Abstract
Non-adherence to medications is an important cause of poor blood pressure control. Long-acting antihypertensives (LAs) could theoretically be beneficial in partially adherent patients, who are common in contemporary practice. Little has been reported about the duration of drug holidays (DHs) in treated hypertensives outside of generally compliant subjects in phase 4 clinical trials. We described patterns of non-adherence to single and multiple antihypertensives in a random sample of 120 primary care patients with uncontrolled hypertension. Adherence to up to three antihypertensives was measured by electronic monitoring. We calculated frequencies of single day omissions and DHs of two consecutive days (DH2), three days (DH3) or four days or more (DH≥4) for each drug. Overall, 89 (74%) of patients had at least a one-day omission. A single day omission was found in 61.4% of the patients on one drug, followed by DH≥4 (28.1%), DH2 (26.3%) and DH3 (8.8%). In patients using multiple drugs, single day omissions were also most common, followed by DH≥4, DH2 and DH3. Omissions of three or fewer days comprise on average 74 % of all omissions. Although encouraging full adherence remains important, it may be prudent to prescribe LAs which can compensate for the majority of dose omissions.
Keywords: Hypertension, medication adherence, drug holidays, electronic monitoring
INTRODUCTION
Non-adherence to medications is an important cause of poor blood pressure (BP) control contributing to cardiovascular disease progression, avoidable hospitalizations, disability and death.1–3 In a World Health Organization report adherence to antihypertensives ranged from 52% to 74%.4 In contemporary treated hypertensives those classified as non-adherent are most commonly partially adherent.5, 6 In a study where 819 hypertensives being treated in a system that serves a predominantly low income, minority population were monitored with Medication Event Monitoring System (MEMS), 80% to 93% of doses were taken by the patients in the third quartile of adherence and even patients with the worst adherence generally took approximately half their medication doses.5
“Forgiving” drugs, those with half lives measured in days, can be considered as drugs of choice in partially adherent patients.5, 7–9 Understanding patterns of non-adherence in primary care (PC) will help to establish whether the intervals of non-adherence, referred to as drug holidays (DHs), are short enough to be covered by long-acting antihypertensives (LAs). Characterizing common patterns of non-adherence could also be important for developing tailored interventions that may be more effective than existing strategies.
Little has been reported about the duration of DHs in treated hypertensives.7 In a European study using MEMS on patients who were in phase IV clinical trials of once a day antihypertensives, those who were still engaged with the drug dosing regimen, omitted about 10% of the scheduled doses, of which 42% were of a single day's dose and 43% were part of a sequence of several days.7 However, this sample of generally compliant patients may have limited generalizability for clinical practice.
In contemporary practice, most hypertensives take multiple drugs; however, we can find no reports describing whether patients on multiple drugs take them all or skip them all in unison.
We described patterns of non-adherence in a random sample of predominantly African American (AA) patients with uncontrolled hypertension treated in public and private PC clinics whose adherence on up to three antihypertensives was monitored with MEMS.
METHODS
This study was conducted as part of the baseline data collection for a cluster-randomized trial on clinical inertia and BP control in 10 PC clinics between 2006 and 2007. The details of the trial have been reported.10, 11 Eligible patients were ≥ 21 years, previously diagnosed with hypertension, and uncontrolled BP in their two most recent visits. Patients with cognitive impairment, renal insufficiency, or a serious concomitant illness such as cancer, recent myocardial infarction, or unstable angina, were excluded. A random subsample of patients recruited to the trial was offered an electronic bottle cap monitoring at baseline. The Aardex MEMS 6 Track Cap was used to record the date and time of each bottle cap opening. Up to three antihypertensives were monitored for 30 days. Standardized quality control procedures included testing each device before it was dispensed to the participant (e.g. checking battery status, examining the devices for defects etc.), educating participants in the proper use of the devices, debriefing participants when they return devices, cleaning and analyzing the data.12
Medications were not provided free of charge. This enabled us to study real-life non-adherence patterns, some of which may be related to cost.
We calculated frequencies of single day and sequential days dose omissions i.e. DHs, in patients using a single antihypertensive (n=57), two antihypertensives (n=38), and three antihypertensives (n=25). For the participants using two and three drugs, DH pattern frequencies were analyzed for each monitored drug.
We defined the following non-adherence patterns of interest. A single day omission was defined as no medication intake during one day. A DH2 was defined as no medication intake during 2 consecutive days, during 3 days (DH3) or during 4 days or more (DH≥4). We also calculated the median number of single day omissions and DHs per patient.
We performed an additional analysis pertinent to the participants with multiple drugs. For the participants with two monitored drugs who missed any dose (n=33), we calculated the percentage of days in which both drugs were missed and the percentage of days in which only one of the drugs was missed. There were 16 patients with three monitored drugs who missed any dose. We did not perform detailed analysis of the patterns of missed doses in these patients because of low numbers. If the patient was taking a combination pill that contained two or more antihypertensives it was considered as one drug for adherence calculation purposes.
Statistical Analysis
Data were checked for normality. Descriptive statistics and frequencies were calculated. Data were analyzed using SPSS (version 19) for Windows (SPSS, Inc, Chicago, IL, USA).
RESULTS
The recruitment goal for the substudy was approximately 20% of the entire study sample, or 134 patients. To account for possible non-response, 248 patients were selected to participate in MEMS monitoring substudy, of whom 154 (62%) agreed. Of these, 124 completed MEMS monitoring.
Thirty patients initially agreed to participate but did not complete MEMS monitoring. The reasons for not completing the substudy were: reluctance to change pill containers, not being prescribed antihypertensive medications, lost to follow up, and low battery of the monitor.
In general, all characteristics of the monitored sample were similar to those of the 665 patients enrolled in the clinical trial. Age, race/ethnicity, presence of diabetes, education, smoking status and BMI were similar between the patients who participated in our substudy and those who declined to participate. Those who declined to participate were more likely to be male (44% vs. 23%), employed (68% vs. 50%) and private practice patients (55% vs. 40%).
We excluded three participants because they admitted using a different drug container for some period of time. We also excluded one participant who was erroneously monitored for anti-diabetic drugs.
Of those monitored (120 patients), 75 (62.5%) took ≥80% of prescribed doses with the mean adherence to dose of 82% (median 93%, interquartile range 69% to 100%). Of those using a single drug, two drugs and three drugs 64.9%, 60.5% and 60%, respectively, took ≥80% of prescribed doses.
Table 1 shows characteristics of the 120 patients included in the study. The mean age of the participants was 55 years; 75.8% were female. The most commonly used medication classes were diuretics and angiotensin-converting enzyme (ACE) inhibitors.
Table 1.
Patient Characteristics (n = 120)
| Female sex | 91 (75.8) | |
| Age yrs | 54.9±10.3 | |
| Race/ethnicity | ||
| Hispanic | 26 (21.7) | |
| African American | 80 (66.7) | |
| Non-Hispanic | 14 (11.7) | |
| Diabetes mellitus | 57 (47.5) | |
| Current smoking* | 37 (31.1) | |
| Education# | ||
| Less than high school | 37 (31.4) | |
| High school or GED | 35 (29.7) | |
| Some college and above | 46 (39.0) | |
| Employment status* | ||
| Employed | 59 (49.6) | |
| Retired | 25 (21.0) | |
| Not working | 35 (29.4) | |
| Clinic type | ||
| Private clinic patients | 47 (39.2) | |
| Public clinic patients | 73 (60.8) | |
| Body Mass Index (kg/m2) | 34.0 (21.3–57.9) | |
| Systolic Blood pressure (average of most recent two visits) | 150.0 (121.0–200.5) | |
| Diastolic Blood pressure (average of most recent two visits) | 85 (57–129) | |
| Number of monitored medications | ||
| 1 Drug | 57 (47.5) | |
| 2 Drugs | 38 (31.7) | |
| 3 Drugs | 25 (20.8) | |
| Number of monitored days | 32 (14–83) | |
| Number of pills per 24h | 2 (1–5) | |
| Class of monitored antihypertensive drugs¶ | ||
| Diuretic | 54 (45.0) | |
| ACE inhibitor | 46 (38.3) | |
| Beta-blocker | 43 (35.8) | |
| Calcium channel blocker | 29 (24.2) | |
| Other** | 8 (6.7) | |
| ARBs | 7 (5.8) | |
| Combination ACE inhibitor/Calcium channel blocker | 8 (6.7) | |
| Combination ACE inhibitor/Diuretic | 5 (4.2) | |
| Combination Beta-blocker/Diuretic | 3 (2.5) | |
| Combination ARB/Diuretic | 3 (2.5) | |
Values are presented as n (%), mean ± sd or median (range).
data missing for 1 participant;
data missing for 2 participants;
Total percentage exceeds 100 because some participants used more than one antihypertensive drug class;
includes α2-agonists (clonidine), vasodilators.
Table 2 shows the patterns of drug omissions during the monitoring period. A single day omission was found in 61.4% of the patients on one drug with a median of one (range 0–7) per patient, followed by DH≥4 (28.1%), DH2 (26.3%) and DH3 (8.8%) In patients using two drugs, single day omissions were by far the most common (>55% of the patients) type of drug omissions, followed by DH≥4. Similarly, in patients using three drugs, the most common were single day omissions, followed by DH≥4, DH2 and DH3 (table 2).
Table 2.
Patterns of drug omissions by number of drugs
| 1 drug n=57 |
2 drugs n=38 |
3 drugs n=25 |
||||
|---|---|---|---|---|---|---|
| 1st drug | 2nd drug | 1st drug | 2nd drug | 3d drug | ||
| Single day omissions, n (%)*# | 35 (61.4) | 22 (57.9) | 21 (55.3) | 15 (60.0) | 11 (44.0) | 14 (56.0) |
| DH 2 days, n (%)*# | 15 (26.3) | 8 (21.1) | 7 (18.4) | 3 (12.0) | 3 (12.0) | 3 (12.0) |
| DH 3 days, n (%)*# | 5 (8.8) | 3 (7.9) | 8 (21.1) | 0 | 2 (8.0) | 2 (8.0) |
| DH≥4days, n (%)*# | 16 (28.1) | 18 (47.4) | 16 (42.1) | 5 (20.0) | 6 (24.0) | 5 (20.0) |
| Median number of single day omissions per person (range) | 1 (0–7) | 1 (0–7) | 1 (0–6) | 1 (0–9) | 0 (0–8) | 1 (0–8) |
| Median number of DH2days per person (range) | 0 (0–5) | 0 (0–4) | 0 (0–2) | 0 (0–2) | 0 (0–1) | 0 (0–1) |
| Median number of DH3days per person (range) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 | 0 (0–1) | 0 (0–2) |
| Median number of DH≥4days per person (range) | 0 (0–3) | 0 (0–4) | 0 (0–4) | 0 (0–1) | 0 (0–4) | 0 (0–1) |
Total percentage exceeds 100 because some participants have drug omissions of different length
Percentages presented are per drug and not per patient
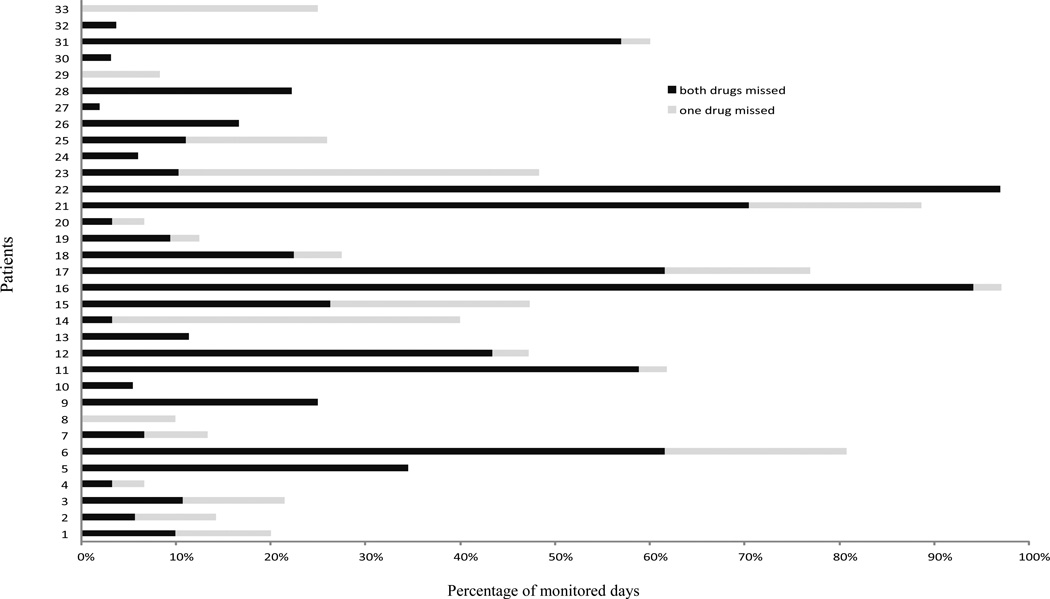
Figure 1 shows the results of the additional analysis including only patients using two drugs who missed any dose. 11 (33%) of the patients on two drugs who missed any dose, always skipped both their drugs during their DHs (figure 1).
Figure 1.
Percentage of days in which each drug was missed among the participants on two drugs who missed any dose (n=33)*
*For example, patient 1 missed doses in 20% of monitored days of which half of the time (10% of monitored days) both drugs were missed and the other half only one of the drugs was missed.
DISCUSSION
In this study of PC patients with uncontrolled hypertension, overall adherence as a dichotomous construct was high. The mean adherence of 82 % (median 93%) was similar to the MEMS-measured mean adherence of 85% (median 94%) in a primary care-based study.5
However, we found that some degree of non-adherence, which we refer to as “partial adherence” was common, with 89 (74%) of the participants having at least a one-day drug omission during the monitored period. Only 14 (12%) missed more than half of their doses.
To the best of our knowledge, this is the first study to provide details of the pattern of missed doses using MEMS in a hypertensive PC population. This information is more useful than the single summary measure for understanding and addressing the behavioral aspects of non-adherence. Single day drug omissions were the most frequent drug omissions in patients using both single and multiple drug regimens. Omissions of three or fewer days made up on average 74% of all omissions. This suggests that LAs could have a substantial benefit for many hypertensives whose blood pressure is not consistently below recommended targets, as the clinical consequences of these gaps in dosing can be minimized by prescribing these drugs. In studies comparing calcium channel blockers with different elimination half-lives, the antihypertensive effect of amlodipine, a long-acting calcium antagonist, persisted for three days after discontinuation of therapy.13–16 Similarly, the multiday half-life of chlorthalidone may explain the apparent superiority of this agent over hydrocholorothiazide in controlling blood pressure.17–19 In a simulation study, using published patterns of missed doses in clinical trials, Lowy and colleagues showed that the use of LAs may mitigate the effect of imperfect adherence to an extent that is clinically meaningful.9 Although improving adherence remains an important priority, it may be prudent to prescribe LAs in partially adherent patients with uncontrolled hypertension.
LAs can compensate for one, two and three day DHs, but not for longer DHs. Although much less frequent in the aggregate, DHs of four or more days were the second most common single pattern of DHs. More research is needed to understand the predictors of long gaps compared to shorter DHs in order to refine adherence interventions.
Combining two or more antihypertensives in the same pill has been proposed as a strategy to improve adherence. We found that only one-third of the patients on a two-drug regimen who missed taking any drug dose, always skipped both their drugs during their DHs, Therefore, two-thirds had some level of therapeutic coverage during the periods when only one of the drugs was missed. Switching this group of patients to one pill combinations would not necessarily improve their therapeutic coverage.
A strength of our study is that our sample is comprised of socio-economically and ethnically diverse hypertensives undergoing routine care in both public and private PC clinics. Another strength is that it contained 67% AAs, a group that is still more likely to have BP control and compliance issues.20–22 However, the sample may not be representative of the overall U.S. population of treated hypertensives, and thus inferences to a broader group are limited.
Our sample size was too small for detailed analysis of the patterns of missed doses in those taking three drugs. Another limitation that we did not have detailed patient self-reports on reasons for the drug omissions.
In conclusion, our study is the first that we are aware of that examines patterns of non-adherence to multiple antihypertensives. We found that, regardless of the number of medications, non-adherence to antihypertensives is usually partial, with most patients engaging in drug holidays of less than four days. The clinical consequences of the majority of dosing gaps may be mitigated by prescribing LAs, but different strategies need to be used for patients taking longer DHs (four or more days). Although counseling patients to achieve 100% adherence is important, it may be may be more practical to preferentially prescribe LAs which can compensate for most dose omissions in partially adherent patients.
Acknowledgments
Funding/Support: This study was supported by grant R01 HL078589 from the National Heart, Lung and Blood Institute, National Institutes of Health.
Footnotes
Financial Disclosure: None
REFERENCES
- 1.Moser M, Setaro JF. Clinical practice. Resistant or difficult-to-control hypertension. N Engl J Med. 2006;355:385–392. doi: 10.1056/NEJMcp041698. [DOI] [PubMed] [Google Scholar]
- 2.Shah NR, Hirsch AG, Zacker C, Wood GC, Schoenthaler A, Ogedegbe G, Stewart WF. Predictors of first-fill adherence for patients with hypertension. Am J Hypertens. 2009;22:392–396. doi: 10.1038/ajh.2008.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43:521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 4.Sabate E. Adherence to long-term therapies: Evidence for action. Geneva, Switzerland: World Health Organization; 2003. [Accessed August 22, 2012]. http://apps.who.int/medicinedocs/en/d/Js4883e. [Google Scholar]
- 5.Rose AJ, Berlowitz DR, Manze M, Orner MB, Kressin NR. Intensifying therapy for hypertension despite suboptimal adherence. Hypertension. 2009;54:524–529. doi: 10.1161/HYPERTENSIONAHA.109.133389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rose AJ, Glickman ME, D'Amore MM, Orner MB, Berlowitz D, Kressin NR. Effects of daily adherence to antihypertensive medication on blood pressure control. J Clin Hypertens (Greenwich) 2011;13:416–421. doi: 10.1111/j.1751-7176.2011.00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M. Adherence to prescribed antihypertensive drug treatments: Longitudinal study of electronically compiled dosing histories. BMJ. 2008;336:1114–1117. doi: 10.1136/bmj.39553.670231.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnier M. Compliance in hypertension. EDTNA ERCA J. 2005;31:152–155. doi: 10.1111/j.1755-6686.2005.tb00417.x. [DOI] [PubMed] [Google Scholar]
- 9.Lowy A, Munk VC, Ong SH, Burnier M, Vrijens B, Tousset EP, Urquhart J. Effects on blood pressure and cardiovascular risk of variations in patients' adherence to prescribed antihypertensive drugs: Role of duration of drug action. Int J Clin Pract. 2011;65:41–53. doi: 10.1111/j.1742-1241.2010.02569.x. [DOI] [PubMed] [Google Scholar]
- 10.Pavlik VN, Greisinger AJ, Pool J, Haidet P, Hyman DJ. Does reducing physician uncertainty improve hypertension control?: Rationale and methods. Circulation. Cardiovasc Qual Outcomes. 2009;2:257–263. doi: 10.1161/CIRCOUTCOMES.109.849984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyman DJ, Pavlik VN, Greisinger AJ, Chan W, Bayona J, Mansyur C, Simms V, Pool J. Effect of a physician uncertainty reduction intervention on blood pressure in uncontrolled hypertensives--a cluster randomized trial. J Gen Intern Med. 2012;27:413–419. doi: 10.1007/s11606-011-1888-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riekert KA, Rand CS. Electronic monitoring of medication adherence: When is high-tech best? J Clin Psychol Med Settings. 2002;9:25–34. [Google Scholar]
- 13.Biston P, Melot C, Degaute JP, Clement D, Quoidbach A. Prolonged antihypertensive effect of amlodipine: A prospective double-blind randomized study. Blood press. 1999;8:43–48. doi: 10.1080/080370599438383. [DOI] [PubMed] [Google Scholar]
- 14.Leenen FH, Fourney A, Notman G, Tanner J. Persistence of anti-hypertensive effect after 'missed doses' of calcium antagonist with long (amlodipine) vs short (diltiazem) elimination half-life. Br J Clin Pharmacol. 1996;41:83–88. doi: 10.1111/j.1365-2125.1996.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 15.Elliott HL, Elawad M, Wilkinson R, Singh SP. Persistence of antihypertensive efficacy after missed doses: Comparison of amlodipine and nifedipine gastrointestinal therapeutic system. J Hypertens. 2002;20:333–338. doi: 10.1097/00004872-200202000-00025. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez RH, Armas-Hernandez MJ, Chourio JA, Armas-Padilla MC, Lopez L, Alvarez M, Pacheco B. Comparative effects of amlodipine and nifedipine gits during treatment and after missing two doses. Blood press Monit. 2001;6:47–57. doi: 10.1097/00126097-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Dorsch MP, Gillespie BW, Erickson SR, Bleske BE, Weder AB. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: A retrospective cohort analysis. Hypertension. 2011;57:689–694. doi: 10.1161/HYPERTENSIONAHA.110.161505. [DOI] [PubMed] [Google Scholar]
- 18.Rosendorff C. Why are we still using hydrochlorothiazide? J Clin Hypertens (Greenwich) 2011;13:867–869. doi: 10.1111/j.1751-7176.2011.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flack JM, Sica DA, Nesbitt S. Chlorthalidone versus hydrochlorothiazide as the preferred diuretic: Is there a verdict yet? Hypertension. 2011;57:665–666. doi: 10.1161/HYPERTENSIONAHA.110.164566. [DOI] [PubMed] [Google Scholar]
- 20.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 21.Ogedegbe G, Chaplin W, Schoenthaler A, Statman D, Berger D, Richardson T, Phillips E, Spencer J, Allegrante JP. A practice-based trial of motivational interviewing and adherence in hypertensive african americans. Am J Hypertens. 2008;21:1137–1143. doi: 10.1038/ajh.2008.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grigoryan L, Pavlik VN, Hyman DJ. Predictors of antihypertensive medication adherence in two urban health-care systems. Am J Hypertens. 2012;25:735–738. doi: 10.1038/ajh.2012.30. [DOI] [PubMed] [Google Scholar]