Summary
Diffusion-weighted imaging (DWI) is a powerful MRI method, which probes abnormalities of tissue structure by detecting microscopic changes in water mobility at a cellular level beyond what is available with other imaging techniques. Accordingly, DWI has the potential to identify pathology before gross anatomic changes are evident on standard anatomical brain images. These features of tissue characterization and earlier detection are what make DWI particularly appealing for the evaluation of gliomas and the newer therapies where standard anatomical imaging is proving insufficient. This article focuses on the basic principles and applications of DWI, and its derived parameter, the apparent diffusion coefficient, for the purposes of diagnosis and evaluation of glioma, especially in the context of monitoring response to therapy.
Traditionally, brain tumor response to treatment has been assessed using the MacDonald criterion [1], which is based on the two-dimensional measurement of enhancing tumor on MRI or computed tomography. Given that tumor enhancement primarily reflects the breakdown of the blood–brain barrier, it may not reflect true changes in tumor growth. For example, for patients treated with chemoradiation, ‘pseudoprogression’ can occur where there is an increase in tumor enhancement on MRI suggestive of tumor progression, but without increased tumor activity [2]. Pseudoprogression is confirmed later by a stabilization or decrease in tumor enhancement on imaging or with tissue obtained at surgery. An imaging biomarker capable of distinguishing these effects early on would be of tremendous benefit for these patients. Similarly, radiation necrosis, occurring 3–12 months after radiotherapy, can mimic tumor recurrence with increased enhancement, raising similar treatment questions.
For similar reasons use of the MacDonald criteria [3] or, more recently, the Revised Assessment in Neuro-Oncology (RANO) criteria [4], which also incorporate fluid-attenuated inversion recovery imaging (FLAIR), may be insufficient for the full evaluation of response to antiangiogenic therapies, such as bevacizumab [5]. Since these agents also act as powerful steroids, decreasing the permeability of the blood–brain barrier, rapid decreases in the degree of contrast enhancement and extent of surrounding hyper-intense T2-weighted signal intensity on FLAIR images may not reflect true changes in tumor activity. This phenomenon, termed ‘pseudoresponse’ [2], may explain why an improvement in progression-free-survival, but not overall survival, has been observed for patients treated with these agents, in that standard imaging is not an accurate representation of both enhancing and nonenhancing tumor growth. Related to this, there is evidence from both preclinical [6–8] and recent clinical studies [9,10] concerning a possible relationship between antiangiogenic drugs and increased tumor cell invasion. Clearly there is a need for imaging biomarkers that provide objective information related to tumor growth and invasion.
Diffusion-weighted imaging (DWI) has the potential to serve as an imaging biomarker for these purposes, since it can provide objective information about tissue and tumor cell density, and thus cell death and tumor cell invasion. Therefore, the focus of this review will be on DWI and its derived parameters, the most important of which is apparent diffusion coefficient (ADC), and its utility as an imaging biomarker in glioma. Specifically, this article will include a brief review of the basic principles of DWI followed by a description of the relationship between ADC and cell density, the potential of ADC and related parameters to distinguish tumor types and grades, and their ability to provide important information regarding response to treatment.
Basic principles of DWI
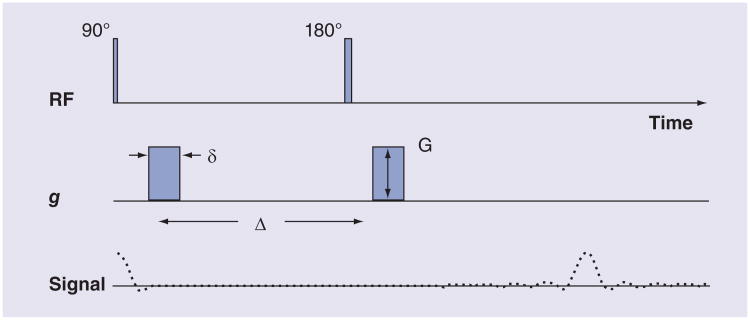
In MRI, the unique spatial information necessary to produce images is generated through the application of imaging gradients, which are magnetic fields that vary with position. MRI is made sensitive to tissue water diffusion by adding additional imaging gradients, called diffusion gradients, which are much larger than the standard imaging gradients. More specifically, as shown in Figure 1, the images are made sensitive to diffusion by adding two identical magnetic field gradients, one on each side of the 180° ‘refocusing’ radiofrequency pulse. These two gradients, separated in time, are effectively the inverse of each other due to the 180° pulse applied between. Water ‘spins’ accumulate spatially dependent phase during the first gradient pulse. The second gradient pulse reverses the phase introduced by the first gradient pulse. Any translation of the spins (i.e., diffusion), during and between the two gradients, results in a phase offset proportional to the distance traveled. This results in an attenuation of the measured diffusion-weighted signal as defined by the following equation:
Figure 1. A diffusion-weighted imaging sequence.
Typical timing of events that occur with a diffusion-weighted imaging sequence. They include application of r.f. pulses, consisting of 90° and 180° (inversion) pulses, and gs. The sequence of signals generated is also shown. Not shown are the imaging gradients, which are required for the measurement of spatially localized magnetic resonance signals. The diffusion weighting or b-value is defined by δ, G and Δ.
δ: Diffusion gradient duration; Δ: Time between application of identical diffusion gradients; g: Diffusion gradient; G: Gradient amplitude; RF: Radiofrequency.
| (Equation 1) |
where ADC is given in units of mm2/s, TE is the echo time, T2 is the tissue transverse relaxation time and b is the diffusion weighting or ‘b-value’. Note that since the diffusing water is not freely diffusing but instead is restricted by many different barriers, such as cell membranes, the measured diffusion coefficient is referred to as the ADC rather than simply D, the diffusion coefficient. The b-value is a function of the gyromagnetic ratio, γ, for a given nucleus (which in this case is water) that relates the nuclear magnetic resonance frequency and the external magnetic field, diffusion gradient strength (G), diffusion gradient duration (δ) and diffusion gradient separation (Δ). For the DWI sequence shown in Figure 1 the equation describing the b-value is:
| (Equation 2) |
From Equation 1 it is clear that when either ADC or the b-value increases, the signal decreases. Conversely, if the ADC decreases, as can occur with a more restricted tissue environment, the DWI signal will increase. However, changes in tissue T2 that might occur, for example, with edema, also influence the DWI signal resulting in what is termed a ‘T2 shine-through’ effect. Consequently, to determine changes in ADC alone, the DWI signal is measured at a minimum of two different b-values and ADC computed:
| (Equation 3) |
In the brain, the b-values that are most commonly used for determination of ADC are b = 0 and b = 1000 s/mm2. These values were chosen to maximize the signal to noise of the computed ADC image map. However, more recent recommendations suggest that the minimum b-value be a nonzero value. Choosing b-values of the order of 50–200 s/mm2 will minimize contributions from fast-diffusing components that may result from microvascular perfusion rather than true tissue water diffusion. These intravoxel incoherent motion effects have been well studied in the brain in the past [11,12], and are being addressed again in several more recent studies [13].
It should be noted that DWI images are generated from the average of three diffusion-weighted signals, each obtained by applying the diffusion gradients in three orthogonal directions. This results in the voxel-wise display of an isotropic diffusion-weighted signal, which by definition is independent of direction. A related technique called diffusion tensor imaging (DTI) enables the calculation of both the direction and magnitude of the water diffusion. This technique is designed to probe the directional differences of diffusion in the brain's gross microstructure, such as in white matter tracts. DTI requires the acquisition of at least six DWI images each with different diffusion gradient directions, but a common diffusion time that is long enough to allow a residing proton to probe the microstructure for the purpose of obtaining the diffusion tensor matrix [14]. DTI has primarily played a role in the presurgical mapping of brain tumors in an effort to avoid damage to these tracts [15 –17]. There have also been studies demonstrating that fractional anisotropy, a parameter that is derived from DTI data, may provide information about tumor invasion into white matter tracts [18,19]. However, DTI is beyond the scope of the present review, and therefore the remainder of this review will continue to focus on DWI and its applications for evaluating glioma.
DWI for brain tumors
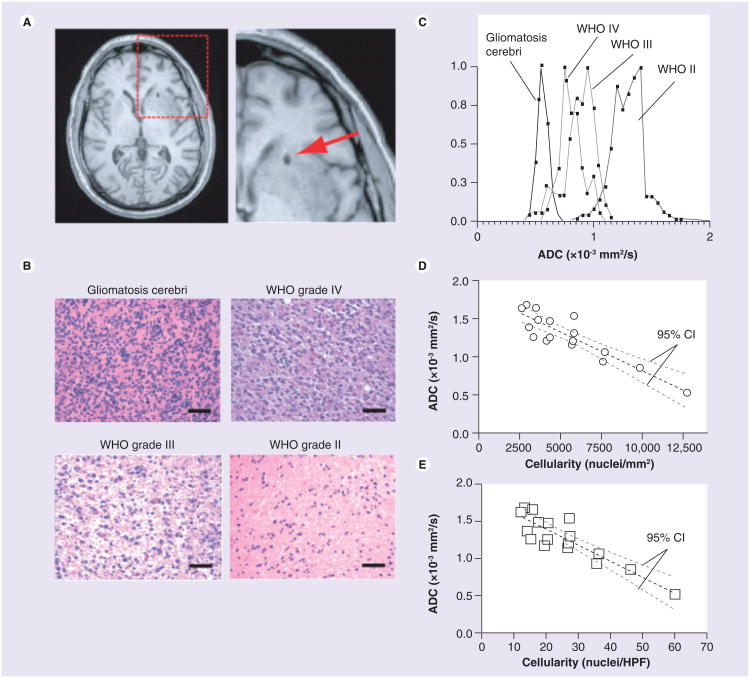
DWI is a rather recent addition to the magnetic resonance sequences conventionally employed to diagnose and follow brain tumors. Early on DWI was used most frequently in the evaluation of stroke. Within seconds of stroke onset, cytotoxic edema occurs, where there is a shift of water from the extracelluar to cellular space, resulting in a greater restriction of water movement. This restriction is visible as increased signal on DWI (Equation 1). Likewise for densely cellular neoplasms, which have a greater restriction of water diffusion compared with less cellular tissues, this gives rise to increased signal on DWI and decreased ADC. The relationship between ADC and tumor cellularity has been demonstrated in several studies [20–22]. The results from one such study [22] are shown in Figure 2 where cell density, measured via histological analysis, was compared with the ADC values computed in the corresponding location.
Figure 2. Correlation between spatially matched apparent diffusion coefficient measurements and cell density from stereotactic biopsy samples.
(A) Postoperative, high-resolution 3D T1-weighted anatomical MRI showing the biopsy location (arrow) in single patients. (B) Representative histological images (hematoxylin and eosin, ×20 magnification) showing how cell density increases with an increase in tumor grade (scale bars: 50 μm). (C) Spatially matched ADC measurements taken from the biopsy location in the same four patients as in (B), showing a decrease in ADC with an increase in tumor grade and cell density. (D) Scatter plot of average ADC within stereotactic biopsy locations and average cellularity for 17 patients (circles) shows a significant linear correlation (Pearson's correlation coefficient, r2 = 0.7933; p < 0.0001) between mean ADC and mean cell density in nuclei/mm2. (E) Correlation between mean ADC and mean cell density in nuclei/HPF. (D & E) Dashed black line indicates the linear regression while the dashed gray lines indicate the 95% CIs for the regression.
ADC: Apparent diffusion coefficient; HPF: High power field. Reproduced with permission from John Wiley and Sons (License No. 2901470746078) [22].
In this context, additional studies demonstrated that the ADC correlated inversely with tumor proliferation rates as measured by the Ki-67 labeling index [23], and was predictive of prognosis in a manner independent of tumor grade [24]. Note that the tumor classification and grading labels used throughout this review are based on those defined by WHO in their 1993 classification [25]. With respect to astrocytoma/glioma, a four-tiered system is applied, where low-grade tumors refer to both grade I and II tumors, and high-grade tumors refer to those diagnosed as grade III or grade IV tumors. The validity of these relationships was further supported by yet another study showing that the diagnosis of early stages of malignant glioma can be aided with DWI, such that areas of low ADC, suggestive of high cellularity, are more consistent with high-grade glioma [26]. As an example, Figure 3 shows DWI and ADC maps for two patients, one with a grade II tumor and another with a glioblastoma (grade IV glioma). For the grade II tumor, the enhancing tumor, indicated by the arrow, corresponds to an area of high signal on both DWI and ADC maps, consistent with a tumor that may be hypocellular compared with normal-appearing brain. Conversely, areas of enhancing glioblastoma correspond to areas of low ADC, suggesting a more densely cellular tumor.
Figure 3. Diffusion-weighted images and apparent diffusion coefficient images for low- and high-grade glioma.
(A & D) Postcontrast T1-weighted images, (B & E) diffusion-weighted images obtained at b = 1000 s/mm2 and (C & F) the corresponding apparent diffusion coefficient maps for patients with (A–C) grade II astrocytoma and (D–F) recurrent glioblastoma, respectively. The bright signal on diffusion-weighted images can result from some combination of restricted diffusion and T2 hyperintensity, the latter of which can occur within edematous tissue, for example. The apparent diffusion coefficient maps eliminate T2 contributions so that restricted diffusion, shown as areas of darker image intensity, can be more clearly delineated. In turn, restricted diffusion may represent areas of increased tumor cell density.
Despite these promising results, other investigators have questioned the relationship between ADC and tumor cell density, and have highlighted the considerable overlap between low- and high-grade gliomas. It was suggested that this overlap may be attributed to the focal necroses commonly found in grade IV gliomas, which cause the overall ADC values to be higher [27,28]. This observation also raises the issue of using mean ADC values to grade gliomas, and underscores the need for new analysis methods that do not characterize the entire, often very heterogeneous tumor, by one averaged value for a region of interest. In addition, the relationship between ADC and tumor cell density in treated tumor, for which treatment effects may confound the relationship, requires further evaluation.
Finally, since diagnosis and tumor grade are established by histopathologic evaluation of tumor biopsy samples or surgical resection, an accurate diagnosis is very dependent on the accurate spatial sampling of the most aggressive parts of the tumor. In this context, information derived from diffusion imaging could be used to better guide such sampling with the potential for greater accuracy in sampling and diagnosis [29]. Thus, more accurate preoperative grading of primary brain tumors could increase the diagnostic yield of brain biopsies, thereby improving patient management.
DWI metrics for the evaluation of treatment response
As previously stated, conventional imaging is often not able to provide an early indication of the effectiveness of radiation therapy, chemotherapy and/or targeted therapies. Clearly biomarkers that can provide more specific information about tumor biology within both contrast-enhancing and nonenhancing areas are needed. Diffusion MRI has this potential, examples of which are described next.
Apparent diffusion coefficient
Consistent with the idea that ADC inversely correlates with tumor cell density, several studies have demonstrated that a decrease in cellularity, and thus increase in ADC, is observed with successful treatment [30,31]. Likewise, DWI was used to evaluate the effectiveness of the radiosensitizer gemcitabine in a mouse glioma model [32], where it was demonstrated that tumor diffusion values increased prior to detectable changes in tumor volume following treatment. Yet another study demonstrated that areas of restricted diffusion in patients with glioblastoma predicted sites that later enhanced, regardless of antiangiogenic therapy with bevacizumab [33]. More recently, a histogram analysis of ADC values was used to stratify progression-free and overall survival in patients with recurrent glioblastoma treated with bevacizumab in a multicenter study. Consequently, ADC alone has a demonstrated promise to predict both treatment response and failure. The potential is further demonstrated when ADCs collected over time are used to create functional diffusion maps (fDMs), the principles of which are described next, followed by examples demonstrating their potential as a biomarker to predict treatment response.
Functional diffusion maps
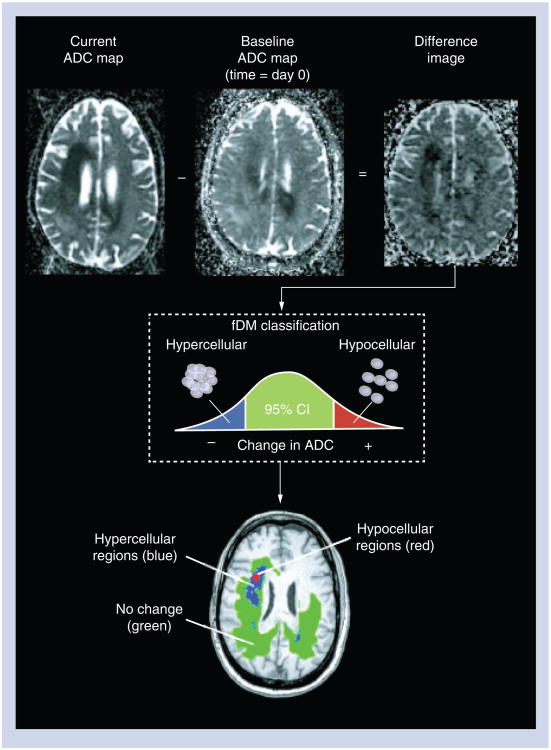
First developed by Moffat et al.[34–36], fDMs display changes in ADC that occur over time, and have demonstrated great promise as a new therapeutic biomarker for glioma. The creation of fDMs first requires image registration between a current ADC map and the baseline ADC map. After proper registration is verified, voxel-by-voxel subtraction is performed between ADC maps acquired at subsequent time points and the baseline, postsurgical and pretreatment ADC maps to create ΔADC images. Individual voxels are then stratified into three categories based on the change in ADC relative to baseline ADC maps. Red voxels represent areas where ADC increased beyond the ΔADC threshold (i.e., ‘hypocellular’ voxels), blue voxels represent areas within the tumor where ADC decreased beyond the ΔADC threshold or confidence limits (i.e., ‘hypercellular’ voxels) and green voxels represent no change in ADC above or below the ΔADC threshold. This process is summarized in Figure 4. Note that the terms ‘hypocellularity’ and ‘hypercellularity’ are used instead of increasing and decreasing ADC for ease of reference, but are given in quotation marks since there exist many tissue conditions other than changes in cell density, such as edema, gliosis, infection and ischemia, that can contribute to such changes. These must all be considered in the context of a given case before concluding that true changes in cell density have occurred.
Figure 4. Calculation of functional diffusion maps from sequential apparent diffusion coefficient maps.
For each postbaseline time point, the baseline ADC map is subtracted from the ADC map from the current day. Each voxel within an ADC difference image is stratified into three categories based on the magnitude of the ADC change: a decrease in ADC suggestive of increased cellularity (blue voxels); an increase in ADC suggestive of decreased cellularity (red voxels); and those with no significant change in ADC (green voxels).
ADC: Apparent diffusion coefficient; fDM: Functional diffusion map. Reproduced with permission from John Wiley and Sons [22].
Early on, the fDM analysis was applied to ADC image voxels within the contrast-enhancing tumor only [34–36], and a graphical method was developed to show the portion of voxels within each category. An example of this graphical representation is shown in Figure 5. In these studies it was demonstrated that the volume of voxels with increasing ADC (i.e., red voxels) or the total volume of voxels with both increasing and decreasing values (red plus blue voxels) were predictive of response to chemotherapy.
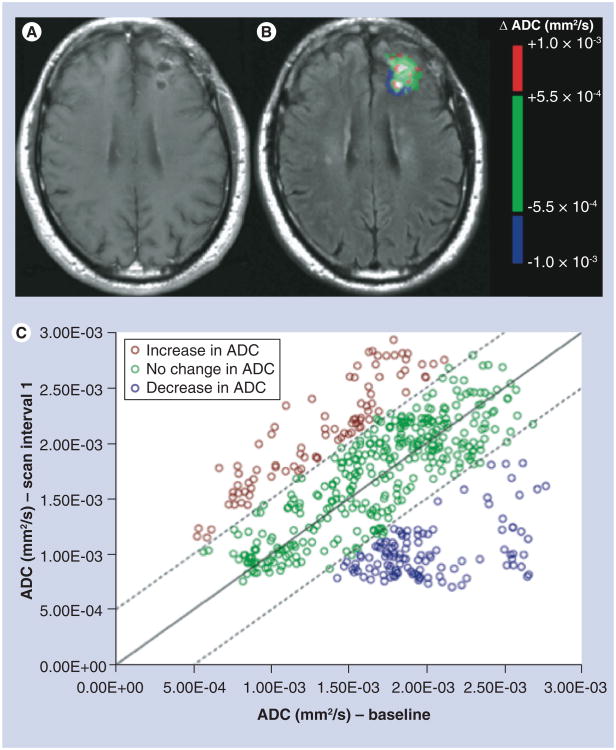
Figure 5. Example of voxel-wide analysis of diffusion changes within the contrast-enhancing tumor region of interest for a 55-year-old male patient, with a diagnosis of anaplastic oligoastrocytoma, after completing radiation therapy.
(A) A postcontrast image, (B) the colorized ADC threshold map (i.e., functional diffusion map) and (C) a graph showing the ADC changes of all pixels within the enhancing region of interest, with red, green and blue voxels representing increases (>5.5E-4), no change and decreases in ADC (<-5.5E-4), respectively.
ADC: Apparent diffusion coefficient.
Further validation of the fDM technique was carried out in a study by Ellingson et al.[22]. In this study, a comprehensive evaluation of the thresholds used to create the fDM categories was performed. Here it was demonstrated that the 95% confidence limits to categorize tissue as having no change in diffusion depend on whether the change is defined as relative to the range of values of white matter alone, gray matter, cerebrospinal fluid or a mixture of some or all tissues. Not surprisingly, the sensitivity and specificity to detect disease progression will change with the chosen ‘normal’ confidence limits. Still, with each limit chosen the distinction was always better than chance. In practice, a threshold of ±0.40 × 10−3 mm2/s is used, based on the 95% confidence limit of normal-appearing white and gray matter. This choice is based on the results of the receiver operating characteristic analysis, confirming that this threshold gave the best sensitivity and specificity for distinguishing stable from progressive disease. Finally, this validation study also led to the development of graded fDMs [37], which enable one to simultaneously view the fDM results using several different thresholds, each with a slightly different accuracy as defined by the validation study [22].
Using ADC & fDMs to evaluate response to cytotoxic & antiangiogenic therapies
To further explore the potential of fDMs to predict response to therapy, Ellingson et al.[38] evaluated fDMs in glioma patients undergoing either cytotoxic or antiangiogenic therapies. In contrast to many previous fDM studies, which focused on changes in diffusion within contrast-agent enhancing tumor [34,35], the focus of this study was on changes in diffusion within abnormalities apparent on FLAIR images. These abnormalities, which are evident as bright areas compared with the surrounding normal-appearing brain, typically consist of enhancing tumor and a region much beyond that. In glioma, the FLAIR image abnormalities have been shown to represent some mixture of edema and invading tumor cells [39].
The results from this study, performed in a total of 50 patients with confirmed glioma, demonstrated that the rate of change in fDM ‘hypercellular’ volumes within FLAIR abnormalities predicted tumor progression, time to progression and overall survival for both antiangiogenic and cytotoxic treatments earlier than standard anatomical imaging. Two examples of this are shown in Figures 6 & 7 for patients undergoing antiangiogenic and cytotoxic therapy, respectively. In each case the increase in the volume of blue ‘hypercellular’ voxels preceded changes observed on standard anatomical imaging. Thus, functional diffusion mapping, applied to both enhancing and nonenhancing tumors, clearly has the potential to serve as an imaging biomarker to track treatment response in glioma.
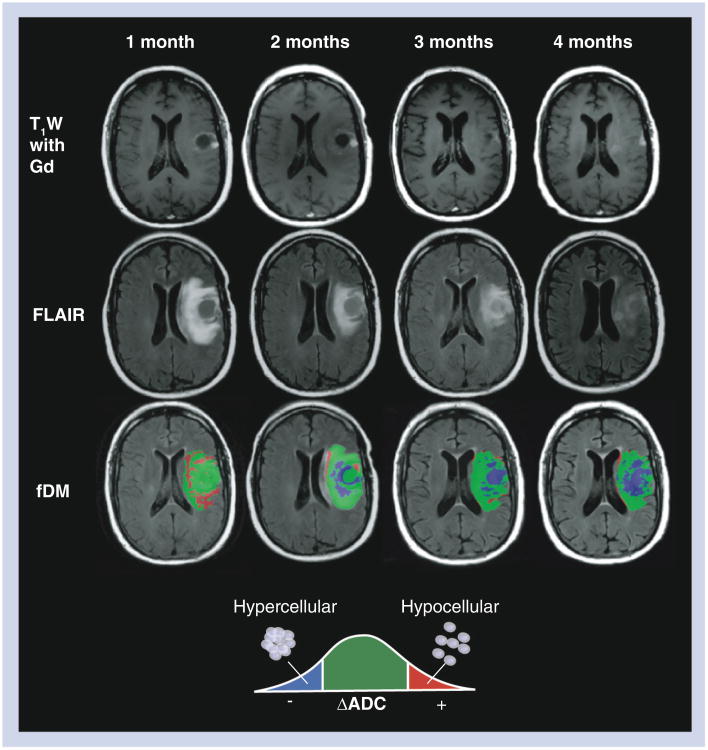
Figure 6. Standard MRI and functional diffusion maps in a patient with progressive disease after treatment with bevacizumab.
A 47-year-old male with a history of glioblastoma multiforme completed radiotherapy with concurrent temozolomide, followed by adjuvant temozolomide. His tumor recurred radiographically just prior to baseline ADC maps. The patient was then changed to bevacizumab monotherapy, and initially contrast enhancement and FLAIR signal abnormality improved substantially. The patient declined neurologically over 4 months of bevacizumab treatment, despite a positive radiographic response on postcontrast T1W (top row) and FLAIR images (middle row). The patient expired 2 months from the last fDM time point (6 months after start of bevacizumab treatment). During bevacizumab treatment, fDMs showed a rapid increase in the volume of hypercellularity (blue voxels), indicative of failed treatment.
ADC: Apparent diffusion coefficient; fDM: Functional diffusion map;
FLAIR: Fluid-attenuated inversion recovery; T1W: T1 weighted. Reproduced with permission from Springer (License No 2898871275280) [43].
Figure 7. Changes in apparent diffusion coefficient predict progression in patient with suspected pseudoprogession.
Shown are postcontrast T1-weighted images (A–C), FLAIR images (D–F) and ADC changes (G–I) for a 52-year-old male patient diagnosed with a glioblastoma at 1 month (column 1), 7 months (column 2) and 9 months (column 3) post radiation therapy plus temozolomide. Based on standard imaging, pseudoprogression and radiation effect were presumed since enhancement appeared and then disappeared. Although true progression was not officially ruled out, radiology reports and discussions leaned more heavily towards a likely diagnosis of pseudoprogression and radiation effect. However, the ADC was progressively decreasing following radiotherapy, suggesting an increase in tumor cellularity, and thus true progression. The clinical course was consistent with true progression since the subject expired months following the scan shown in column 3. ADC: Apparent diffusion coefficient; fDM: Functional diffusion map; FLAIR: Fluid-attenuated inversion recovery.
Current issues & future considerations
As advanced imaging technologies, such as DWI, are being used more routinely to monitor therapies new demands regarding consistency of measurements over time and image registration requirements are arising. First, because of differences regarding how different vendors collect and process their DWI and thus ADC data, it is becoming increasingly difficult to accurately compare ADC across time when the data is obtained on different platforms for the same patient. In this regard, many have attempted or proposed homogenization of data collection and processing. However, this is always a ‘moving target’ given continual technology improvements, upgrades and simple issues of intellectual property and institutional preference. Instead it might be most reasonable to determine a way to standardize the images as part of the postprocessing workflow, as has been previously proposed for postcontrast and perfusion imaging [40–42]. Or, more simply and potentially more practically, each institution could implement the requirement to have each patient consistently scanned on the same platform over time. While this will not solve the problems that may occur with technology upgrades, for the most part it will ameliorate the majority of the inconsistencies that one would otherwise encounter.
Registration of diffusion images over time is also required for longitudinal assessment. In this regard, a wealth of image registration algorithms exist and have been applied. Typically, the higher resolution images are directly registered across time points. Then, assuming no motion occurred between the collection of the high-resolution image and the DWI data, for a given scanning session, the registration transformation is applied to the DWI and ADC data. This in general has been successful for registering DWI and ADC data over time, if the underlying DWI data is sufficiently similar as just discussed.
Additional issues of characterizing and optimizing the diffusion signal and the information that can be derived also need to be addressed. For example, while the focus of this paper was on DWI signal, which is typically obtained at two different b-values, there are studies to suggest that many more b-values, especially of higher value, can be of use. For example, if multiple b-values are collected that are sufficiently high, a sub-voxel diffusion heterogeneity index, termed ‘α’, can be determined [43]. This parameter may be a very early marker of tumor cell invasion owing to its sensitivity to the breakdown of the extracellular matrix. Early results obtained in animals [43] and patients [44] support the potential of this approach. A similar approach, using a diffusion kurtosis model, has also been developed with promise for evaluating gliomas [45,46]. Alternatively, an analytical solution to a glioma growth model was developed. This model allows for direct spatial quantification of microscopic tumor proliferation and migration based on serial diffusion MRI scans [47]. Although this approach appears promising, issues that affect accuracy, such as time interval between diffusion scans and noise levels, require further characterization.
As MRI technology continues to improve, new opportunities regarding how we measure the diffusion signal arise, which have important implications for how best to characterize tissue. For example, referring to Equation 2, the generation of diffusion weighting or b-values depends on many different gradient timing parameters. These, in turn, will dictate what ranges of water diffusion, and thus what types and sizes of cellular components, we are sensitive to. Consequently, by setting these timings differently, new and different information about the pathology can be derived, as demonstrated early on in animal models [48]. These possibilities must always be taken into consideration as we strive to move the field of DWI and evaluation of gliomas forward.
Finally, despite much promise, before DWI and its derived parameters can be used routinely to distinguish progression from treatment response and thus make treatment decisions, it is imperative that rigorous pathological validation be carried out. While initial studies, referred to earlier, demonstrate a strong correlation between tumor cell density and ADC (Figure 2) [22], these studies were performed in untreated patients. It is therefore necessary to perform similar studies in treated patients where spatially accurate correlations between imaging and tissue parameters can be assessed. Of note, such studies are currently underway [49,50].
Conclusion & future perspective
In general, it is becoming well known that conventional radiologic imaging tends to significantly underestimate the extent of diffuse infiltrative glioma growth [51], and as such is proving insufficient for the complete evaluation of treatment response in glioma. Given the sensitivity of DWI and its derived parameters to tissue microstructure and cell density, they clearly have the potential to provide important biomarkers for treatment response in glioma. This was demonstrated by studies showing the ability of ADC to distinguish tumor grade and predict sites of recurrence; new methods such as functional diffusion mapping, for monitoring changes in ADC over time, may provide an even earlier and more accurate predictor of response. Specifically, according to the NIH, a biomarker is defined as ‘a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes or pharmacologic responses to a therapeutic intervention’. Thus, DWI-derived parameters clearly fit this criterion.
Some technical and physiologic issues still need to be addressed. These include ensuring the consistency of data quality and information over time for a given patient, optimization of DWI parameters to most appropriately probe tissue structure, and further exploration and validation of both current and new analysis models.
Despite the necessity for further research and validation in multicenter settings, we suggest that it is already quite clear that DWI and its derived parameter ADC are playing, and will continue to play, an important role in the evaluation of treatment response in glioma.
Practice Points.
Diffusion-weighted imaging (DWI) is a powerful MRI method that is sensitive to microscopic changes in water mobility (i.e., water diffusion).
Image maps of the apparent diffusion coefficient (ADC), derived from DWI, give absolute measures of tissue water diffusion in units of mm2/s on a per-voxel basis.
ADC is inversely correlated with tumor and tissue cell density, and as such can provide information about tumor cell density, and potentially tumor grade.
Because DWI and its derived parameter, ADC, are correlated with cell density, DWI has the potential to detect invading tumor cells, which are often invisible on standard anatomical imaging.
Functional diffusion maps (fDMs) are one approach for showing changes in ADC over time, and thus have the potential to predict and evaluate response to treatment.
The utility of fDMs as a marker of response to chemotherapy has shown promise when looking at ADC changes within enhancing lesions.
The fDM information obtained from both enhancing and nonenhancing lesions has demonstrated a potential to predict response to newer therapeutic regimens earlier than standard radiologic assessment criteria.
Acknowledgments
Funding support was provided through NIH/NCI CA082500 and Advancing a Healthier Wisconsin Partnership Program. K Schmainda has ownership interest and spouse salary in Imaging Biometrics LLC.
Footnotes
Financial & competing interests disclosure: The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.MacDonald DR, Cascino TL, Schold SC, Cairncross JG. Response criteria for Phase II studies of malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 2.Brandsma D, van den Bent MJ. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol. 2009;22:633–638. doi: 10.1097/WCO.0b013e328332363e. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald DR, Cascino TL, Schold SC, Calmcross JG. Response criteria for Phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 4.Wen PY, MacDonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 5•.Henson JW, Ulmer S, Harris GJ. Brain tumor imaging in clinical trials. Am J Radiol. 2008;29:419–424. doi: 10.3174/ajnr.A0963. Presents a nice overview of the current limitations with standard anatomical imaging methods for the assessment of treatment response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamszus K, Kunkel P, Westphal M. Invasion as limitation to anti-angiogenic glioma therapy. Acta Neurochir Suppl. 2003;88:169–177. doi: 10.1007/978-3-7091-6090-9_23. [DOI] [PubMed] [Google Scholar]
- 7.Giese A, Bjerkvig R, Berens ME, Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol. 2003;21:1624–1636. doi: 10.1200/JCO.2003.05.063. [DOI] [PubMed] [Google Scholar]
- 8.Kunkel P, Ulbricht U, Bohlen P, et al. Inhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor 2. Cancer Res. 2001;61:6624–6628. [PubMed] [Google Scholar]
- 9.Tuettenberg J, Grobholz R, Seiz M, et al. Recurrence pattern in glioblastoma multiforme patients treated with anti-angiogenic chemotherapy. J Cancer Res Clin Oncol. 2009;135(9):1239–1244. doi: 10.1007/s00432-009-0565-9. [DOI] [PubMed] [Google Scholar]
- 10.Iwamoto FM, Abrey LE, Beal K, et al. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology. 2009;73:1200–1206. doi: 10.1212/WNL.0b013e3181bc0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lebihan D. IVIM method measures diffusion and perfusion. Diagn Imaging. 1990;12(6):133–136. [PubMed] [Google Scholar]
- 12.Lebihan D, Breton E, Lallemand D, Aubin M, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168:497–505. doi: 10.1148/radiology.168.2.3393671. [DOI] [PubMed] [Google Scholar]
- 13.Lemke A, Stieltjes B, Schad LR, Laun FB. Toward an optimal distribution of b values for intravoxel incoherent motion imaging. Magn Reson Imaging. 2011;29(6):766–776. doi: 10.1016/j.mri.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Basser PJ, Jones DK. Diffusion-tensor MRI: theory, experimental design and data analysis – a technical review. NMR Biomed. 2002;15(7–8):456–467. doi: 10.1002/nbm.783. [DOI] [PubMed] [Google Scholar]
- 15.Ulmer JL, Salvan CV, Mueller WM, et al. The role of diffusion tensor imaging in establishing the proximity of tumor borders to functional brain systems: implications for preoperative risk assessments and postoperative outcomes. Technol Cancer Res Treat. 2004;3(6):567–576. doi: 10.1177/153303460400300606. [DOI] [PubMed] [Google Scholar]
- 16.Cruz LC, Jr, Sorensen AG. Diffusion tensor magnetic resonance imaging of brain tumors. Mag Reson Clin N Am. 2006;14(2):183–202. doi: 10.1016/j.mric.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Ferda J, Kastner J, Mukensnabl P, et al. Diffusion tensor magnetic resonance imaging of glial brain tumors. Eur J Radiol. 2010;74(3):428–436. doi: 10.1016/j.ejrad.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 18.Stadlbauer A, Polking E, Prante O, et al. Detection of tumour invasion into the pyramidal tract in glioma patients with sensorimotor deficits by correlation of 18F-fuoroethyl-l-tyrosine PET and magnetic resonance diffusion tensor imaging. Acta Neurochir. 2009;151(9):1061–1069. doi: 10.1007/s00701-009-0378-2. [DOI] [PubMed] [Google Scholar]
- 19.Deng Z, Yan Y, Zhong D, et al. Quantitative analysis of glioma cell invasion by diffusion tensor imaging. J Clin Neurosci. 2010;17(12):1530–1536. doi: 10.1016/j.jocn.2010.03.060. [DOI] [PubMed] [Google Scholar]
- 20.Sugahara T, Korogi Y, Kochi M, et al. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging. 1999;9(1):53–60. doi: 10.1002/(sici)1522-2586(199901)9:1<53::aid-jmri7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 21.Hayashida Y, Hirai T, Morishita S, et al. Diffusion-weighted imaging of metastatic brain tumors: comparison with histologic type and tumor cellularity. Am J Neuroradiol. 2006;27:1419–1425. [PMC free article] [PubMed] [Google Scholar]
- 22••.Ellingson BM, Malkin MG, Rand SD, et al. Validation of functional diffusion maps (fDMs) as a biomarker for human glioma cellularity. J Magn Reson Imaging. 2010;31(3):538–548. doi: 10.1002/jmri.22068. Presents comprehensive studies to characterize the thresholds used for the creation of functional diffusion maps (fDMs) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simon D, Fritzsche KH, Thieke C, et al. Diffusion-weighted imaging-based probabilistic segmentation of high- and low-proliferative areas in high-grade gliomas. Cancer Imaging. 2012;12:89–99. doi: 10.1102/1470-7330.2012.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higano S, Yun X, Kumabe T, et al. Malignant astrocytic tumors: clinical importance of apparent diffusion coefficient in prediction of grade and prognosis. Radiology. 2006;241(3):839–846. doi: 10.1148/radiol.2413051276. [DOI] [PubMed] [Google Scholar]
- 25.Kleihues P, Burger PC, Scheithauer BW. Histological Typing of Tumours of the Central Nervous System. 2nd. Springer-Verlag; NY, USA: 1993. [Google Scholar]
- 26.Baehring JM, Bi WL, Bannykh S, Piepmeier JM, Fulbright RK. Diffusion MRI in the early diagnosis of malignant glioma. J Neurooncol. 2007;82(2):221–225. doi: 10.1007/s11060-006-9273-3. [DOI] [PubMed] [Google Scholar]
- 27.Sadeghi N, D'haene N, Decaestecker C, et al. Apparent diffusion coefficient and cerebral blood volume in brain gliomas: relation to tumor cell density and tumor microvessel density based on stereotactic biopsies. AJNR. 2008;29(3):476–482. doi: 10.3174/ajnr.A0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lam WW, Poon WS, Metreweli C. Diffusion MR imaging in glioma: does it have any role in the pre-operation determination of grading of glioma? Clin Radiol. 2002;57(3):219–225. doi: 10.1053/crad.2001.0741. [DOI] [PubMed] [Google Scholar]
- 29.Maia AC, Malheiros SM, Darocha AJ, et al. MR cerebral blood volume maps correlated with vascular endothelial growth factor expression and tumor grade in nonenhancing gliomas. AJNR. 2005;26:777–783. [PMC free article] [PubMed] [Google Scholar]
- 30.Chenevert TL, McKeever PE, Ross BD. Monitoring early response of experimental brain tumors to therapy using diffusion magnetic resonance imaging. Clin Cancer Res. 1997;3:1457–1466. [PubMed] [Google Scholar]
- 31.Chenevert TL, Stegman LD, Taylor JM, et al. Diffusion magnetic resonance imaging: an early surrogate marker of therapeutic efficacy in brain tumors. J Natl Cancer Inst. 2000;92:2029–2036. doi: 10.1093/jnci/92.24.2029. [DOI] [PubMed] [Google Scholar]
- 32.Galban S, Lemasson B, Williams TM, et al. DW-MRI as a biomarker to compare therapeutic outcomes in radiotherapy regimens incorporating temozolomide or gemcitabine in glioblastoma. PLoS ONE. 2012 doi: 10.1371/journal.pone.0035857. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta A, Young RJ, Karimi S, et al. Isolated diffusion restriction precedes the development of enhancing tumor in a subset of patients with glioblastoma. AJNR. 2011;32(7):1301–1306. doi: 10.3174/ajnr.A2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moffat BA, Chenevert TL, Lawrence TS, et al. Functional diffusion map: a noninvasive MRI biomarker for early stratification of clinical brain tumor response. Proc Natl Acad Sci USA. 2005;102(15):5524–5529. doi: 10.1073/pnas.0501532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moffat BA, Chenevert TL, Meyer CR, et al. The functional diffusion map: an imaging biomarker for the early prediction of cancer treatment outcome. Neoplasia. 2006;8(4):259–267. doi: 10.1593/neo.05844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36••.Hamstra DA, Chenevert TL, Moffat BA, et al. Evaluation of the functional diffusion map as an early biomarker of time-to-progression and overall survival in high-grade glioma. Proc Natl Acad Sci USA. 2005;102(46):16759–16764. doi: 10.1073/pnas.0508347102. Describes one of the seminal studies demonstrating the utility of changes in diffusion MRI measurements to monitor treatment response in brain tumors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37•.Ellingson BM, Cloughesy TF, Lai A, et al. Graded functional diffusion map-defined characteristics of apparent diffusion coefficients predict overall survival in recurrent glioblastoma treated with bevacizumab. Neuro Oncol. 2011;13(10):1151–1161. doi: 10.1093/neuonc/nor079. Extends fDMs to a multithreshold technique, which may prove to be more informative. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38••.Ellingson BM, Malkin MG, Rand SD, et al. Volumetric analysis of functional diffusion maps is a predictive imaging biomarker for cytotoxic and anti-angiogenic treatments in malignant gliomas. J Neurooncol. 2011;102(1):95–103. doi: 10.1007/s11060-010-0293-7. Demonstrates the ability of fDMS to predict response to both chemotherapy and targeted therapies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelly PJ, Daumas-Duport C, Kispert DB, Kall BA, Scheithauer BW, Illig JJ. Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg. 1987;66(6):865–874. doi: 10.3171/jns.1987.66.6.0865. [DOI] [PubMed] [Google Scholar]
- 40.Nyul LG, Udupa JK. On standardizing the MR image intensity scale. Magn Reson Med. 1999;42:1072–1081. doi: 10.1002/(sici)1522-2594(199912)42:6<1072::aid-mrm11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 41.Nyul LG, Udupa JK, Zhang X. New variants of a method of MRI scale standardization. IEEE Trans Med Imaging. 2000;19:143–150. doi: 10.1109/42.836373. [DOI] [PubMed] [Google Scholar]
- 42.Bedekar D, Jensen TR, Schmainda KM. Standardization of relative cerebral blood volume (rCBV) image maps for ease of both inter and intra-patient comparisons. Magn Reson Med. 2010;64(3):907–913. doi: 10.1002/mrm.22445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43••.Bennett KM, Hyde JS, Rand SD, et al. Introvoxel distribution of DWI decay rates reveals C6 glioma invasion in rat brain. Magn Reson Med. 2004:52, 994–1004. doi: 10.1002/mrm.20286. One of the first demonstrations of a new diffusion-weighted imaging-derived metric, termed α-diffusion, with the potential to detect invading tumor based on sub-voxel heterogeneity. [DOI] [PubMed] [Google Scholar]
- 44.Kwee TC, Galban CJ, Tsien C, et al. Intravoxel water diffusion heterogeneity imaging of human high-grade gliomas. NMR Biomed. 2010;23(2):179–187. doi: 10.1002/nbm.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peled S, Whalen S, Jolesz FA, Golby AJ. High b-value apparent diffusion-weighted images from CURVE-ball DTI. J Magn Reson Imaging. 2009;30(1):243–248. doi: 10.1002/jmri.21808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raab P, Hattingen E, Franz K, Zanella FE, Lanfermann H. Cerebral gliomas: diffusional kurtosis imaging analysis of microstructural differences. Radiology. 2010;254(3):876–881. doi: 10.1148/radiol.09090819. [DOI] [PubMed] [Google Scholar]
- 47.Ellingson BM, Cloughesy TF, Lai A, Nghiemphu PL, Pope WB. Cell invasion, motility, and proliferation level estimate (CIMPLE) maps derived from serial diffusion MR images in recurrent glioblastoma treated with bevacizumab. J Neurooncol. 2011;105(1):91–101. doi: 10.1007/s11060-011-0567-8. [DOI] [PubMed] [Google Scholar]
- 48•.Niendorf T, Dijkhuizen RM, Norris DG, Vanlookeren-Campagne M, Nicolay K. Biexponential diffusion attenuation in various states of brain tissue: implications for diffusion-weighted imaging. Magn Reson Med. 1996:26, 847–857. doi: 10.1002/mrm.1910360607. Illustrates how diffusion imaging sequences can be designed to be more or less sensitive to certain tissue compartments. [DOI] [PubMed] [Google Scholar]
- 49.Hu LS, Baxter LC, Smith KA, et al. Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. AJNR. 2009;30(3):552–558. doi: 10.3174/ajnr.A1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50•.Laviolette PS, Cochran EJ, Cohen AD, et al. Precise ex-vivo histological validation of heightened cellularity in regions of dark ADC in three cases of high-grade glioma; Presented at: 20th Annual Meeting of the International Society of Magnetic Resonance in Medicine; Melbourne, Australia. 5–11 May 2012; Describes a way to precisely validate diffusion metrics with tissue analysis, where the tissue and images correspond spatially. [Google Scholar]
- 51.Claes A, Idema AJ, Wesseling P. Diffuse glioma growth: a guerilla war. Acta Neuropathol. 2007;114(5):443–458. doi: 10.1007/s00401-007-0293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]