Abstract
Epstein-Barr virus-induced gene 3 (EBI3) associates with p28 and p35 to form the immunomodulatory cytokines IL-27 and IL-35, respectively. Infection of EBI3−/− mice with the neuroadapted JHM strain of mouse hepatitis virus (JHMV) resulted in increased mortality that was not associated with impaired ability to control viral replication but enhanced T cell and macrophage infiltration into the CNS. IFN-γ secretion from virus-specific CD4+ and CD8+ T cells isolated from infected EBI3−/− mice was augmented while IL-10 expression muted in comparison to infected WT mice. These data demonstrate a regulatory role for EBI3-associated cytokines in controlling host responses following CNS viral infection.
1. Introduction
Inoculation of the neurotropic JHM strain of mouse hepatitis virus (JHMV) into the CNS of susceptible strains of mice provides an excellent model in which to examine host response mechanisms responsible for both control of viral replication within distinct cell lineages present in the brain as well as host-derived factors regulating neuroinflammation (Bergmann et al., 2006). JHMV infection results in an acute encephalomyelitis characterized by wide-spread replication primarily in astrocytes and oligodendrocytes with relatively few neurons being infected (Bergmann et al., 2006; Buchmeier and Lane, 1999; Knobler et al., 1981; Parra et al., 1999; Perlman et al., 1999). Control of viral replication during acute disease relies on virus-specific CD8+ T cells, which function by two different effector mechanisms within the CNS: IFN-γ secretion is responsible for controlling viral replication in oligodendrocytes whereas a perforin-dependent mechanism promotes viral clearance from astrocytes (Lin et al., 1997; Parra et al., 1999). Infiltrating CD4+ T cells provide a supporting role for the maintenance and expansion of CTLs within the CNS, as well as enhancing effector responses (Phares et al., 2012; Stohlman et al., 1998). Although a robust cell-mediated immune response occurs during acute disease, sterilizing immunity is not achieved, resulting in viral persistence (Stohlman and Weiner, 1981). Histological features associated with viral persistence include the development of an immune-mediated demyelinating disease with both T cells and macrophages being important in amplifying disease severity by contributing to myelin damage (Cheever et al., 1949; Lane et al., 2000; Perlman et al., 1999; Pewe et al., 2002; Pewe and Perlman, 2002).
EBV-induced gene-3 (EBI3) encodes a 34 kDa protein that is similar to the p40 subunit of IL-12 (Collison and Vignali, 2008; Wojno and Hunter, 2012). EBI3 is capable of binding subunits p28 or p35 (a subunit of IL-12) to form the cytokines IL-27 and IL-35, respectively (Collison and Vignali, 2008; Wojno and Hunter, 2012). Both IL-27 and IL-35 have important immunoregulatory roles controlling cytokine secretion and inflammation (Collison and Vignali, 2008; Wojno and Hunter, 2012; Yoshida and Miyazaki, 2008). Recent studies have highlighted that genetic ablation of EBI3 results in altered T cell-mediated cytokine expression (Yang et al., 2008) associated with increased inflammation as well as changes in the composition of the cellular infiltrate in experimental models of delayed-type hypersensitivity (Tong et al., 2010), autoimmune inflammation (Igawa et al., 2009; Liu et al., 2012), as well as hepatitis (Siebler et al., 2008). The influence of EBI3 in host defense in response to infection with a neurotropic virus has not been well characterized and was the focus of the present study.
2. Materials and Methods
2.1. Virus and mice
EBI3−/− mice (on the C57BL/6, H-2b background) were generated as previously described (Yang et al., 2008) and generously provided by Centecor. Age-matched EBI3−/− or C57BL/6 control mice (C57BL/6, H-2b, National Cancer Institute, Frederick, MD) were used at 5–8 weeks of age for all experiments. For CNS infection studies, mice were intracranially (i.c.) injected with 250 plaque forming units (PFU) of MHV strain J2.2v-1 (JHMV) suspended in 30µL of sterile HBSS. Animals were sacrificed at defined time points and one-half of each brain at each time point was used for plaque assay on a mouse astrocytoma cell line (Held et al., 2008; Hirano et al., 1978; Lane et al., 2000; Liu et al., 2000). For systemic MHV infection, mice were intraperitoneally (i.p.) injected with 2.5×105 PFU of MHV strain DM suspended in 500uL HBSS. Control (sham) mice were injected with sterile HBSS alone. All experiments were approved by the University of California, Irvine Institutional Animal Care and Use Committee.
2.2. Flow cytometry
Mononuclear cells were obtained from tissues (brains and spleens) at defined times post- infection with JHMV using previously described methods (Held et al., 2008; Lane et al., 2000; Stiles et al., 2006). In brief, single cell suspensions of experimental tissues (all depleted of red blood cells) were stained with combinations of antibodies directed against the following surface markers: CD4, CD8a, CD44, CD25, CD62L, IA-IE (BD Biosciences), CD45 (eBiosciences), and F4/80 (Ab Direct). In all cases, isotype-matched control antibodies were used. Virus-specific CD4+ and CD8+ T cells recognizing their respective immunodominant epitope between amino acids 133–147 of the membrane (M) glycoprotein (M133–147) and surface (S) glycoprotein (S510–518) were determined by intracellular IFN-γ staining using previously described methods (Glass et al., 2002; Glass and Lane, 2003). The presence of virus-specific CD8+ T cells was also evaluated using S510–518 MHC class I tetramers as previously described (Stiles et al., 2006). Frequency data are presented as the percentage of positive cells within the gated population. Total cell numbers were calculated by multiplying these values by the total number of live cells isolated.
2.3. Quantitative real-time PCR
Total RNA from brains of experimental mice was extracted in Trizol (Invitrogen, Carlsbad, CA) and purified from infected EBI3−/− and WT mice as well as sham-infected mice (Ambion). cDNA synthesis was performed using Superscript VILO cDNA Synthesis Kit (Invitrogen). Real-Time PCR analysis was completed using a LightCycler 480 Instrument II (Roche). IL-10 mRNA levels were assessed using specific primers: forward primer – AGCCGGGAAGACAATAACTG; reverse primer - GGAGTCGGTTAGCAGTATGTTG. Amplicon expression was normalized to GAPDH (Invitrogen). LightCycler 480 SYBR Green I Master (Roche) was used in all reactions with the following assay conditions: 10 min initial denaturation at 95°C, and 45 cycles of 30 sec at 95°C and 1 min at 60°C. Data were analyzed using LightCycler 480 software (Roche).
2.4. Ex vivo cytokine production
C57BL/6 mice were injected via i.p. with 2.5×105 PFU of MHV strain DM suspended in 500uL HBSS. Mice were sacrificed at day 7 p.i. and splenocytes stimulated with 5 µM OVA, M133, or S510 peptides for 48 hours were collected and determined for IL-10 and IFN-γ secretion using the respective R&D DuoSet ELISA kits.
2.5. CTL assay
Spleen-derived CD8+ T cells were analyzed for lytic activity at day 7 following i.p. infection of WT and EBI3−/− mice with 2.5x105 PFU of the DM strain of MHV. Numbers of S510–518-specific CD8+ T cells were determined by intracellular IFN-γ staining following exposure to the S510–518 peptide. CTL assays were performed with RMA-s (H-2b) target cells preincubated with 5 µM S510–518 peptide or OVA peptide as a control that were combined with immune CD8+ T cells at various effector:target ratios. Amounts of lactate dehydrogenase (LDH) released from lysed cells were determined by using a CytoTox 96® Non-Radioactive Cytotoxicity Assay (Promega, Madison, WI). Percentage lysis was determined as specified by manufacturer’s protocols. Percentage lysis of OVA peptide-pulsed co-cultures was subtracted from the percentage lysis of S510–518 pulsed co-cultures to eliminate background CTL activity.
2.6. Histology and immunohistochemistry
Brains and spinal cords from experimental mice were harvested from PBS-perfused mice and fixed in 4% paraformaldehyde overnight. Brain tissues were embedded in paraffin and stained with hematoxylin and eosin (H&E). Spinal cords were fixed in normal balance formalin for 24 hours and then embedded in paraffin. Sections of spinal cords were stained with Luxol Fast Blue (LFB) to identify myelin (blue) and counterstained with Harrison Hematoxylin and Eosin to visualized cellular inflammation (Lane et al., 2000; Liu et al., 2001). Rabbit anti-mouse GFAP (Invitrogen, Carlsbad, CA) (1:500 dilution in phosphate-buffered saline containing 10% normal rabbit serum [NRS]) used for detection of activated astrocytes. A biotinylated secondary antibody (1:400 dilution, Vector Laboratories, Burlingame, CA) was used for detection. Staining was performed on 6µm-thick frozen sections fixed with 4% paraformaldehyde. The ABC Elite (Vector Laboratories) staining system was used according to the manufacturer’s instructions, and diaminobenzidine (DAB) was used as a chromogen. All slides were counterstained with hematoxylin, dehydrated, and mounted. Staining controls were (i) omission of primary antibodies from the staining sequence and (ii) treatment of sham-infected mice with primary and secondary antibodies.
2.7. Statistical Analysis
2-tailed unpaired Student’s T-Test was conducted to determine statistical significance for all assays.
3. Results
3.1. Survival and histopathology
Wildtype (WT) and EBI3−/− mice were infected intracranially (i.c.) with 250 PFU of JHMV to evaluate the contributions of EBI3 in host defense. Genetic ablation of EBI3 signaling was associated with increased (p≤0.01) susceptibility to JHMV-induced death with ~70% of infected EBI3−/− mice dying by day 12 p.i. whereas only ~ 30% of WT mice died (Figure 1A). Examination of brains of JHMV-infected mice revealed that EBI3−/− mice displayed overall increased thickening of the meninges as well as increased perivascular cuffing when compared to infected WT mice indicating enhanced neuroinflammation in response to JHMV infection in the absence of EBI3 expression (Figure 1B). Additionally, increased astrogliosis, as determined by GFAP staining, was evident in JHMV-infected EBI3−/− mice in comparison to WT mice (Figure 1C).
Figure 1. Mortality and neuroinflammation following JHMV infection of the CNS.
(A) WT and EBI3−/− mice were infected i.c. with 250 PFU of JHMV and survival determined. Data presented is representative of 3 independent experiments with a minimum of 6 mice/experiment. (B) Representative H&E staining showing increased perivascular cuffing (top panels) and increased meningeal inflammation in the brains of JHMV-infected WT and EBI3−/− mice at day 7 p.i. (C) Representative GFAP staining showing increased astrogliosis within the brains of JHMV-infected EBI3−/− mice compared to WT mice at day 7 p.i.
3.2. CNS viral titers and neuroinflammation
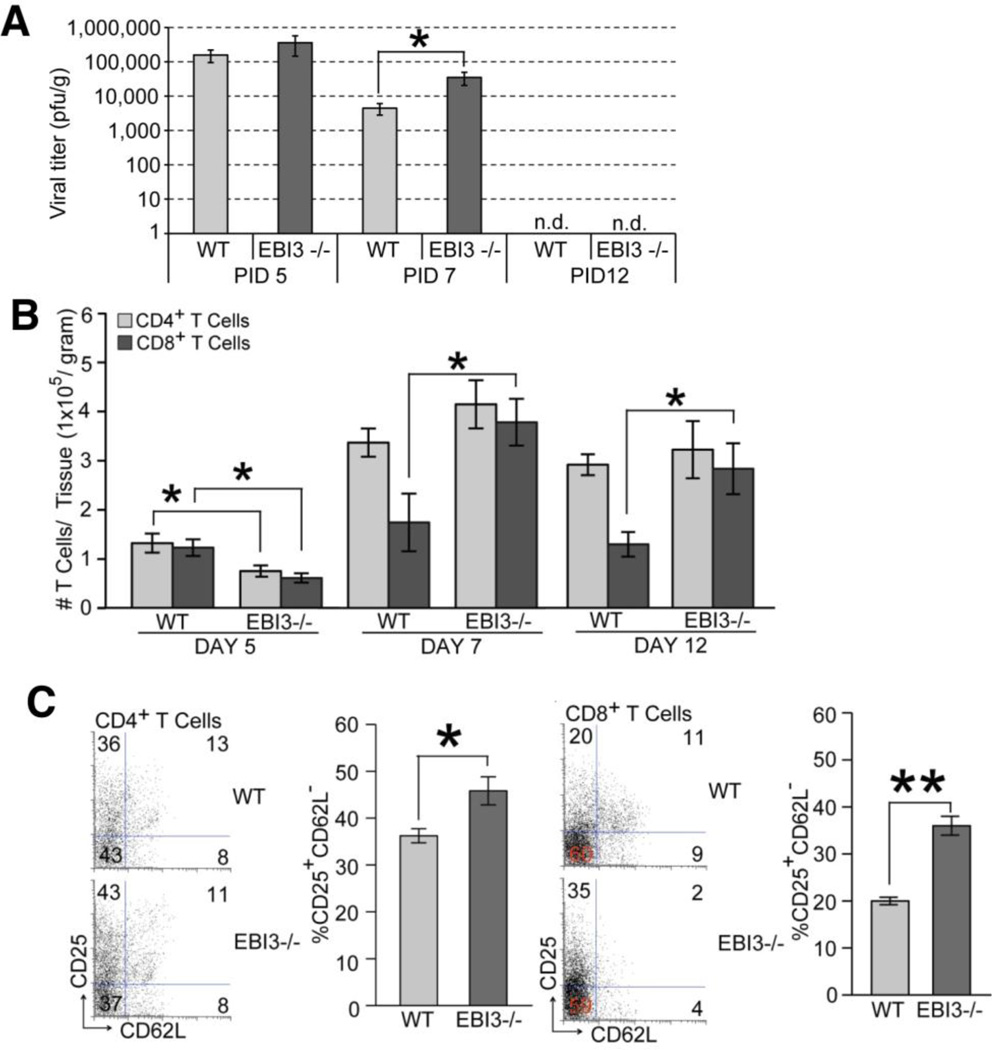
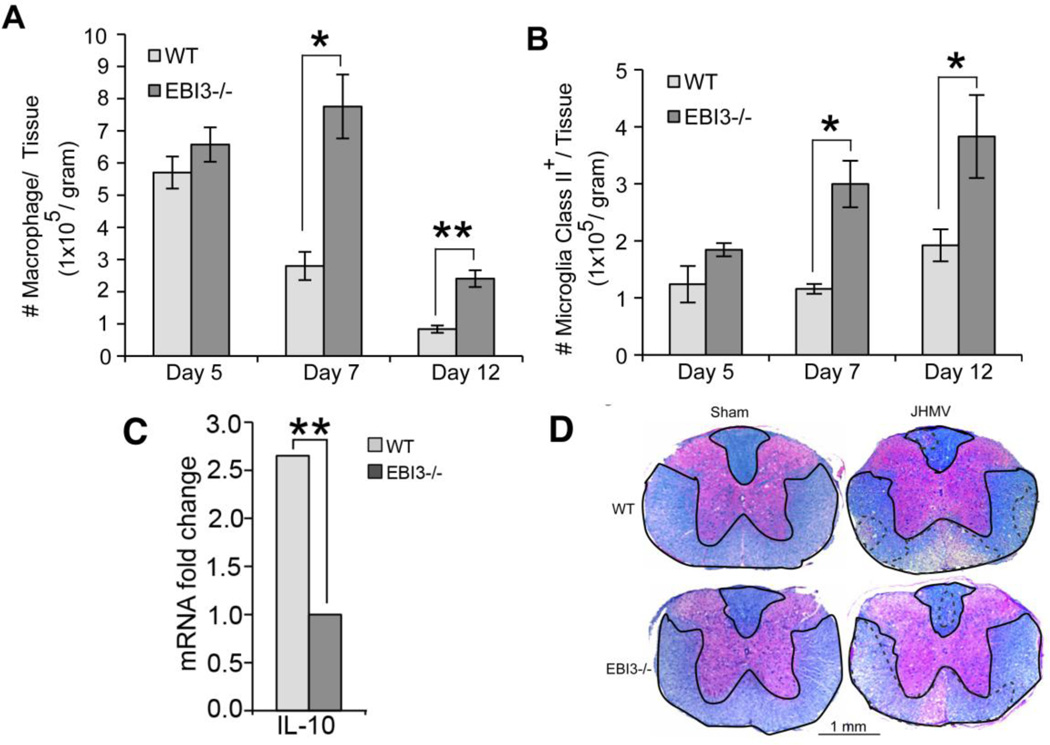
To determine if increased death was the result of an inability to control viral replication, viral titers within the brains of infected WT and EBI3−/− mice were determined. As shown in Figure 2A, similar viral titers were present within the brains of both WT and EBI3 −/− mice at day 5 p.i. By day 7 p.i., EBI3−/− mice displayed elevated titers (p≤0.05) compared to WT mice yet both groups of mice reduced titers below the level of detection (~100 PFU/g tissue) at day 12 p.i. Flow analysis of T cell infiltration into the CNS of infected animals revealed reduced (p≤0.05) numbers of CD4+ and CD8+ T cells present within the brains of infected EBI3−/− mice compared to WT mice at day 5 p.i. (Figure 2B). However, by day 7 p.i., there were increased numbers of CD8+ T cells present in the CNS of infected EBI3−/− mice compared to WT mice and this was sustained out to day 12 p.i. (Figure 2B). In contrast, there were no differences in numbers of inflammatory CD4+ T cells within the CNS of infected WT and EBI3−/− mice at days 7 and 12 p.i. (Figure 2B). Further examination revealed enhanced activation (CD62L−CD25+) states in both CD4+ and CD8+ T cell (Figure 2C) subsets present within the CNS of JHMV-infected EBI3−/− mice compared to WT mice. Macrophage (CD45highF4/80+) infiltration, as determined by flow cytometric analysis, was elevated in infected EBI3−/− mice at days 7 (p≤0.05) and 12 p.i. (p≤0.01) when compared to infected WT mice (Figure 3A). In addition, flow analysis revealed increased (p≤0.05) MHC class II expression on activated microglia at days 7 and 12 p.i. suggesting increased IFN-γ levels within the CNS of JHMV-infected EBI3−/− mice when compared to infected WT mice (Figure 3B). Earlier studies indicate that the absence of EBI3 alters the immunomodulatory cytokine IL-10 levels in models of inflammation (Hausding et al., 2007; Tong et al., 2010; Zahn et al., 2005). In accordance with these earlier studies, we detected significantly (p≤0.01) lower levels of mRNA transcripts specific for IL-10 within the brains of infected EBI3−/− mice compared to WT mice (Figure 3C). Evaluation of demyelination revealed comparable levels of white matter damage in both infected EBI3−/− and WT mice (Figure 3D).
Figure 2. Brain viral titers and T cell infiltration.
(A) WT and EBI3−/− mice were i.c. infected with 250 PFU of JHMV and viral titers determined at days 5, 7, and 12 p.i. (B) T cell infiltration into the CNS of JHMV-infected mice was determined by flow cytometry at days 5, 7, and 12 p.i. (C) Activation status of T cells was determined by measuring CD62L and CD25 expression on T cells infiltrating into the CNS of JHMV-infected mice by flow cytometry. Representative dot blots are shown for CD4+ T cells and CD8+ T cells; and the overall increase in CD62LloCD25hi CD4+ and CD8+ T cell subsets in infected EBI3−/− mice compared to WT mice is shown. For all experiments, data is representative of 2 independent experiments with a minimum of 3 mice per time point; data is presented as average±SEM; * indicates p≤0.05.
Figure 3. Macrophage infiltration and demyelination.
(A) Macrophage (CD45highF480+) infiltration into the CNS of infected mice was determined at days 5, 7, and 12 p.i. by flow cytometry. (B) MHC class II expression on resident microglia (CD45loF480+) from infected EBI3−/− and WT mice was determined at days 5, 7, and 12 p.i. by flow cytometry. Data for A and B is derived from 2 independent experiments with a minimum of 3 mice per time point; data is presented as average+SEM and * indicates p≤0.05. (C) Expression of mRNA transcripts specific for IL-10 within the brains of JHMV-infected EBI3−/− and WT mice at day 7 p.i. was performed by qPCR analysis. Data is derived from 4 mice from each experimental group and presented as average±SEM fold change compared to sham-infected mice; ** indicates p≤0.01. (D) Representative spinal cord sections from JHMV-infected EBI3−/− and WT mice isolated at day 12 p.i. and stained with luxol fast blue to assess the severity of demyelination (areas of white matter pathology are outlined by hashmarks).
3.3. Virus-specific T cells
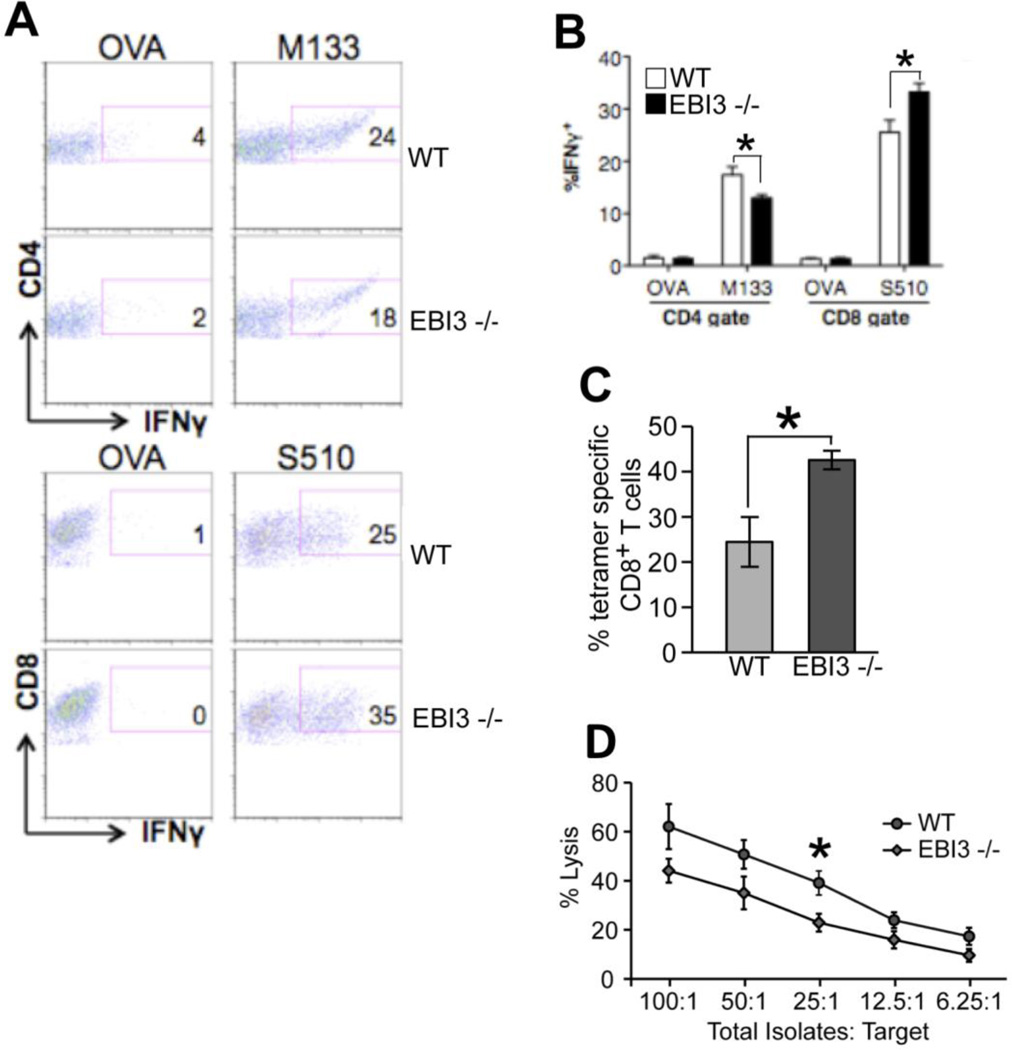
Intracellular IFN-γ staining in response to defined CD4 [Matrix (M) protein spanning epitopes 133–144] and CD8 [Spike (S) glycoprotein spanning epitopes 510–518] viral epitopes was employed to measure infiltration of virus-specific T cells into the CNS of JHMV-infected mice. Analysis at day 7 p.i. revealed reduced (p<0.05) numbers of M133–144-specific CD4+ T cells in the brains of infected EBI3−/− mice compared to WT mice while infiltration of S510–518-specific CD8+ T cells was significantly (p<0.05) increased in EBI3−/− mice compared to WT mice (Figures 4A and B). S510–518 MHC class I tetramer staining confirmed increased numbers (p≤0.05) of virus-specific CD8+ T cells within the CNS of EBI3−/− mice when compared to WT mice (Figure 4C). By day 12 p.i., there were increased (p≤0.001) numbers of virus-specific CD4+ and CD8+ T cells present in the brains of EBI3−/− mice compared to WT mice (not shown). To determine if CTL activity was influenced by loss of EBI3 expression, ex vivo CTL assays were performed. WT and EBI3−/− mice were infected i.p. with MHV and spleens were removed at day 7 p.i. Similar frequencies of virus-specific CD8+ T cells were present within the spleens of infected WT and EBI3−/− mice as determined by intracellular IFN-γ staining in response to S510–518 as well as S510–518 MHC class I tetramer staining (not shown). As shown in Figure 4D, CTL activity is slightly muted at all E:T ratios tested arguing for a potential role for EBI3 in augmenting CTL activity.
Figure 4. Anti-viral T cell responses.
WT and EBI3−/− mice were i.c. infected with 250 PFU of JHMV and generation of virus-specific T cells measured by intracellular IFN-γ staining in response to the CD4+ T cell epitope M133–147 and CD8+ T cell epitope S510–518. As controls, ovalbumin (OVA) peptides corresponding to defined CD4+ and CD8+ T cell subsets were also included. (A) Representative dot blots showing increased IFN-γ staining in CD4+ and CD8+ T cell subsets in infected EBI3−/− mice compared to WT mice at day 7 p.i. (B) Quantification of intracellular IFN-γ expression in response to viral peptides reveals increased frequencies of virus-specific CD4+ and CD8+ T cells within the brains of JHMV-infected EBI3−/− mice compared to WT mice. (C) Staining with the S510–518-MHC class I tetramer reveals increased staining within the brains of infected EBI3−/− mice compared to WT mice at day 7 p.i. Data in B and C is derived from 2 independent experiments with a minimum of 3 mice per group and presented as average±SEM; * indicates p<0.05 (D) WT and EBI3−/− mice were infected i.p. with JHMV and splenocytes removed at day 7 p.i. CTL activity to S510–518-pulsed target cells was performed as previously described (Stiles et al., 2006) using different E:T ratios and * indicates p≤0.05.
3.4. T cell cytokine production
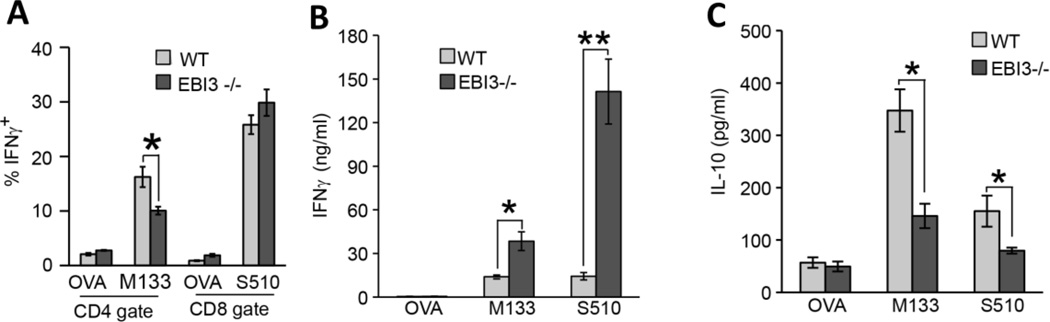
We next interrogated how genetic ablation of EBI3 influenced T cell responses following JHMV infection. Both WT and EBI3−/− mice were challenged by i.p. injection with virus and splenocytes isolated at day 7 p.i. Intracellular IFN-γ staining demonstrated decreased (p≤0.05) numbers of M133–147-specific CD4+ T cells isolated from the spleens of EBI3−/− mice compared to WT mice while comparable numbers of S510–518-positive CD8+ T cells were present in both WT and EBI3−/− mice (Figure 5A). Cultured T cells were stimulated with either M133–147 or S510–518 and IFN-γ levels determined by ELISA. As shown in Figure 5B, T cells from EBI3−/− mice produced significantly more IFN-γ in response to peptide stimulation in comparison to WT mice. There was an ~ 2-fold increase (p≤0.05) in IFN-γ production by M133–147-specific CD4+ T cells from EBI3−/− mice while IFN-γ levels were increased >2.5-fold (p≤0.01) by S510–518-specific CD8+ T cells isolated from EBI3−/− mice. In addition, IL-10 production was significantly (p≤0.05) reduced in antigen-stimulated virus-specific EBI3−/− T cells compared to WT cells (Figure 5C).
Figure 5. T cell cytokine production.
WT and EBI3−/− mice were injected i.p. with JHMV and splenocytes isolated at day 7 p.i. to evaluate expression of IFN-γ and IL-10 in response to viral peptide stimulation. (A) Intracellular IFN-γ staining reveals diminished frequency of M133–147-specific CD4+ T cells in EBI3−/− mice compared to WT whereas there were similar frequencies of virus-specific CD8+ T cells. Splenocytes from infected WT and EBI3−/− mice were stimulated with either 5 µM M133–147 or S510–518 peptides for 48 hours and secretion of IFN-γ (B) or IL-10 (C) determined by ELISA. * indicates p≤0.05 and ** indicates p≤0.01.
4. Discussion
In this study, we demonstrate that EBI3 plays an important role in controlling neuroinflammation and T cell responses in response to JHMV infection of the CNS. Specifically, JHMV infection of EBI3-deficient mice increased mortality during the acute stage of the disease and this not the result of impaired ability to control viral replication. Rather, increased disease severity was associated with enhanced leukocyte entry into the CNS with greater numbers of both CD8+ T cells and virus-specific CD8+ T cells when compared to WT mice. In addition, IFN-γ secretion was increased following viral peptide stimulation of EBI3-deficient CD4+ and CD8+ T cells isolated from JHMV-infected mice. Although IFN-γ production was elevated in CD8+ T cells, CTL activity was muted indicating differential roles for EBI3 in contributing to antiviral effector functions. These findings highlight that within the context of JHMV infection of the CNS, EBI3 is important in influencing virus-specific CD8+ T cell effector function yet the overall impact of how this contributes to increased mortality remains enigmatic and requires continued investigation.
Previous studies indicate that genetic ablation of EBI3 influences cytokine production by T cells. For example, delayed-type hypersensitivity is dramatically exacerbated in EBI3−/− associated with enhanced infiltration of inflammatory cells into the footpad of antigen-challenged mice as compared to control animals (Tong et al., 2010). In addition, EBI3 negatively regulates T cell production of IL-17 and IL-22 and this correlated with enhanced expression of the transcription factor RORgT (Yang et al., 2008). Experimental infection of EBI3-deficient mice with Leishmania demonstrated muted Th1 responses indicating a potential role in regulating immune responses following microbial infection (Zahn et al., 2005). The majority of studies examining the importance of EBI3 in tailoring T cell responses have characterized a role within the context of IL-27 (EBI3/p28) signaling. These studies have highlighted an important role for IL-27 in modulating T cell as well as inflammatory responses. IL-27 signals through a heterodimeric receptor consisting of IL-27Ra (WX1) and gp130 (Pflanz et al., 2004). Originally characterized as a proinflammatory cytokine that was necessary for initiation of Th1 differentiation (Pflanz et al., 2002), IL-27 expression was shown to be critical in generation of protective T cell responses in response to infection with Leishmania major (Zahn et al., 2005). However, additional studies have shown that loss of IL-27 signaling through genetic ablation of either EBI3 or IL27Ra does not diminish the ability to generate a protective Th1 response (Batten and Ghilardi, 2007). Rather, animals lacking these genes have exhibited enhanced immune responses associated with accelerated tissue damage that correlates with elevated levels of proinflammatory cytokines including IFN-γ following infection with Toxoplamsa gondii and Mycobacterium tuberculosis (Holscher et al., 2005; Villarino et al., 2003). With regards to viral infection, IL-27 has been shown to exert an anti-viral effect on HIV and evidence suggests this is mediated, in part, by activating multiple interferon-inducible genes (Fakruddin et al., 2007; Imamichi et al., 2008).
Whether increased neuroinflammation and IFN-γ secretion by T cells in response to JHMV infection of the CNS is due to impaired signaling through either IL-27 or IL-35 remains to be determined. However, a recent study by Liu and colleagues (Liu et al., 2012) examined the influence of EBI3 within the context of experimental autoimmune encephalomyelitis (EAE), an autoimmune model of neuroinflammation and demyelination. Similar to our findings, there was increased neuroinflammation in MOG-immunized EBI3−/− mice compared to WT controls and this was accompanied by increased Th1 responses (Liu et al., 2012). However, production of IFN-γ was not affected in EBI3−/− mice but IL-2 and IL-17 levels were dramatically elevated. Although neuroinflammation was increased in EBI3−/− mice with EAE, the severity of disease was only marginally enhanced compared to WT mice and this may be the result of increased numbers of CD4+Foxp3+ Treg’s that exhibited potent suppressive functions (Liu et al., 2012). IL-17-producing T cells are not detected within the CNS of JHMV-infected mice so it is unlikely that the absence of EBI3 affects secretion of IL-17 in this model (Held et al., 2008; Kapil et al., 2009). Whether Tregs are increased in number and/or exhibit enhanced suppressor functions in response to JHMV infection of EBI3−/− mice is unknown at this time. It is interesting to speculate that a reason that the severity of demyelination is not dramatically increased in JHMV-infected EBI3−/− mice compared to WT mice even in the face of increased neuroinflammation may be the result of enhanced suppressor activity by Tregs and this is currently under investigation.
Our findings that EBI3 deficiency increases IFN-γ secretion are consistent with other studies examining how EBI3/IL-27 controls T cell responses following microbial infection (Stumhofer et al., 2006; Villarino et al., 2003). Moreover, a recent study by Sauer and colleagues (Stumhofer et al., 2006) has demonstrated enhanced anti-tumor responses by CD8+ T cells in EBI3−/− mice associated with increased IFN-γ production. Therefore, loss of EBI3 expression is not restricted to altered effector functions in CD4+ T cell subsets but can also include CD8+ T cell subsets. An important question that remains to be resolved is whether the change in disease course and T cell responses in JHMV-infected EBI3−/− mice reflects deficiencies in IL-27 or IL-35 expression.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Batten M, Ghilardi N. The biology and therapeutic potential of interleukin 27. J Mol Med (Berl) 2007;85:661–672. doi: 10.1007/s00109-007-0164-7. [DOI] [PubMed] [Google Scholar]
- Bergmann CC, Lane TE, Stohlman SA. Coronavirus infection of the central nervous system: host-virus stand-off. Nat Rev Microbiol. 2006;4:121–132. doi: 10.1038/nrmicro1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier MJ, Lane TE. Viral-induced neurodegenerative disease. Curr Opin Microbiol. 1999;2:398–402. doi: 10.1016/S1369-5274(99)80070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheever FS, Daniels JB, Pappenheimer AM, Bailey OT. A murine virus (JHM) causing disseminated encephalomyelitis with extensive destruction of myelin. Journal of Exerimental Medicine. 1949;90:181–194. doi: 10.1084/jem.90.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collison LW, Vignali DA. Interleukin-35: odd one out or part of the family? Immunol Rev. 2008;226:248–262. doi: 10.1111/j.1600-065X.2008.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakruddin JM, Lempicki RA, Gorelick RJ, Yang J, Adelsberger JW, Garcia-Pineres AJ, Pinto LA, Lane HC, Imamichi T. Noninfectious papilloma virus-like particles inhibit HIV-1 replication: implications for immune control of HIV-1 infection by IL-27. Blood. 2007;109:1841–1849. doi: 10.1182/blood-2006-02-001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass WG, Chen BP, Liu MT, Lane TE. Mouse hepatitis virus infection of the central nervous system: chemokine-mediated regulation of host defense and disease. Viral Immunol. 2002;15:261–272. doi: 10.1089/08828240260066215. [DOI] [PubMed] [Google Scholar]
- Glass WG, Lane TE. Functional analysis of the CC chemokine receptor 5 (CCR5) on virus-specific CD8+ T cells following coronavirus infection of the central nervous system. Virology. 2003;312:407–414. doi: 10.1016/S0042-6822(03)00237-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausding M, Karwot R, Scholtes P, Lehr HA, Wegmann M, Renz H, Galle PR, Birkenbach M, Neurath MF, Blumberg RS, Finotto S. Lung CD11c+ cells from mice deficient in Epstein-Barr virus-induced gene 3 (EBI-3) prevent airway hyper-responsiveness in experimental asthma. Eur J Immunol. 2007;37:1663–1677. doi: 10.1002/eji.200636675. [DOI] [PubMed] [Google Scholar]
- Held KS, Glass WG, Orlovsky YI, Shamberger KA, Petley TD, Branigan PJ, Carton JM, Beck HS, Cunningham MR, Benson JM, Lane TE. Generation of a protective T-cell response following coronavirus infection of the central nervous system is not dependent on IL-12/23 signaling. Viral Immunol. 2008;21:173–188. doi: 10.1089/vim.2008.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano N, Murakami T, Fujiwara K, Matsumoto M. Utility of mouse cell line DBT for propagation and assay of mouse hepatitis virus. Jpn J Exp Med. 1978;48:71–75. [PubMed] [Google Scholar]
- Holscher C, Holscher A, Ruckerl D, Yoshimoto T, Yoshida H, Mak T, Saris C, Ehlers S. The IL-27 receptor chain WSX-1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. J Immunol. 2005;174:3534–3544. doi: 10.4049/jimmunol.174.6.3534. [DOI] [PubMed] [Google Scholar]
- Igawa T, Nakashima H, Sadanaga A, Masutani K, Miyake K, Shimizu S, Takeda A, Hamano S, Yoshida H. Deficiency in EBV-induced gene 3 (EBI3) in MRL/lpr mice results in pathological alteration of autoimmune glomerulonephritis and sialadenitis. Mod Rheumatol. 2009;19:33–41. doi: 10.1007/s10165-008-0117-1. [DOI] [PubMed] [Google Scholar]
- Imamichi T, Yang J, Huang DW, Brann TW, Fullmer BA, Adelsberger JW, Lempicki RA, Baseler MW, Lane HC. IL-27, a novel anti-HIV cytokine, activates multiple interferon-inducible genes in macrophages. AIDS. 2008;22:39–45. doi: 10.1097/QAD.0b013e3282f3356c. [DOI] [PubMed] [Google Scholar]
- Kapil P, Atkinson R, Ramakrishna C, Cua DJ, Bergmann CC, Stohlman SA. Interleukin-12 (IL-12), but not IL-23, deficiency ameliorates viral encephalitis without affecting viral control. J. Virology. 2009;83(12):5978–5986. doi: 10.1128/JVI.00315-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobler RL, Dubois-Dalcq M, Haspel MV, Claysmith AP, Lampert PW, Oldstone MB. Selective localization of wild type and mutant mouse hepatitis virus (JHM strain) antigens in CNS tissue by fluorescence, light and electron microscopy. J Neuroimmunol. 1981;1:81–92. doi: 10.1016/0165-5728(81)90010-2. [DOI] [PubMed] [Google Scholar]
- Lane TE, Liu MT, Chen BP, Asensio VC, Samawi RM, Paoletti AD, Campbell IL, Kunkel SL, Fox HS, Buchmeier MJ. A central role for CD4(+) T cells and RANTES in virus-induced central nervous system inflammation and demyelination. J Virol. 2000;74:1415–1424. doi: 10.1128/jvi.74.3.1415-1424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Stohlman SA, Hinton DR. Mouse hepatitis virus is cleared from the central nervous systems of mice lacking perforin-mediated cytolysis. J Virol. 1997;71:383–391. doi: 10.1128/jvi.71.1.383-391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JQ, Liu Z, Zhang X, Shi Y, Talebian F, Carl JW, Jr, Yu C, Shi FD, Whitacre CC, Trgovcich J, Bai XF. Increased Th17 and regulatory T cell responses in EBV-induced gene 3-deficient mice lead to marginally enhanced development of autoimmune encephalomyelitis. J Immunol. 2012;188:3099–3106. doi: 10.4049/jimmunol.1100106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MT, Chen BP, Oertel P, Buchmeier MJ, Armstrong D, Hamilton TA, Lane TE. The T cell chemoattractant IFN-inducible protein 10 is essential in host defense against viral-induced neurologic disease. J Immunol. 2000;165:2327–2330. doi: 10.4049/jimmunol.165.5.2327. [DOI] [PubMed] [Google Scholar]
- Liu MT, Keirstead HS, Lane TE. Neutralization of the chemokine CXCL10 reduces inflammatory cell invasion and demyelination and improves neurological function in a viral model of multiple sclerosis. J Immunol. 2001;167:4091–4097. doi: 10.4049/jimmunol.167.7.4091. [DOI] [PubMed] [Google Scholar]
- Parra B, Hinton DR, Marten NW, Bergmann CC, Lin MT, Yang CS, Stohlman SA. IFN-gamma is required for viral clearance from central nervous system oligodendroglia. J Immunol. 1999;162:1641–1647. [PubMed] [Google Scholar]
- Perlman SR, Lane TE, Buchmeier MJ. Coronaviruses: Hepatitis, peritonitis, and central nervous system disease. In: Cunningham MW, Fujinami RS, editors. Effects of Microbes on the Immune System. Philadelphia: Lippincott Williams & Wilkins; 1999. pp. 331–348. [Google Scholar]
- Pewe L, Haring J, Perlman S. CD4 T-cell-mediated demyelination is increased in the absence of gamma interferon in mice infected with mouse hepatitis virus. J Virol. 2002;76:7329–7333. doi: 10.1128/JVI.76.14.7329-7333.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pewe L, Perlman S. Cutting edge: CD8 T cell-mediated demyelination is IFN-gamma dependent in mice infected with a neurotropic coronavirus. J Immunol. 2002;168:1547–1551. doi: 10.4049/jimmunol.168.4.1547. [DOI] [PubMed] [Google Scholar]
- Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, Phillips JH, McClanahan TK, de Waal Malefyt R, Kastelein RA. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, Blumenschein WM, Mattson JD, Wagner JL, To W, Zurawski S, McClanahan TK, Gorman DM, Bazan JF, de Waal Malefyt R, Rennick D, Kastelein RA. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- Phares TW, Stohlman SA, Hwang M, Min B, Hinton DR, Bergmann CC. CD4 T cells promote CD8 T cell immunity at the priming and effector site during viral encephalitis. J Virol. 2012;86:2416–2427. doi: 10.1128/JVI.06797-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebler J, Wirtz S, Frenzel C, Schuchmann M, Lohse AW, Galle PR, Neurath MF. Cutting edge: a key pathogenic role of IL-27 in T cell- mediated hepatitis. J Immunol. 2008;180:30–33. doi: 10.4049/jimmunol.180.1.30. [DOI] [PubMed] [Google Scholar]
- Stiles LN, Hardison JL, Schaumburg CS, Whitman LM, Lane TE. T cell antiviral effector function is not dependent on CXCL10 following murine coronavirus infection. J Immunol. 2006;177:8372–8380. doi: 10.4049/jimmunol.177.12.8372. [DOI] [PubMed] [Google Scholar]
- Stohlman SA, Bergmann CC, Lin MT, Cua DJ, Hinton DR. CTL effector function within the central nervous system requires CD4+ T cells. J Immunol. 1998;160:2896–2904. [PubMed] [Google Scholar]
- Stohlman SA, Weiner LP. Chronic central nervous system demyelination in mice after JHM virus infection. Neurology. 1981;31:38–44. doi: 10.1212/wnl.31.1.38. [DOI] [PubMed] [Google Scholar]
- Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJ, O'Shea JJ, Hennighausen L, Ernst M, Hunter CA. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- Tong H, Miyazaki Y, Yamazaki M, Hara H, Waldmann H, Hori S, Yoshida H. Exacerbation of delayed-type hypersensitivity responses in EBV-induced gene-3 (EBI-3)-deficient mice. Immunol Lett. 2010;128:108–115. doi: 10.1016/j.imlet.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H, Kastelein RA, Saris C, Hunter CA. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645–655. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- Wojno ED, Hunter CA. New directions in the basic and translational biology of interleukin-27. Trends Immunol. 2012;33:91–97. doi: 10.1016/j.it.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Yang M, Htut TM, Ouyang X, Hanidu A, Li X, Sellati R, Jiang H, Zhang S, Li H, Zhao J, Ting AT, Mayer L, Unkeless JC, Labadia ME, Hodge M, Li J, Xiong H. Epstein-Barr virus-induced gene 3 negatively regulates IL-17, IL-22 and RORgamma t. Eur J Immunol. 2008;38:1204–1214. doi: 10.1002/eji.200838145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Miyazaki Y. Regulation of immune responses by interleukin-27. Immunol Rev. 2008;226:234–247. doi: 10.1111/j.1600-065X.2008.00710.x. [DOI] [PubMed] [Google Scholar]
- Zahn S, Wirtz S, Birkenbach M, Blumberg RS, Neurath MF, von Stebut E. Impaired Th1 responses in mice deficient in Epstein-Barr virus-induced gene 3 and challenged with physiological doses of Leishmania major. Eur J Immunol. 2005;35:1106–1112. doi: 10.1002/eji.200425926. [DOI] [PubMed] [Google Scholar]