Abstract
Rett syndrome is the leading genetic cause of mental retardation in females. Most cases of Rett are due to loss of function mutations in the gene coding for the transcriptional regulator methyl-CpG binding protein 2 (MeCP2), but despite much effort it remains unclear how a loss of MeCP2 function generates the neurological deficits of Rett. Here we show that MeCP2 plays an essential and cell-autonomous role in homeostatic synaptic scaling up in response to reduced firing or reduced sensory drive in rat visual cortical pyramidal neurons. We found that acute RNAi knockdown of MeCP2 blocked synaptic scaling within targeted neocortical pyramidal neurons. Further, MeCP2 knockdown decreased excitatory synapse number without affecting basal mEPSC amplitude or AMPAR accumulation at spared synapses, demonstrating that MeCP2 acts cell-autonomously to maintain both excitatory synapse number and synaptic scaling in individual neocortical neurons. Finally, we used a mouse model of Rett to show that MeCP2 loss prevents homeostatic synaptic scaling up in response to visual deprivation in vivo, demonstrating for the first time that MeCP2 loss disrupts homeostatic plasticity within the intact developing neocortex. Our results establish MeCP2 as a critical mediator of synaptic scaling, and raise the possibility that some of the neurological defects of Rett arise from a disruption of homeostatic plasticity.
Introduction
The neurodevelopmental disorder Rett Syndrome (Rett) is the leading genetic cause of mental retardation in females. Most cases of Rett are caused by loss of function mutations in the X-linked gene coding for methyl-CpG binding protein 2 (MeCP2) and are neurologically characterized by epileptic seizures and mental retardation. Mutations in MeCP2 have also been identified in other neurodevelopmental disorders including autism and Angelman syndrome (Chahrour and Zoghbi, 2007; Guy et al., 2011; Banerjee et al., 2012). The severe neurological symptoms of Rett suggest that transcriptional regulation by MeCP2 is required for proper neuronal function, but despite much effort it remains unclear how a loss of MeCP2 generates the neurological deficits of Rett. Here we show that MeCP2 plays an essential role in homeostatic synaptic scaling up in neocortical neurons in response to reduced firing or reduced sensory drive.
Homeostatic plasticity mechanisms are believed to be necessary for proper development and function of neuronal networks (Turrigiano and Nelson, 2004). The best-characterized form of homeostatic plasticity is synaptic scaling expressed at glutamatergic synapses (Turrigiano, 2008; Pozo and Goda, 2010). Synaptic scaling bidirectionally regulates synaptic strengths in response to chronic changes in network activity, in the correct direction to stabilize firing rates. While these homeostatic changes are transcription-dependent, the relevant transcriptional regulators and their targets are not known (Turrigiano, 2011). Previously we found an imbalance of synaptic excitation and inhibition within neocortical circuits in a mouse model of Rett, without significant defects in Hebbian plasticity (Dani et al., 2005; Dani and Nelson, 2009). This excitation/inhibition balance is thought to be maintained by homeostatic mechanisms, raising the interesting possibility that homeostatic plasticity might be disturbed in Rett. Consistent with this a recent study found that MeCP2 is required for synaptic scaling down induced by elevated network activity in dissociated hippocampal cultures (Qiu et al., 2012). The molecular pathways mediating scaling up and down are in many cases distinct (Rutherford et al., 1998; Shepherd et al., 2006; Peng et al., 2009; Anggono et al., 2011; Sun and Turrigiano, 2011), so here we investigated whether MeCP2 is also necessary for synaptic scaling up, both in vitro and in the intact neocortex.
Here we show that sparse and acute knockdown of MeCP2 blocks synaptic scaling up of miniature excitatory postsynaptic currents (mEPSCs), and the associated increase in synaptic AMPAR accumulation, within targeted neurons. Further, MeCP2 knockdown decreased excitatory synapse number without affecting basal mEPSC amplitude or AMPAR accumulation at spared synapses. These data demonstrate that MeCP2 acts in a cell-autonomous way to maintain both excitatory synapse number and synaptic scaling in individual neocortical neurons. Finally, we used a mouse model of Rett to show that MeCP2 loss prevents homeostatic synaptic scaling up in response to visual deprivation in vivo, demonstrating for the first time that MeCP2 loss disrupts homeostatic plasticity within the intact developing neocortex. Our results establish MeCP2 as a critical mediator of synaptic scaling, and raise the possibility that some of the neurological defects of Rett arise from a disruption of homeostatic plasticity.
Materials and Methods
All experimental procedures were approved by the Animal Care and Use Committee of Brandeis University and followed the guidelines of the National Institutes of Health.
Neuronal Cultures and transfection
Neuronal dissociated cultures were prepared from primary visual cortex of postnatal day 0–2 Long-Evans rats of either sex, and plated on a bed of confluent astrocytes, as previously described (Pratt et al., 2003). Cultures were used for imaging or electrophysiology after 7–9 days in vitro (DIV). Electrophysiology and immunocytochemistry were performed on morphologically identified pyramidal neurons, as previously described (Pratt et al., 2003). All experimental conditions were compared with age-matched sister cultures. All transfections were done using Lipofectamine 2000 (Invitrogen) according to manufacturer instructions; cultures were transfected 3d before the experimental procedure. Two short hairpin RNAs (shRNAs) directed against two different sequences of MeCP2 were used. The targeted sequences were: hp1 5′CTAAAGTAG-3′ described (Jin et al., 2008) and hp2 5′GTCAGAAGACCAGGATCTC-3′ described (Zhou et al., 2006; Wood et al., 2009). Empty vectors (ev) were identical to the hp vectors except for absence of the shRNA insert. Chronic activity blockade was accomplished by adding 20μM DNQX or 5μM TTX as indicated in the text to the culture dishes 1d before the experiment (and 2d after transfection).
Immunostaining
To quantify nuclear MeCP2 levels immunostaining was performed using the following antibodies: NeuN (1:500, MAB377, Millipore), MeCP2 (1:800, 07–013, Millipore). To visualize surface AMPAR immunostaining was performed under non-permeant conditions using antibodies against GluR1 (1:10, PC246, Calbiochem) or GluR2 (1: 200, MAB397, Millipore) as previously described (Wierenga et al., 2005); neurons were counterstained with the presynaptic marker VGLUT-1 (1:200, 135 304, Synaptic Systems). Alexa Fluor 568, 647, Texas Red and Cascade Blue (1:500, Invitrogen) were used as secondary antibodies. All images were acquired on an Olympus IX-81 microscope using a 60X oil-immersion objective and an Orca ER camera (Hamamatsu). Images were obtained with either Openlab or Volocity (Improvision) and analyzed with Metamorph software (Molecular Devices) as described (Sun and Turrigiano, 2011).
Culture electrophysiology
Whole-cell AMPA mediated miniature excitatory post-synaptic currents (mEPSC) were recorded and analyzed as previously described (Turrigiano et al., 1998; Wierenga et al., 2005). MEPSCs were recorded from pyramidal neurons voltage clamped at −70mV using an Axopatch 200B amplifier (Molecular Devices) and perfused at 25 °C in the following artificial CSF (ACSF; in mM): 126 NaCl, 5.5 KCl, 2 MgSO4, 1 NaH2PO4, 25 NaHCO3, 2 CaCl2, 14 dextrose, 0.1μM TTX, 50μM APV and 25μM picrotoxin. Internal recording solution contained the following (in mM): 120 KMeSO4, 10 KCl, 2 MgSO4, 10 K-HEPES, 0.5 EGTA, 3 K2ATP, 0.3 Na-GTP, 10 Na2 phosphocreatine. Recordings with Vm > −50mV, Rs > 20MΩ, Rin <125 MΩ and < 25 mEPSCs were excluded. In-house Igor Pro (Wave Metrics) software was used to detect and measure mEPSCs.
Visual deprivation and slice electrophysiology
Mecp2 Knockout (KO) mice (Mecp2tm1.1Jae) were bred as described previously (Chen et al., 2001; Dani et al., 2005). Male wildtype (WT) and Mecp2 KO mice were raised in a normal 12:12 light/dark cycle. Dark exposure (DE) was started on postnatal day (P) P20–21 for a period of 4d. At P24–25 DE or control (Ctl) animals were sacrificed and coronal brain slices containing primary visual cortex were obtained as described previously (Dani et al., 2005). Whole cell recordings were obtained from layer 2/3 pyramidal neurons of monocular visual cortex using a Multiclamp 700B amplifier (Molecular Devices), and AMPA mediated mEPSCs were recorded as described (Desai et al., 2002; Maffei and Turrigiano, 2008). Pyramidal neurons were identified using DIC/IR optics and morphology verified by post hoc reconstitution of biocytin fills as described (Desai et al., 2002). Internal recording solution contained the following (in mM):100 K-gluconate, 20 KCl, 10 K-HEPES, 0.3 Na-GTP, 4 Mg-ATP, 10 Na-phosphocreatine and 0.1% biocytin. Neurons were voltage clamped to −70mV in ACSF containing the following (in mM): 126 NaCl, 3 KCl, 2 MgSO4, 1 NaH2PO4, 26 NaHCO3, 2 CaCl2 and 20 dextrose and 1μM TTX, 50μM APV and 20μM picrotoxin. Recordings were excluded if the Vm >−60mV, Rs > 25MΩ and Rin < 50 MΩ or if any of these properties changed by more than 10% during the course of the recording.
Scaled cumulative distributions
Cumulative mEPSC amplitude distributions were constructed by including the first 25 (culture) and 100 (slice) minis recorded from each neuron for each condition. Scaling was analyzed as described (Turrigiano et al., 1998); briefly, the rank-ordered control and experimental distributions were interpolated and plotted against each other, the best linear fit to the data obtained, and the experimental distribution transformed by this equation. A Kolmogorov-Smirnov (KS) test was then used to determine if the control and linearly scaled experimental distributions were different from each other.
Statistics
All data are expressed as the mean ± SEM for the number of neurons indicated, n= number of neurons unless otherwise noted in the text. Statistical comparisons were made using unpaired student’s t test; for multiple comparisons single factor ANOVAs followed by corrected t tests were performed. For comparisons of cumulative distributions a KS test was used. A p value of <0.05 was considered statistically significant.
Results
It remains unclear to what extent the synaptic defects that contribute to Rett are due to cell-autonomous loss within particular cell types, or global and prolonged loss throughout development. To determine whether MeCP2 is necessary for cell-autonomous synaptic scaling up in neocortical pyramidal neurons, we began by taking an RNAi approach that allowed us to acutely and sparsely decrease MeCP2 levels within individual pyramidal neurons in vitro, and measure the impact on basal synaptic properties and synaptic scaling.
MeCP2 knockdown does not affect basal quantal amplitude
To investigate whether MeCP2 plays a cell-autonomous role in synaptic scaling up, cultured neocortical neurons were transiently transfected with an shRNA construct targeted against MeCP2 (Jin et al., 2008); vectors also expressed soluble EGFP to allow visualization of transfected neurons. This allowed us to reduce MeCP2 protein levels in a small number of neurons (5 – 15 per culture dish) in an otherwise unaffected network. To characterize the efficiency of knockdown (KD), cultures transfected with either the hairpin (hp1) or the empty vector (ev) were immunolabeled with antibodies against MeCP2 and the pan neuronal marker NeuN (Fig. 1A). Quantification of the fluorescent intensity of the MeCP2 signal in the nucleus showed that expression of hp1 for three days reduced endogenous MeCP2 protein levels to ~40 % of untransfected control neurons. Expression of the empty vector had no significant effect on MeCP2 levels (Fig. 1B). Here and below all experiments were performed on neurons with a pyramidal morphology (Fig. 1A) as described previously (Rutherford et al., 1998; Watt et al., 2000; Pratt et al., 2003).
Figure 1.
MeCP2 KD does not affect basal quantal amplitude. A, Pyramidal neurons transfected for 3d with empty vector (ev) or MeCP2 shRNA1 (hp1) and untransfected control neurons (unt), fixed and stained with antibodies against endogenous MeCP2 (red) and NeuN (orange). Ev and hp1 constructs also express soluble EGFP (green). White triangle shows the position of the same neuron’s cell body in each panel. Bottom panel, yellow triangle indicates a nearby untransfected neuron. Scale bar, 20μm. B, Quantification of the nuclear MeCP2 fluorescent intensity for unt, ev and hp1 expressing neurons (n=164, 48, 39). C, Left: Example raw traces of mEPSCs recorded from ev and hp1 expressing neurons. Right: Average mEPSC waveforms for the same conditions. D, Cumulative histograms of mEPSC amplitudes for ev and hp1 expressing neurons. Inset: Average mEPSC amplitude for ev (n=22) and hp1 (n=20) expressing neurons. E, Peak scaled average mEPSCs waveform for the same conditions. **Different from control, p<0.001.
Previous studies have shown that MeCP2 KO reduces excitatory synaptic transmission in L5 neocortical pyramidal neurons, both through a small reduction in mEPSC amplitude and through a reduction in connectivity (Dani et al., 2005; Dani and Nelson, 2009). To determine whether an acute and cell autonomous loss of MeCP2 is able to reduce baseline mEPSC amplitude we recorded AMPA-mediated mEPSC from neurons expressing either hp1 or the ev for 3 days. MeCP2 knockdown had no significant effect on mEPSC amplitude (Fig. 1C–D). MEPSC frequency was variable, and although there was a trend toward reduction, this was not significant (ev, 0.63 ± 0.10 Hz; hp1 0.58 ± 0.08 Hz; p=0.67). In addition there were no significant changes in passive neuronal properties, and mEPSC kinetics were also unaffected, as can be seen from a comparison of the peak-scaled average mEPSC waveforms (Fig. 1E). These data indicate that an acute and cell autonomous reduction of MeCP2, unlike a chronic and global loss, does not reduce baseline mEPSC amplitude.
MeCP2 knockdown reduces excitatory synapse number without affecting receptor accumulation at spared synapses
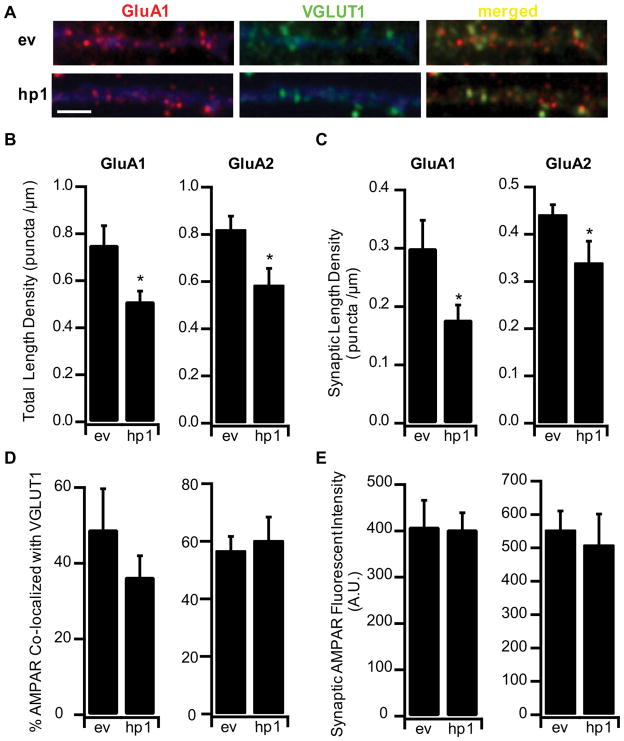
Previous studies have shown that MeCP2 KO reduces the number of glutamatergic synapses (Nelson et al., 2006; Chao et al., 2007; Tropea et al., 2009). However, it was not clear if this reduction in synapse number was a direct result of postsynaptic MeCP2 loss, pre and postsynaptic loss together (Chao et al., 2007), or changes in network function due to global MeCP2 KO (Tropea et al., 2009). To investigate this we examined the effects of acute and sparse MeCP2 KD on excitatory synapse number. Three days after transfection cultures were surface labeled with an antibody against the GluA1 subunit of the AMPA receptor, and VGLUT1 (to label presynaptic excitatory terminals), as described (Wierenga et al., 2005); sites where postsynaptic GluA1 puncta were apposed to or co-localized with VGLUT1 puncta were considered putative synapses (Fig. 2A). MeCP2 KD significantly reduced both total GluA1 puncta, and the number of co-localized GluA1/VGLUT1 puncta, by ~ 30% (Fig. 2B, C), without significantly affecting the co-localization rate between GluA1 and VGLUT1 (Fig. 2D). In keeping with the observation that MeCP2 KD did not affect mEPSC amplitude, there was no change in the fluorescent intensity of the GluA1 signal at synaptic sites, indicating that GluA1 accumulation at spared synapses is unaffected by MeCP2 loss (Fig. 2E). A similar result was obtained when GluA2 was used as the postsynaptic marker (Fig. 2B–E). Interestingly, although MeCP2 has been implicated in the transcriptional regulation of GluA2 (Qiu et al., 2012), MeCP2 KD had no impact on baseline synaptic GluA2 levels. Taken together, these data indicate that MeCP2 plays a critical and cell-autonomous role in the maintenance of excitatory synapse density, but not baseline quantal amplitude at spared synapses.
Figure 2.
MeCP2 KD reduces excitatory synapse number. A, Example dendrites from ev or hp1 expressing neurons, stained against endogenous surface GluA1 (red) and the excitatory synapse marker VGLUT-1 (green); EGFP shown in pseudo color blue. Scale bar, 2μm B. Length density for total GluA1 and GluA2 puncta in ev and hp1 expressing cells, ev (n=5) and hp1 (n=7) for GluA1; ev (n=12) and hp1 (n=6) for GluA2. C, Synaptic length density for GluA1 and GluA2 puncta in ev and hp1 expressing cells. D, Co-localization of GluA1 and GluA2 with VGLUT-1 in ev and hp1 expressing cells. E, Synaptic GluA1 and GluA2 surface receptor fluorescent intensity in ev and hp1 expressing cells. *Different from ev, p<0.05.
MeCP2 knockdown blocks or attenuates synaptic scaling up
Neocortical neurons compensate for changes in synapse number or sensory drive by homeostatically regulating synaptic strength (Desai et al., 2002; Maffei et al., 2006; Goel and Lee, 2007; Maffei and Turrigiano, 2008), yet the reduction in synapse number induced by MeCP2 KD did not result in a compensatory increase in mEPSC amplitude, either in vitro (Fig. 1C–D) or in vivo (Dani et al., 2005). This raised the possibility that synaptic scaling up is impaired following MeCP2 KD. To determine if acute loss of MeCP2 impairs scaling up, transfected cultures were treated with the AMPAR antagonist DNQX for 24 hr to block activity, and AMPA mEPSC were recorded from ev and hp1 expressing neurons. As described previously (Turrigiano et al., 1998; Thiagarajan et al., 2005; Sun and Turrigiano, 2011) DNQX treatment significantly increased mEPSC amplitude in ev expressing neurons, and scaled up the entire distribution of mEPSC amplitudes (Fig. 3A, B; DNQX scaled not different from control, KS test=0.89). In marked contrast, DNQX had no impact on mEPSC amplitude in neurons expressing hp1 (Fig. 3A, C). TTX treatment similarly increased mEPSC amplitude in untransfected neurons (TTX 119% of ctl, p=0.008) but not hp1-expressing neurons (TTX 105% of ctl, p=0.51.
Figure 3.
MeCP2 KD blocks or attenuates synaptic scaling up. A, Top: Example raw traces of mEPSCs recorded from ev (left) and hp1 (right) expressing neurons in untreated control (ctl; top) and DNQX treated (bottom) conditions. Bottom: Average mEPSC waveform for each condition. B, Cumulative histograms of mEPSC amplitudes from ev expressing neurons in ctl and DNQX treated conditions. C, Cumulative histograms of mEPSC amplitudes from hp1 expressing neurons in ctl and DNQX treated conditions. D, Average amplitude for ctl and DNQX treated cultures for neurons expressing ev1 (n=22,10), hp1 (n=20,10), ev2 (n=13,6) and hp2 (n=15,7). E, Plot comparing the % change in mEPSC amplitude during scaling (DNQX as % control) versus the % of MeCP2 remaining after KD for unt, ev1, hp1 and hp2 neurons. *Different from ctl, p<0.05. **Different from ctl, p<0.005.
To control for potential off-target effects of hp1 we repeated these experiments with a second hairpin (hp2) targeted against a different unique sequence of MeCP2 (Zhou et al., 2006; Wood et al., 2009). Hp2 greatly attenuated (but did not completely block) the normal increase in mEPSC amplitude induced by DNQX (Fig. 3D). To determine whether the impact on scaling was related to the efficacy of the two hps at knocking down MeCP2, we compared the increase in mEPSC amplitude induced by DNQX (DNQX as % control) with the fraction of MeCP2 protein remaining after KD. Hp2 reduced MeCP2 levels significantly less (to 60% of control) than hp1 (to 40% of control), and there was an approximately linear relationship between the degree of synaptic scaling and the fraction of MeCP2 remaining (Fig. 3E). These data establish an important role for MeCP2 in synaptic scaling up, and suggest that there is a graded relationship between the amount of scaling and the levels of MeCP2 in the nucleus. On the other hand, our data also suggest there is a threshold level of MeCP2 below which synaptic scaling is completely blocked. These data support the emerging view that MeCP2 levels must be tightly controlled to allow for normal neural circuit function (Chahrour and Zoghbi, 2007).
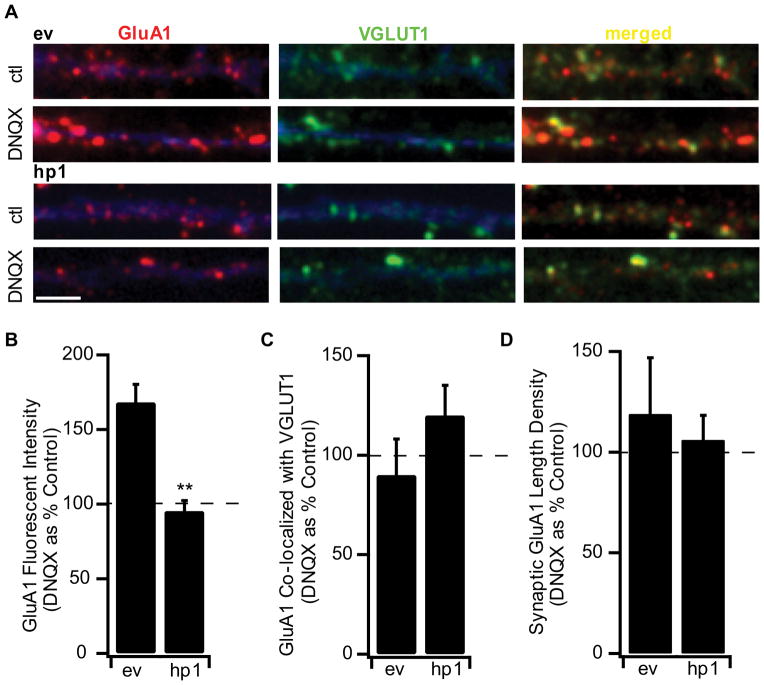
Previous studies have established that synaptic scaling is associated with an increase in AMPAR accumulation at synapses (Lissin et al., 1998; O’Brien et al., 1998; Turrigiano et al., 1998; Wierenga et al., 2005; Wierenga et al., 2006; Ibata et al., 2008). Therefore as a second means of assessing synaptic scaling we compared the fluorescent intensity of the synaptic GluA1 signal in control and DNQX-treated transfected cultures (Fig. 4A). Surface synaptic GluA1 was quantified as described previously, using an antibody directed against an extracellular epitope of GluA1 under non-permeant conditions (Wierenga et al., 2005). DNQX treatment causes a significant increase in the fluorescent intensity of synaptic GluA1 in ev-expressing neurons, but not in neurons expressing hp1 (Fig. 4A–B). DNQX treatment had no significant effect on the percentage of GluA1 puncta co-localized with VGLUT1, or on the synaptic length density in either ev or hp1-expressing neurons (Fig. 4C, D). These data indicate that although MeCP2 knockdown does not affect baseline AMPAR accumulation (Fig. 2E), it prevents the regulated enhancement of synaptic AMPAR that underlies scaling up.
Figure 4.
MeCP2 KD prevents the inactivity-induced accumulation of AMPAR at synapses. A, Examples of staining for endogenous GluA1 (red) and VGLUT-1 (green) for ev (top) and hp1 (bottom) expressing neurons treated with DNQX for 24h. Scale bar, 2μm. B, Fluorescence intensity of synaptic GluA1 puncta for ev and hp1 expressing neurons treated with DNQX. Here and below values from DNQX-treated neurons expressed as a percentage of untreated control neurons. Ev (n=5,8), hp1 (n=7,9). C, Co-localization of GluA1 with VGLUT-1 for ev and hp1 expressing neurons. D, Length density for synaptic GluA1 for ev and hp1 expressing neurons. **Different from ev, p<0.005.
MeCP2 knockout blocks homeostatic synaptic scaling in vivo
Synaptic scaling up can be induced in the intact visual cortex in response to visual deprivation, suggesting that synaptic scaling plays an important role in experience-dependent neocortical development (Desai et al., 2002; Maffei et al., 2006; Goel and Lee, 2007; Maffei and Turrigiano, 2008). In particular, placing animals in the dark for several days (dark exposure, DE) during the classical visual system critical period scales up mEPSC amplitude onto layer 2/3 pyramidal neurons (Goel and Lee, 2007). To determine whether MeCP2 is required for synaptic scaling in vivo, WT or Mecp2 KO mice were visually deprived using 4 days of DE starting at P20/21. We chose to use this KO mouse model, rather than KD in vivo, in order to test whether synaptic scaling is absent in this widely-used mouse model of Rett. Acute cortical slices from primary visual cortex were then obtained from DE and normally reared animals, and AMPA mEPSCs recorded from layer 2/3 pyramidal neurons as described (Desai et al., 2002; Maffei and Turrigiano, 2008). Mecp2 KO mice were compared to age matched normally reared control (Ctl) mice. As previously described (Goel and Lee, 2007) 4 days of DE caused a significant increase in mEPSC amplitude in WT mice (Fig. 5A, B). In contrast, in KO mice DE had no impact on mEPSC amplitude (Fig. 5A, C). As for synaptic scaling in culture, DE scaled up the entire mEPSC amplitude distribution in WT mice (Fig. 5B; WT_DE scaled not different from WT_ctl, KS test=0.52), as expected (Desai et al., 2002; Goel and Lee, 2007). This increase in mEPSC amplitude was not associated with significant changes in mEPSC frequency (WT_Ctl, 1.84 ± 0.22; WT_DE, 2.30 ± 0.23; KO_Ctl, 1.20 ± 0.19; KO_DE, 1.79 ± 0.21) or kinetics. Further, there was no difference in baseline mEPSC amplitude, frequency or passive neuronal properties between WT and KO mice (Fig. 5D, E). These data demonstrate that MeCP2 is essential for visual-deprivation induced synaptic scaling in vivo, suggesting that MeCP2 likely plays a role in experience-dependent visual cortical plasticity.
Figure 5.
MeCP2 knockout blocks homeostatic synaptic scaling in vivo. A, Average waveform for mEPSCs recorded from WT (left) and KO (right) neurons from dark exposed (DE) and normal 12:12 light/dark cycle control (Ctl) mice WT_Ct1 (n=5 animals), WT_DE (n=4 animals), KO_Ctl (n=2 animals) and KO_DE (n=2 animals). B, Cumulative histogram of mEPSC amplitudes recorded from Ctl (n=23) and DE (n=32) WT mice. Inset: Average mEPSC amplitudes for the same conditions C, Cumulative histogram of mEPSC amplitudes recorded from Ctl (n=24) and DE (n=35) KO mice. Inset: Average mEPSC amplitudes for the same conditions. D, Cumulative histogram of mEPSC amplitudes for WT and KO neurons from Ctl mice. Inset: Average mEPSC amplitude for the same conditions. E, Average frequency (left) and input resistance (right) for WT and KO neurons from Ctl mice. *Different from WT_Ctl, p<0.05.
Discussion
Although Rett arises from loss of function of a single gene, MeCP2, the nature of the synaptic defects underlying the neurological symptoms of Rett remain unclear. Here we investigated the role of MeCP2 in synaptic scaling, a form of homeostatic synaptic plasticity widely believed to contribute to the maintenance of the proper excitation-inhibition balance within central neural circuits. We find that acute and cell-autonomous loss of MeCP2 in neocortical pyramidal neurons is sufficient to prevent scaling up of synaptic strengths in response to activity-blockade. Further, we show that MeCP2 is required for synaptic scaling in the intact neocortex in response to sensory deprivation. These data demonstrate that the correct expression level of MeCP2 within individual neurons is essential for the expression of homeostatic synaptic scaling, and suggest that circuit defects arising from loss of scaling contribute to the pathogenesis of Rett.
A hotly debated question in the field is when and where MeCP2 is needed for proper brain function. MeCP2 levels increase postnatally (Shahbazian et al., 2002; Balmer et al., 2003; Cohen et al., 2003; Kishi and Macklis, 2004; Mullaney et al., 2004; Skene et al., 2010), prenatal and early postnatal development is largely normal in Rett and in mouse models of Rett (Chen et al., 2001; Guy et al., 2001; Schule et al., 2008), and loss of MeCP2 in adult mice generates neurological symptoms (McGraw et al., 2011), suggesting that MeCP2 plays an important role in postnatal and mature brain function. Our data provide strong support for the idea that MeCP2 is essential for the ongoing maintenance of synaptic function and plasticity. First, we found that brief (3d) postnatal knockdown of MeCP2 was sufficient to induce a 30% reduction in excitatory synapse density, an effect comparable in magnitude to that observed for knockout throughout development (Nelson et al., 2006; Chao et al., 2007). Thus, in neocortical pyramidal neurons, normal MeCP2 expression is critical for the ongoing maintenance of excitatory synapses. This likely accounts for the consistent observation that excitatory connectivity within neocortical circuits is reduced upon loss of MeCP2 function (Dani et al., 2005; Dani and Nelson, 2009; Wood et al., 2009; Stuss et al., 2012). Second, we found that an acute reduction of MeCP2 was sufficient to block or attenuate synaptic scaling up in response to activity blockade. A recent study showed that more prolonged (9 d) knockdown of MeCP2 in postnatal hippocampal neurons was able to block the reduction in mEPSC amplitude induced by elevated activity (Qiu et al., 2012); taken together with this previous study our data demonstrate that MeCP2 plays a critical role in both directions of synaptic scaling. Postnatal expression of MeCP2 is thus critical for several important aspects of central excitatory synaptic function, including synapse maintenance and bi-directional homeostatic synaptic plasticity. Whether these two aspects of MeCP2 function are mechanistically linked or operate through separate regulatory pathways remains unclear.
Interestingly, we found that basal quantal amplitude and AMPA receptor abundance at spared synapses were normal following MeCP2 loss, both in dissociated cultures following MeCP2 knock down, and in L2/3 pyramidal neurons in acute slices of primary visual cortex of MeCP2 knockout mice. These data are consistent with several studies demonstrating no effect of postnatal MeCP2 loss on mEPSC amplitude in neocortical and hippocampal neurons (Nelson et al., 2006; Chao et al., 2007; Qiu et al., 2012). However, our slice data are in contrast to an earlier study using the same mouse model where acute slice recordings from L5 pyramidal neurons in somatosensory cortex demonstrated a small reduction in mEPSC amplitude (Dani et al., 2005). This could be due to cell-type specific differences (L2/3 vs L5 pyramidal neurons), brain region differences (visual vs. somatosensory cortex), or to differences in age, since we recorded at postnatal day 24–25 rather than P28–35 as previously (Dani et al., 2005). While prolonged loss of MeCP2 may eventually impair the maintenance of baseline postsynaptic properties, our data strongly suggest that acute loss of MeCP2 does not impair baseline AMPAR accumulation, but specifically prevents activity-dependent homeostatic adjustments in quantal amplitude. This in turn suggests that other processes independent of MeCP2 function help to set baseline quantal amplitude, but what these processes might be are currently unknown.
MeCP2 is widely expressed throughout the central nervous system, and is X-linked, so due to X chromosome inactivation females with Rett are mosaic for MeCP2 expression. Further, the severity of the disease is thought to correlate with the fraction of cells expressing the mutated gene (Hoffbuhr et al., 2002; Chahrour and Zoghbi, 2007; Guy et al., 2011). In contrast, most mouse models of Rett involve global loss of function in male mice, and as a consequence the relative contribution of cell-autonomous and non-cell autonomous effects of MeCP2 loss are poorly understood. It has recently become clear that MeCP2 expression in glia is significant, and that loss of this expression can itself induce non-cell autonomous defects in neuronal morphology and synaptogenesis (Ballas et al., 2009); further, specific loss of MeCP2 in GABAergic interneurons recapitulates some aspects of global loss (Chao et al., 2010). These studies make it clear that understanding the cell-type specific functions of MeCP2, and the degree to which loss of MeCP2 induces cell-autonomous defects, is critical for understanding the consequences of mosaic MeCP2 loss in Rett. Here we used sparse knock down in cultured neocortical pyramidal neurons to show that acute MeCP2 loss regulates synaptic scaling up in a cell-autonomous manner, demonstrating for the first time that normal expression level of MeCP2 within individual postnatal pyramidal neurons is essential for the expression of synaptic plasticity. To determine whether synaptic scaling is also blocked by global and prolonged MeCP2 KO we used a common mouse model of Rett (Chen et al., 2001; Dani et al., 2005) and a standard in vivo model of synaptic scaling (Desai et al., 2002; Goel and Lee, 2007) to show that synaptic scaling up in response to sensory deprivation is also blocked in the intact MeCP2-lacking neocortex. These data indicate that neurons are unable to compensate for prolonged developmental loss of MeCP2 to regain the ability to scale synaptic strength, and suggest that loss of synaptic scaling is a major synaptic defect contributing to Rett syndrome. Loss of MeCP2 has recently been reported to disrupt experience-dependent changes at the retinogeniculate synapse (Noutel et al., 2011), supporting the idea that MeCP2 is critical for several aspects of normal experience-dependent plasticity in the visual system.
It is currently unclear how loss of MeCP2 prevents neurons from expressing synaptic scaling. Synaptic scaling up and down are both transcription-dependent (Ibata et al., 2008; Goold and Nicoll, 2010), suggesting that MeCP2 may disrupt scaling by disrupting the regulation of transcription. What the targets of MeCP2 might be, however, is unclear. Synaptic scaling up and down are both critically dependent on trafficking steps involving the GluA2 subunit of the AMPAR (Ibata et al., 2008; Goold and Nicoll, 2010, and it was recently shown that elevated activity enhanced MeCP2 expression and suppressed the expression of GluA2 (Qiu et al., 2012) but because directly reducing GluA2 through RNAi does not impact mEPSC amplitude (Gainey et al., 2009) it is unlikely that this activity-dependent reduction in GluA2 expression is sufficient to drive scaling down. Along the same lines, MeCP2 loss had no significant impact on baseline GluA2 expression (Qiu et al., 2012) or on GluA2 abundance at spared synapses (Fig. 2E), strongly suggesting that block of scaling up is not a simple result of loss of GluA2. Disruption of MeCP2 has recently been shown to disregulate the induction of the immediate early gene Arc (Su et al., 2012), which by influencing AMPAR internalization can occlude synaptic scaling (Rial Verde et al., 2006; Shepherd et al., 2006), suggesting another possible route by which MeCP2 disruption could attenuate synaptic scaling. Alternatively, rather than regulating the transcription of specific scaling-dependent genes, MeCP2 could play a more general role in chromatin remodeling during activity-dependent changes in transcription (Chahrour and Zoghbi, 2007; Skene et al., 2010; Cohen et al., 2011; Guy et al., 2011; Banerjee et al., 2012).
In summary, we demonstrate that MeCP2 acts in a cell-autonomous and acute manner to maintain both excitatory synapse number and synaptic scaling in individual neocortical pyramidal neurons. Further, we show that MeCP2 is critical for the expression of synaptic scaling during postnatal development in vivo. Our results establish MeCP2 as a critical mediator of bi-directional synaptic scaling, and raise the possibility that a disruption of homeostatic plasticity contributes to the imbalance in excitation and inhibition within neocortical circuits induced by MeCP2 loss.
Acknowledgments
Support was provided by National Institutes of Health Grant NS 36853 (G.G.T), and the International Rett Syndrome Foundation (G.G.T). M.P.B was supported by fellowships from the American Psychological Association Diversity Program in Neuroscience and the American Physiological Society Porter Physiology Development Fellowship. We thank Lirong Wang and Bryan Baxter for technical assistance and Xinhua Bao and Michael E. Greenberg for kindly providing DNA constructs.
Literature Cited
- Anggono V, Clem RL, Huganir RL. PICK1 loss of function occludes homeostatic synaptic scaling. J Neurosci. 2011;31:2188–2196. doi: 10.1523/JNEUROSCI.5633-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballas N, Lioy DT, Grunseich C, Mandel G. Non-cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology. Nat Neurosci. 2009;12:311–317. doi: 10.1038/nn.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmer D, Goldstine J, Rao YM, LaSalle JM. Elevated methyl-CpG-binding protein 2 expression is acquired during postnatal human brain development and is correlated with alternative polyadenylation. J Mol Med (Berl) 2003;81:61–68. doi: 10.1007/s00109-002-0396-5. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Castro J, Sur M. Rett syndrome: genes, synapses, circuits, and therapeutics. Front Psychiatry. 2012;3:34. doi: 10.3389/fpsyt.2012.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron. 2007;56:422–437. doi: 10.1016/j.neuron.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Chao HT, Zoghbi HY, Rosenmund C. MeCP2 controls excitatory synaptic strength by regulating glutamatergic synapse number. Neuron. 2007;56:58–65. doi: 10.1016/j.neuron.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, Neul JL, Gong S, Lu HC, Heintz N, Ekker M, Rubenstein JL, Noebels JL, Rosenmund C, Zoghbi HY. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468:263–269. doi: 10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet. 2001;27:327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- Cohen DR, Matarazzo V, Palmer AM, Tu Y, Jeon OH, Pevsner J, Ronnett GV. Expression of MeCP2 in olfactory receptor neurons is developmentally regulated and occurs before synaptogenesis. Mol Cell Neurosci. 2003;22:417–429. doi: 10.1016/s1044-7431(03)00026-5. [DOI] [PubMed] [Google Scholar]
- Cohen S, Gabel HW, Hemberg M, Hutchinson AN, Sadacca LA, Ebert DH, Harmin DA, Greenberg RS, Verdine VK, Zhou Z, Wetsel WC, West AE, Greenberg ME. Genome-wide activity-dependent MeCP2 phosphorylation regulates nervous system development and function. Neuron. 2011;72:72–85. doi: 10.1016/j.neuron.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani VS, Nelson SB. Intact long-term potentiation but reduced connectivity between neocortical layer 5 pyramidal neurons in a mouse model of Rett syndrome. J Neurosci. 2009;29:11263–11270. doi: 10.1523/JNEUROSCI.1019-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani VS, Chang Q, Maffei A, Turrigiano GG, Jaenisch R, Nelson SB. Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proc Natl Acad Sci U S A. 2005;102:12560–12565. doi: 10.1073/pnas.0506071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai NS, Cudmore RH, Nelson SB, Turrigiano GG. Critical periods for experience-dependent synaptic scaling in visual cortex. Nat Neurosci. 2002;5:783–789. doi: 10.1038/nn878. [DOI] [PubMed] [Google Scholar]
- Gainey MA, Hurvitz-Wolff JR, Lambo ME, Turrigiano GG. Synaptic scaling requires the GluR2 subunit of the AMPA receptor. J Neurosci. 2009;29:6479–6489. doi: 10.1523/JNEUROSCI.3753-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, Lee HK. Persistence of experience-induced homeostatic synaptic plasticity through adulthood in superficial layers of mouse visual cortex. J Neurosci. 2007;27:6692–6700. doi: 10.1523/JNEUROSCI.5038-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goold CP, Nicoll RA. Single-cell optogenetic excitation drives homeostatic synaptic depression. Neuron. 2010;68:512–528. doi: 10.1016/j.neuron.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Cheval H, Selfridge J, Bird A. The role of MeCP2 in the brain. Annu Rev Cell Dev Biol. 2011;27:631–652. doi: 10.1146/annurev-cellbio-092910-154121. [DOI] [PubMed] [Google Scholar]
- Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- Hoffbuhr KC, Moses LM, Jerdonek MA, Naidu S, Hoffman EP. Associations between MeCP2 mutations, X-chromosome inactivation, and phenotype. Ment Retard Dev Disabil Res Rev. 2002;8:99–105. doi: 10.1002/mrdd.10026. [DOI] [PubMed] [Google Scholar]
- Ibata K, Sun Q, Turrigiano GG. Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron. 2008;57:819–826. doi: 10.1016/j.neuron.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Jin J, Bao X, Wang H, Pan H, Zhang Y, Wu X. RNAi-induced down-regulation of Mecp2 expression in the rat brain. Int J Dev Neurosci. 2008;26:457–465. doi: 10.1016/j.ijdevneu.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Kishi N, Macklis JD. MECP2 is progressively expressed in post-migratory neurons and is involved in neuronal maturation rather than cell fate decisions. Mol Cell Neurosci. 2004;27:306–321. doi: 10.1016/j.mcn.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Lissin DV, Gomperts SN, Carroll RC, Christine CW, Kalman D, Kitamura M, Hardy S, Nicoll RA, Malenka RC, von Zastrow M. Activity differentially regulates the surface expression of synaptic AMPA and NMDA glutamate receptors. Proc Natl Acad Sci U S A. 1998;95:7097–7102. doi: 10.1073/pnas.95.12.7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Turrigiano GG. Multiple modes of network homeostasis in visual cortical layer 2/3. J Neurosci. 2008;28:4377–4384. doi: 10.1523/JNEUROSCI.5298-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Nataraj K, Nelson SB, Turrigiano GG. Potentiation of cortical inhibition by visual deprivation. Nature. 2006;443:81–84. doi: 10.1038/nature05079. [DOI] [PubMed] [Google Scholar]
- McGraw CM, Samaco RC, Zoghbi HY. Adult neural function requires MeCP2. Science. 2011;333:186. doi: 10.1126/science.1206593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullaney BC, Johnston MV, Blue ME. Developmental expression of methyl-CpG binding protein 2 is dynamically regulated in the rodent brain. Neuroscience. 2004;123:939–949. doi: 10.1016/j.neuroscience.2003.11.025. [DOI] [PubMed] [Google Scholar]
- Nelson ED, Kavalali ET, Monteggia LM. MeCP2-dependent transcriptional repression regulates excitatory neurotransmission. Curr Biol. 2006;16:710–716. doi: 10.1016/j.cub.2006.02.062. [DOI] [PubMed] [Google Scholar]
- Noutel J, Hong YK, Leu B, Kang E, Chen C. Experience-dependent retinogeniculate synapse remodeling is abnormal in MeCP2-deficient mice. Neuron. 2011;70:35–42. doi: 10.1016/j.neuron.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien RJ, Kamboj S, Ehlers MD, Rosen KR, Fischbach GD, Huganir RL. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron. 1998;21:1067–1078. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- Peng YR, He S, Marie H, Zeng SY, Ma J, Tan ZJ, Lee SY, Malenka RC, Yu X. Coordinated changes in dendritic arborization and synaptic strength during neural circuit development. Neuron. 2009;61:71–84. doi: 10.1016/j.neuron.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo K, Goda Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron. 2010;66:337–351. doi: 10.1016/j.neuron.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt KG, Watt AJ, Griffith LC, Nelson SB, Turrigiano GG. Activity-dependent remodeling of presynaptic inputs by postsynaptic expression of activated CaMKII. Neuron. 2003;39:269–281. doi: 10.1016/s0896-6273(03)00422-7. [DOI] [PubMed] [Google Scholar]
- Qiu Z, Sylwestrak EL, Lieberman DN, Zhang Y, Liu XY, Ghosh A. The Rett syndrome protein MeCP2 regulates synaptic scaling. J Neurosci. 2012;32:989–994. doi: 10.1523/JNEUROSCI.0175-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rial Verde EM, Lee-Osbourne J, Worley PF, Malinow R, Cline HT. Increased expression of the immediate-early gene arc/arg3.1 reduces AMPA receptor-mediated synaptic transmission. Neuron. 2006;52:461–474. doi: 10.1016/j.neuron.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford LC, Nelson SB, Turrigiano GG. BDNF has opposite effects on the quantal amplitude of pyramidal neuron and interneuron excitatory synapses. Neuron. 1998;21:521–530. doi: 10.1016/s0896-6273(00)80563-2. [DOI] [PubMed] [Google Scholar]
- Schule B, Armstrong DD, Vogel H, Oviedo A, Francke U. Severe congenital encephalopathy caused by MECP2 null mutations in males: central hypoxia and reduced neuronal dendritic structure. Clin Genet. 2008;74:116–126. doi: 10.1111/j.1399-0004.2008.01005.x. [DOI] [PubMed] [Google Scholar]
- Shahbazian MD, Antalffy B, Armstrong DL, Zoghbi HY. Insight into Rett syndrome: MeCP2 levels display tissue- and cell-specific differences and correlate with neuronal maturation. Hum Mol Genet. 2002;11:115–124. doi: 10.1093/hmg/11.2.115. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52:475–484. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene PJ, Illingworth RS, Webb S, Kerr AR, James KD, Turner DJ, Andrews R, Bird AP. Neuronal MeCP2 is expressed at near histone-octamer levels and globally alters the chromatin state. Mol Cell. 2010;37:457–468. doi: 10.1016/j.molcel.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DP, Boyd JD, Levin DB, Delaney KR. MeCP2 mutation results in compartment-specific reductions in dendritic branching and spine density in layer 5 motor cortical neurons of YFP-H mice. PLoS One. 2012;7:e31896. doi: 10.1371/journal.pone.0031896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su D, Cha YM, West AE. Mutation of MeCP2 alters transcriptional regulation of select immediate-early genes. Epigenetics. 2012;7:146–154. doi: 10.4161/epi.7.2.18907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Turrigiano GG. PSD-95 and PSD-93 play critical but distinct roles in synaptic scaling up and down. J Neurosci. 2011;31:6800–6808. doi: 10.1523/JNEUROSCI.5616-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 2005;47:725–737. doi: 10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Tropea D, Giacometti E, Wilson NR, Beard C, McCurry C, Fu DD, Flannery R, Jaenisch R, Sur M. Partial reversal of Rett Syndrome-like symptoms in MeCP2 mutant mice. Proc Natl Acad Sci U S A. 2009;106:2029–2034. doi: 10.1073/pnas.0812394106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu Rev Neurosci. 2011;34:89–103. doi: 10.1146/annurev-neuro-060909-153238. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Watt AJ, van Rossum MC, MacLeod KM, Nelson SB, Turrigiano GG. Activity coregulates quantal AMPA and NMDA currents at neocortical synapses. Neuron. 2000;26:659–670. doi: 10.1016/s0896-6273(00)81202-7. [DOI] [PubMed] [Google Scholar]
- Wierenga CJ, Ibata K, Turrigiano GG. Postsynaptic expression of homeostatic plasticity at neocortical synapses. J Neurosci. 2005;25:2895–2905. doi: 10.1523/JNEUROSCI.5217-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CJ, Walsh MF, Turrigiano GG. Temporal regulation of the expression locus of homeostatic plasticity. J Neurophysiol. 2006;96:2127–2133. doi: 10.1152/jn.00107.2006. [DOI] [PubMed] [Google Scholar]
- Wood L, Gray NW, Zhou Z, Greenberg ME, Shepherd GM. Synaptic circuit abnormalities of motor-frontal layer 2/3 pyramidal neurons in an RNA interference model of methyl-CpG-binding protein 2 deficiency. J Neurosci. 2009;29:12440–12448. doi: 10.1523/JNEUROSCI.3321-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Hong EJ, Cohen S, Zhao W-n, Ho H-yH, Schmidt L, Chen WG, Lin Y, Savner E, Griffith EC, Hu L, Steen JAJ, Weitz CJ, Greenberg ME. Brain-Specific Phosphorylation of MeCP2 Regulates Activity-Dependent Bdnf Transcription, Dendritic Growth, and Spine Maturation. Neuron. 2006;52:255–269. doi: 10.1016/j.neuron.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]