Abstract
The hemolytic phospholipase C/sphingomyelinase PlcH from the opportunistic pathogen Pseudomonas aeruginosa represents the founding member of a growing family of virulence factors identified in a wide range of bacterial and fungal pathogens. In P. aeruginosa PlcH is co-expressed with a 17kDa chaperone (PlcR2) and secreted as a fully folded heterodimer (PlcHR2) of approximately 95 kDa, by the Twin Arginine Translocase (TAT) via the cytoplasmic membrane and through the outer membrane, by the Xcp (TypeII) secretory system. PlcHR2 has been shown to be an important virulence factor in model P. aeruginosa infections and is selectively cytotoxic, at picomolar concentrations to mammalian endothelial cells. Here we report how the various challenges starting from protein overexpression in the native organism P. aeruginosa, the use of detergents in the crystallization and data collection using the most advanced micro-focus synchrotron beam lines were overcome. Native diffraction data of this heterodimeric protein complex were collected up to a resolution of 4 Å, whereas needle-shaped crystals of L-selenomethionine substituted PlcHR2 with a maximum diameter of 10 micron were used to collect data sets with a maximum resolution of 2.75 Å.
Keywords: Pseudomonas aeruginosa, phospholipase C, sphingomyelinase, crystallization, L-selenomethionine substitution, micro-crystal
Introduction
Pseudomonas aeruginosa is an opportunistic bacterial pathogen that poses a lethal threat to patients with open injuries and burn wounds, immunocompromised patients and above all, cystic fibrosis sufferers (Donaldson and Boucher 2003; Holden, Feil et al. 2004; George, Jones et al. 2009). During infection the pathogen produces and secrets a variety of virulence factors including Exotoxin A, and at least four different phospholipases C (PLC) (Barker, Vasil et al. 2004; Vasil 2006) including the recently discovered P. aeruginosa phospholipases PlcA and PlcB that belong to the well-characterized family of Zn-dependent PLCs (Barker, Vasil et al. 2004). The biological functions of these PLCs however, are not well understood, although PlcB has been shown to be required in the chemotaxis of P. aeruginosa towards phospholipids (e.g. phosphatidylcholine a major component of lung surfactant) (Barker, Vasil et al. 2004; Miller, Tomaras et al. 2008). Two extracellular PLCs of P. aeruginosa were identified earlier (Vasil, Graham et al. 1991). These are the hemolytic phospholipase C (PlcH) and its closely related non-hemolytic ortholog PlcN, which are almost twice as large as PlcA and PlcB, respectively, and show no significant sequence similarity to the Zn-dependent class of PLCs (Stonehouse, Cota-Gomez et al. 2002; Vasil 2006). PlcH is co-expressed with two in-phase overlapping genes plcR1/plcR2 that are located downstream to the plcH gene. The 23KDa PlcR1 and the 17kDa PlcR2 are believed to act as co-chaperones in the secretion of PlcH by forming heterodimeric PlcHR1 and PlcHR2 complexes (Cota-Gomez, Vasil et al. 1997). These complexes are secreted into the extracellular environment in a folded state via the twin-arginine translocation (TAT) pathway through the inner membrane (Berks, Sargent et al. 2000; Voulhoux, Ball et al. 2001) and subsequently through the lipopolysaccharide containing outer membrane of P. aeruginosa via the Xcp (Type II) system (Vasil 2006).
More recently it has become clear that PlcH is the prototype of a growing superfamily of enzymes, which include a range of extracellular toxins with phospholipase C and sphingomyelinase activities. Consequently, PlcH does not merely represent a single extracellular virulence factor produced by only a single opportunistic pathogen. In fact, some fungal and numerous bacterial pathogens express one or more multiple orthologs. For example, Aspergillus fumigatas caries one PlcH homologue (Vasil, Stonehouse et al. 2009), individual Burkholderia pseudomallei possess three genes encoding PlcH orthologs (Korbsrisate, Tomaras et al. 2007) and some Mycobacterium tuberculosis strains express as many as 4 different orthologs (Viana-Niero, de Haas et al. 2004). The emerging opportunistic pathogen Acinetobacter baumannii, as well as the Select Agent Burkholderi mallei each carry two genes encoding members of PlcH superfamily (Antunes, Imperi et al. 2011). Finally, there is one member of this superfamily with a known crystal structure, AcpA from Franicsella tularensis, which, based on sequence comparison would be situated just at the evolutionary border between known PLC/sphingomyelinase members and those enzymes, which only have phosphatase activity (Felts, Reilly et al. 2006). AcpA, which shares approximately 22% sequence identity with the catalytic domain of PlcH is a periplasmic protein with acid phosphatase activity that can utilize an assortment of biologically important phosphorylated compounds (e.g. ATP and tyrosine-PO4) (Mohapatra, Balagopal et al. 2007).
The PlcHR2 complex secreted by P. aeruginosa has profound biological functions as summarized below. First of all, the enzyme has been shown to be highly active on sphingomyelin, as well as phosphatidylcholine, but it is much less active on other phospholipids that do not contain choline (e.g. phosphatidylethanolamine, phosphatidylglycerol), and it is not active on phosphatidylserine (Lopez, Collado et al. 2011). Accordingly PlcHR2 catalyzes the hydrolysis of sphingomyelin to phosphocholine and ceramide, as well as it hydrolyzes phosphatidylcholine to phosphocholine and diacylglycerol (DAG) (Luberto, Stonehouse et al. 2003). Ceramide and DAG are important eukaryotic secondary messenger molecules implicated in a wide array of functions ranging from inflammation to apoptosis (Ohanian and Ohanian 2001), but remarkably they exert very distinct effects. For example, generation of ceramide from sphingomyelin is pro-apoptotic while increased production of DAG induces a proliferative or transformation response in eukaryotic cells. It is important to note in this regard that this enzyme exhibits a highly selective cytotoxicity to human endothelial cells (Vasil, Stonehouse et al. 2009).
The three-dimensional structure of PlcHR2 will help to decipher the catalytic mechanism and hence inform about the molecular basis of enzymatic activity and endothelial cell interactions (e.g. binding to integrin receptors). Crystallographic studies on PlcHR2, however, are hampered by a multitude of difficulties on several levels. To start with, Escherichia coli has a significantly lower G+C content than P. aeruginosa (50% vs 67%) and it lacks a functional Xcp secretory system (Koster, Bitter et al. 2000). Accordingly, the PlcHR2 complex is much more efficiently translated and the heterodimeric complex is secreted as soluble protein into the extracellular compartment (i.e. culture supernatant), using the native P. aeruginosa expression system. Moreover, although PlcHR2 itself is not a membrane protein its substrates are membrane associated, hence the protein shows a high degree of affinity for phospholipids and membrane structures further exacerbating purification as well as crystallization. Finally, L-selenomethionine substituted protein had to be produced in order to solve the crystallographic phase problem
Here we describe the methods we developed to overcome the intrinsic problems towards obtaining diffracting crystals. These include: (i) the optimization of protein preparation (ii) the development of an over-expression system in a methionine auxotroph to produce L-selenomethionine substituted protein samples and (iii) the use of additives and detergents for crystal optimization. Finally, we present the usage of the μ-focus beam line X06SA at the Swiss Light Source (SLS) that enabled us to collect complete diffraction data sets to 2.75 Å resolution using crystals with dimensions of less than 10 μm. The strategies and methods we describe here are applicable to the challenges protein crystallographers face today and in the future.
Materials and Methods
Protein expression in P. aeruginosa, purification and characterization
Our efforts were focused on the native PlcHR2 protein complex as the most stable and soluble complex (Luberto, Stonehouse et al. 2003). Due to the fact that the protein failed to localize extracellularly in recombinant E. coli expression systems, we resorted to expression in the environment of its natural host as previously described (Stonehouse, Cota-Gomez et al. 2002). Briefly, the protein was overexpressed in P. aeruginosa strain PA01 derivative ADD1976 carrying a chromosomal T7 polymerase gene under the control of the lacUV5 promoter. The expression of plcHR2 is controlled by the T7 promotor on the pADD3268 vector. P. aeruginosa was grown at 37°C in minimal M9 media to an optical density of OD590 ≈ 0.6, induced with isopropylthio-β-galactopyranoside (IPTG) and then kept at 32°C for an additional 12h. The use of 32°C is based on the temperature typically used for optimal expression of extracellular factors of P. aeruginosa (Berka and Vasil 1982) and hence no increase of protein yield was observed at 30°C or 37°C (unpublished results). Cells were first separated from the supernatant by centrifugation at 10000g. Then 1.5 times volume of cold ddH2O was added to the supernatant containing PlcHR2 in order to reduce ionic strength before applying the solution to micro granular anion exchanger diethylaminoethyl cellulose DE52. The protein was eluted with 500 mM NaCl and after further concentration and dialysis applied to a BioRad Model 491 prep cell which was used to separate culture supernatant proteins by continuous elution native gel electrophoresis (7.5 % non-denaturing polyacrylamide). The prep cell was run at constant power of 12 W, and protein fractions were eluted at pH 7.2 with a flow rate of 0.35 ml/min. Separated proteins were delivered to a fraction collector and pooled in 2 ml fractions. Peak fractions were pooled, concentrated and frozen at −80°C. All purification procedures were carried out at 4° C. Successive matrix-assisted laser desorption ionization (MALDI) and electron spray mass spectrometric analysis (ESI-MS) confirmed the identity of the heterodimeric PlcHR2 complex as reported earlier (Stonehouse, Cota-Gomez et al. 2002). The overall yield was improved to 1.6 mg/L culture by increasing the aeration after IPTG induction.
L-selenomethionine substituted protein
The plcHR2 genes were expressed in the same native P. aeruginosa T7 expression system (Stonehouse, Cota-Gomez et al. 2002) with the important addition that the metZ gene in P. aeruginosa PAO1 ADD1976 was mutated using ethylmethansulfonate (EMS). Thus, this strain is no longer capable of L-methionine biosynthesis. The mutation (TGG⇒TAG leading to Trp⇒STOP) in the metZ gene was verified by sequencing of PCR products from the amplified gene in this strain. For L-selenomethionine substituted protein production, the plcHR2 genes were expressed in minimal media with L-selenomethionine at 50μg/ml. The protein was purified using the same protocols described above. The yield was significantly lower compared to the native protein with approximately 0.6 mg/L culture.
Crystallization of the native PlcHR2
Initial crystallization experiments were performed with native PlcHR2 in various buffer compositions and concentrations, however the most promising inital results were obtained with the protein at 1.5 mg/ml in 50 mM Tris pH 7.4, 50 mM NaCl, 0.1 mM TCEP. Early crystallization screens using standard manual vapor diffusion setup and commercially available screens (Jancarik and Kim 1991) led to two crystal morphologies. The first crystals were obtained with 10% dioxane, 0.1M MES buffer, pH 6.5 and 1.65 M ammonium sulfate (AS). The second crystal form was obtained with 30% PEG3350, 70 mM BisTris, pH 6.5 and 0.45 AS. Crystallization optimization strategies ranged from different experimental set-ups including hanging and sitting drops at different temperatures, batch crystallization, to the addition of commercially available and in-house additive screens. Tungstate and vanadate were used as these anions have been successfully used in crystallization where they occupy phosphate sites (Davies and Hol 2004). Because PlcHR2 has a high affinity for membranes (i.e. phospholipids) and therefore behaves like a membrane associated protein, the membrane protein additive screen was used to improve crystal reproducibility and quality (Cudney, Patel et al. 1994). The best native crystals were obtained with the protein concentrated to approximately 9 mg/ml in sitting drop vapor diffusion experiments with a reservoir solution of 10% dioxane, 0.1 M MES, pH 6.75 and 1.5 M AS. The drop was setup with 2 μl protein solution and mixed with 0.7 μl reservoir solution and 0.3 μl 300 mM zwittergent.
L-selenomethioine substituted PlcHR2
Initially crystallization was attempted using conditions close to those that were successful with the native PlcHR2 sample, however, no crystals were obtained with the dioxane containing solution. The condition based on PEG3350 led to a large number of very small crystals that typically showed multiple diffraction patterns to a maximum resolution of approximately 5Å. In order to deal with the extensive nucleation observed in many drops various seeding techniques were employed (Bergfors 2003). The best crystals were obtained with a protein solution at 9mg/mL in 50 mM Tris pH 7.4, 50 mM NaCl, 0.1 mM TCEP filtered through a 0.22 μm filter and centrifuged for ten minutes. The crucial step in reproducibly obtaining crystals was streak-seeding using a cat-whisker from micro-crystals obtained under similar conditions.
Diffraction experiments
The first native crystals were tested at the EMBL Hamburg Outstation wiggler beam line BW7B (Pohl, Ristau et al. 2004) located at the 2nd generation synchrotron DORIS at DESY. All consecutive diffraction experiments were performed at the 3rd generation synchrotron, the Swiss Light Source (SLS). Experiments were performed either on beam line X10SA which features a focused beam size of 50 × 10 μm (Pohl, Pradervand et al. 2006; Russo, Schweitzer et al. 2009) or in case of L-selenomethionine substituted PlcHR2 on the μ-focus beam line X06SA. This beam line is equipped with the high-precision microdiffractometer MD2 and allows a focus of 25 × 6 μm (Wagner, Diez et al. 2009). The small beam focus matches the size of these protein crystals much better, which significantly increases signal-to-background ratio.
Native protein crystals were typically fished directly from the drop using standard nylon loops and frozen in the cold nitrogen stream (Teng 1990). L-selenomethionine substituted crystals were extremely fragile and hence mounting only succeeded using MiTeGen MicroLoops E which also showed the lowest background on the diffraction pattern (Thorne, Stum et al. 2003). All diffraction data were collected using the rotation methods with crystals cooled to 100K.
Indexing, integration and scaling
All diffraction data were carefully indexed, integrated and scaled using XDS (Kabsch 2010) and/or HKL2000 (Otwinowski and Minor 1997).
Results and Discussion
High-yield expression and purification
Using P. aeruginosa as the natural host we were able to obtain mg amounts of pure and active PlcHR2. The single step purification resulted in >99% pure protein as judged from SDS PAGE analysis (Figure 1) suitable for crystallization. L-selenomethionine substituted protein was expressed and purified in order to obtain crystals suitable to solve the crystallographic phase problem using MAD techniques (Hendrickson 1991). The expression was thus successfully adapted by disrupting the inherent methionine pathway to incorporate L-selenomethionine from the medium. Although L-selenomethionine containing proteins have been expressed in the past in an auxotrophic P. aeruginosa strain (Frank, Licht et al. 1985), as well as P. fluorescence (Madduri, Badger et al. 2009), the method described here is tailor-made for the expression of extracellular virulence of P. aeruginosa that may be toxic for other expression hosts. ESI-MS analysis was used to verify that both components (PlcH and PlcR2) are present in the complex, and to assess the level of L-selenomethionine substitution. Given the size of the complex and the likely heterogeneity of substitution the molecular masses are not expected not be very accurate, however, peaks at 16878 Da, 16925 and 16996 may correspond to the 1-, 2-, and 3-fold substitution of methionine by L-selonomethionine (mass difference 47 Da) in PlcHR2 (ESI-MS of the native protein 16831 Da). In addition, there is a series of peaks with molecular weight of 78706 Da, 78745 and 78800 Da, which may represent 7-, 8-, and 9-fold substitution with an expected molecular weight of 78386 Da the unmodified PlcH. (Figure S1 in supplementary material).
Figure 1.
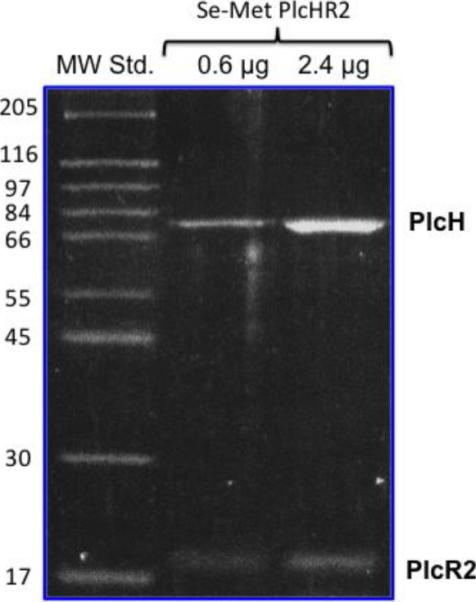
SDS-PAGE analysis of purified Pseudomonas aeruginosa PlcHR2 showing two distinct bands for the separated PlcH and the PlcR2 proteins.
Crystallization
Native PlcHR2 crystals typically grew over a period of one to two months with maximum dimensions up to approximately 20 × 100 × 100 μm3 (Figure 2a). Although the crystals were highly reproducible diffraction properties of crystals differed significantly not only from protein batch to batch but also between crystals from the same crystallization tray. Initial diffraction on the second-generation synchrotron beam line was limited to lower than 10Å resolution, which proved the sample to be protein however, was not sufficient for a unit-cell determination. L-selenomethionine substituted crystal grew typically for a period of two months to thin needles of maximal dimensions of 10 × 10 × 200 μm3. These crystals were hardly visible in sitting drop trays (data now shown) and proved to be extremely fragile (Figure 3).
Figure 2.
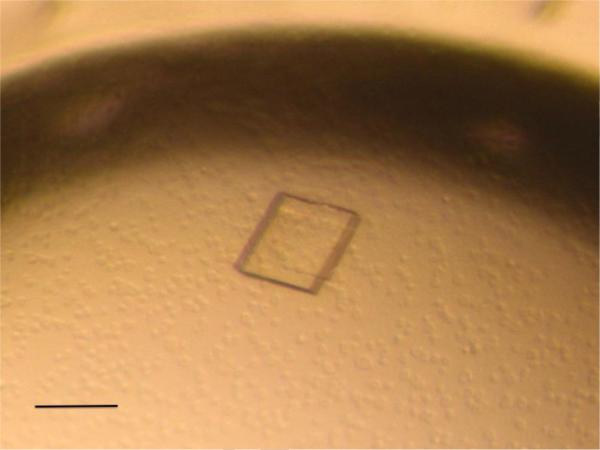
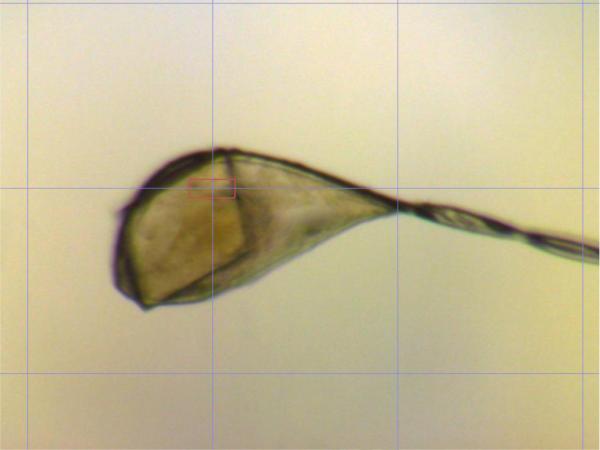
(a) Native crystals of PlcHR with approximate dimensions of 100 ×100× 30 μm in the crystallization droplet. The scale bar represent approximately 100 μm (b) Typical native crystal mounted in a nylon loop on the high-resolution diffractometer of beam line X10SA (Russo, Schweitzer et al. 2009).
Figure 3.
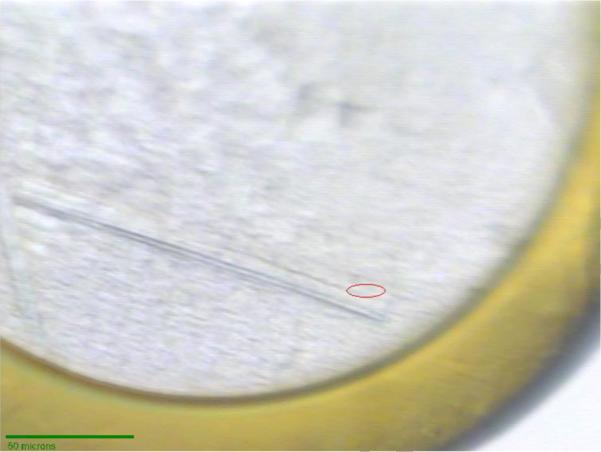
L-selenomethioine crystals mounted on the microdiffractometer MD2 at beam line X06SA. The red ellipsoid corresponds to the focused beam size of 25 × 6 μm (Full width at half maximum).
All attempts to increase diffraction quality of either native protein or Se-Met substituted crystals by the various post-crystallization techniques including various stabilizing solutions (Heras and Martin 2005), dehydration and crystal annealing (Yeh and Hol 1998; Kuo, Bowler et al. 2003; Abergel 2004) did not improve diffraction properties and more often than not quickly destroyed the crystals.
Diffraction experiments
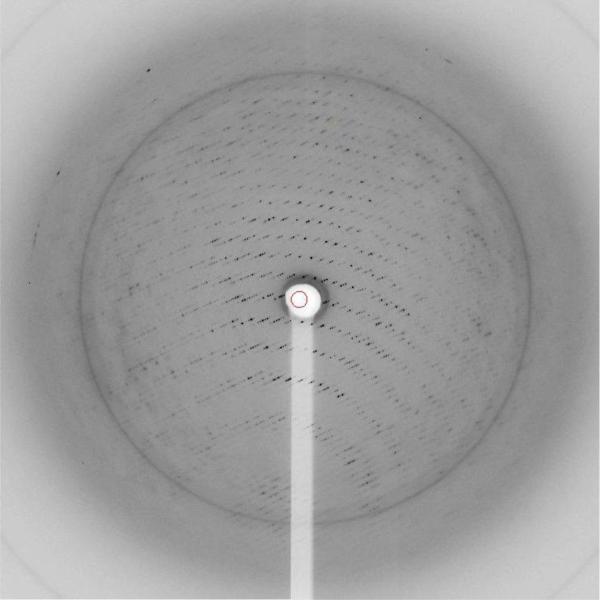
Although the crystal diffracted to reasonable resolution on the SLS beam lines, radiation damage poses a major challenge (Garman 2010). Native PlcHR2 crystals typically showed noticeable signs of severe damage after less than 30 seconds of exposure. However, due to the relative large crystal size compared to beam focus complete data sets to medium resolution could be collected from one single crystal. The best diffraction was recorded to a Bragg spacing of about 3.5 Å, the diffraction data however are anisotropic with significantly higher mosaicity and lower resolution in one direction (Figure 4).
Figure 4.
Diffraction pattern native crystals. Diffraction spots are clearly visible beyond the water ring at approximately 3.5Å resolution.
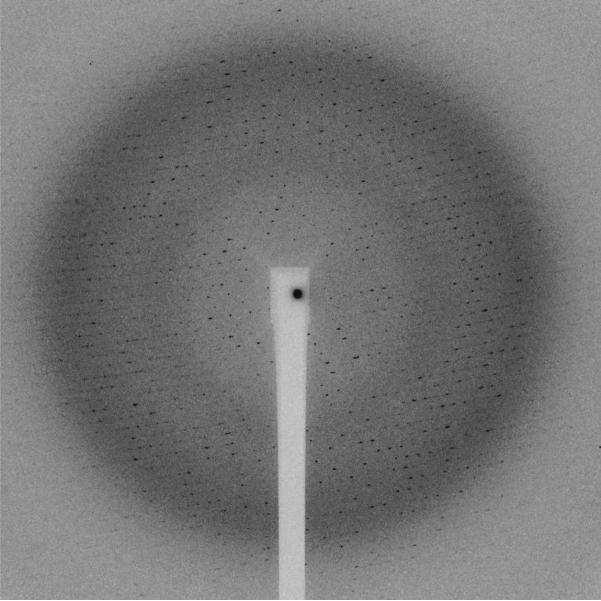
The Se-Met crystals diffracted surprisingly well to a maximum Bragg spacing of 2.2 Å (Figure 5). However, the smaller Se-Met crystals proved to be much more radiation sensitive. Due to the higher cross-section of Selenium compared to Sulfur in particular on the peak of the absorption edge, the crystals absorb more strongly and hence deteriorate much quicker. Clear signs of radiation damage were visible after 3 seconds of exposure with the full beam and hence a maximum of 20 degree of rotation only could be collected by careful attenuation. Beam attenuation of more than 50% led to an unusable weak diffraction pattern. In order to collect a complete data set the crystals were shifted manually by approximately 20 μm along the longest needle dimension after 20 degrees of rotation. For each of these 20 degrees sweep the crystal position was optimized manually by diffraction-based alignment. This was of particular importance in the position where the loop was oriented in the plane of the on-axis microscope. The best diffraction data were obtained by this manual mode of helical data collection where the effects of radiation damage are mitigated by exposing a fresh part of the needle before the exposed part has already been completely destroyed. Helical data collection modes have been automated at the ESRF and the Diamond Light Source where the starting and end points for translation can be picked by the user and the optimal translation for a given oscillation range is calculated (Flot, Mairs et al. 2010; Evans, Axford et al. 2011).
Figure 5.
Diffraction pattern of L-selenomethioine PlcHR2 crystal. The edge of the detector corresponds to approximately 2.5Å resolution.
The optimal wavelength for data collection on L-selenomethionine substituted crystals was determined by performing an X-ray emission fluorescence scan which clearly indicated the optimal energy for the collection of single-anomalous diffraction (SAD) phasing (Figure 6). The scan also confirmed the incorporation of L-seleniummethionine in place of methionine, at sufficient levels for structure solution. Data statistics from the highest resolution data sets are summarized in Table 1.
Figure 6.
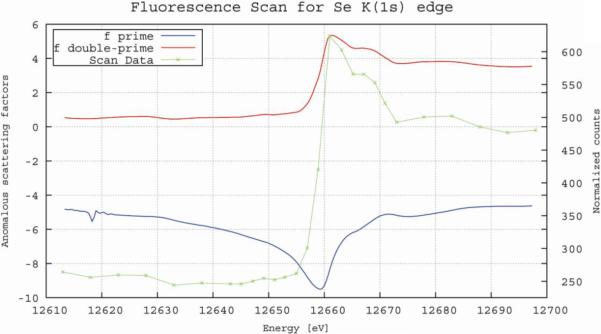
Typical fluorescence emission spectrum recorded from a L-selenomethionine PlcHR2 crystal on beam line X06SA. The values for f' and f" were calculated with CHOOCH (Evans and Pettifer 2001).
Table 1.
Data collection and processing parameter
| Native PlcHR2 | Se-Met PlcHR2 | |
|---|---|---|
| Beam line | X10SA | X06SA |
| Wavelength [Å] | 1.000 | 0.95370 |
| Temperature [K] | 100 K | 100 K |
| Oscillation range [°] | 1 | 1 |
| No. of frames | 180 | 170 |
| Unit cell dimensions [Å], [°] | 175.5, 196.4, 325.3 | 157.9, 75.4, 141.0 β = 93.2 |
| Space group | C2221 | C2 |
| max. resolution [Å] | 4.0 | 2.75 |
| No. observed reflections | 150 089 | 150 083 |
| No unique reflections | 45 739 | 43 080 |
| Completeness (last shell) | 95.6 (92.2) | 99.7 (99.3) |
| R sym* (last shell) | 0.103 (0.352) | 0.130 (0.308) |
| I/σ (last shell) | 8.3 (2.7) | 9.3 (4.7) |
R = SUM (I-<I>)2/SUM(I2)
Data analysis
The native protein crystallized in the orthorhombic space group C2221 with unit cell dimensions of a = 175.5, b = 196.4, and c = 325.3 Å. Given the limited diffraction properties it is reasonable to assume a higher than average solvent content which leads to between four and six PlcHR2 complexes in the asymmetric unit. Six complexes would translate to a solvent content of 50% with a Matthews factor of 2.46 Å3/Da whereas four molecules results in 67% slovent content and VM = 3.7 Å3/Da (Matthews 1968; Kantardjieff and Rupp 2003). The L-selenomethionine substituted protein crystallized in a much smaller unit cell with space group C2 and a = 158.4, b = 74.3, and c = 141.4 Å, β = 93.2°. The asymmetric unit is most likely to contain two independent complexes with a solvent content of 43 % and VM = 2.2 Å3/Da. The smaller unit-cell and the lower solvent content may explain the better diffraction properties of these crystals compared to the native protein.
Molecular replacement
Structure solution attempts by molecular replacement were performed with a number of programs but mainly using PHASER (McCoy, Grosse-Kunstleve et al. 2007) and Phenix/Rosetta (DiMaio, Terwilliger et al. 2011). Initial trials used the native data, but later when the higher resolution L-selenomethionine crystals became available the best data set as given in Table 1 was used. Several reduced search models based on the phosphodiesterase domain of the crystal structure of AcpA were constructed. Models were limited to the most similar part by removing all insertions and to the least flexible model where all flexible loops were also taken out. Models included multi-alanine structures where all residues that are different were changed to alanines and poly-alanine models. In addition, a second search fragment based on a very low similarity of the C-terminal domain-of-unknown function with the IG-like domain of human carcinoembryonic antigen cell adhesion molecule (pdb-code: 2DKS) was constructed and molecular replacement with two models was attempted. In spite of exhaustive efforts no convincing structure solution was obtained. It should be noted that given the low sequence identity of the search fragment constituting only about half of the scattering mass and the overall quality of the diffraction data this failure of molecular replacement trials had to be expected.
Anomalous data and MAD phasing
In spite of the small crystals size (Figure 3) the fluorescence emission scan depicted in Figure 6 showed a clear signal and unambiguously confirmed the substitution of methionine by L-selenomethionine. However, due to the severe radiation damage it was not possible to collect diffraction data at more than one X-ray energy from a single crystal. Even the highest resolution and most complete data set collected from one crystal at the high-energy remote wavelength (summarized in Table 1) resulted in a relatively low overall I/sigma (I) of 9.3 with an Rsym of 0.13. Considering the additional low crystallographic symmetry, which inherently results in low redundancy, it is no surprise that even at low resolution no useful anomalous signal was detected and the sub-structure of Se-positions could not be determined. Further data sets were collected from other crystals at the peak and inflection point determined by the fluorescence emission. The optimization of scaling procedures is currently underway in order to construct a useful 3-wavelength data set that will enable to solve the phase problem in the near future.
Conclusions
In this paper we describe significant progress towards the crystal structure determination of the PlcHR2 complex from P. aeruginosa. Diffracting crystals of the native protein were obtained with detergents typically used for the crystallization of membrane proteins. However, since molecular replacement trials with native data to medium resolution failed, novel methods to over-express and purify L-selenomethionine substituted protein from its natural source P. aeruginosa were developed. These methods will have wider application for proteins that are less amenable for recombinant overexpression in E. coli due to toxicity and the typical inability of that organism to secret extracellular proteins. Further crystallization and the application of seeding techniques led to L-selenomethionine substituted micro-crystals that diffracted, albeit weakly to better than 2.5 Å resolution. Diffraction to this high resolution could only be recorded using the leading micro-focus beam lines highlighting once again the impact of 3rd generation synchrotron sources on macromolecular crystallography. Due to the limited crystal size and radiation sensitivity, the best crystals resulted in a data set to a resolution of 2.75 Å. Molecular replacement attempts have failed also for the higher resolution data set obtained from L-selenomethionine substituted crystals, presumably due to a combination of limited data quality and the lack of a sufficiently similar search model for structure solution. Successful structure solution will therefore require the application of improved data collection methods including optimized mounts for crystal positioning and the helical method where the crystal is rotated and shifted along the longest crystal dimension to minimize and smoothen the effects of radiation damage. Improved scaling procedures will help to merge data collected from different crystals in order to obtain a complete single-anomalous or if possible multiple anomalous data set required to solve the crystallographic phase problem.
Supplementary Material
Hightlights
-
➢
Overexpression of the secreted phospholipase C/sphingomyelinase PlcHR from P. aeruginosa in native and Se-methionine substituted form
-
➢
Purification and crystallisation of PlcHR
-
➢
Crystallographic analysis and data collection of PlcHR micro-crystals
Acknowledgements
We would like to thank C. Pradervand, S. Russo and V. Olieric for their help on the beam line and many fruitful discussions, and M. Groftehauge and P. Denny for critically reading the manuscript. We gratefully acknowledge financial support from the Wellcome Trust to E.P. (WT 094759/Z/10Z) and an NIH grant from the National Heart, Lung and Blood Institute (HL062608) to M.L.V.. This paper is dedicated to the memory of Martin Stonehouse, deceased in October 2011.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abergel C. Spectacular improvement of X-ray diffraction through fast desiccation of protein crystals. Acta Crystallogr D Biol Crystallogr. 2004;60(Pt 8):1413–1416. doi: 10.1107/S0907444904013678. [DOI] [PubMed] [Google Scholar]
- Antunes LC, Imperi F, et al. Deciphering the multifactorial nature of Acinetobacter baumannii pathogenicity. PLoS One. 2011;6(8):e22674. doi: 10.1371/journal.pone.0022674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker AP, Vasil AI, et al. A novel extracellular phospholipase C of Pseudomonas aeruginosa is required for phospholipid chemotaxis. Mol Microbiol. 2004;53(4):1089–1098. doi: 10.1111/j.1365-2958.2004.04189.x. [DOI] [PubMed] [Google Scholar]
- Bergfors T. Seeds to crystals. J Struct Biol. 2003;142(1):66–76. doi: 10.1016/s1047-8477(03)00039-x. [DOI] [PubMed] [Google Scholar]
- Berka RM, Vasil ML. Phospholipase C (heat-labile hemolysin) of Pseudomonas aeruginosa: purification and preliminary characterization. J Bacteriol. 1982;152(1):239–245. doi: 10.1128/jb.152.1.239-245.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berks BC, Sargent F, et al. The Tat protein export pathway. Mol Microbiol. 2000;35(2):260–274. doi: 10.1046/j.1365-2958.2000.01719.x. [DOI] [PubMed] [Google Scholar]
- Cota-Gomez A, Vasil AI, et al. PlcR1 and PlcR2 are putative calcium-binding proteins required for secretion of the hemolytic phospholipase C of Pseudomonas aeruginosa. Infect Immun. 1997;65(7):2904–2913. doi: 10.1128/iai.65.7.2904-2913.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudney R, Patel S, et al. Screening and optimization strategies for macromolecular crystal growth. Acta Crystallogr D Biol Crystallogr. 1994;50(Pt 4):414–423. doi: 10.1107/S0907444994002660. [DOI] [PubMed] [Google Scholar]
- Davies DR, Hol WG. The power of vanadate in crystallographic investigations of phosphoryl transfer enzymes. Febs Letters. 2004;577(3):315–321. doi: 10.1016/j.febslet.2004.10.022. [DOI] [PubMed] [Google Scholar]
- DiMaio F, Terwilliger TC, et al. Improved molecular replacement by density- and energy-guided protein structure optimization. Nature. 2011;473(7348):540–543. doi: 10.1038/nature09964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson SH, Boucher RC. Update on pathogenesis of cystic fibrosis lung disease. Curr Opin Pulm Med. 2003;9(6):486–491. doi: 10.1097/00063198-200311000-00007. [DOI] [PubMed] [Google Scholar]
- Evans G, Axford D, et al. The design of macromolecular crystallography diffraction experiments. Acta Crystallogr D Biol Crystallogr. 2011;67(Pt 4):261–270. doi: 10.1107/S0907444911007608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G, Pettifer RF. CHOOCH: a program for deriving anomalous-scattering factors from X-ray fluorescence spectra. Journal of Applied Crystallography. 2001;34:82–86. [Google Scholar]
- Felts RL, Reilly TJ, et al. Structure of Francisella tularensis AcpA: prototype of a unique superfamily of acid phosphatases and phospholipases C. J Biol Chem. 2006;281(40):30289–30298. doi: 10.1074/jbc.M606391200. [DOI] [PubMed] [Google Scholar]
- Flot D, Mairs T, et al. The ID23-2 structural biology microfocus beamline at the ESRF. Journal of Synchrotron Radiation. 2010;17:107–118. doi: 10.1107/S0909049509041168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank P, Licht A, et al. A selenomethionine-containing azurin from an auxotroph of Pseudomonas aeruginosa. J Biol Chem. 1985;260(9):5518–5525. [PubMed] [Google Scholar]
- Garman EF. Radiation damage in macromolecular crystallography: what is it and why should we care? Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 4):339–351. doi: 10.1107/S0907444910008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George AM, Jones PM, et al. Cystic fibrosis infections: treatment strategies and prospects. FEMS Microbiol Lett. 2009;300(2):153–164. doi: 10.1111/j.1574-6968.2009.01704.x. [DOI] [PubMed] [Google Scholar]
- Hendrickson WA. Determination of macromolecular structures from anomalous diffraction of synchrotron radiation. Science. 1991;254(5028):51–58. doi: 10.1126/science.1925561. [DOI] [PubMed] [Google Scholar]
- Heras B, Martin JL. Post-crystallization treatments for improving diffraction quality of protein crystals. Acta Crystallogr D Biol Crystallogr. 2005;61(Pt 9):1173–1180. doi: 10.1107/S0907444905019451. [DOI] [PubMed] [Google Scholar]
- Holden MT, Feil EJ, et al. Complete genomes of two clinical Staphylococcus aureus strains: evidence for the rapid evolution of virulence and drug resistance. Proc Natl Acad Sci U S A. 2004;101(26):9786–9791. doi: 10.1073/pnas.0402521101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancarik J, Kim SH. Sparse-Matrix Sampling - a Screening Method for Crystallization of Proteins. Journal of Applied Crystallography. 1991;24:409–411. [Google Scholar]
- Kabsch W. Xds. Acta Crystallographica Section D-Biological Crystallography. 2010;66:125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantardjieff KA, Rupp B. Matthews coefficient probabilities: Improved estimates for unit cell contents of proteins, DNA, and protein-nucleic acid complex crystals. Protein Sci. 2003;12(9):1865–1871. doi: 10.1110/ps.0350503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korbsrisate S, Tomaras AP, et al. Characterization of two distinct phospholipase C enzymes from Burkholderia pseudomallei. Microbiology. 2007;153(Pt 6):1907–1915. doi: 10.1099/mic.0.2006/003004-0. [DOI] [PubMed] [Google Scholar]
- Koster M, Bitter W, et al. Protein secretion mechanisms in Gram-negative bacteria. Int J Med Microbiol. 2000;290(4–5):325–331. doi: 10.1016/S1438-4221(00)80033-8. [DOI] [PubMed] [Google Scholar]
- Kuo A, Bowler MW, et al. Increasing the diffraction limit and internal order of a membrane protein crystal by dehydration. J Struct Biol. 2003;141(2):97–102. doi: 10.1016/s1047-8477(02)00633-0. [DOI] [PubMed] [Google Scholar]
- Lopez DJ, Collado MI, et al. Multiple phospholipid substrates of phospholipase C/sphingomyelinase HR(2) from Pseudomonas aeruginosa. Chemistry and Physics of Lipids. 2011;164(1):78–82. doi: 10.1016/j.chemphyslip.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Luberto C, Stonehouse MJ, et al. Purification, characterization, and identification of a sphingomyelin synthase from Pseudomonas aeruginosa. PlcH is a multifunctional enzyme. J Biol Chem. 2003;278(35):32733–32743. doi: 10.1074/jbc.M300932200. [DOI] [PubMed] [Google Scholar]
- Madduri K, Badger M, et al. Development of stable isotope and selenomethionine labeling methods for proteins expressed in Pseudomonas fluorescens. Protein Expr Purif. 2009;65(1):57–65. doi: 10.1016/j.pep.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Matthews BW. Solvent content of protein crystals. J Mol Biol. 1968;33(2):491–497. doi: 10.1016/0022-2836(68)90205-2. [DOI] [PubMed] [Google Scholar]
- McCoy AJ, Grosse-Kunstleve RW, et al. Phaser crystallographic software. Journal of Applied Crystallography. 2007;40(Pt 4):658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RM, Tomaras AP, et al. Pseudomonas aeruginosa twitching motility-mediated chemotaxis towards phospholipids and fatty acids: specificity and metabolic requirements. J Bacteriol. 2008;190(11):4038–4049. doi: 10.1128/JB.00129-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra NP, Balagopal A, et al. AcpA is a Francisella acid phosphatase that affects intramacrophage survival and virulence. Infect Immun. 2007;75(1):390–396. doi: 10.1128/IAI.01226-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohanian J, Ohanian V. Sphingolipids in mammalian cell signalling. Cell Mol Life Sci. 2001;58(14):2053–2068. doi: 10.1007/PL00000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Macromolecular Crystallography, Pt A. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Pohl E, Pradervand C, et al. The new protein crystallography beam lines X10SA at the Swiss Light Source. Synchrotron Rad News. 2006;19(1):24–26. [Google Scholar]
- Pohl E, Ristau U, et al. Automation of the EMBL Hamburg protein crystallography beamline BW7B. J Synchrotron Radiat. 2004;11(Pt 5):372–377. doi: 10.1107/S090904950401516X. [DOI] [PubMed] [Google Scholar]
- Russo S, Schweitzer JE, et al. Crystal structure of the caseinolytic protease gene regulator, a transcriptional activator in actinomycetes. J Biol Chem. 2009;284(8):5208–5216. doi: 10.1074/jbc.M806591200. [DOI] [PubMed] [Google Scholar]
- Stonehouse MJ, Cota-Gomez A, et al. A novel class of microbial phosphocholine-specific phospholipases C. Mol Microbiol. 2002;46(3):661–676. doi: 10.1046/j.1365-2958.2002.03194.x. [DOI] [PubMed] [Google Scholar]
- Teng TY. Mounting of Crystals for Macromolecular Crystallography in a Freestanding Thin-Film. Journal of Applied Crystallography. 1990;23:387–391. [Google Scholar]
- Thorne RE, Stum Z, et al. Microfabricated mounts for high-throughput macromolecular cryocrystallography. Journal of Applied Crystallography. 2003;36:1455–1460. [Google Scholar]
- Vasil ML. Pseudomonas aeruginosa Phospholipases and Phospholipids. In: Ramos J-L, R.C. L, editors. Pseudomonad v4., Molecular Biology and Emerging Issues. Springer; 2006. pp. 69–97. [Google Scholar]
- Vasil ML, Graham LM, et al. Phospholipase C: molecular biology and contribution to the pathogenesis of Pseudomonas aeruginosa. Antibiot Chemother. 1991;44:34–47. doi: 10.1159/000420295. [DOI] [PubMed] [Google Scholar]
- Vasil ML, Stonehouse MJ, et al. A complex extracellular sphingomyelinase of Pseudomonas aeruginosa inhibits angiogenesis by selective cytotoxicity to endothelial cells. PLoS Pathog. 2009;5(5):e1000420. doi: 10.1371/journal.ppat.1000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana-Niero C, de Haas PE, et al. Analysis of genetic polymorphisms affecting the four phospholipase C (plc) genes in Mycobacterium tuberculosis complex clinical isolates. Microbiology. 2004;150(Pt 4):967–978. doi: 10.1099/mic.0.26778-0. [DOI] [PubMed] [Google Scholar]
- Voulhoux R, Ball G, et al. Involvement of the twin-arginine translocation system in protein secretion via the type II pathway. EMBO J. 2001;20(23):6735–6741. doi: 10.1093/emboj/20.23.6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A, Diez J, et al. Crystal structure of ultralente--a microcrystalline insulin suspension. Proteins-Structure Function and Bioinformatics. 2009;74(4):1018–1027. doi: 10.1002/prot.22213. [DOI] [PubMed] [Google Scholar]
- Yeh JI, Hol WG. A flash-annealing technique to improve diffraction limits and lower mosaicity in crystals of glycerol kinase. Acta Crystallogr D Biol Crystallogr. 1998;54(Pt 3):479–480. doi: 10.1107/s0907444998004697. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.