Abstract
Background
In people with diabetes, blood glucose levels should be regularly monitored to prevent serious complications associated with diabetes. This involves invasive method of withdrawing blood which causes inconvenience to patients. The objective of the study was to investigate the efficiency of noninvasive Electroporation and Transcutaneous Sampling (ETS) technique for predicting blood glucose levels.
Methods
In vitro studies were carried out in Franz diffusion cells using porcine epidermis to assess the feasibility of transcutaneous sampling of glucose. In vivo, the ETS technique was assessed in diabetes induced sprague dawley rat model. Glucose was sampled following the application of 30 electrical pulses of 1ms duration at 120 V/cm2, 1 Hz. Clarke Error Grid Analysis was carried out for the venous blood glucose levels that were determined by ETS with reference to that measured by glucose meter.
Results
The amount of glucose sampled by the ETS method in both in vitro and in vivo was proportional to the dermal glucose concentration. All the data points from in vivo studies were in A and B zones of Clarke error grid analysis and the mean absolute relative error was found to be 12.8%.
Conclusions
The results of the present study demonstrate that ETS technique could be developed as a noninvasive method of predicting venous blood glucose levels in diabetics.
Keywords: Electroporation and Transcutaneous Sampling (ETS), Glucose, In vitro, In vivo
1. INTRODUCTION
Diabetes mellitus is a major health concern. Diabetes can lead to chronic complications such as heart disease, blindness, renal failure, peripheral vascular disease and limb amputation [1, 2]. For proper diabetes management, it is very important that blood glucose levels are checked regularly. This involves the invasive method of collecting blood through a finger stick system. This has been major inconvenience for patients [3]. Some patients avoid glucose measurements due to needle phobia. To overcome this problem different noninvasive blood glucose monitoring methods were developed including near-infrared (NIR) spectroscopy, far-infrared (FIR) spectroscopy, radio wave impedance, reverse iontophoresis, ultrasound, blister technique and microneedles [4–8]. However none of the methods are in clinical use. ETS is a noninvasive method of sampling drugs and analytes from the dermal extracellular fluid by reversible electrical permeabilization of the skin [9]. ETS technique involves application of few short electrical pulses on the surface of the skin which permeabilize the stratum corneum by creating transient aqueous pathways. The dermal glucose diffuses into the sampling fluid which is in contact with the permeabilized region of the skin. The objective of the present study was to investigate the feasibility of utilizing ETS technique to sample glucose in the dermal extracellular fluid that correlate with blood glucose levels.
2. MATERIALS AND METHODS
2.1. Chemicals
Glucose, Alloxan monohydrate, Glucose assay kit (GAHK-20) were purchased from Sigma-Aldrich Inc, MO; 10X Phosphate buffered saline (PBS) premixed powder was obtained from EMD chemicals, NJ.
2.2. Skin
Porcine belly skin was obtained from a local abattoir. Pieces of skin wrapped in aluminum foil were heated to 60°C for 2 min and the epidermis was gently peeled off the skin. The fresh epidermis was used for in vitro glucose sampling by electroporation.
2.3. In Vitro experimental arrangement
The in vitro diffusion studies were carried out in Franz diffusion cells (Logan Instruments Ltd, Somerset, NJ) using porcine epidermis collected from three animals. The stratum corneum side of the skin was in contact with upper sampling compartment and ventral side with reservoir compartment. The active diffusion area was 0.64 cm2. Ag-AgCl electrode wires of 2mm diameter (obtained from In Vivo Metric, CA) made in form of circular ring were placed 2mm away from skin in both the sampling and reservoir compartments. The upper sampling compartment and the reservoir compartment were will filled with 0.4 and 5ml PBS respectively. The electrical resistance of the epidermis was measured by placing a load resistor RL (100 kΩ) in series with the epidermis. The voltage drops across the whole circuit (VO) and across the epidermis (VS) were measured using multimeter (Agilent Technologies, Santa Clara, CA). Epidermis resistance in kΩ was approximated from the formula:
| (1) |
Where RS is the epidermis resistance and RL is the load resistor in kΩ. The piece of porcine epidermis, which had a resistance greater than 20 kΩ.cm2, was used for the experiment.
2.4. In Vitro glucose sampling by electroporation
The upper sampling compartment was filled with 0.4 ml of PBS, pH 7.4 and lower reservoir compartment was filled with 5ml of glucose solution in PBS of concentrations between 50–400 mg/dl. Electroporation was carried out using an ECM 830 electrosquare porator (BTX Harvard apparatus, Holliston, USA). The electroporation protocol was 30 pulses each of 1ms duration at 120V/cm2 of active diffusion area. PBS from the sampling compartment was withdrawn 15 min after application of electrical pulses and the amount of glucose was measured using the Glucose assay kit by UV at 340nm. The in vitro permeability coefficient, Pin vitro (cm/min) was calculated using the formula:
| (2) |
2.5 In Vivo experimental arrangement
The in vivo experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Mississippi (Protocol # 07–028). The in vivo studies were carried out in Sprague-Dawley rats (Harlan Sprague-Dawley, Indianapolis, IN) (200–224 g) under ketamine (80mg/kg) and xylazine (10mg/kg) anesthesia.
The moisture content in the epidermal layers and in the dermis of each rat was measured using Delfin moisture meter-SC and Delfin moisture meter-D (Delfin Technologies Ltd, Kuopio, Finland) before sampling to ensure the absence of edema or inflammation (10).
Glucose sampling by ETS was carried out using a custom made sampling cell. The back portion of rats was shaved and a custom made in vivo electroporation cell was fixed using an adhesive (Krazy glue, Elmers products Inc, Ohio). The cell contains a sample collection chamber in which one of the Ag-AgCl electrode was placed and other electrode which acts as counter electrode was fixed just adjacent to the cell on the surface of skin using a micropore surgical tape (3M Healthcare, MN) (Figure 1). The skin was hydrated with 100μl of saline for 5 minutes before each sampling and was replaced with 400μl of sampling buffer. Thirty electrical pulses each of 1ms duration at 120V/cm2 were applied and the sampling fluid remained in the chamber for 15 minutes after pulsing. The sampling fluid was withdrawn and the amount of glucose present was measured using the Glucose assay kit (the limits of detection were 0.1–5 mg/dl)
Figure 1.
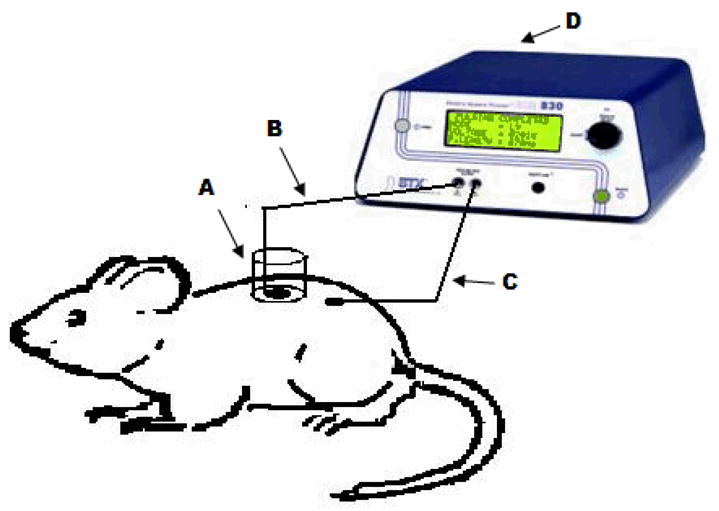
Diagram representing the in vivo experimental set up is shown in this figure. The sampling chamber (A) was glued on the skin surface of the sprague dawley rat. Ag-Agcl electrodes (B and C) are placed in the sampling chamber and secured on the skin surface respectively. 0.4 ml of sampling buffer was placed in the chamber. The two electrodes were connected to BTX 830M electrosquare porator and electrical pulses were applied. The sampling buffer was collected after 15 minute and the amount of glucose present was measured.
In vivo permeability coefficient
The calibration to convert sampling chamber glucose to venous equivalent values is nothing but determination of permeability coefficient in rats having steady normal glucose levels. The constant venous glucose levels were confirmed by triplicate samples. Venous glucose levels and corresponding ETS glucose levels of 18 data points in normal rats was used for calibration. The normal venous glucose range in Sprague Dawley rats is ~150–180 mg/dl (11). The in vivo permeability coefficient, Pin vivo (cm/min) of rat skin was calculated using the formula:
| (3) |
Sampling in diabetes induced rats
Nine rats were used, of which 3 were in control group and 6 in test group. Transcutaneous sampling of glucose by ETS was carried out in the test group only. In the control group transcutaneous sampling was carried out without the application of electrical pulses. All the 9 rats were injected with alloxan (200mg/kg in saline) intraperitoneally. Generally the duration required to induce irreversible diabetes with alloxan is ~24 hours (12). The blood and transcutaneous samples were obtained in all the rats before injecting alloxan and also after 24hrs and 36hrs after injection of alloxan. The venous blood glucose was measured using a Glucose meter (True Track Smart System, Walgreens, IL).
From the ETS glucose levels, the venous blood glucose concentrations are predicted using the formula:
| (4) |
2.6. Data analysis
The clinical utility of the method was determined based on the plot of the data on a Clarke Error Grid and determining the percentage of points that are located in the clinically acceptable A and B zones. Mean absolute relative error was also calculated to estimate the clinical accuracy [10] using the formula:
| (5) |
3. RESULTS AND DISCUSSION
Blood glucose is in homoeostasis with the peripheral tissue extracellular fluid. Therefore any change in the blood glucose is reflected in the glucose levels in the tissue extracellular fluid. Transdermal permeability of a polar diffusant like glucose is limited due to the barrier properties of stratum corneum. Therefore transcutaneous sampling of glucose requires a technique that can reversibly permeabilize the stratum corneum and facilitate rapid diffusion of glucose from dermal fluid into the sampling fluid. In vitro diffusion studies were carried out across the porcine epidermis to assess the feasibility of ETS for sampling glucose. Sampling without the application of electrical pulses (control) was not possible as the glucose transfer by passive diffusion was below the detectable levels, whereas, significant amount of glucose diffused following electroporation of the porcine epidermis. The electrical resistance of the epidermis dropped by an average of 70 ± 6% by application of electrical pulses indicating permeabilization of the skin. The in vitro ETS data is represented in figure 2. The rate of diffusion is predominantly governed by the concentration of glucose in the dermal extracellular fluid (presuming that the concentration of glucose in the sampling compartment is negligible to the total glucose concentration in the reservoir compartment) along with other factors. Therefore, the amount of glucose sampled by ETS was proportional to the concentration of glucose in the reservoir compartment fluid (R2 = 0.93). The in vitro permeability coefficient (Pin vitro) of porcine epidermis permeabilized by electroporation was 1.9 ± 0.3 × 10−4 cm/min.
Figure 2.
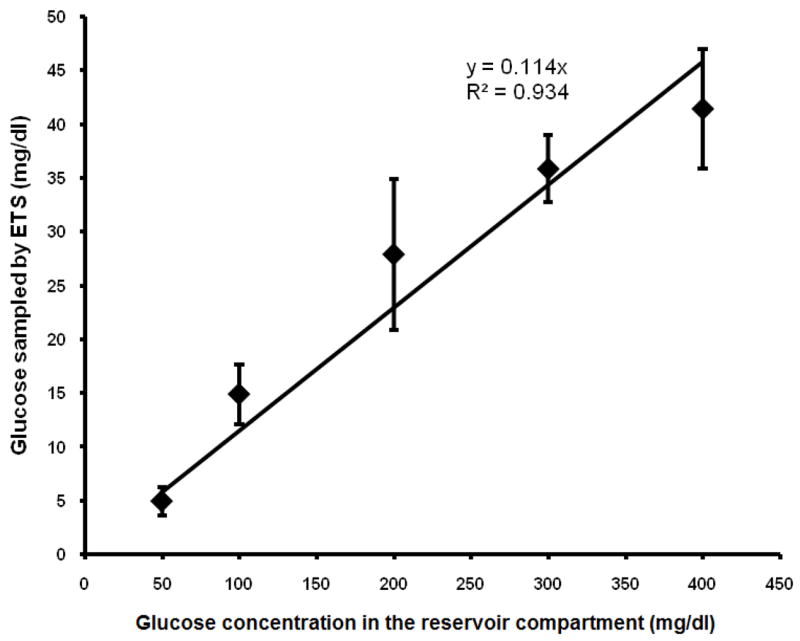
Relationship between glucose concentration in reservoir compartment and glucose sampled by ETS across porcine epidermis in vitro.
Preclinical studies were carried out in a diabetes- induced Sprague Dawley rat model to assess the workability of the technique in vivo. The moisture content in the rat skin was measured to ensure that there was no edema-inflammation (10) in the region of glucose sampling. The measurement also confirms the uniformity of degree of hydration of epidermal layers of skin. The Delfin moisture meters measure the skin moisture contents in terms of dielectric constant of the skin. The average dielectric constant values from moisture meter SC was 9.94± 1.65 and from moisture meter D was 20.67± 3.31. The in vivo permeability coefficient (Pin vivo) of rat skin for glucose was found to be 1.95 ± 0.15 × 10−5 cm/min. Again a good linear correlation between the venous glucose levels and the amount of glucose sampled by ETS was observed (R2=0.87). Generally, Clarke error grid analysis is carried out to assess the clinical utility of glucose monitoring devices. The analysis divides the reference and measured glucose into five zones A, B, C, D and E. Values in zones A and B are considered clinically acceptable, whereas values in zones C, D, E lead to significant errors [13]. At this stage we do not know if one can translate the results from rat model experiments to human application. However, assessing the preclinical data from clinical perspective would provide some insight into the clinical applicability of the technique. The venous blood glucose determined by blood sampling (x-axis) (reference values) and that predicted by ETS techniques (y-axis) are shown in figure 3. All the data points were within Clarke Error Grid A and B zones (Fig. 3). A mean absolute relative error of 12.8% was found for all measurements (n=18) from the in vivo data.
Figure 3.
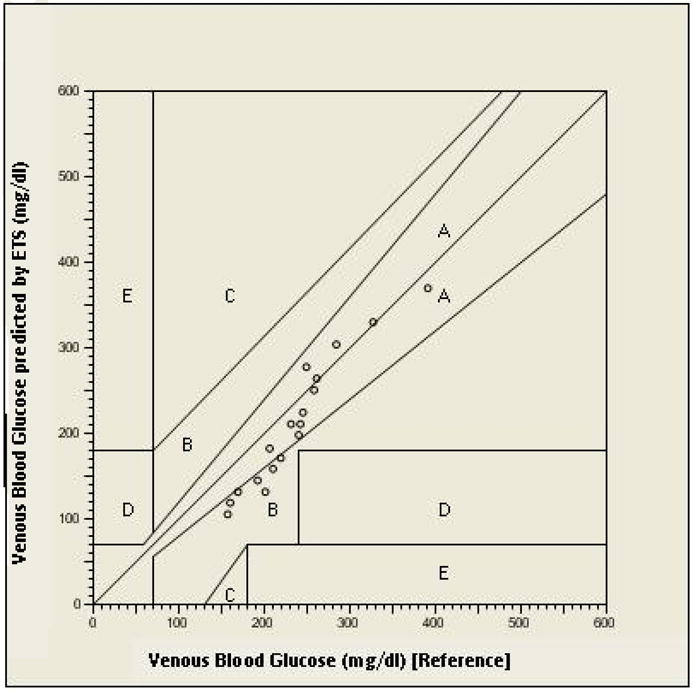
Clarke Error Grid analysis of venous blood glucose (reference) and venous blood glucose predicted by ETS in sprague dawley rats.
4. CONCLUSION
The results support our hypothesis that the concentration of glucose sampled by ETS would be proportional to the concentration of glucose in the dermal extracellular fluid. ETS could be developed as a noninvasive method of blood sampling for glucose monitoring in people with diabetes. However, clinical studies need to be carried out to assess the workability of ETS technique for human applications.
Acknowledgments
This work has been supported by Graduate Student Council Research Grant awarded by GSC, The University of Mississippi. This work was also partially supported by NIAMS, Grant # AR053097. We would like to acknowledge “The Epsilon Group”, Virginia for their support in carrying out Clarke Error Grid analysis. We also would like to acknowledge Delfin Technologies Ltd, Kuopio, Finland for lending us the moisture meters.
Funding source
Graduate Student Council (GSC), The University of Mississippi. National Institute of Arthritis, Musculoskeletal and Skin diseases (NIAMS).
References
- 1.Lee S, Nayak V, Dodds J, Pishko M, Smith Nb. Glucose measurements with sensors and ultrasound. Ultrasound in Med & Biol. 2005;31:971–977. doi: 10.1016/j.ultrasmedbio.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Sieg A, Guy RH, Delgado-Charro MB. Noninvasive glucose monitoring by reverse iontophoresis in vivo: Application of the internal standard concept. Clinical Chemistry. 2004;50(8):1383–1390. doi: 10.1373/clinchem.2004.032862. [DOI] [PubMed] [Google Scholar]
- 3.Sieg A, Guy RH, Delgado-Charro MB. Reverse iontophoresis for noninvasive glucose monitoring: The internal standard concept. J Pharm Sci. 2003;92(11):2295–2302. doi: 10.1002/jps.10492. [DOI] [PubMed] [Google Scholar]
- 4.Klonoff DC. Noninvasive blood glucose monitoring. Diabetes Care. 1997;20(3):433–437. doi: 10.2337/diacare.20.3.433. [DOI] [PubMed] [Google Scholar]
- 5.Sieg A, Guy RH, Delgado-Charro MB. Noninvasive and minimally invasive methods for transdermal glucose monitoring. Diabetes Technology & Therapeutics. 2005;7(1):174–196. doi: 10.1089/dia.2005.7.174. [DOI] [PubMed] [Google Scholar]
- 6.Rong L, Bin D, Wenliang C, Kevin X. Next step of noninvasive glucose monitor by NIR technique from the well controlled measuring condition and results. Opt Quant Electron. 2005;37:1305–1317. [Google Scholar]
- 7.Mitragotri S, Coleman M, Kost J, Langer R. Analysis of ultrasonically extracted interstitial fluid as a predictor of blood glucose levels. J Appl Physiol. 2000:961–966. doi: 10.1152/jappl.2000.89.3.961. [DOI] [PubMed] [Google Scholar]
- 8.Wang PM, Cornwell M, Prausnitz MR. Minimally invasive extraction of dermal interstitial fluid for glucose monitoring using microneedles. Diabetes Technol Ther. 2005;7(1):131–141. doi: 10.1089/dia.2005.7.131. [DOI] [PubMed] [Google Scholar]
- 9.Murthy SN, Zhao YL, Hui SW, Sen A. Electroporation and Transcutaneous extraction (ETE) for pharmacokinetic studies of drugs. J Control Release. 2005;105:132–141. doi: 10.1016/j.jconrel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 10.Miettenen M, Monkkonen J, Lahtinen MR, Nuutinen J, Lahtinen T. Measurement of edema in irritant-exposed skin by a dielectric technique. Skin Res Technol. 2006 Nov;12(4):235–40. doi: 10.1111/j.0909-752X.2006.00162.x. [DOI] [PubMed] [Google Scholar]
- 11.Micheal W, Brands Timothy E. Hopkins: Poor glycemic control induces hypertension in diabetes mellitus. Hypertension. 1996;27:735–739. doi: 10.1161/01.hyp.27.3.735. [DOI] [PubMed] [Google Scholar]
- 12.Carvalho EN, Carvalho NAS, Ferreira LM. Acta Cir Bras [serial online] Special edition Vol. 18. 2003. Experimental model of induction of diabetes mellitus in rats. [Google Scholar]
- 13.Tamada JA, Bohannon NJV, Potts RO. Measurement of glucose in diabetic subjects using noninvasive transdermal extraction. Nature Medicine. 1995;1(11):1198–1201. doi: 10.1038/nm1195-1198. [DOI] [PubMed] [Google Scholar]


