Abstract
The tumor-associated carbohydrate antigen/hapten Thomsen-nouveau (Tn; α-D-GalpNAc-ONH2) was conjugated to a zwitterionic capsular polysaccharide, PS A1, from commensal anaerobe Bacteroides fragilis ATCC 25285/NCTC 9343 for the development of an entirely carbohydrate cancer vaccine construct and probed for immunogenicity. This communication discloses that murine anti-Tn IgG3 antibodies both bind to and recognize human tumor cells that display the Tn hapten. Furthermore, the sera from immunization of mice with Tn-PS A1 contain cytokine interleukin 17 (IL-17A), which is known to possess anti-tumor function and represents a striking difference to an IL-2, and IL-6 profile obtained with anti-PS A1 sera.
Keywords: Carbohydrates, Antigen, Tumor, Vaccine, Interleukin 17, Flow cytometry
Introduction
The study of carbohydrate immune processing has remained an active research area for 75 years dating back to Landsteiner, Avery, and Goebel who discovered that azoproteins could be used as carriers to overcome carbohydrate T cell independence [1, 2]. Exhaustive studies led to the development of glycoprotein bacterial-based vaccines that are currently commercialized [3]. Consequently, these glycoprotein conjugate approaches also led to numerous carbohydrate-based vaccine design and development strategies to eradicate cancer [4]. Although no antitumor vaccine currently exists that specifically targets cancer cells, it is important to note that after antigen-presenting cell (APC) recognition and proteolytic processing, glycopeptide and polysaccharide fragments can be presented for the identification by T cells via the major histocompatibility complex (MHC) class II binding proteins [5]. This then leads to the secretion of cytokines, which are small cell-signaling proteins, from immune cells which can reinforce specific Ab production against the antigenic fragment displayed on the surface of APCs [6].
Paradoxically, the use of carrier proteins for the assembly of carbohydrate-based cancer glycoconjugate vaccines can elicit an immune response against the carrier protein itself resulting in the suppression of carbohydrate-specific Ab production [7]. The large protein will contain immunosuppressive epitopes, which have been shown to reduce the effectiveness of the construct altogether. In addition, glycoprotein conjugation chemistry is often difficult to control leading to constructs with ambiguities in structure and composition affecting reproducibility [8].
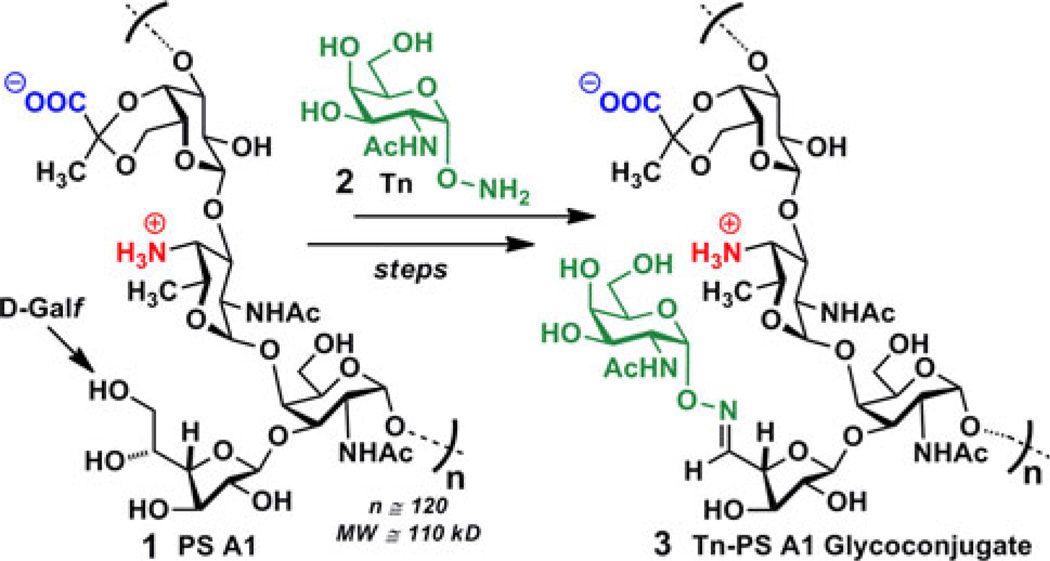
In the search for new, effective carbohydrate-based cancer vaccines that circumvent a myriad of glycoprotein obstacles, we have elected to pursue an alternative strategy for carbohydrate-specific T cell stimulation that aims to eliminate the use of carrier proteins. Upon the discovery that an alternating charge character on adjacent monosaccharide units in a repeating tetrasaccharide capsular polysaccharide PS A1 (1) from Bacteroides fragilis (B. fragilis) ATCC 25285/NCTC 9343 could elicit an MHC II T cell-dependent immune response in the absence of proteins/peptides or lipids [9], we recognized the potential of a new T cell stimulating immunogen that could overcome the aforementioned challenges and concomitantly increase immunogenicity of the target carbohydrate Ag. Our previous work demonstrated that a Tn-PS A1 immunogen could indeed elicit murine Abs that were specific and selective for the Tn hapten [10]. Our findings were highlighted by the fact that murine IgG3 Abs are known to result from an all important isotype-switching event [11] and are carbohydrate specific [12]. They are also a highly potent complement activator of their class [13, 14]. Our earlier publication eluded to the current studies we are disclosing herein, in which FACS was used to determine IgG3 Ab binding to human cancer cell lines known to possess the Tn surface Ag [15–17]. Furthermore, analysis of murine sera, using a BD™ Biosciences Cytometric Bead Array, allowed for the determination of a cytokine profile, beneficial for immune mechanistic insights into Tn-PS A1 glycoconjugate 3. The latter was prepared by selective periodate oxidation of the D-Galf units of PS A1 followed by condensation with Tn derivative 2 [10].
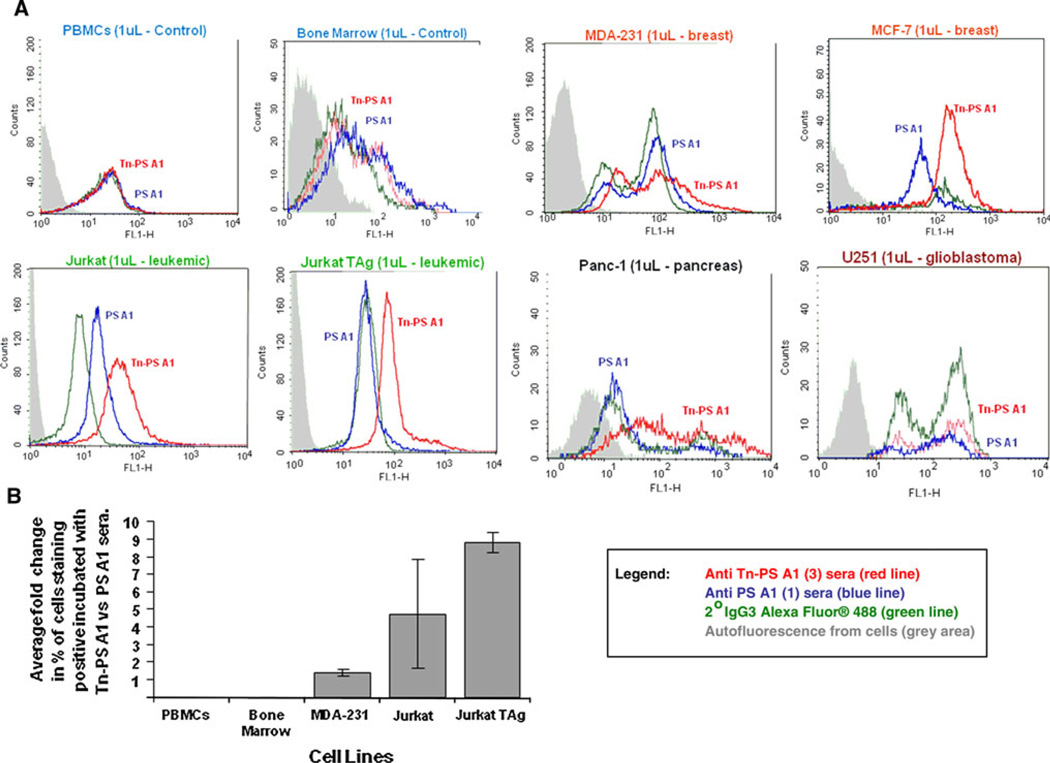
The anti-sera were tested against Tn-expressing human breast cancer cell lines MCF-7 and MDA-231 using FACS. In addition, cell lines Jurkat/JurkatTAg (leukemia), Panc-1 (pancreas), and U251 (glioblastoma) were also examined for Ab binding. As negative controls, human peripheral blood mononuclear cells (PBMCs) and human bone marrow cells were employed. Cells were first incubated for 30 min with anti-sera, diluted 1:250, at 4°C in respective growth media containing 5% FCS and 0.1% sodium azide. Cells were incubated (30 min, on ice, in the dark) with a secondary goat anti-murine IgG3 monoclonal Ab conjugated with the fluorochrome Alexa Fluor® 488. Following incubation, cells were fixed using 1% paraformaldehyde and analyzed on a LSR flow cytometer. Cells were gated based on light scatter properties using forward versus side scatter, and a total of 10,000 events were collected and analyzed using CellQuest software V5.2. Figure 1 shows that anti-Tn sera (red line) from C57BL/6-primed mice with Tn-PS A1 (3) binds, to varying degrees, in five of the six cancer cells examined. Both the PBMCs and bone marrow cells, which do not contain tumor-associated carbohydrate antigens (TACAs), showed negligible Ab binding. More importantly, there was also negligible binding to cancer cells when anti-sera (blue line) from 1 were used as a comparison to 3. When the percentage of positive stained cells, incubated with sera from Tn-PS A1-inoculated mice versus PS A1-treated mice, was compared, a greater than 1 to nine fold increase in staining was observed (Fig. 1b). Although the results did not meet statistical significance, t test, the trend is suggestive that the staining was not due to nonspecific binding of antibody present in sera from Tn-PS A1-injected animals. In combination with previous inhibitory experiments from our group, these studies provide strong evidence for an IgG3 Tn-selective immune response [10], and based on literature precedent regarding the IgG3 Ab, it is well-known to be carbohydrate selective [12] and arises as a result of isotype switching [11]. This result also establishes that an entirely glyco-comprised conjugate 3 will elicit a specific immune response against the Tn antigen as compared to the anti-sera from PS A1-primed mice, in which structure contains a GalpNAc unit in the repeating tetrasaccharide (see Scheme 1, 1). Furthermore, the “small” carbohydrate Tn hapten appended to the adjuvant/carrier PS A1 does not hinder an immune response, and apparent from the data is that 3 can elicit an Ab response that targets surface Tn as indicated by our FACS studies. Importantly, invasive U251 glioblastoma cells contain a substantial amount of surface polysialiac acid, rather than other commonly known TACAs, such as the Tn antigen, and therefore, no noticeable IgG3 binding was observed [18].
Fig. 1.
a Flow cytometry results using human tumor cell lines expressing the Tn hapten (2). Anti-sera (diluted at 1:250) from Tn-PS A1 (3)-primed C57BL/6 mice (red trace) and anti-sera (diluted at 1:250) from PS A1 (1)-primed C57BL/6 mice (blue trace) along with staining agent 2° IgG3 Ab conjugated with Alexa Fluor® 488 dye (green trace). FACS analysis shows Ab binding to MDA-231, MCF-7, Jurkat, JurkatTAg, and Panc-1 but not glioblastoma U251 (see discussion). PBMCs and bone marrow cells were used as controls. Results are representative of two replicate experiments. b Percentage of cells staining positive treated with sera from Tn-PS A1-inoculated animals compared with the percentage of cells staining with sera from PS A1 vaccinated animals. Percent of positive stained cells incubated with Tn-PS A1 sera were divided by those obtained from cells incubated with PS A1. Results represent the fold change from the average of two replicate experiments
Scheme 1.
The Tn-PS A1 construct (3) from the glycoconjugation of PS A1 (1) with Tn hapten (2)
We next examined the cytokine profile for both the anti-Tn-PS A1 sera and the anti-PS A1 sera to ascertain important insights related to the general immune response and the IgG3 Ab. We focused on seven important T cell immune cytokines, IL-10, IL-17A, TNF, IFN-γ, IL-6, IL-4, and IL-2 as noted in Table 1. This grouping of cytokines allows for the determination of T cell polarization toward a Th1, Th2, or a Th17 direction. Based on our findings, we noted that anti-sera from PS A1 immunizations had a significant amount of pro-inflammatory IL-2 (118 pg/mL) and anti/pro-inflammatory IL-6 (62 pg/mL) plus notable amounts of pro-inflammatory IL-17A and anti-inflammatory IL-10. This result infers a Th1/Th2 paradigm for inflammation and coincides well with those results published by Mazmanian et al. describing anti-inflammatory IL-10-producing CD4+ T cells from administered PS A1 in mice for the potential treatment of colitis [19, 20]. Remarkably, anti-sera from Tn-PS A1 immunizations had an entirely different profile as the notable pro-inflammatory cytokine IL-17A (58 pg/mL), which is known to be produced in CD4+ effector T cells, was observed in significant concentration (Table 1). This result implies that a primarily Th17 response was being activated, although IL-17A is produced in invariant NKT cells, CD8+ T cells and γδ-T cells; albeit not as common [21]. Murine γδ-T cell receptors can recognize nonpeptide compounds in analogy to pattern recognition receptors rather than conventional recognition by α/β-TCRs of peptide antigens presented by MHC class I or II molecules [22]. Since most of the natural murine γδ antigens/ligands are unknown, one possibility could be that zwitterionic polysaccharides are a natural ligand for these cells. It is known, however, that zwitterionic polysaccharides activate CD4+ T cells and are presented on MHC II as well as interact with TLR-2 [9]. Unrelated studies have shown that the polysaccharide chitin interacts with TLR-2 and results in a IL-17-dependent immune activation mechanism [23].
Table 1.
Cytokine profile (pg/mL) of anti-Tn-PS A1 (3) sera and anti-PS A1 (1) sera as measured by FACS using a BD™ biosciences cytometric bead array. And while IL-2 and IL-6 dominate in the PS A1-primed sera, there is a distinct shift to IL-17A in Tn-PS A1-primed sera
Our findings also suggest that IL-17A, which is observed in anti-Tn-PS A1 sera, could be a strong link in the eradication of cancer as others have reported impressive antitumor functions for IL-17A [24, 25]. While the concentration of IL-10 is similar in both anti-sera, the significant decrease in pro-inflammatory IL-2 and the disappearance of the pro/anti-inflammatory IL-6 altogether in immunizations with Tn-PS A1 (3) imply a unique inflammatory response—one that may be beneficial at the site of attack.
In conclusion, we have demonstrated that an entirely carbohydrate cancer immunogen can elicit a T cell-mediated immune response and selectively distinguish human tumor cells that are known to possess the Tn antigen. Carbohydrate-specific IgG3 Abs, raised from Tn-PS A1 murine immunization selectively bind to tumor cells that are known to possess surface Tn antigen. Furthermore, the cytokine profile from anti-Tn-PS A1 sera contained a significant IL-17A response that argues for a unique Th17 mode of action. Interestingly, the cytokine profile from anti-PS A1 sera showed a different convention and arguably one that coincides with a Th1/Th2 paradigm for inflammatory immune response. These studies illustrate that zwitterionic polysaccharide PS A1 can be used as a T cell-dependent “carrier/adjuvant” to elicit a specific and selective immune response likely consequent to the Th17-associated tumor killing. Future studies are ongoing to explore the cellular origination of the cytokines and the efficacy of the sera in treating tumor compromised mice.
Supplementary Material
Acknowledgments
Authors thank Dr. Mary Kay Pflum for Jurkat and JurkatTAg cell lines. P.R.A acknowledges Wayne State University for financial support and the National Institutes of Health/National Cancer Institute for an R01 CA 156661 award.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Ravindra A. De Silva, Department of Chemistry, Wayne State University, 5100 Cass Avenue, Detroit, MI 48202, USA
Dananjaya K. Appulage, Department of Chemistry, Wayne State University, 5100 Cass Avenue, Detroit, MI 48202, USA
Halina Pietraszkiewicz, Division of Hematology and Oncology, Department of Internal Medicine, Henry Ford Health System, Detroit, MI, USA; The Josephine Ford Cancer Center, 440 Burroughs, Room 415, Detroit, MI 48202, USA.
Kevin R. Bobbitt, Department of Public Health Sciences Henry Ford Hospital, 1 Ford Place, 1D, Detroit, MI 48202, USA
Joe Media, Division of Hematology and Oncology, Department of Internal Medicine, Henry Ford Health System, Detroit, MI, USA; The Josephine Ford Cancer Center, 440 Burroughs, Room 415, Detroit, MI 48202, USA.
JiaJiu Shaw, 21st Century Therapeutics, Inc., 52623 Seven Oaks Dr. Shelby Township, Detroit, MI 48316, USA.
Fred A. Valeriote, Division of Hematology and Oncology, Department of Internal Medicine, Henry Ford Health System, Detroit, MI, USA The Josephine Ford Cancer Center, 440 Burroughs, Room 415, Detroit, MI 48202, USA.
Peter R. Andreana, Email: pra@chem.wayne.edu, Department of Chemistry, Wayne State University, 5100 Cass Avenue, Detroit, MI 48202, USA.
References
- 1.Avery OT, Goebel WF. Chemo-immunological studies on conjugated carbohydrate-proteins: II. Immunological specificity of synthetic sugar-protein antigens. J Exp Med. 1929;50:533–550. doi: 10.1084/jem.50.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Landsteiner K, van der Scheer J. On the influence of acid groups on the serological specificity of azoproteins. J Exp Med. 1927;45:1045–1056. doi: 10.1084/jem.45.6.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Astronomo RD, Burton DR. Carbohydrate vaccines: developing sweet solutions to sticky situations? Nat Rev Drug Discov. 2010;9:308–324. doi: 10.1038/nrd3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galonic DP, Gin DY. Chemical glycosylation in the synthesis of glycoconjugate antitumor vaccines. Nature. 2007;446:1000–1007. doi: 10.1038/nature05813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Velez CD, Lewis CJ, Kasper DL, Cobb BA. Type I streptococcus pneumonia carbohydrate utilizes a nitric oxide and MHC II-dependent pathway for antigen presentation immunology. 2009;127:73–82. doi: 10.1111/j.1365-2567.2008.02924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esplugues E, et al. Control of TH17 cells occurs in the small intestine. Nature. 2011;475:514–518. doi: 10.1038/nature10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jegerlehner A, et al. Carrier induced epitomic suppression of antibody responses induced by virus-like particles is a dynamic phenomenon caused by carrier-specific antibodies. Vaccine. 2010;28:5503–5512. doi: 10.1016/j.vaccine.2010.02.103. [DOI] [PubMed] [Google Scholar]
- 8.Zijlstra A, Testa JE, Quigley JP. Targeting the proteome/epitome, implementation of subtractive immunization. Biochem Biophys Res Commun. 2003;303:733–744. doi: 10.1016/s0006-291x(03)00357-7. [DOI] [PubMed] [Google Scholar]
- 9.Cobb BA, Wang Q, Tzianabos AO, Kasper DL. Polysaccharide processing and presentation by the MHCII pathway. Cell. 2004;117:677–687. doi: 10.016/j.cell.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Silva RA, Wang Q, Chidley T, Appulage DK, Andreana PR. Immunological responses from an entirely carbohydrate antigen: design of synthetic vaccines based on Tn-PS A1 conjugates. J Am Chem Soc. 2009;131:9622–9623. doi: 10.1021/ja902607a. [DOI] [PubMed] [Google Scholar]
- 11.Markham RB, Pier GB, Schreiber JR. The role of cytophilic IgG3 antibody in T cell-mediated resistance to infection with the extracellular bacterium, Pseudomonas aeruginosa. J Immunol. 1991;146:316–320. [PubMed] [Google Scholar]
- 12.Perlmutter RM, Hansburg D, Briles DE, Nicolotti RA, Davie JM. Subclass restriction of murine anti-carbohydrate antibodies. J Immunol. 1978;121:566–572. [PubMed] [Google Scholar]
- 13.Azeredo da Silveira S, et al. Complement activation selectively potentiates the pathogenicity of the IgG2b and IgG3 isotypes of a high affinity anti-erythrocyte autoantibody. J Exp Med. 2002;195:665–672. doi: 10.1084/jem.20012024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.An Z. Therapeutic monoclonal antibodies: from bench to clinic. Ch 16. New Jersey: Wiley; 2009. [Google Scholar]
- 15.Carcel-Trullols J. Characterization of the glycosylation profile of the human breast cancer cell line, MDA-231, and a bone colonizing variant. Int J Oncol. 2006;28:1173–1183. [PubMed] [Google Scholar]
- 16.Fernsten P, Shaw M, Hocker S, Fulghum R, Winfield J. Expression of the sialosyl-Tn epitope on CD45 derived from activated peripheral blood T cells. Immunol Invest. 1998;27:323–338. doi: 10.3109/08820139809022707. [DOI] [PubMed] [Google Scholar]
- 17.Thurnher M, Rusconi S, Berger EG. Persistent repression of a functional allele can be responsible for galactosyltransferase deficiency in Tn syndrome. J Clin Invest. 1993;91:2103–2110. doi: 10.1172/JCI116434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki M, et al. Polysialic acid facilitates tumor invasion by glioma cells. Glycobiology. 2005;15:887–894. doi: 10.1093/glycob/cwi071. [DOI] [PubMed] [Google Scholar]
- 19.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 20.Chung DR, et al. CD4+ T cells mediate abscess formation in intra-abdominal sepsis by an IL-17-dependent mechanism. J Immunol. 2003;170:1958–1963. doi: 10.4049/jimmunol.170.4.1958. [DOI] [PubMed] [Google Scholar]
- 21.Michel ML, et al. Critical role of ROR-γt in a new thymic pathway leading to IL-17-producing invariant NKT cell differentiation. Proc Natl Acad Sci USA. 2008;105:19845–19850. doi: 10.1073/pnas.0806472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ness-Schwickerath KJ, Morita CT. Regulation and function of IL-17A- and IL-22-producing γδ T cells. Cell Mol Life Sci. 2011;68:2371–2390. doi: 10.1007/s00018-011-0700-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Da Silva CA, Hartl D, Liu W, Lee GL, Elias JA. TLR-2 and IL-17A in chitin-induced macrophage activation and acute inflammation. J Immunol. 2008;181:4279–4286. doi: 10.4049/jimmunol.181.6.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muranski P, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benchetrit F, et al. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99:2114–2121. doi: 10.1182/blood.v99.6.2114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.