Abstract
The most conserved part of the vertebrate dopaminergic system is the orthopedia (otp)-expressing diencephalic neuronal population that constitutes the dopaminergic diencephalospinal tract (DDT). While studies in the neonatal murine spinal cord in vitro suggest an early locomotor role of the DDT, the function of the DDT in developing vertebrates in vivo remains unknown. Here, we investigated the role of the DDT in the locomotor development of zebrafish larvae. To assess the development of the behavioral and neural locomotor pattern, we employed high-throughput video-tracking in combination with peripheral nerve recordings. We found a behavioral and neural correspondence in the developmental switch from an immature to mature locomotor pattern. Blocking endogenous dopamine receptor 4 (D4R) signaling in vivo either before or after the developmental switch prevented or reversed the switch, respectively. Spinal transections of post-switch larvae reestablished the immature locomotor pattern, which was rescued to a mature-like pattern via spinal D4R agonism. Selective chemogenetic ablation of otp b (otpb) neurons that contribute to the DDT perpetuated the immature locomotor pattern in vivo. This phenotype was recapitulated by diencephalic transections that removed the dopaminergic otpb population and which were rescued to a mature-like locomotor pattern by D4R agonism. We conclude that the dopaminergic otpb population, via the DDT, is responsible for spinal D4R signaling to mediate the developmental switch to the mature locomotor pattern of zebrafish. These results, integrated with the mammalian literature, suggest that the DDT represents an evolutionarily conserved neuromodulatory system that is necessary for normal vertebrate locomotor development.
INTRODUCTION
Noteworthy similarities and differences are present among the dopaminergic (DAergic) systems across vertebrates. While midbrain DAergic neurons appear to be a more recent evolutionary development (Reiner et al., 1998; Smeets and Gonzalez, 2000), the DAergic diencephalospinal tract (DDT), which provides the exclusive source of spinal dopamine (DA) in most vertebrates, is highly conserved and may represent an ancient neuromodulatory system of functional relevance to both zebrafish and mammals (Tay et al., 2011). The diencephalic DAergic neurons that comprise the DDT have a conserved somatic location, ascending and descending projections (Takada et al., 1988; Qu et al., 2006; Kastenhuber et al., 2010) and require the transcription factor orthopedia (otp) for the development of cellular identity in both zebrafish and mammals (Ryu et al., 2007). While the function of the zebrafish DDT is unknown, the adult mammalian DDT is implicated in human restless leg syndrome (Paulus et al., 2007; Qu et al., 2007; Zhao et al., 2007) and exerts direct antinociceptive actions in the rat spinal cord in vivo (Taniguchi et al., 2011). Conversely, the function of the DDT in developing vertebrates in vivo is unknown, but in vitro and anatomical evidence suggests that the DDT may serve an early role in locomotor development (McEwen et al., 1997; Zhu et al., 2007; Tay et al., 2011).
Zebrafish exhibit a locomotor pattern of episodic beat-and-glide swimming, which consists of discrete swimming episodes interposed with passive gliding. A developmental switch in the episodic locomotor pattern from long to short swimming episodes is observed between 3 and 4 days post fertilization (dpf) (Buss and Drapeau, 2001), which is concurrent with increased locomotion and the advent of foraging (Borla et al., 2002). Interestingly, the DDT does not yet exert a locomotor effect in the spinal cord at 3 dpf (Thirumalai and Cline, 2008), even though, at this stage, the otp cells that comprise the DDT are positive for tyrosine hydroxylase (TH) and DA transporter mRNA (dat; slc6a3) and send robust projections across the entire rostral-caudal extent of the spinal cord (McLean and Fetcho, 2004b; Kastenhuber et al., 2010; Fujimoto et al., 2011) that, by 4 dpf, form putative synapses with spinal motor neurons (McLean and Fetcho, 2004a).
We postulated that the DDT becomes functional in the zebrafish spinal cord at 4 dpf to confer the mature locomotor pattern of short swimming episodes that is observed into adulthood (Fuiman and Webb, 1988). To assay the development of the episodic locomotor pattern, the present study introduces a novel adaptation of a high-throughput video-tracking algorithm (Ctrax; Branson et al., 2009) to compare the free-swimming locomotor pattern with peripheral nerve recordings of the neural locomotor output of the spinal cord (Masino and Fetcho, 2005). Through pharmacological perturbations, demarcated transections and selective chemogenetic ablation of otp neurons, we demonstrate that the conserved DAergic otp neurons that comprise the DDT provide the impetus for spinal D4R signaling to mediate the developmental switch to the mature episodic locomotor pattern. These findings suggest that the DDT may serve an evolutionarily conserved function in mediating vertebrate locomotor development.
MATERIALS AND METHODS
Animals
All experiments were performed on zebrafish (Danio rerio) larvae between 1-7 days post fertilization (dpf). Wild-type (wt) larvae were obtained from a laboratory stock (Segrest) of adults at the University of Minnesota. Embryos and larvae were raised in an incubator at 28.5°C under a 14:10 light:dark cycle until the start of behavioral or neural recordings between 3 and 7 dpf. Larvae were kept at room temperature during all manipulations and recording sessions and returned to the incubator during interims.
The Tg(otpb.A:nfsB-egfp)zc77 line was generated at the University of Utah. In the Tg(otpb.A:nfsB-egfp)zc77 line, the otpb.A promoter drives expression of the nfsB enzyme nitroreductase (Ntr). Specific plasmids used for cloning were: p5E-otpb.A (Fujimoto et al., 2011), pME-nsfB (no stop codon) (kind gift of C. Seiler), p3E-EGFPpA and pDestTol2pA2 (Kwan et al., 2007). Injection of DNA constructs and husbandry of stable transgenic lines were performed as previously described (Bonkowsky et al., 2008). All procedures were approved by the Institutional Animal Care and Use Committees (IACUC) at the University of Minnesota and the University of Utah.
Immunohistochemistry, In Situ Hybridization and TUNEL Staining
Whole-mount immunohistochemistry was performed as previously described (Bonkowsky et al., 2008; Fujimoto et al., 2011). Embryos were raised in phenylthiourea (0.003%) in E3 media to inhibit pigment formation beginning at 24 hours post-fertilization (hpf). Antibodies used were: rabbit polyclonal anti-tyrosine hydroxylase (anti-TH) 1:400 (Millipore), mouse monoclonal anti-GFP 1:250 (Millipore), Cy-3 anti-rabbit 1:400 (Invitrogen), Alexa 488 donkey anti-mouse 1:400 (Invitrogen). Whole-mount in situ labeling for dat (Holzschuh et al., 2001), was performed as previously described (Bonkowsky and Chien, 2005; Fujimoto et al., 2011).
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) was performed on whole-mount larvae (ApopTag Fluorescein In Situ Apoptosis Detection Kit; Chemicon). Following standard fixation and dehydration of larvae in 100% methanol, larvae were rehydrated stepwise into PBS with 0.1% Tween-20 (PBST), permeabilized with 10 mg/ml Proteinase K in PBST at 28°C, washed twice with PBST, re-fixed for 20 min with 4% paraformaldehyde (PFA), and washed with PBST. Subsequently, 75 μl of equilibration buffer was added to the larvae for one hour, then removed and replaced with 55 μl of “working strength” (per Apoptosis Detection kit instructions) terminal deoxynucleotidyl transferase (TdT) enzyme overnight at 37°C. To avoid drying out the larvae, eppendorf tubes were sealed with parafilm. Prior to use, the anti-digoxigenin conjugate was warmed to room temperature. The end-labeling reaction was stopped by washing the embryos three times for 15 min each with 2 mL of the stop/wash buffer, followed by three 1 min washes with PBS. Then 65 ml of working strength sheep anti-digoxigenin rhodamine was added to the embryos overnight at 4°C. Double immunohistochemistry for GFP and TUNEL was performed by TUNEL staining; following washes with PBST, larvae were fixed for 20 min in 4% PFA at RT, and washed again with PBST. Larvae were then permeabilized and antibody stained per our routine protocol (Bonkowsky et al., 2008; Fujimoto et al., 2011).
Microscopy and image analysis
Image acquisition and analysis were performed essentially as described previously (Fujimoto et al., 2011). Images of embryos/larvae processed for in situ or immunohistochemistry were taken using a confocal microscope or bright-field microscope. Embryos/larvae were taken step-wise into a solution of 80% glycerol / 20% PBST, then mounted on a glass slide with a #0 coverslip fixed into place over a well-made using electrical tape. NIH ImageJ software was used for image processing.
Metronidazole Treatments
Tg(otpb.A:nfsB-egfp)zc77 embryos were dechorionated manually at 24 hpf and placed in 5 mM metronidazole (Mtz) (Vetranal from Sigma Chemical, St. Louis, MO) dissolved in embryo media (0.03% Instant Ocean in dH2O). These larvae were then transferred to fresh 10 mM Mtz and incubated from 48 to 96 hpf, after which Mtz was removed through a series of washes in embryo media. Untreated siblings, also dechorionated at 24 hpf, remained in embryo media continuously, with fresh media being given at 24, 48 and 96 hpf. Following Mtz washout at 96 hpf in Mtz-treated larvae, untreated and Mtz-treated larvae were given a day in embryo media before behavioral and neural locomotor activity was assessed at 5 dpf. Genotypes for untreated and Mtz-treated larvae were screened as Ntr− (egfp−) or Ntr+ (egfp+) via an epifluorescent stereoscope (Leica, MZ16FA). Only larvae that exhibited an inflated swim bladder at 5 dpf were included in analyses for all experimental groups.
Pharmacology
All drug concentrations were determined in preliminary concentration-response experiments (data not shown). For each pharmacologic agent, we determined the lowest drug concentration that achieved a maximal locomotor effect rather than the IC50 or EC50 concentration that would only induce a half-maximal effect (e.g. a half-maximal effect would not demonstrate the necessity of endogenous D4R signaling as effectively as the lowest maximal concentration that completely prevented the developmental switch). As such, the lowest concentration for each drug that achieved a maximal locomotor effect was subsequently employed for the reported experiments. For each DA receptor (DAR)-specific antagonist, for example, this concentration was determined to be 10 μM, which is consistent with the range of efficacious concentrations determined during high-throughput behavioral screening of 5,280 (10-30 μM, Rihel et al., 2010) and 14,000 (10-100 μM, Kokel et al., 2010) compounds in zebrafish larvae and embryos, respectively. N-methyl-D-aspartate (NMDA), dimethyl sulfoxide (DMSO), DA, Mtz, DAR-specific antagonists (D1R: SCH-23390, D2/D3R: raclopride, D4R: L-745,870) and DAR-specific agonists (D1-like: SKF-38393, D2-like: quinpirole, D4R: PD168,077) were obtained from Sigma Chemical (St. Louis, MO).
Chronic Drug Incubations
For chronic incubations of DAR-specific antagonists to wt larvae, drugs were dissolved at stated concentrations in embryo media containing 0.1% DMSO; low concentrations of DMSO facilitate in aqueous drug delivery through the skin without affecting behavior (Rihel et al., 2010). Larvae subjected to embryo media containing 0.1% DMSO served as vehicle controls. Baseline free-swimming behavior of all groups was video recorded prior to the start of drug incubations and comprises the control data reported for 3 and 4 dpf larvae. Chronic drug incubations began at 7 PM for behavioral and neural assessments on the following day. All experimental groups remained in their respective drugs throughout the recording day, which spanned between 9 AM and 6 PM. During all behavioral recording sessions, experimental groups remained in their respective drugs. For neural recording sessions of pilot experiments, preparations were superfused in their respective drugs and it was determined that the effect of chronic drug incubations did not wash out upon removal of the drug, via superfusion in extracellular solution alone (see Electrophysiology), during the ~30 min recording session. As such, neural recording sessions occurred in the presence of extracellular solution without drug. For each experimental condition, behavioral and neural recordings were each performed on a minimum of 3 different recording days, coming from a minimum of 3 different clutches. Behavioral and neural recordings for each experimental condition were equally sampled across the recording day and were pooled irrespective of recording time.
Video Acquisition
A group of ten larvae in embryo media or embryo media containing drug were transferred to a 50 mm watch glass (Fisher Scientific) positioned atop a transmitted light stage (Schott TLS and MC-1500 LED Controller). The light intensity at the level of the arena was set to 15,000 lux in order to maximize contrast and facilitate tracking of dark targets on a light background. The aqueous depth was 10 mm at the center of the arena and became progressively shallower towards the periphery. The larvae acclimated to the recording arena for 5 minutes prior to the start of video recording. Spontaneous free-swimming was recorded for ten minutes at 60 frames per second with a digital CMOS camera (Point Grey, Firefly MV) with an attached 50 mm macro lens (Sigma Corporation, Ronkonkoma, NY). Videos were acquired, via Fview (open-source, Straw and Dickinson, 2009), in uncompressed Fly Movie Format (FMF).
Video Analysis
The FMF files generated by Fview were imported into the Cal Tech Fly Tracker (Ctrax) (open-source, Branson et al., 2009) to obtain independent trajectories of each target within the arena. We used the open-source Fix Errors Matlab Toolbox (FEMT), provided by the creators of Ctrax (Branson et al., 2009), to identify and fix tracking errors (such as swapping of target identities). The total number of errors per ten-minute video was 3.89 +/− 0.38 (n = 100 videos) and all errors were corrected via the FEMT.
Subsequently, scripts from the open-source Behavioral Microarray Matlab Toolbox (BMMT), provided by the creators of Ctrax (Branson et al., 2009), were implemented to compute descriptive statistics of a suite of behavioral parameters for each of the individual targets from the fixed Ctrax trajectories. The target speed function, velmag, was extracted for each target to define and detect event onsets and offsets by thresholding the speed function at 1.5 mm/sec. This threshold was sufficiently above most baseline noise, which was, on average, < 0.15 mm/sec. To filter out transient high amplitude noise (i.e. greater than 1.5 mm/sec and less than 3.0 mm/sec), only events with an average speed of 3 mm/sec or greater were classified as swimming episodes. Finally, 3 dpf-like swimming consisted of long episodes characterized by continuous active undulations when viewing the video frame by frame, but larvae sometimes exhibited periods of the active swimming episode with little or no displacement. Consequently, such periods during an active swimming episode are not captured in the unfiltered speed function. To better capture the entire continuous swimming episode, events that occurred within an 18 frame (300 ms) inter-event interval were concatenated into a single swimming episode. This classification system did not erroneously concatenate multiple individual swimming episodes since the shortest latencies between successive free-swimming episodes of zebrafish larvae between 3 and 9 dpf exceed 300 ms and on average exceed 800 ms (Fuiman and Webb, 1988; Müller et al., 2000; Farrell et al., 2011). Identical filters were applied for all videos across all ages and experimental groups.
Once all swimming episodes were identified, we quantified the total distance traveled per individual in cm (sum of instantaneous speeds during swimming episodes divided by the frame rate) and mean swimming episode duration per individual in ms (mean duration from the onset to the offset of each individual episode) over the ten-minute recording period. Sample sizes were ultimately smaller for quantifying episode duration, compared to total distance traveled, since episode duration could not be obtained from animals that did not move during the recording session. Values for total distance traveled and episode duration are expressed as Mean ± SD.
Electrophysiology
Neural recordings from paralyzed preparations (i.e. fictive swimming) were assessed via peripheral nerve recordings in vivo as previously described (Masino et al., 2005). Larvae were anesthetized with 0.02% Tricaine-S (Western Chemical, Ferndale, WA) in extracellular recording solution that contained (in mM) 134 NaCl, 3 KCl, 1.2 MgCl2, 2.1 CaCl2, 10 HEPES buffer, and 10 glucose, adjusted to pH 7.8 with NaOH and osmolarity of 290 mOSM with sucrose. Larvae were transferred to Sylgard®-lined dissecting dishes and pinned on their sides through the notochord using short pieces of fine tungsten wire (0.001” diameter). In order to access the peripheral motor nerves, the skin was removed between the tungsten pins using a sharp tungsten probe and fine forceps (Fine Science Tools, Foster City, CA). In order to prevent muscle contractions during recording, larvae were paralyzed using 5 μl of 0.1 mM α-bungarotoxin (Tocris, Ellisville, MO) added to the small amount (~15 μL) of extracellular solution in the dissection dish. Subsequently, larvae were transferred to the stage of an Olympus BX51 WI microscope, (Center Valley, PA) and extracellular recording solution was superfused at room temperature. Suction electrodes (6-15 μm tip diameter), filled with extracellular recording solution, were placed in an electrode holder and positioned over the peripheral nerves using micromanipulators (Siskiyou, Grants Pass, OR). All recordings were between midbody segments 10 and 20 and were acquired using a Multi-Clamp 700B amplifier (Molecular Devices), a Digidata series 1440A digitizer (Molecular Devices) and pClamp 10.2 software (Molecular Devices). Extracellular voltage was monitored in current clamp mode at a gain of 2,000 (Rf =50 MOhm) with the low- and high-frequency cut-off at 300 and 1,000 Hz, respectively.
For all preparations in which spontaneous fictive swimming was assessed, spontaneous fictive activity was recorded for a minimum of 30 minutes. For acute drug administration (~30 min) to fictive preparations, drugs were dissolved at stated concentrations in extracellular recording solution and superfused at 1 ml/min. NMDA (50 μM) was superfused for 15-20 minutes to elicit a baseline of NMDA-induced fictive swimming prior to application of DA or DAR agonists. DA or DAR agonists were superfused with NMDA for 10-15 minutes before returning to an NMDA solution (washout).
Analysis of Peripheral Nerve Activity
A program written in Matlab (Mathworks, Natick, MA) was used to analyze extracellular peripheral nerve voltage recordings. The program detected the presence or absence of activity at each voltage sample (v(n)). For each v(n), the algorithm determined a voltage autocorrelation (cn(k)) over a small window (3 ms) centered at v(n). These “windowed” autocorrelations were computed as
| (Eq. 1) |
where 3 ms windowing was implemented in Eq. 1 by setting N0 = (3 ms * fsam)/2, where fsam is the sampling frequency, and by setting v(j) = 0 for j outside the interval [n-N0, n+N0].
A subset of the autocorrelation values (lags) from Eq. 1 were used to compute a test-statistic for each v(n) with the same lags used for all voltage samples. Building on Eq. 1, for each v(n), a test-statistic cn was computed as
| (Eq. 2) |
where Eq. 2 is the sum of the cn(k) from Eq. 1 specified by . k was set at k=[1,2] to separate the distributions of the test statistics {cn} in the cases of noise versus activity.
Finally, activity was considered present at v(n) only when cn was greater than a detection threshold T. T was set as the maximum of a set of {cn} corresponding to the {v(n)} in one contiguous second of the voltage recording where activity was absent (typically the first second of the recording), and was set this way for each individual voltage recording to account for differences in gain settings and/or baseline noise levels. Fictive locomotor bursts were detected, grouped into episodes and the burst and episode properties (duration, frequency, and inter-burst or inter-episode intervals) for each voltage trace were determined as described previously (Masino and Fetcho, 2005). Values for episode duration are expressed as Mean ± SD.
Transections of Nervous System
Transections at various levels of the nervous system were made with a fine razor blade (FA-10 Feather S, Ted Pella, Redding, CA) held with a blade breaker and holder (F.S.T 10053-09). Larvae were anesthetized and prepared for electrophysiological recordings as described above. Spinalized preparations were generated in both wt and Tg(otpb.A:nfsB-egfp)zc77 larvae between 4-7 dpf by transecting the nervous system between body segments 3 and 4, just caudal to the hindbrain-spinal cord junction (between body segments 2 and 3). Spinalization completely separated the brain from the spinal cord, ensuring that all descending inputs to the transected spinal cord were eliminated.
To target mid-diencephalic and caudal-diencephalic transections, Tg(otpb.A:nfsB-egfp)zc77 larvae between 4-7 dpf were used to visualize egfp+ otpb cell populations using an epifluorescent stereoscope (Leica, MZ16FA). Following preparation for electrophysiological recordings as described above, larvae were reoriented dorsoventrally to visualize egfp+ otpb populations. Mid-diencephalic transections were made between rostral, non-DAergic and caudal, DAergic diencephalic otpb populations. Caudal-diencephalic transections were made just posterior to the caudal DAergic diencephalic otpb population. Confirmation of successful transections was visually verified by the presence of only the egfp+ DAergic otpb population in mid-diencephalic-transected preparations and the absence of all egfp+ otpb populations in caudal-diencephalic-transected preparations.
Statistical Analyses
Statistical analyses were performed with SigmaPlot 11.0 (Systat). Data were analyzed using Student t-tests (2 conditions only), one-way ANOVAs (more than 2 conditions) or two-way ANOVAs (1st factor: condition (either age or drug-exposure) assayed by 2 different locomotor outputs (2nd factor: free-swimming or fictive swimming)). These statistical tests accounted for repeated measures when appropriate, namely when comparisons incorporated intra-animal baseline and post-drug data sets. For ANOVAs, post-hoc (Holm-Sidak) pairwise multiple comparisons were performed to identify which groups were significantly different from one another. Significance was established using an α criterion of p = 0.05. In the figures, *,** and *** denote p < 0.05, < 0.01 and < 0.001, respectively. In the text, reported sample sizes denote the number of fish for each experimental group, which is also indicated by the numbers at the base of the bars in bar graphs within the figures.
RESULTS
Behavioral and fictive correspondence in developmental switch of locomotor pattern
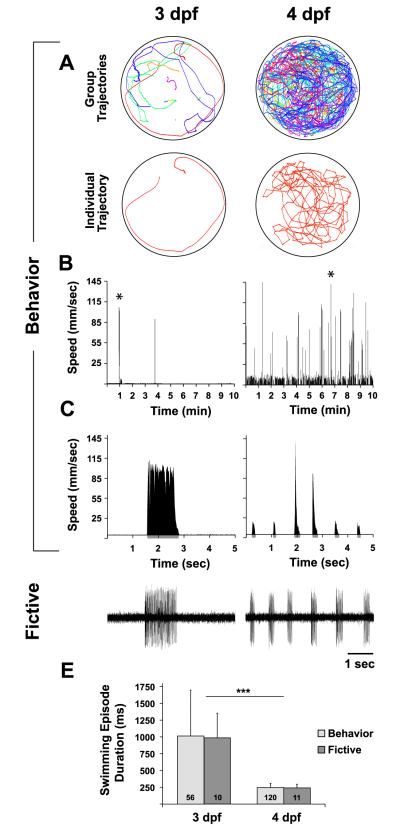
The developmental switch in the locomotor pattern from long to short swimming episodes has been demonstrated via peripheral nerve recordings in paralyzed larvae (Thirumalai and Cline, 2008), which is termed a fictive preparation. However, this switch has been characterized (Buss and Drapeau, 2001) but not quantified in free-swimming larvae. To this aim, we adapted open-source software, Ctrax (Branson et al., 2009), to individually track multiple zebrafish larvae in a group setting (Fig 1A). This high-throughput tracking algorithm allows for the assessment of a suite of behavioral classifications that include an array of locomotor parameters (Branson et al., 2009). We focused on two behavioral locomotor parameters for each individual larva: 1) total distance traveled (cm per 10 min), obtained from the sum of instantaneous speeds during swimming episodes divided by the frame rate (Fig 1B), to assess the amount of free-swimming activity (Fig 1B) and 2) mean swimming episode duration (milliseconds (ms)), obtained as the mean duration from the onset to offset of each swimming episode (Fig 1C, expansion of 5 sec from * in 1B) across all identified swimming episodes (see methods) in the speed function (Fig 1B), to assess the locomotor pattern during free-swimming activity (Fig 1C). The goal of these behavioral assessments was to compare the developmental switch in the free-swimming episodic locomotor pattern (Buss & Drapeau, 2001) to that of neural activity during fictive swimming.
Figure 1. Behavioral and fictive correspondence in developmental switch of locomotor pattern.
A-E, Comparison of behavioral (A-C) and fictive (D) swimming patterns between 3 dpf (left column) and 4 dpf (right column). A, Free-swimming trajectory plots. Top row, group trajectories from a group of 10 larvae in the same 50 mm arena. Bottom row, unique trajectory of an individual larva from the group trajectory above. B, Speed function for an individual larva over the course of the 10 min record, from which total distance traveled and swimming episode durations were calculated. C, Expansion of 5 sec from * in B to show the locomotor pattern of discrete swimming episodes. Grey regions below x-axis represent episode durations detected from filter settings (see methods). D, Voltage traces of fictive swimming from extracellular peripheral nerve recordings. E, Mean behavioral (light grey bars) and fictive (dark grey bars) swimming episode durations (ms) at 3 and 4 dpf. Numbers at the base of bars in bar graphs denote the sample sizes of fish for each experimental group. Asterisks in E indicate significance (*** = p < 0.001).
Free-swimming larvae at 3 dpf traveled significantly less distance (4.7 ± 11 cm, n = 210) than did 4 dpf larvae (38 ± 42 cm, n = 170; t (378) = −11.2, p < 0.001). In terms of the locomotor pattern, we found a correspondence between behavioral (Beh) (Fig 1C) and fictive (Fic) (Fig 1D) episode durations in the developmental switch from long to short swimming episodes between 3 dpf (Beh: 1,014 ± 684 ms, n = 56; Fic: 987 ± 364 ms, n = 10) and 4 dpf (Beh: 248 ± 59 ms, n = 120; Fic: 241 ± 57 ms, n = 11). A two-way ANOVA revealed a significant effect of age (F(1, 193) = 74, p < 0.001) but not preparation type (Beh vs. Fic, F(1, 193) = 0.03, p = 0.85) (Fig 1E). This behavioral and fictive correspondence suggests that the difference in the episodic locomotor pattern between 3 and 4 dpf is due to an intrinsic development of the nervous system rather than a byproduct of extrinsic behavioral factors, which are not present in the fictive preparation.
The developmental switch from long to short swimming episodes did not begin over the time course of our recording sessions (9 AM – 6 PM) at 3 dpf, as the immature behavioral phenotype of long episode durations remained unchanged (t (21) = 0.78, p = 0.45) from the morning (9-11 AM: 1,228 ± 893 ms, n = 11) to early evening (4-6 PM: 1,024 ± 315 ms, n = 12). Strikingly, the switch occurred by the morning at 4 dpf, as the short episode durations at this time were not significantly different from those at the end of the recording day at 4 dpf (9-11 AM: 270 ± 59 ms, n = 24; 4-6 PM: 255 ± 55 ms, n = 30; t (52) = 0.97, p = 0.34). These data demonstrate that the developmental switch in the episodic locomotor pattern is rapid, occurring at some time window between the late evening at 3 dpf and the early morning at 4 dpf.
Blocking endogenous D4 receptor signaling prevents maturation of the locomotor pattern
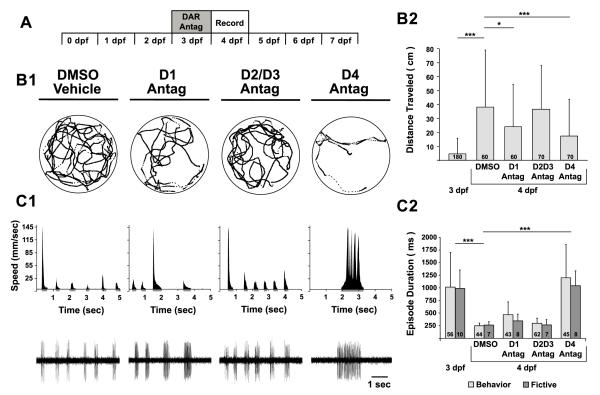
To initially investigate whether the DDT is involved in conferring the mature episodic locomotor pattern, we tested the hypothesis that endogenous DAergic signaling is required to initiate the developmental switch to short swimming episodes. We systemically incubated larvae in specific DAR antagonists (D1R: SCH-23390; D2/D3R: raclopride; D4R: L-745,870) prior to and during the natural timing of the switch (3-4 dpf) and assessed resultant behavioral and fictive locomotor patterns at 4 dpf (Fig 2A). All DAR antagonists were administered at 10 μM, informed by initial concentration-response experiments (data not shown), in the presence of 0.1% DMSO to facilitate drug delivery (Rihel et al., 2010).
Figure 2. Blocking endogenous D4 receptor signaling prevents maturation of the locomotor pattern.
A, Timeline of experimental paradigm, where larvae are incubated in DAR antagonists at 3 dpf and behavioral and fictive locomotor patterns are assessed at 4 dpf. B1, Representative trajectory plot of an individual larva (from a group of 10 larvae in the same arena) over 10 min, depicting total activity per individual for each experimental group (DMSO, D1 antagonist, D2/D3 antagonist, D4 antagonist). Labels for experimental groups also correspond to data in C1. Circles surrounding trajectories denote the periphery of the 50 mm diameter arena. B2, Total distance traveled per individual (cm). C1, Behavioral (top row) and fictive (bottom row) locomotor pattern of discrete swimming episodes. Top row, speed function from individual in B1. Grey regions below x-axis represent resultant episode durations detected from filter settings (see methods). Bottom row, voltage traces of fictive swimming from extracellular peripheral nerve recordings. C2, Mean behavioral (lights grey bars) and fictive (dark grey bars) swimming episode durations (ms) at 3 dpf and after pharmacological treatments at 4 dpf. Numbers at the base of bars in bar graphs denote the sample sizes of fish for each experimental group. Asterisks indicate significance (* = p < 0.05; *** = p < 0.001).
The DAR antagonists differentially affected the total distance traveled by 4 dpf larvae (one-way ANOVA; F(3, 256) = 6.85, p < 0.001) (Fig 2B1, B2). Compared to DMSO vehicles (38 ± 41 cm, n = 60), there was no change in the total distance traveled by blocking D2/D3Rs (37 ± 31 cm, n = 70; p = 0.79) (Fig 2B1, B2). Conversely, blocking either D1Rs or D4Rs reduced the total distanced traveled (D1R: 24 ± 30 cm, n = 60; p < 0.05; D4R: 17 ± 26 cm, n = 70; p < 0.001) (Fig 2B1, B2); note that both groups traveled significantly more than untreated 3 dpf larvae (D1R: p < 0.001; D4R: p < 0.001). This demonstrates that endogenous D1R and D4R signaling contribute to increased locomotion at 4 dpf, but that other contributions, such as from serotoninergic signaling (Brustein et al., 2003), likely contribute to the differences in the amount of free-swimming of 3 and 4 dpf larvae.
A two-way ANOVA revealed a significant effect on episode durations of 4 dpf larvae for DAR antagonist incubations (F(3, 216) = 40, p < 0.001) but not preparation type (Beh vs. Fic, F(3, 216) = 1.30; p = 0.26) (Fig. 2C1, C2). The episode durations produced by larvae incubated in D1R antagonists (Beh: 470 ± 249 ms, n = 43; Fic: 348 ± 134 ms, n = 8) or D2/3R antagonists (Beh: 298 ± 104 ms, n = 62; Fic: 268 ± 108 ms, n = 7) were not significantly different from DMSO vehicles (Beh: 253 ± 52 ms, n = 44; Fic: 264 ± 67 ms, n = 7; p = 0.11 and p = 0.79, respectively) (Fig. 2C1, C2). In contrast, larvae incubated in D4R antagonists produced significantly longer episode durations (Beh: 1,199 ± 657 ms, n = 52; Fic: 1,043 ± 294 ms, n = 8) than DMSO vehicles (p < 0.001) (Fig. 2C1, C2). Moreover, the D4R antagonist completely prevented the maturation of the locomotor pattern, as the induced long episode durations were indistinguishable (p = 0.47) from that of 3 dpf larvae (Fig 2C2). These results suggest that endogenous D4R signaling mediates the advent of short swimming episodes.
Blocking endogenous D4 receptor signaling reverts the mature locomotor pattern to an immature pattern
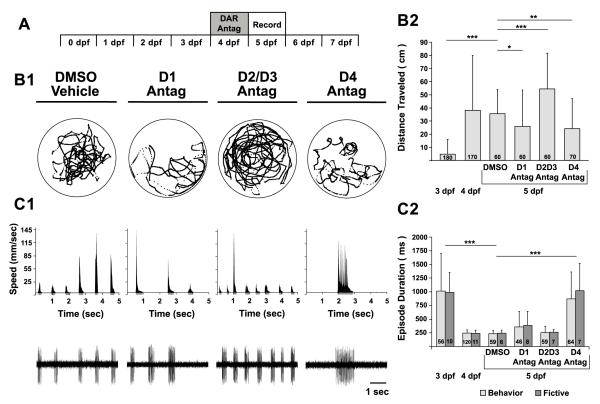
We next examined whether endogenous D4R signaling is required, not only to initiate, but also to maintain the mature locomotor pattern. To test this hypothesis, we systemically incubated larvae in specific DAR antagonists subsequent to the developmental switch (4-5 dpf) (Fig 3A), such that larvae were already exhibiting increased locomotion in the form of short and directed swimming episodes. Resultant behavioral and fictive locomotor patterns were assessed at 5 dpf (Fig 3A).
Figure 3. Blocking endogenous D4 receptor signaling reverts the mature locomotor pattern to an immature pattern.
A, Timeline of experimental paradigm, where larvae are incubated in DAR antagonists at 4 dpf and resultant behavioral and fictive locomotor patterns are assessed at 5 dpf. B1, Representative trajectory plot of an individual larva (from a group of 10 larvae in the same arena) over 10 min, depicting total activity per individual for each experimental group (DMSO, D1 antagonist, D2/D3 antagonist, D4 antagonist). Labels for experimental groups also correspond to data in C1. Circles surrounding trajectories denote the periphery of the 50 mm diameter arena. B2, Total distance traveled per individual (cm). C1, Behavioral (top row) and fictive (bottom row) locomotor pattern of discrete swimming episodes. Top row, speed function from individual in B1. Grey regions below x-axis represent resultant episode durations detected from filter settings (see methods). Bottom row, voltage traces of fictive swimming from extracellular peripheral nerve recordings. C2, Mean behavioral (lights grey bars) and fictive (dark grey bars) swimming episode durations (ms) at 3 dpf, 4 dpf and after pharmacological treatments at 5 dpf. Numbers at the base of bars in bar graphs denote the sample sizes of fish for each experimental group. Asterisks indicate significance (* = p < 0.05; ** = p < 0.01 *** = p < 0.001).
The DAR antagonists differentially affected the total distance traveled by 5 dpf larvae (one-way ANOVA, F(3, 246) = 20; p < 0.001) (Fig 3B1, B2). Compared to DMSO vehicles (36 ± 18 cm, n = 60), blocking D2/D3Rs after the switch increased the total distanced traveled (54 ± 27 cm, n = 60; p < 0.001) (Fig 3B1, B2), which was not observed when administered before the switch (Fig 2B1, B2). Conversely, blocking either D1Rs or D4Rs reduced the total distanced traveled (D1R: 26 ± 27 cm, n = 60; p < 0.05; D4: 24 ± 23 cm, n = 70; p < 0.01) (Fig 3B1, B2), similar to when the antagonists were given before the switch (Fig 2B1, B2). These data suggest that, at 5 dpf, endogenous D1R and D4R signaling contribute to increased locomotion while endogenous D2/D3R signaling suppresses the initiation of locomotion.
A two-way ANOVA revealed an effect on episode durations of 5 dpf larvae for DAR antagonist incubations (F(3, 246) = 31, p < 0.001) but not preparation type (Beh vs. Fic, F(3, 246) = 0.65, p = 0.42) (Fig. 3C1, C2). The episode durations produced by larvae incubated in D1R antagonists (Beh: 357 ± 286 ms, n = 46; Fic: 383 ± 256 ms, n = 7) or D2/3R antagonists (Beh: 253 ± 114 ms, n = 59; Fic: 261 ± 57 ms, n = 7) were not significantly different from DMSO vehicles (Beh: 234 ± 60 ms, n = 59; Fic: 245 ± 53 ms, n = 6; p = 0.14 and p = 0.84, respectively) (Fig. 3C1, C2). Conversely, larvae incubated in D4R antagonists produced significantly longer episode durations (Beh: 871 ± 492 ms, n = 64; Fic: 1,022 ± 493 ms, n = 7) than DMSO vehicles (p < 0.001) (Fig. 3C1, C2). Moreover, the D4R antagonist completely reversed the developmental switch in the locomotor pattern, relegating 5 dpf larvae to long episode durations that were not significantly different from that of 3 dpf larvae (p = 0.72) (Fig 2C2). These results demonstrate that D4R signaling is necessary to maintain the mature locomotor pattern of short swimming episodes at 5 dpf.
D4 receptor signaling in the spinal cord is sufficient to induce the mature locomotor pattern of short swimming episodes
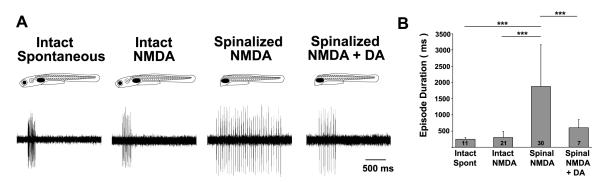
Having elucidated that systemic block of D4R signaling can both prevent (Fig 2) and reverse (Fig 3) the developmental switch in the locomotor pattern, we next investigated whether the anatomical site of action is in the spinal cord. To test this, we compared the fictive locomotor pattern of intact animals at 4-7 dpf compared to those that were spinalized (i.e. spinal-transection), in which the spinal cord was isolated from the brain to remove all descending inputs, including the DDT. Since fictive swimming in spinalized preparations does not occur spontaneously but can be elicited with NMDA (McDearmid and Drapeau, 2006), we added NMDA (50 μM) to induce fictive locomotor output and subsequently tested the effects of exogenous application of DAergic agonists. To more directly compare intact and spinalized fictive swimming, we assessed NMDA-induced, in addition to spontaneous, fictive swimming in intact animals.
There was a significant difference in episode durations among the experimental groups (F( 3, 65) = 23, p < 0.001). In intact preparations, episode durations were not significantly different (p = 0.61) between spontaneous (241 ± 57 ms, n = 11) and NMDA-induced fictive swimming (300 ± 186 ms, n = 21) (Fig. 4A, B). Conversely, significantly longer episode durations were produced by spinalized preparations (1,871 ± 1,285 ms, n = 30; p < 0.001) (Fig. 4A, B), reminiscent of the immature locomotor pattern of intact 3 dpf larvae (Fig. 1D, E). This demonstrates that some descending input from the brain is required to confer short NMDA-induced swimming episodes. Next, we tested whether supplying exogenous DA to the putatively DA-deprived transected spinal cord could sculpt NMDA-induced locomotor activity to better resemble that of the intact animal. Application of exogenous DA (1 μM) to spinalized preparations significantly shortened NMDA-induced episode durations (601 ± 251 ms, n = 7) when compared to NMDA application alone (p < 0.001) (Fig 4A, B). This result demonstrates that DA can act directly in the spinal cord to shorten fictive swimming episodes.
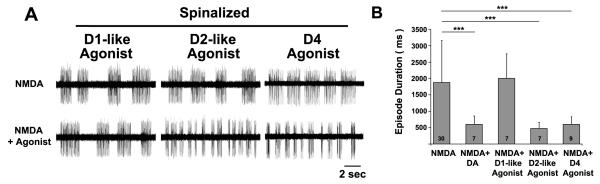
Figure 4. Dopaminergic signaling in the spinal cord is sufficient to induce the mature locomotor pattern of short swimming episodes.
A, Voltage traces of fictive swimming from extracellular peripheral nerve recordings from intact and spinalized preparations. B, Mean fictive swimming episode durations (ms). Numbers at the base of bars in bar graphs denote the sample sizes of fish for each experimental group. Asterisks indicate significance (*** = p < 0.001).
To determine whether DA acts through excitatory (D1-like: D1R, D5R) or inhibitory (D2-like: D2R, D3R, D4R) DARs in the spinal cord to shorten NMDA-induced fictive episode durations, DAR-specific agonists (D1-like: SKF-38393, D2-like: quinpirole or D4R: PD168077) were applied to spinalized preparations of 4-7 dpf wt larvae. There was a significant difference among the pharmacological treatments (F(4, 55) = 8.4, p < 0.001). Compared to NMDA alone, the D1-like agonist (1 μM) had no effect on episode duration (1,999 ± 750 ms, n = 7; p = 0.34) while the D2-like agonist (1 μM) significantly shortened episode durations (475 ± 183 ms, n = 7; p < 0.001) (Fig. 5A, B), similar to exogenous DA (Fig 5B). Moreover, application of a specific D4R agonist was sufficient to significantly shorten episode durations (598 ± 234 ms, n = 9; p < 0.001) (Fig. 5A, B), similar to that of exogenous DA and the broad-spectrum D2-like agonist (Fig 5B). This result demonstrates that D4R signaling in the spinal cord is sufficient to shorten fictive episodes.
Figure 5. D4 receptor signaling in the spinal cord is sufficient to induce the mature locomotor pattern of short swimming episodes.
A, Voltage traces of fictive swimming from extracellular peripheral nerve recordings from spinalized preparations in NMDA alone (top traces) and NMDA + agonist (bottom traces). B, Mean fictive swimming episode durations (ms). Numbers at the base of bars in bar graphs denote the sample sizes of fish for each experimental group. Asterisks indicate significance (*** = p < 0.001).
Selective chemogenetic ablation of otpb neurons
The evidence that DAergic signaling in the spinal cord is sufficient to shorten fictive swimming episodes (Fig 4, 5) suggests a critical role for otp neurons in sculpting the mature episodic locomotor pattern, since otp neurons provide the exclusive source of spinal DA (Kastenhuber et al., 2010; Tay et al., 2011). To explore this possibility, we used a transgenic line that drives expression in DAergic otpb neurons in the caudal ventral diencephalon, which co-expresses TH and dat, and forms part of the DDT (Fujimoto et al., 2011). In addition, this transgenic line drives expression in a non-DAergic neurosecretory otpb population in the rostral diencephalon (Fujimoto et al., 2011). To test the hypothesis that otpb cells contribute to conferring the mature episodic locomotor pattern, we performed selective, cell-autonomous chemogenetic ablation of otpb cells in Tg(otpb.A:nfsB-egfp)zc77 larvae via the nitroreductase-metronidazole (Ntr:Mtz) system (Curado et al., 2008; Pisharath and Parsons, 2009).
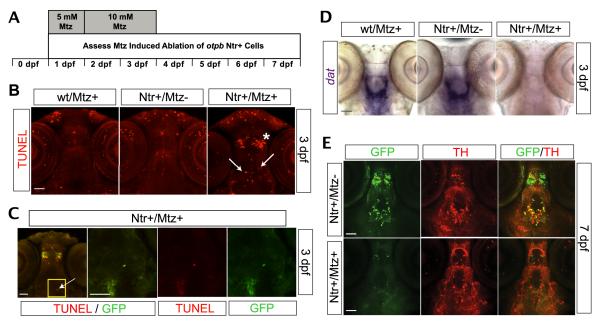
Application of Mtz to the aqueous environment from 1-4 dpf (Fig 6A) induced selective cell death of otpb neurons in Tg(otpb.A:nfsB-egfp)zc77 larvae (Fig. 6B, C). A marked increase of TUNEL staining in Mtz treated Ntr+ larvae (Ntr+/Mtz+) was observed when compared to untreated Ntr+ siblings (Ntr+/Mtz-) or Mtz treated wt larvae (wt/Mtz+) (Fig 6B). Moreover, the increased TUNEL staining in Ntr+/Mtz+ larvae colocalized to both rostral non-DAergic and caudal DAergic diencephalic GFP+ populations (Fig 6C). By 3 dpf, wt/Mtz+ and Ntr+/Mtz− larvae showed robust dat mRNA expression in the ventral diencephalon (Fig 6D), which colocalizes with the DAergic otpb population (Fujimoto et al., 2011). Conversely, there was a marked absence of dat mRNA expression in the ventral diencephalon of Ntr+/Mtz+ larvae (Fig 6D), coincident spatially with the Ntr+/Mtz+ induced TUNEL staining (Fig 6B, arrows in right panel). This indicates an Ntr+/Mtz+ mediated loss of putatively functional DAergic otpb cells in the ventral diencephalon. Additionally, by 7dpf there was an absence of GFP+ cells in Ntr+/Mtz+ larvae when compared to Ntr+/Mtz− siblings (Fig 6E, left panels), suggesting an Mtz mediated loss of Ntr+ cells. Many of the GFP+ cells in the ventral diencephalon of Ntr+/Mtz− siblings colocalize with TH+ cells in the ventral diencephalon (Fig 6E, top-right panel), which suggests that Mtz treatment induced a loss of double-labeled TH+ and Ntr+ (GFP+) otpb DAergic cells (Fig 6E). To determine the efficacy of Ntr+/Mtz+ mediated ablation of DAergic otpb cells, we counted the number of cells in the diencephalon that expressed both TH and GFP, in Ntr+/Mtz− compared to Ntr+/Mtz+ larvae. At 7dpf, Ntr+/Mtz− larvae had 26 ± 3 TH+/GFP+ DAergic otpb cells, which was significantly reduced to 3 ± 0.9 TH+/GFP+ DAergic otpb cells in Ntr+/Mtz+ larvae (p < 0.0001; n = 11 larvae per group; Mean ± SEM). Taken together, these results demonstrate that the Mtz regimen was effective in selectively ablating otpb Ntr+ cells in Tg(otpb.A:nfsB-egfp)zc77 larvae.
Figure 6. Selective chemogenetic ablation of otpb.
neurons. A, Timeline of experimental paradigm, where larvae were incubated in 5 mM Mtz from 24-48 hpf and 10 mM Mtz from 48-96 hpf; assessment of cell death was investigated from 1-7 dpf. B-E, Ventral views in whole-mount Tg(otpb.A:nfsB-egfp)zc77 larvae, rostral to the top, scale bars 50 μm. B, TUNEL signal in confocal z-stacks following Mtz treatment; asterisk denotes region of rostral non-DAergic otpb+ population and arrows denote caudal DAergic otpb+ population. C, Confocal images showing co-localization of TUNEL signal and GFP in larvae treated with Mtz. First panel shows z-stack image; yellow box indicates ventral diencephalon shown in inset in subsequent panels as single confocal slices; arrow denotes a GFP+ and TUNEL stained cell. D, Brightfield images of loss of slc6a3 (dat) mRNA expression via ISH following Mtz treatment in whole-mount embryos. E, Confocal z-stack images of double-labeling for GFP and TH immunohistochemistry at 7dpf.
Loss of otpb neurons prevents maturation of the locomotor pattern
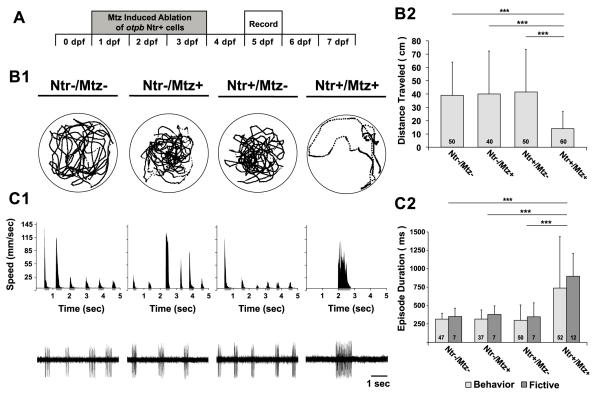
To test whether otpb neurons contribute to the developmental switch to the mature episodic locomotor pattern, we assessed the effect of ablating otpb neurons on the behavioral and fictive locomotor patterns at 5 dpf (Fig 7A). There was a significant difference in the total distance traveled among the experimental groups (F(3,206) = 15, p < 0.001). There were no significant differences among the total distance traveled by Ntr−/Mtz− (39 ± 32 cm, n = 50), Ntr-/Mtz+ (40 ± 32 cm, n = 40) and Ntr+/Mtz− (42 ± 25 cm; n = 50) larvae (p > 0.76 for all pairwise comparisons) (Fig 7B1, B2). Conversely, selective chemogenetic ablation of otpb cells in Ntr+/Mtz+ larvae significantly reduced the total distance traveled (14 ± 13 cm, n = 60) compared to all other groups (p < 0.001) (Fig 7B1, B2). In terms of the locomotor pattern, a two-way ANOVA revealed an effect on episode duration for treatment condition (F(3, 211) = 15, p < 0.001) but not preparation type (Beh vs. Fic, F(3, 211) = 1.49, p = 0.22). The stage-appropriate expression of short episodes was observed in both behavioral and fictive preparations from Ntr−/Mtz− (Beh: 315 ± 82 ms, n = 47; Fic: 351 ± 112 ms, n = 7), Ntr-/Mtz+ (Beh: 317 ± 124 ms, n = 37; Fic: 379 ± 116 ms, n = 7) and Ntr+/Mtz− (Beh: 298 ± 212 ms, n = 50; Fic: 349 ± 186 ms, n = 7) larvae (p > 0.75 for all pairwise comparisons) (Fig 7C1, C2). Conversely, a significant increase in the durations of behavioral and fictive episodes was observed in Ntr+/Mtz+ larvae (Beh: 738 ± 697 ms, n = 56; Fic: 897 ± 311 ms, n = 12) compared to all other groups (p < 0.001) (Fig 7C1, C2). These results indicate that otpb cells contribute to the mature locomotor pattern of short episodes since their specific ablation perpetuates long episodes in 5 dpf larvae.
Figure 7. Loss of otpb neurons prevents maturation of the locomotor pattern.
A, Timeline of experimental paradigm, where otpb+ cells are chemogenetically ablated via Mtz treatment from 1-4 dpf in Tg(otpb.A:nfsB-egfp)zc77 larvae and behavioral and fictive locomotor patterns are assessed at 5 dpf. B1, Representative trajectory plot of an individual larva (from a group of 10 larvae in the same arena) over 10 min, depicting total activity per individual for each experimental group (Ntr-/Mtz-, Ntr-/Mtz+, Ntr+/Mtz-, Ntr+/Mtz+). Labels for experimental groups also correspond to data in C1. Circles surrounding trajectories denote the periphery of the 50 mm diameter arena. B2, Total distance traveled per individual (cm). C1, Behavioral (top row) and fictive (bottom row) locomotor pattern of discrete swimming episodes. Top row, speed function from individual in B1. Grey regions below x-axis represent resultant episode durations detected from filter settings (see methods). Bottom row, Voltage traces of fictive swimming from extracellular peripheral nerve recordings. C2, Mean behavioral (lights grey bars) and fictive (dark grey bars) swimming episode durations (ms) at 5 dpf. Numbers at the base of bars in bar graphs denote the sample sizes of fish for each experimental group. Asterisks indicate significance (*** = p < 0.001).
Removal of the caudal diencephalon reverts the mature locomotor pattern to an immature pattern that is rescued by D4 receptor agonism
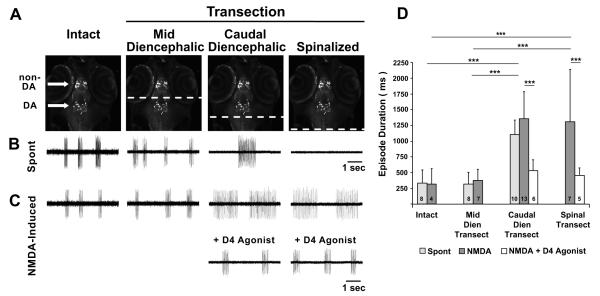
Specific chemogenetic ablation of otpb cells included the rostral non-DAergic and caudal DAergic otpb populations. To determine the relative contributions of DAergic and non-DAergic otpb+ cells to the generation of short episodes, Tg(otpb.A:nfsB-egfp)zc77 larvae at 4-7 dpf were used to produce reduced preparations in which the nervous system was transected either just rostral to or just caudal to the DAergic otpb population. Mid-diencephalic-transected (MDT; Fig. 8A) preparations lack non-DAergic otpb cells and the ascending projections from DAergic otpb cells, but retain DAergic otpb somata and their descending projections (i.e. including the DDT). Caudal-diencephalic-transected (CDT; Fig. 8A) preparations lack both DAergic and non-DAergic otpb neurons, but retain midbrain and hindbrain structures. The consequences of these transections were examined by measuring episode durations in fictive preparations.
Figure 8. Removal of the caudal diencephalon reverts the mature locomotor pattern to an immature pattern that is rescued by D4 receptor agonism.
A, Schematic of demarcated transections, denoted by broken lines in white, with respect to the rostral non-DAergic and caudal DAergic otpb+ populations in Tg(otpb.A:nfsB-egfp)zc77 larvae. Repeated image is from a confocal z-stack projection with a ventral view in whole-mount embryos, rostral to the top. B-C, Voltage traces from extracellular peripheral nerve recordings showing fictive swimming that is spontaneous in B and NMDA-induced in C. D, Mean fictive swimming episode durations (ms) for spontaneous and NMDA-induced activity in intact, mid-diencephalic, caudal-diencephalic and spinalized-transected preparations. Numbers at the base of bars in bar graphs denote the sample sizes of fish for each experimental group. Asterisks indicate significance (*** = p < 0.001).
Intact, MDT and CDT preparations exhibited spontaneous fictive swimming while spinalized preparations did not (Fig 8B). Spontaneous episode durations were significantly different among the intact, MDT and CDT groups (F(2, 23) = 41, p < 0.001). MDT preparations, which retain the DAergic otpb population and the DDT, produced short spontaneous episode durations (320 ± 187 ms, n = 8) that were not significantly different from intact larvae (333 ± 211 ms, n = 8; p = 0.91) (Fig 8B, D). CDT preparations, which lack DAergic otpb neurons, produced significantly longer spontaneous episode durations (1,105 ± 231 ms, n = 10; p < 0.001) (Fig 8B, D) that were not significantly different (p = 0.40) from the episodes observed in 3 dpf wt larvae (Fig. 1D). These results demonstrate that neither the non-DAergic otpb population nor the ascending projections of the DAergic otpb population are necessary for the generation of short episodes. Only the caudal diencephalic region, where DAergic otpb neurons reside, is necessary for the production of short episodes.
Next, we tested the hypothesis that D4R agonism would shorten the long episodes of CDT preparations to stage-appropriate short episodes and that this would be recapitulated at the level of the spinal cord via spinalized preparations. Since spinalized preparations do not exhibit spontaneous fictive swimming (Fig. 8B), we compared NMDA-induced locomotor activity from intact, MDT, CDT and spinalized preparations in Tg(otpb.A:nfsB-egfp)zc77 larvae (Fig. 8C). There was a significant difference in the NMDA-induced episode durations among these preparations (F(5, 36) = 9.18, p < 0.001). The short NMDA-induced episode durations of intact (317 ± 250 ms, n = 4) and MDT (379 ± 172 ms, n = 7) preparations were not significantly different from one another (p = 0.82) or compared to spontaneous episode durations of intact and MDT preparations (p= 0.91 and p= 0.53, respectively) (Fig 8C, D). Conversely, the duration of the NMDA-induced episodes generated by CDT (1,355 ± 432 ms, n = 12) and spinalized (1,310 ± 830 ms, n = 7) preparations were significantly longer (p < 0.001 and p < 0.001, respectively) when compared to intact or MDT preparations (Fig. 8C,D). However, the long NMDA-induced episodes of CDT and spinalized preparations were not significantly different from one another (p = 0.85) or compared to spontaneous episodes of CDT preparations (p = 0.11 and p = 0.47, respectively) (Fig 8D). Subsequent to NMDA-induced locomotor activation, the D4R agonist was exogenously applied and episode durations were measured in CDT and spinalized preparations (Fig. 8C, D). A change in the locomotor pattern from long to short episode durations was observed in both preparations (CDT: 533 ± 172 ms, n = 6; p < 0.001; spinalized: 458 ± 117 ms, n = 5; p < 0.001). This result demonstrates that, in the absence of the caudal diencephalon, D4R agonism is sufficient to produce short episodes and suggests that the DAergic otpb population is the critical caudal diencephalic component for conferring the mature episodic locomotor pattern.
DISCUSSION
We explored the function of the evolutionarily conserved vertebrate DDT and found that it mediates the progression of locomotor development in zebrafish larvae. The developmental switch to the mature episodic locomotor pattern requires spinal D4R signaling that is driven by DAergic otpb cells comprising the DDT. Loss of dopaminergic input prior to the developmental switch led to a maintained immature locomotor pattern. Further, continued dopaminergic input is necessary, as loss of D4R signaling after the developmental switch led to a reversion to the immature pattern. Our findings demonstrate a tonic requirement for central sources of dopaminergic signaling that act in the spinal cord, with implications for understanding the normal development of locomotion in vertebrates, or effects from disease states.
Behavioral and fictive correspondence in the locomotor pattern
While a myriad of behavioral studies in zebrafish larvae have assayed the amount of free-swimming activity under an array of paradigms (reviewed by Tierney, 2011), only a few have assessed the actual locomotor pattern of swimming episodes during free-swimming (Fuiman and Webb, 1988; Müller et al., 2000; Buss and Drapeau, 2001). Of these, only Buss and Drapeau (2001) quantitatively compared free-swimming and fictive locomotor patterns and did so just at 4 dpf, reporting a free-swimming and fictive correspondence that our study corroborates. Expanding upon this, we found a strong correspondence between free-swimming and fictive locomotor patterns in the developmental switch from long to short swimming episodes between 3 and 4 dpf (Fig 1). Furthermore, there was correspondence between the altered free-swimming and fictive locomotor patterns resulting from blocking specific DARs (Fig 2, 3) or from chemogenetic ablation of otpb cells (Fig 7). Interestingly, behavioral and morphological factors in the free-swimming assay that could plausibly influence the locomotor pattern are not present in the immobilized fictive preparation. These factors include: 1) group interactions, since our assay tracked multiple larvae in the same arena, and 2) the absence or presence of an inflated swim bladder, which dramatically affect buoyancy and resultant free-swimming trajectories (Lindsey et al., 2010). Hence, the strong correspondence between the free-swimming and fictive locomotor patterns under all of our experimental conditions demonstrates that behavioral and morphological factors are not responsible for the developmental switch from the immature to mature episodic locomotor pattern, suggesting that the mechanisms underlying this switch are intrinsic to the nervous system.
Role for spinal D4R signaling in sculpting mature locomotor pattern
We found that specifically D4R signaling in the spinal cord is required for the advent and maintenance of the mature episodic locomotor pattern. Conversely, we demonstrate that all DAR subtypes play a role in influencing the amount of free-swimming activity. The specific DAR antagonists that we administered elicited unique locomotor phenotypes (Fig 2, 3), indicating that each drug concentration was sufficient to differentially modulate behavior. The D4R antagonist group was hypoactive, which is consistent with other studies (Giacomini et al., 2006; Boehmler et al., 2007), and exhibited the immature pattern of long swimming episodes. The D1R antagonist group was also hypoactive while the D2/D3R antagonist group was hyperactive, which is consistent with evidence for such opposing roles of these receptors (Souza et al., 2011), yet both of these groups exhibited the mature pattern of short swimming episodes. Therefore, we demonstrate that the amount of swimming activity is separable from the locomotor pattern during swimming activity, suggesting that non-overlapping neural mechanisms exist for these two phenomena. This interpretation is supported by evidence that, at 3 dpf, DAergic signaling in the brain influences the amount of swimming activity but not the locomotor pattern (Thirumalai and Cline, 2008), which also suggests that our results of systemic DAR-mediated changes in the amount of swimming activity may be due to blocking DARs in the brain.
We elucidated that the site of action of systemic D4R-mediated changes in the locomotor pattern is likely in the spinal cord, since spinalization of 4-7 dpf larvae reverted the fictive locomotor pattern to immature-like long swimming episodes (Fig 4) that were transformed to mature-like short episodes via spinal D4R agonism (Fig 5). The ability of D4R antagonism to both prevent and reverse the developmental switch in the locomotor pattern suggests that the D4R-dependent expression of short swimming episodes is not due to D4R signaling simply providing a transient role in transforming neural circuit function. Rather, it is indicates a requirement for ongoing D4R-mediated maintenance of the swimming pattern, possibly in the form of providing neuromodulatory tone (Hauber, 2010) to spinal locomotor circuits.
Our functional evidence for spinal D4R signaling is supported by previous in situ hybridization studies of DAR mRNA expression patterns and suggests a distinctive functional role for a specific D4R subtype. Among D2-like DARs, three D2Rs (drd2a,b,c), one D3R (drd3) and three D4Rs (drd4a,b,c) have been identified in zebrafish larvae. Interestingly, all of these DAR mRNAs are expressed in the zebrafish spinal cord by 2 dpf (Boehmler et al., 2004; Boehmler et al., 2007) except drd4b, which is present at 5 dpf (Boehmler et al., 2007). Since these studies did not examine expression patterns at 3 and 4 dpf, it is possible that the advent of spinal drd4b expression correlates with, and may underlie, the developmental switch in the locomotor pattern between 3 and 4 dpf.
Role for DAergic otpb population, via the DDT, in driving spinal D4R-mediated sculpting of mature locomotor pattern
Among the diencephalic DAergic populations in zebrafish (DC1-DC6), the otp populations comprise four of the six (DC2, DC4, DC5 and DC6) (Kastenhuber et al., 2010). Of these otpb populations, three of four (DC2, DC4 and DC5) exclusively comprise the DDT (Tay et al., 2011), and we hypothesized that some or all of these populations were involved in the spinal DAergic-mediated shortening of swimming episodes since the DDT provides the exclusive source of DA to the spinal cord (Kastenhuber et al., 2010). Since the Tg(otpb.A:nfsB-egfp)zc77 line that we employed (Fig 6-8) drives expression in the majority of DC4, DC5 and DC6 populations (Fujimoto et al., 2011), we were able to selectively chemogenetically ablate two out of the three (DC4, DC5), otp neuronal populations that comprise the DDT. Since this chemogenetic ablation was sufficient to perpetuate the immature locomotor pattern, it suggests that DC4 and/or DC5 DAergic otpb populations are necessary for the developmental switch to short swimming episodes.
Conversely, we demonstrated that the non-DAergic otpb population is not necessary to confer the mature episodic locomotor pattern, which persisted following removal of these cells by way of mid-diencephalic transections (Fig 8). Importantly, this transection also removed the majority of DAergic non-otpb populations, including a robust local subpallial DAergic system (Tay et al., 2011), as well as ascending subpallial projections of the DAergic otpb population (Tay et al., 2011), suggesting that these components are also not necessary for the expression of the mature episodic locomotor pattern. Removal of the DAergic otpb population by transecting at the caudal diencephalon reestablished the immature locomotor pattern (Fig 8), demonstrating that the caudal diencephalon, which distinguishes MDT from CDT preparations, plays a role in conferring the expression of the mature locomotor pattern. Since the hindbrain remains intact in CDT preparations, and these larvae are unable to maintain short swimming episodes, it suggests that serotonergic and noradrenergic projections into the spinal cord, which exclusively originate in the hindbrain (McLean and Fetcho, 2004b; Kastenhuber et al., 2010), are not sufficient for or do not contribute to the mature pattern of short swimming episodes. This interpretation is consistent with a previous study that showed that serotonin reduces the quiescent periods between swimming episodes but does not influence the active properties of swimming episodes themselves, including episode duration (Brustein et al., 2003). Although the literature is lacking on any role for noradrenaline on the pattern of swimming episodes, the number of noradrenergic axonal projections to the spinal cord of zebrafish larvae is markedly small compared to that of DAergic axonal projections via the DDT, as demonstrated by a largely unaffected descending catecholaminergic tract following genetic knockdown of the noradrenaline system (Kastenhuber et al., 2010).
Although many cell populations and fibers of passage are contained within the caudal diencephalon, the physical removal of this region resulted in a similar locomotor phenotype as that induced from selective chemogenetic ablation of otpb cells, suggesting that DAergic otpb cells are the critical caudal diencephalic component. Consistent with this conclusion, in the absence of the caudal diencephalon, the reestablished immature locomotor pattern was rescued to a mature-like pattern via D4R agonism (Fig 8). Moreover, the equitable efficacy of D4R agonism to rescue the locomotor pattern in CDT and spinalized preparations (Fig 8) indicates the sufficiency of spinal D4R signaling to confer the mature episodic locomotor pattern. This provides further credence to the conclusion that caudal diencephalic DAergic otpb cells comprising the DDT act on D4Rs in the spinal cord, rather than the brain, to confer the mature episodic locomotor pattern.
Conserved functional role of the vertebrate DDT
This study is the first to elucidate the role of the DDT in a developing vertebrate in vivo. The conserved transcriptional identity (Ryu et al., 2007), somatic location, morphology, and projection pattern (Takada et al., 1988; Tay et al., 2011) of the DAergic otp population and DDT suggest that it has a conserved locomotor function across vertebrates. In support of this rationale in developing mammals, all DAR mRNAs are expressed across the dorsal-ventral axis of the neonatal mouse spinal cord and specifically in motor neurons (Zhu et al., 2007), which are modulated by DA during fictive locomotion in vitro as early as post-natal day 2 (PN2) (Han et al., 2007). Moreover, before neonatal rodents can support their body weight to start walking, they are capable of coordinated locomotion in the form of L-dopa-induced air-stepping that is dependent on DAR signaling (Sickles et al., 1992; McCrea et al., 1997). Spinalized in vitro preparations have demonstrated that this DAR signaling acts at the level of the spinal cord (McEwen et al., 1997). This early DAR-dependent signaling in the spinal cord may play a developmental role, since L-dopa-induced air-stepping develops from being bipedal, at PN0, to quadrupedal, by PN5 (Van Hartesveldt et al., 1991). In disease states, for example, hypoxic-ischemic injury to the developing brain, dopaminergic cell populations are at risk and may contribute to the impaired motor development following these injuries (Bax et al., 2006). Hence, the evolutionarily conserved anatomical, genetic, and in vitro evidence suggests that, similar to our findings in zebrafish, the mammalian DDT may also contribute to locomotor development.
Acknowledgements
We thank Drs. Teresa Nick and Martha Flanders for helpful comments on this manuscript and John Eian for writing Matlab code for analysis. This work was supported by National Institutes of Health TRINOD Training Grant T32-DA022616 (A.M.L.), National Institutes of Health K08 DA024753 (J.L.B.), PCMC Foundation Grant (J.L.B.), National Institutes of Health Grant R01-NS65054 (M.A.M.), Minnesota Medical Foundation Grant 4052-9238-11 (M.A.M.) and Office of the Dean of the Graduate School of the University of Minnesota (Grant-in-Aid of Research, Artistry and Scholarship 21934; M.A.M.).
Footnotes
Conflict of Interest: None
REFERENCES
- Bax JJ, Poldermans D, Schuijf JD, Scholte AJ, Elhendy A, van der Wall EE. Imaging to differentiate between ischemic and nonischemic cardiomyopathy. Heart Fail Clin. 2006;2:205–214. doi: 10.1016/j.hfc.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Boehmler W, Obrecht-Pflumio S, Canfield V, Thisse C, Thisse B, Levenson R. Evolution and expression of D2 and D3 dopamine receptor genes in zebrafish. Dev Dyn. 2004;230:481–493. doi: 10.1002/dvdy.20075. [DOI] [PubMed] [Google Scholar]
- Boehmler W, Carr T, Thisse C, Thisse B, Canfield VA, Levenson R. D4 Dopamine receptor genes of zebrafish and effects of the antipsychotic clozapine on larval swimming behaviour. Genes Brain Behav. 2007;6:155–166. doi: 10.1111/j.1601-183X.2006.00243.x. [DOI] [PubMed] [Google Scholar]
- Bonkowsky JL, Chien CB. Molecular cloning and developmental expression of foxP2 in zebrafish. Dev Dyn. 2005;234:740–746. doi: 10.1002/dvdy.20504. [DOI] [PubMed] [Google Scholar]
- Bonkowsky JL, Wang X, Fujimoto E, Lee JE, Chien CB, Dorsky RI. Domain-specific regulation of foxP2 CNS expression by lef1. BMC Dev Biol. 2008;8:103. doi: 10.1186/1471-213X-8-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borla MA, Palecek B, Budick S, rsquo, Malley DM. Prey Capture by Larval Zebrafish: Evidence for Fine Axial Motor Control. Brain, Behavior and Evolution. 2002;60:207–229. doi: 10.1159/000066699. [DOI] [PubMed] [Google Scholar]
- Branson K, Robie AA, Bender J, Perona P, Dickinson MH. High-throughput ethomics in large groups of Drosophila. Nat Methods. 2009;6:451–457. doi: 10.1038/nmeth.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustein E, Chong M, Holmqvist B, Drapeau P. Serotonin patterns locomotor network activity in the developing zebrafish by modulating quiescent periods. J Neurobiol. 2003;57:303–322. doi: 10.1002/neu.10292. [DOI] [PubMed] [Google Scholar]
- Buss RR, Drapeau P. Synaptic drive to motoneurons during fictive swimming in the developing zebrafish. J Neurophysiol. 2001;86:197–210. doi: 10.1152/jn.2001.86.1.197. [DOI] [PubMed] [Google Scholar]
- Curado S, Stainier DY, Anderson RM. Nitroreductase-mediated cell/tissue ablation in zebrafish: a spatially and temporally controlled ablation method with applications in developmental and regeneration studies. Nat Protoc. 2008;3:948–954. doi: 10.1038/nprot.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell TC, Cario CL, Milanese C, Vogt A, Jeong JH, Burton EA. Evaluation of spontaneous propulsive movement as a screening tool to detect rescue of Parkinsonism phenotypes in zebrafish models. Neurobiol Dis. 2011;44:9–18. doi: 10.1016/j.nbd.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuiman LA, Webb PW. Ontogeny of Routine Swimming Activity and Performance in Zebra Danios (Teleostei, Cyprinidae) Anim Behav. 1988;36:250–261. [Google Scholar]
- Fujimoto E, Stevenson TJ, Chien CB, Bonkowsky JL. Identification of a dopaminergic enhancer indicates complexity in vertebrate dopamine neuron phenotype specification. Dev Biol. 2011;352:393–404. doi: 10.1016/j.ydbio.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomini NJ, Rose B, Kobayashi K, Guo S. Antipsychotics produce locomotor impairment in larval zebrafish. Neurotoxicol Teratol. 2006;28:245–250. doi: 10.1016/j.ntt.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Han P, Nakanishi ST, Tran MA, Whelan PJ. Dopaminergic modulation of spinal neuronal excitability. J Neurosci. 2007;27:13192–13204. doi: 10.1523/JNEUROSCI.1279-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber W. Dopamine release in the prefrontal cortex and striatum: temporal and behavioural aspects. Pharmacopsychiatry. 2010;43(Suppl 1):S32–41. doi: 10.1055/s-0030-1248300. [DOI] [PubMed] [Google Scholar]
- Holzschuh J, Ryu S, Aberger F, Driever W. Dopamine transporter expression distinguishes dopaminergic neurons from other catecholaminergic neurons in the developing zebrafish embryo. Mech Dev. 2001;101:237–243. doi: 10.1016/s0925-4773(01)00287-8. [DOI] [PubMed] [Google Scholar]
- Kastenhuber E, Kratochwil CF, Ryu S, Schweitzer J, Driever W. Genetic dissection of dopaminergic and noradrenergic contributions to catecholaminergic tracts in early larval zebrafish. J Comp Neurol. 2010;518:439–458. doi: 10.1002/cne.22214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokel D, Bryan J, Laggner C, White R, Cheung CY, Mateus R, Healey D, Kim S, Werdich AA, Haggarty SJ, Macrae CA, Shoichet B, Peterson RT. Rapid behavior-based identification of neuroactive small molecules in the zebrafish. Nat Chem Biol. 2010;6:231–237. doi: 10.1038/nchembio.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, Parant JM, Yost HJ, Kanki JP, Chien CB. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn. 2007;236:3088–3099. doi: 10.1002/dvdy.21343. [DOI] [PubMed] [Google Scholar]
- Lindsey BW, Smith FM, Croll RP. From inflation to flotation: contribution of the swimbladder to whole-body density and swimming depth during development of the zebrafish (Danio rerio) Zebrafish. 2010;7:85–96. doi: 10.1089/zeb.2009.0616. [DOI] [PubMed] [Google Scholar]
- Masino MA, Fetcho JR. Fictive swimming motor patterns in wild type and mutant larval zebrafish. J Neurophysiol. 2005;93:3177–3188. doi: 10.1152/jn.01248.2004. [DOI] [PubMed] [Google Scholar]
- McCrea AE, Stehouwer DJ, Van Hartesveldt C. Dopamine D1 and D2 antagonists block L-DOPA-induced air-stepping in decerebrate neonatal rats. Brain Res Dev Brain Res. 1997;100:130–132. doi: 10.1016/s0165-3806(97)00027-8. [DOI] [PubMed] [Google Scholar]
- McDearmid JR, Drapeau P. Rhythmic motor activity evoked by NMDA in the spinal zebrafish larva. J Neurophysiol. 2006;95:401–417. doi: 10.1152/jn.00844.2005. [DOI] [PubMed] [Google Scholar]
- McEwen ML, Van Hartesveldt C, Stehouwer DJ. L-DOPA and quipazine elicit air-stepping in neonatal rats with spinal cord transections. Behav Neurosci. 1997;111:825–833. doi: 10.1037//0735-7044.111.4.825. [DOI] [PubMed] [Google Scholar]
- McLean DL, Fetcho JR. Relationship of tyrosine hydroxylase and serotonin immunoreactivity to sensorimotor circuitry in larval zebrafish. J Comp Neurol. 2004a;480:57–71. doi: 10.1002/cne.20281. [DOI] [PubMed] [Google Scholar]
- McLean DL, Fetcho JR. Ontogeny and innervation patterns of dopaminergic, noradrenergic, and serotonergic neurons in larval zebrafish. J Comp Neurol. 2004b;480:38–56. doi: 10.1002/cne.20280. [DOI] [PubMed] [Google Scholar]
- Müller UK, Stamhuis EJ, Videler JJ. Hydrodynamics of unsteady fish swimming and the effects of body size: comparing the flow fields of fish larvae and adults. J Exp Biol. 2000;203:193–206. doi: 10.1242/jeb.203.2.193. [DOI] [PubMed] [Google Scholar]
- Paulus W, Dowling P, Rijsman R, Stiasny-Kolster K, Trenkwalder C, de Weerd A. Pathophysiological concepts of restless legs syndrome. Mov Disord. 2007;22:1451–1456. doi: 10.1002/mds.21533. [DOI] [PubMed] [Google Scholar]
- Pisharath H, Parsons MJ. Nitroreductase-mediated cell ablation in transgenic zebrafish embryos. Methods Mol Biol. 2009;546:133–143. doi: 10.1007/978-1-60327-977-2_9. [DOI] [PubMed] [Google Scholar]
- Qu S, Ondo WG, Zhang X, Xie WJ, Pan TH, Le WD. Projections of diencephalic dopamine neurons into the spinal cord in mice. Exp Brain Res. 2006;168:152–156. doi: 10.1007/s00221-005-0075-1. [DOI] [PubMed] [Google Scholar]
- Qu S, Le W, Zhang X, Xie W, Zhang A, Ondo WG. Locomotion is increased in a11-lesioned mice with iron deprivation: a possible animal model for restless legs syndrome. J Neuropathol Exp Neurol. 2007;66:383–388. doi: 10.1097/nen.0b013e3180517b5f. [DOI] [PubMed] [Google Scholar]
- Reiner A, Medina L, Veenman CL. Structural and functional evolution of the basal ganglia in vertebrates. Brain Res Brain Res Rev. 1998;28:235–285. doi: 10.1016/s0165-0173(98)00016-2. [DOI] [PubMed] [Google Scholar]
- Rihel J, Prober DA, Arvanites A, Lam K, Zimmerman S, Jang S, Haggarty SJ, Kokel D, Rubin LL, Peterson RT, Schier AF. Zebrafish behavioral profiling links drugs to biological targets and rest/wake regulation. Science. 2010;327:348–351. doi: 10.1126/science.1183090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu S, Mahler J, Acampora D, Holzschuh J, Erhardt S, Omodei D, Simeone A, Driever W. Orthopedia homeodomain protein is essential for diencephalic dopaminergic neuron development. Curr Biol. 2007;17:873–880. doi: 10.1016/j.cub.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Sickles AE, Stehouwer DJ, Van Hartesveldt C. Dopamine D1 and D2 antagonists block L-dopa-elicited air-stepping in neonatal rats. Brain Res Dev Brain Res. 1992;68:17–22. doi: 10.1016/0165-3806(92)90243-p. [DOI] [PubMed] [Google Scholar]
- Smeets WJ, Gonzalez A. Catecholamine systems in the brain of vertebrates: new perspectives through a comparative approach. Brain Res Brain Res Rev. 2000;33:308–379. doi: 10.1016/s0165-0173(00)00034-5. [DOI] [PubMed] [Google Scholar]
- Souza BR, Romano-Silva MA, Tropepe V. Dopamine D2 receptor activity modulates Akt signaling and alters GABAergic neuron development and motor behavior in zebrafish larvae. J Neurosci. 2011;31:5512–5525. doi: 10.1523/JNEUROSCI.5548-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straw AD, Dickinson MH. Motmot, an open-source toolkit for realtime video acquisition and analysis. Source Code Biol Med. 2009;4:5. doi: 10.1186/1751-0473-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada M, Li ZK, Hattori T. Single thalamic dopaminergic neurons project to both the neocortex and spinal cord. Brain Res. 1988;455:346–352. doi: 10.1016/0006-8993(88)90093-5. [DOI] [PubMed] [Google Scholar]
- Taniguchi W, Nakatsuka T, Miyazaki N, Yamada H, Takeda D, Fujita T, Kumamoto E, Yoshida M. In vivo patch-clamp analysis of dopaminergic antinociceptive actions on substantia gelatinosa neurons in the spinal cord. Pain. 2011;152:95–105. doi: 10.1016/j.pain.2010.09.034. [DOI] [PubMed] [Google Scholar]
- Tay TL, Ronneberger O, Ryu S, Nitschke R, Driever W. Comprehensive catecholaminergic projectome analysis reveals single-neuron integration of zebrafish ascending and descending dopaminergic systems. Nat Commun. 2011;2:171. doi: 10.1038/ncomms1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirumalai V, Cline HT. Endogenous dopamine suppresses initiation of swimming in prefeeding zebrafish larvae. J Neurophysiol. 2008;100:1635–1648. doi: 10.1152/jn.90568.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney KB. Behavioural assessments of neurotoxic effects and neurodegeneration in zebrafish. Biochim Biophys Acta. 2011;1812:381–389. doi: 10.1016/j.bbadis.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Van Hartesveldt C, Sickles AE, Porter JD, Stehouwer DJ. L-dopa-induced air-stepping in developing rats. Brain Res Dev Brain Res. 1991;58:251–255. doi: 10.1016/0165-3806(91)90012-8. [DOI] [PubMed] [Google Scholar]
- Zhao H, Zhu W, Pan T, Xie W, Zhang A, Ondo WG, Le W. Spinal cord dopamine receptor expression and function in mice with 6-OHDA lesion of the A11 nucleus and dietary iron deprivation. J Neurosci Res. 2007;85:1065–1076. doi: 10.1002/jnr.21207. [DOI] [PubMed] [Google Scholar]
- Zhu H, Clemens S, Sawchuk M, Hochman S. Expression and distribution of all dopamine receptor subtypes (D(1)-D(5)) in the mouse lumbar spinal cord: a real-time polymerase chain reaction and non-autoradiographic in situ hybridization study. Neuroscience. 2007;149:885–897. doi: 10.1016/j.neuroscience.2007.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]