Abstract
The emerging role of microRNAs (miRNAs) in the epigenetic regulation of many cellular processes has become recognized in both basic research and translational medicine as an important way that gene expression can be fine-tuned. Breast cancer is the most frequent cancer in women, with about one million new cases diagnosed each year worldwide. Starting with the early work of miRNA profiling, more effort has now been put on functions of miRNAs in normal mammary stem cells, breast cancer initiating cells and metastatic cells, and therapy-resistant cancer cells. Future translational studies may focus on identifying miRNA signatures as cancer biomarkers and developing miRNA-based targeted therapeutics.
Keywords: MicroRNAs, Breast cancer stem cells, Metastasis, Therapy resistance, MiRNA biomarkers, MiRNA therapeutics
Introduction
The discovery in the 1990s of microRNAs (miRNAs, miRs), powerful epigenetic regulators that have an overall inhibitory effect on expression of genes, opened a new layer of regulation in understanding both normal and cancer development. This also initiated the exploration for the use of miRNAs in therapeutic applications. With a length of 20–22 nucleotides, miRNAs are small non-coding single-stranded RNAs transcribed from genomic DNA and processed to mature miRNAs by Drosha in the nucleus and subsequently Dicer in the cytoplasm [1, 2]. Although most miRNAs are yet to be characterized in terms of function and the signaling pathways that they regulate, certain mammalian miRNAs have emerged as critical regulators of stem cell function, self-renewal, epithelial–mesenchymal transition (EMT), cancer initiation, therapy resistance, and metastasis [3–15].
Based on American Cancer Society data (http://www.cancer.org), breast cancer still remains the most frequent cancer among American women, with an estimated 230,000 (29 %) new cases diagnosed and about 40,000 (14 %) deaths in 2012. It is therefore essential to better understand the underlying molecular mechanisms and to develop more effective treatments. Translational medicine is needed to efficiently bridge basic research and clinical medicine with novel biomarkers and therapeutics.
In this review, we will focus on the expression and function of miRNAs in normal mammary development, breast cancer initiation and progression, and the potential clinical applications using miRNAs as novel biomarkers and therapeutics.
MicroRNAs in normal mammary stem cells
In the mammary gland, multi-potent epithelial stem cells and progenitors are thought to generate and maintain both myoepithelial and luminal epithelial lineages, each with a distinct expression status of cytokeratins (e.g., CK14 and CK8) and estrogen receptor (ER) [16]. Mouse and human multi-potent mammary epithelial stem cells have been identified based on in vitro and in vivo functional assays of sorted cells using cell surface markers, such as CD24 and CD49f [17], CD24 and CD29 [18], CD49f and EpCAM [19], or ALDH1 [20]. Utilizing genetic lineage-tracing approaches, Blanpain et al. [21] recently demonstrated that unipotent K14+ myoepithelial stem cells and K8+ luminal stem cells are able to maintain their respective postnatal lineages during development, adulthood, and pregnancy.
The Clarke laboratory discovered that the clustered miR-200 family members (miR-200a, -200b, -200c, -141, -182, -183, and -96) are down-regulated in mouse mammary stem cells (CD24medCD49fhighLineage−) compared to that of luminal progenitor cells (CD24highCD49flowLineage−) [12]. Functional studies support a pivotal role of miR-200c in regulating self-renewal of mammary stem cells through a direct targeting of BMI-1 [12], which encodes a subunit of the polycomb repressor complex 1 (PRC1) and functions as a self-renewal regulator. Expression of the self-renewal regulator BMI-1 is inhibited by miR-200c through direct interactions between the seeding sequence of miR-200c and the 3′UTR of BMI-1 [12, 22]. The miR-200 family also promotes mesenchymal–epithelial transition (MET) by targeting ZEB1 and ZEB2 with increased expression levels of E-cadherin in various cell lines [9–11]. In development, conserved miR-200 family members target FOG2 and the downstream PI3K and AKT pathway in many tissues [23].
The mammary progenitor population (ALDHbrightSca-1high) isolated from Comma-Dβ cells exhibits high expression levels of miR-205 and miR-22, but low let-7 (let-7b and -7c) and miR-93 levels [24]. Hannon et al. [24] constructed a let-7c sensor with its perfect complement introduced into the 3′UTR of DsRed, thereby marking let-7 low cells DsRed-positive and let-7 high cells DsRed-negative. They found that DsRed+ (let-7low) cells can mark or enrich self-renewing populations, and enforced let-7 expression removes self-renewing cells from mixed cultures [24]. As one of the earliest identified miRNAs, let-7 is conserved between C. elegans [25] and mammals, and functions as a fundamental stem cell/differentiation regulator and tumor suppressor gene [26] by targeting let-60/RAS [27].
In addition to an up-regulation in progenitor-like AldefluorhiSca-1hi or Sca-1+ cells [24], miR-205 is also highly expressed in normal adult mammary stem cell populations (Lin−CD24+/loSca-1−, Lin−CD29hiCD24+, or Lin−CD49fhiCD24med) and myoepithelial cells [28, 29]. Other miRNAs, such as miR-138 and miR-431, are down-regulated whereas miR-133a and miR-133b are up-regulated in mammary glands during pregnancy and lactation compared to virgin and involuting mammary glands [30]. However, the functions of these miRNAs in mammary stem cells or differentiation have not been well characterized.
MicroRNA signatures as biomarkers in tumor diagnosis and prognosis
MicroRNA profiling methodology
The detection techniques for genome-wide miRNA expression profiling include oligonucleotide miRNA microarray analysis [31], bead-based flow cytometry [32], miRNA serial analysis of gene expression (miRAGE) [33], the RNA-primed, array-based Klenow enzyme (RAKE) assay, quantitative real-time PCR [34], and RNA deep sequencing [35]. Among these methods, microarray is the most frequently used and commercialized high-throughput technology, but it cannot distinguish mature miRNAs from pre-miRNAs or pri-miRNAs. Real-time PCR greatly improves detection specificity for mature miRNAs, and certain platforms are also available for high-throughput profiling. It is also used as a complementary validation approach after other high-throughput screenings. RNA sequencing represents the most advanced and expensive technology, which provides detailed information about mature sequences, precursors, genome locations, maturation processes, inferred transcriptional units, and conservation patterns [35].
MiRNA profile data analyses are very similar to those for mRNA or DNA profile data, such as hierarchical clustering, significance analysis of microarrays (SAM) for gene and outcome association analyses, and prediction analysis of microarrays (PAM) for signature identifications [36]. The logistic regression and Kaplan–Meier survival estimator have been applied to identify prognostic biomarkers.
MicroRNA signatures of tumor tissues
Given the greater stability of miRNAs relative to mRNAs and the easier detection of miRNAs in circulation and paraffin-embedded tissues, miRNA signatures emerge as novel biomarkers for various tumors [36, 37].
Using bead-based flow cytometric assays, Lu et al. [32] first profiled aberrant expression patterns of miRNAs in a large set of multiple human tumor tissues (n = 334), including breast cancer. They found that more than half of measured miRNAs were down-regulated in tumors compared to normal tissues. Hierarchical clustering of miRNA expression profiles for these tumors also implicated the developmental lineages and differentiation states of the tumors, suggesting a diagnostic advantage of miRNA profiles over mRNA expression [32]. However, this study, done a few years ago, only measured expression levels of the 217 miRNAs then known. With over 1,000 human miRNAs identified so far, new technologies for miRNA profiling will provide more comprehensive analyses of known or unknown miRNAs.
To identify miRNA signatures in solid tumors, Volinia et al. [38] profiled tumors of breast and other tissues using global miRNA microarrays. They found that miR-17-5p, miR-20a, miR-21, miR-92, miR-106a, and miR-155 are strongly associated with tumor development. These are possibly oncogenic and tumor-suppressor-like miRNAs that target tumor suppressors and oncogenes, respectively, such as miR-106a targeting RB1 and miR-20a targeting TGF-βR2, with inverse expression correlations between miRNA::mRNA pairs [38]. Clustering analyses identified shared miRNA signatures across 6 solid tumors, and 15 up-regulated miRNAs and 12 down-regulated miRNAs were used to classify normal and cancerous breast tissues [38].
In another study using miRNA microarray technology, Croce and colleagues identified a group of miRNAs differentially expressed in breast cancer versus normal breast tissue, including down-regulated let-7, miR-125, miR-10b, and miR-145, and up-regulated miR-155, miR-191, miR-21, miR-206, miR-210, and miR-213 [39]. Furthermore, a group of miRNAs were found to be associated with ER status (miR-26, miR-30, miR-185, miR-191, miR-206, and miR-212), progesterone receptor (PR) status (let-7c, miR-26a, miR-29b, and miR-30), tumor stage (miR-21, miR-181a, miR-30a–s, miR-213, etc.), lymph node metastasis (let-7 and miR-9-3), vascular invasion (miR-10b, miR-123, miR-29a, miR-205, etc.), and proliferation index (let-7, miR-26, miR-30a-5p, miR-102, and miR-145) [39]. MiR-145promotes ES cell differentiationbya direct targeting of the core pluripotency factors OCT4, SOX2, and KLF4, thereby repressing the self-renewal program [40].
Similar to mRNA expression profiling, which have been used to classify breast tumors into four or five intrinsic subtypes [41, 42], miRNA expression profiles may also be applied to classify breast tumors, such as luminal A, luminal B, basal-like, HER2+, and normal-like [43]. Individual miRNAs, such as let-7 family members, have been found to be associated with tumor subtype, ER status, and tumor grade [43].
Recent studies by Buffa et al. [44] integrated mRNA and miRNA expression profiling (n = 207) to identify miRNA-associated progression pathways in breast cancer. In Tamoxifen-treated ER+ breast tumors, up-regulated miR-135a in combination with a few down-regulated miRNAs (miR-128a, miR-767-3p, and miR-769-3p) represented a favorable low-risk signature that correlated with prolonged distant relapse-free survival (DRFS) [44]. In ER− breast tumors, the low-risk signature for DRFS integrated up-regulated miRNAs (miR-342, miR-30c, miR-150, and miR-768-3p) along with down-regulated miRNAs (miR-27b, miR-144, and miR-210) [44]. Bockhorn et al. [45] demonstrated that miR-30c is an individual prognostic marker for breast tumor DRFS.
Predicted miRNA target genes (e.g., MAPK14, MAPK8, and SPRY2) inversely correlated with miRNAs (miR-128a, miR-144, and miR-210, respectively) can further strengthen the prognostic power of the miRNA–mRNA combined signature and may indicate a role in regulating tumor growth and metastasis [44]. In triple-negative cancers, decreased miR-150 is associated with increased expression of multiple predicted targets including AKT2, ERBB3, S6K, and MAPK pathways. A similar pattern was found for miR-342 expression and expression of RRM2, G1,6-BPS, and TLE-1 [44]. The role of these genes in growth control may be relevant to the poor prognosis of many triple-negative breast cancers.
The above studies provided useful biomarker information for breast cancer. However, all these miRNA profiling studies were based on mixed populations of tumor and stromal cells. Additional studies will need to focus on further specific profiling for purified tumor cells and stromal cells, and even subpopulations of cancer cells, such as tumor initiating cells or cancer stem cells as discussed below. Furthermore, combined profiling at genomic, epigenetic, and protein modification levels will be necessary for personalized cancer medicine. Except for a few miRNAs, the functions of most prognostic miRNAs in breast cancer initiation and progression remain to be elucidated.
Role of microRNAs in breast cancer initiation and progression
Many miRNAs have consistent functions in a broad spectrum of tissues and tumors while others may have tissue-specific, tumor-specific, or tumor progression stage-specific roles. In addition to functional dissection of miRNAs in cancer, identification of their downstream target genes, especially direct targets in the functional signaling pathway, becomes one of the most essential aspects of miRNA characterization studies. Overall, most miRNAs inhibit target genes by interacting with the 3′UTRs of coding genes. Experimental validations include reduced target gene expression at the mRNA and/or protein levels and inhibitory effects of miRNAs on a luciferase reporter gene located upstream of the candidate gene 3′UTR. The functional importance of a target gene is usually examined by evaluating the effects of gene overexpression in miRNA phenotype rescue studies or gene knockdown in miRNA phenotype copying evaluations.
Introduction of breast cancer stem cells (BCSCs)
Increasing evidence suggests that cancer stem cells (CSCs) or tumor initiating cells (TICs) are a portion of tumor cells with stem cell-like properties, which can propagate human tumors with heterogeneous tumor populations in immunodeficient mice [46–60]. Based on cell surface markers CD44 and CD24, Clarke and colleagues [46] first identified human breast tumor initiating cells (BTICs) or breast cancer stem cells (BCSCs) that are able to regenerate breast tumors in vivo. They also discovered an “invasiveness” gene signature of BCSCs correlating with metastasis and poor prognosis [61]. Recent studies suggest that pancreatic CSCs mediate cancer metastasis [53]. Liu et al. [62] demonstrated that BCSCs are involved in spontaneous metastases of human breast cancer in orthotopic xenograft models. BCSCs are intrinsically resistant to traditional cancer therapies, such as chemotherapy and radiotherapy [63, 64]. While the epithelial–mesenchymal transition (EMT) marks invasive cells, Weinberg et al. [65] demonstrated that EMT induces stem-cell like properties in immortalized cells. However, it remains unclear what mechanisms underlie CSC-mediated tumor initiation, metastasis, and therapy resistance in vivo. MiRNAs function as a new mechanism to modulate multiple processes as discussed below.
MicroRNAs in breast cancer initiation
The family members of miR-200 (miR-200a, -200b, -200c, -141, -182, -183, and -96) have been shown to be down-regulated in CD44+CD24−Lin− human primary BCSCs compared to non-tumorigenic cancer cells [12]. Expression of the let-7 family (let-7a, b, c, d, e, f, g, and i) is reduced in both SKBR3 mammospheric cells and clinically isolated BCSCs, with a similar expression pattern compared to that of the miR-200 family [13]. From breast tumor profiling, these two miRNA families often share similar expression patterns, with differential expression across breast tumor subtypes [45]. The pivotal roles of miR-200 and let-7 are relatively well characterized in breast cancer initiation and self-renewal in addition to their general regulatory functions in normal development.
Shimono et al. [12] demonstrated that enforced expression of miR-200c disrupts BCSCs-mediated colony formation in vitro and inhibits tumorigenesis in vivo. Overexpression of 3′UTR-deficient BMI-1 rescues the phenotype caused by miR-200c, suggesting the importance of BMI-1 as a direct target in the miR-200c-mediated pathway regulating breast cancer initiation [12]. MiR-200b also regulates BCSC growth by directly targeting Suz12, which is a subunit of PRC2 and regulates EMT by repressing E-cadherin [66]. Many other groups have reported that miR-200 family members (miR-141, miR-200a, b and c, and miR-429) regulate EMT by targeting the transcriptional repressors ZEB1 and ZEB2, thereby increasing E-cadherin expression [8–11].
Let-7 is a known tumor suppressor that targets oncogenes RAS and HMGA2 [13, 27, 67]. Yu et al. [13] reported that reduced let-7 is required for functional SKBR3 mammospheric cells (SK-3rd), as enforced expression of let-7 suppresses self-renewal and tumor initiation, while reduced let-7 levels, mediated by a let-7a antisense oligonucleotide, inhibit cell differentiation without affecting proliferation of these cells.
Similar to let-7, miR-30e was also shown to be down-regulated in BCSCs and SK-3rd mammospheres compared to non-tumorigenic cells or more differentiated cells, respectively [68]. Enforced expression of miR-30e inhibits mammosphere formation and tumorigenesis of SK-3rd cells in vitro and in vivo, respectively, by targeting ITGB3 and UBC9 [68].
Other miRNAs, such as the miR-181 family members (miR-181a and miR-181b) and miR-155, were reported as onco miRs which promote self-renewal, sphere formation, colony formation, or tumor development of breast cancer cells [69, 70]. The tumor suppressor ATM is a direct target of miR-181 and inhibits sphere formation of BT474 and MDA-MB 361 breast cancer cells by phosphorylating CHK2 [69]. Consistently, knockdown of ATM accelerates in vitro sphere formation and in vivo tumorigenesis of MDA-MB 361 breast cancer cells [69]. Notably, inflammation-associated miR-155 directly inhibits the tumor suppressor gene suppressor of cytokine signaling 1 (socs1) as well as activating the JAK-STAT3 signaling pathway in breast cancer cells [70].
MicroRNAs in breast cancer metastasis
Metastasis causes 90 % of solid tumor-related mortality. In this cancer progression process or distant relapse, cancer cells need to accomplish five sequential steps: invade surrounding tissue, intravasate into tumor-associated vasculature or lymphatic vessels, circulate via the bloodstream or lymphatics, extravasate to, and then colonize at distant sites in the body [71]. Different cancers may metastasize to different organs, and breast cancer frequently colonizes the lungs, bones, and brain [72, 73]. Metastatic cancers are often resistant to traditional therapies and not curable in the clinic, thus requiring more comprehensive understanding of the molecular mechanisms involved. Research in the past five years has identified a number of miRNAs that regulate metastasis, especially breast cancer metastasis, such as miR-10b [5], miR-335 [6], miR-373, and miR-520 [4]. In addition, miR-200 and let-7 families are also involved in the regulation of metastasis [9, 11, 13].
Although miR-10b was found to be down-regulated in breast cancer compared to normal tissue [39], Ma et al. [5] reported miR-10b as a metastasis promoter in breast cancer cells, with higher expression in metastatic MDA-MB-231 cells compared to non-metastatic MCF-7 or MCF-10A cells. Anti-miR-10b inhibitor suppresses invasion of MDA-MB-231 cells, while enforced miR-10b expression enhances migration and invasion of non-metastatic HMEC and SUM149 cells as well as intravasation and lung metastases of SUM149 breast cancer cells in vivo [5]. MiR-10b directly targets HOXD10, which further suppresses the downstream oncogenic RHOC, suggesting a signaling cascade mediated by miR-10b, HOXD10, and RHOC in breast cancer cell migration and invasion [39]. However, in a separate study, the clinical profile of down-regulated miR-10b in breast tumors was not convincing to support a role for miR-10b in promoting human cancer metastasis [74].
By profiling the MDA-MB-231 parental cell line and its derived bone/lung-metastatic cell lines (BoM1 and LM2), Tavazoie et al. [6] identified eight miRNAs (miR-335, miR-199a*, miR-122a, miR-126, miR-206, miR-203, miR-489, and miR-127) down-regulated in derived metastatic cells. Three of them, miR-335, miR-126, and miR-206, showed a similar reduced expression in other patient tumor-derived malignant cell lines (CN34-LM1 and CN34-BoM1) compared to parental cancer cells [6]. The restoration of these three miRNAs significantly inhibited dissemination and metastases of metastatic cell populations, where miR-335 and miR-206 suppressed migration and morphology but miR-126 reduced tumor growth and proliferation [6]. Expression levels of miR-335 and miR-126 are significantly associated with metastasis-free survival of breast cancer patients [6]. Based on microarray analyses of miR-335-expressing cells and LM2/BoM1 cells, the overlapping metastasis-relevant target gene signature of miR-335 includes SOX4, TNC, PTPRN2, MERTK, PLCB1, and COL1A1, the first four of which are direct targets [6]. SOX4 and TNC are important functional targets of miR-335, as knockdown of SOX4 or TNC decreases the migration, invasion, and lung colonization of LM2 cells [6].
Huang et al. identified migration-promoting oncomiRs, miR-373 and miR-520, by employing a forward screening approach with a retroviral miRNA expression library (n = 450) to transduce MCF-7 cells [4]. These two miRNAs also enhance MCF-7 cell invasion in vitro and tail vein-inoculated lung metastasis in vivo [4]. Microarray analysis combined with TargetScan prediction and experimental validations demonstrated that CD44 is a direct functional target of miR-373 and miR-520c in metastasis regulation [4]. However, CD44 is a breast cancer stem cell marker [46], and CD44 knock-out and other studies support the role of CD44 in promoting tumorigenesis and/or metastasis (see review [75] and other references [76–78]). As MCF-7 cells may have cell-specific CD44 splicing variants or post-translational modifications, functions of the signaling cascade mediated by miR-373/miR-520-CD44 in metastasis regulation may also be cell context-dependent.
In human breast cancer, high levels of miR-103/107 are associated with poor outcome and promote metastasis and mesenchymal fate by targeting Dicer, resulting in down-regulation of many miRNAs, including miR-200 levels [79].
Other miRNAs involved in breast cancer metastasis include miR-21 [80], miR-193b [81], and miR-31 [82]. Mo [80] and colleagues reported that miR-21 promotes invasion and metastasis of MDA-MB-231 cells by a direct targeting of tumor suppressor genes TPM1, PDCD4, and maspin. MiR-31 inhibits MDA-MB-231 breast cancer cell-mediated metastasis by targeting a cohort of prometastatic genes Fzd3, ITGA5, RDX, and RhoA [82].
In addition to the regulatory role in normal development and tumorigenesis, the well-characterized miR-200 family and let-7 family also inhibit invasion, metastasis, and EMT of breast cancer [8, 9, 11, 13, 67]. However, while EMT is a critical feature for initiation of metastasis (invasion), homing of metastatic cells to distant organs and colonization may require a mesenchymal–epithelial transition (MET). One recent study argued that miR-200a might promote metastasis by accelerating the last step of metastasis colonization [83].
Therefore, it is important to reconcile the roles of miRNAs at different stages of metastasis. Because invasion is the rate-limiting step of metastasis, much attention has been drawn to prevent and inhibit cancer invasion. However, the dynamics between EMT and MET during early and late stages of metastasis create an even more complicated situation for understanding and for formulating translational applications. How to effectively target meta-static cells at different stages requires more comprehensive investigations.
MicroRNAs in therapy resistance and tumor progression
As breast tumors or tumor-initiating cells may exhibit intrinsic resistance to cancer therapies [64, 84], locally and distantly relapsed tumors often show acquired resistance to therapy. Therapy resistance is closely related to tumor progression and patient prognosis. The role of miRNAs in therapy resistance of breast cancer remains poorly characterized. A few miRNAs are listed below with their functions in regulating therapy resistance in vitro, but their role in therapy resistance has not yet been demonstrated in breast cancer models in vivo. Our recent studies, which take advantage of primary tumor-derived xenograft models labeled with a Luc2-eGFP or Luc2-tdTomato reporter, have facilitated the functional study of miRNAs in regulating chemo-resistance and tumor progression in vivo [45, 62].
Expression of miR-221/222 has been reported to be up-regulated in tamoxifen-resistant MCF-7 cells or ERα-negative breast cancer cells, as well as in endocrine therapy-resistant HER2+ or ERα− primary breast cancer tissues [85, 86]. In addition, ectopic expression of miR-221/222 can confer resistance to tamoxifen in MCF-7 cells by targeting the cell cycle inhibitor p27Kip1, resulting in increased proliferation and reduced apoptosis [85], whereas knockdown of miR-221/222 restored tamoxifen sensitivity in MDA-MB-468 cells by increasing the expression of the direct target ERα [86].
Up-regulation of miR-221/222 and miR-125b was also found in paclitaxel-resistant MDA-MB-435 breast cancer cells [87]. As one of the additional chemo-resistance regulators, miR-125b confers paclitaxel resistance in multiple breast cancer cell lines by a direct targeting of apoptosis-inhibitor BAK1 [87].
By miRNA array profiling, Kovalchuk et al. [88] identified 63 up-regulated and 75 down-regulated miR-NAs in doxorubicin-resistant MCF-7 cells (MCF-7/DOX) compared with the parental cells. In MCF-7/DOX cells, down-regulated miR-127, miR-34a, miR-27b, and let-7 were associated with increased anti-apoptotic targets BCL6, NOTCH1, CYP1B1, and K-RAS, while up-regulated miR-206, miR-106a, miR-21, and miR-214 correlated with decreased levels of ERα, RB1, and PTEN target proteins [88]. However, the role of each miRNA in the resistance to doxorubicin therapy was not characterized. Although these authors demonstrated that overexpressed miR-451 sensitizes MCF-7 cells to doxorubicin treatment by a direct targeting of the Multi-Drug Resistance 1 (mdr1) gene, miR-451 was not shown to be among the down-regulated miRNAs of MCF-7/DOX cells [88].
Acquired resistance to cisplatin in MCF-7 cells correlated with altered miRNAs and their targets, such as up-regulation of miR-146, miR-10a/b, miR-221/222, miR-29a/b, and miR-206, as well as down-regulation of miR-200b/c, miR-345, miR-126, miR-205, and miR-342 [89]. MiR-345 and miR-7 were shown to sensitize MCF-7 cells to cisplatin treatment by a direct targeting of the MRP1 (Abcc1) gene, which encodes the efflux-pump multi-drug resistance protein 1 [89].
Many miRNAs also regulate therapy resistance in other cancer types. For instance, miR-214 induces cisplatin resistance by targeting PTEN in ovarian cancer [90]. In lung cancer cells, elevated levels of let-7 family members cause radiosensitization, whereas down-regulation of let-7 results in radioprotection [91]. In a C. elegans-based in vivo model, inhibition of the let-7 targets RAS and DNA damage response pathway components (RAD51, RAD21, FANCD2, and CDC25) mimicked the ability of let-7 to decrease radio resistance [91].
Regulation of microRNAs in cancer
As aberrant expression of miRNAs contributes to development and progression of various cancers including breast cancer, it is important to understand the mechanisms regulating miRNA expression. Similar to protein coding genes, miRNA expression is regulated at genomic, epigenetic, and post-transcriptional levels (see review [36]).
Calin et al. [92] reported that 98 of 186 miRNA genes (52.5 %) are located in cancer-associated genomic regions, such as fragile sites, minimal regions of loss of heterozygosity, minimal regions of amplification (minimal amplicons), and common breakpoint regions. For example, a few tumor suppressor miRNAs (miR-15a, miR-16-1, let-7 family members, and miR-29a/b) are located in regions (13q14, 302, 9d22.3, 11q23–q24, 21q21, and 7q32) susceptible to chromosome deletions in prostate cancer, leukemia, breast cancer, and other tumors [92, 93]. Other onco miRs, such as miR-17–92 (13q31.3) and miR-155 (21q23), are overexpressed in lymphoma and many other tumors due to amplifications [92]. Sequence mutations have also been identified in the loci of miR-15a-16-1 and miR-29 family members [92].
Altered miRNA levels in breast cancer cells, such as SKBR3, might be due to epigenetic deacetylations on histones, as HDAC inhibition increased expression of over 20 miRNAs and decreased expression of a few other miRNAs [94]. DNA methylation also plays an important role in down-regulating miRNAs in solid tumors [94].
Specifically, Png et al. [95] reported both genetic and epigenetic mechanisms that contribute to decreased expression of the miR-335 metastasis suppressor in meta-static breast cancer cells. First, copy number loss of the miR-335 gene at the 7q32.2 locus was found in more metastatic derivatives of MDA-MB-231 cells compared to the parental cell line [95]. Second, methylation-specific PCR and pyrosequencing revealed hyper-methylated CpG islands at the Mest/miR-335 promoter, while inhibition of DNA methylation with 5-aza-deoxycytidine (5-Aza) increased endogenous miR-335 expression [95].
Transcription factor-mediated regulation has been demonstrated for a few miRNAs that regulate metastasis or stem cell functions, such as miR-10b promoted by TWIST [5], the miR-17-92 cluster directly transactivated by c-Myc [96], and miR-200 and miR-203 repressed by ZEB1 [8, 22]. A recent study reported that ERα positively regulates miR-200c expression by directly binding to the miR-200c promoter, whereas the ERα promoter is repressed by IL-6-activated STAT3 [97]. Experimental evidence for direct transcriptional regulation include altered effects of modified transcription factors (TFs) on miRNA expression, endogenous interactions between TFs and the miRNA promoters examined by chromatin-immunoprecipitation, functional interactions between TFs and promoters by luciferase reporter assays, and specific binding by gel-shift assays.
The differential expression profiles or miRNAs across breast tumor subtypes might correlate with genomic loss or gain, primary transcription, and biogenesis or processing pathway components, such as DICER1 and AGO2 [43]. Deficiency or down-regulation of Drosha and Dicer has been found in triple-negative and basal-like breast tumors with poor prognosis [98]. For instance, the processing of let-7 family members was inhibited at the step of Drosha cleavage by LIN28 and LIN28B, two homologs of the C. elegans heterochronic gene lin-28, in both stem cells and primary tumors [99, 100].
Lastly, functional inhibition of miRNAs by saturated binding to introduced substrates or sponges has been utilized in experimental studies [101].
Future direction: microRNAs as cancer therapeutics
Due to their relatively small molecular weight (~6,000) and the endogenous power of targeting broad-range regulators, miRNAs have emerged as promising therapeutics—miRNA inhibition (antagomirs) for onco miRs and miRNA replacement therapy for tumor suppressor-like miRNAs.
Because miRNA therapeutic development is relatively new, it can be facilitated by the more established siRNA therapeutics, since miRNAs share many common molecular properties and cellular processing machinery with siRNAs. MiRNAs target a broad range of target genes in a context-dependent manner. Thus, while miRNAs are powerful regulators, they may also elicit unexpected effects in certain cells or tissues. The promise of miRNA therapeutics in pre-clinical tumor models has been investigated by several research groups and has stimulated industry investment within the past few years. Since there are still no specific treatments available for triple-negative breast cancers beyond conventional therapies, we anticipate that novel therapeutics, including miRNAs and others, will provide additional options and pave the way for more personalized cancer medicine. However, the limitations of miRNA therapeutics must be overcome. It is still very challenging to deliver tumor cell-specific miRNA therapeutics via an affordable and convenient approach.
Delivery, stability, and tissue bioavailability
Although synthetic miRNAs might be locally or systemically (intravenously) administered, negatively charged miRNAs have difficulty in penetrating hydrophobic cellular membranes, therefore requiring assisted cellular delivery. One approach is the use of lentiviral or adenoviral vectors expressing miRNA precursors or inhibitors (antagomirs) [102, 103]. However, the production of mature miRNAs is dependent on endogenous miRNA processing machinery, which is often compromised in tumor cells. A related concern is the safety issue of viral vectors in clinical applications. Other delivery approaches include nanoparticles or liposomes, which are among the very active forefront technologies for future translational medicine. Physiologically and pathologically, one type of small secreted membranous vesicles, called exosomes, may deliver RNAs including miRNAs, proteins, and lipids among different cells, such as tumor cells and stem cells [104, 105] (see review [106]). However, it remains unclear and challenging how to engineer exosomes for delivering miRNA therapeutics. Free miRNAs may also bind to high-density lipoproteins for transportation in plasma and possibly delivery to the cells in the liver tissue [107].
MiRNAs are relatively more stable than mRNAs, but the half-life in circulation is still too short for practical therapeutic applications. Chemical modifications to stabilize miRNA inhibitors or antagomirs include (1) 2′-O-methyl (2′-O-M) ribose modification on each nucleotide, (2) modified phosphorothioate linkage, and (3) cholesterol functionality at the 3′ end [108]. These can prolong the half-life of miRNAs and promote tissue distribution in liver, heart, kidney, adipose, and lungs [108]. Nanoparticle encapsulations of miRNAs would protect the stability and prolong half-life time of miRNAs in circulation.
Tissue-specific and cell-specific delivery is the goal of all targeted therapies. For miRNA therapeutics, approaches for targeted delivery may include conjugating miRNAs/antagomirs or conjugating encapsulating particles to tissue/cell-specific ligands or antigen-recognizing antibodies.
MicroRNA inhibition
Endogenous miRNAs can be silenced by antisense oligo-nucleotides (ASO) or antagomirs with chemical modifications, such as 2′-O-M ribose, phosphorothioate linkage, and cholesterol functionality [108]. Davis et al. [109] reported that 2′-O-methoxyethyl (2′-O-MOE) substituted RNA oligonucleotides, as well as a mixed locked nucleic acid (LNA)–DNA ASO and 2′-flouro (2′F) substitution improved targeting affinity for better efficiency than other modifications in inhibiting miR-21. Synthetic LNA-antimiR-122 was effectively administered via intravenous or intraperitoneal injections at doses of 1–10 mg/kg to antagonize the liver-expressed miR-122 in non-human primates [110]. However, for tumor treatment, it may require higher doses as the liver, kidney, and other organs may take up a large portion of antagomirs nonspecifically.
To inhibit oncomiR miR-10b’s functions in breast tumor metastasis, Ma et al. [101] utilized modified antagomir-10b to inhibit cell motility and invasion of mouse breast tumor 4T1 cells in vitro at 50 μg/ml and in vivo at 50 mg/kg. No obvious side effect of the antagomir-10b was observed in the mouse model studies [101]. In neuroblastoma progression, the miRNA 17-5p-92 cluster functions as oncomiRs to promote cell growth and antagomir-17-5p abolishes therapy resistance by restoring p21 and BIM [111]. Antagomirs have also been used to treat other diseases, such as antagomir-133 for cardiac hypertrophy [112].
MicroRNA replacement therapy
As breast cancer and many other tumors show overall down-regulated miRNAs expression compared to normal tissues [32], miRNA replacement therapy is intended to restore the levels and functions of down-regulated tumor suppressor-like miRNAs, thereby inhibiting tumor initiation and progression. Distinct from the single-strand antagomirs, miRNA replacement therapy requires double-stranded miRNAs for functional recruitment to the RNA-induced silencing complex (RISC). The doubled molecular weight of miRNAs may be a further hurdle to overcome for efficient cellular delivery. Another big challenge is targeted tumor distribution with minimized uptake by the liver and other organs. Theoretically, viral vectors, liposomes, or nanoparticles designed for targeted delivery of siRNA therapeutics may also be appropriate for miRNA encapsulation and delivery.
Experimental miRNA replacement therapies have been tested in many solid tumor models, and these miRNA delivery approaches could possibly be applied to breast cancer treatment. A few examples are listed below using adenoviral vectors, nanoparticles and lipid-based materials to deliver miRNAs in vivo.
Kota et al. [103] utilized adeno-associated virus (AAV) to systemically deliver miR-26a (1012 AAV vector genomes per animal by tail vein injection), which directly targets cyclins D2 and E2, for treating liver cancer with inhibited cancer cell proliferation and induced tumor-specific apoptosis. In this study, normal tissues tolerated the treatment well [103], suggesting the promise of miRNA replacement therapy with minimal side effects.
Ibrahim et al. [113] took advantage of siRNA-delivering nanoparticle material polyethylenimines (PEI) F25-LMW to encapsulate non-modified miR-145 and miR-33 (double-stranded Dicer processing product) at an N/P ratio = 33. When systemically or locally administered, both PEI/miR-145 and PEI/miR-33a showed significant tumor-inhibiting effects on a colon cancer model [113].
Complexed with a lipid-based transfection reagent si-PORT amine (siPORT), let-7b was shown to inhibit lung tumor growth when repeatedly administered to mice by intratumoral injections (6.25 μg miR per injection to subcutaneous tumors) [114]. An intranasal delivery of let-7a to lung tumors was facilitated by lentiviral vectors (106 infectious units per injection) [114].
Using a lipid-based delivery tool, Wiggins et al. [115] reported that intratumoral or systemic injections of miR-34a (100 μg of oligo) induced growth-inhibiting and apoptosis-inducing effects in cultured cancer cells and in vivo by repression of c-Met and Bcl-2, as well as partial repression of CDK4.
However, no study has yet achieved targeted delivery of miRNA therapeutics. Future directions will need to focus on developing tumor-specific and cancer stem cell-targeted miRNA therapeutics to combine with other cancer therapies, thereby contributing to the prevention and eradication of breast and other kinds of cancer.
Conclusions
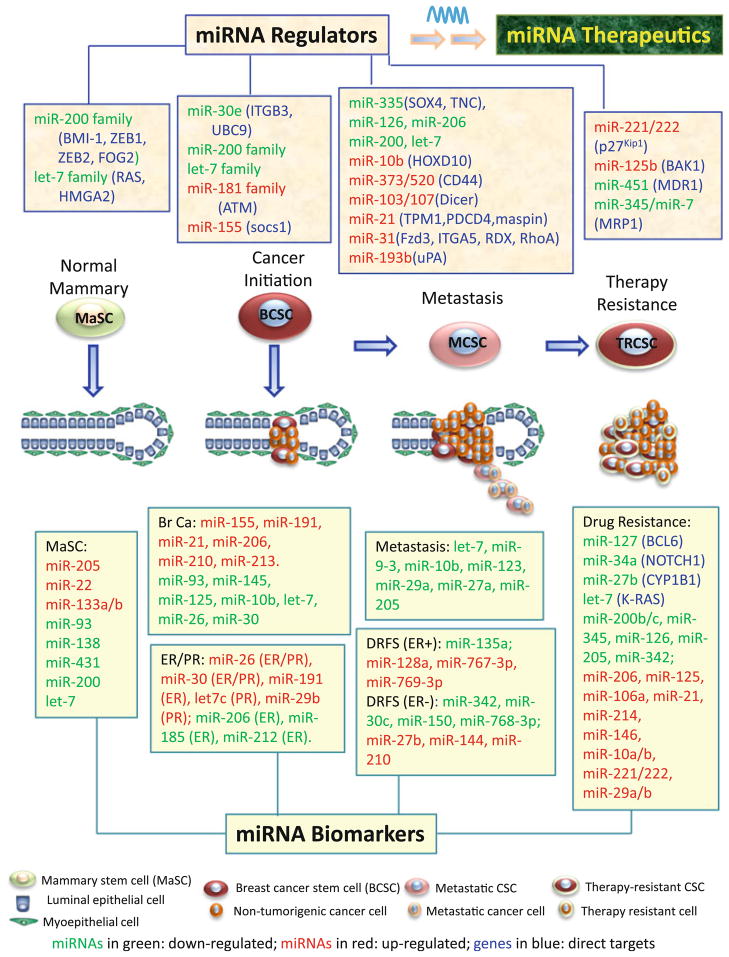
Overall, as summarized in Fig. 1, many miRNAs have been identified as biomarkers and/or characterized as indispensable regulators in both normal mammary, and breast cancer development, including cancer initiation, metastasis, and therapy resistance. The functional miRNA regulators hold the potential for development of novel miRNA therapeutics, including both antagomirs to inhibit oncomiRs and miRNA replacement therapy to restore levels of tumor suppressor-like miRNAs.
Fig. 1.
A summary of miRNA regulators and biomarkers in the development of the normal mammary gland, breast cancer initiation, metastasis, and therapy resistance. In these four processes, mammary stem cells (MaSC), breast cancer stem cells (BCSCs), metastatic CSCs (MCSCs), and therapy-resistant CSCs (TRCSCs) are pivotal originators and/or critical cell players. The top panels list miRNA regulators, including suppressor-miRs in green and oncomiRs in red, in normal mammary gland development (MaSCs-originated), breast cancer initiation (BCSCs-mediated), breast cancer metastasis, and therapy resistance, respectively. These tumor-inhibiting suppressor-miRs and tumor-promoting oncomiRs are promising candidates for therapeutic intervention as miR replacement therapy or antagomirs. The bottom panels list miRNA biomarkers, coded as up-regulated miRs in red and down-regulated miRs in green, which showed differential expression in normal MaSCs/mammary glands and primary breast tumors compared to distinct counterparts: (1) In MaSC panel, let-7b/c, miR-205, -22, -93, and -200 are differentially expressed in MaSCs and/or mammary progenitors compared to differentiated epithelial cells; miR-138, -431, and -133a/b are related to mammary glands during pregnancy and lactation, compared to virgin and involuting glands. (2) Br Ca (breast cancer) panel presents differentially expressed miRs in breast cancer versus normal breast tissue. (3) ER/PR panel displays miRs related to ER or PR status (ER+ or PR+ tumors vs. ER− or PR− tumors respectively). (4) Invasion panel shows down-regulated miRs in tumors with lymph node metastasis (let-7 and miR-9-3) or vascular invasion (miR-10b, miR-123, miR-29a, miR-205, etc.). (5) DRFS panel includes up- and down-regulated miRs related to high risk or poor prognosis of ER+ and ER− breast tumor patients. (6) Drug resistance panel lists miRs (a few with direct target genes) related to drug resistance of breast cancer cells or primary tumors, such as tamoxifen resistance of MCF-7 cells and HER2+/ER− tumors (miR-221/222), paclitaxel resistance of MDA-MB-435 cells 468 cells (miR-221/222, -125b), doxorubicin resistance of MCF-7 cells (miR-127, -34a, -27b, -206, -106a, -21, -214, and let-7), and cisplatin resistance of MCF-7 cells (miR-146, -10a/b, -221/222, -29a/b, -206, -200b/c, -345, -126, -205, and -342)
Future miRNA biomarker studies will need to specify tumor cells and stromal cells and distinguish subpopulations of tumor cells, especially CSCs and non-tumorigenic cells. Advancement in technology for miRNA profiling of individual cancer patients will facilitate personalized cancer medicine which must be based on comprehensive miRNA functional studies. It is of particular interest to identify more novel prognostic markers which are also critical regulators of breast cancer because these miRNAs hold great potential for applications in translational medicine.
Given the continued high incidence and death rates for breast cancer, it is essential for researchers, physicians, and advocates to work together to develop novel approaches for the prevention, treatment, and management of breast cancer.
Acknowledgments
I am thankful to Dr. Geoffrey Greene, Jessica Bockhorn and Simo Huang in the Greene laboratory, Dr. Yinyuan Mo, Dr. John Kokontis, and Dr. Richard Hiipakka who read and edited the manuscript. This was supported in part by Department of Defense Breast Cancer Research Program W81XWH-09-1-0331, Paul Calabresi K12 Award 1K12CA139160-02, Chicago Fellows Program at the University of Chicago, and the University of Chicago Clinical and Translational Science Award (UL1 RR024999).
References
- 1.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. pii:S0092867404000455. [DOI] [PubMed] [Google Scholar]
- 2.Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 3.Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, Allgayer H. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene. 2008;27(15):2128–2136. doi: 10.1038/sj.onc.1210856. [DOI] [PubMed] [Google Scholar]
- 4.Huang Q, Gumireddy K, Schrier M, le Sage C, Nagel R, Nair S, Egan DA, Li A, Huang G, Klein-Szanto AJ, Gimotty PA, Katsaros D, Coukos G, Zhang L, Pure E, Agami R. The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat Cell Biol. 2008;10(2):202–210. doi: 10.1038/ncb1681. [DOI] [PubMed] [Google Scholar]
- 5.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449(7163):682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 6.Tavazoie SF, Alarcon C, Oskarsson T, Padua D, Wang Q, Bos PD, Gerald WL, Massague J. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451(7175):147–152. doi: 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10(5):593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 8.Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, Brabletz T. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9(6):582–589. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22(7):894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korpal M, Lee ES, Hu G, Kang Y. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem. 2008;283(22):14910–14914. doi: 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 12.Shimono Y, Zabala M, Cho RW, Lobo N, Dalerba P, Qian D, Diehn M, Liu H, Panula SP, Chiao E, Dirbas FM, Somlo G, Pera RA, Lao K, Clarke MF. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138(3):592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, Huang Y, Hu X, Su F, Lieberman J, Song E. Let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131(6):1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 14.Hurst DR, Edmonds MD, Welch DR. Metastamir: the field of metastasis-regulatory microRNA is spreading. Cancer Res. 2009;69(19):7495–7498. doi: 10.1158/0008-5472.CAN-09-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo X, Wu Y, Hartley RS. MicroRNA-125a represses cell growth by targeting HuR in breast cancer. RNA Biol. 2009;6(5):575–583. doi: 10.4161/rna.6.5.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shipitsin M, Polyak K. The cancer stem cell hypothesis: in search of definitions, markers, and relevance. Lab Invest. 2008;88(5):459–463. doi: 10.1038/labinvest.2008.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439(7079):993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 18.Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439(7072):84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 19.Eirew P, Stingl J, Raouf A, Turashvili G, Aparicio S, Emerman JT, Eaves CJ. A method for quantifying normal human mammary epithelial stem cells with in vivo regenerative ability. Nat Med. 2008;14(12):1384–1389. doi: 10.1038/nm.1791. [DOI] [PubMed] [Google Scholar]
- 20.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1(5):555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Keymeulen A, Rocha AS, Ousset M, Beck B, Bouvencourt G, Rock J, Sharma N, Dekoninck S, Blanpain C. Distinct stem cells contribute to mammary gland development and maintenance. Nature. 2011;479(7372):189–193. doi: 10.1038/nature10573. [DOI] [PubMed] [Google Scholar]
- 22.Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, zur Hausen A, Brunton VG, Morton J, Sansom O, Schuler J, Stemmler MP, Herzberger C, Hopt U, Keck T, Brabletz S, Brabletz T. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11 (12):1487–1495. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- 23.Hyun S, Lee JH, Jin H, Nam J, Namkoong B, Lee G, Chung J, Kim VN. Conserved microRNA miR-8/miR-200 and its target USH/FOG2 control growth by regulating PI3K. Cell. 2009;139(6):1096–1108. doi: 10.1016/j.cell.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 24.Ibarra I, Erlich Y, Muthuswamy SK, Sachidanandam R, Hannon GJ. A role for microRNAs in maintenance of mouse mammary epithelial progenitor cells. Genes Dev. 2007;21(24):3238–3243. doi: 10.1101/gad.1616307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403(6772):901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 26.Bussing I, Slack FJ, Grosshans H. Let-7 microRNAs in development, stem cells and cancer. Trends Mol Med. 2008;14(9):400–409. doi: 10.1016/j.molmed.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D, Slack FJ. RAS is regulated by the let-7 microRNA family. Cell. 2005;120(5):635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 28.Greene SB, Gunaratne PH, Hammond SM, Rosen JM. A putative role for microRNA-205 in mammary epithelial cell progenitors. J Cell Sci. 2010;123(Pt 4):606–618. doi: 10.1242/jcs.056812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greene SB, Herschkowitz JI, Rosen JM. The ups and downs of miR-205: identifying the roles of miR-205 in mammary gland development and breast cancer. RNA Biol. 2010;7(3):300–304. doi: 10.4161/rna.7.3.11837. pii:11837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang C, Li Q. Identification of differentially expressed microRNAs during the development of Chinese murine mammary gland. J Genet Genomics. 2007;34(11):966–973. doi: 10.1016/S1673-8527(07)60109-X. [DOI] [PubMed] [Google Scholar]
- 31.Liu CG, Calin GA, Meloon B, Gamliel N, Sevignani C, Ferracin M, Dumitru CD, Shimizu M, Zupo S, Dono M, Alder H, Bullrich F, Negrini M, Croce CM. An oligonucleotide microchip for genome-wide microRNA profiling in human and mouse tissues. Proc Natl Acad Sci USA. 2004;101(26):9740–9744. doi: 10.1073/pnas.0403293101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435(7043):834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 33.Cummins JM, He Y, Leary RJ, Pagliarini R, Diaz LA, Jr, Sjoblom T, Barad O, Bentwich Z, Szafranska AE, Labourier E, Raymond CK, Roberts BS, Juhl H, Kinzler KW, Vogelstein B, Velculescu VE. The colorectal microRNAome. Proc Natl Acad Sci USA. 2006;103(10):3687–3692. doi: 10.1073/pnas.0511155103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 35.Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M, Lin C, Socci ND, Hermida L, Fulci V, Chiaretti S, Foa R, Schliwka J, Fuchs U, Novosel A, Muller RU, Schermer B, Bissels U, Inman J, Phan Q, Chien M, Weir DB, Choksi R, De Vita G, Frezzetti D, Trompeter HI, Hornung V, Teng G, Hartmann G, Palkovits M, Di Lauro R, Wernet P, Macino G, Rogler CE, Nagle JW, Ju J, Papavasiliou FN, Benzing T, Lichter P, Tam W, Brownstein MJ, Bosio A, Borkhardt A, Russo JJ, Sander C, Zavolan M, Tuschl T. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129(7):1401–1414. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev. 2006;6(11):857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 37.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev. 2009;10(10):704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006;103(7):2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M, Menard S, Palazzo JP, Rosenberg A, Musiani P, Volinia S, Nenci I, Calin GA, Querzoli P, Negrini M, Croce CM. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65(16):7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- 40.Xu N, Papagiannakopoulos T, Pan G, Thomson JA, Kosik KS. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell. 2009;137(4):647–658. doi: 10.1016/j.cell.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 41.Prat A, Perou CM. Deconstructing the molecular portraits of breast cancer. Mol Oncol. 2011;5(1):5–23. doi: 10.1016/j.molonc.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prat A, Parker JS, Karginova O, Fan C, Livasy C, Herschkowitz JI, He X, Perou CM. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12(5):R68. doi: 10.1186/bcr2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blenkiron C, Goldstein LD, Thorne NP, Spiteri I, Chin SF, Dunning MJ, Barbosa-Morais NL, Teschendorff AE, Green AR, Ellis IO, Tavare S, Caldas C, Miska EA. MicroRNA expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol. 2007;8(10):R214. doi: 10.1186/gb-2007-8-10-r214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buffa FM, Camps C, Winchester L, Snell CE, Gee HE, Sheldon H, Taylor M, Harris AL, Ragoussis J. MicroRNA-associated progression pathways and potential therapeutic targets identified by integrated mRNA and microRNA expression profiling in breast cancer. Cancer Res. 2011;71(17):5635–5645. doi: 10.1158/0008-5472.CAN-11-0489. [DOI] [PubMed] [Google Scholar]
- 45.Bockhorn J, Dalton R, Nwachukwu C, Prat A, Yee K, Chang Y-F, Huo D, Wen Y, Huang S, Swanson KE, Qiu T, Lu J, Park SY, Dolan ME, Perou CM, Olopade OI, Clarke MF, Greene GL, Liu H. MicroRNA-30c inhibits human breast tumor chemotherapy resistance. Nat Commun. 2012 doi: 10.1038/ncomms2393. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 48.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 49.Cho RW, Wang X, Diehn M, Shedden K, Chen GY, Sherlock G, Gurney A, Lewicki J, Clarke MF. Isolation and molecular characterization of cancer stem cells in MMTV-Wnt-1 murine breast tumors. Stem Cells. 2008;26(2):364–371. doi: 10.1634/stemcells.2007-0440. [DOI] [PubMed] [Google Scholar]
- 50.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65(23):10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 51.Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, Shelton AA, Parmiani G, Castelli C, Clarke MF. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104(24):10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE, Herlyn M. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65(20):9328–9337. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- 53.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1(3):313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 54.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67(3):1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 55.Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, Zheng BJ, Guan XY. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132(7):2542–2556. doi: 10.1053/j.gastro.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 56.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445(7123):106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 57.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. 2007;104(3):973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445(7123):111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 59.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–5828. [PubMed] [Google Scholar]
- 60.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 61.Liu R, Wang X, Chen GY, Dalerba P, Gurney A, Hoey T, Sherlock G, Lewicki J, Shedden K, Clarke MF. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med. 2007;356(3):217–226. doi: 10.1056/NEJMoa063994. [DOI] [PubMed] [Google Scholar]
- 62.Liu H, Patel MR, Prescher JA, Patsialou A, Qian D, Lin J, Wen S, Chang YF, Bachmann MH, Shimono Y, Dalerba P, Adorno M, Lobo N, Bueno J, Dirbas FM, Goswami S, Somlo G, Condeelis J, Contag CH, Gambhir SS, Clarke MF. Cancer stem cells from human breast tumors are involved in spontaneous metastases in orthotopic mouse models. Proc Natl Acad Sci USA. 2010;107(42):18115–18120. doi: 10.1073/pnas.1006732107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC, Wong H, Rosen J, Chang JC. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100(9):672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 64.Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, Joshua B, Kaplan MJ, Wapnir I, Dirbas FM, Somlo G, Garberoglio C, Paz B, Shen J, Lau SK, Quake SR, Brown JM, Weissman IL, Clarke MF. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458(7239):780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iliopoulos D, Lindahl-Allen M, Polytarchou C, Hirsch HA, Tsichlis PN, Struhl K. Loss of miR-200 inhibition of Suz12 leads to polycomb-mediated repression required for the formation and maintenance of cancer stem cells. Mol Cell. 2010;39(5):761–772. doi: 10.1016/j.molcel.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee YS, Dutta A. The tumor suppressor microRNA let-7 represses the HMGA2 oncogene. Genes Dev. 2007;21(9):1025–1030. doi: 10.1101/gad.1540407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu F, Deng H, Yao H, Liu Q, Su F, Song E. Mir-30 reduction maintains self-renewal and inhibits apoptosis in breast tumor-initiating cells. Oncogene. 2010;29(29):4194–4204. doi: 10.1038/onc.2010.167. [DOI] [PubMed] [Google Scholar]
- 69.Wang Y, Yu Y, Tsuyada A, Ren X, Wu X, Stubblefield K, Rankin-Gee EK, Wang SE. Transforming growth factor-beta regulates the sphere-initiating stem cell-like feature in breast cancer through miRNA-181 and ATM. Oncogene. 2011;30(12):1470–1480. doi: 10.1038/onc.2010.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jiang S, Zhang HW, Lu MH, He XH, Li Y, Gu H, Liu MF, Wang ED. MicroRNA-155 functions as an OncomiR in breast cancer by targeting the suppressor of cytokine signaling 1 gene. Cancer Res. 2010;70(8):3119–3127. doi: 10.1158/0008-5472.CAN-09-4250. [DOI] [PubMed] [Google Scholar]
- 71.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12(8):895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 72.Lee YT. Breast carcinoma: pattern of metastasis at autopsy. J Surg Oncol. 1983;23(3):175–180. doi: 10.1002/jso.2930230311. [DOI] [PubMed] [Google Scholar]
- 73.Weigelt B, Peterse JL, van ‘t Veer LJ. Breast cancer metastasis: markers and models. Nat Rev. 2005;5 (8):591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 74.Gee HE, Camps C, Buffa FM, Colella S, Sheldon H, Gleadle JM, Ragoussis J, Harris AL. MicroRNA-10b and breast cancer metastasis. Nature. 2008;455(7216):E8–E9. doi: 10.1038/nature07362. author reply E9. [DOI] [PubMed] [Google Scholar]
- 75.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4(1):33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 76.Gunthert U, Hofmann M, Rudy W, Reber S, Zoller M, Haussmann I, Matzku S, Wenzel A, Ponta H, Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991;65(1):13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- 77.Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12(10):1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 78.Weber GF, Bronson RT, Ilagan J, Cantor H, Schmits R, Mak TW. Absence of the CD44 gene prevents sarcoma metastasis. Cancer Res. 2002;62(8):2281–2286. [PubMed] [Google Scholar]
- 79.Martello G, Rosato A, Ferrari F, Manfrin A, Cordenonsi M, Dupont S, Enzo E, Guzzardo V, Rondina M, Spruce T, Parenti AR, Daidone MG, Bicciato S, Piccolo S. A MicroRNA targeting dicer for metastasis control. Cell. 2010;141(7):1195–1207. doi: 10.1016/j.cell.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 80.Zhu S, Wu H, Wu F, Nie D, Sheng S, Mo YY. MicroRNA-21 targets tumor suppressor genes in invasion and metastasis. Cell Res. 2008;18(3):350–359. doi: 10.1038/cr.2008.24. [DOI] [PubMed] [Google Scholar]
- 81.Li XF, Yan PJ, Shao ZM. Downregulation of miR-193b contributes to enhance urokinase-type plasminogen activator (uPA) expression and tumor progression and invasion in human breast cancer. Oncogene. 2009;28(44):3937–3948. doi: 10.1038/onc.2009.245. [DOI] [PubMed] [Google Scholar]
- 82.Valastyan S, Reinhardt F, Benaich N, Calogrias D, Szasz AM, Wang ZC, Brock JE, Richardson AL, Weinberg RA. A pleiotropically acting microRNA, miR-31, inhibits breast cancer metastasis. Cell. 2009;137(6):1032–1046. doi: 10.1016/j.cell.2009.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 83.Korpal M, Ell BJ, Buffa FM, Ibrahim T, Blanco MA, Celia-Terrassa T, Mercatali L, Khan Z, Goodarzi H, Hua Y, Wei Y, Hu G, Garcia BA, Ragoussis J, Amadori D, Harris AL, Kang Y. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nat Med. 2011;17(9):1101–1108. doi: 10.1038/nm.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A, Herschkowitz JI, Fan C, Zhang X, He X, Pavlick A, Gutierrez MC, Renshaw L, Larionov AA, Faratian D, Hilsenbeck SG, Perou CM, Lewis MT, Rosen JM, Chang JC. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci USA. 2009;106(33):13820–13825. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miller TE, Ghoshal K, Ramaswamy B, Roy S, Datta J, Shapiro CL, Jacob S, Majumder S. MicroRNA-221/222 confers tamoxifen resistance in breast cancer by targeting p27Kip1. J Biol Chem. 2008;283(44):29897–29903. doi: 10.1074/jbc.M804612200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhao JJ, Lin J, Yang H, Kong W, He L, Ma X, Coppola D, Cheng JQ. MicroRNA-221/222 negatively regulates estrogen receptor alpha and is associated with tamoxifen resistance in breast cancer. J Biol Chem. 2008;283(45):31079–31086. doi: 10.1074/jbc.M806041200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 87.Zhou M, Liu Z, Zhao Y, Ding Y, Liu H, Xi Y, Xiong W, Li G, Lu J, Fodstad O, Riker AI, Tan M. MicroRNA-125b confers the resistance of breast cancer cells to paclitaxel through suppression of pro-apoptotic Bcl-2 antagonist killer 1 (Bak1) expression. J Biol Chem. 2010;285(28):21496–21507. doi: 10.1074/jbc.M109.083337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kovalchuk O, Filkowski J, Meservy J, Ilnytskyy Y, Tryndyak VP, Chekhun VF, Pogribny IP. Involvement of microR-NA-451 in resistance of the MCF-7 breast cancer cells to chemotherapeutic drug doxorubicin. Mol Cancer Ther. 2008;7(7):2152–2159. doi: 10.1158/1535-7163.MCT-08-0021. [DOI] [PubMed] [Google Scholar]
- 89.Pogribny IP, Filkowski JN, Tryndyak VP, Golubov A, Shpyleva SI, Kovalchuk O. Alterations of microRNAs and their targets are associated with acquired resistance of MCF-7 breast cancer cells to cisplatin. Int J Cancer. 2010;127(8):1785–1794. doi: 10.1002/ijc.25191. [DOI] [PubMed] [Google Scholar]
- 90.Yang H, Kong W, He L, Zhao JJ, O’Donnell JD, Wang J, Wenham RM, Coppola D, Kruk PA, Nicosia SV, Cheng JQ. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 2008;68(2):425–433. doi: 10.1158/0008-5472.CAN-07-2488. [DOI] [PubMed] [Google Scholar]
- 91.Weidhaas JB, Babar I, Nallur SM, Trang P, Roush S, Boehm M, Gillespie E, Slack FJ. MicroRNAs as potential agents to alter resistance to cytotoxic anticancer therapy. Cancer Res. 2007;67(23):11111–11116. doi: 10.1158/0008-5472.CAN-07-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, Croce CM. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci USA. 2004;101(9):2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, Rassenti L, Kipps T, Negrini M, Bullrich F, Croce CM. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci USA. 2002;99(24):15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Scott GK, Mattie MD, Berger CE, Benz SC, Benz CC. Rapid alteration of microRNA levels by histone deacetylase inhibition. Cancer Res. 2006;66(3):1277–1281. doi: 10.1158/0008-5472.CAN-05-3632. [DOI] [PubMed] [Google Scholar]
- 95.Png KJ, Yoshida M, Zhang XH, Shu W, Lee H, Rimner A, Chan TA, Comen E, Andrade VP, Kim SW, King TA, Hudis CA, Norton L, Hicks J, Massague J, Tavazoie SF. MicroRNA-335 inhibits tumor reinitiation and is silenced through genetic and epigenetic mechanisms in human breast cancer. Genes Dev. 2011;25(3):226–231. doi: 10.1101/gad.1974211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.O’Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435(7043):839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 97.Rokavec M, Wu W, Luo JL. IL6-mediated suppression of miR-200c directs constitutive activation of inflammatory signaling circuit driving transformation and tumorigenesis. Mol Cell. 2012;45(6):777–789. doi: 10.1016/j.molcel.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dedes KJ, Natrajan R, Lambros MB, Geyer FC, Lopez-Garcia MA, Savage K, Jones RL, Reis-Filho JS. Down-regulation of the miRNA master regulators Drosha and Dicer is associated with specific subgroups of breast cancer. Eur J Cancer. 2011;47(1):138–150. doi: 10.1016/j.ejca.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 99.Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science. 2008;320 (5872):97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Newman MA, Thomson JM, Hammond SM. Lin-28 interaction with the Let-7 precursor loop mediates regulated microRNA processing. RNA. 2008;14 (8):1539–1549. doi: 10.1261/rna.1155108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ma L, Reinhardt F, Pan E, Soutschek J, Bhat B, Marcusson EG, Teruya-Feldstein J, Bell GW, Weinberg RA. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat Biotechnol. 2010;28(4):341–347. doi: 10.1038/nbt.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Scherr M, Venturini L, Battmer K, Schaller-Schoenitz M, Schaefer D, Dallmann I, Ganser A, Eder M. Lentivirus-mediated antagomir expression for specific inhibition of miRNA function. Nucleic Acids Res. 2007;35(22):e149. doi: 10.1093/nar/gkm971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kota J, Chivukula RR, O’Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P, Torbenson M, Clark KR, Mendell JR, Mendell JT. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137(6):1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Collino F, Deregibus MC, Bruno S, Sterpone L, Aghemo G, Viltono L, Tetta C, Camussi G. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One. 2010;5(7):e11803. doi: 10.1371/journal.pone.0011803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen X, Liang H, Zhang J, Zen K, Zhang CY. Secreted microRNAs: a new form of intercellular communication. Trends Cell Biol. 2012;22(3):125–132. doi: 10.1016/j.tcb.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 107.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13(4):423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005;438(7068):685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 109.Davis S, Lollo B, Freier S, Esau C. Improved targeting of miRNA with antisense oligonucleotides. Nucleic Acids Res. 2006;34(8):2294–2304. doi: 10.1093/nar/gkl183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Elmen J, Lindow M, Schutz S, Lawrence M, Petri A, Obad S, Lindholm M, Hedtjarn M, Hansen HF, Berger U, Gullans S, Kearney P, Sarnow P, Straarup EM, Kauppinen S. LNA-mediated microRNA silencing in non-human primates. Nature. 2008;452(7189):896–899. doi: 10.1038/nature06783. [DOI] [PubMed] [Google Scholar]
- 111.Fontana L, Fiori ME, Albini S, Cifaldi L, Giovinazzi S, Forloni M, Boldrini R, Donfrancesco A, Federici V, Giacomini P, Peschle C, Fruci D. Antagomir-17-5p abolishes the growth of therapy-resistant neuroblastoma through p21 and BIM. PLoS One. 2008;3(5):e2236. doi: 10.1371/journal.pone.0002236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Care A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, Elia L, Latronico MV, Hoydal M, Autore C, Russo MA, Dorn GW, 2nd, Ellingsen O, Ruiz-Lozano P, Peterson KL, Croce CM, Peschle C, Condorelli G. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007;13(5):613–618. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- 113.Ibrahim AF, Weirauch U, Thomas M, Grunweller A, Hartmann RK, Aigner A. MicroRNA replacement therapy for miR-145 and miR-33a is efficacious in a model of colon carcinoma. Cancer Res. 2011;71(15):5214–5224. doi: 10.1158/0008-5472.CAN-10-4645. [DOI] [PubMed] [Google Scholar]
- 114.Trang P, Medina PP, Wiggins JF, Ruffino L, Kelnar K, Omotola M, Homer R, Brown D, Bader AG, Weidhaas JB, Slack FJ. Regression of murine lung tumors by the let-7 microR-NA. Oncogene. 2010;29(11):1580–1587. doi: 10.1038/onc.2009.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wiggins JF, Ruffino L, Kelnar K, Omotola M, Patrawala L, Brown D, Bader AG. Development of a lung cancer therapeutic based on the tumor suppressor microRNA-34. Cancer Res. 2010;70(14):5923–5930. doi: 10.1158/0008-5472.CAN-10-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]