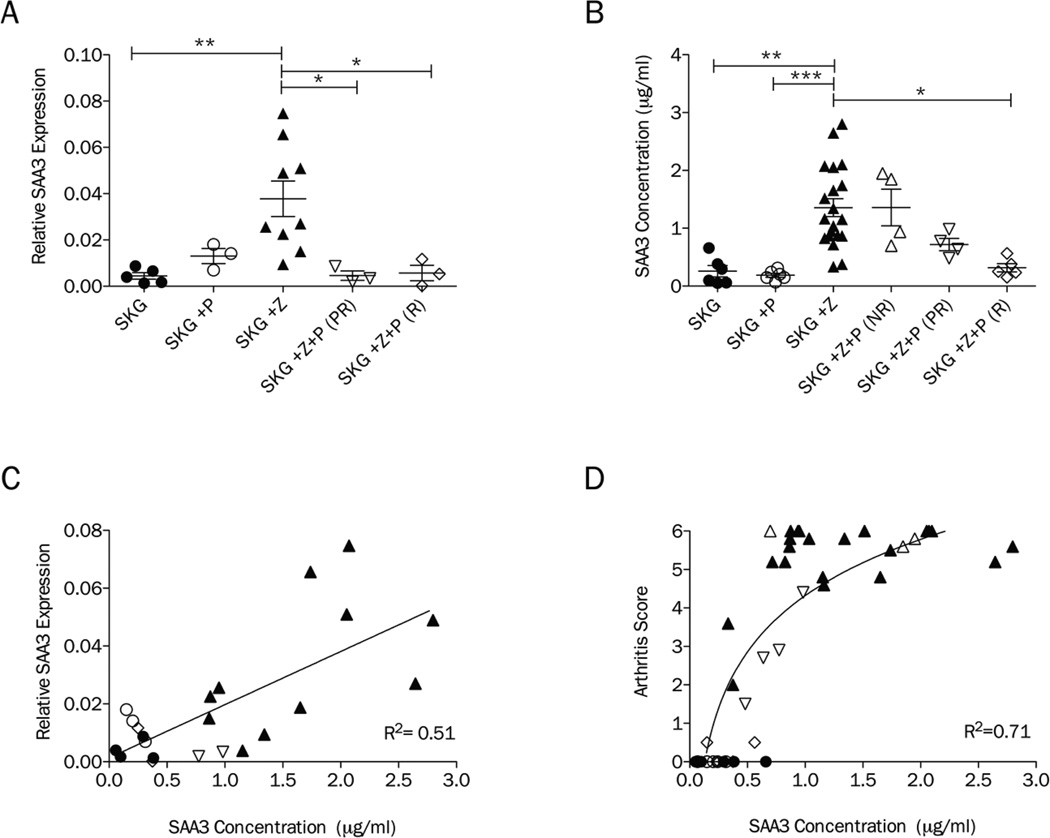
To confirm and expand the SAA3 microarray results across all treatment groups, qRT-PCR was performed using RNA isolated from PBMCs of SKG, SKG+P, SKG+Z, SKG+Z+P(PR) and SKG+Z+P(R) mice. Sufficient quality RNA was not available from SKG+Z+P(NR) mice for this analysis. Significant differences in medians were detected among treatment groups (p<0.01). SAA3 levels were elevated in SKG+Z mice compared to SKG (p<0.01). SKG+P was not significantly different from SKG showing that pregnancy alone does not increase SAA3 expression in PBMCs. Compared to SKG+Z, both partially [SKG+Z+P(PR)] and completely remitted mice [SKG+Z+P(R)] had significantly decreased expression of SAA3 in the PBMC transcriptome (p<0.05, p<0.05).
As SAA3 can be detected as a secreted molecule in the plasma, we sought to determine if the gene expression alterations in SAA3 in PBMCs would translate to differences in plasma SAA3 levels. Concentrations of SAA3 were determined by ELISA in plasma at E14.5, the same time point when the PBMC microarray and qRT-PCR analyses was performed. Significant differences in medians were detected among treatment groups (p<0.001). The ELISA showed that zymosan-treatment resulted in increased plasma SAA3 5.3 fold (p<0.01) compared to naïve SKG mice and 7.2 fold (p<0.001) compared to SKG+P (Figure 3B). Again there was no significant difference between SKG and SKG+P. SAA3 plasma levels were also not significantly different between SKG+Z and SKG+Z+P mice that did not remit. Similar to the results obtained by qRT-PCR, SKG+Z+P(R) mice had significantly decreased plasma SAA3 compared to SKG+Z (p<0.05). SKG+Z+P(PR) mice had SAA3 levels that were intermediate between the non-remit and remit SKG+Z+P mice.
Figure 3.
SAA3 is a biomarker of inflammatory arthritis remission in SKG mice. A, Relative RNA expression of SAA3 in PBMCs as determined by qRT-PCR in untreated SKG mice (SKG, n= 5; circle), pregnancy only (SKG+P, n=3; open circle), zymosan-treated (SKG+Z, n=9; triangle pointed up), zymosan-treated pregnant partially-remitted (SKG+Z+P (PR), n=3; open triangle pointed down) and zymosan-treated pregnant remitted (SKG+Z+P(R), n=3; open diamond) on E14.5. B, SAA3 plasma concentrations as determined by ELISA in untreated SKG mice (SKG, n= 6; circle), pregnancy only (SKG+P, n=6; open circle), zymosan-treated (SKG+Z, n=20; triangle pointed up), zymosan-treated pregnant non-remitted (SKG+Z+P(NR), n=4; open triangle pointed up), zymosan-treated pregnant partially-remitted (SKG+Z+P(PR), n=4; open triangle pointed down) and zymosan-treated pregnant remitted (SKG+Z+P(R), n=5; open diamond) on E14.5. C, Correlation of relative RNA expression of SAA3 in PBMCs and concentrations of SAA3 in plasma. D, Correlation of arthritis score and plasma concentration of SAA3. Symbols in panels C and D correspond with groups defined in panels A and B. (*p<0.05, **p<0.01, ***p<0.001)
To determine if plasma SAA3 levels correspond with the PBMC expression levels, we analyzed the correlation between these two measures of SAA3 from mice for which we had both types of data (n= 22; Figure 3C) and found a positive correlation (R2=0.51, p<0.001). This suggests that the source of the plasma SAA3 protein levels may be cells within the PBMCs. Next, we examined the correlation of circulating SAA3 levels with arthritis scores regardless of treatment group (n=45; Figure 3D). There was a positive correlation between SAA3 plasma concentration and arthritis score using linear regression (R2=0.59, p<0.001). However, the relationship appears curvilinear and best fits a semi-logarithmic (log-X, linear-Y) model (R2=0.71). This suggests that log increases in circulating SAA3 corresponds to unit changes in arthritis score.
Discussion
Our data demonstrate that pregnancy can cause complete or partial arthritis remission in the SKG mouse model of inflammatory arthritis. The variability in remission recapitulates the pregnancy-associated RA amelioration that has been observed in humans [1–3]. In SKG mice, the relative risk of arthritis was significantly decreased for SKG+Z+P mice compared to SKG+Z mice. Using this model, we confirmed our hypothesis that the peripheral blood transcriptome is modulated in SKG+Z+P(R) mice as compared to SKG+Z mice by performing a microarray which identified 27 differentially expressed genes in maternal PBMCs. Interestingly, SAA3, a known acute phase reactant, was significantly decreased in SKG+Z+P(R) mice as compared to SKG+Z mice. This differential expression of SAA3 in arthritic versus pregnancy remitted mice was confirmed both by qRT-PCR of PBMC RNA and determination of plasma SAA3 protein levels by ELISA. Further our data show that pregnancy alone, although considered an inflammatory state, does not result in elevated SAA3 levels and that plasma SAA3 levels correlate with arthritis severity.
SAA3 is an apolipoprotein and a member of the serum amyloid A family of acute phase reactants, which are secreted under inflammatory conditions [17]. In the mouse, it is the predominant isoform expressed extrahepatically by a variety of cell types including macrophages and adipocytes [17]. This knowledge and the correlation between the PBMC RNA and plasma SAA3 levels suggest that one source of the plasma SAA3 is circulating macrophages. Further work is needed to determine if indeed macrophages or a different cell type are responsible for the plasma SAA3 levels measured. SAA3 is also expressed in chondrocytes and its transcript was found to be up-regulated in the diseased synovium of an Ag-induced arthritis rabbit model and therefore could also contribute to SAA3 plasma levels [18]. However, we suspect that pregnancy alterations in peripheral blood macrophage numbers, development or functional status accounts for a portion of the changes seen in plasma SAA3 levels.
While SAA3 was originally thought to be a pseudogene that is not transcribed in humans [19], recent work has shown that expression of SAA3 transcript is detected in human cells [20]. In response to stimulation with either prolactin or lipopolysaccharide, SAA3 gene expression was detected in mammary gland epithelial cells [20]. Further work is needed to determine if SAA3 is expressed during states of inflammation in humans and if its transcript is translated into protein. However, serum amyloid A (SAA) isoforms (SAA1 and SAA2) have been detected as both transcript and protein in the diseased synovium of human RA patients identified in synoviocytes and macrophages [21]. SAA plasma levels have been shown to be higher in RA patients compared to controls [22]. Whether serum amyloid A can be used to monitor pregnancy amelioration of RA in women is a potential area of investigation based on our results in the SKG mouse model.
This work establishes the SKG mouse as a model system to dissect the molecular and cellular mechanisms by which pregnancy modulates the maternal immune system resulting in arthritis amelioration. Importantly our data demonstrate that SAA3 can serve as a plasma biomarker for arthritis severity. The fact that the SAA3 plasma levels fit a semi-logarithmic model suggests using SAA3 plasma levels to quantify arthritis regression will provide a larger dynamic range for future mechanistic studies using this model. The promise of this work is that understanding the mechanism of pregnancy-induced arthritis amelioration will lead to novel treatment strategies for rheumatoid arthritis and also provide insight into the mechanism of fetal tolerance.
Acknowledgements
We thank members of the Dragone and Winn laboratories especially Samantha Friend and Anita Kramer who contributed technical and intellectual expertise. This work was supported by an NIH/NCRR Colorado CTSI UL1 RR025780 CCTSI co-pilot award (LLD), an American College of Rheumatology Within Our Reach (WOR) grant (LLD), an Arthritis Foundation Arthritis Investigator award (LLD), an University of Colorado Department of OB/GYN Academic Enrichment Fund award (VDW), an NIH/NCRR/NCATS Colorado CTSI TL1 RR025778 (ALS, KKR), and an Arthritis Foundation Postdoctoral Fellowship (LKP).
References
- 1.Ostensen M, Villiger PM. The remission of rheumatoid arthritis during pregnancy. Semin Immunopathol. 2007;29:185–191. doi: 10.1007/s00281-007-0072-5. [DOI] [PubMed] [Google Scholar]
- 2.van Vollenhoven RF. Treatment of rheumatoid arthritis: state of the art 2009. Nat Rev Rheumatol. 2009;10:531–541. doi: 10.1038/nrrheum.2009.182. [DOI] [PubMed] [Google Scholar]
- 3.Golding A, Haque UJ, Giles JT. Rheumatoid arthritis and reproduction. Rheum Dis Clin N Am. 2007;33:319–343. doi: 10.1016/j.rdc.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Hench PS. The ameliorating effect of pregnancy on chronic atrophic (infectious rheumatoid) arthritis, fibrositis, and intermittent hydrarthrosis. Mayo Clin Proc. 1938;13:161–180. [Google Scholar]
- 5.Nelson JL, Hughes KA, Smith AG, Nisperos BB, Branchaud AM, Hansen JA. Maternal-fetal disparity in HLA class II alloantigens and the pregnancy-induced amelioration of rheumatoid arthritis. N Engl J Med. 1993;329:466–471. doi: 10.1056/NEJM199308123290704. [DOI] [PubMed] [Google Scholar]
- 6.Jansson L, Holmdahl R. Oestrogen induced suppression of collagen arthritis. IV: Progesterone alone does not affect the course of arthritis but enhances the oestrogen-mediated therapeutic effect. J Reprod Immunol. 1989;15:141–150. doi: 10.1016/0165-0378(89)90033-8. [DOI] [PubMed] [Google Scholar]
- 7.Waites GT, Whyte A. Effect of pregnancy on collagen-induced arthritis in mice. Clin Exp Immunol. 1987;67:467–476. [PMC free article] [PubMed] [Google Scholar]
- 8.Mattsson R, Mattsson A, Holmdahl R, Whyte A, Rook GA. Maintained pregnancy levels of oestrogen afford complete protection from postpartum exacerbation of collagen-induced arthritis. Clin Exp Immunol. 1991;85:41–47. doi: 10.1111/j.1365-2249.1991.tb05679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakaguchi N, Takahashi T, Hata H, Nomura T, Tagami T, Yamazaki S, Sakihama T, Matsutani T, Negishi I, Nakatsuru S, Sakaguchi S. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature. 2003;426:454–460. doi: 10.1038/nature02119. [DOI] [PubMed] [Google Scholar]
- 10.Peterson LK, Shaw LA, Joetham A, Sakaguchi S, Gelfand EW, Dragone LL. SLAP deficiency enhances number and function of regulatory T cells preventing chronic autoimmune arthritis in SKG mice. J Immunol. 2011;186:2273–2281. doi: 10.4049/jimmunol.1003601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.R Development Core Team. R: a language and environment for statistical computing. R foundation for statistical computing. 2011 [Google Scholar]
- 12.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: a Practical and Powerful Approach to Multiple Testing. J R Statist Soc. 1995;57:289–300. [Google Scholar]
- 15.Wu C, Orozco C, Boyer J, Leglise M, Goodale J, Batalov S, Hodge CL, Haase J, Janes J, i.Huss JW, 3rd, Su AI. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol. 2009;10:R130. doi: 10.1186/gb-2009-10-11-r130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lattin JE, Schroder K, Su AI, Walker JR, Zhang J, Wiltshire T, Saijo K, Glass CK, Hume DA, Kellie S, Sweet MJ. Expression analysis of G Protein-Coupled Receptors in mouse macrophages. Immunome Res. 2008;4:5. doi: 10.1186/1745-7580-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meek RL, Eriksen N, Benditt EP. Murine serum amyloid A3 is a high density apolipoprotein and is secreted by macrophages. Proc Natl Acad Sci. 1992;89:7949–7952. doi: 10.1073/pnas.89.17.7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vallon R, Freuler F, Netsanet DT, Robeva A, Dawson J, Wenner P, Engelhardt P, Boes L, Schnyder J, Tschopp C, Urfer R, Baumann G. Serum amyloid A (apoSAA) expression is up-regulated in rheumatoid arthritis and induces transcription of matrix metalloproteinases. Arthritis Rheum. 2001;62:1842–1848. doi: 10.4049/jimmunol.166.4.2801. [DOI] [PubMed] [Google Scholar]
- 19.Kluve-Beckerman B, Drumm ML, Benson MD. Nonexpression of the human serum amyloid A three (SAA3) gene. DNA Cell Biol. 1991;10:651–661. doi: 10.1089/dna.1991.10.651. [DOI] [PubMed] [Google Scholar]
- 20.Larson MA, Wei SH, Weber A, Weber AT, McDonald TL. Induction of human mammary-associated serum amyloid A3 expression by prolactin or lipopolysaccharide. Biochem Biophys Res Commun. 2003;301:1030–1037. doi: 10.1016/s0006-291x(03)00045-7. [DOI] [PubMed] [Google Scholar]
- 21.O'Hara R, Murphy EP, Whitehead AS, FitzGerald O, Bresnihan B. Acute-phase serum amyloid A production by rheumatoid arthritis synovial tissue. Arthritis Res. 2000;2:142–144. doi: 10.1186/ar78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong M, Toh L, Wilson A, Rowley K, Karschimkus C, Prior D, Romas E, Clemens L, Dragicevic G, Harianto H, Wicks I, McColl G, Best J, Jenkins A. Reduced arterial elasticity in rheumatoid arthritis and the relationship to vascular disease risk factors and inflammation. Arthritis Rheum. 2003;48:81–89. doi: 10.1002/art.10748. [DOI] [PubMed] [Google Scholar]