Abstract
In order to develop agents for early detection and selective treatment of melanomas, high affinity and high specificity molecular tools are required. Enhanced specificity may be obtained by simultaneously binding to multiple cell surface targets via the use of multimeric analogs of naturally occurring ligands. Trimers targeting overexpressed melanocortin receptors have been found to be potential candidates for this purpose. In the present letter, we describe the synthesis and study of multimers based on a dendrimer-like scaffold. The binding affinity and activity results revealed that dendrimers promote multivalent interactions via statistical and/or cooperative effects on binding. Moreover, viability studies showed no significant toxicity at micromolar concentrations, which will allow these molecular complexes to be used in vivo. Finally, imaging studies showed effective internalization for all the molecules confirming their potential as delivery agents.
Keywords: Multivalent interactions, multimers, peptides, dendrimers, cancer, melanoma, targeted therapy, delivery
Melanoma is a type of skin cancer, which develops in melanocytes and accounts for 80% of skin cancer deaths. Once, the tumor metastases and spread through the body, the chances of survival are poor with only 14 % of patient survival in 5 years. Metastatic melanoma being one of the most virulent types of cancer, meaningful tools for its early detection and effective treatment are key to improve chances of patient survival and recovery1, 2. To provide such molecular entities, tools possessing a high affinity and selectivity toward the cancer cells are desirable. In order to obtain selectivity, specific characteristics of the cancer cell must be identified then targeted. G-protein coupled receptors (GPCRs) are involved in a myriad of biological responses within the body and their responses are altered in many diseases3. In cancer, some specific GPCRs are found to be overexpressed at the cancer cell surface4, 5. Therefore, targeting receptor overexpression via the use of multivalent interactions provides an alternative way to enhance selectivity toward these cancer cells. Indeed, multivalent interactions, arising from synergy of binding (or cooperative effect) are known to be much more specific than the corresponding monovalent ligands6–13. In the case of metastatic melanoma, melanocortin 1 receptors (MC1-R) are known to be overexpressed at the melanoma cell surface14–18. Thus, targeting these receptors via the use of synthetic agents composed of multiple copies of a low affinity melanocortin ligand should allow enhancement in affinity and specificity toward metastatic melanoma cells due to the creation of cooperative multivalent interactions. In a previous paper19, we described the design, synthesis and study of trimers bearing copies of the pharmacophore for melanocortin receptors, MSH(4) ligand (His-D-Phe-Arg-Trp-CONH2), on a cancer cell model overexpressing MC4R as a substitute for MC1R. The enhancement in affinity was shown to correlate with the valency and the lead compound, trimer NB341 (designated here as compound B) showed more than 300-fold increases in affinity compared to its monovalent version, therefore resulting in the creation of a 15 nM affinity trimer from a 4.9 µM monovalent ligand. It can also be noted that an order of magnitude in binding affinity was afforded per ligand added, providing evidence for the creation and efficiency of multivalent interactions.
Encouraged by these results, the development of higher order constructs based on dendrimers-like scaffolds was undertaken. Indeed, knowing that dendrimers usually provide statistical binding due to the close proximity of ligands, we were interested in determining their effect on binding and the resulting biological properties of multimer combinations. Also, the highly branched and globular structure of dendrimers makes them attractive for their delivery properties as well as for their tumor passive targeting via enhanced permeability and retention effect (EPR effect) which consists of the accumulation of macromolecules within tumor tissues 20–22. Thus, in the present letter, we report the synthesis and in vitro analysis of multimers resulting from the combination of trimers on an optimal established scaffold in order to determine if these macromolecules can promote multivalent interactions.
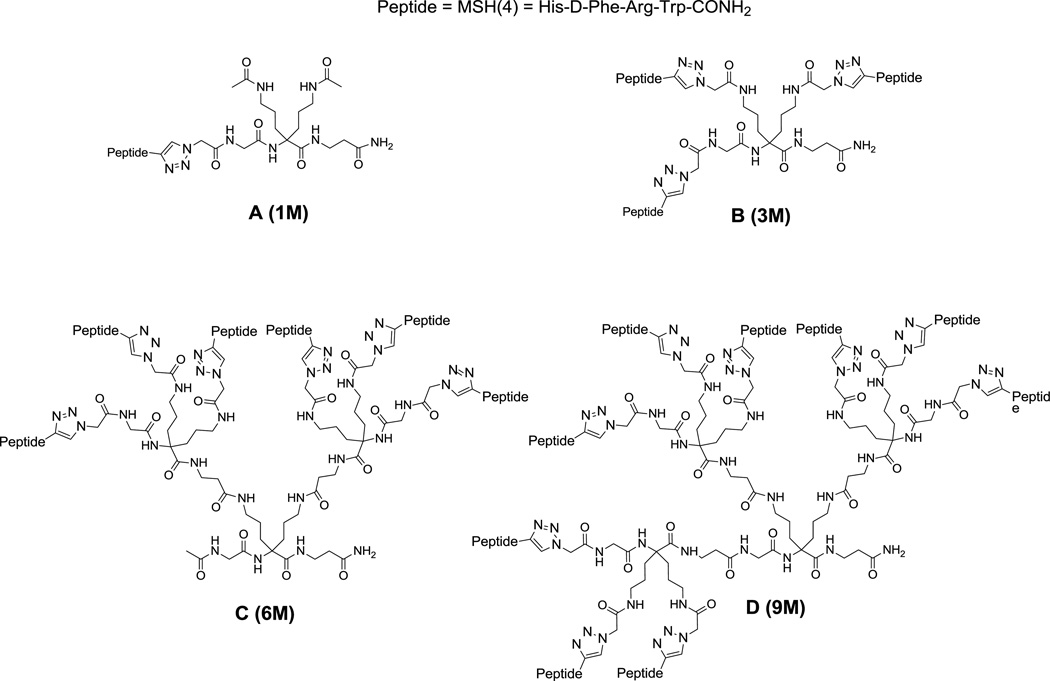
The multimers investigated in this study are represented in Figure 1 and were synthesized following the procedures described in the Supporting Information. The scaffolds previously described19 were attached to the resin followed by deprotection and coupling of azido acetic acid to prepare the precursor for the click chemistry reaction. Upon peptide attachment, cleavage and purification were performed to provide the desired compounds.
Figure 1.
Sequences of multimers investigated.
The affinity of the multimers was evaluated using a time resolved fluorescence (TRF) binding assay23–25. The multimers were competed against Eu-NDP-α-MSH (previously described19) and were evaluated on a cancer cell model overexpressing the melanocortin 4 receptor (MC4-R), constituted of HEK293 cells transfected with the MC4R. These multimers were able to efficiently displace the Eu-NDP-α-MSH analog revealing their potency; the results are summarized in Table 1. The multimers C and D exhibit a 100-fold enhancement in affinity compared to the monomer. However, the trimer B exhibits the lowest IC50 although the multimer IC50 values remain within the same order of magnitude as the trimer. The similarity of IC50 values for the higher order multimers compared to the trimer suggest that some limit to the effect of multivalent interactions on apparent binding affinity is reached at the trimer stage. The trimer is considered to bind by a cluster/chelate mechanism where each ligand binds to a different receptor resulting in cooperative binding. This is supported by our previous studies19 which showed a correlation between valency and enhanced binding affinity and functional activity. On the other hand, the limit on binding of the higher order dendrimers may result from a mixture of statistical binding and cluster/chelate binding, or limits placed on binding of individual recognition elements due to steric effects.
Table 1.
Multimer binding affinities. Competition experiments were performed using a time resolved fluorescence based assay23–25. Ligands were competed against Eu-NDP-α-MSH on HEK293 cells overexpressing the MC4R and IC50’s values are averaged from four experiments performed in quadruplicate.
| Name | Valency | IC50 (nM) | Relative potency to A |
|---|---|---|---|
| A (1M) | 1 | 4900 ± 760 | - |
| B (3M) | 3 | 14 ± 2 | 350 |
| C (6M) | 6 | 46 ± 8 | 104 |
| D (9M) | 9 | 49 ± 9 | 98 |
Even though increasing the valency above three did not result in further affinity enhancement, the macromolecules still possess nanomolar affinity and remain interesting tools for targeting especially due to their larger size and higher branches which theoretically should increase the EPR effect.
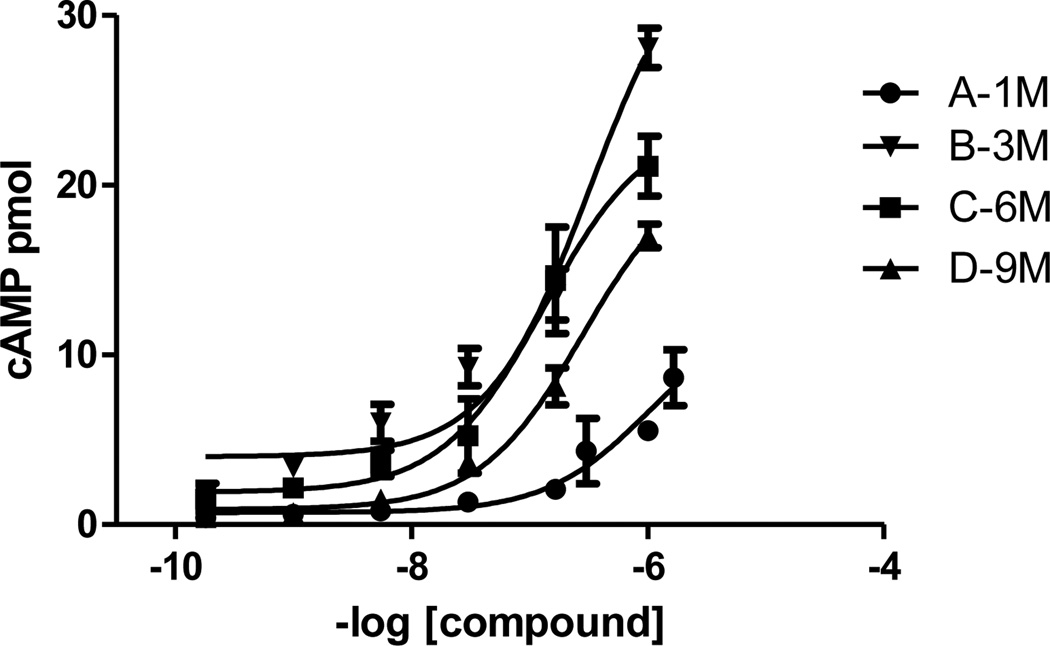
Functional activity measurements were also performed using a chemiluminescent immunoassay. The melanocortin receptors activate cAMP production upon stimulation by an agonist ligand. As observed in Figure 2, the larger molecules activate cAMP production, and therefore remain functional agonists. Interestingly, the same correlation observed for the binding is also observed for activity in that the larger multimers produce cAMP less efficiently when compared to the trimer. Also, the multimer D, possessing nine MSH(4) binding sequences, activates cAMP production less efficiently than C, possessing six ligands, lending evidence for the presence of a statistical effect with higher ligand density.
Figure 2.
cAMP accumulation in response to dendrimers. cAMP accumulation in hMC4R cells in response to multimers was quantified using a chemiluminescent immunoassay described on the Supporting information.
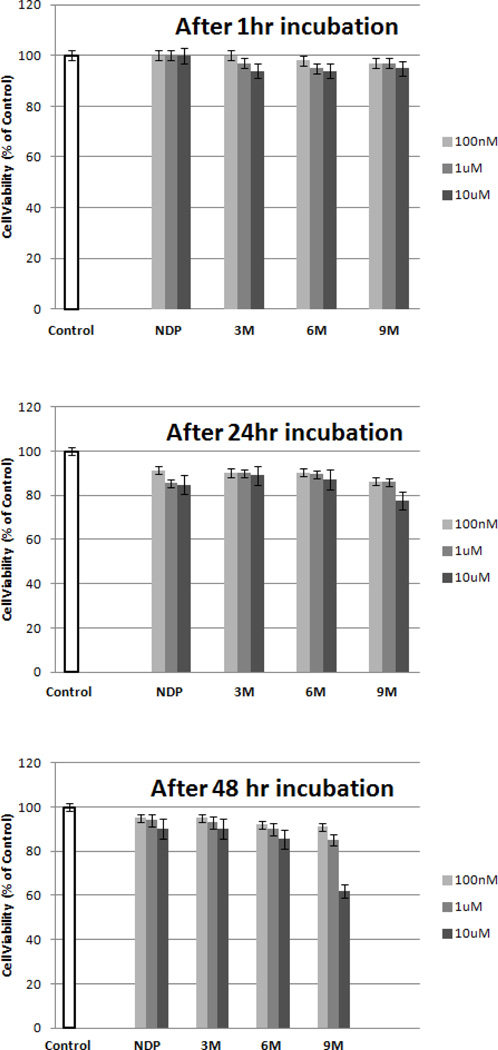
To further investigate the potential use of these compounds for in vivo studies, the toxicity of the multimers was investigated using a cell viability assay. Cell viability was measured at three different concentrations over three different periods of time. The results are illustrated in Figure 3. By comparison to NDP-α-MSH, a well-known melanotropin ligand used as a drug in some countries, no significant toxicity was observed for all the multimeric constructs at 1 µM and up to 48 hrs. Although no toxicity was observed at 10 µM after two days for the smaller constructs, partial toxicity was observed for the largest molecule bearing nine peptides at 10 µM. However, since these compounds possess high nanomolar affinity, such high concentrations would not be necessary for in vivo applications. Therefore, the constructs show a low cytotoxicity in the range of interest, which supports their potential as candidates for efficient in vivo use.
Figure 3.
Toxicity studies. Cell viability was measured and represented as a % of control. The results are an average of three independent experiments done in triplicates.
The significant size of the multimeric agonist molecules may preclude normal processing of the ligand-receptor complex. To study if these compounds internalize, a fluorescent tag was added to facilitate high-resolution imaging. The chosen tag, composed of a triarginine linker and a Cy5 NIR dye, was added to the C-terminus of the multimers (See Supporting information for structure and synthesis). Cy5 was selected for its commercial availability as an NHS ester and the low autofluorescence observed at its emission wavelength. Even though the multimers were soluble in water, a triarginine linker was chosen to increase the length between the fluorescent probe and the molecules as well as to prevent the use of costly PEG linkers. A model molecule was synthesized and evaluated by TRF assay, and confirmed that the linker addition did not affect the compound binding properties (See Supporting information for details.). Images were recorded using an epifluorescence microscope. The labeled multimers (3M*, 6M*and 9M*) were evaluated as well as a Cy5 labeled NDP-α-MSH molecule (NDP*).
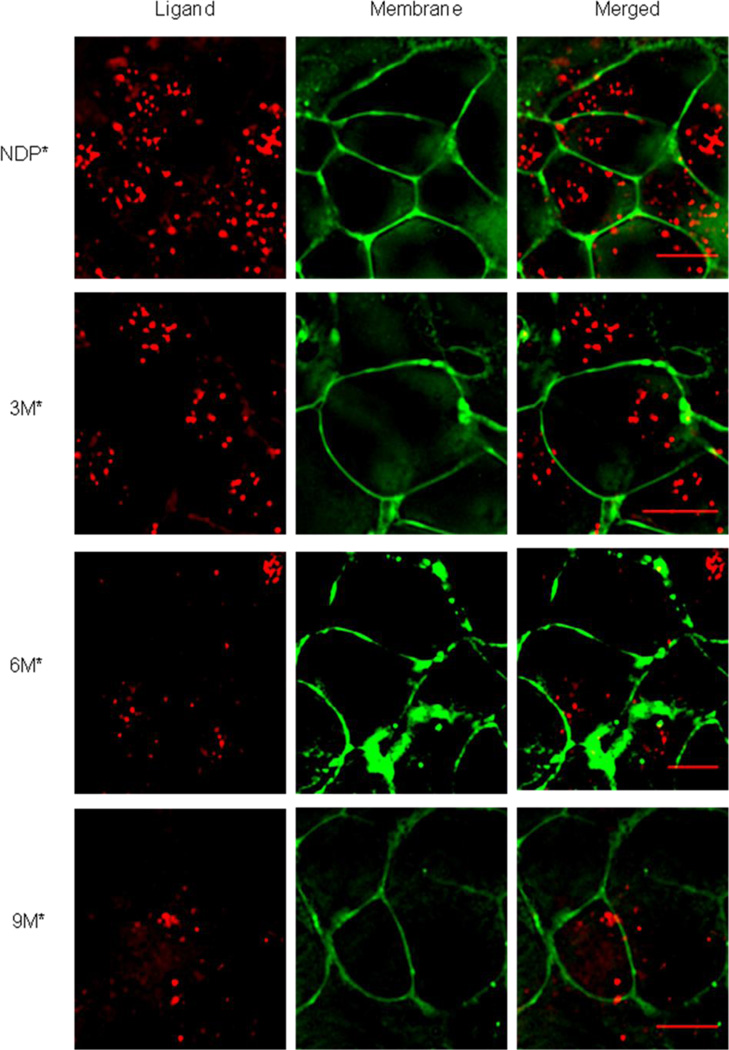
At first, a time course study was performed to evaluate initial processing. All compounds initially localized to the cell surface at three minutes, then began to localize within small punctate structures within five minutes (See Supporting information). To confirm internalization, membrane staining was performed by treatment with FM1–43. The data shown in Figure 4 demonstrate that no ligand was localized at the membrane after 90 minutes. Therefore, these compounds do internalize and potentially could be used for drug delivery purposes. In summary, these studies revealed the applicability of our established synthetic scheme to the creation of a new generation of higher order constructs using dendrimers as scaffolds.
Figure 4.
Localization of Cy5 labeled molecules (Red) at the membrane 90 minutes after initiation of incubation. The membrane probe, FM 1–43 (Green) was added two minutes prior to image acquisition (Scale bar, 10 µm).
These new ligands also provided new insight into the mechanism by which these macromolecules bind to cells via multivalent interactions. Moreover, we have reported novel tools that possess a high affinity for the targeted receptor, no toxicity and which could be used as a prodrug for targeted cancer therapy.
Supplementary Material
ACKNOWLEDGMENT
Authors are thankful to James P. Cain for helpful discussions and critical review of the manuscript.
Funding Sources
This research was supported in parts by grants from CNRS, U-S. Public Health Service, National Institutes of Health RO1 CA097360. N. Brabez is a BDI recipient from CNRS.
ABBREVIATIONS
- GPCR
G-protein coupled receptor
- MC1-R
melanocortin receptor 1
- EPR effect
enhanced permeation and retention effect
- TRF
time resolved fluorescence
- MC4-R
melanocortin receptor 4
- Eu-NDP-α-MSH
Eu labeled [Nle7-D-Phe7]-α-melanocyte stimulating hormone
- cAMP
cyclic adenosine monophosphate
- Cy5
Cyanine dye 5
- NIR
Near infrared
- NHS
N-hydroxy succinimide
- PEG
polyethylene glycol
- FM 1–43
Fey Mao dye 1–43
Footnotes
ASSOCIATED CONTENT
Supporting Information. Experimental details for synthesis, characterization of compounds and the experimental procedures for biological studies. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Miller AJ, Mihm MC., Jr Melanoma. The New England journal of medicine. 2006;355(1):51–65. doi: 10.1056/NEJMra052166. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia S, Tykodi SS, Thompson JA. Treatment of metastatic melanoma: an overview. Oncology. 2009;23:488–496. [PMC free article] [PubMed] [Google Scholar]
- 3.Spiegel AM, Weinstein LS. Inherited diseases involving G-proteins and G-protein coupled receptors. Annu. Rev. Med. 2004;55:27–39. doi: 10.1146/annurev.med.55.091902.103843. [DOI] [PubMed] [Google Scholar]
- 4.Li S, Huang S, Peng SB. Overexpression of G protein-coupled receptors in cancer cells: involvement in tumor progression. International journal of oncology. 2005;27(5):1329–1339. [PubMed] [Google Scholar]
- 5.Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat. Rev. Cancer. 2007;7:79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- 6.Mammen M, Choi S-K, Whitesides GM. Polyvalent Interactions in Biological Systems: Implications for Design and Use of Multivalent Ligands and Inhibitors. Angewandte Chemie International Edition. 1998;37(20):2754–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Handl HL, Vagner J, Han H, Mash E, Hruby VJ, Gillies RJ. Hitting multiple targets with multimeric ligands. Expert opinion on therapeutic targets. 2004;8(6):565–586. doi: 10.1517/14728222.8.6.565. [DOI] [PubMed] [Google Scholar]
- 8.Andre S, Pieters RJ, Vrasidas I, Kaltner H, Kuwabara I, Liu FT, Liskamp RM, Gabius HJ. Wedge-like glycodendrimers as inhibitors of binding of mammalian galectins to glycoproteins, lactose maxiclusters, and cell surface glycoconjugates. Chem. bio. chem. 2001;2(11):822–830. doi: 10.1002/1439-7633(20011105)2:11<822::AID-CBIC822>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 9.Gestwicki JE, Cairo CW, Strong LE, Oetjen KA, Kiessling LL. Influencing receptor-ligand binding mechanisms with multivalent ligand architecture. Journal of the American Chemical Society. 2002;124(50):14922–14933. doi: 10.1021/ja027184x. [DOI] [PubMed] [Google Scholar]
- 10.Humphries HE, Williams JN, Blackstone R, Jolley KA, Yuen HM, Christodoulides M, Heckels JE. Multivalent liposome-based vaccines containing different serosubtypes of PorA protein induce cross-protective bactericidal immune responses against Neisseria meningitidis. Vaccine. 2006;24(1):36–44. doi: 10.1016/j.vaccine.2005.07.056. [DOI] [PubMed] [Google Scholar]
- 11.Terskikh AV, Le Doussal JM, Crameri R, Fisch I, Mach JP, Kajava AV. "Peptabody": a new type of high avidity binding protein. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(5):1663–1668. doi: 10.1073/pnas.94.5.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vadas O, Hartley O, Rose K. Characterization of new multimeric erythropoietin receptor agonists. Biopolymers. 2008;90(4):496–502. doi: 10.1002/bip.20959. [DOI] [PubMed] [Google Scholar]
- 13.Vassiliou G, Jakobsen K, Parish CR. Detection of low-affinity adhesion ligands by linking recombinant cell adhesion molecules in uniform orientation to a fluorescently labelled dextran molecule by means of hexahistidine tagging: the case of multimeric CD40. Journal of immunological methods. 1998;215(1–2):9–15. doi: 10.1016/s0022-1759(98)00030-1. [DOI] [PubMed] [Google Scholar]
- 14.Bagutti C, Oestreicher M, Siegrist W, Oberholzer M, Eberle AN. Alpha-MSH receptor autoradiography on mouse and human melanoma tissue sections and biopsies. Journal of receptor and signal transduction research. 1995;15(1–4):427–442. doi: 10.3109/10799899509045231. [DOI] [PubMed] [Google Scholar]
- 15.Loir B, Perez Sanchez C, Ghanem G, Lozano JA, Garcia-Borron JC, Jimenez-Cervantes C. Expression of the MC1 receptor gene in normal and malignant human melanocytes. A semiquantitative RT-PCR study. Cellular and molecular biology (Noisy-le-Grand, France) 1999;45(7):1083–1092. [PubMed] [Google Scholar]
- 16.Salazar-Onfray F, Lopez M, Lundqvist A, Aguirre A, Escobar A, Serrano A, Korenblit C, Petersson M, Chhajlani V, Larsson O, Kiessling R. Tissue distribution and differential expression of melanocortin 1 receptor, a malignant melanoma marker. Br. J. Cancer. 2002;87(4):414–422. doi: 10.1038/sj.bjc.6600441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wit NJ, van Muijen GN, Ruiter DJ. Immunohistochemistry in melanocytic proliferative lesions. Histopathology. 2004;44(6):517–541. doi: 10.1111/j.1365-2559.2004.01860.x. [DOI] [PubMed] [Google Scholar]
- 18.Xia Y, Skoog V, Muceniece R, Chhajlani V, Wikberg JE. Polyclonal antibodies against human melanocortin MC1 receptor: preliminary immunohistochemical localisation of melanocortin MC1 receptor to malignant melanoma cells. Eur. J. Pharmacol. 1995;288(3):277–283. doi: 10.1016/0922-4106(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 19.Brabez N, Lynch RM, Xu L, Gillies RJ, Chassaing G, Lavielle S, Hruby VJ. Design, Synthesis, and Biological Studies of Efficient Multivalent Melanotropin Ligands: Tools toward Melanoma Diagnosis and Treatment. J. Med. Chem. 2011;54(20):7375–7384. doi: 10.1021/jm2009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox ME, Szoka FC, Frechet JMJ. Soluble polymer carriers for the treatment of cancer: the importance of molecular architecture. Accounts of Chemical Research. 2009;42(8):1141–1151. doi: 10.1021/ar900035f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker JR. Dendrimer-based nanoparticles for cancer therapy. Hematology. 2009:708–719. doi: 10.1182/asheducation-2009.1.708. [DOI] [PubMed] [Google Scholar]
- 22.Wolinsky JB, Grinstaff MW. Therapeutic and diagnostic applications of dendrimers for cancer treatment. Adv. Drug Deliv. Rev. 2008;60(9):1037–1055. doi: 10.1016/j.addr.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Handl HL, Gillies RJ. Lanthanide-based luminescent assays for ligand-receptor interactions. Life Sci. 2005;77(4):361–371. doi: 10.1016/j.lfs.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Handl HL, Vagner J, Yamamura HI, Hruby VJ, Gillies RJ. Lanthanide-based time-resolved fluorescence of in cyto ligand-receptor interactions. Anal Biochem. 2004;330(2):242–250. doi: 10.1016/j.ab.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 25.Handl HL, Vagner J, Yamamura HI, Hruby VJ, Gillies RJ. Development of a lanthanide-based assay for detection of receptor-ligand interactions at the delta-opioid receptor. Anal Biochem. 2005;343(2):299–307. doi: 10.1016/j.ab.2005.05.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.