Abstract
We studied three forms of dyadic communication involving theory of mind (ToM) in 82 children with traumatic brain injury (TBI) and 61 children with orthopedic injury (OI): Cognitive (concerned with false belief), Affective (concerned with expressing socially deceptive facial expressions), and Conative (concerned with influencing another’s thoughts or feelings). We analyzed the pattern of brain lesions in the TBI group and conducted voxel-based morphometry for all participants in five large-scale functional brain networks, and related lesion and volumetric data to ToM outcomes. Children with TBI exhibited difficulty with Cognitive, Affective, and Conative ToM. The perturbation threshold for Cognitive ToM is higher than that for Affective and Conative ToM, in that Severe TBI disturbs Cognitive ToM but even Mild-Moderate TBI disrupt Affective and Conative ToM. Childhood TBI was associated with damage to all five large-scale brain networks. Lesions in the Mirror Neuron Empathy network predicted lower Conative ToM involving ironic criticism and empathic praise. Conative ToM was significantly and positively related to the package of Default Mode, Central Executive, and Mirror Neuron Empathy networks and, more specifically, to two hubs of the Default Mode network, the posterior cingulate/retrosplenial cortex and the hippocampal formation, including entorhinal cortex and parahippocampal cortex.
1 Introduction
Humans are social animals whose cognitive activity often occurs within an interpersonal context. While social competence continues to improve into the teen years, most typically developing children, by the time they attend school, have mastered a palette of social cognitive skills that allow them to act as partners in social dyads and groups in which social-cognitive-affective information flows back and forth.
A component of social cognition is Theory of Mind (ToM), which involves mentalizing (Frith and Frith, 2003), the ability to think about mental states in oneself and others and to use this information to understand what other people know and predict how they will act. The term ToM emphasizes that individuals see themselves and others in terms of mental states that result in (and from) human action (Wellman et al., 2001). In the paradigmatic false belief ToM task (Wimmer and Perner, 1983), people entertain beliefs that contradict reality and act in accordance with their beliefs. For example, Child A exhibits ToM if he or she judges that Child B will search for a candy in a red cup because Child B believes it to be hidden there, even if Child A knows that it is actually hidden in a blue cup.
As interest in ToM grew, the term was applied to an ever broadening range of constructs, from inferences about other’s judgments regarding object locations to self-reports of lending money to others as a measure of empathy. In part as a result of new behavioral and neuroimaging evidence (Shamay-Tsoory and Aharon-Peretz, 2007; Hein and Singer, 2008), ToM was partitioned into cognitive ToM, concerned with cognitive beliefs and reading the content of people’s minds, and affective ToM, concerned with emotional states and functions involving affective influence, such as empathy.
While a separation of cognition from emotion is useful, we also distinguish between expressing what feels or wishes to appear to feel (affective ToM) and exerting influence on what someone else feels (conative ToM). Pragmatic linguistics of the Prague school identified a conative communicative function concerned with exerting social influence (Jakobson, 1960) to make others feel good or bad about themselves.
1.1 A Model of ToM
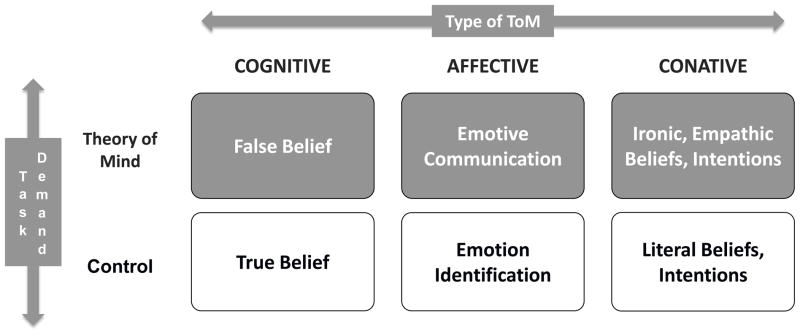
We propose a tripartite model of ToM (Fig. 1) that distinguishes three types of ToM: cognitive, affective, and conative.
Figure 1.
Tripartite Theory of Mind (ToM) Model.
Cognitive ToM. This is the original mindreading sense of ToM, concerned with false belief.
Affective ToM. Facial emotion expresses feelings (emotional expression), but also what we want people to think we feel (emotive communication, in which the expression on the face is consciously pantomimed or even deceptive). Emotive communication is a form of affective ToM (Hein and Singer, 2008).
Conative ToM. This refers to forms of social communication in which one person tries to influence the mental and emotional state of another. Ironic criticism and empathic praise are prototypical forms of conative ToM.
For each type of ToM, a key task requires ToM and a control task involves the same task demands but without the ToM construct of interest. For example, Cognitive ToM is measured by false belief and the control condition of the same form involves true belief. Table 1 shows the link of ToM type to specific ToM and Control tasks.
Table 1.
Three types of theory of mind: Cognitive, affective, and conative.
| Type of ToM | Basis of ToM | ToM Task | Control Task |
|---|---|---|---|
| Cognitive ToM | False belief | What A thinks B thinks based on B’s false belief | What A thinks B thinks based on B’s true belief |
| Affective ToM | Facial deception | What A wants B to think A feels | What A feels |
| Conative ToM | Referentially opaque beliefs and intentions | What A wants B to think about B’s actions and self where what is said is not literally what is meant | What A wants B to think about B’s actions and self where what is said is literally what is meant |
1.2 Neurological Bases of ToM
The brain is organized in terms of a number of large-scale functional networks involving distributed brain regions (e.g., Menon, 2011), and any complex cognitive activity such as social cognition must involve large-scale functional brain networks. Neuroimaging evidence has implicated several brain regions in a range of social cognitive tasks (e.g., Lieberman, 2007). In approaching the neural basis of social cognition, we consider the effects of damage to and post-injury volumes of the three main large-scale brain networks (the Default Mode Network, DMN; the Central Executive Network, CEN; and the Salience Network, SN) and two social cognitive networks identified from lesion deficit and neuroimaging studies (a Mentalizing Network, MN, and a Mirror Neuron/Empathy Network, MNEN). Table 2 defines and describes the function of and neural structures within each network.
Table 2.
Neural networks.
| Neural Network | Description |
|---|---|
| Default mode network (DMN) |
|
| Central executive network (CEN) |
|
| Salience network (SN) |
|
| Mentalizing network (MN) |
|
| Mirror Neuron Empathy Network (MNEN) |
|
1.3 ToM in Children with Traumatic Brain Injury
ToM has been studied in typically developing preschoolers, in adults with behavioural and neurological pathologies (Bibby & McDonald, 2005), and in children with neurodevelopmenal and acquired brain disorders. ToM, broadly construed, is impaired in various developmental disorders that are associated with poor social function, including autism (Hill & Frith, 2003), ADHD (Uekermann et al., 2010), spina bifida (Dennis & Barnes, 1993), and fetal alcohol spectrum disorder (Greenbaum, Stevens, Nash, Koren, & Rovet, 2009). Poor ToM has also been reported in acquired brain disorders of childhood, including traumatic brain injury (TBI). Children with TBI display impairments in social-affective functions, including pragmatic language, the understanding of mental state language, the production of speech acts, the understanding of the social function of emotional expressions, the comprehension of the intentions involved in exerting social influence through sarcastic or empathic communications, and the ability to produce coherent social discourse (Chapman et al., 2004; Dennis, Wilkinson, & Humphreys, 1998; Dennis & Barnes, 2000, 2001; Dennis, Purvis, Barnes, Wilkinson, & Winner, 2001; Yeates et al., 2007).
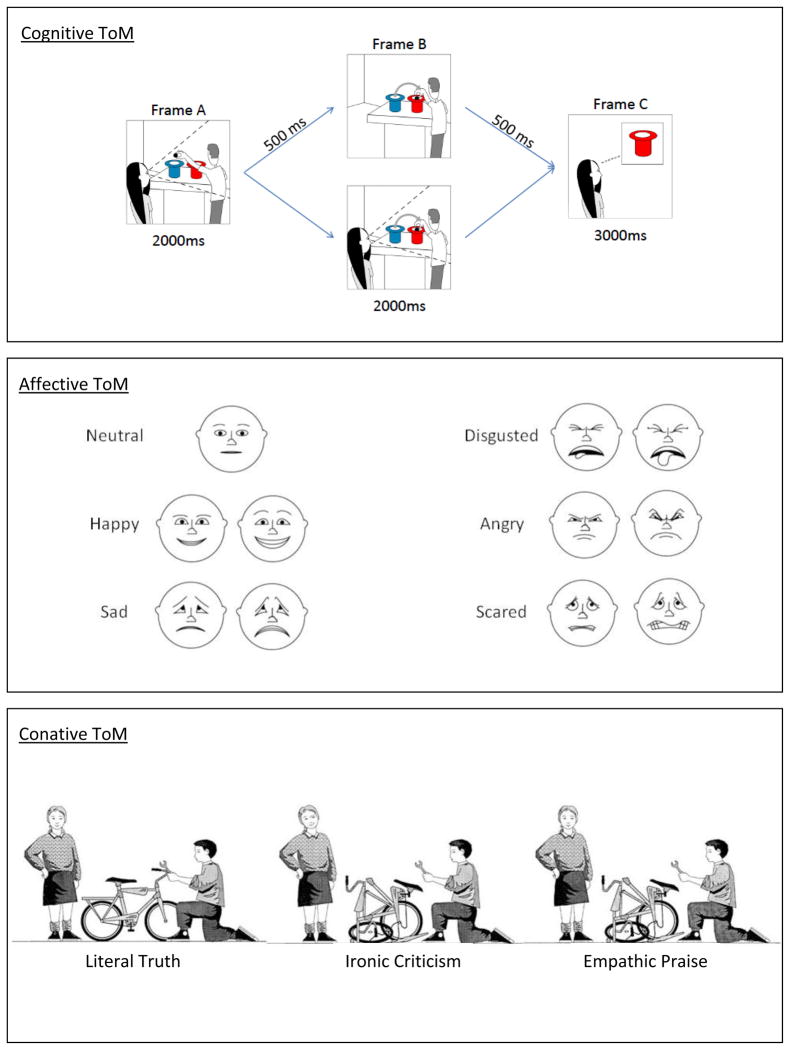
Recently, we have studied social cognition in children with traumatic brain injury (TBI) using the three tasks in Figure 1 and Table 1 (Dennis et al., 2012; Dennis et al., in press.a; Dennis et al., in press.b). The tasks are illustrated in Figure 2.
Figure 2. Sample Stimuli and Descriptions of ToM Tasks.
Top Panel (Jack and Jill Task): Participants were shown three consecutive frames (stimulus duration indicated under each frame; interstimulus interval indicated on arrows) on a computer screen. Each frame included a character (Jack and/or Jill), two hats (red and blue), and a ball. In Frame A of each sequence, Jack is preparing to drop a ball into either a blue or red hat (here, blue) while Jill watches. In Frame B, Jack either moves the ball further into the blue hat (unswitched trials; not shown) or switches the ball to the red hat (switched trials). Jill is present in half of Frame B trials (witnessed trials) and absent in the other half (unwitnessed trials). In Frame C, participants decide whether Jill’s belief about the location of the ball is correct or incorrect. Jill’s judgment depends on what she believes about the ball’s location, not its actual location: She will choose the original (Frame A) hat if she did not witness the switch. The study consisted of 32 trials, 8 for each of four trial types: Unswitched-Witnessed, Unswitched-Unwitnessed, Switched-Witnessed, Switched-Unwitnessed. The ToM trials involve an unwitnessed switch of hat color; control trials are those in which the switch was witnessed.
Middle Panel (Emotional and Emotive Faces Task): Participants listen to 25 short narratives, five for each involving happiness, sadness, fear, disgust, and anger, involving a discrepancy between Terry’s “inside” feeling and her facial expression (e.g., “Terry woke up with a tummy ache. If her mom knew about the tummy ache she wouldn’t let Terry go out to play.”) Participants were asked how Terry felt inside (Emotional condition) and how she looked on her face (Emotive condition) by selecting a face from the display. The ToM condition is the Emotive Communication score (“Look on Face” questions), which measures the emotion on the face as a deceptive representation of what is felt inside. The control condition is the Emotional Expression score (“Feel Inside” questions), which measures the emotion on the face as a transparent read-out of the emotion experienced.
Bottom Panel (Ironic Criticism and Empathic Praise Task): Two social dyads are shown in six situations (fixing a bicycle is pictured), with simultaneous presentation of a picture, a narrative, and an audiotape of the speaker’s utterances recorded with neutral, ironic, or empathic intonation (totaling 18 trials). In all three bicycle scenarios, Sally tells John he has done a good job fixing the bicycle. In the Literal Truth scenario, this matches the actual job. In the Ironic Criticism scenario, Sally believes John has done a poor job and her intent is to convey a negative evaluation. In the Empathic Praise scenario, Sally believes John has done a poor job but her intent is to convey a positive, comforting evaluation. Participants were told the task goal (e.g., to repair a bicycle), shown the outcome (e.g., “the bicycle was…”), and informed about the speaker’s character (e.g., “she liked to chat and talk to people”; “she liked to bug and annoy people”; “she liked to cheer people up”) and what the speaker said to the hearer (e.g., “You did a great job”). Questions probed beliefs (what the speaker thought about the event, what the speaker thought about the hearer) and intentions (what the speaker wanted the hearer to think about the event, what the speaker wanted the hearer to think about him- or herself). The key measures are Literal Truth (control task), Ironic Criticism (conative ToM task, with an opaque relation between words and meaning, and a negative second-order intention towards the hearer), and Empathic Praise (conative ToM task, with an opaque relation between words and meaning, and a positive second-order intention towards the hearer). Scores for irony and empathy were combined for the purposes of the current paper.
Children with TBI, compared to peers with orthopedic injuries (OI), exhibited difficulty on all three ToM tasks (Dennis et al., 2012; Dennis et al., in press.a; Dennis et al., in press.b). Presently, we report on the behavioral and neural bases of these difficulties.
1.4 Study Objectives
This paper investigates aspects of the behavioral and neural basis of the tripartite ToM model in Figure 1 and Table 1, using children with TBI and OI controls who were tested on each form of ToM, described above. We have three specific aims:
-
To compare cognitive, affective, and conative ToM in children with TBI and OI controls. We predict:
a group main effect whereby children with TBI perform more poorly overall than those with OI;
a task demand main effect whereby measures requiring ToM (Switched Unwitnessed trials on the Cognitive ToM task, Emotive Communication items on the Affective ToM task, and Ironic Criticism and Empathic Praise items on the Conative ToM task) will be performed more poorly by all children than control items of the same format (Switched Witnessed trials on the Cognitive ToM task, Emotional Expression items on the Affective ToM task, and Literal Truth items on the Conative ToM task);
a main effect of ToM type whereby Cognitive ToM will be performed better than either Affective or Conative ToM because the latter require more effortful, conscious processing to override habitual modes of communication (letting the face express true feeling in the Affective ToM task and saying something different from what is meant in the Conative ToM task);
a group by ToM main effect whereby children with TBI will find ToM tasks disproportionately more difficult than will children with OI.
To report patterns of damage to components of five functional brain networks in children with TBI compared to OI controls. Given the vulnerability to TBI of frontal, temporal, and limbic areas because of their proximity to skull bones (Yeates et al., 2007), we expected that lesions in the five functional networks (which have frontal, temporal, and limbic hubs) will be common for children with TBI, particularly those with severe injuries. We also expected volumetric reductions in any or all of the networks.
-
To test specific hypotheses about the relation of ToM type in children with TBI and OI controls to damage to functional brain networks. We test the following specific predictions:
Cognitive ToM will be related to the MN; b) Affective ToM will be related to the SN; c) Conative ToM will be related to the MNEN network; and; d) Affective and Conative ToM require conscious manipulation of thought and feeling, so they will be related to the integrity of the CEN, whereas Cognitive ToM, which has a more automatic character (see, e.g., Senju, 2012), will not; and e) All three forms of ToM, involving reacting to outside stimuli as well as to self-reflection, will be related to the DMN.
2 Material and methods
2.1 Participants
Participants included children previously hospitalized for either a TBI or OI who were 8 to 13 years of age and who were injured between 6 and 48 months before testing. All children were injured after 3 years of age, most after 4 years of age.
For both TBI and OI groups, we applied the following exclusion criteria: (a) history of more than one serious injury requiring medical treatment; (b) premorbid neurological disorder or mental retardation; (c) any injury resulting from child abuse or assault; (d) a history of severe psychiatric disorder requiring hospitalization prior to the injury; (e) sensory or motor impairment that prevented valid administration of study measures; (f) primary language other than English; and (g) medical contraindication to MRI or behavioral study. Children in full-time special education classrooms were excluded (in all but one case), although those with a history of premorbid learning or attention problems were not excluded.
Recruitment occurred in three metropolitan sites: Toronto (Canada), Columbus (US) and Cleveland (US). Among children eligible to participate and approached about the study, 82 (47%) of those with TBI and 61 (26%) of those with OI agreed to enroll. The participation rate was significantly higher for TBI than OI participants. However, participants and non-participants in both groups did not differ in age at injury, age at initial contact about the study, sex, race, or census tract measures of socioeconomic status (SES; i.e., mean family income, percentage of minority heads of household, and percentage of households below the poverty line). Participants and non-participants also did not differ on measures of injury severity (i.e., mean length of stay, median Glasgow Coma Scale (GCS; Teasdale and Jennett, 1974) score for children with TBI).
The human data included in this manuscript were obtained in compliance with formal ethics review committees at the participating institutions in Columbus, Toronto, and Cleveland. Parent consent and child assent was obtained prior to testing. The TBI group had a lowest GCS score of 12 or less after resuscitation, or 13–15 score with positive imaging for brain insult or depressed skull fracture. The children with TBI were grouped by injury severity: GCS scores 9–15 defined a Complicated Mild/Moderate TBI group (n=57) and GCS scores 3–8 defined a Severe TBI group (n= 25). The OI group (n= 61) consisted of children who sustained fractures that involved hospital admission but that were not associated with any loss of consciousness or other risks or indications of brain injury (e.g., skull or facial fractures).
Table 3 shows participant demographics, including sex, race, socioeconomic status (Yeates and Taylor, 1997), estimated full-scale IQ obtained using the Wechsler Abbreviated Scale of Intelligence (Stano, 1999), age at injury, age at time of test, and time since injury, mechanism of injury, and day-of-injury CT information. The socioeconomic composite index (SCI, Yeates and Taylor, 1997) was significantly higher for OI than for either TBI group, with the Severe TBI having the lowest mean SCI. The groups also differed in the distribution of mechanism of injury, with injuries arising from motorized vehicles being most common among Severe TBI and those arising from sports and recreational events being most common among OI participants. Because group differences in SCI were no longer significant when injury mechanism was taken into account, we did not treat SCI as a covariate in data analyses, because the SCI differences appeared to be intrinsic to the injury groups. When a covariate is an attribute of a disorder, or is intrinsic to the condition, it is not meaningful and can be potentially misleading to “adjust” for differences in the covariate (Dennis et al., 2009). Our findings are consistent with epidemiological studies showing that the risk of TBI, particularly those linked to motorized vehicles, is highest for children of lower SCI and minority status (Brown, 2010; Howard et al., 2005; Langlois et al., 2005; McKinlay et al., 2010; Parslow et al., 2005; Yates et al., 2006).
Table 3.
Demographic characteristics of entire sample.
| Characteristic | OI (n=61) | TBI-Mild/Moderate (n=57) | TBI-Severe (n=25) | F (χ2) | |||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | ||
| Age At Injury (years) | 7.8 | 1.8 | 8.0 | 1.9 | 7.5 | 2.1 | 0.611 |
| Age At Testing (years) | 10.6 | 1.7 | 10.5 | 1.5 | 9.9 | 1.5 | 1.752 |
| Time from Injury To Testing (years) | 2.8 | 1.0 | 2.6 | 1.2 | 2.5 | 1.2 | 1.162 |
| SES | 0.30 | 1.01 | −0.14 | 1.00 | −0.41 | 0.77 | 5.779*** |
| WASI IQ | 109.9 | 13.4 | 99.8 | 14.6 | 97.7 | 14.3 | 10.306*** |
| Glasgow Coma Scale | 15 | 0 | 13.8 | 2.0 | 4.0 | 1.7 | - |
| Sex (female; male) | 24 female, 37 male | 19 female, 38 male | 9 female, 16 male | (0.462) | |||
| Ethnicity | 54 Caucasian, 3 African/Caribbean descent, 3 Multiracial, 1 Unspecified | 45 Caucasian, 6 African/Caribbean descent, 4 Multiracial, 2 Unspecified | 19 Caucasian, 3 African/Caribbean descent, 0 Multiracial, 3 Unspecified | - | |||
p<0.01.
p<0.05
2.2 Behavioral Measures
Sample stimuli for the three types of ToM are illustrated in Figure 2 and task details are described in the figure caption. The Jack and Jill task (Dennis et al., 2012) measures cognitive ToM with a series of switched unwitnessed trials that measure false belief, compared to a series of control (switched witnessed) trials that measure true belief. The Emotional and Emotive Faces task (Dennis, et al., in press.a) measures affective ToM with trials requiring identification of emotions expressed for social purposes (Emotive communication) compared to control trials requiring identification of emotions actually felt (Emotional expression). The Ironic Criticism and Empathic praise task (Dennis et al., 2001; Dennis, et al., in press.b) measures conative ToM with trials requiring the child to identify the beliefs and conative intentions underlying referentially opaque communications involving irony and empathy, compared to control trials probing for beliefs and intentions in literally true statements.
2.3 MRI Brain Imaging
A research-quality MRI was obtained for each child (details in Bigler et al., submitted). Magnetic field strength was 1.5 Tesla for all studies. The Toronto and Columbus sites used GE Signa Excite scanners and the Cleveland site used a Siemens Symphony scanner. All sites acquired the following sequences on each participant: thin slice, volume acquisition T1-weighted ultrafast 3D Gradient Echo, commonly referred to as MPRAGE or FSPGR (depending on scanner manufacturer); a dual-echo proton density (PD)/T2 weighted sequence; fluid attenuated inversion recovery (FLAIR); and gradient recalled echo (GRE). For the current study, MRI images were reviewed by author EDB for evidence of lesions within the large-scale networks of interest. Lesions were defined as areas of old cortical contusion and/or focal region of encephalomalacia (based primarily on the T1, PD/T2 and/or GRE sequences), hemosiderin deposit reflecting prior hemorrhagic lesions, (identified on the PD/T2 and GRE sequences), or prominent focal white matter hyperintensity (based upon the PD/T2 and FLAIR sequences). Volumetric data were obtained using Freesurfer version 5.1 (http://surfer.nmr.mgh.harvard.edu/) following methods previously outlined (see Bigler et al., 2010). Total volumes for each of the five networks were computed using regions of interest (ROI) defined in Freesurfer variables (see Desikan et al., 2006) (Table 4).
Table 4.
Neural networks and associated Freesurfer regions.
| Neural Network | Fresurfer regions |
|---|---|
| Default Mode Network (DMN) | |
| Ventromedial prefrontal cortex (vmPFC) | Frontal pole + medial orbito-frontal cortex |
| Posterior cingulate cortex (PCC) | Posterior cingulate + cingulate isthmus |
| Inferior parietal lobule (IPL) | Inferior parietal lobe + precuneus |
| Hippocampal formation (HF) | Hippocampus + entorhinal cortex + parahippocampal cortex |
| Central Executive Network (CEN) | |
| Dorsolateral prefrontal cortex (dlPFC) | Superior frontal cortex + caudal middle frontal cortex + rostral middle frontal cortex |
| Posterior parietal cortex (PPC) | Inferior parietal cortex + superior parietal cortex + precuneus |
| Caudate nucleus (CN) | Caudate volume |
| Thalamus (TH) | Thalamus volume |
| Salience Network (SN) | |
| Ventrolateral prefrontal cortex (vlPFC) | Pars orbitalis |
| Insula (I) | Insula short volume + insula large central volume |
| Anterior cingulate cortex (ACC) | Rostral anterior cingulate + caudal anterior cingulate |
| Amygdala (A) | Amygdala volume |
| Mentalizing Network (MN) | |
| Dorsomedial prefrontal cortex (dmPFC) | Caudal middle frontal cortex + rostral middle frontal cortex |
| Superior temporal sulcus (STS) | Superior temporal gyrus + bank superior temporal sulcus + middle temporal gyrus |
| Temporo-parietal junction (TPJ) | Supramarginal gyrus |
| Temporal pole (TP) | Temporal pole |
| Mirror Neuron Empathy Network (MNEN) | |
| Premotor area (PMA) | Caudal middle frontal |
| Inferior parietal lobule (IPL) | Inferior parietal lobe + precuneus |
| Inferior frontal gyrus, pars opercularis (IFG, po) | Pars opercularis |
| Inferior frontal gyrus, pars triangularis (IFG, pt) | Pars triangularis |
2.4 Data Analysis
2.4.1 Behavior
All data were converted to percentage of correct responses and entered into a repeated measures analysis of variance (ANOVA) with group membership (OI vs. Mild/Moderate TBI vs. Severe TBI) as a between-subjects factor, and Type of ToM (Cognitive vs. Affective vs. Conative) and Task Demand (ToM vs. Control) as within-subjects factors.
2.4.2 MRI
Chi-square analyses were used to compare the presence or absence of network lesions between each TBI group and the OI group, and to compare the two TBI groups to each other. To study the relationship between behavioral and MRI lesion results for the TBI participants, the five networks were entered into simple linear regressions by hypotheses, for the key ToM constructs (Switched Unwitnessed, Emotive, and Ironic Criticism + Empathic Praise), with lesions within each network coded as absent (0), unilateral (1), or bilateral (2). Volumetric data for the entire sample were analyzed using multivariate analyses of variance (MANOVA) with Group membership as the between-subjects factor and network (DMN vs. CEN vs. SN vs. MN vs. MNEN) and region of interest (entered into separate MANOVAs as in Table 4) as the within-subjects factors. Regression analyses were conducted to examine the relationship of the 5 network volumes to the key ToM constructs by hypotheses.
3 Results
3.1 Behavior
120 of the 143 participants enrolled in the study (52 OI, 48 Mild/Moderate TBI, and 20 Severe TBI) completed all three behavioral ToM tasks, so only their data were used for the repeated measures ANOVA.
The main effect of Group was significant, F(2,117)=9.573, p<.001, with the OI group performing marginally better than Mild/Moderate TBI group (p=.070) and significantly better than the Severe TBI group (p<.001); accuracy across tasks was 76% versus 71% versus 62%, respectively. The difference between the two TBI groups was significant (p=.004). The main effect of Type of ToM also was significant, F(2,116)=6.199, p=.003, with marginally higher accuracy on the Cognitive than Affective ToM (p=.094) and significantly lower accuracy on the Affective than Conative ToM (p=.001) (70% vs. 67% vs. 72%, respectively); Cognitive and Conative ToM did not differ (p=.496). The main effect of Task Demand was significant, F(1,117)=247.73, p<.001, such that accuracy was higher on control than ToM communications (81% versus 59%).
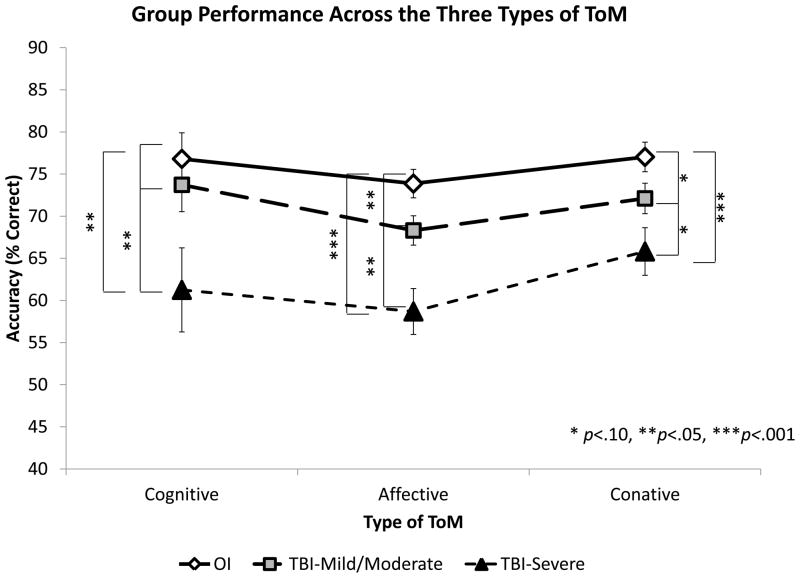
The interaction between Group and Type of ToM (Figure 3) was not significant, F(4,232)=.417, p=.796.
Figure 3. Accuracy by group over three types of ToM.
Mild/Moderate TBI did not differ from OI on Cognitive ToM (p=.490) but did differ on Affective (p=.024) and Conative ToM (p=.053). Severe TBI differed on all three types of ToM (p=.009, <.001, .001, respectively).
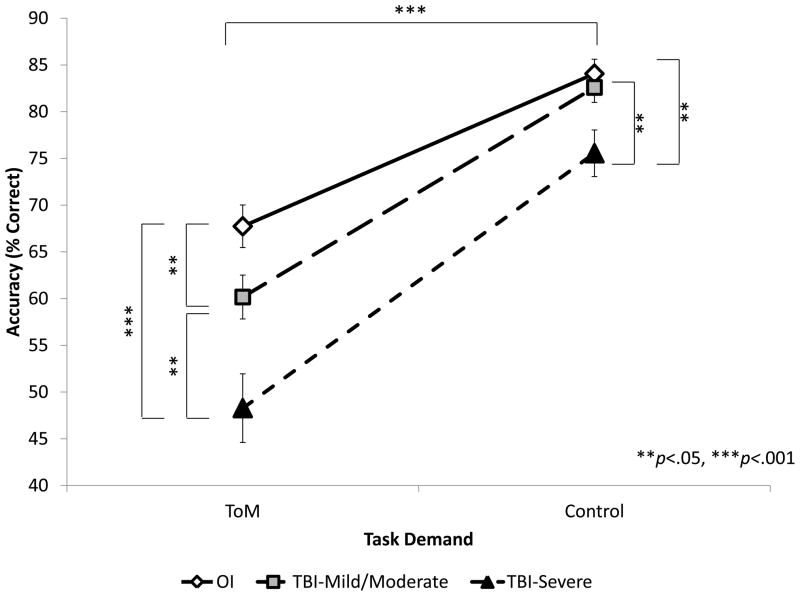
The interaction between Group and Task Demand, illustrated in Figure 4, was significant, F(2,117)=5.268, p=.006. Accuracy was higher on Control versus ToM condition for all three groups but the two TBI groups had different performance profiles from the OI group: on the Control condition, the Mild/Moderate TBI group did not differ from the OI group (p=.597), whereas the Severe TBI group was significantly poorer than the OI group (p=.004). On the ToM condition, both TBI groups were significantly poorer than the OI group (p<.01).
Figure 4. Accuracy by group on ToM versus Control tasks.
Performance was significantly better on Control than on ToM conditions within each of the three groups (p<.001). Within ToM conditions, the OI group was significantly more accurate than the Mild/Moderate (p=.022) and Severe (p<.001) TBI groups; the Mild/Moderate TBI group was significantly more accurate than the Severe TBI group (p=.007). Within Control conditions, the OI and Mild/Moderate TBI groups did not differ (p=.508), but the Severe TBI group was significantly less accurate than either OI (p=.004) or Mild/Moderate TBI (p=.019) group.
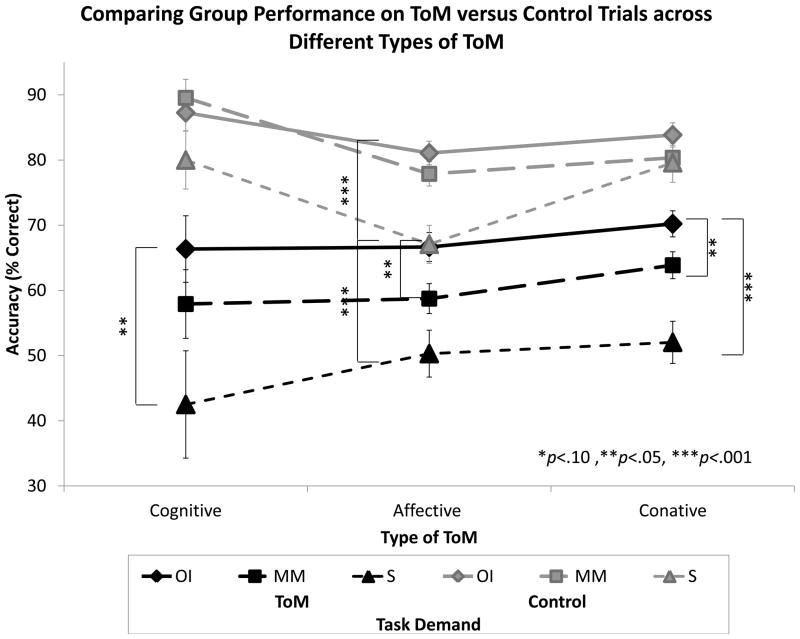
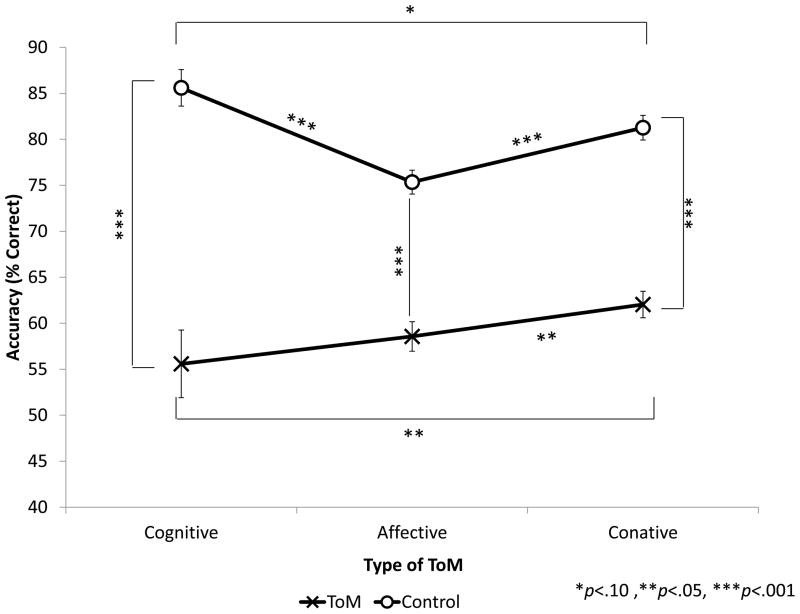
The Type of ToM × Task Demand interaction, illustrated in Figure 5, was significant, F(2,116)=4.671, p=.011. Accuracy was affected by type of ToM (Cognitive vs. Affective vs. Conative), but the way in which accuracy was affected was different in the Control versus ToM conditions. Comparing cognitive and affective results, on the ToM condition accuracy was similar (p=.398) on the Cognitive and Affective tasks, but on the Control condition accuracy was significantly higher on Cognitive than Affective (p<.001). Comparing cognitive and conative results, on the ToM condition accuracy was significantly higher on the Conative than on the Cognitive task (p=.05), but on the Control condition accuracy was marginally higher on Cognitive than on Conative (p=.055). Finally, comparing affective and conative results, accuracy was significantly higher on the Conative than on the Affective task on both the ToM (p=.049) and Control (p<.001) conditions.
Figure 5. Accuracy by Type of ToM and Task Demand (ToM versus Control).
Within all three types of ToM, performance was significantly better on Control than on ToM conditions (p<.001 for each domain). Within Control conditions, performance was significantly better on Cognitive than either Affective (p<.001) or Conative (p=.044) ToM, and better on the Conative than the Affective domain (p<.001). Within ToM conditions, performance was similar on Cognitive and Affective domains (p=.427), but marginally better for Conative than either Cognitive (p=.071) or Affective (p=.075) ToM.
The three-way interaction between Group, Type of ToM, and Task Demand (illustrated in Figure 6) was not significant, F(4,232)=1.947, p=.103, indicating that the Group × Task Demand interaction did not differ significantly across Type of ToM.
Figure 6. Performance Across the Three ToM Tasks Broken Down by Group and Task Demand (ToM versus Control).
Within the Cognitive task, Severe TBI was significantly worse than OI on ToM trials (p=.015) but not on Control trials (p=.168), whereas Mild/Moderate TBI did not differ on either ToM (p=.252) or Control (p=.565) trials. Within the Affective task, Severe TBI was significantly worse than OI on both ToM (p<.001) and Control trials (p<.001), whereas Mild/Moderate TBI was significantly worse than OI only on ToM (p=.015) but not Control trials (p=219). Within the Conative task, Severe TBI and Mild/Moderate TBI were significantly worse than OI on ToM trials (p<.001 and p=.003) but not on Control trials (p=.229 and p=.193).
3.2 MRI: Lesion
MRI scans were available for 111 participants (46 OI, 45 Mild/Moderate TBI, and 20 Severe TBI).
Results for the lesion sample are in Table 5. Relative to the Mild-Moderate TBI group, the Severe TBI group sustained significantly more frequent lesions to all 5 networks, and had more bilateral lesions.
Table 5.
Percentage of each group with lesions in the neural networks of interest.
| Neural Network | OI (n=61) | Mild-Moderate TBI (n=45) | Severe TBI (n=20) |
|---|---|---|---|
| DMN | -- | 18%, 2%†# | 30%, 20%‡# |
| CEN | -- | 24%, --†# | 40%, 10%‡# |
| SN | -- | 7%, -# | 30%, 5%‡# |
| MN | -- | 27%, 2%† | 50%, 5%‡ |
| MNEN | -- | 2%, --# | 15%, 5%†# |
Note: Significant group difference for the presence of any lesion, regardless of whether lesion was unilateral or bilateral, at p<.05:
OI vs. TBI-Mild/Moderate,
OI vs. TBI-Severe,
TBI-Mild/Moderate vs. TBI-Severe.
Of interest, 48% of the sample sustained lesions in the DMN.
3.3 Lesion and Behavior
The regression models (Table 6) were not significant for Cognitive (R2=.001, F(2,60) = 0.024, p = .976) or Affective (R2=.061, F(3,59) = 1.272, p = .292) ToM. The regression model for Conative ToM was significant (R2=.183, F(3,53) = 3.969, p = .013), with the only significant regressor being the MNEN network (p =.005), such that fewer MNEN lesions were related to better irony and empathy scores.
Table 6.
Regression models predicting Cognitive, Affective, and Conative ToM based on lesion data.
| ToM Domain | B | SE B | β | p-value |
|---|---|---|---|---|
| Cognitive | ||||
| DMN | 1.758 | 8.038 | .029 | .828 |
| MN | −.406 | 9.127 | −.006 | .965 |
|
| ||||
| Affective | ||||
| DMN | −.022 | 4.312 | −.001 | .996 |
| CEN | 9.413 | 5.218 | .253 | .076 |
| SN | −.708 | 7.108 | −.015 | .921 |
|
| ||||
| Conative | ||||
| DMN | −1.491 | 3.422 | −..055 | .665 |
| CEN | 7.080 | 4.153 | .213 | .094 |
| MNEN | −32.937 | 11.239 | −.374 | .005 |
Note: In Conative ToM, only lesions in the MNEN significantly predicted performance.
3.4 MRI: Volume
MRI scans for 106 participants (47 OI, 47 Mild/Moderate TBI, and 18 Severe TBI) were of sufficient quality for Freesurfer analyses to produce volumetric data. One OI was an extreme outlier in terms of volume and his data were excluded from subsequent analyses.
The three groups did not differ in total intracranial volume, F(2,99)=1.071, p=.347, nor were there any significant interactions between Group and Sex, F(2,99)=.995, p=.373. Therefore, total intracranial volume was not used as a covariate in subsequent analyses.
For the network analyses, the overall MANOVA was significant, F(5,99)=3.320, p=.008 as were the MANOVAs within in the individual networks. Group differences in network and region volume are presented in Table 7.
Table 7.
Group differences in volumes of neural networks and regions of interest.
| Network/Region | OI | TBI-Mild/Moderate | TBI-Severe | F | p-value |
|---|---|---|---|---|---|
| DMN | 134711 | 140238 | 126797‡# | 5.915 | 0.004 |
| vmPFC | 22887 | 23253 | 22384 | 0.651 | ns |
| PCC | 28469 | 29240 | 26510‡# | 4.665 | 0.012 |
| IPL | 61444 | 65304† | 58003# | 6.693 | 0.002 |
| HF | 21911 | 22442 | 19900‡# | 7.899 | 0.001 |
| CEN | 204155 | 208326 | 190645‡# | 4.693 | 0.011 |
| dlPFC | 71930 | 73443 | 67548# | 2.695 | 0.072 |
| PPC | 109967 | 113072 | 102313‡# | 5.254 | 0.007 |
| CN | 7766 | 7539 | 7491 | 0.630 | ns |
| TH | 14492 | 14272 | 13293‡# | 3.985 | 0.022 |
| SN | 63904 | 64305 | 59963‡# | 3.384 | 0.038 |
| vlPFC | 8364 | 8499 | 7986 | 1.900 | ns |
| I | 31950 | 31729 | 29885‡ | 2.361 | 0.099 |
| ACC | 20569 | 21037 | 19368# | 2.786 | 0.066 |
| A | 3021 | 3041 | 2724‡# | 4.895 | 0.009 |
| MN | 234098 | 237463 | 218811‡# | 4.153 | 0.018 |
| dmPFC | 93214 | 95411 | 88038# | 2.775 | 0.067 |
| STS | 91364 | 91760 | 83799‡# | 4.741 | 0.011 |
| TPJ | 42899 | 43668 | 40534 | 1.774 | ns |
| TP | 6620 | 6624 | 6441 | 0.356 | ns |
| MNEN | 156148 | 161272 | 149199# | 3.298 | 0.041 |
| PMA | 27154 | 27001 | 26071 | 0.430 | ns |
| IPL | 95933 | 101453† | 90896# | 6.386 | 0.002 |
| iFG, po | 17150 | 17001 | 16814 | 0.154 | ns |
| iFG, pt | 15912 | 15817 | 15418 | 0.357 | ns |
Note: Significant (p<.05) analyses comparing
OI vs. TBI-Mild/Moderate,
OI vs. TBI-Severe,
TBI-Mild/Moderate vs. TBI-Severe. TBI-Mild/Moderate has marginally larger DMN (p=.069) volumes than OI. TBI-Severe has marginally smaller I (p=.066) volumes than TBI-Mild-Moderate and marginally smaller dlPFC (p=.083), ACC (p=.087), and dmFPC (p=.096) volumes than OI.
3.5 Volume and Behavior
The regression models (Table 8) were not significant for Cognitive (R2=.010, F(2,101) = .520, p = .596) or Affective (R2=.054, F(3,97) = 1.855, p = .142), but were significant for Conative (R2=.098, F(3,91) = 3.294, p = .024) ToM.
Table 8.
Regression models predicting Cognitive, Affective, and Conative ToM based on volumetric data.
| ToM Domain | B | SE B | β | p-value |
|---|---|---|---|---|
| Cognitive | ||||
| DMN | 000 | .000 | −.080 | .631 |
| MN | .000 | .000 | .153 | .358 |
|
| ||||
| Affective | ||||
| DMN | .000 | .000 | .249 | .406 |
| CEN | .000 | .000 | .134 | .634 |
| SN | −.001 | .001 | −.187 | .359 |
|
| ||||
| Conative | ||||
| DMN | .000 | .000 | .108 | .755 |
| CEN | .000 | .000 | .439. | .171 |
| MNEN | .000 | .000 | −.262 | .377 |
Note: In Conative ToM, no individual predictor reached significance, although the overall model was significant. We explored the individual predictors further with correction for multiple comparisons.
We explored the relation between Conative ToM and specific regions of interest in the DMN, CEN, and MNEN, the package of which was significantly related to function. Of 12 regions, 8 were significantly related to Conative ToM, all in a positive manner such that greater volume was related to better performance. Correcting for multiple comparisons with p=.004, two significant structure-function relations emerged involving posterior cingulate/retrosplenial cortex (r=.317, p=.002) and hippocampal formation, including entorhinal cortex and parahippocampal cortex (r=.310, p=.002).
4 Discussion
Study results support the hypothesis that children with TBI have difficulty in Cognitive, Affective, and Conative ToM. Affective and Conative ToM have a lower threshold for perturbation than does Cognitive ToM, in that these types of ToM are vulnerable to even milder forms of TBI. Childhood TBI damaged both large scale brain networks and networks concerned with mentalizing and empathy. Lesions of the MNEN network disrupted Conative ToM. Conative ToM was also related to the overall status of DMN, CEN, and MNEN, and to two hubs of the DMN, in particular: the posterior cingulate/retrosplenial cortex, and the hippocampal formation, including entorhinal cortex and parahippocampal cortex.
4.1 Cognitive architecture of social cognition
The ToM effect was robust. All groups found control conditions easier than ToM conditions. For each type of ToM, the difference between ToM and control conditions was largest for Severe TBI, intermediate for Mild/Moderate TBI, and smallest for OI, showing that children with Severe TBI were more generally challenged by these tasks, whereas those with Mild/Moderate TBI had more specific difficulty with ToM requirements.
What is difficult about tasks requiring ToM? For one thing, ToM tasks like irony and empathy require referentially opacity, whereby what is said is not what is meant. An opaque context represents someone’s beliefs, not about a referent, but about a referent represented in a particular manner (Kamawar and Olson, 2011). Epistemic opacity (involving constructions using the verb know) predicts false belief in 3-to 7-year old children (Kamawar and Olson, 2009). Opacity, then, is a medium through which ToM is expressed, and children with TBI, even those with TBI of a milder character, have difficulty with opaque communications carrying ToM information, such as irony and empathy.
Cognition, affect, and conation have long been regarded as separable components of mental function, from German faculty psychology of the 18th century and Scottish and British association psychology of the 19th century to more recent trilogies of mind (Hilgard, 1980). Evidence from our sample of children with TBI supports the idea of at least partial separability of the three forms of ToM.
Long ago, MacLean (1949) noticed the dissociation of cognition and affect in brain-injured patients. The separability of Cognitive ToM from Affective and Conative ToM is supported by the fact that children with Severe TBI were impaired on all three forms of ToM, whereas children with Mild-Moderate TBI were impaired on Affective and Conative ToM. This data suggests that the threshold for perturbation for Affective and Conative ToM is lower than that for Cognitive ToM, a conclusion supported by recent animal research arguing for a stronger relation of TBI severity to cognitive outcome than to social-affective outcomes: In mice, all levels of TBI severity disrupt affective outcome, but only Severe TBI impairs cognitive outcome (Washington et al., 2012).
Despite variability in definitions of conativeness (Militello et al., 2006), the consensus is that it refers to a separable component of the mind (Hilgard, 1980). We found some support for the separability of Cognitive ToM from Affective and Conative ToM. Comparing performance on the Affective and Conative tasks, we found accuracy to be significantly higher on Conative than on Affective ToM.
4.2 Social cognitive challenges of children with TBI
Participants in a social dyad must develop an on-line representation of a dynamic and changing mental model of each other’s beliefs, expectations, emotions, and desires (Ybarra and Winkielman, 2012) and then update this model with a representation of their personal history of social-affective influence on (and from) that person. Many children with TBI may lack key ToM skills needed for dyadic participation.
Although children with severe TBI had more severe ToM deficits, even milder forms of TBI disrupted ToM tasks involving affect and conation. Assertions that milder forms of TBI have no lasting functional consequences need to be moderated in light of new evidence about the vulnerability of children with milder TBI to social cognitive impairment.
The ecological relevance of social cognitive deficits to social skills, social problem solving, and real world, real time social relationships remains to be fully established. It is known, for instance, that the number of close friends is correlated with individual differences in mentalizing skills (Stiller and Dunbar, 2007). Facial expressions provide an overt cue about others’ intentions; for example, anger and fear result in a “vigilant” style of scanning compared to non-threat facial expressions (e.g., sad, happy, and neutral) (Green et al., 2003). Detecting others’ intention to approach or avoid may shape social interactions (Adams et al., 2006). Sharing others’ emotional states enhances the synchrony of brain events across individuals, which may promote understanding of their intentions and actions (Nummenmaa et al., 2012).
Children with TBI who are insensitive to affective and conative information may face negative social consequences. In our TBI sample, children with severe TBI were rated higher in rejection-victimization than children with OI, and were less likely than children with OI to have a mutual friendship in their classroom; in addition, children with TBI without a mutual friend were rated lower than those with a mutual friend on sociability-popularity and prosocial behavior and higher on rejection-victimization, and had lower peer acceptance ratings (Yeates. et al., in press).
Childhood TBI, even of relatively mild degree, is associated with de novo changes in personality and behavior (Dennis et al., 2001a, 2001b; Levin et al., 2004, 2007; Max et al., 2005, 2006, 2011, 2012), as well as in inhibitory control (Leblanc et al., 2005; Sinopoli, et al., 2011; Sinopoli and Dennis, 2012). It remains to be understood how post-injury changes in personality and inhibitory control are related to social cognition and peer relationships.
Domain-general processes like inhibitory control and working memory develop in concert with ToM. In one view, poor inhibitory control reduces the child’s experience of the volitional nature of mental states and thereby limits the emergence of ToM (Russell, 1997); in an alternative view, the emergence of ToM precedes inhibitory control (Perner, 1998; Perner & Lang, 2000) because ToM provides a suitable platform for novel, misleading, and interfering schemes. In a study modeling the causal paths linking working memory, inhibitory control, and ToM in a smaller group of children with TBI (Dennis, Agostino, Roncadin, & Levin, 2009), we found that the relation between inhibition and ToM was fully mediated by working memory, although working memory did not mediate the relation between anatomical frontal lobe injury and ToM. How domain-general processes and ToM are causally related in the present TBI cohort is a topic of current investigation.
Irony and empathy are social skills that lubricate social discourse. Irony and empathy modulate social distance: Irony mutes criticism and establishes social distance, while empathy gives comfort and maintains connectedness. Irony and empathy express social rules, and allow the social modulation of emotional expression. Irony allows a social evaluation of praise, blame, and responsibility to be communicated without angry confrontation. Deficits in irony and empathy are likely to affect a child’s success in key life situations in the home, classroom, playground, and sports arena (McCauley et al., 2012).
Social deficits after TBI may have a negative effect on the development of more general cognitive skills. It is known that the ability to sustain a mismatch between experienced and communicated emotion not only provides social advantages, but also provides a cognitive advantage because it expands the boundaries of cognitive categories to include atypical exemplars when the environment becomes atypical (Huang and Galinsky, 2011).
4.3 Neural networks supporting social cognition
Lesions in the neural networks important for social cognition are common in this cohort of children with TBI. Significant volume differences assumed to reflect atrophic change (Bigler et al., 2010) were consistently observed in the Severe TBI group across all regions of interest. New information is that lesions occur both in the most common large-scale brain networks (DMN, CEN, SN) and in two networks implicated more specifically in the social functions of mentalizing and empathy. Also new is the finding that lesions in large-scale neural networks occur not only in children with severe TBI, but also in those with TBI of lesser severity. To be sure, TBI produces lesions in several brain networks with overlapping anatomy and physiology, and the meaning of patterns of TBI damage remains to be more fully understood.
Nearly one-quarter of the TBI sample sustained lesions in the DMN, a finding of interest in light of recent proposals that the DMN overlaps with brain regions involved in social cognition (Schilbach et al., 2008). In looking at brain volume-function correlations, we found that greater volumes in two hubs of the DMN, the posterior cingulate cortex and the hippocampal formation, predicted better Conative ToM. The mechanism of this relation remains to be established. It is possible that social cognitive impairments occur in part because of failure to deactivate the DMN in synchrony with activation of task-relevant networks, an issue to be explored further in functional imaging studies.
Lesions in the MNEN were significantly related to poorer Conative ToM. These data extend the range of neuroimaging data relating relating empathy to components of the MNEN (e.g., Gallese & Goldman, 1998; Rizzolatti & Sinigaglia, 2010; Schulte-Rüther, Markowitsch, Fink, & Piefke, 2007). However, Cognitive and Affective ToM were not significantly related to lesions or brain networks, although on fMRI studies, similar tasks have shown network activations. Structural lesion and volumetrics may be less related than fMRI measures to ToM outcomes; for example, while children with TBI show reduced white matter and problems in mental state attributions, the correlations between DTI and behavioral measures are similar in TBI and control groups (Levin et al., 2011). Long-term structural changes after childhood TBI (Wu et al., 2010), including alterations in the expected pattern of brain development (Wilde et al., 2012), may produce a functional reorganization of the social brain; for example, patterns of brain activation in a social cognition task change after adolescent TBI (Newsome et al., 2012). Functional imaging of ToM measures remains to be fully explored.
Some of the networks or network hubs damaged in children with TBI may be related to social functions we did not assess. Social network size correlates with amygdala volume and the volume of brain regions implicated in ToM (Bickart et al., 2011; Dunbar, 2012). Relative to adults, children have weaker intrinsic integration and segregation of amygdala circuits with subcortical, paralimbic, limbic, polymodal association and ventromedial prefrontal cortex (Qin et al., 2012). One entailment of this is that damage to the SN, including to the amygdala, may easily disrupt function in a weakly developed network. Lesions and volume reduction in the SN occur with some frequency in our children with TBI, and we found group differences in amygdala volume, which may have consequences for friends and social networks, not tested here.
Social challenges increase in adolescence. To support the increased number and complexity of social relationships, the adolescent brain undergoes considerable structural maturation (Gogtay et al., 2004), particularly in regions implicated in social cognition (Choudhury et al., 2006). Over development, a dynamic reconfiguration of both structural and functional connectivity occurs in the core networks, involving increased integrity along within-and between-network pathways (Sommer et al., 2010; Vogel et al., 2010; Uddin et al., 2011). In adolescence, efficiency of emotional ToM develops in parallel with brain maturation (Choudhury et al., 2006).
Our pre-adolescent participants will soon emerge into a challenging adolescence in which they will struggle to acquire a full complement of adolescent social cognitive skills. As adolescents, their social cognition will be doubly fractured, with dysfunctional childhood skills and a failure to develop adolescent skills. As pre-adolescents, they have damage to key regions of the social brain, which will not only limit social cognition, as we have shown, but also truncate or slow normal structural maturation of their adolescent brains. The TBI groups in the present cohort, if followed longitudinally, may experience considerable difficulty meeting the social demands of adolescence.
Highlights.
Children with TBI have difficulty with ToM in cognitive, affective, & conative ToM
Even mild-moderate TBI disrupts affective & conative ToM
TBI affects default mode, executive, salience, mentalizing & empathy brain networks
Acknowledgments
Preparation of this paper was supported by National Institute of Neurological Diseases and Stroke Grant 1 RO1 HD 04946,“Social Outcomes in Pediatric Traumatic Brain Injury.” We have no conflicts of interest to declare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams R, Ambady N, Macrae CN, Kleck R. Emotional expressions forecast approach-avoidance behavior. Motiv Emot. 2006;30(2):177–186. [Google Scholar]
- Adolphs R. What does the amygdala contribute to social cognition? Ann N Y Acad Sci. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibby H, McDonald S. Theory of mind after traumatic brain injury. Neuropsychologia. 2005;43(1):99–114. doi: 10.1016/j.neuropsychologia.2004.04.027. [DOI] [PubMed] [Google Scholar]
- Bickart KC, Wright CI, Dautoff RJ, Dickerson BC, Barrett LF. Amygdala volume and social network size in humans. Nat Neurosci. 2011;14(2):163–164. doi: 10.1038/nn.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler ED, Abildskov TJ, Petrie J, Dennis M, Simic N, Taylor HG, Yeates KO. Brain lesions in pediatric traumatic brain injury (TBI) have common features, but are also heterogeneous by group and variable within individuals: Implications for neuropsychological investigations. 2012 Manuscript submitted for publication. [Google Scholar]
- Bigler ED, Abildskov TJ, Wilde EA, McCauley SR, Li X, Merkley TL, et al. Diffuse damage in pediatric traumatic brain injury: a comparison of automated versus operator-controlled quantification methods. Neuroimage. 2010;50(3):1017–1026. doi: 10.1016/j.neuroimage.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Bonnelle V, Ham TE, Leech R, Kinnunen KM, Mehta MA, Greenwood RJ, et al. Salience network integrity predicts default mode network function after traumatic brain injury. Proc Natl Acad Sci U S A. 2012;109(12):4690–4695. doi: 10.1073/pnas.1113455109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RL. Epidemiology of injury and the impact of health disparities. Curr Opin Pediatr. 2010;22:321–325. doi: 10.1097/MOP.0b013e3283395f13. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Chapman SB, Sparks G, Levin HS, Dennis M, Roncadin C, Zhang L, Song J. Discourse macrolevel processing after severe pediatric traumatic brain injury. Developmental Neuropsychology. 2004;25:37–60. doi: 10.1080/87565641.2004.9651921. [DOI] [PubMed] [Google Scholar]
- Choudhury S, Blakemore SJ, Charman T. Social cognitive development during adolescence. Soc Cogn Affect Neurosci. 2006;1(3):165–174. doi: 10.1093/scan/nsl024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes CT, Loewen PJ, Schreiber D, Simmons AN, Flagan T, McElreath R, et al. Neural basis of egalitarian behavior. PNAS. 2012;109(17):6479–6483. doi: 10.1073/pnas.1118653109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84(6):3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Dennis M, Agostino A, Roncadin C, Levin H. Theory of mind depends on domain general executive functions of working memory and cognitive inhibition in children with traumatic brain injury. Journal of Clinical and Experimental Neuropsychology. 2009;31(7):835–847. doi: 10.1080/13803390802572419. [DOI] [PubMed] [Google Scholar]
- Dennis M, Barnes M. Oral discourse skills in children and adolescents after early-onset hydrocephalus: Linguistic ambiguity, figurative language, speech acts, and script-based inferences. Journal of Pediatric Psychology. 1993;18:639–652. doi: 10.1093/jpepsy/18.5.639. [DOI] [PubMed] [Google Scholar]
- Dennis M, Barnes MA. Speech acts after mild or severe childhood head injury. Aphasiology. 2000;14(4):391–405. [Google Scholar]
- Dennis M, Barnes MA. Comparison of literal, inferential, and intentional text comprehension in children with mild or severe closed head injury. Journal of Head Trauma Rehabilitation. 2001;16:1–14. doi: 10.1097/00001199-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Dennis M, Barnes MA, Wilkinson M, Humphreys RP. How children with head injury represent real and deceptive emotion in short narratives. Brain Lang. 1998;61:450–483. doi: 10.1006/brln.1997.1886. [DOI] [PubMed] [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. J Int Neuropsychol Soc. 2009;15:331–343. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Guger S, Roncadin C, Barnes M, Schachar R. Attentional-inhibitory control and social-behavioral regulation after childhood closed head injury. Do biological developmental and recovery variables predict outcome? J Int Neuropsychol Soc. 2001a;7:683–692. doi: 10.1017/s1355617701766040. [DOI] [PubMed] [Google Scholar]
- Dennis M, Guger S, Roncadin C, Barnes M, Schachar R. Attentional control and social discourse after childhood closed head injury: Are frontal contusions, age at injury and time since injury predictive? Brain Cogn. 2001b;48:197–200. [Google Scholar]
- Dennis M, Purvis K, Barnes MA, Wilkinson M, Winner E. Understanding of literal truth, ironic criticism, and deceptive praise after childhood head injury. Brain Lang. 2001;78:1–16. doi: 10.1006/brln.2000.2431. [DOI] [PubMed] [Google Scholar]
- Dennis M, Agostino A, Taylor HG, Bigler ED, Rubin K, Vannatta K, et al. Emotional expression and socially modulated emotive communication in children with traumatic brain injury. J Int Neuropsychol Soc. doi: 10.1017/S1355617712000884. in press.a. [DOI] [PubMed] [Google Scholar]
- Dennis M, Simic N, Agostino A, Taylor HG, Bigler ED, Rubin K, et al. Irony and empathy in children with traumatic brain injury. J Int Neuropsychol Soc. doi: 10.1017/S1355617712001440. in press.b. [DOI] [PubMed] [Google Scholar]
- Dennis M, Simic N, Taylor HG, Bigler ED, Rubin KR, Vannatta K, Yeates KO. Theory of mind in children with traumatic brain injury. J Int Neuropsychol Soc. 2012;18:1–9. doi: 10.1017/S1355617712000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Wilkinson M, Humphreys RP. How children with head injury represent real and deceptive emotion in short narratives. Brain and Language. 1998;61:450–483. doi: 10.1006/brln.1997.1886. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dunbar RI. The social brain meets neuroimaging. Trends Cogn Sci. 2012;16(2):101–102. doi: 10.1016/j.tics.2011.11.013. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B Biol Sci. 2003;358(1431):459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallese V, Goldman A. Mirror neurons and the simulation theory of mind-reading. Trends Cogn Sci. 1998;2(12):493–501. doi: 10.1016/s1364-6613(98)01262-5. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101(21):8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MJ, Williams LM, Davidson D. In the face of danger: Specific viewing strategies for facial expressions of threat? Cogn Emot. 2003;17:779–786. [Google Scholar]
- Greenbaum RL, Stevens SA, Nash K, Koren G, Rovet J. Social cognitive and emotion processing abilities of children with fetal alcohol spectrum disorders: A comparison with attention deficit hyperactivity disorder. Alcoholism, Clinical and Experimental Research. 2009;33(10):1656–1670. doi: 10.1111/j.1530-0277.2009.01003.x. [DOI] [PubMed] [Google Scholar]
- Gu X, Liu X, Van Dam NT, Hof PR, Fan J. Cognition-emotion integration in the anterior insular cortex. Cereb Cortex. 2012 doi: 10.1093/cercor/bhr367.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H. Anatomical differences in the mirror neuron system and social cognition network in autism. Cereb Cortex. 2006;16(9):1276–1282. doi: 10.1093/cercor/bhj069. [DOI] [PubMed] [Google Scholar]
- Hein G, Singer T. I feel how you feel but not always: the empathic brain and its modulation. Curr Opin Neurobio. 2008;18:153–158. doi: 10.1016/j.conb.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Hilgard ER. The trilogy of mind: cognition, affection, and conation. J Hist Behav Sci. 1980;16(2):107–117. doi: 10.1002/1520-6696(198004)16:2<107::aid-jhbs2300160202>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Hill EL, Frith U. Understanding autism: Insights from mind and brain. Philosophical Transactions: Biological Sciences. 2003;358:281–289. doi: 10.1098/rstb.2002.1209. 1430, Autism: Mind and Brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard I, Joseph JG, Natale JE. Pediatric traumatic brain injury: do racial/ethnic disparities exist in brain injury severity, mortality, or medical disposition? Ethn Dis. 2005;15:51–56. [PubMed] [Google Scholar]
- Huang L, Galinsky AD. Mind-body dissonance: conflict between the senses expands the mind’s horizon. Soc Psychol Personal Sci. 2011;2:351–359. [Google Scholar]
- Jakobson R. Linguistics and Poetics. In: Sebeok T, editor. Style in Language. Cambridge, MA: M.I.T. Press; 1960. pp. 350–377. [Google Scholar]
- Kalbe E, Schlegel M, Sack AT, Nowak DA, Dafotakis M, Bangard C, et al. Dissociating cognitive from affective theory of mind: a TMS study. Cortex. 2010;46(6):769–780. doi: 10.1016/j.cortex.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Kamawar D, Olson DR. Children’s understanding of referentially opaque contexts: The role of metarepresentational and metalinguistic ability. J Cogn Dev. 2009;10(4):285–305. [Google Scholar]
- Kamawar D, Olson DR. Thinking about representations: the case of opaque contexts. J Exp Child Psychol. 2011;108(4):734–746. doi: 10.1016/j.jecp.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42(2):998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Thomas KE. The incidence of traumatic brain injury among children in the United States: Differences by race. J Head Trauma Rehabil. 2005;20:229–238. doi: 10.1097/00001199-200505000-00006. [DOI] [PubMed] [Google Scholar]
- Leblanc N, Chen S, Swank P, Ewing-Cobbs L, Barnes M, Dennis M, Schachar R. Response inhibition after traumatic brain injury (TBI) in children: Impairment and recovery. Dev Neuropsychol. 2005;28:829–848. doi: 10.1207/s15326942dn2803_5. [DOI] [PubMed] [Google Scholar]
- Levin H, Hanten G, Max J, Li X, Swank P, Ewing-Cobbs L, Schachar R. Symptoms of attention-deficit/hyperactivity disorder following traumatic brain injury in children. JDBP. 2007;28(2):108–118. doi: 10.1097/01.DBP.0000267559.26576.cd. [DOI] [PubMed] [Google Scholar]
- Levin HS, Wilde EA, Hanten G, Li X, Chu ZD, Vásquez AC, et al. Mental state attributions and diffusion tensor imaging after traumatic brain injury in children. Developmental Neuropsychology. 2011;36(3):273–287. doi: 10.1080/87565641.2010.549885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin HS, Zhang L, Dennis M, Ewing-Cobbs L, Schachar R, Max J, Hunter JV. Psychosocial outcome of TBI in children with unilateral frontal lesions. J Int Neuropsychol Soc. 2004;10:305–316. doi: 10.1017/S1355617704102129. [DOI] [PubMed] [Google Scholar]
- Levin H, Hanten G, Max J, Li X, Swank P, Ewing-Cobbs L, Schachar R. Symptoms of attention-deficit/hyperactivity disorder following traumatic brain injury in children. JDBP. 2007;28(2):108–118. doi: 10.1097/01.DBP.0000267559.26576.cd. [DOI] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience: a review of core processes. Annu Rev Psychol. 2007;58:259–289. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- MacLean P. Psychosomatic disease and the visceral brain; recent developments bearing on the Papez theory of emotion. Psychosom Med. 1949;11(6):338–353. doi: 10.1097/00006842-194911000-00003. [DOI] [PubMed] [Google Scholar]
- Max JE, Levin HS, Landis J, Schachar R, Saunders AE, Ewing-Cobbs L, Dennis M. Predictors of personality change due to traumatic brain injury in children and adolescents in the first six months after injury. J Am Acad Child Adolesc Psychiatry. 2005;44:435–442. doi: 10.1097/01.chi.0000156280.66240.61. [DOI] [PubMed] [Google Scholar]
- Max JE, Levin HS, Schachar R, Landis J, Saunders AE, Ewing-Cobbs L, Dennis M. Predictors of personality change due to traumatic brain injury in children and adolescents 6 to 24 months after injury. J Neuropsychiatry Clin Neurosci. 2006;18:21–32. doi: 10.1176/jnp.18.1.21. [DOI] [PubMed] [Google Scholar]
- Max JE, Keatley E, Wilde EA, Bigler ED, Levin HS, Schachar RJ, et al. Anxiety disorders in children and adolescents in the first six months after traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2011;23(1):29–39. doi: 10.1176/jnp.23.1.jnp29. [DOI] [PubMed] [Google Scholar]
- Max JE, Keatley E, Wilde EA, Bigler ED, Schachar RJ, Saunders AE, Levin HS. Depression in children and adolescents in the first six months after traumatic brain injury. Int J Dev Neurosci. 2012;30:239–245. doi: 10.1016/j.ijdevneu.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley SR, Wilde EA, Anderson VA, Bedell G, Beers SR, Campbell TF, et al. Recommendations for the use of common outcome measures in pediatric traumatic brain injury research. J Neurotrauma. 2012;29(4):678–705. doi: 10.1089/neu.2011.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinlay A, Kyonka EGE, Grace RC, Horwood LJ, Fergusson DM, MacFarlane MR. An investigation of the pre-injury risk factors associated with children who experience traumatic brain injury. Inj Prev. 2010;16:31–35. doi: 10.1136/ip.2009.022483. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Militello LG, Gentner FC, Swindler SD, Beisner GI. Conation: Its Historical Roots and Implications for Future Research. Paper presented at the Collaborative Technologies and Systems, CTS 2006. International Symposium.2006. May 14–17, [Google Scholar]
- Molenberghs P, Cunnington R, Mattingley JB. Brain regions with mirror properties: a meta-analysis of 125 human fMRI studies. Neurosci Biobehav Rev. 2012;36(1):341–349. doi: 10.1016/j.neubiorev.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Newsome MR, Scheibel RS, Chu Z, Hunter JV, Li X, Wilde EA, et al. The relationship of resting cerebral blood flow and brain activation during a social cognition task in adolescents with chronic moderate to severe traumatic brain injury: A preliminary investigation. International Journal of Developmental Neuroscience. 2012;30(3):255–266. doi: 10.1016/j.ijdevneu.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa L, Glerean E, Viinikainen M, Jaaskelainen IP, Hari R, Sams M. Emotions promote social interaction by synchronizing brain activity across individuals. Proc Natl Acad Sci U S A. 2012;109(24):9599–9604. doi: 10.1073/pnas.1206095109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304(5669):452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- Parslow RC, Morris KP, Tasker RC, Forsyth RJ, Hawley CA. Epidemiology of traumatic brain injury in children receiving intensive care in the UK. Arch Dis Child. 2005;90(11):1182–1187. doi: 10.1136/adc.2005.072405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perner J. The meta-intentional nature of executive functions and theory of mind. In: Carruthers P, Boucher J, editors. Language and thought. Cambridge, UK: Cambridge University Press; 1998. pp. 270–283. [Google Scholar]
- Perner J, Lang B. Theory of mind and executive function: Is there a developmental relationship? In: Baron-Cohen S, Tager-Flusberg H, Cohen D, editors. Understanding other minds: Perspectives from autism and developmental cognitive neuroscience. Oxford, UK: Oxford University Press; 2000. pp. 150–181. [Google Scholar]
- Qin S, Young CB, Supekar K, Uddin LQ, Menon V. Immature integration and segregation of emotion-related brain circuitry in young children. Proc Natl Acad Sci U S A. 2012;109(20):7941–7946. doi: 10.1073/pnas.1120408109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameson LT, Lieberman MD. Empathy: A social cognitive neuroscience approach. Soc Personal Psychol Compass. 2009;3(1):94–110. [Google Scholar]
- Rameson LT, Morelli SA, Lieberman MD. The neural correlates of empathy: Experience, automaticity, and prosocial behavior. J Cogn Neurosci. 2012;24(1):235–245. doi: 10.1162/jocn_a_00130. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Sinigaglia C. The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nat Rev Neurosci. 2010;11(4):264–274. doi: 10.1038/nrn2805. [DOI] [PubMed] [Google Scholar]
- Russell J. How executive disorders can bring about an inadequate “theory of mind”. In: Russell J, editor. Autism as an executive disorder. Oxford, UK: Oxford University Press; 1997. pp. 256–299. [Google Scholar]
- Schilbach L, Eickhoff SB, Rotarska-Jagiela A, Fink GR, Vogeley K. Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Conscious Cog. 2008;17:457–467. doi: 10.1016/j.concog.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Schulte-Ruther M, Markowitsch HJ, Fink GR, Piefke M. Mirror neuron and theory of mind mechanisms involved in face-to-face interactions: a functional magnetic resonance imaging approach to empathy. J Cogn Neurosci. 2007;19(8):1354–1372. doi: 10.1162/jocn.2007.19.8.1354. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J. Dissociable prefrontal networks for cognitive and affective theory of mind: A lesion study. Neuropsychologia. 2007;45(13):3054–3067. doi: 10.1016/j.neuropsychologia.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Firth CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty JP, Stephan KE, Dolan RJ, Frith CD. Empathic neural responses are modulated by the perceived fairness of others. [10.1038/nature04271] Nature. 2006;439(7075):466–469. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinopoli KJ, Dennis M. Inhibitory control after traumatic brain injury in children. I J Dev Neurosci. 2012;30:207–215. doi: 10.1016/j.ijdevneu.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinopoli KJ, Schachar RS, Dennis M. Traumatic brain injury and secondary attention-deficit/hyperactivity disorder in children and adolescents: The effect of reward on inhibitory control. J Clin Exp Neuropsychol 2011b. 2011 doi: 10.1080/13803395.2011.562864.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer M, Meinhardt J, Eichenmuller K, Sodian B, Dohnel K, Hajak G. Modulation of the cortical false belief network during development. Brain Res. 2010;1354:123–131. doi: 10.1016/j.brainres.2010.07.057. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stano JF. Wechsler Abbreviated Scale of Intelligence. 3. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Stiller J, Dunbar RIM. Perspective-taking and memory capacity predict social network size. Soc Networks. 2007;29(1):93–104. [Google Scholar]
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Supekar KS, Ryali S, Menon V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J Neurosci. 2011;31(50):18578–18589. doi: 10.1523/JNEUROSCI.4465-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uekermann J, Kraemer M, Abdel-Hamid M, Schimmelmann BG, Hebebrand J, Daum I, Kis B. Social cognition in attention-deficit hyperactivity disorder (ADHD) Neuroscience and Biobehavioral Reviews. 2010;34(5):734–743. doi: 10.1016/j.neubiorev.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Vogel AC, Power JD, Petersen SE, Schlaggar BL. Development of the brain’s functional network architecture. Neuropsychol Rev. 2010;20(4):362–375. doi: 10.1007/s11065-010-9145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington PM, Forcelli PA, Wilkins T, Zapple DN, Parsadanian M, Burns MP. Effect of injury severity on behavior: A phenotypic study of cognitive and emotional deficits after mild, moderate, and severe controlled cortical impact injury in mice. Journal Of Neurotrauma. 2012;29:2283–2296. doi: 10.1089/neu.2012.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman HM, Cross D, Watson J. Meta-analysis of theory-of-mind development: The truth about false belief. Child Dev. 2001;72(3):655–684. doi: 10.1111/1467-8624.00304. [DOI] [PubMed] [Google Scholar]
- Wilde EA, Merkley TL, Bigler ED, Max JE, Schmidt AT, Ayoub KW, et al. Longitudinal changes in cortical thickness in children after traumatic brain injury and their relation to behavioral regulation and emotional control. International Journal of Developmental Neuroscience. 2012;30(3):267–276. doi: 10.1016/j.ijdevneu.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer H, Perner J. Beliefs about beliefs: Representation and constraining function of wrong beliefs in young children’s understanding of deception. Cognition. 1983;13(1):103–128. doi: 10.1016/0010-0277(83)90004-5. [DOI] [PubMed] [Google Scholar]
- Wu TC, Wilde EA, Bigler ED, Li X, Merkley TL, Yallampalli R, et al. Longitudinal changes in the corpus callosum following pediatric traumatic brain injury. Developmental Neuroscience. 2010;32(5–6):361–373. doi: 10.1159/000317058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates PJ, Williams WH, Harris A, Round A, Jenkins R. An epidemiological study of head injuries in a UK population attending an emergency department. J Neurol Neurosurg Psychiatry. 2006;77:699–701. doi: 10.1136/jnnp.2005.081901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ybarra O, Winkielman P. On-line social interactions and executive functions. Front Hum Neurosci. 2012;6(75):1–6. doi: 10.3389/fnhum.2012.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates KO, Gerhardt CA, Bigler ED, Dennis M, Rubin KR, Stancin T, Taylor HG, Vannatta K. Peer relationships of children with traumatic brain injury. Journal of the International Neuropsychological Society. doi: 10.1017/S1355617712001531. in press. [DOI] [PubMed] [Google Scholar]
- Yeates KO, Taylor HG. Predicting premorbid neuropsychological functioning following pediatric traumatic brain injury. J Clin Exp Neuropsychol. 1997;19:825–837. doi: 10.1080/01688639708403763. [DOI] [PubMed] [Google Scholar]
- Yeates K, Bigler E, Dennis M, Gerhardt C, Rubin K, Stancin T, Vannatta K. Social outcomes in childhood brain disorder: A heuristic integration of social neuroscience and developmental psychology. Psych Bull. 2007;133(3):535–556. doi: 10.1037/0033-2909.133.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaitchik D, Walker C, Miller S, LaViolette P, Feczko E, Dickerson BC. Mental state attribution and the temporoparietal junction: an fMRI study comparing belief, emotion, and perception. Neuropsychologia. 2010;48(9):2528–2536. doi: 10.1016/j.neuropsychologia.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]