Abstract
For animals inhabiting multiple environments, the ability to select appropriate behaviors is crucial as their adaptability is often context dependent. Caenorhabditis elegans uses distinct gaits to move on land and in water. Gait transitions can potentially coordinate behaviors associated with distinct environments. We investigated whether land and water differentially affect the behavioral repertoire of C. elegans. Swimming worms interrupted foraging, feeding, egg-laying and defecation. Exogenous dopamine induced bouts of these land-associated behaviors in water. Our finding that worms do not drink fluid while immersed may explain why higher drug doses are required in water than on land to elicit the same effects. C. elegans is a valid model to study behavioral hierarchies and how environmental pressures alter their balance.
Keywords: environmental transitions, swimming, crawling, egg-laying, pharyngeal pumping, defecation rhythm, foraging, omega bends, reversals
Introduction
Key for the transition to land by many aquatic species was their ability to respond to the different selective pressures posed by very different physical environments.1 Many animals (e.g., arthropods, amphibians, birds, etc.) possess adaptations that allow them to exploit and thrive in multiple physical environments. Indeed, many animals use distinct environmental niches to satisfy distinct survival requirements. For example, many birds hunt underwater, breed on land, and fly to travel between locations. Animals often have adaptations that modify, or even restrict the production of certain behaviors to its most advantageous environment. In each of these, different subsets of behaviors performed by a given animal will be adaptive. The ability to determine which behaviors are adaptive (or maladaptive) when transitioning between physical environments can be crucial for survival. The concept of behavioral hierarchies, pioneered by Timbergen and later refined by Davis, explains how animals choose between the production of different possible behaviors based on the integration of sensory information.2-5 How sets of behaviors become activated or inhibited as animals transition between environments remains poorly understood. Distinct physical limitations posed by different environments may effect selective pressure on behavioral repertoires so that different motor patterns may become inhibited, enhanced, or modified to maximize fitness.
The nematode Caenorhabditis elegans is a free-living worm capable of locomotion in water and on land. Recently, we showed that C. elegans uses distinct gaits for swimming in water and for crawling on land.6 While crawling on an agar plate (what we will call “land”), C. elegans engages in a number of adaptive behaviors. These include sensory sampling by the tip of its nose (foraging) ingestion of food (and liquid) by its pharynx (pharyngeal pumping) and intestine defecation, and egg-laying.7 Because these behaviors are under neural control and are genetically tractable they present a unique opportunity to study how motor patterns are modulated when animals transition between distinct physical environments.8,9 Here we investigated how aquatic and terrestrial environments (in the lab) affect the performance of different motor programs in C. elegans.
Results and Discussion
Worms reversibly transition from crawling on land to swimming in water (Fig. 1). We previously observed that crawling was accompanied by sub-behaviors, including foraging and pharyngeal pumping, that were not observed during swimming. Moreover, we found that inducing worms to ectopically crawl in water (by experimental manipulations) also triggered additional behavioral changes.6 We set out to determine whether land and water environments influence the incidence of foraging, egg-laying, defecation and feeding behaviors for C. elegans.
Figure 1.
Caenorhabditis elegans employs distinct motor patterns to crawl on land and to swim in water. Time lapse montage showing the characteristic sinusoidal shape of worm as it crawls toward a 3 μl drop of NGM buffer (left). Once it enters the drop it first abandons crawling and then begin swimming during which it alternates between dorsal and ventral “C” shapes (middle). After the liquid is absorbed by the agar, worms cease swimming and begin emerging from the remaining puddle, resuming crawling once they completely exit it (right).
Foraging
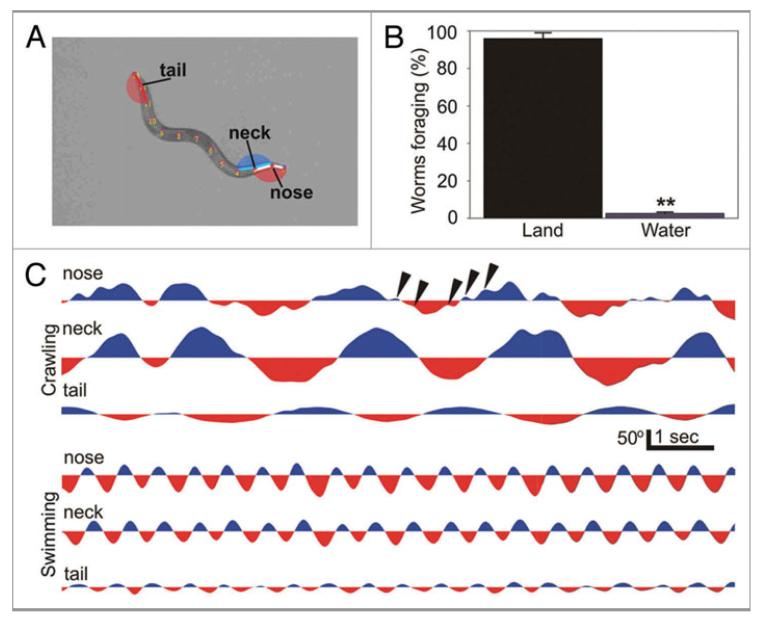
Foraging involves the high frequency (~10 Hz) oscillation of the tip of the head of crawling worms.10 Foraging is readily seen in plots of body curvature obtained by filming freely crawling animals (Fig. 2 and Vid. S1). The variations in body curvature at the tip of the head appeared to be due to foraging and not due to noise in image analysis because body curvature plots of the tip of the tail revealed no high frequency bending (Fig. 2C). As described previously, foraging did not occur during backward crawling.11 We observed worms foraging while crawling forward or stopped on an agar surface (both on and off food), however once worms entered a liquid puddle and began swimming, foraging terminated (Fig. 2B).
Figure 2.
Foraging behavior is suppressed during swimming. (A) Worms crawling on land and swimming in water were filmed and digitized using a custom algorithm. This allowed us to obtain the angular excursions in the dorsal-ventral plane for different points along their body. Foraging excursions in the left-right dimension could not be recorded. (B) While the majority of worms forage while they crawl on land, most cease foraging as they begin to swim in water. (C) Examples of angular excursions for three points along the body of a worm (A) showing the foraging behavior during crawling and not swimming (arrowheads).
Egg-laying
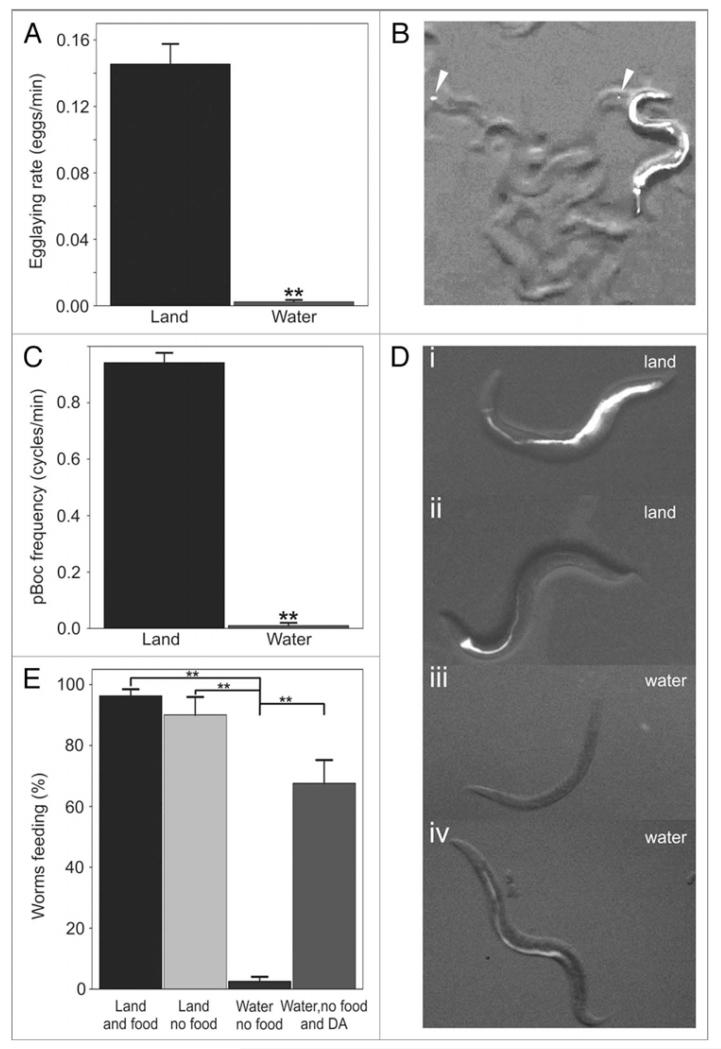
Egg-laying rate by wild-type worms crawling on a bacterial lawn has been previously reported as 0.07–0.17 eggs/minute.12 We found that well-fed first-day adults laid eggs at a similar rate on a lawn of OP50 bacteria (0.14 eggs/min, Fig. 3A) but that upon entering a puddle of NGM buffer their egg-laying rate decreased nearly to a stop (0.002 eggs/min) for the ten minute assay (Fig. 3A).
Figure 3.
Swimming inhibits multiple behaviors. (A) Swimming worms reduced the rate at which they laid eggs. (B) Feeding worms with DiI allowed us to observe alimentary behaviors as well as their fluorescent excreta (arrowheads). (C) Swimming worms interrupted their defecation cycles. (D) DiI was also used to ascertain the feeding behavior of worms crawling on (i) and off food (ii), as well as those swimming in water (iii) or dopamine solutions (iv). (E) Worms crawling on and off food readily ingested the DiI solution. However, worms swimming in a DiI solution did not ingest the dye unless dopamine was also present.
Defecation
Defecation was studied by first feeding worms the vital fluorescent dye DiI mixed with bacteria and then transferring them to different environmental conditions (Fig. 3B and C). The defecation cycle was characterized by the clearly visible contraction of the posterior intestine (pBoc).13 Worms transferred to dry agar plates with bacteria as food showed a defecation cycle frequency of roughly 56 sec (Vid. S2). However, worms transferred to water above bacterial food arrested their defecation rhythm (Fig. 3B).
Feeding
Feeding was also assayed with the help of fluorescent dye. Worms were incubated on agar plates that contained the fluorescent dye DiI (with or without bacterial food, OP50). Successful ingestion of the plate contents was obvious by the appearance of DiI within the lumen of the gut of worms harvested from the plate after 10 min (Fig. 3D, i). Although C. elegans has been reported to stop pumping its pharynx upon removal from a food patch, we also observed that worms crawling on agar plates devoid of bacteria took up DiI in the gut showing that feeding behavior occurs also in the absence of bacteria—a behavior we propose calling “drinking” (Fig. 3D, ii).14 To rule out passive uptake of the DiI into the gut, we placed freshly killed worms onto the same plates for the same period of time. Although some individuals showed dye uptake into sensory neurons exposed to the external environment as expected, none of the dead worms showed dye uptake into the pharynx or gut.15
Dopamine induction of land-associated behaviors in water
We previously showed that dopamine induces certain land-associated behaviors in water.6 To investigate the ability of dopamine to induce feeding behaviors in worms immersed in water, we placed animals into a liquid mixture of dopamine and DiI. Dopamine-treated worms displayed uptake of the dye in the gut (Fig. 3D, iv); however, untreated worms showed no dye uptake in the gut after ten minutes of swimming (Fig. 3D, iii).
We further investigated the ability of dopamine to stimulate additional behaviors in worms immersed in water. We found that it was also sufficient to induce foraging, feeding and defecation motor programs in water in a dose dependent manner (Fig. 4A, C and D) but had limited effect on inducing egg laying (Fig. 4B).
Figure 4.
Dopamine triggers land-associated behaviors in swimming animals. Worms swimming in dopamine solutions began foraging (A), laying eggs (B), feeding (C) and defecating (D) in a dose-dependent manner.
Sharp turns
Sharp turns (omega bends and reversals) are the main way by which C. elegans changes its heading.16 We found that on land, and consistent with previous reports, crawling worms most often changed direction by using reversals (Fig. 5D, i).17,18 In water, however, swimming worms maintained the average turning rate observed on land (about five sharp turns per minute) but did so primarily by using omega bends (Fig. 5D, ii and iii; Vids. S3 and 4).
Figure 5.
Swimming and crawling worms maintain a constant turning rate by distinct means. (A) Moving worms change their heading principally by using omega bends (i) and reversals (ii). (B) Body curvature matrix showing a ten second period during which a swimming worm performed an omega bend resulting in a change in heading (arrowhead). (C) Curvature matrix showing a crawling worm performing a reversal resulting in a change in heading. In the plots blue, white and red denote body angles of 50, 0 and −50 degrees respectively, a means anterior and p posterior. (D) On land, worms used mostly reversals to change heading (i) while in water, the use of omega bends predominated (ii). Interestingly, the rate at which the animals turned remained constant in both environments (iii).
C. elegans shows specific subsets of behaviors while swimming and while crawling
As worms transitioned from crawling on land to swimming in water they ceased to perform many behaviors such as foraging (Fig. 2), feeding as well as egg laying and defecation (Fig. 3). We have previously shown that, like many animals, C. elegans has distinct gaits dedicated to terrestrial or aquatic locomotion and that in water worms can travel faster than they can on land.6 Previous work also showed that swimming C. elegans can chemotax in water which is reflected by our observations that animals were able to maintain their turning rate in water by increasing the use of omega turns over reversals (Fig. 5).19 The results of the pharmacological experiments demonstrate that dopamine can trigger the foraging, defecation and ingestion motor patterns in swimming worms, eliminating the possibility that swimming and/or immersion in water mechanically preclude the performance of these sub-behaviors (Fig. 4). This is in keeping with our previous findings where we observed worms deficient in serotonergic signaling engaged in foraging even while still swimming.6 Because these motor patterns can occur on land during active crawling or even in periods of immobility (“dwelling”), it seems likely that foraging, feeding and defecation are inhibited once worms enter water (as opposed to being triggered by contact with land).
Although the balance between serotonin and dopamine appears to be important in determining the permissibility of motor outputs performed by C. elegans, the mechanisms by which this gating takes place remain elusive and likely involve additional amines.6 The remaining biogenic amines in C. elegans, tyramine and octopamine, have been shown to inhibit egg-laying, foraging, reversals and pharyngeal pumping and other serotonin-associated behaviors on land.20,21
While we saw foraging and pharyngeal pumping for octopaminergic and tyraminergic-related mutants (tbh-1, octr-1, tdc-1, tyra-3 and ser-2 mutants) both on water and land, we did not observe eggs laying nor defecation in swimming (data not shown). This suggests that these amines likely gate behaviors in a combinatorial way.
In the wild, C. elegans is proposed to be a free-living nematode that inhabits soil and feeds on the bacterial lawns associated with decaying vegetation.22 Although C. elegans needs moisture to survive, it is likely that its food source is most abundant in close association with its solid decaying substrate. Indeed worms have been reported to lay eggs adjacent to food sources and to inhibit pharyngeal pumping and defecation while away from food.14,23 This would represent a remarkable parallel to other organisms such as the leech, where serotonin has also been shown to inhibit foraging, feeding and enteric processes in swimming animals.24
Recent work showed that nictating worms can be transported to distant and potentially more appealing environments by riding on other invertebrates.25 Our previous observation that worms can locomote faster in water than on land, coupled with the often translational nature of aquatic environments, could potentially provide additional means of dispersal for this fragile and yet ubiquitous, species.
Fluorescein dyes have been previously used in C. elegans research.15 We found that feeding worms small quantities of DiI (diluted in OP50 bacterial lawns) was an excellent way to image and quantify the different motor programs associated with feeding, digestion and defecation in C. elegans. The highly fluorescent dye could label the food as it was ingested by the corpus, masticated by the posterior bulb and swallowed by the intestinal valve. Furthermore, the muscular contractions leading to defecation could be clearly imaged (Vid. S2) even at low magnification. The number and spatial distribution of fluorescent excreta on the agar plate could also be easily monitored in water and on land. We therefore believe that this approach would prove amenable to high throughput techniques applied to the study of alimentary processes.
Our findings have additional implications for studies that expose animals to different physical environments. Since the cuticle and the gut are two of the largest organs in C. elegans, animals feeding on a bacterial lawn are likely to absorb diluted chemicals at a faster rate (and extent) than those that stop feeding swimming in liquid. Furthermore, our observation that worms on empty plates ingest dye into their guts suggests that they are capable of drinking the fluid on the substrate. By contrast, our dye experiments indicated that worms did not feed nor drink when swimming. Pharmacological studies therefore can yield widely different potencies for drugs depending on whether they are delivered on dry agar or in liquid. This is illustrated by the finding that C. elegans requires 400 mM ethanol on land vs. over 800 mM ethanol in water to become immobilized.26,27
Over 60 years ago, Nikko Tinbergen proposed a model for the production of animal behavior in which the hormonal state of an animal (under the influence of external and internal signals) established a decision hierarchy dictating which behaviors an animal would choose to perform.2 Jack Davis and others later furthered Tinbergen’s work by showing that one mechanism by which this model could work was by inhibitory interactions in which one behavior always inhibited the production of another, thus setting the stage for the construction of hierarchical charts describing the interaction between unrelated motor networks in an animal.3 Originally these interactions were believed to be exclusively mediated by dedicated command neurons.28 Evidence now suggests that these decisions may be the result of combinatorial codes in which the combination of neurons recruited decides the behavioral output.4 Our finding that environmental transitions hormonally modulate the production of groups of motor programs in C. elegans is evidence of the existence of behavioral hierarchies. Specifically, swimming inhibits feeding-associated behaviors and sharp reversal turns while crawling promotes these behaviors and inhibits omega turns. The genetic tractability, complete neural wiring diagram and behavioral repertoire of C. elegans make it an attractive system for the study of the mechanisms responsible for the coordination of adaptive hierarchical behaviors.
Materials and Methods
Wild-type C. elegans were grown on nematode growth media (NGM) agar plates seeded with OP50 bacteria at 20°C as previously described.29
Statistical analysis
For all comparisons in this study, 30 < n < 60. All the bars report means and variation is given as SEM throughout. All statistical analysis was performed using SigmaStat 3.5 (Aspire Software). Comparisons between different experimental groups were performed by planned, two-tailed paired or unpaired t-tests to compare different groups that were normally distributed. Differences between non-normally distributed groups (or those with unequal variances) were compared using the Mann-Whitney ranked sum test. In all cases, p values are reported following the convention: *p < 0.05 and **p < 0.001.
Behavioral assays
Each assay was conducted on never-starved, young adult worms over several trials. Worms were cleaned of bacteria by allowing them to crawl on an empty plate before each experiment. After transfer to the assay plate, worms were allowed a two-min acclimation period. Movie recordings were made at 30 fps, 344 pixels/mm using a Flea2 camera (Point Grey Research) and StreamPix software (NorPix).
Foraging
We tested foraging by filming groups of 20 worms in a copper ring (see below) and counting the fraction of worms showing the typical high-frequency oscillations of the tip of the head. Additionally, we digitally filmed individual crawling and swimming worms and analyzed as previously described19 (Movie S1). Angular data from the apical ends of the worms were used to quantify high frequency oscillations during foraging (Fig. 2).
Feeding
We placed groups of 20 worms on an agar plate surrounded by a copper ring. To assay feeding behavior in the presence of food we placed worms inside a ring containing OP50 bacteria stained with a fluorescent dye (2 μM DiI, Sigma). We also measured feeding behavior in crawling worms in the absence of food by placing them in a ring without bacteria but where 10 μl of 2 μM DiI was placed allowing the agar to absorb the liquid and leave the dye behind. To assess if worms fed while swimming in water we placed them in a ring filled with 300 μl of liquid NGM buffer (with 2 μM DiI). We tested the potential role of dopamine in gating feeding behavior by placing worms in a ring with 300 μl of dopamine dissolved in liquid NGM buffer (DA: 5 mg/ml, or 33 mM; DiI: 2 μM). After 10 min of incubation in each of these conditions, we observed worms under UV light and counted those with fluorescent DiI in their intestine.
Egg-laying
The rate of egg laying was assayed by placing ten first-day adult worms in a copper ring (measuring 1.4 cm in diameter) over a bacterial lawn. Worms were allowed to lay eggs for ten minutes after which they were removed and the number of eggs counted. To measure egg-laying behavior in water worms were assayed as above but in a ring that was prefilled with 0.4 ml of liquid NGM buffer. We used liquid NGM buffer to ensure that the osmolarity of the solution and that of the plates were comparable, thus controlling for potential osmolarity effects on this behavior.
Defecation
We incubated worms in a mixture of OP50 bacteria and 2 μM DiI for three hours. Worms with guts filled with this mix were then selected for transfer to a new OP50 seeded plate (this time without DiI) where their defecation behavior could easily be assayed using UV light to visualize the contractions of their (now fluorescent) gut and excreta (Movie S2). We assayed worms individually and in groups of eight as follows: Worms were individually transferred (n = 20) to a seeded plate and following a two-minute acclimation period the time elapsed between posterior body wall contractions (pBoc) was measured as previously described for 10 min.30 Additionally, groups of eight worms were selected as above and transferred to seeded plates. The total number of fluorescent excreta was counted at t = 2 min (after the initial two-minute acclimation period) and at t = 12 min. The difference between these numbers was used to calculate the average egestion frequency. Because the mean frequencies obtained by these two methods were similar, these data were pooled in the comparisons. To assay worms in water, a copper ring was placed above a bacterial lawn and prefilled with liquid NGM buffer (as described above). The measurements were conducted as described for groups of worms on dry agar. The stained bacteria were sufficiently bright and cohesive to sink and remain visible for the duration of the assay. Each assay was conducted four times (n > 40).
Spontaneous reversal and omega turn rates
The centroid tracks from crawling and swimming worms were obtained as previously described.6 Briefly, 15 worms were placed in a copper ring and filmed as they first crawled on agar and later swam in liquid NGM buffer. The centroids of the animals, as well as their trajectories were obtained using ImagePro (Media Cybernetics). The number of reversal and omega turning events were then obtained from the digitized centroid tracks and confirmed from the movies. For each animal, the number of individual omega turns and reversals events were counted as they swam or crawl. Only tracks from unobstructed animals (either by the copper ring or another animal) were used.
Supplementary Material
Acknowledgments
Worms were kindly provided by the Caenorhabditis Genetics Center, which is funded by the National Institute of Health (NIH) National Center for Research Resources. This work was supported by the Alcohol Beverage Medical Research Foundation, NIH NINDS Grant R01NS075541 and NIH NIAAA Grant R03AA020195.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/worm/article/19148
References
- 1.Wilson HM, Anderson LI. Morphology and taxonomy of Paleozoic millipedes (Diplopoda: Chilognatha: Archilopoda) from Scotland. J Paleontol. 2004;78:169–84. http://dx.doi.org/10.1666/0022-3360(2004)078, 0169:MATOPM.2.0.CO;2. [Google Scholar]
- 2.Timbergen N. The study of instinct. Clarenden Press; Oxford: 1951. [Google Scholar]
- 3.Davis WJ, Mpitsos GJ, Siegler VS, Pinneo JM, Davis KB. Neuronal substrates of behavioral hierarchies and associative learning in Pleurobranchaea. Am Zool. 1974;14:1037–50. [Google Scholar]
- 4.Esch T, Kristan WB., Jr. Decision-making in the leech nervous system. Integr Comp Biol. 2002;42:716–24. doi: 10.1093/icb/42.4.716. PMID:21708768; http://dx.doi.org/10.1093/icb/42.4.716. [DOI] [PubMed] [Google Scholar]
- 5.Kovac MP, Davis WJ. Behavioral choice: neural mechanisms in Pleurobranchaea. Science. 1977;198:632–4. doi: 10.1126/science.918659. PMID:918659; http://dx.doi.org/10.1126/science.918659. [DOI] [PubMed] [Google Scholar]
- 6.Vidal-Gadea AG, Topper S, Young L, Crisp A, Kressin L, Elbel E, et al. Caenorhabditis elegans selects distinct crawling and swimming gaits via dopamine and serotonin. Proc Natl Acad Sci U S A. 2011;108:17504–9. doi: 10.1073/pnas.1108673108. PMID:21969584; http://dx.doi.org/10.1073/pnas.1108673108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hart AC, editor. WormBook. The C. elegans Research Community, WormBook; Behavior. doi/10.1895/wormbook.1.87.1, http://www.wormbook.org. [Google Scholar]
- 8.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. http://dx.doi.org/10.1098/rstb.1986.005. [DOI] [PubMed] [Google Scholar]
- 9.C. elegans Sequencing Consortium Genome sequence of the nematode C. elegans: a platform for investigating biology. Science. 1998;282:2012–8. doi: 10.1126/science.282.5396.2012. PMID:9851916; http://dx.doi.org/10.1126/science.282.5396.2012. [DOI] [PubMed] [Google Scholar]
- 10.Croll NA. Components and patterns in the behaviour of the nematode Caenorhabditis elegans. J Zool. 1975;175:159–76. [Google Scholar]
- 11.Hart AC, Sims S, Kaplan JM. Synaptic code for sensory modalities revealed by C. elegans GLR-1 glutamate receptor. Nature. 1995;378:82–5. doi: 10.1038/378082a0. PMID:7477294; http://dx.doi.org/10.1038/378082a0. [DOI] [PubMed] [Google Scholar]
- 12.Waggoner LE, Zhou GT, Schafer RW, Schafer WR. Control of alternative behavioral states by serotonin in Caenorhabditis elegans. Neuron. 1998;21:203–14. doi: 10.1016/s0896-6273(00)80527-9. PMID:9697864; http://dx.doi.org/10.1016/S0896-6273(00)80527-9. [DOI] [PubMed] [Google Scholar]
- 13.Thomas JH. Genetic analysis of defecation in Caenorhabditis elegans. Genetics. 1990;124:855–72. doi: 10.1093/genetics/124.4.855. PMID: 2323555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raizen DM, Lee RYN, Avery L. Interacting genes required for pharyngeal excitation by motor neuron MC in Caenorhabditis elegans. Genetics. 1995;141:1365–82. doi: 10.1093/genetics/141.4.1365. PMID:8601480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hedgecock EM, Culotti JG, Thomson JN, Perkins LA. Axonal guidance mutants of Caenorhabditis elegans identified by filling sensory neurons with fluorescein dyes. Dev Biol. 1985;111:158–70. doi: 10.1016/0012-1606(85)90443-9. PMID:3928418; http://dx.doi.org/10.1016/0012-1606(85)90443-9. [DOI] [PubMed] [Google Scholar]
- 16.Croll NA. Components and patterns in the behavior of the nematode Caenorhabditis elegans. J Zool. 1975;176:159–76. http://dx.doi.org/10.1111/j.1469-7998.1975.tb03191.x. [Google Scholar]
- 17.Pierce-Shimomura JT, Morse TM, Lockery SR. The fundamental role of pirouettes in Caenorhabditis elegans chemotaxis. J Neurosci. 1999;19:9557–69. doi: 10.1523/JNEUROSCI.19-21-09557.1999. PMID: 10531458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gray JM, Hill JJ, Bargmann CI. A circuit for navigation in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2005;102:3184–91. doi: 10.1073/pnas.0409009101. PMID:15689400; http://dx.doi. org/10.1073/pnas.0409009101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pierce-Shimomura JT, Chen BL, Mun JJ, Ho R, Sarkis R, McIntire SL. Genetic analysis of crawling and swimming locomotory patterns in C. elegans. Proc Natl Acad Sci U S A. 2008;105:20982–7. doi: 10.1073/pnas.0810359105. PMID:19074276; http://dx.doi.org/10.1073/pnas.0810359105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alkema MJ, Hunter-Ensor M, Ringstad N, Horvitz HR. Tyramine Functions independently of octopamine in the Caenorhabditis elegans nervous system. Neuron. 2005;46:247–60. doi: 10.1016/j.neuron.2005.02.024. PMID:15848803; http://dx.doi.org/10.1016/j.neuron.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 21.Wragg RT, Hapiak V, Miller SB, Harris GP, Gray J, Komuniecki PR, et al. Tyramine and octopamine independently inhibit serotonin-stimulated aversive behaviors in Caenorhabditis elegans through two novel amine receptors. J Neurosci. 2007;27:13402–12. doi: 10.1523/JNEUROSCI.3495-07.2007. PMID:18057198; http://dx.doi.org/10.1523/JNEUROSCI.3495-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Félix M-A, Braendle C. The natural history of Caenorhabditis elegans. Curr Biol. 2010;20:R965–9. doi: 10.1016/j.cub.2010.09.050. PMID:21093785; http://dx.doi.org/10.1016/j.cub.2010.09.050. [DOI] [PubMed] [Google Scholar]
- 23.Hardaker LA, Singer E, Kerr R, Zhou G, Schafer WR. Serotonin modulates locomotory behavior and coordinates egg-laying and movement in Caenorhabditis elegans. J Neurobiol. 2001;49:303–13. doi: 10.1002/neu.10014. PMID: 11745666; http://dx.doi.org/10.1002/neu.10014. [DOI] [PubMed] [Google Scholar]
- 24.Kristan WB, Jr., Calabrese RL, Friesen WO. Neuronal control of leech behavior. Prog Neurobiol. 2005;76:279–327. doi: 10.1016/j.pneurobio.2005.09.004. PMID:16260077; http://dx.doi.org/10.1016/j.pneurobio.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Lee H, Choi M-K, Lee D, Kim H-S, Hwang H, Kim H, et al. Nictation, a dispersal behavior of the nematode Caenorhabditis elegans, is regulated by IL2 neurons. Nat Neurosci. 2011;15:107–12. doi: 10.1038/nn.2975. PMID: 22081161; http://dx.doi.org/10.1038/nn.2975. [DOI] [PubMed] [Google Scholar]
- 26.Davies AG, Pierce-Shimomura JT, Kim H, VanHoven MK, Thiele TR, Bonci A, et al. A central role of the BK potassium channel in behavioral responses to ethanol in C. elegans. Cell. 2003;115:655–66. doi: 10.1016/s0092-8674(03)00979-6. PMID:14675531; http://dx.doi.org/10.1016/S0092-8674(03)00979-6. [DOI] [PubMed] [Google Scholar]
- 27.Morgan PG, Sedensky MM. Mutations affecting sensitivity to ethanol in the nematode, Caenorhabditis elegans. Alcohol Clin Exp Res. 1995;19:1423–9. doi: 10.1111/j.1530-0277.1995.tb01002.x. PMID: 8749805; http://dx.doi.org/10.1111/j.1530-0277.1995.tb01002.x. [DOI] [PubMed] [Google Scholar]
- 28.Wiersma CAG, Ikeda K. Interneurons commanding swimmeret movements in the crayfish, Procambarus clarkii (Girard) Comp Biochem Physiol. 1964;12:509–25. doi: 10.1016/0010-406x(64)90153-7. PMID:14206963; http://dx.doi.org/10.1016/0010-406X(64)90153-7. [DOI] [PubMed] [Google Scholar]
- 29.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. PMID:4366476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwasaki K, Liu DW, Thomas JH. Genes that control a temperature-compensated ultradian clock in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1995;92:10317–21. doi: 10.1073/pnas.92.22.10317. PMID:7479775; http://dx.doi.org/10.1073/pnas.92.22.10317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.