Abstract
Introduction
Cream of tartar (potassium bitartrate) has a long history as a cooking aid and medicinal purgative. Despite containing large amounts of potassium, there are no well-documented cases of it causing toxicity. We report two cases in which intentional ingestions of cream of tartar resulted in life-threatening hyperkalemia. In addition, we briefly review the use of cream of tartar as a historical purgative.
Case Reports
In both cases, individuals ingested a large quantity of cream of tartar in an effort to “clean themselves out”. They manifested similar initial symptoms (vomiting), abnormal serum potassium (>8.0 mmol/L), and EKG’s with peaked T waves. Both patients were treated for hyperkalemia and recovered without complication. A search for articles on an academic internet database failed to identify any cases specifically dealing with ill effects of potassium bitartrate and numerous websites continue to purport its beneficial health effects.
Conclusion
Ingestion of cream of tartar can potentially result in life-threatening hyperkalemia.
Keywords: Cream of tartar, Potassium bitartrate, Hyperkalemia, Purgative
Introduction
Cream of tartar (potassium bitartrate) is a common baking ingredient, which can be used to stabilize whipped egg whites (e.g., in meringues) or when combined with baking powder can be used as a leavening agent. It has also been used to make homemade play dough and as an environmentally friendly cleaning agent. Cream of tartar has long been used as a remedy for a number of ailments. In medical studies, it has been shown to be an effective stool softener and, when combined with sodium bicarbonate in a polyethylene glycol-based suppository, as a treatment for chronic constipation [1, 2]. In addition, many websites recommend cream of tartar as “natural”remedy for a variety of conditions including cystitis, smoking cessation, and as a laxative [3–5]. Despite its myriad uses, there are no cases in the literature describing toxicity from ingesting cream of tartar. We report two cases in which ingestion of cream of tartar, as a purgative, resulted in life-threatening hyperkalemia
Case Reports
Case #1
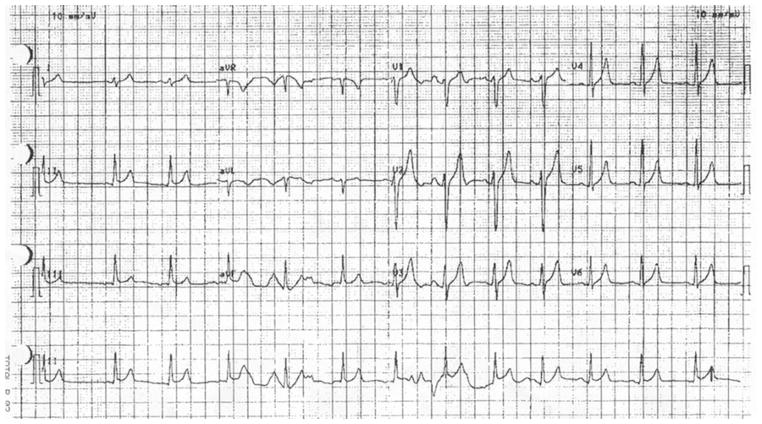
On the advice of a fellow body builder, a 16-year-old man ingested approximately six tablespoons of cream of tartar mixed in a soft drink to “clean himself out.” Four hours later, his sister called our poison center reporting that he was vomiting and nauseated. On our advice, she called EMS, which transported him to a local emergency department (ED). An EKG, obtained shortly after his arrival showed a normal sinus rhythm with a heart rate (HR) of 79 bpm, flattened P waves, and peaked T waves (Fig. 1). His initial laboratory values (5 to 6 h after ingestion) revealed marked hyperkalemia [8.5 mmol/L; reference range (RR) (3.5–5.1)], a mildly elevated chloride level [121 mmol/L; RR (95–105)], and evidence of a mild acidemia [CO2=20 mmol/L; RR (21.0–32.0)]. His renal function was reported as normal. Recommendations were made for treating the patient for hyperkalemia with intravenous (iv) sodium bicarbonate (50 meq), regular insulin (10 units), calcium gluconate (4.65 meq), nebulized albuterol (20 mg), and sodium polystyrene sulfonate (15 g). Shortly after treatment (~2 h after arrival), his potassium was 7.2 mmol/L and subsequently normalized (4.4 mmol/L) within 4 h of arrival. His EKG was also reported as normal after treatment and the patient was discharged the following morning.
Fig. 1.
Case 1 EKG
Case #2
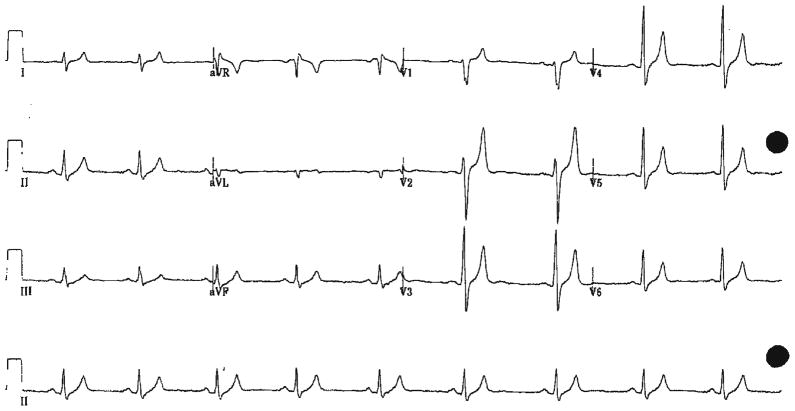
In an effort to “clean himself out” a 32-year-old previously healthy man ingested approximately six tablespoons of cream of tartar mixed in water. Four hours later he developed diarrhea followed by persistent non-bloody vomiting. The patient developed muscle weakness and difficulty ambulating by the next morning and sought care at the local ED. On arrival, approximately 24 h from the time of ingestion, his EKG showed a sinus bradycardia (HR 55 bpm) with peaked T waves (Fig. 2). The patient’s labs showed marked hyperkalemia (8.7 mmol/L) and mild renal insufficiency with a BUN of 10.7 mmol/L [RR (2.9–8.6)] and a creatinine of 168 μmol/L (RR [53–115]). He was hydrated with normal saline and his hyperkalemia was treated with iv sodium bicarbonate (50 meq), regular insulin (10 units), and calcium chloride (13 meq). Within 4 h of arrival, and after treatment, potassium had decreased to 5.9 mmol/L and by 24 h it was 5.4 mmol/L. Similarly, his BUN (8.57 mmol/L) and creatine (115 μmol/L) returned to normal by the next day and the patient was discharged without symptoms.
Fig. 2.
Case 2 EKG
Discussion
Cream of tartar has a long history as a medicinal purgative. For instance, included among Lewis and Clark’s medicinal supplies was two pounds of cream of tartar; in his diary Clark describes treating a “dangerously ill” child with cream of tartar [6]. Other historical publications also extolled the usefulness of cream of tartar as a diuretic for the treatment of edema [7] and as a cathartic and laxative [8].
In spite of its high potassium content and its widely reported beneficial effects, a search of an academic internet database using the terms cream of tartar or potassium bitartrate revealed no cases of poisoning. However, using a generic internet search engine, we were able to find one case in the medical literature. In 1837, the London Medical Gazette included a case report of the death of an employee of a company manufacturing ‘Morrison’s Pills’. These pills were said to be a “cure for all curable diseases,” and were peddled with the philosophy that the more you take the sooner you would get better. The report describes the worker having ingested four to five tablespoons of cream of tartar over the period of a day to “cool his stomach.” The patient developed vomiting and diarrhea (“…had been severely purged”) and developed symptoms of dehydration and muscle weakness (“The thighs and legs appeared paralysed”). Despite “treatment” with an opiate, he died approximately 36 h after ingestion [9]. The ingredients of these pills were later analyzed and found to include aloe, colocynth, gamboge, ginger, and the main ingredient cream of tartar [10].
Although our report and this one are separated by 175 years, they are very similar. Both cases describe persons ingesting cream of tartar for supposed health reasons and suffering GI symptoms and muscle weakness. While the cause of death in the 1837 report could not be determined, we can infer that the death was secondary to hyperkalemia.
Based on its formula (C4H5KO6), cream of tartar is 20 % potassium. A consumer bottle of cream of tartar usually contains about 28 g/oz; therefore, six tablespoons would be 3 oz or 84 g, of which 16.8 g (430 mmol) is potassium. To put this number in perspective, the Food and Nutrition Board of the Institute of Medicine recommend adults have at least 4.7 g (120 mmol) of potassium a day [11]. Therefore, our patients ingested 3.5 times than this daily recommendation.
Patients who have mild to moderate renal insufficiency and patients taking drugs which can increase serum potassium, like potassium-sparing diuretics or angiotensin-converting-enzyme inhibitors, are routinely told to avoid ingesting potassium supplements and dietary salt substitutes to prevent hyperkalemia [12]. Cream of tartar should be added to the list of agents these patients should avoid. Although it is rare, persons without renal insufficiency or on drugs that increase potassium can also develop hyperkalemia by ingesting potassium. These cases, similar to ours, typically involve the acute ingestion of massive amounts of potassium [13–18].
Footnotes
One of the cases outlined in this paper was presented at the 2000 NACCT conference.
Contributor Information
Daniel E. Rusyniak, Email: drusynia@iupui.edu, Department of Emergency Medicine, Indiana University School of Medicine, 1050 Wishard Blvd, RG R2200, Indianapolis, IN 46202, USA.
Pamela J. Durant, Department of Emergency Medicine, Indiana University School of Medicine, 1050 Wishard Blvd, RG R2200, Indianapolis, IN 46202, USA
James B. Mowry, Department of Emergency Medicine, Indiana University School of Medicine, 1050 Wishard Blvd, RG R2200, Indianapolis, IN 46202, USA. Indiana Poison Center, Indiana University School of Medicine, Indianapolis, IN 46202, USA
Jo A. Johnson, Indiana Poison Center, Indiana University School of Medicine, Indianapolis, IN 46202, USA
Jayne A. Sanftleben, Indiana Poison Center, Indiana University School of Medicine, Indianapolis, IN 46202, USA
Joanne M. Smith, Indiana Poison Center, Indiana University School of Medicine, Indianapolis, IN 46202, USA
References
- 1.Spiller GA, Story JA, Furumoto EJ, Chezem JC, Spiller M. Effect of tartaric acid and dietary fibre from sundried raisins on colonic function and on bile acid and volatile fatty acid excretion in healthy adults. Br J Nutr. 2007;90:803–807. doi: 10.1079/bjn2003966. [DOI] [PubMed] [Google Scholar]
- 2.Lazzaroni M, Casini V, Bianchi Porro G. Role or carbon dioxide-releasing suppositories in the treatment of chronic functional constipation: a double-blind, randomised, placebo-controlled trial. Clin Drug Investig. 2005;25:499–505. doi: 10.2165/00044011-200525080-00002. [DOI] [PubMed] [Google Scholar]
- 3.GrannyMed.com. [Accessed 5 July 2012];Cream of tartar for bladder infection (cystitis) Available at http://www.grannymed.com/remedies/conditions/bladder-infection/cream-of-tartar-for-bladder-infection-cystitis.
- 4.Hull JS. [Accessed 5 July 2012];No-smokin’ the cream of tartar. 2011 Available at http://www.janethull.com/newsletter/0807/nosmokin_the_cream_of_tartar.php.
- 5.Stuhler R. [Accessed 5 July 2012];Cream of tartar health uses. 2012 eHow Health:// http://www.ehow.com/list_5910562_cream-tartar-health-uses.html.
- 6.University of Nebraska-Lincoln Libraries-Electronic Text Center. [Accessed 6 July 2012];The journals of the Lewis and Clark expedition. 2005 From http://lewisandclarkjournals.unl.edu/
- 7.Eberle J. A treatise of the materia medica and therapeutics. 6. Grigg, Elliot & Co; Philadelphia: 1847. [Google Scholar]
- 8.Wood GB. A treatis on therapeutics and pharmacology or materia medica. 3. Lippincott, Philadelphia: 1868. [Google Scholar]
- 9.Tyson WT. On poisoning with cream of tartar. Lond Med Gaz. 1837;517(21):177–178. [Google Scholar]
- 10.Anonymous. Quacks and quack medicines. Penny Mag Soc Diff Useful Knowl. 1837;431:495. [Google Scholar]
- 11.Panel on Dietary Reference Intakes for Electrolytes and Water. Food and Nutrition Board, Institute of Medicine. Dietary reference intakes for water, potassium, sodium, chloride, and sulfate. National Academies; Washington: 2004. [Google Scholar]
- 12.Palmer BF. Metabolic complications associated with use of diuretics. Semin Nephrol. 2011;31:542–552. doi: 10.1016/j.semnephrol.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Bosse GM, Platt MA, Anderson SD, Presley MW. Acute oral potassium overdose: the role of hemodialysis. J Med Toxicol. 2011;7:52–56. doi: 10.1007/s13181-010-0106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Illingworth RN, Proudfoot AT. Rapid poisoning with slow-release potassium. Br Med J. 1980;281:485–486. doi: 10.1136/bmj.281.6238.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.John SK, Rangan Y, Block CA, Koff MD. Life-threatening hyperkalemia from nutritional supplements: uncommon or undiagnosed? Am J Emerg Med. 2011;29(1237):e1–e2. doi: 10.1016/j.ajem.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 16.Kallen RJ, Rieger CH, Cohen HS, Sutter MA, Ong RT. Near-fatal hyperkalemia due to ingestion of salt substitute by an infant. JAMA. 1976;235:2125–2126. [PubMed] [Google Scholar]
- 17.Steedman DJ. Poisoning with sustained release potassium. Arch Emerg Med. 1988;5:206–211. doi: 10.1136/emj.5.4.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su M, Stork C, Ravuri S, Lavoie T, Anguish D, Nelson LS, Hoffman RS. Sustained-release potassium chloride overdose. J Toxicol Clin Toxicol. 2001;39:641–648. doi: 10.1081/clt-100108499. [DOI] [PubMed] [Google Scholar]