Abstract
BACKGROUND AND OBJECTIVES
Obstructive sleep apnea (OSA) causes increased cardiovascular morbidity and mortality, including systemic arterial hypertension, coronary heart disease, heart rhythm and conduction disorders, heart failure and stroke. In our study, we aimed to assess left ventricular mass and myocardial performance index (MPI) in OSA patients.
DESIGN AND SETTING
A cross-sectional study conducted between May 2007 and August 2009 in a tertiary hospital in Istanbul, Turkey.
PATIENTS AND METHODS
Forty subjects without any cardiac or pulmonary disease referred for evaluation of OSA had overnight polysomnography and echocardiography. According to the apnea-hypopnea index (AHI), subjects were classified into three groups; mild OSA (AHI: 5–14/h; n=7), moderate OSA (AHI: 15–29/h; n=13), and severe OSA (AHI: ≥30/h; n=20). The thickness of the interventricular septum (IVS) and left ventricular posterior wall (LVPW) were measured by M-mode along with left ventricular mass (LVM) and LVM index (LVMI). The left ventricular MPI was calculated as (isovolumic contraction time + isovolumic relaxation time)/aortic ejection time by Doppler echocardiography.
RESULTS
No differences were observed in age or body mass index among the groups, but blood pressures were higher in severe OSA compared with moderate and mild OSA. In severe OSA, the thickness of the IVS (11.6 [1.7 mm]), LVPW (10.7 [1.7 mm]), LVM (260.9 [50.5 g]), and LVMI (121.9 [21.1 g/m2]) were higher than in moderate OSA (9.4 [1.3 mm]; 9.9 [1.6]; 196.4 [35.2]; 94.7 [13.2 g/m2], respectively) and mild OSA (9.8 [2.4 mm], 8.9 [2.0 mm], 187.6 [66.2 g], 95.8 [28.6 g/m2], respectively). In severe OSA, MPI (0.8 [0.2]) was significantly higher than in mild OSA (0.5 [P<.01]) but not significantly higher than moderate OSA (0.8 [0.1]).
CONCLUSIONS
OSA patients have demonstrable cardiac abnormalities that worsen with the severity of apnea. The MPI may have utility in subsequent OSA studies, possibly as a surrogate outcome measure.
Obstructive sleep apnea (OSA) is a serious, underdiagnosed disease1,2 associated with increased cardiovascular morbidity and mortality, including systemic hypertension and pulmonary hypertension, coronary heart disease, heart rhythm and conduction disorders, and heart failure and stroke.2–6 Given the strong link between OSA and hypertension, both left ventricular hypertrophy and left ventricular diastolic dysfunction (LVDD) are common echocardiographic abnormalities in OSA.7
Repeated episodes of hypoxemia, hypercapnia, and microarousal plus intrathoracic pressure fluctuations trigger mechanisms such as sympathetic hyperactivity,8,9 oxidative stress,10 systemic inflammation,11 hypercoagulability, 12 and even endothelial dysfunction.13 The prevailing hypothesis is that these abnormalities combine chronically to yield vascular lesions. The large arteries play a crucial role in cardiac structure, so increased arterial stiffness contributes independently to arterial pressure and to an increase in left ventricular afterload, thereby promoting left ventricular hypertrophy (LVH).14
Left ventricular diastolic dysfunction precedes left ventricular systolic impairment and accounts for 30% to 40% of overall patients with left ventricular failure.15–17 Since systolic and diastolic dysfunctions frequently coexist, combined measures of left ventricular performance might be more reflective of overall cardiac dysfunction than systolic or diastolic measures alone. The Doppler-derived myocardial performance index (MPI also denoted the TEI-Doppler index), an index of combined systolic and diastolic functions, is defined as the sum of isovolumic contraction time and isovolumic relaxation time (IVRT) divided by the ejection time. Thus, MPI reflects global LV function as opposed to other measurements that reflect mainly either LV systolic or diastolic function.18 The MPI is a sensitive index of symptomatic heart failure and predicts future development of heart failure independent of other echocardiographic measurements.18 The MPI is a non-invasive, quick, and reproducible technique that can be used to evaluate left ventricular global function.19
Based on the above findings, we evaluated left ventricular mass (LVM) and left ventricular global function in OSA patients, including the relationship between apnea-hypopnea index (AHI) and MPI. This aim would allow us to test the hypothesis that OSA had important influences on cardiac functions as assessed by MPI. These data would allow us to explore the potential utility of the MPI in future OSA studies, eg, as a biomarker or to assess response to therapy or possibly as a robust marker of disease severity.
PATIENTS AND METHODS
Forty patients were referred to the sleep clinic between May 2007 and August 2009 with symptoms of nocturnal snoring and/or excessive daytime sleepiness. Patients who had known cardiac (including angina or arrhythmia) or lung disease, diabetes mellitus, angina pectoris, chronic renal and hepatic diseases, and serum electrolytes imbalances were excluded from the study. The Epworth sleepiness scale20 was used to assess all participants; patients with a score above 10/24 (indicative of excessive daytime sleepiness) were recruited into the study. Blood pressure was measured according to European Society of Hypertension-European Society of Cardiology guidelines.21 Hypertension was defined as a blood pressure >140/90 mm Hg. The heart rate (HR) per minute was measured in the sitting position and the body mass index (BMI) of the patients was calculated as weight divided by height squared (kg/m2). A 12-lead surface electrocardiogram was taken from every subject to ensure normal sinus rhythm.
Overnight-attended polysomnography was performed in our sleep laboratory during which we obtained a 3-channel electroencephalogram (EEG), an electro-oculogram, and a submental electromyogram using surface electrodes. The airflow was measured by monitoring nasal pressure through nasal cannulae (Ultima Dual Pressure Sensor 0585. Braebon Medical Corporation. Carp. Ontario, Canada). The respiratory effort was measured by inductance plethysmography with transducers placed on the chest and abdomen (Respitrace, Ambulatory Monitoring, Ardsley, New York, USA). The arterial oxyhemoglobin saturation (SaO2) was recorded with a pulse oximeter (Biox 3740, Ohmeda. Boulder, Colorado, United States). All variables were recorded continuously by a computerized data-acquisition system and stored electronically for later analysis (Sandman, Tyco Healthcare, Kanata, Ontario, Canada). All-night polysomnographic recordings were scored visually according to the Rechtschaffen and Kales (R and K) rules by experienced sleep scorers.22 Briefly, sleep was scored as stage 1 to 4 non-rapid eye movement (NREM) or REM. Total sleep time (TST) was defined as the duration of sleep from “lights out” at the beginning of the study to “lights on” the following morning. Sleep latency was defined as the time from lights out to the first 30-second period of sleep. Sleep efficiency was defined as the TST expressed as a percent of the total study duration. Apnea was defined as the absence of airflow for ≥10 seconds associated with continued respiratory efforts (obstructive), absence of respiratory efforts (central), or features of central apnea followed by obstructive apnea (mixed). Hypopnea was defined as a 50% reduction in airflow for ≥10 seconds or a marked reduction in airflow associated with 4% oxygen desaturation and/or arousal from sleep. The number of apneas and hypopneas per hour of sleep were expressed as AHI. Arousal was defined as an abrupt shift of EEG frequency including alpha, theta, and/or frequencies greater than 16 Hz (but not spindles), lasting at least 3 seconds in duration and preceded by at least 10 seconds of stable sleep. Desaturations were accepted as a ≥4% decrease in oxygen saturation. Desaturation index (DI) was defined as the number of oxygen desaturation events per hour of sleep.23
All echocardiographic measurements were performed with the subjects in the lateral decubitus position using a 2.5 MHz probe. All echocardiographic examinations were performed by the same cardiologist who was blinded to the presence or absence of OSA. Ventricular diameters, volumes, and functions were measured according to the recommendations of the American Society of Echocardiography.24 Basic measurements of left ventricular dimensions in diastole and systole, and thicknesses of interventricular septum (IVS), left ventricular posterior wall (LVPW), and LVM were measured by the M-mode technique; LVM was divided by body surface area to obtain LVM index (LVMI). The left ventricular ejection fraction (LVEF) was calculated by the Simpson method as (diastolic volume − systolic volume)/diastolic volume.
The early (E) and atrial (A) transmitral maximal flow velocities, the ratio (E/A), and the deceleration time of E-wave were registered. The IVRT was measured by the continuous wave Doppler technique. The velocity of mitral flow propagation was estimated using color Doppler M-mode. The left ventricular MPI was calculated as: (isovolumic contraction time + IVRT)/aortic ejection time. The global left ventricular dysfunction was defined as an MPI ≥0.50 [0.39 (0.05) normal level].25
Data were presented as mean (standard deviation), and the Kruskal-Wallis test was used for group comparisons. The Mann-Whitney U test was used when only two groups were compared. A P value <.05 was considered statistically significant.
RESULTS
A total of 31 males (77.5%) and 9 females (22.5%) were included in the study. Patients were classified into three subtypes according to their AHI scores; mild (AHI 5–14 events/h), moderate (AHI 15–29 events/h), and severe (AHI ≥30 events/h). Twelve percent of patients were using alcohol and 42% of patients were cigarette smokers. Basic characteristics of the patients with OSA are shown in Table 1. No significant differences were observed in their sex, BMI, and HR among the OSA patients (P>.05). However, mean of age, systolic blood pressure, and diastolic blood pressure were higher in severe OSA patients than in moderate and mild OSA patients. Of the 40 OSA patients, 15 were hypertensive and the majority (50%) were in the severe group. As expected, AHI and DI were highest in patients with severe OSA (P<.0001), and these patients had both the lowest average and nadir oxygen saturation (SaO2) (P<.0001). The percentage of sleep duration at <90% SaO2 was significantly higher in the severe OSA group, while it was the lowest in the mild OSA group.
Table 1.
Basic characteristics of patients with obstructive sleep apnea (OSA) syndrome.
| Patients with OSA | AHI 5–14/h | AHI 15–29/h | AHI ≥ 30/h | P |
|---|---|---|---|---|
|
| ||||
| No. of subjects | 7 | 13 | 20 | |
| Mean age (years) | 38.3 (6.1) | 42.7 (9.1) | 48.9 (7.4) | .01 |
| Male, n (%) | 4 (57.1) | 10 (76.9) | 17 (85.0) | NS |
| Female, n (%) | 3 (42.9) | 3 (23.1) | 3 (15.0) | NS |
| Body mass index (kg/m2) | 27.5 (5.1) | 31.4 (4.6) | 31.5 (4.9) | .275 |
| Systolic blood pressure (mm Hg) | 122.1 (12.7) | 127.6 (17.6) | 136.5 (18.2) | .005 |
| Diastolic blood pressure (mm Hg) | 75.3 (10.0) | 78.5 (9.9) | 81.4 (8.7) | .016 |
| Heart rate (pulse/min) | 92.9 (12.9) | 86.4 (9.9) | 87.7 (12.0) | .579 |
| Hypertension, n (%) | 1 (14.3) | 4 (30.8) | 10 (50.0) | .005 |
| Apnea-hypopnea index (AHI) (per hour) | 8.5 (2.2) | 21.5 (3.8) | 50.5 (15.3) | .001 |
| Desaturation index (per hour) | 9.8 (8.4) | 22.0 (6.0) | 39.7 (18.4) | .001 |
| Minimum saturation of nocturnal arterial oxygen (SaO2) (%) | 87.3 (8.9) | 85.3 (4.2) | 75.7 (8.3) | .001 |
| Average SaO2 (%) | 96.1 (1.8) | 94.2 (1.9) | 91.6 (4.2) | .001 |
| Sleep duration (%) a | 8.6 (11.9) | 2.5 (5.0) | 18.5 (22.7) | .002 |
Data are presented as n, n (%), or mean SD.
At SaO2 <90%
Basic echocardiographic measurements of the left ventricle in patients with OSA are shown in Table 2. The left atrial diameter and left ventricular end-diastolic and end-systolic diameters were not statistically different among groups of OSA patients in severe OSA patients. The thickness of IVS, LVPW, LVM, and LVMI were higher than both the moderate and mild OSA patients. Basic measurements in the different OSA groups are shown in Table 3.
Table 2.
Echocardiographic measurements of the left ventricle in patients with obstructive sleep apnea (OSA) syndrome.
| Patients with OSA | AHI 5–14/h | AHI 15–29/h | AHI ≥ 30/h | P |
|---|---|---|---|---|
|
| ||||
| No. of subjects | 7 | 13 | 20 | |
|
| ||||
| Thickness and diameters | ||||
| Left atrium (19–40 mm)a | 37.0 (5.1) | 36.2 (3.4) | 37.5 (4.1) | .619 |
| Interventricular septum thickness in diastole (6–11 mm)a | 9.8 (2.4) | 9.4 (1.3) | 11.6 (1.7) | .004 |
| Posterior left ventricular wall thickness in diastole (37–56 mm)a | 8.9 (2.0) | 9.9 (1.6) | 10.7 (1.7) | .033 |
| Left ventricular end-diastolic diameter (37–56 mm)a | 46.2 (4.7) | 46.7 (6.3) | 49.5 (4.2) | .278 |
| Left ventricular end-systolic diameter (19–40 mm)a | 28.7 (4.4) | 29.5(4.4) | 31.2 (3.4) | .383 |
| Left ventricular mass | ||||
| Left ventricular massb | 187.6 (66.2) | 196.4 (35.2) | 260.9 (50.5) | .001 |
| Left ventricular mass indexb | 95.8 (28.6) | 94.6 (13.2) | 121.9 (21.1) | .020 |
Data are presented as mean (SD) or n. AHI: Apnea-hypopnea index
Normal values; <198 g in females, <294 g in males;
<110 g/m2 in females, <134 g/m2 in males.
Table 3.
Statistical comparison of the basic measurements in the different obstructive sleep apnea syndrome groups.
| Group 1 vs 2a | Group 2 vs 3a | Group 1 vs 3a | |
|---|---|---|---|
|
| |||
| Systolic blood pressure | .145 | .017 | .006 |
| Diastolic blood pressure | .281 | .042 | .011 |
| Interventricular septum thickness in diastole | .498 | .001 | .090 |
| Posterior left ventricular wall thickness in diastole | .150 | .111 | .019 |
| Left ventricular mass | .452 | .001 | .017 |
| Left ventricular mass index | .937 | .001 | .043 |
| Myocardial performance index | .001 | .495 | .001 |
Data are presented as P values obtained using the Mann-Whitney U test.
Group 1: apnea-hypopnea index (AHI)=5–14, Group 2: AHI=15–29, Group 3: AHI=≥30.
The left ventricular systolic and diastolic functions in patients with OSA are shown in Table 4. The left ventricular systolic function was not significantly different in the three groups of OSA patients and was within normal limits. While mild OSA patients had normal left ventricular diastolic function, moderate and severe OSA patients had LVDD. The left ventricular MPI was significantly higher in severe OSA patients than in mild OSA patients (P=.01), but it was not statistically different between mild and moderate OSA patients (P>.05).
Table 4.
Left ventricular functions by echocardiography in patients with obstructive sleep apnea syndrome.
| AHI 5–14/h | AHI 15–29/h | AHI ≥ 30/h | P | |
|---|---|---|---|---|
|
| ||||
| N | 7 | 13 | 20 | |
| Left ventricular ejection fraction in two dimensions by Simpson’s method 55–75%a | 67.4 (7.9) | 67.6 (4.9) | 66.1 (5.6) | .514 |
| Early mitral flow velocity (m/sec) | 0.8 (0.1) | 0.7 (0.2) | 0.7 (0.2) | .601 |
| Atrial mitral flow velocity (m/sec) | 0.7 (0.2) | 0.6 (0.2) | 0.7 (0.2) | .481 |
| Ratio of early and atrial mitral flow velocity >1a | 1.2 (0.3) | 1.2 (0.4) | 1.0 (0.3) | .369 |
| Mitral deceleration time, <220# (m/sec)a | 220.6 (46.5) | 224.3 (54.5) | 225.8 (64.0) | .965 |
| Isovolumic relaxation time, <100# (m/sec)a | 75.1 (10.2) | 100.4 (19.7) | 105.0 (19.3) | .002 |
| Myocardial performance index, 0.39 (0.05)a | 0.5 (0.1) | 0.8 (0.2) | 0.8 (0.2) | .001 |
Data presented as mean (SD).
AHI: Apnea-hypopnea index
Normal values.
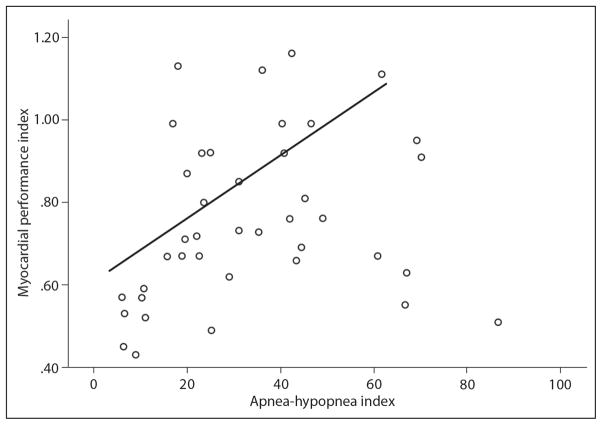
A positive correlation was shown between left ventricular MPI and AHI reflecting the severity of OSA (P=.001, r = 0.33). The correlation between MPI and AHI in OSA patients is shown in Figure 1.
Figure 1.
Correlation between myocardial performance index (MPI) and apnea-hypopnea index (AHI) in obstructive sleep apnea syndrome patients (r=0.33; P<.05).
DISCUSSION
OSA has important influences on cardiac functions such as left/right ventricular dysfunction, cardiac arrhythmia, and pulmonary hypertension.26 Since these dysfunctions are closely related with cardiac and systemic complications, we need a reproducible, widely applicable, and a simple noninvasive method for the estimation of left ventricular global function in patients with OSA. For this purpose, we investigated LVM and left ventricular global function in OSA patients by MPI including the relationship between AHI and MPI. These data would allow us to explore the potential utility of the MPI in future OSA studies.
Although the mechanism of impairment in myocardial contraction and relaxation seen in patients with OSA is poorly understood, OSA may increase cardiac risk due to an imbalance of myocardial oxygen demand and supply as a result of hypoxemia, hypercapnia, and increased sympathetic activation occurring during apnea. 27 Diseases, such as hypertension, obesity, and diabetes mellitus, which often accompany OSA, also contribute to the development of LVH. In our study, diabetes mellitus and coronary artery disease were excluded and no significant differences were observed in BMI between the patient groups. However, in our study, 15 of 40 OSA patients were hypertensive, and the majority (50%) were in the severe OSA group. Because of the compelling evidence of a causal link between OSA and hypertension, we believe that it would be inappropriate to exclude hypertensive patients from these analyses since the causal pathway underlying LVDD from OSA may well involve hypertension. In a study by Lavie et al28 in 2677 people attending a sleep clinic, the relationship between the severity of OSA significantly contributed to hypertension independent of all known confounding variables. Each apneic event per hour of sleep added about 1% to the risk of having hypertension. Apnea and hypopnea cause temporary elevations in blood pressure in association with blood oxygen desaturation, arousal, and sympathetic activation and may cause elevated blood pressure during the daytime and ultimately sustained hypertension.29 In our patients, the percentage of sleep duration <90% SaO2 was the highest in the severe OSA group, while it was the lowest in the mild OSA group. The severe OSA patients had more hypoxic duration in their sleep compared with the moderate and mild OSA groups. Because OSA may cause hypertension that may then lead to LVH, we do not view the imbalance of blood pressure between groups as a confounder, but rather an important factor on the causal pathway of interest.
In this study, IVS and LVPW diameters and LVM and LVMI were slightly higher in severe OSA patients, whereas they were within normal limits in mild and moderate OSA patients. Moreover, severe OSA patients had slight LVH. Our study did not define the mechanism underlying LVH; however, LVH could be caused by hypertension, intermittent blood pressure surges, and/or nocturnal hypoxemia. Noda et al30 reported echocardiographic evidence of LVH in 50% of patients with an AHI >20 per hour compared with 21.4% in those with an AHI <20 per hour.
In our study, we found that moderate and severe OSA patients had LVDD and also had global dysfunction diagnosed with increased MPI, although they had normal LVEF. In contrast, Laaban et al31 suggested that OSA may be a direct cause of daytime LV systolic dysfunction. However, several cross-sectional studies32,33 concordant with our results have demonstrated that the daytime LVEF was normal in patients with OSA and did not significantly differ between patients with OSA and control subjects. In theory, the discordance between our study and Laaban’s study may be because of our relatively small sample size or perhaps because of a high risk of technical failure of echocardiography in patients with severe obesity. We would advocate for future studies using radionuclide angiography or cardiac magnetic resonance in a large group of OSA patients.
The mechanisms underlying diastolic dysfunction in OSA patients are not clear; however, elevations in nocturnal blood pressure and sympathetic nervous system activity34 in OSA subjects create ventricular pressure overload.35 It could be speculated that as it occurs in other processes, such as chronic hypertension and aortic stenosis, increased pressure overload at the cellular level would mainly result in decreased levels of sarcoplasmic reticulum calcium adenosine triphosphotase pump, and increased phospholamban.36 This process could slow the removal of calcium from the cytosol, which leads to impaired ventricular relaxation. However, the pressure overload causes activation of multiple cellular signals that create myocardial tissue hypertrophy and interstitial fibrosis, increasing passive stiffness.37 Indeed an impaired coronary flow reserve would cause silent ischemia, worsening ventricular active relaxation when left ventricular diastolic pressure begins to rise.
Another plausible mechanism to explain the presence of diastolic dysfunction is related to futile inspiratory efforts.38 These efforts result in exaggerated negative intrathoracic pressure, which leads to an increase in left ventricular transmural pressure and hence after-load without increasing blood pressure. Another consequence of the increased negative intrathoracic pressure is the leftward shift of the IVS related to enhanced venous return and right ventricle dilatation. All of the aforementioned effects of the enhanced negative intrathoracic pressure have been demonstrated to affect left ventricular filling.35 It is difficult to define how each of those mechanisms affects diastolic function in a single OSA patient because of their complex interactions. However, in the initial stages of the disease, we hypothesize that mechanical effects of obstructive events mainly create ventricular pressure overload that by itself might lead to slowed ventricular relaxation.
Our study has strengths, including its novelty, but we acknowledge a number of limitations. We had a relatively small sample size and thus we were likely underpowered for some of our assessments particularly in subgroup analyses. We also realize that a number of covariates are present such that we are unable to distinguish the effects of severe OSA from those of hypoxemia. Although hypertension is quite prevalent among those with severe OSA, we would argue that hypertension may be on the causal pathway from OSA to LVH, such that it may be inappropriate to control for hypertension or to exclude it. That is, if we were to select severe OSA patients without hypertension, we may be choosing those patients who are somewhat resistant to OSA consequences. Ultimately, we acknowledge that control of BMI and hypertension in future studies as well as controlled interventional studies will be required to show the isolated effect of AHI on the measured parameters and to draw rigorous conclusions. However, we believe that our data represent an important addition to the published studies based on their novelty and the hypotheses that we have generated for subsequent research. We believe the MPI may have utility in subsequent OSA research, potentially as a surrogate outcome measure.
In conclusion, we found that the OSA may result in left ventricular dysfunction. A strong positive correlation was observed between MPI and severity of OSA. The MPI is a reproducible, widely applicable, and a simple noninvasive method for the estimation of left ventricular global function in patients with OSA. Since the ventricular function provides prognostic information in patients, the results from this study should be further confirmed with several longitudinal studies.
References
- 1.Hwang KO, Hamadah AM, Johnson CW, Thomas EJ, Goodrick GK, Bernstam EV. Screening for obstructive sleep apnea on the internet: Randomized trial. Am J Med. 2009;122:961.e1–6. doi: 10.1016/j.amjmed.2009.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuniyoshi FH, Pusalavidyasagar S, Singh P, Somers VK. Cardiovascular consequences of obstructive sleep apnea. Indian J Med Res. 2010;131:196–205. [PubMed] [Google Scholar]
- 3.Vanderveken OM, Boudewyns A, Ni Q, Kashyap B, Verbraecken J, De Backer W, et al. Cardiovascular implications in the treatment of obstructive sleep apnea. J Cardiovasc Transl Res. 2011;4:53–60. doi: 10.1007/s12265-010-9238-y. [DOI] [PubMed] [Google Scholar]
- 4.Drager LF, Genta PR, Pedrosa RP, Nerbass FB, Gonzaga CC, Krieger EM, et al. Characteristics and predictors of obstructive sleep apnea in patients with systemic hypertension. Am J Cardiol. 2010;105:1135–9. doi: 10.1016/j.amjcard.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 5.Di Guardo A, Profeta G, Crisafulli C, Sidoti G, Zammataro M, Paolini I, et al. Obstructive sleep apnea in patients with obesity and hypertension. Br J Gen Pract. 2010;60:325–8. doi: 10.3399/bjgp10X484174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373:82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 7.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, et al. Sleep apnea and cardiovascular disease: An American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council On Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing in collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health) Circulation. 2008;118:1080–111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 8.Leuenberger U, Jacob E, Sweer L, Waravdekar N, Zwillich C, Sinoway L. Surges of muscle sympathetic nerve activity during obstructive apnea are linked to hypoxemia. J Appl Physiol. 1995;79:581–8. doi: 10.1152/jappl.1995.79.2.581. [DOI] [PubMed] [Google Scholar]
- 9.Narkiewicz K, Somers VK. The sympathetic nervous system and obstructive sleep apnea: Implications for hypertension. J Hypertens. 1997;15:1613–9. doi: 10.1097/00004872-199715120-00062. [DOI] [PubMed] [Google Scholar]
- 10.Arnardottir ES, Mackiewicz M, Gislason T, Teff KL, Pack AI. Molecular signatures of obstructive sleep apnea in adults: A review and perspective. Sleep. 2009;32:447–70. doi: 10.1093/sleep/32.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shamsuzzaman AS, Winnicki M, Lanfranchi P, Wolk R, Kara T, Accurso V, et al. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation. 2002;105:2462–4. doi: 10.1161/01.cir.0000018948.95175.03. [DOI] [PubMed] [Google Scholar]
- 12.Von Kanel R, Loredo JS, Ancoli-Israel S, Mills PJ, Natarajan L, Dimsdale JE. Association between polysomnographic measures of disrupted sleep and prothrombotic factors. Chest. 2007;131:733–9. doi: 10.1378/chest.06-2006. [DOI] [PubMed] [Google Scholar]
- 13.Nieto FJ, Herrington DM, Redline S, Benjamin EJ, Robbins JA. Sleep apnea and markers of vascular endothelial function in a large community sample of older adults. Am J Respir Crit Care Med. 2004;169:354–60. doi: 10.1164/rccm.200306-756OC. [DOI] [PubMed] [Google Scholar]
- 14.Roman MJ, Ganau A, Saba PS, Pini R, Pickering TG, Devereux RB. Impact of arterial stiffening on left ventricular structure. Hypertension. 2000;36:489–94. doi: 10.1161/01.hyp.36.4.489. [DOI] [PubMed] [Google Scholar]
- 15.Dursunoglu D, Dursunoglu N, Evrengül H, Ozkurt S, Kuru O, Kiliç M, et al. Impact of obstructive sleep apnea on left ventricular mass and global function. Eur Respir J. 2005;26:283–8. doi: 10.1183/09031936.05.00038804. [DOI] [PubMed] [Google Scholar]
- 16.Bonow RO, Udelson JE. Left ventricular diastolic dysfunction as a cause of congestive heart failure. Mechanisms and management. Ann Intern Med. 1992;117:502–10. doi: 10.7326/0003-4819-117-6-502. [DOI] [PubMed] [Google Scholar]
- 17.Fischer M, Baessler A, Hense HW, Hengstenberg C, Muscholl M, Holmer S, et al. Prevalence of left ventricular diastolic dysfunction in the community. Results from a Doppler echocardiographic-based survey of a population sample. Eur Heart J. 2003;24:320–8. doi: 10.1016/s0195-668x(02)00428-1. [DOI] [PubMed] [Google Scholar]
- 18.Arnlov J, Ingelsson E, Riserus U, Andren B, Lind L. Myocardial performance index, a Doppler-derived index of global left ventricular function, predicts congestive heart failure in elderly men. Eur Heart J. 2004;25:2220–5. doi: 10.1016/j.ehj.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 19.Tei C, Nishimura RA, Seward JB, Tajik AJ. Non-invasive Doppler-derived myocardial performance index: correlation with simultaneous measurements of cardiac catheterization measurements. J Am Soc Echocardiogr. 1997;10:169–78. doi: 10.1016/s0894-7317(97)70090-7. [DOI] [PubMed] [Google Scholar]
- 20.Johns MW. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 21.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25:1105–87. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 22.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Washington DC: Public Health Service, U.S. Government Printing Office; 1968. [Google Scholar]
- 23.Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 24.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards. Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–67. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 25.Tei C, Ling LH, Hodge DO, Bailey KR, Oh JK, Rodeheffer RJ, et al. New index of combined systolic and diastolic myocardial performance: A simple and reproducible measure of cardiac function a study in normals and dilated cardiomyopathy. J Cardiol. 1995;26:357–66. [PubMed] [Google Scholar]
- 26.Hedner J, Grote L. The link between sleep apnea and cardiovascular disease: Time to target the nonsleepy sleep apneics? Am J Respir Crit Care Med. 2001;163:5–6. doi: 10.1164/ajrccm.163.1.ed1400d. [DOI] [PubMed] [Google Scholar]
- 27.Leung RS, Bradley TD. Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med. 2001;164:2147–65. doi: 10.1164/ajrccm.164.12.2107045. [DOI] [PubMed] [Google Scholar]
- 28.Lavie P, Herer P, Hoffstein V. Obstructive sleep apnea syndrome as a risk factor for hypertension: Population study. BMJ. 2000;320:479–82. doi: 10.1136/bmj.320.7233.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 30.Noda A, Okada T, Yasuma F, Nakashima N, Yokota M. Cardiac hypertrophy in obstructive sleep apnea syndrome. Chest. 1995;107:1538–44. doi: 10.1378/chest.107.6.1538. [DOI] [PubMed] [Google Scholar]
- 31.Laaban JP, Pascal-Sebaoun S, Bloch E, Orvoën-Frija E, Oppert JM, Huchon G. Left ventricular systolic dysfunction in patients with obstructive sleep apnea syndrome. Chest. 2002;122:1133–8. doi: 10.1378/chest.122.4.1133. [DOI] [PubMed] [Google Scholar]
- 32.Alchanatis M, Paradellis G, Pini H, Tourkohoriti G, Jordanoglou J. Left ventricular function in patients with obstructive sleep apnoea syndrome before and after treatment with nasal continuous positive airway pressure. Respiration. 2000;67:367–71. doi: 10.1159/000029532. [DOI] [PubMed] [Google Scholar]
- 33.Laaban JP, Cassuto D, Orvoën-Frija E, Iliou MC, Mundler O, Léger D, et al. Cardiorespiratory consequences of sleep apnoea syndrome in patients with massive obesity. Eur Respir J. 1998;11:20–7. doi: 10.1183/09031936.98.11010020. [DOI] [PubMed] [Google Scholar]
- 34.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bradley TD, Hall MJ, Ando S, Floras JS. Hemodynamic effects of simulated obstructive apneas in humans with and without heart failure. Chest. 2001;119:1827–35. doi: 10.1378/chest.119.6.1827. [DOI] [PubMed] [Google Scholar]
- 36.Arai M, Alpert NR, MacLennan DH, Barton P, Periasamy M. Alterations in sarcoplasmic reticulum gene expression in human heart failure. A possible mechanism for alterations in systolic and diastolic properties of the failing myocardium. Circ Res. 1993;72:463–9. doi: 10.1161/01.res.72.2.463. [DOI] [PubMed] [Google Scholar]
- 37.Gaasch WH, Blaustein AS, Andrias CW, Donahue RP, Avitall B. Myocardial relaxation. II. Hemodynamic determinants of rate of left ventricular isovolumic pressure decline. Am J Physiol. 1980;239:1–6. doi: 10.1152/ajpheart.1980.239.1.H1. [DOI] [PubMed] [Google Scholar]
- 38.Lorell BH, Carabello BA. Left ventricular hypertrophy: Pathogenesis, detection and prognosis. Circulation. 2000;102:470–9. doi: 10.1161/01.cir.102.4.470. [DOI] [PubMed] [Google Scholar]