Abstract
The study of bone and immunology (termed osteoimmunology) has led to the discovery of many important similarities between the two systems including shared niches, mechanisms, cytokines and receptors. The bone marrow provides a niche for hematopoietic cells including those of the lymphoid and myeloid lineage. Osteoclasts, specialized polykarons arising from myeloid precursors, bind to bone and resorb the organic and inorganic components through secretion of acid and proteases. Osteoclasts are differentiated and activated by cytokines that can be produced by immune cells and osteoclast activity can be dysregulated in states of autoimmunity or high inflammation. Similar to B and T cells, osteoclasts require coordinated co-stimulation of signaling pathways provided in the form of receptor-associated immunoreceptor tyrosine-based activation motif adaptor proteins, DAP12 and FcRγ, to drive differentiation and activation. In this review, we will cover the differentiation process of osteoclasts from the earliest precursors shown to have differentiation potential and the signals needed to drive these cells into osteoclast commitment and activation.
Osteoclast Precursors: Monocytes, Macrophages or Dendritic Cells (DCs)
Despite many attempts to identify a specific precursor population for osteoclasts, studies have revealed that multiple cell surface markers can identify cells with osteoclast potential. Early work attempting to decipher the osteoclast precursor focused on osteopetrotic mouse models and discovered that osteoclasts arose from a myeloid cell pool of hematopoietic origin that could be expanded in vivo by treatment with recombinant human macrophage colony-stimulating factor (rhM-CSF) to increase osteoclastogenesis.1-3 Several groups have further explored cell surface markers on myeloid pools to determine the best marker of bone marrow-derived osteoclast progenitors. Arai et al.4 found osteoclast potential in cell populations identified by c-Kit+Mac-1dullc-Fms+ (CD117+CD11b−/loCD115+) and c-Kit+Mac-1dullc-Fms− (CD117+CD11b−/loCD115−). They showed the expression of c-Fms, the M-CSF receptor, priming of cells by M-CSF and induction of RANK expression by M-CSF were prerequisites to osteoclastogenesis.4 Still others have identified additional osteoclast precursor populations as CD45R−CD11bhighCD3−.5 Yao et al.6 showed that blood osteoclast precursors are CD11b−GR-1−lo and that this population is increased by tumor necrosis factor-alpha (TNFα). Data from these groups reveal the complex expression pattern of the most accepted marker of pre-osteoclast precursors, CD11b or Mac-1, where CD11b−/lo correlates with earliest cells with osteoclast potential, CD11bhi identifies more mature osteoclast precursors, and finally mature, multinucleated osteoclasts become CD11b−.4-6 Additionally, Mizoguchi et al.7 identified c-Fms+/RANK+ osteoclast precursor population that they termed cell cycle-arrested quiescent osteoclast precursors (QuOPs), named so because of their gradual loss of cell cycle proteins (Cyclin D1-D3 or Cdk2/4/6) and increased expression of cell cycle inhibitor p27Kip1. QuOPs differentiate into osteoclasts in vivo upon stimulation; yet when BrdU, a thymidine analog, was co-administered with osteoclastogenic stimuli, only a low percentage of osteoclast nuclei incorporated BrdU, indicating that there is very low proliferation. Interestingly, it appears QuOPs are maintained, or at least associated with alkaline phosphatase-expressing osteoblasts, as shown by immunohistochemistry.
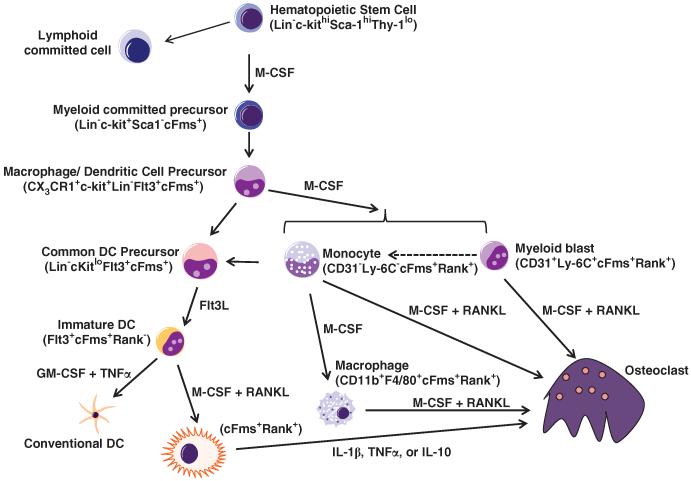
More recently, de Vries et al.8 have identified a common myeloid progenitor that gives rise to monocytes, macrophages and DCs, termed myeloid blasts (CD31+/Ly-6C+), as the myeloid cell most equipped to rapidly differentiate into functional osteoclasts (Figure 1). This myeloid blast arises from macrophage and DC precursor cell (MDP), identified as CX3 CR1+CD117+Lin−, that will differentiate into either monocytes or DCs while excluding other cell lineages in vitro and in vivo.9 Expression status of RANK tracks with osteoclast potential and negatively influences DC development. C-Fms+RANK− myeloid precursors stimulated with granulocyte macrophage colony-stimulating factor (GM-CSF), M-CSF and RANKL develop in DCs, whereas osteoclast differentiation is inhibited.10 However, if RANK is upregulated by priming cells with M-CSF before GM-CSF, cells lose their DC differentiation capacity.10 Recently, Muto et al.11 showed that circulating osteoclast precursors, CD11b+GR1loRANKhi/loc-Fms+, lose their capacity to become DC when they become RANKhic-Fmslo. However, some plasticity exists between immature human and mouse DCs, as they can trans-differentiate into TRAP+, resorbing osteoclasts in vitro.12,13 Whether trans-differentiation occurs in vivo is still controversial. Overall, the current state of the field reveals that osteoclast potential exists in multiple myeloid populations, and the diversity of osteoclastogenic stimuli and micro-environmental influences that occur in vivo likely regulate differing pools of osteoclast precursors. Given the myeloid nature of osteoclasts, it is not surprising that osteoclast differentiation and activation is regulated by immune receptors found on monocytes, macrophages and DCs.
Figure 1.
Osteoclast precursor development and differentiation. Hematopoietic stem cells give rise to lymphoid and myeloid committed precursors. Myeloid precursors generate MDPs. MDPs give rise to monocytes, macrophages and common DC precursor (CDP). Monocytes (Ly-6C−) stimulated by M-CSF mature into macrophages, but addition of RANKL drives monocytes into osteoclast commitment. Earlier stage Ly-6C+ monocytes, termed myeloid blasts, show strong osteoclast commitment potential when stimulated with M-CSF and RANKL but still retain the ability to become monocytes (Ly-6C−). Macrophages stimulated with M-CSF and RANKL can fuse to form osteoclasts. CDPs differentiate into immature DCs under stimulation with Fms-related tyrosine kinase 3 ligand (FLT3L) and become mature conventional DCs with the addition of GM-CSF and TNFα. Before GM-CSF stimulation, immature DC can trans-differentiate into an osteoclast under the influence of M-CSF and RANKL.
RANKL–RANK–Osteoprotegerin (OPG) Axis in Osteoclastogenesis
The differentiation, survival and activation of myeloid precursors into multinucleated, bone resorbing osteoclasts is tightly controlled by the RANKL–RANK–OPG axis.14 The regulation and manipulation of this system have elucidated several key findings about normal bone homeostasis requirements, pathological bone turnover, therapeutic targets for bone disease and interactions with the immune system. The master osteoclast differentiation stimulus in vitro and in vivo is RANKL (TRANCE, OPGL, ODF, TNFSF11), which is widely expressed on the cell surface of activated CD4+ and CD8+ T cells, B cells, osteoblasts, stromal cells, chondrocytes, macrophages, megakaryocytes and synoviocytes. RANKL binds to its receptor, RANK (TRANCE-R, ODAR, ODFR, CD265, TNFRSF11A), on the surface of myeloid precursors, mature osteoclasts, DCs and mature T cells, and initiates signaling cascades that lead to NF-κB, NFATc1 and JNK activation (Figure 2). OPG (OCIF, TNFRSF11B, TR1,FDRC1) acts as a soluble receptor for RANKL that competitively binds RANKL and potently inhibits osteoclastogenesis. OPG is produced by osteoblasts, stromal cells, endothelial cells, vascular smooth muscle cells and follicular DCs. Both RANKL−/− and RANK−/− mice suffer from severe osteopetrosis with defective tooth eruption due to the inability to generate osteoclasts, whereas OPG−/− mice show an opposite osteoporotic phenotype secondary to excessive osteoclasts.15
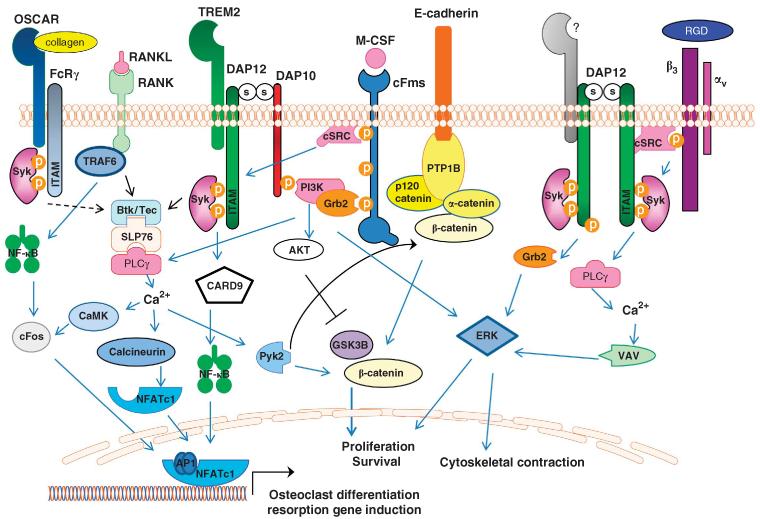
Figure 2.
Synergy of osteoclast signaling pathways. Osteoclast differentiation is induced by integration of M-CSF, RANKL and ITAM signaling. DAP12 co-stimulates RANK signaling by Syk-induced activation of BTK and Tec kinases, as well as PI3K activation leading to PLCγ and calcium oscillations. Ca2+-stimulated calcineurin dephosphorylates NFATc1, enabling nuclear translocation of NFATc1. DAP12-induced Ca2+ drives CaMKIV (calcium/calmodulin-dependent protein kinase type IV), which further activates RANK-induced cFos to translocate to the nucleus and form AP-1 dimers that work with NFATc1 to activate osteoclast-specific genes. Binding of M-CSF to its receptor, cFms, leads to recruitment of cSrc that phosphorylates the DAP12 ITAM, inducing Syk binding and activation. Syk subsequently activates PI3K, likely recruited to the membrane predominately via DAP10, leading to activation of PLCγ, VAV and ERK, culminating in actin cytoskeletal rearrangements. Activation of DAP12 by c-Fms-recruited cSrc induces Ca2+-stimulated Pyk2 release of β-catenin from an E-cadherin complex, thus allowing the release and nuclear accumulation of β-catenin to drive osteoclast cell proliferation and survival. DAP12-induced AKT activation further facilitates this process by inhibiting GSK3B. Integrin αvβ3 recruits cSrc to activate DAP12 leading to activation of ERK, PLCγ and VAV to mediated cytoskeleton rearrangement. OSCAR, an FcRγ-associated receptor, acts as a collagen receptor and can provide necessary ITAM co-stimulation of RANK in the absence of DAP12.
Exciting contributions to our understanding of in vivo production of RANKL have recently been clarified through work on osteocytes, matrix-embedded osteoblast cells that act as mechanosensors for bone. Osteocytes have been shown to induce TRAP+ multinucleated osteoclasts either through direct or indirect stimulation.16,17 However, the question remained whether this was relevant in vivo. To address this question, two groups simultaneously utilized Dmp1-Cre mice crossed with Tnfsf11flox/flox that targeted ablation of RANKL in osteocytes, and showed increased bone mass secondary to decreased osteoclast numbers and resorption.18,19 Furthermore, osteocyte-mediated RANKL production drives mechanical stress-induced bone loss and pathological bone turnover.18-20 These studies suggest that osteocytes are the major source of RANKL during in vivo bone remodeling in a sclerostin-mediated pathway.18,19,21 These conclusions are based on the finding that the DMP-1 promoter construct that was used in these studies is specifically expressed only in osteocytes. Thus, mechanosensing osteocytes have a critical role in RANKL-induced osteoclastogensis.
Costimulatory Adapters and Osteoclastogenesis
The master osteoclast differentiation cytokine, RANKL, is insufficient to drive osteoclastogenesis without costimulatory receptor engagement (Figure 2). Several small, transmembrane adapter proteins, including DAP12, FcRγ and DAP10, and their receptors provide co-stimulation to RANKL-induced osteoclastogenesis. DAP12 and FcRγ have intracellular tyrosine-based activation motifs (ITAM) with the consensus sequence YxxI/Lx(6– 12)YxxI/L (x denotes any amino acid) that provides docking sites for kinases and phosphatases. Although the extracellular domains of DAP12 or FcRγ are small and do not directly interact with any ligands, each adapter associates with cell surface receptors via paired charged residues in the transmembrane regions of each adapter (Tables 1 and 2).22 In myeloid cells, receptor crosslinking induces phosphorylation of the tyrosine residues in the ITAM, recruitment and activation of Syk, with subsequent activation of PI3K, MAPK and calcium flux (Figure 2).22 In osteoclasts, DAP12 and FcRγ are required for co-stimulation of RANK signaling, driving calcium oscillations necessary for NFATc1 production required for osteoclastogenesis.23,24 Mice deficient in both ITAM adapters have significant osteopetrosis, though not as severe as RANK- or RANKL-deficient mice, with mononuclear osteoclasts in vivo and in vitro.24,25 Interestingly, DAP12−/− FcRγ−/− mice respond normally to ovariectomy with profound bone loss coupled with the generation of multinucleated osteoclasts in vivo, indicating that under some conditions osteoclasts can differentiate and function in the absence of DAP12 and FcRγ.25 Additional studies have identified downstream signaling effectors of DAP12 and FcRγ, including Syk, PLCγ and VAV, as critical for normal osteoclast development and actin ring formation.23,26,27 These studies underscore the importance of ITAM signaling in osteoclast fusion, actin cytoskeleton organization and bone resorption.
Table 1.
DAP12-associated receptors
| Name | Species | Cell expression | Expression in osteoclast | Ligands |
|---|---|---|---|---|
| KIR2DS1, KIR2DS2, KIR2DS3 | Human | NK cells, T-cell subsets | ? | HLA class I (KIR2DS1) |
| CD94-NKG2C, CD94-NKG2E | Human, mouse | NK cells, T-cell subsets | ? | HLA-E (human), Qa-1b (mouse) |
| Ly49Db6a, Ly49Hb6, Ly49R129/J, Ly49U129/J |
Mouse | NK cells, T-cell subsets | ? | H-2Dd, M157 (CMV protein) |
| NKG2D-Sa (short isoform) | Mouse | NK cells, T-cell subsets, macrophages | + | Rae-1, H60, Mult I |
| NKp44 | Human | NK cells, γδ T cells, pDCs | ? | Viral HA? PCNA, proliferating cell nuclear antigen |
| TREM1, TREM3b | Human, mouse | Monocyte, neutrophils, macrophages, osteosteoclasts |
TREM3 + by RTPCR | |
| TREM2a | Human, mouse | Macrophages, DCs, mast cells, osteoclasts, microglia |
+ | Anionic ligands (dextran slufate and bacteria), HSP60, apoptotic membranes |
| TLT4 | Mouse | ? | ||
| MDL1a | Human, mouse | Monocytes, macrophages, DCs |
+ | Dengue virion |
| SIRPβ | Human, mouse | Neutrophils, monocytes, macrophages, DCs |
+ | |
| CD300C, CD300Db, CD300Eb | Human, mouse | Myeloid cells | ? | |
| CD200R3, CD200R4 | Human, mouse | Myeloid cells | + | CD200 |
| PILRβ | Human, mouse | Leukocytes, NK cells, macrophages, neutrophils, DCs |
+ | PILR-β ligand (CD99-like), sialylated O-linked sugars |
| SIGLEC-H | Mouse | pDCs | ? | |
| SIGLEC14 | Human, mouse | Hematopoietic cells | ? | A2-8-linked oligo Neu5A |
Abbreviations: DCs, dendritic cells; NK cells, natural killer cells; PCNA, proliferating cell nuclear antigen; pDC, plasmacytoid dendritic cell.
Indicates receptor can also associate with DAP10.
Indicates receptor is in mouse only.
Indicates expression in osteoclasts.
Indicates osteoclast expression unknown.
Table 2.
FcRγ-associated receptors
| Name | Species | Cell expression | Expression in osteoclast |
Ligand |
|---|---|---|---|---|
| DCAR | Human, mouse | DCs | ? | |
| PIR-A multiple isoforms (>six genes) | Mouse | B cells, DCs, monocytes/macrophages, granulocytes, mast cells, megakaryocytes/platelets |
+ | H2-D, H2K |
| ILT/LIR | Human | NK cells, T-cell subsets, B cells, macrophages, mast cells, DCs |
? | |
| mOSCAR ILT1/LIR7/LILRA2 LIR6/ LILRA1 ILT8/LILRB6 ILT10/LILRA5 ILT11/LILRB7 ILT7/LILRA4 |
Mouse | Osteoclasts | + | Collagen |
| hOSCAR | Human | DCs and osteoclasts | + | Collagen |
| FcαR | Eosinopohils, DCs, basophils, monocytes, macrophages, neutrophils |
? | IgA | |
| FcγRIII | Macrophages and NK cells | + | IgG | |
| FcεRI | Mast cells | ? | IgE | |
| KIR2DLY | NK cells and some T cells | ? | ||
| NKp46 | NK cells | ? |
Abbreviations: DCs, dendritic cells; Ig, immunoglobin; IL, interleukin; NK cells, natural killer cells; PIR-A, immunoglobulin-like receptor-A; H2-D/H2K, histocompatability 2, D or K region.
DAP10, another transmembrane adapter protein widely expressed in myeloid, NK and CD8+ T cells, also participates in osteoclastogenesis.28,29 Instead of an ITAM, DAP10 has a tyrosine-based YINM motif that binds the p85 subunit of PI3K and Grb2.30 Mice deficient in DAP10 develop aging-related high bone mass due to decreases in osteoclast numbers.28 DAP10 cooperates with DAP12 to drive osteoclastogenesis when DAP12 is associated with either TREM2, a DAP12-associated receptor, or myeloid DAP12-associating lectin-1 (MDL-1).28,29 In the context of a TREM2–DAP12–DAP10 trimolecular complex, DAP10 is required for recruitment of PI3K and Grb2, as well as ERK and AKT activation downstream of TREM2 receptor crosslinking (Figure 2).29 Although DAP12 deficiency leads to decreases in macrophage and pre-osteoclast proliferation and survival,31 further studies are needed to determine whether the loss of the DAP10–DAP12 complex is responsible for these effects.
Highlighting the importance of DAP12 in osteoclastogenesis, osteoclasts from humans with Nasu–Hakola disease, due to DAP12 deficiency, have small, mononuclear osteoclasts with disorganized actin cytoskeletons and poor resorptive capacity similar to mouse DAP12−/− osteoclasts.32 Nasu–Hakola or polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy is a rare, recessively inherited human disease characterized by multiple bony cysts, rapidly progressive dementia and premature death by age 50.33 Genetic mapping studies revealed that deletion or mutations of TYROBP encoding DAP12 at chromosome 19q13.1 were linked to Nasu–Hakola in most patients and functional mutations in TREM2 in a small subset of patients.34,35 Thus, Nasu–Hakola is associated with mutations that lead to defective signal transduction through TREM2 and DAP12, and these studies suggest that this TREM2–DAP12 signaling is critical for normal function of human bone and brain tissue.35 Differences exist between humans and mice deficient in DAP12, where humans develop osteoporosis and bone cysts, whereas mice develop osteopetrosis without bone cysts. However, TREM2-deficient mice more closely mimic humans with Nasu–Hakola disease and exhibit osteoporosis although they do not develop bone cysts.36 Additional studies are needed to determine whether differences in humans and mice TREM2-deficient osteoclasts are related to alternate DAP12-associated or FcRγ receptors, or alternate expression of ligands for these receptors.
DAP12-associated Receptors in Osteoclasts
Several DAP12-associated receptors are present in osteoclasts, including TREM2, MDL-1 and signaling regulatory protein-beta 1 (SIRP β1) (Table 1). TREM2 is expressed on many myeloid cells and likely functions as a scavenger receptor as it binds to a variety of anionic ligands including Gram-negative and -positive bacteria, heparin and dextran sulfate.37 When expressed on microglia in the brain, TREM2 induces anti-inflammatory clearance of dead neurons when it binds to secreted HSP60 or apoptotic neurons.38,39 In both human and mouse osteoclasts, TREM2 facilitates osteoclast multinucleation and migration.32,40-42 Unlike human TREM2-deficient osteoclasts that fail to multinucleate in vitro, TREM2-deficient mouse osteoclasts have accelerated osteoclastogensis in vitro due to inappropriate activation of β-catenin downstream of M-CSF, leading to a halt in pre-osteoclast proliferation and hastened osteoclast development.36 Other studies have shown that TREM2 crosslinking on mouse osteoclasts inhibits resorption while promoting multinucleation.41 These data suggest that TREM2 functions to both activate and inhibit osteoclasts. Recently, DAP12 was shown to associate with SH2-domain inositol phosphatase (SHIP1) in osteoclasts to mediate inhibitory ITAM signals induced by TREM2 crosslinking or M-CSF but not RANKL.29 This dual action of TREM2–DAP12 complex could be regulated by differing ligands, strength of ligand signals or other co-stimulatory signals.
MDL-1, another DAP12-associated receptor expressed in osteoclasts, has a small impact on basal osteoclastogenesis but a profound impact on inflammatory bone loss.28,43 MDL-1 associates with DAP12 and DAP10 adapters that are needed for full cellular activation downstream of MDL-1.28 MDL-1 is most highly expressed in activated myeloid cells and is potently induced by TNFα.43 Although endogenous ligands for MDL-1 have yet to be identified, MDL-1 serves as receptor for Dengue virus, the cause of ‘break bone’ hemorrhagic fever.44 In mouse models of autoimmune arthritis, agonistic anti-MDL-1 anti-bodies promote synovial inflammation and bone erosions, whereas blocking antibodies prevent bone erosions.43 These studies suggest that endogenous MDL-1 ligands are modulated during inflammatory arthritis. Additional studies are needed to determine whether MDL-1 serves a pathogenic role in human inflammatory arthritis or whether MDL-1 functions in homeostatic bone remodeling.
FcRγ-associated Receptors in Osteoclasts
Several FcRγ-associated receptors are present in pre-osteoclasts and osteoclasts including osteoclast-associated receptor (OSCAR) and paired immunoglobulin-like receptor-A (PIR-A) (Table 2).24,45 OSCAR was recently found to be a collagen receptor and provides the necessary ITAM co-stimulation in human and mouse DAP12−/− osteoclasts generated on extracellular matrix or collagen.46,47 In vivo, OSCAR deficiency alone fails to alter bone mass, similar to FcRγ−/− mice, indicating that DAP12 is the main co-stimulatory pathway in vivo.46 However, OSCAR−/−DAP12−/− mice exhibited significant osteopetrosis compared with DAP12−/− mice due to decreases in osteoclast numbers and function. These data suggest that OSCAR does contribute to ITAM co-stimulation in vivo during basal bone remodeling. On the other hand, TNFα induces the expression of PIR-A on osteoclasts and PIR-A ligands, major histocompatibility complex class 1 molecules, on osteoblasts that participate in TNFα-induced bone loss in vivo.48 Additional studies are needed to identify the role of PIR-A in basal bone remodeling or OSCAR in pathogenic bone remodeling in mice and humans.
ITAM Adapter Associations with Non-immunoreceptors
In addition to RANK co-stimulation, DAP12 cooperates with non-immunoreceptors in osteoclasts including αvβ3 integrin and c-Fms receptor to regulate the osteoclast cytoskeleton, adhesion and proliferation (Figure 2).49,50 DAP12 serves as a docking protein for Syk and c-Src after integrin β3 stimulation in osteoclasts and is required for bone resorption and actin ring formation.50 Likewise, M-CSF stimulation of c-Fms leads to c-Src-induced phosphorylation of DAP12 with subsequent recruitment of Syk and activation of VAV leading to osteoclast spreading.49 Furthermore, M-CSF-induced osteoclast proliferation and survival require nuclear accumulation of β-catenin in a DAP12-dependent manner.31 Interestingly, DAP12−/− osteoclastogenesis is partially rescued by high-dose M-CSF in vitro but fails to correct the abnormal cytoskeleton formation.51 Negative regulation of c-Fms occurs in part by the DAP12-dependent recruitment of SHIP1.29 These studies provided novel insight into the biology of osteoclasts and show that DAP12 ITAM signaling is co-stimulatory for RANKL-, M-CSF- and integrin-mediated signaling in osteoclasts. Thus, the osteoclast deficits seen in mouse and human DAP12−/− osteoclasts are multifactorial and likely change depending on the in vivo microenvironment.
Immunoreceptor Tyrosine-Based Inhibitory Motif (ITIM) Receptors in Osteoclasts
Immune cells are frequently negatively regulated by ITIM receptors, and osteoclasts are no exception. ITIM receptors recruit tyrosine phosphatases, SHP-1 or SHP-2, or lipid phosphatases, SHIP1 and SHIP2, to limit ITAM-mediated signals. Several ITIM receptors negatively regulate osteoclastogenesis including CLM-1, PIR-B, leukocyte immunoglobin-like receptor B (LILRB1), SIRPα and FcγRIIB.52-55 However, two ITIM receptors, Ly49Q and DC-STAMP, positively regulate osteoclastogenesis, revealing the complexities of phosphatase regulation of intracellular signaling as it relates to cellular differentiation.56,57 These studies suggest that the expression of individual ITIM- and ITAM-associated receptors on osteoclasts coupled with the expression of receptor ligands fine tunes osteoclastogenesis and osteoclast activation.
Summary
Osteoclasts remain a critical innate immune cell within bone poised to respond to cellular distress, bacteria, inflammation or microenvironmental stresses by means of a growing number of immunoreceptors. Indeed, more work will elucidate even greater similarities between osteoclasts and immune cells, and how these similarities are intertwined during healthy and disease states. Further understanding of these receptors in normal and pathological bone remodeling will help to identify alternative therapeutic strategies for osteoporosis or pathological bone remodeling.
ACKNOWLEDGMENTS
This work was supported by NIH R01 DE019398 (MBH) and Veterans Affairs PECASE (MBH). We thank Mary Nakamura and Julia Charles for their helpful critical reviews and suggestions.
Footnotes
Conflict of Interest
The author declares no conflict of interest.
References
- 1.Kodama H, Yamasaki A, Nose M, Niida S, Ohgame Y, Abe M, et al. Congenital osteoclast deficiency in osteopetrotic (op/op) mice is cured by injections of macrophage colony-stimulating factor. J Exp Med. 1991;173:269–272. doi: 10.1084/jem.173.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker DG. Congenital osteopetrosis in mice cured by parabiotic union with normal siblings. Endocrinology. 1972;91:916–920. doi: 10.1210/endo-91-4-916. [DOI] [PubMed] [Google Scholar]
- 3.Walker DG. Control of bone resorption by hematopoietic tissue. The induction and reversal of congenital osteopetrosis in mice through use of bone marrow and splenic transplants. J Exp Med. 1975;142:651–663. doi: 10.1084/jem.142.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arai F, Miyamoto T, Ohneda O, Inada T, Sudo T, Brasel K, et al. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J Exp Med. 1999;190:1741–1754. doi: 10.1084/jem.190.12.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacquin C, Gran DE, Lee SK, Lorenzo JA, Aguila HL. Identification of multiple osteoclast precursor populations in murine bone marrow. J Bone Miner Res. 2006;21:67–77. doi: 10.1359/JBMR.051007. [DOI] [PubMed] [Google Scholar]
- 6.Yao Z, Li P, Zhang Q, Schwarz EM, Keng P, Arbini A, et al. Tumor necrosis factor-alpha increases circulating osteoclast precursor numbers by promoting their proliferation and differentiation in the bone marrow through up-regulation of c-Fms expression. J Biol Chem. 2006;281:11846–11855. doi: 10.1074/jbc.M512624200. [DOI] [PubMed] [Google Scholar]
- 7.Mizoguchi T, Muto A, Udagawa N, Arai A, Yamashita T, Hosoya A, et al. Identification of cell cycle-arrested quiescent osteoclast precursors in vivo. J Cell Biol. 2009;184:541–554. doi: 10.1083/jcb.200806139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Vries TJ, Schoenmaker T, Hooibrink B, Leenen PJ, Everts V. Myeloid blasts are the mouse bone marrow cells prone to differentiate into osteoclasts. J Leukoc Biol. 2009;85:919–927. doi: 10.1189/jlb.0708402. [DOI] [PubMed] [Google Scholar]
- 9.Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 10.Miyamoto T, Ohneda O, Arai F, Iwamoto K, Okada S, Takagi K, et al. Bifurcation of osteoclasts and dendritic cells from common progenitors. Blood. 2001;98:2544–2554. doi: 10.1182/blood.v98.8.2544. [DOI] [PubMed] [Google Scholar]
- 11.Muto A, Mizoguchi T, Udagawa N, Ito S, Kawahara I, Abiko Y, et al. Lineage-committed osteoclast precursors circulate in blood and settle down into bone. J Bone Miner Res. 2010;26:2978–2990. doi: 10.1002/jbmr.490. [DOI] [PubMed] [Google Scholar]
- 12.Rivollier A, Mazzorana M, Tebib J, Piperno M, Aitsiselmi T, Rabourdin-Combe C, et al. Immature dendritic cell transdifferentiation into osteoclasts: a novel pathway sustained by the rheumatoid arthritis microenvironment. Blood. 2004;104:4029–4037. doi: 10.1182/blood-2004-01-0041. [DOI] [PubMed] [Google Scholar]
- 13.Speziani C, Rivollier A, Gallois A, Coury F, Mazzorana M, Azocar O, et al. Murine dendritic cell transdifferentiation into osteoclasts is differentially regulated by innate and adaptive cytokines. Eur J Immunol. 2007;37:747–757. doi: 10.1002/eji.200636534. [DOI] [PubMed] [Google Scholar]
- 14.Wright HL, McCarthy HS, Middleton J, Marshall MJ. RANK, RANKL and osteoprotegerin in bone biology and disease. Curr Rev Musculoskelet Med. 2009;2:56–64. doi: 10.1007/s12178-009-9046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards JR, Mundy GR. Advances in osteoclast biology: old findings and new insights from mouse models. Nat Rev Rheumatol. 2010;7:235–243. doi: 10.1038/nrrheum.2011.23. [DOI] [PubMed] [Google Scholar]
- 16.Zhao S, Zhang YK, Harris S, Ahuja SS, Bonewald LF. MLO-Y4 osteocyte-like cells support osteoclast formation and activation. J Bone Miner Res. 2002;17:2068–2079. doi: 10.1359/jbmr.2002.17.11.2068. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka K, Yamaguchi Y, Hakeda Y. Isolated chick osteocytes stimulate formation and bone-resorbing activity of osteoclast-like cells. J Bone Miner Metab. 1995;13:61–70. [Google Scholar]
- 18.Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2010;17:1231–1234. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 19.Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2010;17:1235–1241. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, et al. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5:464–475. doi: 10.1016/j.cmet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Wijenayaka AR, Kogawa M, Lim HP, Bonewald LF, Findlay DM, Atkins GJ. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS One. 2010;6:e25900. doi: 10.1371/journal.pone.0025900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lanier LL, Bakker AB. The ITAM-bearing transmembrane adaptor DAP12 in lymphoid and myeloid cell function. Immunol Today. 2000;21:611–614. doi: 10.1016/s0167-5699(00)01745-x. [DOI] [PubMed] [Google Scholar]
- 23.Mocsai A, Humphrey MB, Van Ziffle JA, Hu Y, Burghardt A, Spusta SC, et al. The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc Natl Acad Sci USA. 2004;101:6158–6163. doi: 10.1073/pnas.0401602101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004;428:758–763. doi: 10.1038/nature02444. [DOI] [PubMed] [Google Scholar]
- 25.Wu Y, Torchia J, Yao W, Lane NE, Lanier LL, Nakamura MC, et al. Bone microenvironment specific roles of ITAM adapter signaling during bone remodeling induced by acute estrogen-deficiency. PLoS One. 2007;2:e586. doi: 10.1371/journal.pone.0000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faccio R, Teitelbaum SL, Fujikawa K, Chappel J, Zallone A, Tybulewicz VL, et al. Vav3 regulates osteoclast function and bone mass. Nat Med. 2005;11:284–290. doi: 10.1038/nm1194. [DOI] [PubMed] [Google Scholar]
- 27.Mao D, Epple H, Uthgenannt B, Novack DV, Faccio R. PLCgamma2 regulates osteoclastogenesis via its interaction with ITAM proteins and GAB2. J Clin Invest. 2006;116:2869–2879. doi: 10.1172/JCI28775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inui M, Kikuchi Y, Aoki N, Endo S, Maeda T, Sugahara-Tobinai A, et al. Signal adaptor DAP10 associates with MDL-1 and triggers osteoclastogenesis in cooperation with DAP12. Proc Natl Acad Sci USA. 2009;106:4816–4821. doi: 10.1073/pnas.0900463106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng Q, Malhotra S, Torchia JA, Kerr WG, Coggeshall KM, Humphrey MB. TREM2- and DAP12-dependent activation of PI3K requires DAP10 and is inhibited by SHIP1. Sci Signal. 2010;3:ra38. doi: 10.1126/scisignal.2000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lanier LL. DAP10- and DAP12-associated receptors in innate immunity. Immunol Rev. 2009;227:150–160. doi: 10.1111/j.1600-065X.2008.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otero K, Turnbull IR, Poliani PL, Vermi W, Cerutti E, Aoshi T, et al. Macrophage colony-stimulating factor induces the proliferation and survival of macrophages via a pathway involving DAP12 and beta-catenin. Nat Immunol. 2009;10:734–743. doi: 10.1038/ni.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paloneva J, Mandelin J, Kiialainen A, Bohling T, Prudlo J, Hakola P, et al. DAP12/TREM2 deficiency results in impaired osteoclast differentiation and osteoporotic features. J Exp Med. 2003;198:669–675. doi: 10.1084/jem.20030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bianchin MM, Capella HM, Chaves DL, Steindel M, Grisard EC, Ganev GG, et al. Nasu-Hakola disease (polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy–PLOSL): a dementia associated with bone cystic lesions. From clinical to genetic and molecular aspects. Cell Mol Neurobiol. 2004;24:1–24. doi: 10.1023/B:CEMN.0000012721.08168.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paloneva J, Kestila M, Wu J, Salminen A, Bohling T, Ruotsalainen V, et al. Loss-of-function mutations in TYROBP (DAP12) result in a presenile dementia with bone cysts. Nat Genet. 2000;25:357–361. doi: 10.1038/77153. [DOI] [PubMed] [Google Scholar]
- 35.Paloneva J, Manninen T, Christman G, Hovanes K, Mandelin J, Adolfsson R, et al. Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am J Hum Genet. 2002;71:656–662. doi: 10.1086/342259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Otero K, Shinohara M, Zhao H, Cella M, Gilfillan S, Colucci A, et al. TREM2 and beta-catenin regulate bone homeostasis by controlling the rate of osteoclastogenesis. J Immunol. 2012;188:2612–2621. doi: 10.4049/jimmunol.1102836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daws MR, Sullam PM, Niemi EC, Chen TT, Tchao NK, Seaman WE. Pattern recognition by TREM-2: binding of anionic ligands. J Immunol. 2003;171:594–599. doi: 10.4049/jimmunol.171.2.594. [DOI] [PubMed] [Google Scholar]
- 38.Stefano L, Racchetti G, Bianco F, Passini N, Gupta RS, Panina Bordignon P, et al. The surface-exposed chaperone, Hsp60, is an agonist of the microglial TREM2 receptor. J Neurochem. 2009;110:284–294. doi: 10.1111/j.1471-4159.2009.06130.x. [DOI] [PubMed] [Google Scholar]
- 39.Hsieh CL, Koike M, Spusta SC, Niemi EC, Yenari M, Nakamura MC, et al. A role for TREM2 ligands in the phagocytosis of apoptotic neuronal cells by microglia. J Neurochem. 2009;109:1144–1156. doi: 10.1111/j.1471-4159.2009.06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cella M, Buonsanti C, Strader C, Kondo T, Salmaggi A, Colonna M. Impaired differentiation of osteoclasts in TREM-2-deficient individuals. J Exp Med. 2003;198:645–651. doi: 10.1084/jem.20022220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Humphrey MB, Daws MR, Spusta SC, Niemi EC, Torchia JA, Lanier LL, et al. TREM2, a DAP12-associated receptor, regulates osteoclast differentiation and function. J Bone Miner Res. 2006;21:237–245. doi: 10.1359/JBMR.051016. [DOI] [PubMed] [Google Scholar]
- 42.Humphrey MB, Ogasawara K, Yao W, Spusta SC, Daws MR, Lane NE, et al. The signaling adapter protein DAP12 regulates multinucleation during osteoclast development. J Bone Miner Res. 2004;19:224–234. doi: 10.1359/JBMR.0301234. [DOI] [PubMed] [Google Scholar]
- 43.Joyce-Shaikh B, Bigler ME, Chao CC, Murphy EE, Blumenschein WM, Adamopoulos IE, et al. Myeloid DAP12-associating lectin (MDL)-1 regulates synovial infl ammation and bone erosion associated with autoimmune arthritis. J Exp Med. 2010;207:579–589. doi: 10.1084/jem.20090516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen ST, Lin YL, Huang MT, Wu MF, Cheng SC, Lei HY, et al. CLEC5A is critical for dengue-virus-induced lethal disease. Nature. 2008;453:672–676. doi: 10.1038/nature07013. [DOI] [PubMed] [Google Scholar]
- 45.Kim N, Takami M, Rho J, Josien R, Choi Y. A novel member of the leukocyte receptor complex regulates osteoclast differentiation. J Exp Med. 2002;195:201–209. doi: 10.1084/jem.20011681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barrow AD, Raynal N, Andersen TL, Slatter DA, Bihan D, Pugh N, et al. OSCAR is a collagen receptor that costimulates osteoclastogenesis in DAP12-deficient humans and mice. J Clin Invest. 2011;121:3505–3516. doi: 10.1172/JCI45913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Merck E, Gaillard C, Gorman DM, Montero-Julian F, Durand I, Zurawski SM, et al. OSCAR is an FcRgamma-associated receptor that is expressed by myeloid cells and is involved in antigen presentation and activation of human dendritic cells. Blood. 2004;104:1386–1395. doi: 10.1182/blood-2004-03-0850. [DOI] [PubMed] [Google Scholar]
- 48.Ochi S, Shinohara M, Sato K, Gober HJ, Koga T, Kodama T, et al. Pathological role of osteoclast costimulation in arthritis-induced bone loss. Proc Natl Acad Sci USA. 2007;104:11394–11399. doi: 10.1073/pnas.0701971104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zou W, Reeve JL, Liu Y, Teitelbaum SL, Ross FP. DAP12 couples c-Fms activation to the osteoclast cytoskeleton by recruitment of Syk. Mol Cell. 2008;31:422–431. doi: 10.1016/j.molcel.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zou W, Kitaura H, Reeve J, Long F, Tybulewicz VL, Shattil SJ, et al. Syk, c-Src, the alphavbeta3 integrin, and ITAM immunoreceptors, in concert, regulate osteoclastic bone resorption. J Cell Biol. 2007;176:877–888. doi: 10.1083/jcb.200611083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Faccio R, Zou W, Colaianni G, Teitelbaum SL, Ross FP. High dose M-CSF partially rescues the Dap12−/− osteoclast phenotype. J Cell Biochem. 2003;90:871–883. doi: 10.1002/jcb.10694. [DOI] [PubMed] [Google Scholar]
- 52.van Beek EM, de Vries TJ, Mulder L, Schoenmaker T, Hoeben KA, Matozaki T, et al. Inhibitory regulation of osteoclast bone resorption by signal regulatory protein alpha. FASEB J. 2009;23:4081–4090. doi: 10.1096/fj.09-131557. [DOI] [PubMed] [Google Scholar]
- 53.Mori Y, Tsuji S, Inui M, Sakamoto Y, Endo S, Ito Y, et al. Inhibitory immunoglobulin-like receptors LILRB and PIR-B negatively regulate osteoclast development. J Immunol. 2008;181:4742–4751. doi: 10.4049/jimmunol.181.7.4742. [DOI] [PubMed] [Google Scholar]
- 54.Shanmugarajan S, Youssef RF, Pati P, Ries WL, Rao DS, Reddy SV. Osteoclast inhibitory peptide-1 (OIP-1) inhibits measles virus nucleocapsid protein stimulated osteoclast formation/activity. J Cell Biochem. 2008;104:1500–1508. doi: 10.1002/jcb.21723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chung DH, Humphrey MB, Nakamura MC, Ginzinger DG, Seaman WE, Daws MR. CMRF-35-like molecule-1, a novel mouse myeloid receptor, can inhibit osteoclast formation. J Immunol. 2003;171:6541–6548. doi: 10.4049/jimmunol.171.12.6541. [DOI] [PubMed] [Google Scholar]
- 56.Chiu YH, Mensah KA, Schwarz EM, Ju Y, Takahata M, Feng C, et al. Regulation of human osteoclast development by dendritic cell-specific transmembrane protein (DC-STAMP) J Bone Miner Res. 2011;27:79–92. doi: 10.1002/jbmr.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hayashi M, Nakashima T, Kodama T, Makrigiannis AP, Toyama-Sorimachi N, Takayanagi H. Ly49Q, an ITIM-bearing NK receptor, positively regulates osteoclast differentiation. Biochem Biophys Res Commun. 2010;393:432–438. doi: 10.1016/j.bbrc.2010.02.013. [DOI] [PubMed] [Google Scholar]