Abstract
Background
A growing body of evidence suggests that caffeinated beverages may impair chronic glucose control in type 2 diabetes. This pilot study tested the chronic effects of caffeine abstinence on glucose control in type 2 diabetic patients who were daily coffee drinkers.
Methods
Twelve coffee drinkers (six males) with established type 2 diabetes participated. Seven (five males) completed 3 months of total caffeine abstinence. Measures of chronic glucose control, long-term (hemoglobin A1c [HbA1c]) and short-term (1,5-anhydroglucitol [1,5-AG]), were collected at baseline and during follow-up. Abstinence was established by diaries confirmed by saliva caffeine assays.
Results
Abstinence produced significant decreases in HbA1c and increases in 1,5-AG, both indicating improvements in chronic glucose control. Fasting glucose and insulin did not change, nor were changes in body weight observed.
Conclusions
Although preliminary, these results suggest that caffeine abstinence may be beneficial for patients with type 2 diabetes. This hypothesis should be confirmed in larger controlled clinical trials.
Introduction
A growing body of research finds that acute administration of caffeine produces a transient decrease in sensitivity to insulin that can impair glucose tolerance.1 Studies of type 2 diabetic coffee drinkers2–4 have found that caffeine exaggerates glucose and insulin responses to a carbohydrate challenge and increases average daytime glucose and postprandial responses to meals in the natural environment.5 This pilot study explored the possibility that caffeine abstinence would lead to beneficial improvements in chronic glucose control for habitual coffee drinkers who had type 2 diabetes.
Materials and Methods
The pilot study employed an open-label, single-arm, pre–post design to test the chronic effects of caffeine abstinence on chronic glucose control over a 3-month follow-up. Twelve adult (six males) habitual coffee drinkers (≥2 cups of coffee daily) who had an established history of type 2 diabetes treated by diet, exercise, and oral agents were enrolled. Following baseline measurements of glucose control, participants began a 3-month period of complete caffeine abstinence. Follow-up after 2 weeks and 1, 2, and 3 months assessed compliance with abstinence and changes in glucose control.
The primary outcome was change in chronic glucose control that was assessed by measurements of hemoglobin A1c (HbA1c), a well-accepted measure of average glucose concentration within the prior 3 to 4 months.6 HbA1c was measured at baseline and the 3-month follow-up visit. A short-term measure of average glucose concentration was provided by the GlycoMark assay of 1,5-anhydroglucitol (1,5-AG) that reflects postprandial hyperglycemia over the prior 1–2 weeks.7,8 1,5-AG was measured in blood at baseline and after 2 weeks and 3 months of abstinence. Fasting glucose and insulin were measured at baseline, 2 weeks, and 3 months to assess changes in these standard clinical measures of glucose control. HbA1c and GlycoMark assays were performed by a commercial laboratory (LabCorp, Burlington, NC). Plasma glucose was measured by machine (Beckman Glucose Analyzer II) and plasma insulin by double-antibody radioimmunoassay (Millipore Corporation) in the laboratory of Dr. Kuhn.
Baseline caffeine intake and compliance with abstinence were assessed by self-report with biochemical validation. At baseline and before each follow-up visit, participants completed a 7-day diary of all beverages and medications consumed. Participants also collected a saliva sample (Salivette; Sarstedt, Inc., Newton, NC) in the afternoon of each day, which provided biochemical validation of caffeine intake or abstinence on that day.9 Diary records were examined to determine initial caffeine intake and to insure against accidental caffeine consumption during abstinence. Caffeine assays were conducted on three of the seven saliva samples, following a bogus pipeline protocol. Body weight was measured at each visit to ensure that weight was maintained during the course of the study.
Results
Of 12 participants enrolled, 1 quit before baseline measurements, 2 moved away during the follow-up, and 2 could not be reached for follow-up visits per the protocol. Seven (five males) completed the protocol, abstaining from caffeine for 3 months. Continued abstinence was confirmed by diary reports and saliva caffeine assays. Repeated-measures analysis of outcome variables was conducted using mixed designs that included all available data (PROC MIXED, SAS ver. 9; SAS Institute, Cary, NC).
Despite the small number of participants, statistical analyses revealed significant improvements in glucose control after initiation of caffeine abstinence. The primary outcome, HbA1c, fell by −0.56% [F(1,6) = 6.57, p = 0.04] from baseline to the 3-month follow-up visit. In addition, measures of 1,5-AG rose by 5.5 µg/mL from baseline to 3 months [F(2,13) = 8.87, p = 0.004]. Changes for individual participants and the group are graphed in Figure 1 below.
FIG. 1.
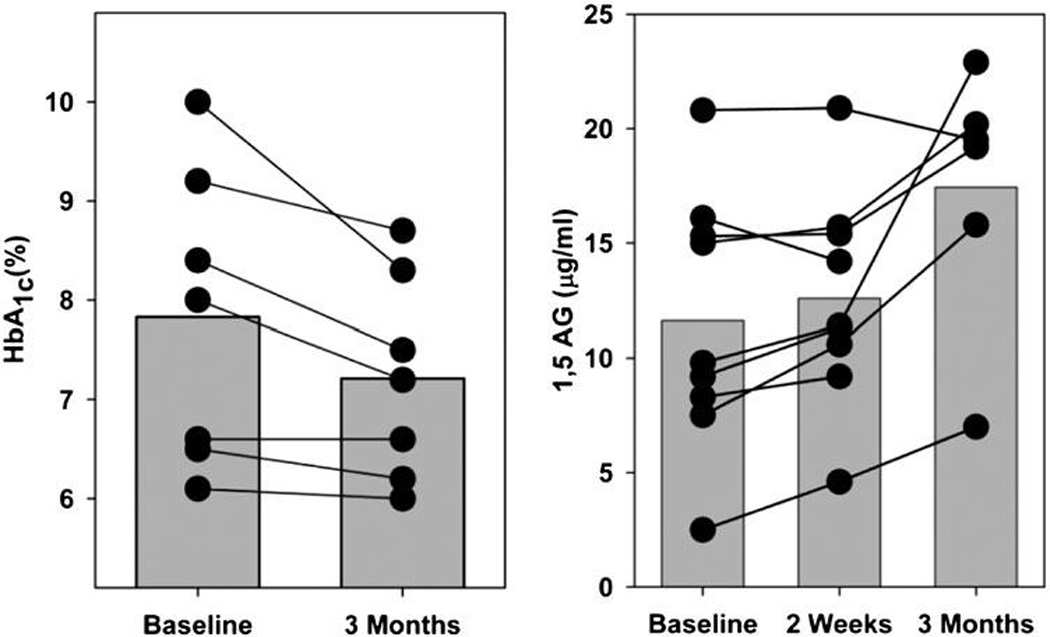
Effects of 3 months of caffeine abstinence on chronic glucose control in type 2 diabetic coffee drinkers. Bars indicate average effects on hemoglobin A1c (HbA1c; left) and 1,5-anhydroglucitol (1,5-AG; right). Lines indicate changes for individual participants.
In contrast, no changes were observed in fasting glucose or fasting insulin across the three time points, baseline, 2 weeks, and 3 months. For fasting glucose F(2,14) = 1.1, p = 0.36, and for fasting insulin F(2,14) = 1.67, p = 0.22. Analysis of body weight and waist circumference revealed no significant changes during abstinence.
Examination of Figure 1 suggests greater improvement in those who began the study with worse glucose control (HbA1c > 8%) and minimal change in those who began the study in relatively good control (HbA1c < 7%). The normal range for 1,5-AG is 10–30 µg/mL, and the diagnostic range is < 2–10 µg/mL. Values < 10 µg/mL indicate that average postprandial maximum glucose is in the hyperglycemic range of 185 mg/dL or higher. Most of the participants began the study in relatively good control by this measure, but all of those who had baseline levels below the 10 µg/mL cut-off showed improvements at 3 months.
Discussion
This was a small pilot study with limited experimental controls. However, the results indicate that habitual caffeine consumption increases chronic glucose levels, and more importantly that caffeine abstinence may lead to beneficial improvements in chronic glucose control in patients with type 2 diabetes who drink coffee daily. The magnitude of HbA1c reductions observed following caffeine abstinence indicates clinically significant improvement in chronic glucose control, especially for those who are not already demonstrating appropriate glucose control. These improvements compare favorably with the reductions in HbA1c obtained from use of oral antidiabetic agents, which range from 0.5% to 1.25%.10
Attrition in this study is a concern, although we have no evidence that any participant dropped out due to failure of compliance. Although attrition could indicate a problem with compliance with long-term caffeine abstinence as an intervention, it does not compromise the positive results obtained from those who maintained caffeine abstinence.
The appearance of treatment effects in HbA1c and 1,5-AG, but not in fasting glucose or insulin, suggests that caffeine abstinence likely exerted its beneficial effects through chronic reductions in postprandial hyperglycemia rather than by changes in fasting levels. This interpretation is consistent with the results of experimental studies that consistently show that the acute effect of caffeine is an exaggeration of glucose and insulin responses to a carbohydrate challenge.2–4
This pilot study did not include nontreatment groups to control for factors other than the caffeine abstinence intervention that could affect glucose. Larger controlled trials are needed to confirm these initial findings and establish the possible benefits of caffeine abstinence for those who have type 2 diabetes.
Conclusions
Despite its limitations, this study provides the first evidence that habitual caffeine consumption may have a detrimental impact on chronic glucose control in patients with type 2 diabetes. The magnitude of these adverse effects is seen in the reductions that follow when caffeine is removed. These results also add to a growing body of evidence that caffeine impairs glucose control in type 2 diabetes and undermines clinical efforts to manage the disease. Caffeine abstinence may prove to be a valuable addition to current recommendations for lifestyle management of type 2 diabetes if future trials support these findings.
Acknowledgments
This research was supported by a grant from the Office of the Director, NIDDK, 2R56DK067486-04A1 “Caffeine and Glucose Regulation.” The study was performed with the assistance of the Duke Clinical Research Unit, a component of the Duke Translational Medicine Institute funded by the NIH CTSA (NCRR, 5UL1RR024128-03).
Footnotes
Author Disclosure Statement
No competing financial interests exist for any of the authors.
References
- 1.Lane JD. Caffeine, glucose metabolism, and type 2 diabetes. J Caffeine Res. 2011;1:23–28. [Google Scholar]
- 2.Lane JD, Barkauskas CE, Surwit RS, Feinglos MN. Caffeine impairs glucose metabolism in type 2 diabetes. Diabetes Care. 2004;27:2047–2048. doi: 10.2337/diacare.27.8.2047. [DOI] [PubMed] [Google Scholar]
- 3.Lane JD, Hwang AL, Feinglos MN, Surwit RS. Exaggeration of postprandial hyperglycemia in patients with type 2 diabetes by administration of caffeine in coffee. Endocr Pract. 2007;13:239–243. doi: 10.4158/EP.13.3.239. [DOI] [PubMed] [Google Scholar]
- 4.Robinson LE, Savani S, Battram DS, et al. Caffeine ingestion before an oral glucose tolerance test impairs blood glucose management in men with type 2 diabetes. J Nutr. 2004;134:2528–2533. doi: 10.1093/jn/134.10.2528. [DOI] [PubMed] [Google Scholar]
- 5.Lane JD, Feinglos MN, Surwit RS. Caffeine increases ambulatory glucose and postprandial responses in coffee drinkers with type 2 diabetes. Diabetes Care. 2008;31:221–222. doi: 10.2337/dc07-1112. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association. Standards of Medical Care in Diabetes—2011. Diabetes Care. 2011;34:S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dworacka M, Winiarska H, Szymanska M, et al. 1,5-anhydro-D-glucitol: a novel marker of glucose excursions. Int J Clin Pract Suppl. 2002;129:40–44. [PubMed] [Google Scholar]
- 8.Dungan KM, Buse JB, Largay J, et al. 1,5-anhydroglucitol and postprandial hyperglycemia as measured by continuous glucose monitoring system in moderately controlled patients with diabetes. Diabetes Care. 2006;29:1214–1219. doi: 10.2337/dc06-1910. [DOI] [PubMed] [Google Scholar]
- 9.James JE, Bruce MS, Lader MH, Scott NR. Self-report reliability and symptomatology of habitual caffeine consumption. Br J Clin Pharmacol. 1989;27:507–514. doi: 10.1111/j.1365-2125.1989.tb05400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sherifali D, Nerenberg K, Pullenayegum E, Cheng JE, Gerstein HC. The effect of oral antidiabetic agents on A1c levels: a systematic review and meta-analysis. Diabetes Care. 2010;33:1859–1864. doi: 10.2337/dc09-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]


