Abstract
Autism spectrum disorders (ASD) are complex neurodevelopmental disorders. Twin studies have provided heritability estimates as high as 90% for idiopathic ASD. Further evidence for the spectrum’s heritability is provided by the presence of the broad autism phenotype (BAP) in unaffected first-degree relatives. Language ability, specifically phonological processing, is proposed to be a core BAP trait. To date, however, no functional neuroimaging investigations of phonological processing in relatives of individuals with ASD have been undertaken. We conducted a functional magnetic resonance imaging (fMRI) study in parents of children with ASD utilizing a priming task probing implicit phonological processing. In our condition that placed heavier demands on phonological recoding, parents exhibited greater hemodynamic responses than controls in a network of cortical regions involved in phonological processing. Across conditions, parents exhibited enhanced priming-induced response suppression suggesting compensatory neural processing. A nonword repetition test used in previous studies of relatives was also administered. Correlations between this measure and our functional measures also suggested compensatory processing in parents. Regions exhibiting atypical responses in parents included regions previously implicated in the spectrum’s language impairments and found to exhibit structural abnormalities in a parent study. These results suggest a possible neurobiological substrate of the phonological deficits proposed to be a core BAP trait. However, these results should be considered preliminary. No previous fMRI study has investigated phonological processing in ASD, so replication is required. Furthermore, interpretation of our fMRI results is limited by the fact that the parent group failed to exhibit behavioral evidence of phonological impairments.
Keywords: functional magnetic resonance imaging (fMRI), autism spectrum disorders (ASD), broad autism phenotype (BAP), phonology, priming, nonword repetition
INTRODUCTION
Autism spectrum disorders (ASD), which include autistic disorder, Asperger’s disorder, and pervasive developmental disorder not otherwise specified (PDD-NOS), are complex neurodevelopmental disorders defined clinically by a triad of impairments in communication, social interaction, and behavioral flexibility. The prevalence of ASD is estimated to be as high as 1 in 110 children (Centers for Disease Control and Prevention, 2009), with the majority of cases classified as idiopathic (Kielinen et al., 2004; Reddy, 2005; Schaefer and Lutz, 2006; Wassink et al., 2001). Based on twin studies, the heritability of idiopathic ASD has been estimated to be as high as 90% (Bailey et al., 1995; Folstein and Rutter, 1977; Steffenburg et al., 1989). Further evidence for the heritability of ASD has been provided by the presence of the broad autism phenotype (BAP), which is a sub-clinical profile of autistic traits including milder traits qualitatively similar to the defining deficits of ASD that is observed in the unaffected first-degree relatives of individuals with ASD. For example, parents of children with ASD have been observed to exhibit increased rates of developmental language delays, anxiety disorders, and certain personality traits such as aloofness, hypersensitivity, and rigidness (Folstein et al., 1999; Losh et al., 2008; Piven et al., 1997a); and unaffected siblings have been observed to exhibit lower receptive language skills, a history of language delay, poor social-emotional functioning, and impairments in adaptive behavior (Constantino et al., 2010; Toth et al., 2007).
Language ability, specifically phonological processing ability, has been proposed to be one of six candidate BAP traits (Dawson et al., 2002). While impairments in language functioning are a hallmark of ASD, the severity of language impairments across individuals with ASD is highly variable (Kjelgaard and Tager-Flusberg, 2001; Lord and Paul, 1997; Tager-Flusberg and Caronna, 2007). At one extreme, language skills may fall within a normal range with quite mild deficits; at the other, there is an absence of language. Most studies of language in ASD have focused on pragmatic communication deficits (i.e., the inappropriate social use of language), an area of impairment that appears consistently across the spectrum of disorders as well as across all developmental stages (Tager-Flusberg, 2005). These deficits, however, have been suggested to be secondary to the social impairments observed in ASD, such as those involving theory of mind (i.e., the ability to attribute mental states to oneself and to others) (Baron-Cohen, 1988; Tager-Flusberg, 1996, 1999) and weak central coherence (i.e., a cognitive style marked by an inability to process information in context for global meaning) (Frith and Happe, 1994).
In contrast to pragmatics, which requires higher order language processing combined with social skills, there is also evidence for lower order structural language deficits in ASD, one of which involves phonology (i.e., the sounds of a language and their organization in that language). In earlier clinical investigations, as many as 63% (N = 299) (Allen and Rapin, 1992) and 59% (N=197) (Tuchman et al., 1991) of children with ASD were found to exhibit deficient phonological processing skills. In more recent investigations, 77% (N = 44) of children with ASD (Kjelgaard and Tager-Flusberg, 2001) were found to exhibit impairments involving phonology, although a recent investigation of school-age children with ASD reported that only 24% (N = 62) of their sample exhibited deficits in phonological processing (Rapin et al., 2009). This study, however, did not measure phonological processing skills using tests of nonword repetition that may reveal persistent, albeit compensated for, underlying deficits (Bishop et al., 1996) and that have been used to exhibit phonological processing deficits in school-age children with ASD (Bishop et al., 2004; Gabig, 2008; Whitehouse et al., 2008).
Given the evidence for phonological processing deficits in ASD, behavioral investigations have been undertaken to examine phonological processing deficits in the unaffected first-degree relatives of individuals with ASD. However, these have resulted in both positive and negative findings (Bishop et al., 2004; Folstein et al., 1999; Lindgren et al., 2009; Piven and Palmer, 1997; Schmidt et al., 2008), indicating that further studies are required. Furthermore, to date no neuroimaging studies have investigated phonological processing in the unaffected relatives of individuals with ASD. Therefore, we conducted a functional magnetic resonance imaging (fMRI) study in parents of children with ASD utilizing a priming task that we developed in order to investigate the automatic, implicit stages of phonological processing. Behaviorally, priming refers to an increased sensitivity to a stimulus following prior experience with that or a related stimulus and has been used in conjunction with fMRI as a tool to identify brain regions associated with the processing of linguistic stimuli and more specifically phonological processing (Chou et al., 2006; Graves et al., 2008; Haist et al., 2001; Kouider et al., 2010; Kouider et al., 2007; Vaden et al., 2010; Wilson et al., 2011). In order to investigate phonological processing, the task in the present study consisted of prime-target word pairs differing in terms of their phonological relatedness including both word-word homophone (e.g., PAUSE-paws) and pseudoword-word pseudohomophone (e.g., JURM-germ) pairs. In addition, we administered a test of nonword repetition in order to obtain a behavioral measure of phonological processing that has been used in previous behavioral studies of phonological processing in unaffected first-degree relatives.
The present study was intended as a preliminary investigation of the possible neurobiological substrates of the phonological processing deficits that have been proposed to be part of the BAP. Parents of children with ASD were recruited specifically to examine phonological processes in persons who exhibit ASD traits (i.e., BAP) without exhibiting the full clinical ASD spectrum. We hypothesized that parents of individuals with ASD would exhibit enhanced hemodynamic responses for pseudohomophone relative to homophone priming, due to the greater phonological recoding demands of the pseudoword primes. Based on previous fMRI studies of visual word recognition in control subjects, we expected to observe these effects primarily in the inferior frontal cortex (IFC) and anterior insular cortex (IC) (Awh et al., 1996; Burton et al., 2005; Chen and Desmond, 2005a; Herbster et al., 1997; Kouider et al., 2007; Newman and Joanisse, 2011; Poldrack et al., 1999). In addition, we predicted that parents of individuals with ASD would exhibit decreased hemodynamic suppression in response to phonological primes as a reflection of reduced automatic prime facilitation of target word processing. We expected this effect to be observed in cortical regions shown to exhibit hemodynamic response suppression induced by phonological priming in controls and found in numerous previous imaging studies to play a key role in phonological processing, in particular the left lateralized superior temporal gyrus (STG) and supramarginal gyrus (SMG) (e.g., Bles and Jansma, 2008; Chou et al., 2006; Hickok and Poeppel, 2007; Jobard et al., 2003; Stoeckel et al., 2009; Turkeltaub and Coslett, 2010; Wilson et al., 2011). These findings would mirror those from imaging studies in individuals with dyslexia, a disorder thought to arise primarily from phonological processing deficits (Shaywitz, 1996, 1998; Vellutino et al., 2004), that using a variety of tasks have found a disruption in posterior cortical regions including the parietotemporal cortex with compensatory engagement of anterior cortical regions including the IFC, IC, and supplementary motor area (SMA) (e.g., Brunswick et al., 1999; Richlan et al., 2009; Shaywitz et al., 2006; Shaywitz et al., 2002; Shaywitz and Shaywitz, 2008; Shaywitz et al., 1998; Sun et al., 2010).
MATERIALS & METHODS
Participants
The study included data from sixteen parents of a child with ASD and eighteen comparison subjects. Data from three additional subjects were excluded: two due to scan acquisition errors and one due to head motion greater than one voxel (3.44 mm) during scanning. Each of the sixteen parents had a child who met the Diagnostic and Statistical Manual, fourth edition (DSM-IV) (APA, 1994) criteria for ASD as determined by consensus of the Autism Diagnostic Observation Schedule (ADOS) (Lord et al., 2000), the Autism Diagnostic Interview, Revised (ADI-R) (Lord et al., 1994), and DSM-IV diagnosis by an experienced clinical psychologist (SH). Since both parents of one proband participated, fifteen probands served to qualify the parent sample. Of the fifteen probands, eleven had a clinical diagnosis of autistic disorder, three of Asperger’s disorder, and one of PDD-NOS. Twelve probands were male and three female. Child proband participation was limited to the qualification of the parent group.
All subjects spoke English as their first language and were classified as right-handed as determined by the Annett Handedness Scale (Annett, 1985). The Autism Spectrum Quotient (AQ) (Baron-Cohen et al., 2001), a self-administered scale of autism symptoms, was completed by subjects in order to obtain a measure of the presence of ASD traits. To obtain a measure of cognitive ability, the four subtests of the Wechsler Abbreviated Scale of Intelligence (WASI) were administered (Wechsler, 1999). All subjects signed informed consent to participate in the study consistent with the guidelines of the Colorado Multiple Institution Review Board.
Nonword repetition: Administration
The nonword repetition subtest of the Comprehensive Test of Phonological Processing (CTOPP) (Wagner et al., 1999) was administered to participants in the current study. This standardized test consists of eighteen nonwords of increasing difficulty, which subjects are required to repeat aloud immediately after hearing each item presented on an audiocassette recorder. The task assesses an individual’s ability to encode phonological information, store it in working memory, and reproduce it.
fMRI: Stimuli Design and Task Procedure
A total of 192 prime-target pairs were developed for the current study. Stimuli were divided into four conditions: 40 homophone, 40 pseudohomophone, 40 unrelated, and 72 word/nonword pairs. All words were matched across conditions for written frequency, bigram sum, bigram mean, bigram frequency by position, number of phonemes, length, and number of syllables with ratings derived from the English Lexicon Project (ELP) Web Site (Balota et al., 2007). All nonwords were formed by rearrangement of the target words appearing in the other three conditions in order that similar phonemes and syllable structures would be maintained across target conditions. All primes were presented in uppercase and all targets in lowercase so that the visual form of primes and targets differed. For additional detail of stimuli, with examples, see Wilson et al. (2011).
Participants performed a lexical decision task (LDT) to ensure proper attention to the stimuli, which were presented using a projector and screen system. They indicated if each target was a real word or nonword by pressing one of two buttons on an MR-compatible response pad. Participants were not informed of the presence of the uppercase prime, which was below perceptual threshold, but were told that they would see a series of number signs followed by a lowercase word to which they were to respond. Prior to the scan, all participants practiced the task on a set of additional trials not repeated in the scanner. A single trial proceeded as follows: 500 ms forward mask consisting of a series of number signs of equal length to the prime, 30 ms prime, 30 ms blank screen, 400 ms target, and 1040 ms blank screen during which participants responded. The preceding design resulted in a prime-target stimulus onset asynchrony (SOA) of 60 ms.
Stimuli were presented in one session of 30 16-second blocks, for a total time of 8 minutes. Each of the four conditions and a rest condition (i.e., fixation on a series of plus signs) were presented six times in the following order: homophone, pseudohomophone, unrelated, word/nonword. Each block of the homophone, pseudohomophone, and unrelated conditions consisted of six pseudorandomized word pairs within the given condition intermixed with two pairs from the word/nonword condition to minimize strategy use. Accordingly, each block of the word/nonword condition contained six pseudorandomized word/nonword pairs intermixed with two pairs randomly chosen from the other three conditions. Rest blocks were of equal duration to trial blocks (i.e., series of plus signs presented for the trial length of 2000 ms repeated eight times). Within blocks, stimuli were pseudorandomized. Stimulus order was the same for all participants.
Behavioral Data Analyses
Statistical analyses of behavioral data were performed using SPSS version 11 (SPSS Inc., Chicago, IL) with a two-tailed alpha criterion of 0.05. Group differences in age, gender, WASI scores, AQ scores, and CTOPP nonword repetition subtest raw scores (i.e., the number of items completed correctly before the discontinue rule of three incorrect items in a row) were examined separately in one-way ANOVAs (i.e., age by group, gender by group, verbal IQ (VIQ) by group, performance IQ (PIQ) by group, full-scale IQ (FSIQ) by group, AQ score by group, and CTOPP raw score by group). Accuracy on the fMRI LDT task was examined by entering the percentage of each condition (i.e., homophone, pseudohomophone, unrelated, and word/nonword) correctly identified into a 4×2 ANOVA (i.e., condition by group). A’ scores were also calculated for each subject in order to obtain a nonparametric measure of signal detection (Stanislaw and Todorov, 1999). Reaction times were examined by entering the mean reaction times of all accurate trials for each condition into a 4×2 ANOVA (i.e., condition by group). For all ANOVAs, assumptions of sphericity were confirmed via Mauchly’s test with degrees of freedom corrected using Greenhouse-Geisser estimates of sphericity as needed. Post hoc comparisons were conducted using the least significant difference (LSD) pairwise multiple comparison test.
MRI/fMRI: Data Acquisition and Analyses
Imaging data were acquired with at 3T GE whole-body MR scanner with an Excite upgrade using an 8-channel head coil. A high-resolution, T1-weighted 3D anatomical scan was acquired for coregistration to functional data (inversion recovery-spoiled gradient-recall acquisition IR-SPGR, TR = 9 ms, TE = 1.9 ms, TI = 500 ms, flip angle = 10°, matrix = 2562, FOV = 220 mm2, 124 1.7-mm-thick coronal slices). Functional images were acquired with a gradient-echo T2* Blood Oxygenation Level Dependent (BOLD) contrast technique with TR = 2000 ms, TE = 30 ms, FOV = 220 mm2, 642 matrix, 32 slices, 3.5 mm thick, 0.5 gap, angled parallel to the planum sphenoidale. Additionally, one IR-EPI (TI = 505 ms) volume was acquired from each subject to improve the spatial normalization of EPIs.
Data were analyzed using SPM8 (Wellcome Department of Imaging Neuroscience, London). The first four image volumes were excluded for saturation effects. Functional data from each subject were realigned to the first volume. The realigned images were then normalized to Montreal Neurological Institute space using the unified segmentation algorithm (Ashburner and Friston, 2005) on the IR-EPI image and applying the resultant estimated warp parameters to the coregistered EPI data. Finally, functional images were smoothed with an 8-mm FWHM Gaussian kernel. All trials belonging to each of the five conditions (i.e., homophone, pseudohomophone, unrelated, word/nonword, and rest) were separately convolved with the canonical hemodynamic response function using the general linear model (e.g., homophone trials within homophone blocks as well as homophone trials within word/nonword blocks, word/nonword trials within word/nonword blocks as well as word/nonword trials within homophone blocks, etc.). Response accuracy within a window of 300 to 1440 ms of target onset was included as an additional regressor at the first level to model and remove variance associated with task performance. A 182-second high-pass filter was applied to remove low-frequency fluctuation in the BOLD signal, and a one-lag autoregression (AR(1)) model was used to correct for temporal autocorrelation.
To account for both within-group and within-subject variance, a whole-brain random-effects analysis was implemented. Parameter estimates for each individual’s first level analysis (SPM contrast images) were entered into second-level two-sample t tests. Contrasts of interest for our group comparisons included: 1) homophone prime vs. pseudohomophone prime and 2) primed (i.e., homophone + pseudohomophone) vs. unrelated. In addition, in order to investigate correlations between the fMRI and CTOPP measures, we computed a regression between each functional contrast of interest and the CTOPP nonword repetition raw scores separately for each group. Multiple comparison correction was performed using the AFNI (Analysis of Functional NeuroImages) program AlphaSim (http://afni.nimh.nih.gov/afni/doc/manual/AlphaSim), which with 10,000 permutations resulted in a combined height threshold of p < 0.01 and cluster size of 57 voxels for a family-wise error (FWE) corrected threshold of p < 0.05. Anatomic localization of all reported clusters meeting the corrected threshold was established using the Automated Anatomical Labeling (AAL) atlas supplemented with visual inspection (Tzourio-Mazoyer et al., 2002). Finally, possible language lateralization differences between our groups were investigated using the lateralization toolbox for SPM (Wilke and Lidzba, 2007). In order to examine overall language dominance, SPM contrast images were computed for each subject of all our language related conditions (i.e., homophone, pseudohomophone, unrelated, nonword) relative to rest. A lateralization index (LI: values between −1 and +1 with −1 indicating purely right and +1 purely left activation) was then calculated using adaptive thresholding methods for each participant for this contrast in the following ROIs: 1) fusiform gyrus (FG), 2) STG, 3) SMG, and 4) IFC. For each ROI, the main effect of task was first examined by entering each participant’s LI into a one-sample t-test to determine if hemodynamic responses related to the task exhibited laterality effects across groups. Group differences were then examined by entering each participant’s LI into a one-way ANOVA (i.e., LI by group). These statistical analyses were performed using SPSS version 11 with a two-tailed alpha criterion of 0.05.
RESULTS
Participant Characteristics
No significant group differences were found for age (F(1,32) = 0.94, p = 0.34), gender (F(1,32) = 0.06, p = 0.81), VIQ (F(1,31) = 1.48, p = 0.23), PIQ (F(1,31) = 0.60, p = 0.45), FSIQ (F(1,31) = 1.16, p = 0.29), AQ (F(1,29) = 0.10, p = 0.76), or the nonword repetition subtest of the CTOPP (F(1,30) = 0.25, p = 0.62). Participant characteristics are listed in Table I.
Table I.
Behavioral characteristics of participants
| Parents of ASD | Control Subjects | |
|---|---|---|
| Age | 43.7 (8.1) | 41.0 (8.1) |
| Women/Men | 10/6 | 12/6 |
| VIQ | 110.8 (9.3) | 115.0 (10.4) |
| PIQ | 113.6 (13.2) | 116.7 (9.0) |
| FSIQ | 113.8 (11.2) | 117.8 (9.8) |
| AQ | 14.9 (5.0) | 15.6 (6.0) |
| NWR CTOPP | 10.8 (1.9) | 11.2 (2.3) |
Note. Numbers in parenthesis are standard deviations. VIQ = verbal IQ. PIQ = performance IQ. FSIQ = full-scale IQ. AQ = Autism-Spectrum Quotient. NWR CTOPP = Nonword repetition subtest of the Comprehensive Test of Phonological Processing. Age and gender include all participants (parents: N = 16; controls: N = 18). IQ measures were collected for all parents and seventeen controls. The AQ was administered to fifteen parents and sixteen controls. The NWR CTOPP was administered to sixteen parents and sixteen controls.
fMRI Reaction Times and Accuracy
For percentage accuracy, the main effect of condition (F(1.44,46.19) = 3.02, p = 0.07), the main effect of group (F(1,32) = 0.03, p = 0.87), and the condition by group interaction (F(1.44,46.19) = 1.21, p = 0.30) were non-significant. In addition, A’ scores for all conditions were greater than 0.8, suggesting the absence of systematic response bias for all subjects. Examination of reaction times revealed a significant main effect of condition (F(1.50,48.04) = 63.59, p < 0.001), but the main effect of group (F(1,32) = 0.34, p = 0.56) and the group by interaction effect (F(1.50,48.04) = 1.76, p = 0.19) were not significant. Post-hoc comparisons indicated reaction times for the word/nonword condition were significantly slower than each of the three other conditions (p < 0.001). Mean accuracy and reaction time data are listed in Table II.
Table II.
fMRI task behavioral results.
| Parents of ASD (N=16) | Control Subjects (N=18) | ||||
|---|---|---|---|---|---|
|
| |||||
| Condition | Percentage Correct | Reaction Time – ms | Percentage Correct | Reaction Time – ms | |
|
| |||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
|
|
|||||
| Homophone | 92.34 (4.52) | 665.92 (79.12) | 93.33 (4.54) | 651.11 (72.66) | |
| Pseudohomophone | 95.16 (5.66) | 667.06 (80.17) | 96.11 (4.39) | 634.83 (63.12) | |
| Unrelated | 95.00 (3.76) | 662.86 (78.73) | 95.83 (4.37) | 645.34 (76.14) | |
| Word/Nonword | 93.84 (7.62) | 752.83 (85.21) | 90.12 (13.21) | 761.95 (75.93) | |
fMRI
Whole-brain FWE corrected fMRI results are listed in Table III. Relative to comparison subjects, the parent group exhibited greater hemodynamic responses for pseudohomophone relative to homophone priming in several cortical regions (Figure 1). These included the bilateral SMA, posterior cingulate gyrus, thalamus, cerebellum, and midbrain; the left lateralized precentral gyrus, postcentral gyrus, middle cingulate gyrus, and IC; and the right lateralized precuneus, STG, and SMG. In addition, the parent group exhibited greater hemodynamic response suppression for unrelated relative to primed stimuli in the left lateralized postcentral gyrus, middle temporal gyrus (MTG), STG, and SMG (Figure 2). No other contrasts were significant in our whole-brain group analyses.
Table III.
Group differences: Parents of individuals with ASD (N=16) and control subjects (N=18). Whole-brain FWE (q < 0.05) corrected fMRI results.
| Brain region | t | Cluster Size | MNI Coordinates | ||
|---|---|---|---|---|---|
|
| |||||
| Parents of ASD > Control Subjects | |||||
| Pseudohomophone > Homophone | |||||
| Left precentral gyrus | 4.30 | 1642 | −27 | −19 | 52 |
| Left postcentral gyrus | 4.24 | −27 | −28 | 49 | |
| Left middle cingulate gyrus | 4.06 | −9 | −1 | 43 | |
| Left supplementary motor cortex | 3.76 | −6 | −7 | 61 | |
| Right supplementary motor cortex | 3.65 | 12 | 2 | 46 | |
| Left midbrain | 4.16 | 516 | −15 | −22 | −8 |
| Left thalamus | 3.70 | −15 | −22 | 13 | |
| Right thalamus | 3.69 | 15 | −13 | 13 | |
| Left cerebellar lobule VI | 3.40 | 136 | −27 | −55 | −35 |
| Right posterior cingulate gyrus | 3.28 | 128 | 9 | −46 | 22 |
| Right precuneus | 2.88 | 15 | −55 | 31 | |
| Left posterior cingulate gyrus | 2.64 | −9 | −43 | 22 | |
| Right midbrain | 3.19 | 118 | 15 | −25 | −11 |
| Right superior temporal gyrus | 3.25 | 109 | 57 | 2 | −8 |
| Right supramarginal gyrus | 3.61 | 83 | 48 | −31 | 37 |
| Left insular cortex | 3.44 | 78 | −33 | −13 | 16 |
| Right cerebellar lobule VI | 3.16 | 65 | 36 | −55 | −26 |
| Unrelated > Primed | |||||
| Left postcentral gyrus | 3.42 | 261 | −30 | −28 | 46 |
| Left middle temporal gyrus | 3.06 | −51 | −61 | −5 | |
| Left superior temporal gyrus | 3.02 | −57 | −49 | 19 | |
| Left supramarginal gyrus | 3.00 | −51 | −34 | 25 | |
Note. All labels are derived from the AAL atlas supplemented with visual inspection. Where cluster size is not indicated, peak voxels represent sub-peaks within the above-labeled cluster.
Figure 1.
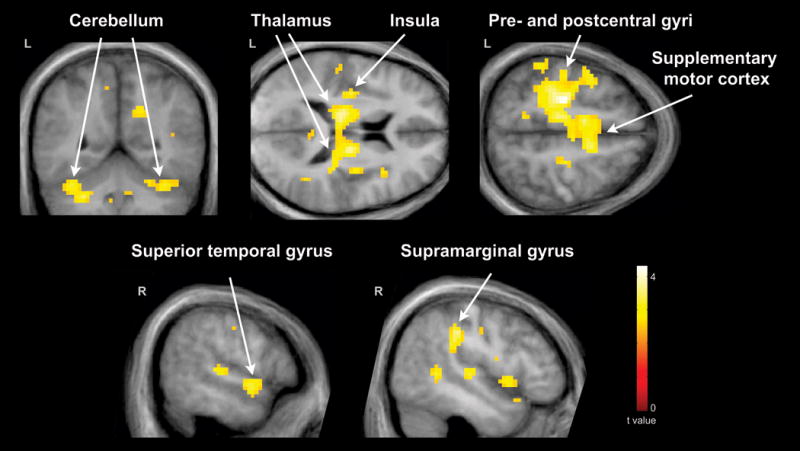
Cortical regions exhibiting greater hemodynamic response enhancement for pseudohomophone relative to homophone priming in parents of children with ASD (N = 16) relative to control subjects (N = 18). These included the bilateral cerebellum; bilateral thalamus and the left insular cortex; the left precentral gyrus, postcentral gyrus, and bilateral supplementary cortex; the right superior temporal gyrus; and the right supramarginal gyrus. Results are whole-brain with statistical maps shown thresholded at p < 0.01 uncorrected, the cluster-defining threshold used in the study. Results are overlaid onto the average T1-weighted image from the study and presented in neurological convention (left hemisphere on the left).
Figure 2.
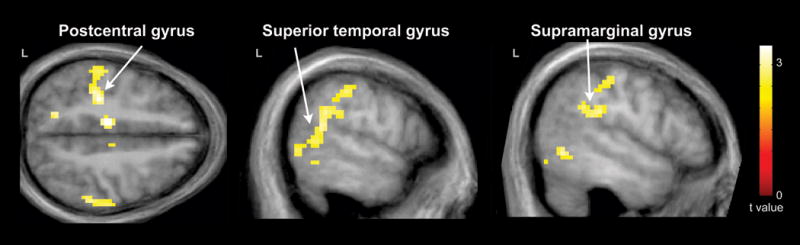
Cortical regions exhibiting greater hemodynamic response suppression due to phonological priming in parents of children with ASD (N = 16) relative to comparison subjects (N = 18). These included the left postcentral, superior temporal, and supramarginal gyri. Results are whole-brain with statistical maps shown thresholded at p < 0.01 uncorrected, the cluster-defining threshold used in the study. Results are overlaid onto the average T1-weighted image from the study and presented in neurological convention (left hemisphere on the left).
Correlations Between CTOPP Raw Scores and fMRI Contrasts
Correlations between SPM functional contrasts of interest and raw scores on the nonword repetition subtest of the CTOPP are listed in Table IV. For the parent group, significant positive correlations were found between CTOPP raw scores and greater hemodynamic responses for pseudohomophone relative to homophone priming in the right lateralized STG; and the left-lateralized IFG and IC. For the control subjects, significant positive correlations were found between CTOPP raw scores and greater hemodynamic responses for pseudohomophone relative to homophone priming in the bilateral occipital gyrus, parietal lobule, postcentral gyrus, lingual gyrus, and IFG; the left lateralized parahippocampal gyrus, cerebellum, and calcarine sulcus; and the right lateralized inferior temporal gyrus (ITG), precentral gyrus, and SMG; and the cerebellar vermis. No other significant correlations between CTOPP subscale raw scores and our functional contrasts of interest were observed.
Table IV.
Significant correlations between functional SPM contrasts of interest and raw scores on the nonword repetition subtest of the Comprehensive Test of Phonological Processing (CTOPP). Parents of individuals with ASD (N=16) and control subjects (N=16). Whole-brain FWE (q < 0.05) corrected results.
| Brain region | t | Cluster Size | MNI Coordinates | ||
|---|---|---|---|---|---|
| Parents of ASD | |||||
| Pseudohomophone > Homophone | |||||
| Right superior temporal gyrus | 3.82 | 80 | 57 | −31 | 4 |
| Left inferior frontal gyrus, triangularis | 3.37 | 57 | −42 | 26 | 7 |
| Left insular cortex | 3.06 | −33 | 26 | 1 | |
| Control Subjects | |||||
| Left middle occipital gyrus | 5.05 | 651 | −42 | −79 | 19 |
| Left superior occipital gyrus | 4.41 | −21 | −88 | 31 | |
| Right inferior temporal gyrus | 6.39 | 390 | 48 | −46 | −26 |
| Right inferior occipital gyrus | 4.98 | 45 | −76 | −2 | |
| Right superior parietal lobule | 6.80 | 376 | 27 | −67 | 55 |
| Right middle occipital gyrus | 3.92 | 45 | −70 | 25 | |
| Right precentral gyrus | 6.06 | 346 | 54 | 2 | 28 |
| Right postcentral gyrus | 5.27 | 57 | −19 | 37 | |
| Right lingual gyrus | 4.85 | 310 | 9 | −31 | −8 |
| Left parahippocampal gyrus | 4.09 | −21 | −40 | −8 | |
| Left postcentral gyrus | 6.32 | 294 | −60 | −1 | 28 |
| Left inferior frontal gyrus, opercularis | 4.15 | −57 | 11 | 19 | |
| Left cerebellar crus I | 4.63 | 248 | −45 | −64 | −23 |
| Left cerebellar lobule VI | 3.74 | −27 | −67 | −23 | |
| Left cerebellar crus II | 3.61 | −12 | −76 | −32 | |
| Cerebellar vermis | 3.42 | 165 | 6 | −67 | −11 |
| Right inferior frontal gyrus, triangularis | 3.87 | 135 | 48 | 35 | 7 |
| Left cerebellar lobule IX | 3.00 | 83 | −12 | −49 | −41 |
| Right supramarginal gyrus | 4.02 | 82 | 42 | −28 | 37 |
| Right inferior parietal lobule | 3.67 | 45 | −49 | 49 | |
| Left inferior frontal gyrus, triangularis | 3.95 | 77 | −42 | 29 | 28 |
| Left lingual gyrus | 3.34 | 74 | −12 | −76 | 1 |
| Left calcarine sulcus | 3.08 | −12 | −88 | −2 | |
| Left inferior parietal lobule | 3.60 | 62 | −51 | −58 | 46 |
Note. The sign of the t-statistic indicates whether the correlations are positive or negative. All labels are derived from the AAL atlas supplemented with visual inspection. Where cluster size is not indicated, peak voxels represent sub-peaks within the above-labeled cluster.
Hemispheric Lateralization Indices
One-sample t tests indicated significant left hemispheric lateralization across groups within our chosen ROIs. The mean LI for each ROI with standard deviations in parenthesis was: 1) FZ: 0.12 (0.28), p = 0.01, 2) STG: 0.15 (0.39), p = 0.04, 3) SMG: 0.47 (.30), p < 0.01, and 4) IFG: 0.14 (0.26), p < 0.01. No significant group LI differences were found for any of our ROIs.
DISCUSSION
Our primary hypothesis was that parents of children with ASD would exhibit greater hemodynamic responses than control subjects for pseudohomophone relative to homophone priming. Pseudohomophones lack an addressed phonology (i.e., phonology activated on the basis of whole word representations in the orthographic lexicon) and require the use of assembled phonology (i.e., phonology activated on the basis of direct grapheme-to-phoneme conversion). As a result, the pseudohomophone primes were expected to place heavier demands than homophone primes on phonological recoding and working memory skills, two skills assessed in the tests of nonword reading and repetition that have been utilized to provide behavioral evidence for phonological processing deficits in the BAP. Greater hemodynamic responses in the parent group were therefore predicted as an index of more effortful processing. As hypothesized, parents of children with ASD exhibited significantly greater hemodynamic responses than controls for pseudohomophone relative to homophone priming. These effects were observed in an extensive network of cortical regions including as hypothesized the left lateralized IC and in addition the bilateral cerebellum and thalamus; left lateralized postcentral gyrus, precentral gyrus, and SMA; and right lateralized STG and SMG.
Cerebellar anomalies are one of most replicated neuroanatomical findings in ASD (e.g., Abell et al., 1999; Courchesne, 1997; Courchesne et al., 1994; Courchesne et al., 1988; Gaffney et al., 1987; Hashimoto et al., 1993; Murakami et al., 1989; Palmen and van Engeland, 2004; Piven et al., 1997b). The cerebellum, previously thought to be primarily involved in aspects of motor function, is now known to play a role in many nonmotor aspects of behavior including language (Ghosh et al., 2008; Marien et al., 2001; Murdoch, 2010) with evidence for a role in phonological processing and verbal working memory (Bohland and Guenther, 2006; Chen and Desmond, 2005a, b; Fulbright et al., 1999; Ghosh et al., 2008). Superior portions of the cerebellum in conjunction with left lateralized frontal cortical regions including the precentral gyrus and SMA, regions in which the parent group also exhibited enhanced responses for pseudohomophone priming, have been suggested to contribute to the articulatory component of the phonological loop as proposed by Baddeley (Baddeley, 1992; Chen and Desmond, 2005a, b). As such, this cerebro-cerebellar network is thought to play a role in subvocal rehearsal mechanisms and speech motor plan representations (Ackermann et al., 2007). Furthermore, the left lateralized IC, a region in which the parent group also exhibited enhanced hemodynamic responses for pseudohomophone relative to homophone priming as hypothesized, is thought to be involved in articulatory processes (for a review see Ackermann and Riecker, 2004). Findings of increased activity with the IC as well as the SMA are also interesting in that they parallel findings in dyslexia in which enhanced activity within these anterior cortical regions has been found relative to controls and has been interpreted as compensatory involvement of articulatory routines to access phonological representations (e.g., Brunswick et al., 1999; Richlan et al., 2009).
Related to these cerebellar and cortical findings, enhanced hemodynamic responses in the parent group for pseudohomophone relative to homophone priming were also observed in the bilateral thalamus. The cerebellum and the cerebral cortex are extensively interconnected through afferent projections from widespread cortical regions relayed through the pontine nuclei to the cerebellum and efferent projections from the cerebellum via thalamic nuclei back to multiple cortical regions (Schmahmann, 1996; Strick et al., 2009). There is some evidence for atypical thalamic volumes in relation to total brain volume as well as for aberrant functioning in thalamo-cortical and cerebello-thalamo-cortical circuits in individuals with ASD (e.g., Hardan et al., 2006; Hardan et al., 2008a; Hardan et al., 2008b; Mizuno et al., 2006; Muller et al., 1998; Thatcher et al., 2009; Tsatsanis et al., 2003). Functional imaging studies of individuals with ASD have also reported atypical activation patterns relative to controls in the SMA (Enticott et al., 2009; Mostofsky et al., 2009; Muller et al., 2001) and the IC (Anderson et al., 2010). In addition, in a recent voxel-based morphometry (VBM) study several cortical regions of atypical gray matter volumes were observed in parents of children with ASD relative to a control group (Peterson et al., 2006). These included both cerebellar enhancements and reductions, although in different cerebellar regions than reported here, in their sample of parents of children with ASD relative to controls. In light of these studies as well those for cerebellar anomalies in ASD, the combined findings of enhanced responses in the cerebellum, premotor cortical regions, IC, and thalamus in parents of children with ASD relative to a control group suggest a network of regions involved in phonological processing that could possibly be neural substrates of the phonological processing deficits that have been proposed to be part of the BAP.
Lastly, parents of children with ASD exhibited greater hemodynamic response enhancements for pseudohomophone relative to homophone priming in the right lateralized STG and SMG. The STG and SMG are both regions known to be involved in phonetic and prelexical phonological processing (e.g., Booth et al., 2002; Hickok and Poeppel, 2007; Jobard et al., 2003; Turkeltaub and Coslett, 2010; Vaden et al., 2010). While there is clear evidence for the involvement of bilateral STG and SMG in language processing (e.g., Benson et al., 2001; Booth et al., 2002; Lee et al., 2007; Vaden et al., 2010), the left hemisphere of the brain is generally accepted to be functionally specialized for linguistic processing in approximately 95% of right-handed individuals (Knecht et al., 2000a; Knecht et al., 2000b; Pujol et al., 1999; Szaflarski et al., 2002). Numerous studies have provided evidence for atypical language lateralization in individuals with ASD (Bigler et al., 2007; Flagg et al., 2005; Herbert et al., 2002; Jou et al., 2010; Kleinhans et al., 2008; Knaus et al., 2010; Knaus et al., 2008; Redcay and Courchesne, 2008; Rojas et al., 2002). The present study included only right-handed individuals, so the finding of greater recruitment of the right lateralized STG and SMG during phonological priming in the parent group possibly suggest familiality of atypical hemispheric lateralization in ASD. However, our examination of hemispheric lateralization related to our language conditions did not indicate greater right hemispheric language dominance in our parent sample. Our results related specifically to phonological processing do suggest greater engagement of right hemispheric parietotemporal regions for parents of ASD relative to controls during this particular aspect of language processing, a finding that has also been found in individuals with dyslexia and that has been interpreted as compensating for a corresponding left hemispheric dysfunction (Richlan et al., 2009).
In addition to our hypothesis about differences in pseudohomophone versus homophone priming, we predicted that parents of children with ASD would exhibit reduced priming-related hemodynamic response suppression across both homophone and pseudohomophone conditions relative to controls as a result of phonological processing impairments and subsequent inefficient implicit activation of phonological representations. In particular, we expected these effects to be greatest in the left lateralized STG and SMG. Contrary to our hypothesis, parents of individuals with ASD exhibited enhanced hemodynamic suppression in response to phonological priming relative to controls in several cortical regions including both the left lateralized STG and SMG. Together with our lack of findings for significant CTOPP nonword repetition subtest scores between groups and in light of findings in which reduced activation in parietotemporal regions is seen as part of a ‘neural signature’ of dyslexia (Shaywitz and Shaywitz, 2008), greater priming-induced response suppression could be an index of compensatory neural processing in the parent group. While unexpected in terms of their directionality, the findings of significant priming-induced hemodynamic response differences within the STG relative to controls is particularly interesting since both volumetric and functional imaging studies have provided evidence for STG abnormalities in individuals with ASD (Amaral et al., 2008; Bigler et al., 2007; Boddaert et al., 2004; Herbert et al., 2002; Hyde et al., 2010; Jou et al., 2010; Kleinhans et al., 2008; Verhoeven et al., 2010; Williams and Minshew, 2007) and these abnormalities have been proposed to contribute to the language and communication deficits that are a core feature of the autism spectrum. Furthermore, Peterson et al. (2006) also observed larger left STG and SMG gray matter volumes in parents of children with ASD relative to a control group in their VBM study, which were suggested to contribute to the etiology of ASD. Furthermore, Dawson et al. 2002 proposed that the STG and parietotemporal cortex were brain regions associated with the phonological processing deficits that they put forth as a core component of the BAP.
In addition to our fMRI task, the nonword repetition subtest of the CTOPP was administered to obtain a behavioral measure of phonological processing in our sample. While we expected the parent group to perform less accurately than controls, no differences between groups were observed for the CTOPP subtest raw scores. Previous behavioral studies of phonological processing using tests of nonword repetition and reading in unaffected first-degree relatives of individuals with ASD have reported significant deficits (Folstein et al., 1999; Schmidt et al., 2008). However, negative findings have also been reported (Bishop et al., 2004; Lindgren et al., 2009; Piven and Palmer, 1997). In the present study, the findings of hemodynamic response differences despite a lack of significant behavioral differences between groups suggests that compared to behavioral studies functional imaging offers a more direct measure of brain activity and a possible measure of the neural mechanisms underlying the phonological processing deficits that have been proposed to be part of the BAP. Examination of correlations between our functional contrasts of interests and the nonword repetition raw scores also revealed significant positive correlations between raw scores and greater hemodynamic responses for pseudohomophone relative to homophone priming in several cortical regions for each group. Of particular interest are the positive correlations that were observed within the left lateralized IFG for both groups (Figure 3) and the IC for the parent group (Figure 4). The left lateralized IFG is a region known to be involved in phonological processing and has been observed to exhibit increased hemodynamic responses for pseudowords and nonwords relative to words thought due to the greater articulatory recoding demands of these stimuli (Burton et al., 2005; Herbster et al., 1997; Newman and Joanisse, 2011; Poldrack et al., 1999). The IC is a region also known to be involved in articulatory processes (Ackermann and Riecker, 2004). In addition, in individuals with dyslexia compensatory engagement within these regions has been found relative to controls (Richlan et al., 2009; Shaywitz and Shaywitz, 2008; Shaywitz et al., 1998). The left IC was also a region in which the parent group as we had hypothesized exhibited greater hemodynamic responses than controls for pseudohomophones relative to homophones. The findings of positive correlations between activation in these regions and raw scores of nonword repetition suggests that greater recruitment of these regions is associated with more effective phonological recoding and are a possible indication that the greater activation observed in the parent group reflects compensatory neural processes.
Figure 3.
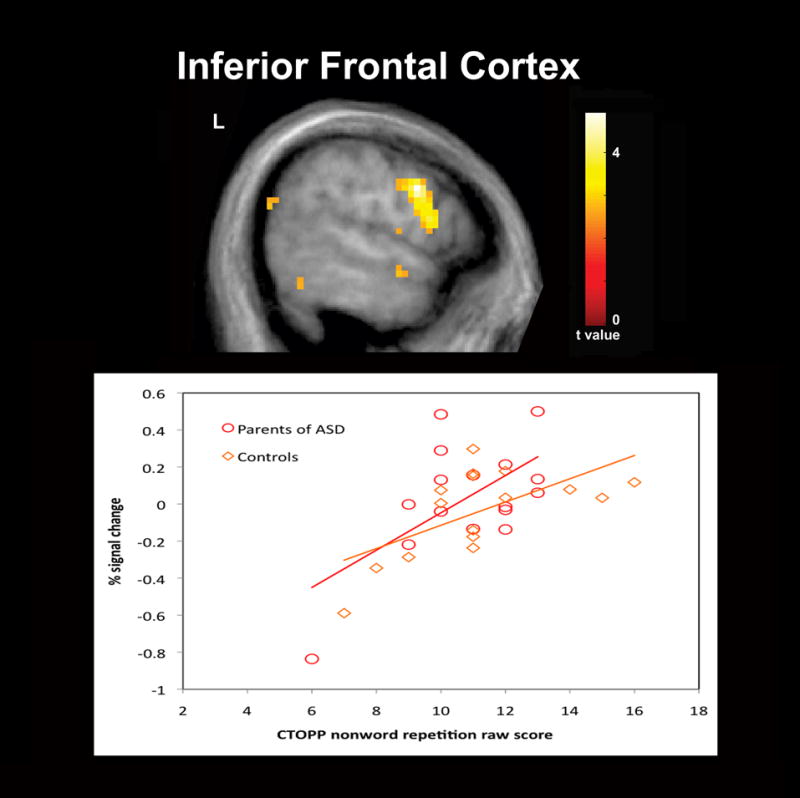
Significant positive correlations between raw scores on the Comprehensive Test of Phonological Processing (CTOPP) nonword repetition subtest and greater hemodynamic responses for pseudohomophone relative to homophone priming for both parents of children with ASD (N = 16) and comparison subjects (N = 16) were observed in several cortical regions including the left inferior frontal cortex. Results are whole-brain with statistical maps shown thresholded at p < 0.01 uncorrected, the cluster-defining threshold used in the study. Results are overlaid onto the average T1-weighted image from the study and presented in neurological convention (left hemisphere on the left).
Figure 4.
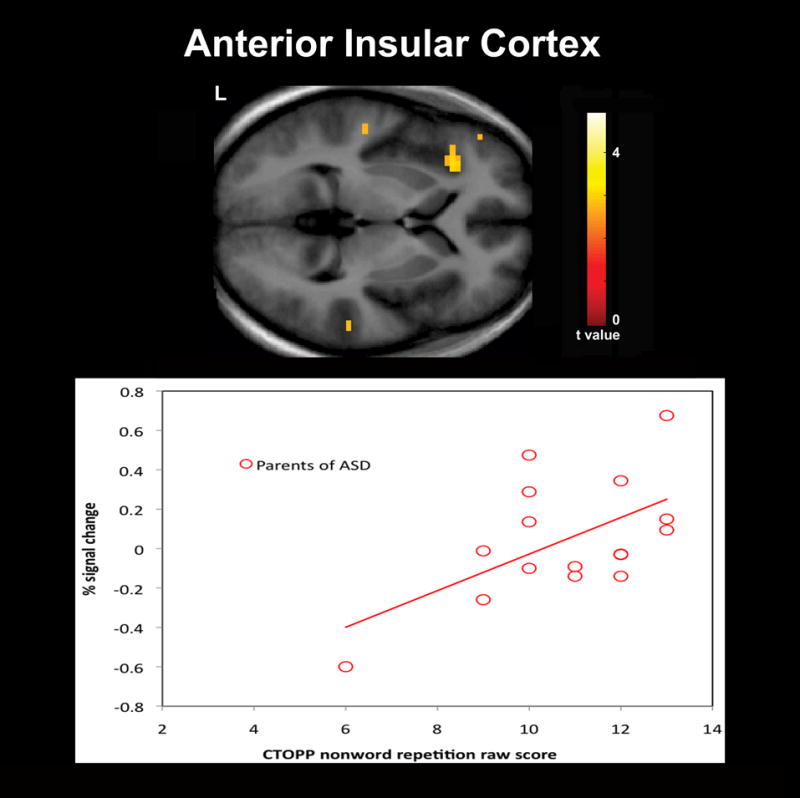
Significant positive correlations between raw scores on the Comprehensive Test of Phonological Processing (CTOPP) nonword repetition subtest and greater hemodynamic responses for pseudohomophone relative to homophone priming for parents of children with ASD (N = 16) were observed in the anterior insular cortex. Results are whole-brain with statistical maps shown thresholded at p < 0.01 uncorrected, the cluster-defining threshold used in the study. Results are overlaid onto the average T1-weighted image from the study and presented in neurological convention (left hemisphere on the left).
The present study provides evidence for possible neural substrates of phonological processing deficits in the unaffected first-degree relatives of children with ASD. Other recent investigations have also begun to utilize functional imaging techniques to investigate the neurobiology underlying the BAP in unaffected first-degree relatives of individuals with ASD (Baron-Cohen et al., 2006; Dalton et al., 2007; Dawson et al., 2005; Elsabbagh et al., 2009; Maziade et al., 2000; Mitchell et al., 2009; Peterson et al., 2006; Rojas et al., 2008; Rojas et al., 2004). One goal of these studies to identify heritable physiological endophenotypes related to distinct pieces of the full autistic clinical syndrome. These in turn could be used to incorporate phenotypically defined subgroups into genetic studies potentially leading to strengthened linkage findings to genes contributing to more narrowly defined components of the disorder. This approach has proven effective in recent studies of ASD, in which incorporation of subgroups based on the behavioral markers of the proband’s history of language delay and a parental history of language difficulties has resulted in strengthened linkage findings on chromosomes 3q, 7q, 13q, and 17q (Alarcon et al., 2002; Alarcon et al., 2005; Bradford et al., 2001). Similarly, a recent combined structural imaging and genetic investigation of CNTNAP2 (contactin-associated like protein-2), which has been implicated as a susceptibility gene for ASD and has been found to be associated with nonword repetition in children with language impairment (Alarcon et al., 2002; Alarcon et al., 2005; Bradford et al., 2001; Vernes et al., 2008), reported morphological brain variations in healthy controls that varied with genotype. These included neural regions implicated in ASD such as the cerebellum. In regards to the language impairments found in ASD, CNTNAP2 is particularly interesting because it encodes a member of the neurexin superfamily of transmembrane proteins that has been implicated in neurodevelopmental processes including cell adhesion, neuronal recognition, and localization/maintenance of voltage-gated potassium channels (Alarcon et al., 2008; Arking et al., 2008; Vernes et al., 2008). In addition, CNTNAP2 expression is regulated by the forkhead box transcription factor FOXP2, the gene for which has been found to be mutated in the KE family, a multigenerational pedigree of over thirty members wherein approximately half display severe impairments in speech and language development (Hurst et al., 1990). These studies suggest the potential of the incorporation of physiological endophenotypes into genetic studies of ASD in order to identify genes contributing to the language impairments found in individuals with ASD rather than attempting to examine the full heterogeneous clinical syndrome.
This study was designed as a first functional imaging investigation of the phonological processing deficits that have been proposed to be a core component of the BAP. Given that no other fMRI studies have investigated phonological processing in first-degree relatives of individuals with ASD, the results should be considered preliminary. While the choice of task is based on previous successful behavioral and imaging studies of phonological processing in controls, replication in other samples of first-degree relatives of individuals with ASD is required. A further limitation of the current study is that the parent sample did not exhibit BAP traits based on the results of the AQ or exhibit phonological processing deficits based on the nonword repetition subtest of the CTOPP. As a result, our neuroimaging results are difficult to interpret. While the imaging data may reflect increased effort in task-related phonological processing in the parent sample, independent replication and use of a more extensive set of behavioral measures of phonological processing are warranted. Additionally, the lack of behavioral evidence for phonological processing deficits in our parent sample could be due to the use of singleton families in the current study, as the BAP as been found to be expressed more strongly in multiplex families (Losh et al., 2008; Virkud et al., 2009). The use of direct tests of phonological processing such as the nonword repetition subtest of the CTOPP have provided inconsistent findings in first-degree relatives, which partly motivated the use of fMRI to identify a more sensitive measure of the phonological processing deficits that have been proposed to be a core BAP trait. Given the lack of CTOPP differences observed between groups and the nature of the fMRI task utilized in the current study that has been shown to probe phonological processing in control samples in both behavioral and imaging studies, our whole-brain and correlation results suggest that parents are engaging different underlying neural mechanisms than controls during phonological processing possibly due to compensatory mechanisms. In addition to a lack of group CTOPP differences, no behavioral differences between groups were observed for the fMRI LDT. However, the lack of behavioral differences for the LDT avoids a potential confound in performance task differences between groups. For any group comparisons in fMRI, it has been suggested that behavioral tasks be chosen that can be performed equally well (i.e., in terms of accuracy and/or response times) by all included groups. If task performance is matched, the differential hemodynamic responses can then be interpreted as differential neural processing as opposed to less successful task performance due to such factors as inattention, error processing, guessing, or misunderstanding of the task (Church et al., 2010; Johnson et al., 2002; Price and Friston, 1999; Schlaggar and McCandliss, 2007). Lastly, further studies will need to be undertaken with larger sample sizes and measures of language functioning within the proband sample. The relatively small sample size of the current study did not allow us to investigate effects of gender as a few BAP studies have suggested that the BAP is more strongly expressed in fathers than mothers (Wheelwright et al., 2010). There is no evidence of which we are aware, however, suggesting that the language aspect of the BAP exhibits a gender difference. In addition, proband participation in the current study was limited to confirmation of autism diagnoses for qualification of the parent sample. Further studies should include proband language measures to examine relationships between parent and child functioning.
Our whole-brain findings of group differences in hemodynamic responses between parents of individuals with ASD and control subjects during a implicit phonological priming task provide preliminary neurobiological evidence of phonological processing deficits within first-degree relatives with ASD. Therefore, our results indicate that these deficits may be part of the BAP. Regions exhibiting atypical hemodynamic response patterns in our parent sample included cortical regions shown previously to be implicated in the language and communication impairments that define ASD as well as regions found to show structural abnormalities in a previous study of parents of children with ASD. Furthermore, our overall findings in our parent sample mirror findings in individuals with dyslexia, a reading disorder that is thought to primarily be due to difficulties in phonological processing. Studies such as these in unaffected first-degree relatives allow for the identification of which anatomical substrates of the disorder may be heritable and thereby may reflect genetic factors.
Acknowledgments
The authors would like to thank Dr. Bruce Pennington for his advice during preparation of this study and Debra Singel for her help in fMRI data acquisition.
FUNDING SOURCES
Funding for this study was provided by Autism Speaks Grant 2090, Autism Speaks Weatherstone Grant 6291, the National Institutes of Health (NIH) Grant PHS R01 MH082020, NIH/NCRR Colorado CTSI Grant TL1 RR025778, and the National Institute of Child Health and Human Development (NICHHD) Grant HD041697.
Contributor Information
Lisa B. Wilson, Email: lisa.wilson@ucdenver.edu.
Jason R. Tregellas, Email: jason.tregellas@ucdenver.edu.
Erin Slason, Email: erin.slason@gmail.com.
Bryce E. Pasko, Email: bryce.pasko@ucdenver.edu.
Susan Hepburn, Email: susan.hepburn@ucdenver.edu.
Donald C. Rojas, Email: don.rojas@ucdenver.edu.
BIBLIOGRAPHY
- Abell F, Krams M, Ashburner J, Passingham R, Friston K, Frackowiak R, Happe F, Frith C, Frith U. The neuroanatomy of autism: a voxel-based whole brain analysis of structural scans. Neuroreport. 1999;10:1647–51. doi: 10.1097/00001756-199906030-00005. [DOI] [PubMed] [Google Scholar]
- Ackermann H, Mathiak K, Riecker A. The contribution of the cerebellum to speech production and speech perception: clinical and functional imaging data. Cerebellum. 2007;6:202–13. doi: 10.1080/14734220701266742. [DOI] [PubMed] [Google Scholar]
- Ackermann H, Riecker A. The contribution(s) of the insula to speech production: a review of the clinical and functional imaging literature. Brain Struct Funct. 2004;214:419–33. doi: 10.1007/s00429-010-0257-x. [DOI] [PubMed] [Google Scholar]
- Alarcon M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, Sebat J, Wigler M, Martin CL, Ledbetter DH, et al. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am J Hum Genet. 2008;82:150–9. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon M, Cantor RM, Liu J, Gilliam TC, Geschwind DH. Evidence for a language quantitative trait locus on chromosome 7q in multiplex autism families. Am J Hum Genet. 2002;70:60–71. doi: 10.1086/338241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon M, Yonan AL, Gilliam TC, Cantor RM, Geschwind DH. Quantitative genome scan and Ordered-Subsets Analysis of autism endophenotypes support language QTLs. Mol Psychiatry. 2005;10:747–57. doi: 10.1038/sj.mp.4001666. [DOI] [PubMed] [Google Scholar]
- Allen D, Rapin I. Autistic children are also dysphasic. In: Naruse H, Ornitz E, editors. Neurobiology of infantile autism. Amsterdam: Excerpta Medica; 1992. pp. 73–80. [Google Scholar]
- Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–45. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Anderson JS, Lange N, Froehlich A, DuBray MB, Druzgal TJ, Froimowitz MP, Alexander AL, Bigler ED, Lainhart JE. Decreased left posterior insular activity during auditory language in autism. AJNR Am J Neuroradiol. 2010;31:131–9. doi: 10.3174/ajnr.A1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annett M. Left, Right, Hand and Brain: The Right Shift Theory. Hillsdale, NJ: Lawrence Erlbaum Associates; 1985. [Google Scholar]
- APA. Diagnostic and statistical manual of mental disorders. Washington, D. C.: American Psychiatric Association; 1994. [Google Scholar]
- Arking DE, Cutler DJ, Brune CW, Teslovich TM, West K, Ikeda M, Rea A, Guy M, Lin S, Cook EH, et al. A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. Am J Hum Genet. 2008;82:160–4. doi: 10.1016/j.ajhg.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Awh E, Jonides J, Smith E, Schumacher E, Koeppe R, Katz S. Dissociation of storage and rehearsal in working memory: PET evidence. Psychological Science. 1996;7:25–31. [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255:556–9. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, Rutter M. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med. 1995;25:63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- Balota DA, Yap MJ, Cortese MJ, Hutchison KA, Kessler B, Loftis B, Neely JH, Nelson DL, Simpson GB, Treiman R. The English Lexicon Project. Behavioral Research Methods. 2007;39:445–59. doi: 10.3758/bf03193014. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Social and pragmatic deficits in autism: cognitive or affective? J Autism Dev Disord. 1988;18:379–402. doi: 10.1007/BF02212194. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring H, Chitnis X, Wheelwright S, Gregory L, Williams S, Brammer M, Bullmore E. fMRI of parents of children with Asperger Syndrome: a pilot study. Brain Cogn. 2006;61:122–30. doi: 10.1016/j.bandc.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord. 2001;31:5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Benson RR, Whalen DH, Richardson M, Swainson B, Clark VP, Lai S, Liberman AM. Parametrically dissociating speech and nonspeech perception in the brain using fMRI. Brain Lang. 2001;78:364–96. doi: 10.1006/brln.2001.2484. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Mortensen S, Neeley ES, Ozonoff S, Krasny L, Johnson M, Lu J, Provencal SL, McMahon W, Lainhart JE. Superior temporal gyrus, language function, and autism. Dev Neuropsychol. 2007;31:217–38. doi: 10.1080/87565640701190841. [DOI] [PubMed] [Google Scholar]
- Bishop DV, Maybery M, Wong D, Maley A, Hill W, Hallmayer J. Are phonological processing deficits part of the broad autism phenotype? Am J Med Genet B Neuropsychiatr Genet. 2004;128B:54–60. doi: 10.1002/ajmg.b.30039. [DOI] [PubMed] [Google Scholar]
- Bishop DV, North T, Donlan C. Nonword repetition as a behavioural marker for inherited language impairment: evidence from a twin study. J Child Psychol Psychiatry. 1996;37:391–403. doi: 10.1111/j.1469-7610.1996.tb01420.x. [DOI] [PubMed] [Google Scholar]
- Bles M, Jansma BM. Phonological processing of ignored distractor pictures, an fMRI investigation. BMC Neurosci. 2008;9:20. doi: 10.1186/1471-2202-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddaert N, Chabane N, Gervais H, Good CD, Bourgeois M, Plumet MH, Barthelemy C, Mouren MC, Artiges E, Samson Y, et al. Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. Neuroimage. 2004;23:364–9. doi: 10.1016/j.neuroimage.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Bohland JW, Guenther FH. An fMRI investigation of syllable sequence production. Neuroimage. 2006;32:821–41. doi: 10.1016/j.neuroimage.2006.04.173. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Functional anatomy of intra- and cross-modal lexical tasks. Neuroimage. 2002;16:7–22. doi: 10.1006/nimg.2002.1081. [DOI] [PubMed] [Google Scholar]
- Bradford Y, Haines J, Hutcheson H, Gardiner M, Braun T, Sheffield V, Cassavant T, Huang W, Wang K, Vieland V, et al. Incorporating language phenotypes strengthens evidence of linkage to autism. Am J Med Genet. 2001;105:539–47. [PubMed] [Google Scholar]
- Brunswick N, McCrory E, Price CJ, Frith CD, Frith U. Explicit and implicit processing of words and pseudowords by adult developmental dyslexics: A search for Wernicke’s Wortschatz? Brain. 1999;122(Pt 10):1901–17. doi: 10.1093/brain/122.10.1901. [DOI] [PubMed] [Google Scholar]
- Burton MW, Locasto PC, Krebs-Noble D, Gullapalli RP. A systematic investigation of the functional neuroanatomy of auditory and visual phonological processing. Neuroimage. 2005;26:647–61. doi: 10.1016/j.neuroimage.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Surveillance Summaries. SS10. Vol. 58. MMWR; 2009. Prevalence of Autism Spectrum Disorders – Autism and Developmental Disabilities Monitoring Network, United Sates, 2006; pp. 1–20. December 18, 2009. [PubMed] [Google Scholar]
- Chen SH, Desmond JE. Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks. Neuroimage. 2005a;24:332–8. doi: 10.1016/j.neuroimage.2004.08.032. [DOI] [PubMed] [Google Scholar]
- Chen SH, Desmond JE. Temporal dynamics of cerebro-cerebellar network recruitment during a cognitive task. Neuropsychologia. 2005b;43:1227–37. doi: 10.1016/j.neuropsychologia.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Chou TL, Davis MH, Marslen-Wilson WD, Booth JR. Phonological priming in visual word recognition for english words: an event-related functional MRI study. Chin J Psychol. 2006;48:1–18. [Google Scholar]
- Church JA, Petersen SE, Schlaggar BL. The “Task B problem” and other considerations in developmental functional neuroimaging. Hum Brain Mapp. 2010;31:852–62. doi: 10.1002/hbm.21036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Zhang Y, Frazier T, Abbacchi AM, Law P. Sibling recurrence and the genetic epidemiology of autism. Am J Psychiatry. 2010;167:1349–56. doi: 10.1176/appi.ajp.2010.09101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E. Brainstem, cerebellar and limbic neuroanatomical abnormalities in autism. Curr Opin Neurobiol. 1997;7:269–78. doi: 10.1016/s0959-4388(97)80016-5. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Saitoh O, Yeung-Courchesne R, Press GA, Lincoln AJ, Haas RH, Schreibman L. Abnormality of cerebellar vermian lobules VI and VII in patients with infantile autism: identification of hypoplastic and hyperplastic subgroups with MR imaging. AJR Am J Roentgenol. 1994;162:123–30. doi: 10.2214/ajr.162.1.8273650. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Yeung-Courchesne R, Press GA, Hesselink JR, Jernigan TL. Hypoplasia of cerebellar vermal lobules VI and VII in autism. N Engl J Med. 1988;318:1349–54. doi: 10.1056/NEJM198805263182102. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Alexander AL, Davidson RJ. Gaze-fixation, brain activation, and amygdala volume in unaffected siblings of individuals with autism. Biol Psychiatry. 2007;61:512–20. doi: 10.1016/j.biopsych.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb S, Schellenberg GD, Dager S, Friedman S, Aylward E, Richards T. Defining the broader phenotype of autism: genetic, brain, and behavioral perspectives. Dev Psychopathol. 2002;14:581–611. doi: 10.1017/s0954579402003103. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, Wijsman E, Schellenberg G, Estes A, Munson J, Faja S. Neurocognitive and electrophysiological evidence of altered face processing in parents of children with autism: implications for a model of abnormal development of social brain circuitry in autism. Dev Psychopathol. 2005;17:679–97. doi: 10.1017/S0954579405050327. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Volein A, Csibra G, Holmboe K, Garwood H, Tucker L, Krljes S, Baron-Cohen S, Bolton P, Charman T, et al. Neural correlates of eye gaze processing in the infant broader autism phenotype. Biol Psychiatry. 2009;65:31–8. doi: 10.1016/j.biopsych.2008.09.034. [DOI] [PubMed] [Google Scholar]
- Enticott PG, Bradshaw JL, Iansek R, Tonge BJ, Rinehart NJ. Electrophysiological signs of supplementary-motor-area deficits in high-functioning autism but not Asperger syndrome: an examination of internally cued movement-related potentials. Dev Med Child Neurol. 2009;51:787–91. doi: 10.1111/j.1469-8749.2009.03270.x. [DOI] [PubMed] [Google Scholar]
- Flagg EJ, Cardy JE, Roberts W, Roberts TP. Language lateralization development in children with autism: insights from the late field magnetoencephalogram. Neurosci Lett. 2005;386:82–7. doi: 10.1016/j.neulet.2005.05.037. [DOI] [PubMed] [Google Scholar]
- Folstein S, Rutter M. Infantile autism: a genetic study of 21 twin pairs. J Child Psychol Psychiatry. 1977;18:297–321. doi: 10.1111/j.1469-7610.1977.tb00443.x. [DOI] [PubMed] [Google Scholar]
- Folstein SE, Santangelo SL, Gilman SE, Piven J, Landa R, Lainhart J, Hein J, Wzorek M. Predictors of cognitive test patterns in autism families. J Child Psychol Psychiatry. 1999;40:1117–28. [PubMed] [Google Scholar]
- Frith U, Happe F. Autism: beyond “theory of mind”. Cognition. 1994;50:115–32. doi: 10.1016/0010-0277(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Fulbright RK, Jenner AR, Mencl WE, Pugh KR, Shaywitz BA, Shaywitz SE, Frost SJ, Skudlarski P, Constable RT, Lacadie CM, et al. The cerebellum’s role in reading: a functional MR imaging study. AJNR Am J Neuroradiol. 1999;20:1925–30. [PMC free article] [PubMed] [Google Scholar]
- Gabig CS. Verbal working memory and story retelling in school-age children with autism. Lang Speech Hear Serv Sch. 2008;39:498–511. doi: 10.1044/0161-1461(2008/07-0023). [DOI] [PubMed] [Google Scholar]
- Gaffney GR, Tsai LY, Kuperman S, Minchin S. Cerebellar structure in autism. Am J Dis Child. 1987;141:1330–2. doi: 10.1001/archpedi.1987.04460120096044. [DOI] [PubMed] [Google Scholar]
- Ghosh SS, Tourville JA, Guenther FH. A neuroimaging study of premotor lateralization and cerebellar involvement in the production of phonemes and syllables. J Speech Lang Hear Res. 2008;51:1183–202. doi: 10.1044/1092-4388(2008/07-0119). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves WW, Grabowski TJ, Mehta S, Gupta P. The left posterior superior temporal gyrus participates specifically in accessing lexical phonology. J Cogn Neurosci. 2008;20:1698–710. doi: 10.1162/jocn.2008.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haist F, Song AW, Wild K, Faber TL, Popp CA, Morris RD. Linking sight and sound: fMRI evidence of primary auditory cortex activation during visual word recognition. Brain Lang. 2001;76:340–50. doi: 10.1006/brln.2000.2433. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Girgis RR, Adams J, Gilbert AR, Keshavan MS, Minshew NJ. Abnormal brain size effect on the thalamus in autism. Psychiatry Res. 2006;147:145–51. doi: 10.1016/j.pscychresns.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Girgis RR, Adams J, Gilbert AR, Melhem NM, Keshavan MS, Minshew NJ. Brief report: abnormal association between the thalamus and brain size in Asperger’s disorder. J Autism Dev Disord. 2008a;38:390–4. doi: 10.1007/s10803-007-0385-1. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Minshew NJ, Melhem NM, Srihari S, Jo B, Bansal R, Keshavan MS, Stanley JA. An MRI and proton spectroscopy study of the thalamus in children with autism. Psychiatry Res. 2008b;163:97–105. doi: 10.1016/j.pscychresns.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Tayama M, Miyazaki M, Murakawa K, Kuroda Y. Brainstem and cerebellar vermis involvement in autistic children. J Child Neurol. 1993;8:149–53. doi: 10.1177/088307389300800207. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Harris GJ, Adrien KT, Ziegler DA, Makris N, Kennedy DN, Lange NT, Chabris CF, Bakardjiev A, Hodgson J, et al. Abnormal asymmetry in language association cortex in autism. Ann Neurol. 2002;52:588–96. doi: 10.1002/ana.10349. [DOI] [PubMed] [Google Scholar]
- Herbster AN, Mintun MA, Nebes RD, Becker JT. Regional cerebral blood flow during word and nonword reading. Hum Brain Mapp. 1997;5:84–92. doi: 10.1002/(sici)1097-0193(1997)5:2<84::aid-hbm2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hurst JA, Baraitser M, Auger E, Graham F, Norell S. An extended family with a dominantly inherited speech disorder. Dev Med Child Neurol. 1990;32:352–5. doi: 10.1111/j.1469-8749.1990.tb16948.x. [DOI] [PubMed] [Google Scholar]
- Hyde KL, Samson F, Evans AC, Mottron L. Neuroanatomical differences in brain areas implicated in perceptual and other core features of autism revealed by cortical thickness analysis and voxel-based morphometry. Hum Brain Mapp. 2010;31:556–66. doi: 10.1002/hbm.20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: a metanalysis of 35 neuroimaging studies. Neuroimage. 2003;20:693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Halit H, Grice SJ, Karmiloff-Smith A. Neuroimaging of typical and atypical development: a perspective from multiple levels of analysis. Dev Psychopathol. 2002;14:521–36. doi: 10.1017/s0954579402003073. [DOI] [PubMed] [Google Scholar]
- Jou RJ, Minshew NJ, Keshavan MS, Vitale MP, Hardan AY. Enlarged right superior temporal gyrus in children and adolescents with autism. Brain Res. 2010;1360:205–12. doi: 10.1016/j.brainres.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielinen M, Rantala H, Timonen E, Linna SL, Moilanen I. Associated medical disorders and disabilities in children with autistic disorder: a population-based study. Autism. 2004;8:49–60. doi: 10.1177/1362361304040638. [DOI] [PubMed] [Google Scholar]
- Kjelgaard MM, Tager-Flusberg H. An Investigation of Language Impairment in Autism: Implications for Genetic Subgroups. Lang Cogn Process. 2001;16:287–308. doi: 10.1080/01690960042000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans NM, Muller RA, Cohen DN, Courchesne E. Atypical functional lateralization of language in autism spectrum disorders. Brain Res. 2008;1221:115–25. doi: 10.1016/j.brainres.2008.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus TA, Silver AM, Kennedy M, Lindgren KA, Dominick KC, Siegel J, Tager-Flusberg H. Language laterality in autism spectrum disorder and typical controls: a functional, volumetric, and diffusion tensor MRI study. Brain Lang. 2010;112:113–20. doi: 10.1016/j.bandl.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus TA, Silver AM, Lindgren KA, Hadjikhani N, Tager-Flusberg H. fMRI activation during a language task in adolescents with ASD. J Int Neuropsychol Soc. 2008;14:967–79. doi: 10.1017/S1355617708081216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht S, Deppe M, Drager B, Bobe L, Lohmann H, Ringelstein E, Henningsen H. Language lateralization in healthy right-handers. Brain. 2000a;123(Pt 1):74–81. doi: 10.1093/brain/123.1.74. [DOI] [PubMed] [Google Scholar]
- Knecht S, Drager B, Deppe M, Bobe L, Lohmann H, Floel A, Ringelstein EB, Henningsen H. Handedness and hemispheric language dominance in healthy humans. Brain. 2000b;123(Pt 12):2512–8. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- Kouider S, de Gardelle V, Dehaene S, Dupoux E, Pallier C. Cerebral bases of subliminal speech priming. Neuroimage. 2010;49:922–9. doi: 10.1016/j.neuroimage.2009.08.043. [DOI] [PubMed] [Google Scholar]
- Kouider S, Dehaene S, Jobert A, Le Bihan D. Cerebral bases of subliminal and supraliminal priming during reading. Cereb Cortex. 2007;17:2019–29. doi: 10.1093/cercor/bhl110. [DOI] [PubMed] [Google Scholar]
- Lee H, Devlin JT, Shakeshaft C, Stewart LH, Brennan A, Glensman J, Pitcher K, Crinion J, Mechelli A, Frackowiak RS, et al. Anatomical traces of vocabulary acquisition in the adolescent brain. J Neurosci. 2007;27:1184–9. doi: 10.1523/JNEUROSCI.4442-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren KA, Folstein SE, Tomblin JB, Tager-Flusberg H. Language and reading abilities of children with autism spectrum disorders and specific language impairment and their first-degree relatives. Autism Res. 2009;2:22–38. doi: 10.1002/aur.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Paul R. Language and communication in autism. In: Cohen DJ, Volkmar FR, editors. Handbook of autism and pervasive developmental disorders. 2. New York: John Wiley & Sons; 1997. [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–23. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Losh M, Childress D, Lam K, Piven J. Defining key features of the broad autism phenotype: a comparison across parents of multiple- and single-incidence autism families. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:424–33. doi: 10.1002/ajmg.b.30612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marien P, Engelborghs S, Fabbro F, De Deyn PP. The lateralized linguistic cerebellum: a review and a new hypothesis. Brain Lang. 2001;79:580–600. doi: 10.1006/brln.2001.2569. [DOI] [PubMed] [Google Scholar]
- Maziade M, Merette C, Cayer M, Roy MA, Szatmari P, Cote R, Thivierge J. Prolongation of brainstem auditory-evoked responses in autistic probands and their unaffected relatives. Arch Gen Psychiatry. 2000;57:1077–83. doi: 10.1001/archpsyc.57.11.1077. [DOI] [PubMed] [Google Scholar]
- Mitchell SR, Reiss AL, Tatusko DH, Ikuta I, Kazmerski DB, Botti JA, Burnette CP, Kates WR. Neuroanatomic alterations and social and communication deficits in monozygotic twins discordant for autism disorder. Am J Psychiatry. 2009;166:917–25. doi: 10.1176/appi.ajp.2009.08101538. [DOI] [PubMed] [Google Scholar]
- Mizuno A, Villalobos ME, Davies MM, Dahl BC, Muller RA. Partially enhanced thalamocortical functional connectivity in autism. Brain Res. 2006;1104:160–74. doi: 10.1016/j.brainres.2006.05.064. [DOI] [PubMed] [Google Scholar]
- Mostofsky SH, Powell SK, Simmonds DJ, Goldberg MC, Caffo B, Pekar JJ. Decreased connectivity and cerebellar activity in autism during motor task performance. Brain. 2009;132:2413–25. doi: 10.1093/brain/awp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RA, Chugani DC, Behen ME, Rothermel RD, Muzik O, Chakraborty PK, Chugani HT. Impairment of dentato-thalamo-cortical pathway in autistic men: language activation data from positron emission tomography. Neurosci Lett. 1998;245:1–4. doi: 10.1016/s0304-3940(98)00151-7. [DOI] [PubMed] [Google Scholar]
- Muller RA, Pierce K, Ambrose JB, Allen G, Courchesne E. Atypical patterns of cerebral motor activation in autism: a functional magnetic resonance study. Biol Psychiatry. 2001;49:665–76. doi: 10.1016/s0006-3223(00)01004-0. [DOI] [PubMed] [Google Scholar]
- Murakami JW, Courchesne E, Press GA, Yeung-Courchesne R, Hesselink JR. Reduced cerebellar hemisphere size and its relationship to vermal hypoplasia in autism. Arch Neurol. 1989;46:689–94. doi: 10.1001/archneur.1989.00520420111032. [DOI] [PubMed] [Google Scholar]
- Murdoch BE. The cerebellum and language: historical perspective and review. Cortex. 2010;46:858–68. doi: 10.1016/j.cortex.2009.07.018. [DOI] [PubMed] [Google Scholar]
- Newman RL, Joanisse MF. Modulation of brain regions involved in word recognition by homophonous stimuli: an fMRI study. Brain Res. 2011;1367:250–64. doi: 10.1016/j.brainres.2010.09.089. [DOI] [PubMed] [Google Scholar]
- Palmen SJ, van Engeland H. Review on structural neuroimaging findings in autism. J Neural Transm. 2004;111:903–29. doi: 10.1007/s00702-003-0068-9. [DOI] [PubMed] [Google Scholar]
- Peterson E, Schmidt GL, Tregellas JR, Winterrowd E, Kopelioff L, Hepburn S, Reite M, Rojas DC. A voxel-based morphometry study of gray matter in parents of children with autism. Neuroreport. 2006;17:1289–92. doi: 10.1097/01.wnr.0000233087.15710.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piven J, Palmer P. Cognitive deficits in parents from multiple-incidence autism families. J Child Psychol Psychiatry. 1997;38:1011–21. doi: 10.1111/j.1469-7610.1997.tb01618.x. [DOI] [PubMed] [Google Scholar]
- Piven J, Palmer P, Landa R, Santangelo S, Jacobi D, Childress D. Personality and language characteristics in parents from multiple-incidence autism families. Am J Med Genet. 1997a;74:398–411. [PubMed] [Google Scholar]
- Piven J, Saliba K, Bailey J, Arndt S. An MRI study of autism: the cerebellum revisited. Neurology. 1997b;49:546–51. doi: 10.1212/wnl.49.2.546. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Scanning patients with tasks they can perform. Hum Brain Mapp. 1999;8:102–8. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<102::AID-HBM6>3.0.CO;2-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Deus J, Losilla JM, Capdevila A. Cerebral lateralization of language in normal left-handed people studied by functional MRI. Neurology. 1999;52:1038–43. doi: 10.1212/wnl.52.5.1038. [DOI] [PubMed] [Google Scholar]
- Rapin I, Dunn MA, Allen DA, Stevens MC, Fein D. Subtypes of language disorders in school-age children with autism. Dev Neuropsychol. 2009;34:66–84. doi: 10.1080/87565640802564648. [DOI] [PubMed] [Google Scholar]
- Redcay E, Courchesne E. Deviant functional magnetic resonance imaging patterns of brain activity to speech in 2–3-year-old children with autism spectrum disorder. Biol Psychiatry. 2008;64:589–98. doi: 10.1016/j.biopsych.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy KS. Cytogenetic abnormalities and fragile-X syndrome in Autism Spectrum Disorder. BMC Med Genet. 2005;6:3. doi: 10.1186/1471-2350-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. Functional abnormalities in the dyslexic brain: a quantitative meta-analysis of neuroimaging studies. Hum Brain Mapp. 2009;30:3299–308. doi: 10.1002/hbm.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas DC, Bawn SD, Benkers TL, Reite ML, Rogers SJ. Smaller left hemisphere planum temporale in adults with autistic disorder. Neurosci Lett. 2002;328:237–40. doi: 10.1016/s0304-3940(02)00521-9. [DOI] [PubMed] [Google Scholar]
- Rojas DC, Maharajh K, Teale P, Rogers SJ. Reduced neural synchronization of gamma-band MEG oscillations in first-degree relatives of children with autism. BMC Psychiatry. 2008;8:66. doi: 10.1186/1471-244X-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas DC, Smith JA, Benkers TL, Camou SL, Reite ML, Rogers SJ. Hippocampus and amygdala volumes in parents of children with autistic disorder. Am J Psychiatry. 2004;161:2038–44. doi: 10.1176/appi.ajp.161.11.2038. [DOI] [PubMed] [Google Scholar]
- Schaefer GB, Lutz RE. Diagnostic yield in the clinical genetic evaluation of autism spectrum disorders. Genet Med. 2006;8:549–56. doi: 10.1097/01.gim.0000237789.98842.f1. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, McCandliss BD. Development of neural systems for reading. Annu Rev Neurosci. 2007;30:475–503. doi: 10.1146/annurev.neuro.28.061604.135645. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum Brain Mapp. 1996;4:174–98. doi: 10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Schmidt GL, Kimel LK, Winterrowd E, Pennington BF, Hepburn SL, Rojas DC. Impairments in phonological processing and nonverbal intellectual function in parents of children with autism. J Clin Exp Neuropsychol. 2008;30:557–67. doi: 10.1080/13803390701551225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz BA, Lyon GR, Shaywitz SE. The role of functional magnetic resonance imaging in understanding reading and dyslexia. Dev Neuropsychol. 2006;30:613–32. doi: 10.1207/s15326942dn3001_5. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, Skudlarski P, Constable RT, Marchione KE, Fletcher JM, Lyon GR, et al. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biol Psychiatry. 2002;52:101–10. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE. Dyslexia. Sci Am. 1996;275:98–104. doi: 10.1038/scientificamerican1196-98. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE. Dyslexia. N Engl J Med. 1998;338:307–12. doi: 10.1056/NEJM199801293380507. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA. Paying attention to reading: the neurobiology of reading and dyslexia. Dev Psychopathol. 2008;20:1329–49. doi: 10.1017/S0954579408000631. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Constable RT, Mencl WE, Shankweiler DP, Liberman AM, Skudlarski P, Fletcher JM, et al. Functional disruption in the organization of the brain for reading in dyslexia. Proc Natl Acad Sci U S A. 1998;95:2636–41. doi: 10.1073/pnas.95.5.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behav Res Methods Instrum Comput. 1999;31:137–49. doi: 10.3758/bf03207704. [DOI] [PubMed] [Google Scholar]
- Steffenburg S, Gillberg C, Hellgren L, Andersson L, Gillberg IC, Jakobsson G, Bohman M. A twin study of autism in Denmark, Finland, Iceland, Norway and Sweden. J Child Psychol Psychiatry. 1989;30:405–16. doi: 10.1111/j.1469-7610.1989.tb00254.x. [DOI] [PubMed] [Google Scholar]
- Stoeckel C, Gough PM, Watkins KE, Devlin JT. Supramarginal gyrus involvement in visual word recognition. Cortex. 2009;45:1091–6. doi: 10.1016/j.cortex.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–34. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Sun YF, Lee JS, Kirby R. Brain imaging findings in dyslexia. Pediatr Neonatol. 2010;51:89–96. doi: 10.1016/S1875-9572(10)60017-4. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Binder JR, Possing ET, McKiernan KA, Ward BD, Hammeke TA. Language lateralization in left-handed and ambidextrous people: fMRI data. Neurology. 2002;59:238–44. doi: 10.1212/wnl.59.2.238. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H. Brief report: current theory and research on language and communication in autism. J Autism Dev Disord. 1996;26:169–72. doi: 10.1007/BF02172006. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H. A psychological approach to understanding the social and language impairments in autism. Int Rev Psychiatry. 1999;11:325–34. doi: 10.1080/09540269974203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager-Flusberg H. Language and communication disorders in autism spectrum disorders. In: Bauman ML, Kemper TL, editors. The neurobiology of autism. 2. Baltimore: Johns Hopkins University Press; 2005. pp. 45–58. [Google Scholar]
- Tager-Flusberg H, Caronna E. Language disorders: autism and other pervasive developmental disorders. Pediatr Clin North Am. 2007;54:469–81. doi: 10.1016/j.pcl.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Thatcher RW, North DM, Neubrander J, Biver CJ, Cutler S, Defina P. Autism and EEG phase reset: deficient GABA mediated inhibition in thalamo-cortical circuits. Dev Neuropsychol. 2009;34:780–800. doi: 10.1080/87565640903265178. [DOI] [PubMed] [Google Scholar]
- Toth K, Dawson G, Meltzoff AN, Greenson J, Fein D. Early social, imitation, play, and language abilities of young non-autistic siblings of children with autism. J Autism Dev Disord. 2007;37:145–57. doi: 10.1007/s10803-006-0336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsatsanis KD, Rourke BP, Klin A, Volkmar FR, Cicchetti D, Schultz RT. Reduced thalamic volume in high-functioning individuals with autism. Biol Psychiatry. 2003;53:121–9. doi: 10.1016/s0006-3223(02)01530-5. [DOI] [PubMed] [Google Scholar]
- Tuchman RF, Rapin I, Shinnar S. Autistic and dysphasic children. I: Clinical characteristics. Pediatrics. 1991;88:1211–8. [PubMed] [Google Scholar]
- Turkeltaub PE, Coslett HB. Localization of sublexical speech perception components. Brain Lang. 2010;114:1–15. doi: 10.1016/j.bandl.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vaden KI, Jr, Muftuler LT, Hickok G. Phonological repetition-suppression in bilateral superior temporal sulci. Neuroimage. 2010;49:1018–23. doi: 10.1016/j.neuroimage.2009.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellutino FR, Fletcher JM, Snowling MJ, Scanlon DM. Specific reading disability (dyslexia): what have we learned in the past four decades? J Child Psychol Psychiatry. 2004;45:2–40. doi: 10.1046/j.0021-9630.2003.00305.x. [DOI] [PubMed] [Google Scholar]
- Verhoeven JS, De Cock P, Lagae L, Sunaert S. Neuroimaging of autism. Neuroradiology. 2010;52:3–14. doi: 10.1007/s00234-009-0583-y. [DOI] [PubMed] [Google Scholar]
- Vernes SC, Newbury DF, Abrahams BS, Winchester L, Nicod J, Groszer M, Alarcon M, Oliver PL, Davies KE, Geschwind DH, et al. A functional genetic link between distinct developmental language disorders. N Engl J Med. 2008;359:2337–45. doi: 10.1056/NEJMoa0802828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virkud YV, Todd RD, Abbacchi AM, Zhang Y, Constantino JN. Familial aggregation of quantitative autistic traits in multiplex versus simplex autism. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:328–34. doi: 10.1002/ajmg.b.30810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner RK, Togesen JK, Rashotte CA. Comprehensive Test of Phonological Processing. Austin, TX: PRO-ED; 1999. [Google Scholar]
- Wassink TH, Piven J, Patil SR. Chromosomal abnormalities in a clinic sample of individuals with autistic disorder. Psychiatr Genet. 2001;11:57–63. doi: 10.1097/00041444-200106000-00001. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Wheelwright S, Auyeung B, Allison C, Baron-Cohen S. Defining the broader, medium and narrow autism phenotype among parents using the Autism Spectrum Quotient (AQ) Mol Autism. 2010;1:10. doi: 10.1186/2040-2392-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse AJ, Barry JG, Bishop DV. Further defining the language impairment of autism: is there a specific language impairment subtype? J Commun Disord. 2008;41:319–36. doi: 10.1016/j.jcomdis.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Wilke M, Lidzba K. LI-tool: a new toolbox to assess lateralization in functional MR-data. J Neurosci Methods. 2007;163:128–36. doi: 10.1016/j.jneumeth.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Williams DL, Minshew NJ. Understanding autism and related disorders: what has imaging taught us? Neuroimaging Clin N Am. 2007;17:495–509. ix. doi: 10.1016/j.nic.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson LB, Tregellas JR, Slason E, Pasko BE, Rojas DC. Implicit phonological priming during visual word recognition. Neuroimage. 2011;55:724–31. doi: 10.1016/j.neuroimage.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]


