Abstract
Premature children born with very low birth weight (VLBW) can suffer chronic hypoxic injury as a consequence of abnormal lung development and cardiovascular abnormalities, often leading to grave neurological and behavioral consequences. Emerging evidence suggests that environmental enrichment improves outcome in animal models of adult brain injury and disease, however, little is known about the impact of environmental enrichment following developmental brain injury. Intriguingly, data on socio-demographic factors from longitudinal studies that examined a number of VLBW cohorts suggest that early environment has a substantial impact on neurological and behavioral outcomes. In the current study, we demonstrate that environmental enrichment significantly enhances behavioral and neurobiological recovery from perinatal hypoxic injury. Using a genetic fate-mapping model that allows us to trace the progeny of GFAP+ astroglial cells, we show that hypoxic injury increases the proportion of astroglial cells that attain a neuronal fate. In contrast, environmental enrichment increases the stem cell pool, both through increased stem cell proliferation and stem cell survival. In mice subjected to hypoxia and subsequent enrichment there is an additive effect of both conditions on hippocampal neurogenesis from astroglia, resulting in a robust increase in the number of neurons arising from GFAP+ cells by the time these mice reach full adulthood.
Introduction
Approximately 1% of children born in the United States are premature and born at a very low birth weight (VLBW). Clinically, VLBW children exhibit a range of neurological and behavioral disturbances, including decreased brain volume, white matter abnormalities, and developmental delays. These perturbations are thought to be a consequence of chronic hypoxic injury due to immature lung development. Remarkably, a substantial portion of VLBW children are able to recover from these abnormalities over time and some of them attain normal cognitive function by the time they reach early adulthood (Saigal and Doyle, 2008; Luu et al., 2011). However, the factors that contribute to heterogeneity in long-term outcomes are not known. The current study attempts to elucidate the underlying cellular mechanisms using a mouse model of perinatal hypoxia that mimics the injury and recovery observed in VLBW children. Emerging evidence suggests that various types of injury, such as hypoxic and/or ischemic insults, induce strong proliferative and neurogenic/gliogenic responses in the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG), a postnatal neurogenic niche (Kuhn et al., 2001; Sharp et al., 2002; Parent, 2003; Fagel et al., 2006; Yang and Levison, 2006; Kernie and Parent, 2010). In the postnatal brain, neural stem cells (NSCs) are a subset of Glial Fibrillary Acidic Protein (GFAP)-expressing astrocytes (Doetsch et al., 1999; Seki and Arai, 1999; Seri et al., 2001; Alvarez-Buylla et al., 2002; Filippov et al., 2003; Imura et al., 2003; Garcia et al., 2004). Neural stem cells give rise to transient-amplifying progenitors that express neuronal lineage makers and generate new neurons throughout life (Belachew et al., 2003; Hack et al., 2005; Hevner et al., 2006; Brill et al., 2008; Rivers et al., 2008).
To study the cellular and molecular mechanisms that confer higher inherent plasticity to the DG, allowing for a more adaptive response to brain injury, we investigated the fate of GFAP+ astroglia in the DG by tagging these cells with heritable reporter genes via tamoxifen-inducible Cre recombinase in GFAP-CreERT2 (GCE) transgenic mice. In addition, because environmental enrichment with exercise has been demonstrated to increase hippocampal neurogenesis in adulthood (Kempermann et al., 1997; van Praag et al., 1999b; Olson et al., 2006), we assessed the influence of enriched environment on the recovery process after perinatal hypoxic injury. Number and self-renewal of hippocampal GFAP+ neural stem cells as well as phenotype and long-term survival of the new neurons arising from these cells were examined in both hypoxic and hypoxic-enriched mice. Finally, we examined the functional outcome of hypoxic injury and subsequent enriched rearing on cognitive and emotive behavioral tasks.
We show that perinatal hypoxia enhances the proportion of new neurons arising from GFAP+ astroglial cells and correspondingly decreases the proportion of GFAP+ astrocytes among fate-mapped cells. The newly-generated new neurons persist long-term in the DG. Rearing the animals in enriched environment induces a large, additive increase in neurons derived from GFAP+ cell within DG. We suggest that the effect of enrichment is attributable to a potent increase in proliferation and survival of neural stem cells.
Materials and Methods
Generation of Mice, Genotyping and Breeding strategy
The GFAPCreERT2 (GCE) mice were generated as previously described and back-crossed to C57/B6 mice 10 generations (Ganat et al., 2006; Bi et al., 2011). GCE transgenic mice carry a Cre recombinase-estrogen receptor type 2 fusion protein (CreErT2) placed under control of the human GFAP promoter, which is active in radial glia, astrocytes and adult neural stem cells (Brenner, 1994; Ganat et al., 2006). Genotyping was done by PCR using primers to the Cre gene (5'-GCAACGAGTGATGAGGTTCGCAAG-3') (forward) and (5'-TCCGCCGCATAACCAGTGAAACAG-3') (reverse) to generate a band of 307 bp (Ganat et al., 2006; Bi et al., 2011). GCE mice were crossed with either the R26R LacZ reporter mice (Soriano, 1999) (available from The Jackson Laboratory, Bar Harbor, ME) or the CAG-eGFP reporter mice (Nakamura et al., 2006). Numbers of animals used in experiments range between 3 and 6 per group, as indicated in the results section. Littermates negative for the CAG-eGFP gene were used for behavioral experiments. All animal experiments comply with Institutional and National policies and guidelines.
Induction of Cre Recombination via Tamoxifen Treatment
In order to induce Cre recombination in GFAP promoter expressing cells, GCE mice crossed with reporter lines were injected daily from P12 to P14 intraperitoneally with tamoxifen at a dosage of 60 mg/kg from a 2 mg/ml stock solution prepared in autoclaved sunflower seed oil and stored at −20 °C.
Hypoxic Rearing
Mice (males and females) were placed in a chamber maintaining a 9.5–10.5% O2 concentration by displacement with N2 as previously described (Fagel et al., 2006). Hypoxia began at postnatal day 3 (P3) for 8 days until P11. A separate group of control (normoxic) mice were matched for strain and age. Mice were sacrificed at P15, P16, P35, or P90. Mice were perfused transcardially with 20 ml of phosphate-buffered saline (PBS) followed by 25 ml of 4% paraformaldehyde (PFA). Brains were post-fixed overnight in 4% PFA, followed by equilibration in a 30% sucrose solution overnight for cryoprotection, and stored at −80°C.
Enriched environment
Following hypoxic or normoxic rearing conditions, male and female mice were placed under either enriched or standard housing until sacrifice. Enrichment was maintained for two weeks, from P21–P35; or for 10 weeks, from P21–P90. All groups were counterbalanced for previous hypoxic experience, therefore four groups of animals were generated: normoxic standard environment (NSE), normoxic enriched (NEn), hypoxic standard environment (HSE) and hypoxic enriched (HEn). Environmental enrichment consisted of a larger cage (10” × 19” × 8”h), a running wheel, several plastic toys including hanging objects, balls and modular plastic tunnels that were changed and reconfigured weekly during routine cage cleaning Animals were group housed in both the standard and enriched paradigms.
Behavioral Testing
In order to assess the functional outcome of both hypoxia and enriched environment, GCE: CAG negative of mice used in the fate-mapping experiments were tested on the open field and Morris water maze tests at P35.
Mice were brought to the behavioral testing room in their home cages at least 30 minutes prior to testing in order to acclimatize to the testing environment. Mice were then placed in an open field (26×48cm) under bright overhead lights and allowed to explore freely for twenty minutes. Distance and time spent in the periphery and center of the open field as well as freezing bouts and immobility were all assessed using AnyMazeTM software tracking system (Stoelting USA). Following this, mice were tested on a shortened Morris water maze protocol adapted from J. Nunez (Nunez, 2008). Briefly, mice were placed in each of the four quadrants of the water maze in random order. The platform remained in a fixed location until the probe trial. Mice received two sets of 6 trials separated by one-hour break. Each trial (inter-trial interval was 10 minutes) ended either when they found the platform or after 60 seconds had elapsed. Mice that did not find the platform during the first trial were gently guided to the platform and removed from the platform after 5 seconds. 10 minutes following the 12th trial, the platform was removed and memory retention for the platform location was tested with a 60-second probe trial. We assessed both the number of trials it took for greater than 50% of the animals in a group to find the platform (the rate of acquisition) and the quadrant preference during the probe trial (memory test - presented as both percent time spent in the target quadrant and a probe preference score).
2-bromodeoxyuridine, chlorodeoxyuridine and iododeoxyuridine treatment
In order to assess the effect of environmental enrichment on cell survival, normoxic mice were injected with the thymidine analog, CldU (100mg/kg) twice on P15 and P16, prior to the onset of enrichment. Cell proliferation was assessed by injecting the thymidine analog, IdU (100mg/kg) one hour prior to sacrifice at P35.
Tissue Preparation and Immunohistochemistry
Serial 20 μm cryosections were obtained as previously described (Ganat et al., 2006). For immunohistochemistry, sections were blocked in PBS containing 0.3% Triton (PBS-T) containing 10% goat serum (10% GS/PBS-T) or 10% donkey serum (10% DS/PBS-T), and then incubated in primary antibody in 10% GS/PBS-T or DS/PBS-T. For a list of primary antibodies, see Table 1. Sections were washed thoroughly and then reacted to the secondary antibody of the appropriate species. The secondary antibodies used were as follows: Alexa 488-, Alexa 594-, Alexa 350-, Alexa 546- and Alexa 632-conjugated species directed IgGs (Molecular Probes), and FITC-, DYlight 549- and 649-conjugated species directed IgGs (Jackson Labs), all at 1:500 dilution. For BrdU/CldU/IdU immunostaining sections were incubated for 45 minutes in 2 N HCl, followed by washes and immunohistochemistry as described above.
Table 1.
Primary Antibodies.
| Antibody | Manufacturer | Species | Dilution |
|---|---|---|---|
| Bgalactosidase | Cappel | Rabbit | 1:10000 |
| Bgalactosidase | Abcam | Chicken | 1:500 |
| BrdU/CldU | Accurate Chemical | Rat | 1:250 |
| BrdU/IdU | Becton-Dickenson | Mouse | 1:100 |
| Doublecortin | Abcam | Rabbit | 1:2000 |
| Doublecortin | Santa Cruz | Goat | 1:500 |
| GFAP | Sigma | Mouse | 1:500 |
| GFAP | Dako | Rabbit | 1:1000 |
| GFP | Molecular Probes | Rabbit | 1:500 |
| GFP | Abcam | Chicken | 1:1000 |
| Ki67 | Vector Laboratories | Rabbit | 1:500 |
| Neuronal Nuclei | Chemicon | Mouse | 1:500 |
| Ng2 | Millipore | Mouse | 1:500 |
| Olig2 | Kindly provided by Drs. Charles Stiles and John Alberta, Dana-Farber Cancer Institute, Boston, MA | Rabbit | 1:20000 |
| Sox2 | Chemicon | Goat | 1:200 |
| Ng2 | Chemicon | Rabbit | 1:500 |
| Sox2 | Chemicon | Goat | 1:200 |
Cell Counting and microscopic analysis
Unbiased estimates of cell number were obtained via a Zeiss Axioskope 2 Mot Plus (Carl Zeiss, Thornwood, NY, USA) attached to a motorized stage and connected to a computer running the Stereoinvestigator Software (MicroBrightfield, Colchester, VT, USA). Serial sagittal sections (one every 600 μm) were used for all counts. Contours of the dentate gyrus were drawn at 10× magnification. Nuclear profiles of stained cells were counted using the optical fractionator probe with a 40× oil-immersion objective. Sampling grids sized 300 μm × 135 μm were used in order to obtain a relatively constant number of cells sampled and obtain a coefficient of error ≤ 0.5. This systematic yet unbiased method provides an estimate of cell density and number that is independent from cell size, shape, orientation, tissue shrinkage and spatial distribution of the cells (Schmitz and Hof, 2005). Tri-dimensional sampling boxes or (100 μm × 100 μm × 10 μm) with 3 out of 6 exclusion borders (Gundersen et al., 1988; West, 1993) were automatically placed by StereoInvestigator at each grid intersection point. The density for each cell type was calculated by dividing the total number of cells by the total volume sampled. Image acquisition and Z-stack analysis was performed on an ApoTome equipped Axiovert 200M with Axiovision 4.5 software (Carl Zeiss, Thornwood, NY, USA).
Statistical Analyses
All data was analysed using Statview 4.51 or SPSS 16.0.2 software. T-tests or factorial analysis of variance were used as necessary, with level of enrichment (enriched or not), and/or hypoxia (hypoxic or normoxic) as independent variables. The density of cells/region, total overall number of cells, and where appropriate, the percent colocalization with βgal or eGFP reporter were analyzed. F values were considered significant when p≤0.05. Post-hoc analyses using Fisher's LSD were conducted when p values reached significance. Planned comparisons were conducted to confirm previously published results when omnibus F scores were not significant.
Results
Characterization of the original population of reporter-tagged cells in the DG
In order to track the fate of GFAP+ astroglial cells after hypoxic injury in the immature brain, we used a genetic fate mapping technique that we have previously employed to characterize cerebral cortical recovery following hypoxic injury (Bi et al., 2011). GCE mice carrying either the R26R or the CAG-CAT-eGFP reporter genes were reared continuously in 10 or 20% oxygen environment (hypoxia and normoxia, respectively) from P3 to P11, then injected daily with tamoxifen (60 mg/kg) from P12 to P14 to induce either β-galactosidase (βgal) or enhanced green fluorescent (eGFP) gene expression in cells with GFAP promoter activity. Tissue was analyzed at P15 (n=4 for both groups)(Ganat et al., 2006; Bi et al., 2011). Although we have previously observed no differences in reporter induction between normoxic and hypoxic groups using this model (Bi et al., 2011), these observations were primarily conducted in the cortex and subventricular zone; therefore, we confirmed that this was also true of the dentate gyrus (DG). As expected, GCE;reporter+ mice that did not receive tamoxifen demonstrated no recombination, irrespective of the survival time after vehicle administration (Fig. 1A, B). One day after the last tamoxifen injection (P15), 95.7±3.1 % and 92.7% ± 0.9 of the cells expressing the βgal reporter also expressed GFAP protein in the DG in normoxic and hypoxic animals, respectively (Fig. 1C–E). At the same age no reporter+ cells were co-labeled with mature markers for neurons (NeuN) or oligodendrocytes (RIP) in the hippocampus of either normoxic or hypoxic conditions (Fig.1F–H and data not shown). These data are consistent with our previous examination of the cerebral cortex of GCE-CAG mice (Bi et al., 2011).
Figure 1. Reporter expression is initiated and maintained in dentate gyrus astrocytes and neural progenitors/stem cells.
GCE;R26R and GCE;CAG-EGFP hypoxic mice were analyzed at P15 after tamoxifen or vehicle injections at P12–P14. (A) Mice that received tamoxifen injections exhibited lacZ positive cells in the hippocampus (indicated with arrows). No cells were observed in response to vehicle only injections (B). (C–E), Over 90% of reporter positive cells (shown in green) are co-localized with GFAP (red) and (F–H), no reporter+ cells were co-localized with NeuN (shown in red). Scale bar, 10 μm.
Hippocampal neurogenesis from fate-mapped astroglial lineage cells increases after hypoxic injury
To assess whether hypoxic rearing alters the fate of GFAP+ cells, GCE;R26R and GCE;CAG-eGFP hypoxic and normoxic mice were injected with tamoxifen at P12–P14 and sacrificed at P35 (n=3 for both groups).
Although the data only approached statistical significance, an increase in density of reporter+Dcx+ neuronal progenitors and percentage Dcx+ cells among reporter+ cells was noted in the DG (Fig.2A–F) at P35; the percentage of βgal+ cells expressing Dcx in the DG increased from 56.4 ± 2.9 in normoxia to 68.3 ± 1.7 in hypoxia (p= 0.058). To further examine the fates of GFAP+ cells after hypoxia, we double stained reporter+ cells at P35 with markers for mature neurons and glia. The number of reporter+ NeuN+ cells in the DG at P35 was 2-fold increased in hypoxic-reared mice, implying that the number of 3-week old DG neurons born after the insult from GFAP+ precursors was significantly enhanced. The total number of fate mapped new DG neurons was 1,860 ± 360 and 3,580 ± 440 in normoxic and hypoxic animals, respectively (p<0.05; Student's t-test) (Fig 2G–L). The proportion of reporter+ cells expressing NeuN also significantly increased after hypoxic rearing, from 55.7 ± 0.5 % in normoxia to 66.2 ± 3.3 % under hypoxia (p<0.05; Student's t-test), and concomitantly the proportion of reporter + cells expressing GFAP decreased from 49.1 ± 2.2 to 35.1 ± 4.8. These data suggest that hypoxic rearing increases the proportion of fate-mapped astroglial cells that differentiate towards a neuronal fate.
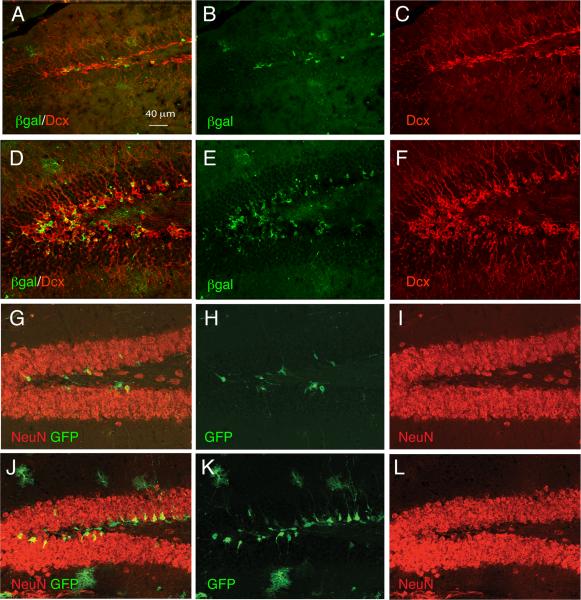
Figure 2. Increased neurogenesis from fate mapped astroglial lineage cells following hypoxia.
GCE;R26R and GCE;eGFP mice were tamoxifen-injected at P12–P14 and analyzed at P35. Hypoxic reared mice had increased numbers of reporter+/DCX+ immature neurons (D–F) and reporter+/NeuN+ mature neurons (J–L) in the dentate gyrus as compared to normoxic mice (A–C, G–I). Scale bar, 40 μm.
Environmental enrichment increases neurogenesis from GFAP+ cells
We next assessed both the ability of newly-generated neurons to survive long term and the effect of environmental enrichment on their development. For these experiments, GCE mice carrying a reporter gene were reared under normoxic or hypoxic conditions until P11 were subsequently returned to standard environment (SE) or placed in enriched environment (En) upon weaning (P21) and then sacrificed at a juvenile/young adult stage (P35) or full adulthood (P90) (see Figure 3A for a schematic outline of the experiment). For each of the 4 groups (normoxic, standard environment, NSE; normoxic, enriched environment, NEn; hypoxic, standard environment, HSE; hypoxic, enriched environment, HEn) we used the following number of mice: P35, βgal reporter: n=4 for all groups; eGFP reporter: NSE n=3; NEn n=4; HSE n= 6; HEn n= 4; P90, n=3 for all groups). We assessed the effect of enrichment on the number of newly generated neuronal cells arising from the GFAP lineage by analyzing Dcx and NeuN double staining of reporter+ cells in the DG at P35. We found that hypoxia and environmental enrichment independently increased neurogenesis from GFAP+ astroglial cells by about 4-fold, and that in hypoxic, enriched mice neurogenesis (both DCX+ and NeuN+ cells) from astroglial cells increased by more than 6-fold with respect to standard rearing (Fig. 3B–I,N,O). These changes were also reflected in the total number of eGFP+ cells, where we observed a similar pattern of results (Fig. 3Q). In addition, hypoxic enriched mice showed a mild increase in the proportion of total granule neurons that expressed GFP from 2.2% (NSE), 3.1%(NEn) and 3.4% in HSE, to 5.6% in Hen mice at P35. Finally, A nearly additive pattern of hypoxia and enrichment on NeuN+ cells was also observed at P90 (Fig. 3J–M,P).
Figure 3. Hypoxia and subsequent environmental enrichment increase the number of neurons arising from GFAP+ cells in an additive way.
(A) Schematic of the time course of hypoxia and enrichment experiment. (B–I), Representative images showing the increase in reporter+/DCX+ neurons in hypoxic, enriched mice (F–I) as compared to hypoxic, standard reared mice (B–E) at P35; stereological quantification of the reporter+/DCX+ and reporter+/NeuN+ cell numbers is shown in (N) and (O), respectively. (J–M), Long-term increase in mature reporter+/NeuN+ neurons in response to hypoxia and enrichment at P90, with stereological quantification of reporter+/NeuN+ cell numbers at P90 shown in (P). (Q), The total number of eGFP+ cells at P35 increases in hypoxic and enriched mice. (R), Proportion of reporter+ cells that attain neuronal (NeuN+), astroglial (GFAP+) and oligodendroglial (Olig2) fate are shown across groups. NSE, normoxic, standard environment; HSE, hypoxic, standard environment; NEnnormoxic, enriched environment; HEn, hypoxic, enriched environment. (*) denote significant increases from NSE, (#), denote significant difference from all other groups.
In order to assess whether there were population fate shifts in response to hypoxia and environmental enrichment, we also examined the proportion of reporter+ cells that co-expressed each of the lineage markers, NeuN, Olig2 and GFAP in the four groups at P35 and found an increase in the proportion of reporter+ cells that expressed NeuN in both hypoxic and enriched mice. This increase in proportion of GFAP+ NSCs attaining neuronal fate seemed to be at the expense of the astroglial population, as there was a concomitant decrease in the proportion of reporter+ cells expressing GFAP (Fig. 3I). In contrast to our previous observations in the cortex (Bi et al., 2011), very few GFAP+ cells attained an oligodendroglial fate in the DG: only 1 or 2 reporter+Olig2+ cells were observed in the DG per animal, and these were only seen in the enriched conditions.
Effect of hypoxia and subsequent enrichment on NSCs
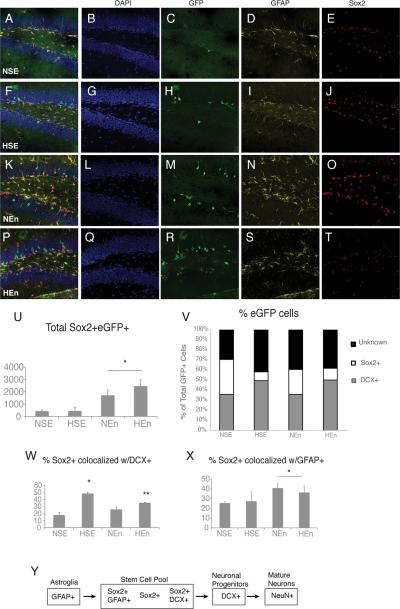
Hypoxia and environmental enrichment increase neurogenesis from GFAP+ astroglial cells in a nearly additive fashion. We wanted to understand if this prolonged increase in neuronal production depletes the DG stem cell pool or if this population was replenished and the number sustained. In order to assess this, we counted the number of reporter+ cells that expressed Sox2, a transcription factor expressed by neural stem cells and, in a minor part, by neuronal progenitors (Avilion et al., 2003; Komitova and Eriksson, 2004; Episkopou, 2005). We found that at two weeks after the onset of enrichment (P35), there was a drastic increase in the total number of fate mapped Sox2+ cells in both normoxic and hypoxic enriched groups (Fig. 4, compare A–J with K–T, quantified in U). By contrast, the total number of fate mapped Sox2+ neural stem cell population did not significantly change due to hypoxia alone (Fig. 4, compare A–E with F–J, quantified in U).
Figure 4. Hypoxia pushes stem cells to a neuronal fate, whereas enrichment increases the total stem cell pool.
(A–T), Representative Sox2/GFAP/eGFP immunostaining for all four groups; note the increase in number of Sox2+ cells expressing GFAP with environmental enrichment. (U), Environmental enrichment also increased the number of reporter+/Sox2+ cells in both normoxic and hypoxic-reared mice. (V) Proportion of reporter+ cells that attain an immature neuronal (DCX+) or stem cell (Sox2+) fate is shown across groups. NSE, normoxic, standard environment; HSE, hypoxic, standard environment; NEn, normoxic, enriched environment; HEn, hypoxic, enriched environment. (W), The percentage of Sox2+ stem cells that were committed to a neuronal lineage (co-localized with DCX) increased with hypoxic rearing, and this effect was attenuated with enrichment. (X), The percentage of Sox2+ stem cells that remained in the NSC lineage (co-localized with GFAP) increased with enrichment. (Y), Schematic representation of cell lineages arising from fate mapped GFAP+ astroglia. (*) denote significant increases from NSE, (**) denote significant increase from both NSE and HSE groups; (—) denote significance from all other groups).
In order to understand if there are fate shifts within the neural stem cell population, we also examined the proportion of eGFP+ cells that expressed neural stem cell and progenitor markers. We found a decrease in the proportion of eGFP+ cells expressing Sox2+ in both hypoxic, standard environment-reared mice and in hypoxic enriched mice. The decrease in the proportion of eGFP+/Sox2+ cells was concomitant to an increase in the proportion of eGFP cells expressing Dcx following hypoxia, with and without enrichment (Fig. 4V). In summary, hypoxia leads to an expansion of DCX+ neuroblast pool but has no effects on the number of Sox2+ cells.
In order to examine whether the shift in Sox2+ cells towards neuronal fate is also true of the total Sox2+ cell population (including both reporter+ and reporter- cells), we counted the proportion of total Sox2+ cells that were co-labelled with GFAP or Dcx (Fig. 4W,X, and scheme in Y). We found that the percentage of Sox2+ cells that were also Dcx+ increased significantly in hypoxic, standard environment-reared mice, as well as in hypoxic enriched mice, although post-hoc analysis showed that this last group remained significantly lower than the hypoxic, standard reared mice (Fig. 4W). In addition, enrichment increased the proportion of Sox2+ cells that were co-labeled with GFAP, suggesting an increase in the stem cell pool (Fig. 4X). Together, it would seem that hypoxia induces a global shift towards a neurogenic fate, that is not specific to the fate mapped population.
Mechanisms of increased stem cell number in environmental enrichment
The increased neural stem cell number observed in response to environmental enrichment in the current study could be attained through increases in stem cell proliferation, stem cell survival or a combination of both. To understand whether the increase in the stem cell pool in response to enrichment was due to changes in stem cell proliferation, we assessed the total number and proportion of Sox2+ cells that co-express Ki67, a marker of cell proliferation at P35. As compared to animals reared in standard conditions, both enriched groups exhibited almost four times the total number of Sox2+Ki67+ cells (Fig. 5A–G), as well as a significant increase in the proportion of proliferating stem cells (Fig. 5H).
Figure 5. Effect of hypoxia and environmental enrichment on stem cell proliferation.

GCE;R26R mice reared under normoxic or hypoxic conditions followed by environmental enrichment as indicated were analyzed at P35. (A–H) Representative double staining of Sox2 and Ki67 at P35. Stereological quantification showed an increase in the total number (G) and proportion (H) of proliferating Sox2+ cells in response to environmental enrichment. No increase in stem cell proliferation was observed in response to hypoxic rearing (G–H). NSE, normoxic, standard environment; HSE, hypoxic, standard environment; NEn, normoxic, enriched environment; HEn, hypoxic, enriched environment. (*) denote significance from all other groups.
To further understand whether increased stem cell proliferation and survival contributed to our observations, we injected normoxic mice with two thymidine analogues: CldU given at P15&P16 and IdU given 1hr prior to sacrifice. CldU, when incorporated in cells during their last mitotic division, allows us to examine the long-term survival of cells labeled prior to enrichment; and IdU, injected one hour prior to sacrifice at P35, was given to examine cell proliferation (NSE, n=4; NEn, n=3). Co-expression of both of these markers in Sox2+ cells (Sox2+CldU+ and Sox2+IdU+) was increased approximately 3-fold in enriched mice at P35, two weeks after the onset of enrichment (Fig. 6). This suggests that both stem cell survival and cell proliferation contribute to the increased neurogenesis observed in response to environmental enrichment and that enhanced neurogenesis did not deplete the Sox2+ progenitor pool.
Figure 6. Environmental enrichment increases stem cell proliferation and survival.

GCE;R26R and GCE;eGFP normoxic-reared mice were tamoxifen injected from P12–14, subsequently injected with CldU from P15–16 (to examine cell survival) (see schematic outline in Fig. 4A), then reared in enriched or standard environment from P21–P35 and injected with IdU one hour before sacrifice (to examine cell proliferation) at P35. Both Sox2+ cell proliferation (A–C) and Sox2+ cell survival (D–F) were significantly increased in enriched mice. (*) denote significant increases from NSE.
Behavioral Testing
The four groups were tested for spatial memory using the Morris water maze task (NSE, n=9; NEn, n=10; HSE, n=6; HEn, n=9). Results showed that the rate of acquisition of new spatial information on the Morris water maze was significantly slower for the hypoxic, standard-reared mice: 50% of the mice were successful in reaching the platform within 60 seconds only by the 7th trial, whereas 50% of normoxic, standard-reared mice were successful by the end of the 4th trial. Interestingly, when hypoxic mice were reared in an enriched environment, they were no different from normoxic, standard-reared mice. The normoxic-enriched group reached criterion the fastest, by the second trial (Fig. 7A). During the probe trial for memory retention, hypoxic, standard-reared mice showed a decreased preference for the target quadrant, indicating decreased recall for the platform location, whereas those hypoxic mice reared in an enriched environment were no different from both normoxic groups (Fig. 7B,C).
Figure 7. Environmental enrichment reverses hypoxia- induced cognitive deficits.
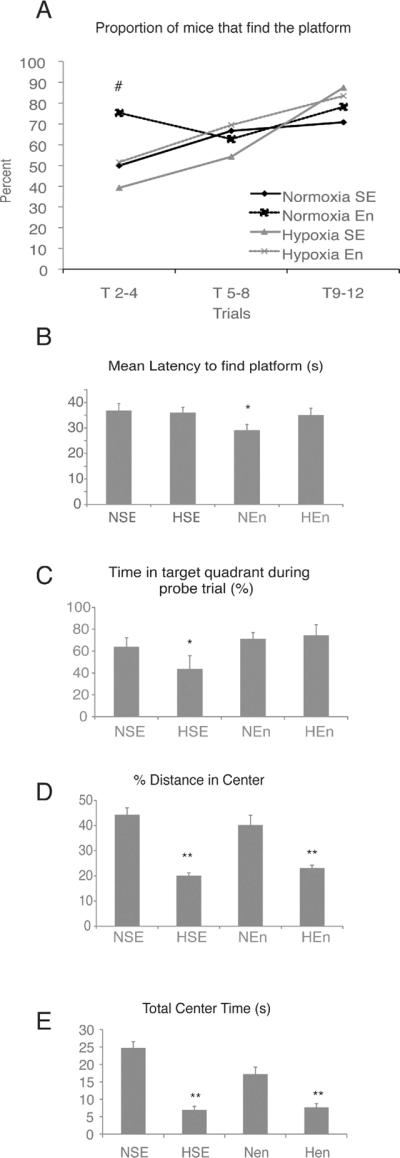
Mice were reared in normoxia or hypoxia form P3–P11, and subsequently in standard or enriched environment from P21 –P35. Behavioral testing was conducted on P35–36. (A–C) show the results of the Morris water maze. (A), displays the proportion of mice that successfully found the platform in under 60 seconds, averaged across six trials, during the acquisition phase on the maze. (B), latency to find the platform across trials and (C), probe trial. (D–E) show results from the open field test. (D), proportion of distance travelled in the center/total distance travelled; (E), time spent in the center. NSE, normoxic, standard environment; HSE, hypoxic, standard environment; NEn, normoxic, enriched environment; HEn, hypoxic, enriched environment. (*) denotes significance from all other groups, (**) denotes significance from NSE and NEn groups, (#) denotes that HSE and NEn are different from all other groups.
In addition to cognitive deficits, VLBW children have also been shown to have an increased risk for anxiety disorders throughout the lifespan (Johnson et al., 2010; Lund et al., 2011). In order to assess whether our model of perinatal hypoxia also results in increased anxiety behavior, we assessed mice in the open field task at P35. We found that mice exposed to hypoxic conditions showed increased anxiety-like behavior, spending less time and travelling less distance in the center of the open field, regardless of whether mice were environmentally enriched subsequent to hypoxic injury (Fig. 7D,E). Taken together, these data demonstrate that hypoxic-rearing results in cognitive and emotional deficits, and that although the memory deficits can be reversed through as little as two weeks of environmental enrichment following hypoxic injury, anxiety behavior remains elevated regardless of enrichment status.
Discussion
The current studies used assays for cell proliferation, BrdU birthdating studies and genetic fate mapping techniques to examine the fate of hippocampal GFAP+ astroglial cells after perinatal hypoxic insult and subsequent rearing in standard or enriched environment. We show that GFAP+ cells in the juvenile period (P12–P15) generate astrocytes and neurons in the hippocampal DG, consistent with previous data indicating that hippocampal NSCs express GFAP. Chronic perinatal hypoxia shifts the long-term differentiation of juvenile GFAP+ cells from an astrocytic to a neuronal fate. Rearing juvenile animals in an enriched environment, regardless of previous hypoxic experience, induced a long-lasting 6-fold increase in the total number of hippocampal neurons generated from astroglial cells in the DG, which persisted until adulthood. We have found that this effect of enrichment could be attributed to an increase in the stem cell pool due to increased NSC proliferation and survival.
The mouse model of perinatal hypoxia that we employ is unique in that is probably the best model for brain injury in VLBW premature children (Vaccarino and Ment, 2004; Scafidi et al., 2009), which are now approximately 1.5% of all children born in the USA (Ment and Constable, 2007; Luu et al., 2009). Unlike models of neonatal stroke, chronic hypoxic injury is a more generalized insult; it does not seem to induce local acute effects such as reactive gliosis or focal leukomalacia, although it does have abnormalities in white matter development including delayed maturation of oligodendrocytes and abnormal myelination (Jablonska et al, submitted). Interestingly, amongst the best predictors of positive outcome in VLBW children are a two-parent household and an increased level of maternal education (Ment et al., 2003). This raises the important question of the mechanisms by which increased environmental stimulation of VLBW children months and years after the perinatal insult improves recovery (Ment et al., 2003). It has been well-documented in adult rodents that environmental enrichment not only increases cell proliferation and neurogenesis, but increases the ability of new neurons to differentiate, integrate and mature in the DG (Kempermann et al., 2004). However, the cognitive and neurobiological effect of environmental enrichment during development, and in particular its ability to interact with prior hypoxic injury, has not been investigated. Our fate mapping studies show that environmental enrichment for 2 weeks increased the number of neurons generated from astroglial cells in the DG at P35, but did not augment the neurogenic effect of hypoxia. Interestingly, extending the enrichment until full adulthood (P90) increased neurogenesis in both normoxic and hypoxic mice, and the effects of hypoxia and enrichment appeared independent and additive. It is likely that the increase in total number of fate-mapped neurons described here is a gross underestimation of the neurogenesis that occurs in response to hypoxia and environmental enrichment. The population of fate-mapped neurons that we observe arise from GFAP+ cells labeled from P12–P14 only; furthermore, the targeting efficiency of the GCE transgene is not 100% (Ganat et al., 2006). Therefore, they likely represent a small fraction of total neurogenesis induced by hypoxia and enrichment in these mice. Indeed, at P35, we show that the proportion of fate-mapped neurons relative to the total number of granule neurons ranges across groups from approximately 2% to 6%, whereas a recent study by Cushman et al (2012) demonstrates that the proportion of total DG neurons born from GFAP+ cells was 24% at 2 months of age, suggesting that we are capturing about 1/4 of the total number of neurons arising from GFAP+ precursors described by (Cushman et al., 2012).
Increased neurogenesis can be attributed to a shift in fate of the GFAP+ stem cells towards the neuronal lineage, or to a change in progenitor proliferation or neuronal survival. Moreover, increased neurogenesis will, over the long term, deplete the astroglial stem cell population, unless these cells self-renew via asymmetric division. We find that hypoxia induced a fate shift in juvenile GFAP+ cells, away from the astroglial lineage and in favor of the DCX neuronal lineage (Fig. 4V). Although a similar increase in total number of DCX+/NeuN+ cells was seen in response to environmental enrichment, no fate shift towards the Dcx lineage was observed in normoxic enriched animals. Our results show that environmental enrichment causes a robust increase in size of the astroglial stem cell population that is attributable to enhanced NSC self-renewal as well as survival, as shown by the Ki67 double labeling and CldU/IdU incorporation experiments. Hypoxic-rearing on its own does not increase the stem cell pool and in fact, hypoxia increases the proportion of GFAP+ and Sox2+ stem cells that co-express the neuronal progenitor marker Dcx (Fig. 4V,W), suggesting that more of these cells are committing to a neuronal fate. Subsequent exposure to environmental enrichment increased the proportion of Sox2+ cells co-expressing GFAP (Fig. 4X) as well as the total number of stem cells (Fig. 4U), suggesting that both a fate shift towards the neuronal lineage and an increase in the number of stem cells may be acting together in hypoxic enriched mice, accounting for the additive effect on the number of newly generated neurons.
Together, our results suggest that hypoxia alone increases neurogenesis by pushing the stem cell population towards a neuronal fate, whereas enrichment increases the total stem cell population. Consequently, hypoxia coupled with enrichment enables neurogenesis from GFAP+ astroglia to rise, without depleting the population of Sox2+ neural stem cells and maintaining their proportion relative to neuroblasts (see Fig. 8). Thus, the robust long-term increase in neurogenesis that we have observed in hypoxic enriched mice can be attributed to the hypoxia-induced neurogenic shift coupled with an overall increase in the stem cell pool observed in response to environmental enrichment. Interestingly, the increase in stem cells we have observed in response to enriched environment may be unique to the early phase of enrichment. In a recent study, Hen and colleagues employed a similar genetic fate mapping model driven by a Nestin-cre promoter and demonstrated that exposing mice to long-term environmental enrichment in adulthood leads to a similar decrease in GFAP+/reporter+ cells while increasing NeuN+/reporter+ cells; however, unlike our current study, they did not observe a significant increase in the total number of reporter+ stem cells in response to environmental enrichment (Dranovsky et al., 2011). The differential response of stem cells to enrichment observed between these studies may reflect differences in the age at which environmental enrichment was commenced (P21 in our study versus adult); however, it is also possible that it is a transient effect in response to the early phase of enrichment, and perhaps, to physical exercise with the running wheel (van Praag et al., 1999a; Kobilo et al., 2011). Indeed, a recent study examining the fate of hippocampal Hes5+ cells in adult mice that were given free access to a running wheel for 12 days showed increased proliferation of reporter+ stem cells in mice with running wheel access as compared to controls (Lugert et al., 2010). Taken together, it seems more likely that at least in the hippocampus, the number of stem cells increase during the early phases of environmental enrichment; however, more detailed future studies examining the relative contribution of hippocampal GFAP+ stem cells in response to environmental enrichment while controlling for age of onset, duration and access to physical activity are needed to answer these questions.
Figure 8. Schematic representation depicting hypoxia and enrichment induced changes in astroglial lineage.
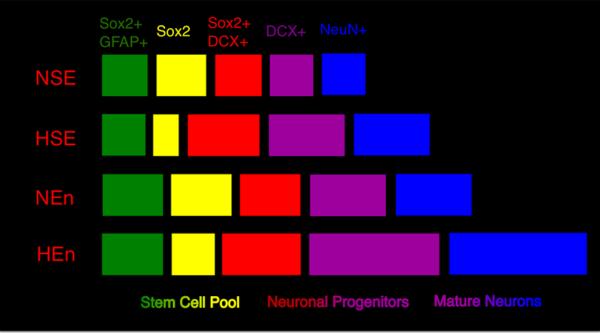
There is an increase in the number of neuronal cells in hypoxic-reared (HSE) as well as normoxic enriched (NEn) mice, shown in purple (DCX+) and blue (NeuN+). HEn mice show the greatest increase in total number of reporter+ neurons, suggesting an additive effect of hypoxia and enrichment on neurogenesis arising from GFAP+ cells. HSE mice show a greater proportion of the stem cell pool that are commited to the neuronal lineage (Sox2+DCX+, shown in red), this effect remains apparent in hypoxic mice that were subsequently enriched. In NEn mice there is no change in fate potential of NSC but rather a robust increase in the total stem/progenitor cell pool (shown in green, yellow, red) which is still present in Hen mice. NSE, normoxic, standard environment; HSE, hypoxic, standard environment; NEn, normoxic, enriched environment; HEn, hypoxic, enriched environment.
The relative contribution of increasing the number of stem cells versus neurons to cognitive amelioration in hypoxic enriched mice remains to be determined. In the current study, hypoxic mice show a similar increase in neurogenesis as do normoxic, enriched mice, yet hypoxic mice show a slower rate of acquisition and impaired retention, suggesting that hippocampal neurogenesis alone cannot account for the differences seen in cognitive performance. These findings are in support of previous work showing that the role of neurogenesis in learning and memory, as revealed by the Morris water maze task, is not clearly defined, with some studies finding a significant relationship and others not (Shors et al., 2002). Furthermore, it is also likely that behavioral changes in response to both hypoxia and environmental enrichment within other brain regions, such as white matter, cortex or the SVZ may contribute to our behavioral findings (Bi et al., 2011).
On the other hand, the increased performance seen in both enriched groups could be related to the increased stem cell pool also observed in these groups, albeit whether this is causally related remains to be determined. Although the current paper has focused on the hippocampus and GFAP+ cell fate, environmental enrichment impacts other mechanisms of neural plasticity in the hippocampus that could also play a causal role in enhanced learning and memory, including synaptogenesis, inhibitory tone and dendritic spine formation (Johansson and Belichenko, 2002; Sale et al., 2007; Bednarek and Caroni, 2011).
Longitudinal studies of VLBW children have shown that these children are more likely to be diagnosed with an anxiety disorder, even decades later (Johnson et al., 2010; Lund et al., 2011). Results from the open field test also showed increased anxiety in our mouse model. Interestingly, these effects were not ameliorated with short-term environmental enrichment, suggesting that anxiety behavior after hypoxic injury is mediated by different neurobiological changes that are not responsive to short-term enrichment.
In conclusion, we demonstrate that subsequent environmental enrichment after hypoxic injury reverses the cognitive deficits consequential to hypoxic injury. Furthermore, hypoxic injury and environmental enrichment together increase neurogenesis in the dentate gyrus in an additive, long-term manner. Environmental enrichment increases the total stem cell population through proliferation and survival, which ultimately leads to sustained increases in neurogenesis in hypoxic, enriched mice.
Acknowledgements
We acknowledge Teresa Sandoval-Minero, Eylem Ocal, Ellen Hoffman, Lauren Provini, Devon Fagel, and Allyson Vermaak for technical assistance and useful discussions. We thank Dr. David Mumby for his advice on alternatives to testing a traditional Morris water maze paradigm. We wish to thank Drs. Charles Stiles and John Alberta, Dana-Farber Cancer Institute, Boston, MA for their gift of the Olig2 antibody. This work was funded by NIH (P01 NS062686 and R21 AG034495 to F.M.V.), a PSD2 Fellowship from the Fonds de recherché en santé du Quebec to N.S, a Swedish Brain Foundation (Hjarnfonden) and a Swedish Medical Association fellowship to M.K.
Footnotes
Conflict of Interest: none
References
- Alvarez-Buylla A, Seri B, Doetsch F. I}dentification of neural stem cells in the adult vertebrate brain. Brain Research Bulletin. 2002;57:751–758. doi: 10.1016/s0361-9230(01)00770-5. [DOI] [PubMed] [Google Scholar]
- Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek E, Caroni P. beta-Adducin is required for stable assembly of new synapses and improved memory upon environmental enrichment. Neuron. 2011;69:1132–1146. doi: 10.1016/j.neuron.2011.02.034. [DOI] [PubMed] [Google Scholar]
- Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S, Gallo V. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol. 2003;161:169–186. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi B, Salmaso N, Komitova M, Simonini MV, Silbereis J, Cheng E, Kim J, Luft S, Ment LR, Horvath TL, Schwartz ML, Vaccarino FM. Cortical glial fibrillary acidic protein-positive cells generate neurons after perinatal hypoxic injury. J Neurosci. 2011;31:9205–9221. doi: 10.1523/JNEUROSCI.0518-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner M. Structure and transcriptional regulation of the GFAP gene. Brain Pathol. 1994;4:245–257. doi: 10.1111/j.1750-3639.1994.tb00840.x. [DOI] [PubMed] [Google Scholar]
- Brill MS, Snapyan M, Wohlfrom H, Ninkovic J, Jawerka M, Mastick GS, Ashery-Padan R, Saghatelyan A, Berninger B, Gotz M. A dlx2- and pax6-dependent transcriptional code for periglomerular neuron specification in the adult olfactory bulb. J Neurosci. 2008;28:6439–6452. doi: 10.1523/JNEUROSCI.0700-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman JD, Maldonado J, Kwon EE, Garcia AD, Fan G, Imura T, Sofroniew MV, Fanselow MS. Juvenile neurogenesis makes essential contributions to adult brain structure and plays a sex-dependent role in fear memories. Front Behav Neurosci. 2012;6:3. doi: 10.3389/fnbeh.2012.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular Zone Astrocytes are Neural Stem Cells in the Adult Mammalian Brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Dranovsky A, Picchini AM, Moadel T, Sisti AC, Yamada A, Kimura S, Leonardo ED, Hen R. Experience dictates stem cell fate in the adult hippocampus. Neuron. 2011;70:908–923. doi: 10.1016/j.neuron.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Episkopou V. SOX2 functions in adult neural stem cells. Trends Neurosci. 2005;28:219–221. doi: 10.1016/j.tins.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Fagel DM, Ganat Y, Silbereis J, Ebbitt T, Stewart W, Zhang H, Ment LR, Vaccarino FM. Cortical neurogenesis enhanced by chronic perinatal hypoxia. Exp Neurol. 2006;199:77–91. doi: 10.1016/j.expneurol.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Filippov V, Kronenberg G, Pivneva T, Reuter K, Steiner B, Wang LP, Yamaguchi M, Kettenmann H, Kempermann G. Subpopulation of nestin-expressing progenitor cells in the adult murine hippocampus shows electrophysiological and morphological characteristics of astrocytes. Mol Cell Neurosci. 2003;23:373–382. doi: 10.1016/s1044-7431(03)00060-5. [DOI] [PubMed] [Google Scholar]
- Ganat YM, Silbereis J, Cave C, Ngu H, Anderson GM, Ohkubo Y, Ment LR, Vaccarino FM. Early postnatal astroglial cells produce multilineage precursors and neural stem cells in vivo. J Neurosci. 2006;26:8609–8621. doi: 10.1523/JNEUROSCI.2532-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B. The new stereological tools: disector, fractionator, nucleator, and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Hack MA, Saghatelyan A, de Chevigny A, Pfeifer A, Ashery-Padan R, Lledo PM, Gotz M. Neuronal fate determinants of adult olfactory bulb neurogenesis. Nat Neurosci. 2005;8:865–872. doi: 10.1038/nn1479. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Hodge RD, Daza RA, Englund C. Transcription factors in glutamatergic neurogenesis: conserved programs in neocortex, cerebellum, and adult hippocampus. Neurosci Res. 2006;55:223–233. doi: 10.1016/j.neures.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Imura T, Kornblum HI, Sofroniew MV. The predominant neural stem cell isolated from postnatal and adult forebrain but not early embryonic forebrain expresses GFAP. J Neurosci. 2003;23:2824–2832. doi: 10.1523/JNEUROSCI.23-07-02824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson BB, Belichenko PV. Neuronal plasticity and dendritic spines: effect of environmental enrichment on intact and postischemic rat brain. Journal of Cerebral Blood Flow & Metabolism. 2002;22:89–96. doi: 10.1097/00004647-200201000-00011. [DOI] [PubMed] [Google Scholar]
- Johnson S, Hollis C, Kochhar P, Hennessy E, Wolke D, Marlow N. Psychiatric disorders in extremely preterm children: longitudinal finding at age 11 years in the EPICure study. J Am Acad Child Adolesc Psychiatry. 2010;49:453–463. e451. [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr Opin Neurobiol. 2004;14:186–191. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Kernie SG, Parent JM. Forebrain neurogenesis after focal Ischemic and traumatic brain injury. Neurobiol Dis. 2010;37:267–274. doi: 10.1016/j.nbd.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilo T, Liu QR, Gandhi K, Mughal M, Shaham Y, van Praag H. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn Mem. 2011;18:605–609. doi: 10.1101/lm.2283011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komitova M, Eriksson PS. Sox-2 is expressed by neural progenitors and astroglia in the adult rat brain. Neurosci Lett. 2004;369:24–27. doi: 10.1016/j.neulet.2004.07.035. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Palmer TD, Fuchs E. Adult neurogenesis: a compensatory mechanism for neuronal damage. European Archives of Psychiatry & Clinical Neuroscience. 2001;251:152–158. doi: 10.1007/s004060170035. [DOI] [PubMed] [Google Scholar]
- Lugert S, Basak O, Knuckles P, Haussler U, Fabel K, Gotz M, Haas CA, Kempermann G, Taylor V, Giachino C. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell. 6:445–456. doi: 10.1016/j.stem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Lugert S, Basak O, Knuckles P, Haussler U, Fabel K, Gotz M, Haas CA, Kempermann G, Taylor V, Giachino C. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell. 2010;6:445–456. doi: 10.1016/j.stem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Lund LK, Vik T, Skranes J, Brubakk AM, Indredavik MS. Psychiatric morbidity in two low birth weight groups assessed by diagnostic interview in young adulthood. Acta Paediatr. 2011;100:598–604. doi: 10.1111/j.1651-2227.2010.02111.x. [DOI] [PubMed] [Google Scholar]
- Luu TM, Vohr BR, Allan W, Schneider KC, Ment LR. Evidence for catch-up in cognition and receptive vocabulary among adolescents born very preterm. Pediatrics. 2011;128:313–322. doi: 10.1542/peds.2010-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu TM, Ment LR, Schneider KC, Katz KH, Allan WC, Vohr BR. Lasting effects of preterm birth and neonatal brain hemorrhage at 12 years of age. Pediatrics. 2009;123:1037–1044. doi: 10.1542/peds.2008-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ment LR, Constable RT. Injury and recovery in the developing brain: evidence from functional MRI studies of prematurely born children. Nat Clin Pract Neurol. 2007;3:558–571. doi: 10.1038/ncpneuro0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ment LR, Vohr B, Allan W, Katz KH, Schneider KC, Westerveld M, Duncan CC, Makuch RW. Change in cognitive function over time in very low-birthweight infants. Jama. 2003;289:705–711. doi: 10.1001/jama.289.6.705. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Colbert MC, Robbins J. Neural crest cells retain multipotential characteristics in the developing valves and label the cardiac conduction system. Circ Res. 2006;98:1547–1554. doi: 10.1161/01.RES.0000227505.19472.69. [DOI] [PubMed] [Google Scholar]
- Nunez J. Morris Water Maze Experiment. J Vis Exp. 2008 doi: 10.3791/897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson AK, Eadie BD, Ernst C, Christie BR. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16:250–260. doi: 10.1002/hipo.20157. [DOI] [PubMed] [Google Scholar]
- Parent JM. Injury-induced neurogenesis in the adult mammalian brain. Neuroscientist. 2003;9:261–272. doi: 10.1177/1073858403252680. [DOI] [PubMed] [Google Scholar]
- Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- Sale A, Maya Vetencourt JF, Medini P, Cenni MC, Baroncelli L, De Pasquale R, Maffei L. Environmental enrichment in adulthood promotes amblyopia recovery through a reduction of intracortical inhibition. Nat Neurosci. 2007;10:679–681. doi: 10.1038/nn1899. [DOI] [PubMed] [Google Scholar]
- Scafidi J, Fagel DM, Ment LR, Vaccarino FM. Modeling premature brain injury and recovery. Int J Dev Neurosci. 2009;27:863–871. doi: 10.1016/j.ijdevneu.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz C, Hof PR. Design-based stereology in neuroscience. Neuroscience. 2005;130:813–831. doi: 10.1016/j.neuroscience.2004.08.050. [DOI] [PubMed] [Google Scholar]
- Seki T, Arai Y. Temporal and spacial relationships between PSA-NCAM-expressing, newly generated granule cells, and radial glia-like cells in the adult dentate gyrus. Journal of Comparative Neurology. 1999;410:503–513. doi: 10.1002/(sici)1096-9861(19990802)410:3<503::aid-cne11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. Journal of Neuroscience. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp FR, Liu J, Bernabeu R. Neurogenesis following brain ischemia. Brain Res Dev Brain Res. 2002;134:23–30. doi: 10.1016/s0165-3806(01)00286-3. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature Gen. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Vaccarino FM, Ment LR. Injury and repair in developing brain. Arch Dis Child Fetal Neonatal Ed. 2004;89:F190–192. doi: 10.1136/adc.2003.043661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999a;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neuroscience. 1999b;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- West MJ. New Stereological Methods for Counting Neurons. Neurobiology of Aging. 1993;14:275–285. doi: 10.1016/0197-4580(93)90112-o. [DOI] [PubMed] [Google Scholar]
- Yang Z, Levison SW. Hypoxia/ischemia expands the regenerative capacity of progenitors in the perinatal subventricular zone. Neuroscience. 2006;139:555–564. doi: 10.1016/j.neuroscience.2005.12.059. [DOI] [PubMed] [Google Scholar]