Abstract
GluA1 subunits of AMPA glutamate receptors are implicated in the synaptic plasticity induced by drugs of abuse for behaviors of drug addiction, but GluA1 roles in emotional learning and memories of drug reward in the development of drug addiction remain unclear. In this study of the central nucleus of the amygdala (CeA), which is critical in emotional learning of drug reward, we investigated how adaptive changes in the expression of GluA1 subunits affected the learning process of opioid-induced context–reward association (associative learning) for the acquisition of reward-related behavior. In CeA neurons, we found that CeA GluA1 expression was significantly increased 2 h after conditioning treatment with morphine, but not 24 h after the conditioning when the behavior of conditioned place reference (CPP) was fully established in rats. Adenoviral overexpression of GluA1 subunits in CeA accelerated associative learning, as shown by reduced minimum time of morphine conditioning required for CPP acquisition and by facilitated CPP extinction through extinction training with no morphine involved. Adenoviral shRNA-mediated downregulation of CeA GluA1 produced opposite effects, inhibiting the processes of both CPP acquisition and CPP extinction. Adenoviral knockdown of CeA GluA2 subunits facilitated CPP acquisition, but did not alter CPP extinction. Whole-cell recording revealed enhanced electrophysiological properties of postsynaptic GluA2-lacking AMPA receptors in adenoviral GluA1-infected CeA neurons. These results suggest that increased GluA1 expression of CeA AMPA receptors facilitates the associative learning of context-drug reward, an important process in both development and relapse of drug-seeking behaviors in drug addiction.
Introduction
Drug addiction is a chronic, relapsing neuropsychiatric disorder characterized by compulsive behaviors of drug seeking and drug taking, driven by positive rewarding effects of drugs and negative emotional state during drug withdrawal (Hyman et al., 2006; Robbins et al., 2008; Koob and Volkow, 2010). The development of drug addiction engages active learning and memories that associate drug stimuli and related environmental cues with positive emotion of reward. The cellular mechanisms for learning and memory involve adaptive changes in glutamate synaptic strength particularly through altered subunit composition of AMPA receptors (Bowers et al., 2010). Thus, activity-driven synapses may be strengthened by membrane insertion of GluA2-lacking (i.e., GluA1/GluA1 or GluA1/GluR3) AMPA receptors, which have higher Ca++ permeability and channel conductance than GluA2-containing AMPA receptors (Isaac et al., 2007; Kauer and Malenka, 2007). GluA1, the predominant subunit of central AMPA receptors, has been shown to mediate an enhanced response to the rewarding effect of opioids in the ventral tegmental area (VTA) (Carlezon et al., 1997); however, direct activation of VTA AMPA receptors does not induce rewarding effect or behaviors of reward (Ikemoto et al., 2004). GluA1 also plays an important role in behaviors of cocaine seeking and reinstatement (Bachtell et al., 2008; Engblom et al., 2008). Nevertheless, it remains unknown how GluA1 may act to influence the contextual learning of rewarding effects of drugs through environmental cues–reward association, an important process in the development of drug addiction.
The central nucleus of the amygdala (CeA), as part of the brain's reward circuitry, is important in processing positive emotions and particularly, in the learning process of stimulus–reward association (Baxter and Murray, 2002; See et al., 2003). Many studies have demonstrated the important role of amygdala, including CeA and basolateral amygdala (BLA), in opioid reward. Activation of CeA NMDA receptors increases low-dose morphine-induced CPP (Rezayof et al., 2007) and the signaling pathway of NMDA receptor–extracellular signal-regulated kinase (ERK) in CeA is critical for expression of morphine CPP and for enhanced morphine CPP (incubation of morphine craving) after morphine withdrawal (Li et al., 2008; Li et al., 2011). Recently, He et al. found that blockade of protein kinase M zeta (PKMzeta) in BLA, but not in CeA, inhibits morphine CPP (He et al., 2011). Additionally, ablation of neurokinin-1 receptor-expressing neurons in amygdala reduces morphine CPP (Gadd et al., 2003).
In adult amygdala neurons, both GluA1 and GluA2 subunits are highly expressed and GluA2-containing (GluA1/GluA2) AMPA receptors are predominant (Zamanillo et al., 1999; Mead and Stephens, 2003a, b; Tye et al., 2011). While activity-dependent synaptic long-term potentiation, a cellular model for learning and memory, can drive GluA1-containing AMPA receptors into synapses in hippocampal neurons (Hayashi et al., 2000), the function of CeA GluA1 subunits in the behavior of context–reward learning is largely unknown. In the current study in rats, we determined the behavioral effects of virally altered GluA1 and GluA2 expression in CeA neurons on the acquisition of morphine-induced CPP behavior, a widely used rodent model for measuring rewarding effects of drugs (Tzschentke, 2007).
Materials and Methods
Animals
All procedures involving the use of animals conformed to the guidelines set by the Institutional Animal Care and Use Committee of MD Anderson Cancer Center. Male Wistar rats (250–300 g) were housed in groups of three with food and water available ad libitum, and a 12 h light/dark cycle. All behavioral trainings and tests were performed between 8:00 am and 18:00 pm.
Cannula implantation and microinjection
General methods for site-specific microinjection were similar to those used in our previous studies (Zhu et al., 2007; Bie et al., 2009). Briefly, rats were anesthetized with sodium pentobarbital (50 mg/kg i.p.) and restrained in a stereotaxic apparatus. A 26-gauge, single guide cannula (Plastic One, Roanoke, VA) was inserted on each side of the brain, aiming at CeA (anteroposterior, –2.3 mm from the Bregma; lateral, ±4.0 mm; ventral, –8.0 mm from dura) (Paxinos and Watson, 1986). The guide cannula was then cemented in place to the skull and capped after placement of a solid dummy cannula with the same length as the guide cannula. The implanted rats were housed individually and allowed to recover from the surgery for at least 1 week before experiments. Bilateral microinjection of a viral vector (1 μl each side) into CeA was made through a 33-gauge injector with an infusion pump at a rate of 0.1 μl/min. All cannula placements for bilateral CeA injections were histologically verified afterwards.
Adeno-associated viral (AAV) vectors
For construction of AAV-GluA1-flip vector, the coding sequence of the rat GluA1 flip version (gene bank accession: M38060.1) (Sommer et al., 1990) was amplified by RT-PCR from rat brain mRNA with the primer pair: 5′-CCGAATTCTATGCCGTACATCTTTGCC-3′ and 5’-CCGTCGACTTACAATCCTGTGGCTCCCAAGG-3′. The PCR product was purified and inserted into pAAV-CMV backbone vector through EcoR I and Sal I sites to obtain the pAAV-CMV-GluA1-flip vector. The vector was analyzed by PCR, restriction endonuclease analysis and sequencing to make sure there was no mutation in the GluA1 coding sequence. The vector was further validated by expression of GluA1 protein in CHO cells and then the validated vector was prepared for adeno virus packaging (Gao et al., 2002). For construction of AAV-GluA1-shRNA-GFP vector, four 21 nt candidate sequences against rat GluA1 mRNA were selected with the BLOCK-iT™ RNAi Designer (Invitrogen) and then cloned into pAAVsc-si-EGFP shRNA expression vector with a loop sequence of TTCAAGAGA. The silence efficiency of these shRNA sequences was evaluated by co-transfection of each shRNA expression vector with pAAV-GluA1 vector into CHO cells. Transfected cells were harvested at 48 h post-transfection. AAV-GluA2-shRNA vectors were similarly constructed, validated and packaged. GluA1 and GluA2 expression levels of each sample were evaluated by Western blot. The most effective vector was selected and marked as AAV-GluA1-shRNA containing the shRNA sequence 5’-GGAATCCGAAAGATTGGTTAC-3’ (M38060.1, 1123-1143), and AAV-GluA2-shRNA with the sequence 5’-GGAGCACACACAGCGACAATT-3’ (NM017261, 1289-1309). Animals were injected with 1 μl (~5×109 GC/μl) AAV-GluA1 virus, or 1 μl (~2×109 GC/μl) AAV-GluA1-shRNA-GFP or 1 μl (1.5×109 GC/μl) AAV-GluA2-shRNA-GFP virus into CeA on each side of the brain. All behavioral experiments were performed 10 days after the virus injection.
CPP and extinction training
The general CPP procedure has been described in our previous studies (Zhu et al., 2007; Bie et al., 2009). With a standard 3-chamber CPP apparatus (MED Associates, St. Albans, VT), each conditioning session, conducted around 6 pm, consisted of one cycle of saline (s.c.) conditioning on one day and morphine (s.c.) conditioning on the following day, and CPP behavior was measured at 9 am in posttests after 1, 2, 4 or 5 sessions of conditioning treatment (45 min each) and 1 day post-conditioning. A saline injection (s.c.) without conditioning was made before each CPP test. In this study, control rats displayed baseline preference for one chamber (equipment bias) and morphine conditioning was mostly paired with the non-preferred chamber. CPP was also consistently induced by pairing with the preferred chamber (Zhu et al., 2007). CPP behavior was defined by statistical difference in times spent in the morphine-paired chamber before conditioning treatment in pretest and in the posttest in the same rats. CPP data were presented as actual times a rat spent in the morphine-paired chamber in pretests and posttests. CPP extinction was achieved by once daily conditioning with saline (s.c.) only and was measured by a CPP test on every other day to minimize its inference with extinction training. After the behavioral experiments, brain slices (600 μM thick) containing the CeA were cut on a vibratome (Leica Microsystems) and CeA tissues were punched out by a pipette tip with an inner diameter of 1.5 mm for biochemical analyses.
Western blot analysis
CHO cells were transfected with plasmids (pAAV-GluA1, pAAV-GluA1-shRNA, pAAV-GluA2-shRNA and control vectors) and harvested after 48 h. The cells were lysed in 200 μl RIPA buffer and supplemented with Complete Protease inhibitor Tablets (Roche). Samples were mixed with 2xSDS loading buffer and denatured at 70 °C for 15 min. For total protein preparation, CeA tissue were homogenized in 150 μl RIPA buffer with fresh protease inhibitor, the lysate was centrifuged and the supernatant was kept as total protein samples. For crude synaptosome preparation (Bie et al., 2009), CeA tissues were gently homogenized in sucrose buffer and centrifuged at 1000×g. The supernatant was centrifuged at 10000g×20 min and the synaptosomal pellet was resuspended in 80 μl RIPA lysis buffer. Protein concentration of each sample was determined by the DC protein assay from Bio-Rad. Equal amounts of protein (25 μg for total protein, 7.5 μg for crude synaptosome) were loaded per lane and separated on an 8% SDS-PAGE gel. The PVDF membranes with transferred protein were incubated overnight at 4°C with the primary antibodies against GluA1 (1:1,000, Millipore, Cat. # 05-855R), phospho-GluA1-Ser845 (1:3,000, Millipore, Cat.# 04-1073), GluA2 (1:1,000, Santa Cruz Biotechnology, sc-7610) and β-actin (1:1,000, Santa Cruz Biotechnology, sc-81178). After washes, the blots were incubated with horseradish peroxidase-conjugated secondary antibodies (1:10,000, Jackson ImmunoResearch) for 1 h. The blots were developed with ECL plus reagent (GE healthcare). The densitometric quantification of immunoreactive bands was performed with the AlphaView software (Alpha Innotech Corp.)
Whole-cell recording
General recording methods have been reported before (Zhang and Pan, 2010; Zhang et al., 2011). Coronal slices (240 μm) containing the amygdala were cut from the brain of virus-injected rats and maintained in a preheated (35 °C) physiological solution containing (in mM): NaCl, 126; KCl, 2.5; NaH2PO4, 1.2; MgCl2, 1.2; CaCl2, 2.4; glucose, 11; NaHCO3, 25, saturated with 95% O2 and 5% CO2, pH 7.2–7.4. Virus-transfected neurons were identified by GFP signals in CeA slices from rats after single-side CeA injection of mixed viruses (AAV-GluA1+AAV-GFP, 3:1) for GluA1-overexpressing cells or AAV-GluA1-shRNA-GFP for cells with GluA1 knockdown. Due to the limited packaging capacity of AAV that prevents expression of both GluA1 and GFP in one vector, the GFP-expressing cells near the injection track in CeA from rats injected with the mixed viruses were approximately considered as GluA1-overexpressing cells, and control cells were those in CeA of the other uninjected side in the slice. Visualized whole-cell voltage-clamp recordings were obtained from GFP-labeled, infected neurons and GFP-negative control neurons in CeA with a glass pipette filled with a solution containing (in mM): cesium methansulphonic acid, 120; HEPES, 20; EGTA, 0.4; MgCl2, 2.0; TEA-Cl, 5; Mg-ATP, 2.5; Na2GTP, 0.25; QX-314-Cl, 1; pH 7.2–7.4, 280-290 mOsm. For rectification analysis, spermine (100 μM) was added to the pipette solution. Electrical stimuli of constant current (0.25 ms, 0.2–0.5 mA) were used to evoke postsynaptic currents with a bipolar stimulating electrode placed in the basolateral amygdala (BLA). All recordings were performed in the presence of picrotoxin (50 μM). To calculate the AMPA/NMDA ratio, the AMPA receptor-mediated excitatory postsynaptic current (EPSC) was determined by the averaged amplitude of ten EPSCs at +40 mV in the presence of D-AP5 (50 μM) and the NMDA receptor-mediated EPSC was obtained by subtracting the AMPA EPSC from the total EPSC. The rectification index (RI) of AMPA receptors was obtained by the equation: RI = [EPSC-70/(70–Erev)]/[EPSC+40/(40–Erev)] (EPSC-70 and EPSC+40, EPSCs recorded at a holding potential of -70 and +40 mV, respectively; Erev, reversal potential calculated from the I-V relationship) (Balland et al., 2006; Conrad et al., 2008). All drugs were applied through the bath solution unless stated otherwise.
Immunohistochemistry
AAV-injected animals were deeply anesthetized with sodium pentobarbital (60 mg/kg i.p.) and perfused transcardinally with heparinized saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer. The brain was extracted and post-fixed overnight at 4 °C, and cryoprotected in 30% sucrose in 0.1 M phosphate buffer. Consecutive 30-μm coronal brain sections were prepared and stored in PBS (containing 0.1% sodium azide). For GFP expression, sections were mounted on slides, dried and coverslipped with ProLong® Gold antifade reagent for DAPI staining (Invitrogen). For GluA1 staining, sections were blocked with 5% normal donkey serum (NDS) in PBS containing 0.3% Triton X-100 and incubated overnight with anti-GluA1 antibody (1:500, Millipore, Cat. #05-855R). Sections were then rinsed and incubated with the Alexa-conjugated secondary antibodies: Alexa Fluro 568 goat anti-rabbit IgG (1:500, Invitrogen). The stained sections were examined with a Nikon E600 fluorescence microscope (Nikon Instech).
Novel object recognition test
This test, consisting of three phases of habituation, acquisition trial and retention trial, was performed to assess learning of novel objects and recognition memory according to the procedures described in a previous report (Bevins and Besheer, 2006). Seven days after viral injection, a rat was habituated to an open-field arena for 20 min on three consecutive days. In an acquisition trial, the rat was given 3 min to explore the arena with two identical objects: two cans of Coca-cola or two plastic water bottles of similar sizes. The exploration time the rat spent on each object was recorded. In a retention trial 4 hours after the acquisition trial, the animal was returned and allowed to explore the arena with one can of Coca-cola and one water bottle for 3 min. The exploration time on the familiar object (presented in the acquisition trial) and on the novel object (not presented in the acquisition trial) was recorded. All objects and the test box were cleaned with 70% ethanol between rats to remove odor cues. Novel preference in percentage was calculated by the time exploring on the novel object divided by total exploration time on both novel and familiar objects.
Data Analysis and materials
Numeral data of CPP and EPSCs before and after a treatment were statistically compared and analyzed with Student's t tests or one-way ANOVA. For behavioral data of CPP and extinction time courses involving multiple treatments or CPP tests, two-way ANOVA for repeated measures with post hoc analysis of the Bonferronic method was used to determine statistical significance in group–treatment and between–group interactions at each time point. Data were expressed as mean ± S.E.M. and a p value < 0.05 was considered statistically significant. All statistical analyses were performed with the Prism software version 5.04 (GraphPad Software). Morphine hydrochloride was kindly supplied by the National Institute on Drug Abuse. All other drugs were purchased from Tocris Bioscience (Ellisville, MO) or from Sigma-Aldrich.
Results
GluA1 upregulation during proposed learning consolidation of morphine reward
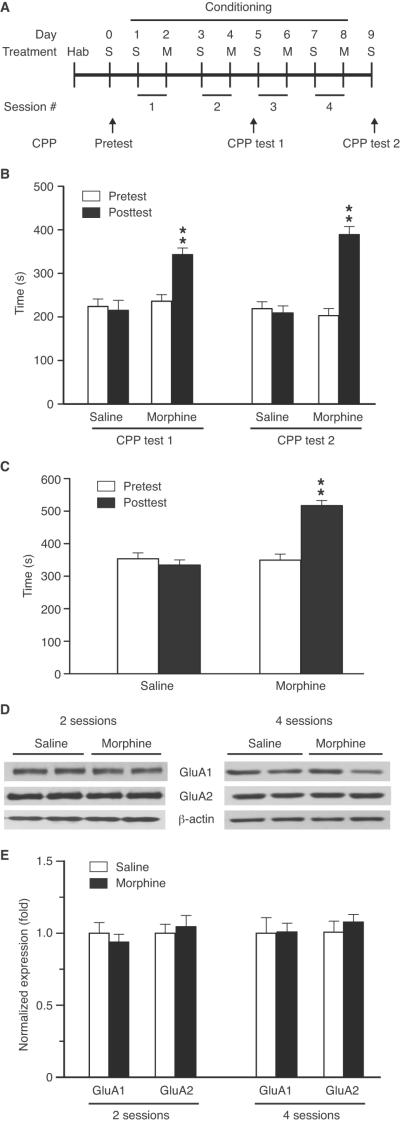
We first examined the expression level of GluA1 in CeA from rats with morphine-induced CPP. Conditioning with morphine pairing with the non-preferred chamber at a dose of 10 mg/kg (s.c.) induced significant CPP behavior after 2 conditioning sessions (t = 6.962, p < 0.01, Fig. 1A,B). Continued conditioning with four such sessions produced CPP with no further increase in CPP magnitude (t = 6.080, p < 0.01). Conditioning with morphine paring with the preferred chamber also consistently induced CPP (t = 4.487, p < 0.01, Fig. 1C). In CeA tissues taken from rats after two or four sessions following posttest 1 or posttest 2 (Fig. 1A), we found no significant change in the levels of synaptosomal GluA1 and GluA2 proteins between control rats and rats with morphine-induced CPP (Fig. 1D,E). No change was found either in total protein levels of CeA GluA1 and GluA2 (data not shown).
Figure 1.
Morphine conditioning induces behavior of conditioned place preference (CPP) without altering protein expression of AMPA receptor GluA1 and GluA2 subunits in the central nucleus of the amygdala (CeA). A, Timeline of experimental protocol for morphine conditioning and CPP tests. Hab, habituation; S, saline; M, morphine. B, CPP in rats induced by conditioning with morphine (10 mg/kg, s.c.) paring with the non-preferred chamber after 2 (CPP test 1) or 4 (CPP test 2) conditioning sessions (n = 6 rats each group). C, CPP induced by morphine (10 mg/kg, s.c.) paring with the preferred chamber after 4 conditioning sessions (n = 6 rats/group). D,E, Western blotting (D) and summarized results (E) of GluA1 and GluA2 protein levels in synaptosomal preparations of CeA from rats (n = 5/group) conditioned with saline or morphine with established CPP after 2 or 4 conditioning sessions. GluR protein levels were normalized to that of β-actin. ** p < 0.01.
We therefore predicted that CeA GluA1 might be important for the learning and consolidation process of environmental cues–reward association in CPP behavior, which occurs several minutes to several hours after conditioning training (McGaugh, 2000). To examine this, rats were then conditioned by a single conditioning session with morphine (10 mg/kg, s.c.), which also induced significant CPP (t = 6.513, p < 0.01, Fig. 2A). In CeA tissues harvested 2 h after morphine conditioning, we found a significant increase (about 34%) in synaptosomal GluA1 when compared to saline-conditioned control rats (t = 2.572, p < 0.05, Fig. 2B). Morphine injection (10 mg/kg, s.c.) in home cage without conditioning (chamber pairing) induced a small increase in the GluA1 protein expression, but it did not reach statistical significance (t = 1.671, p = 0.1332, Fig. 2B). In contrast, the GluA2 level remained unchanged across the three rat groups. In addition, the level of CeA synaptosomal GluA1 expression remained unchanged in single session-conditioned rats with a low dose of morphine (0.5 mg/kg, s.c., Fig. 2C), which failed to induce CPP (see results of Fig. 4A below). Thus, it appears likely that CeA GluA1 plays a role in the learning/consolidation process of context–reward association for morphine-induced CPP behavior.
Figure 2.
Morphine conditioning transiently increases the protein level of synaptosomal GluA1 in CeA. A, Timeline of experimental protocol (upper) and CPP induced by one conditioning session with morphine (10 mg/kg, s.c., lower) (n = 6 rats/group). B,C, Western blot data of CeA synaptosomal GluA1 and GluA2 proteins from rats conditioned with one session of saline (S-cond.) or morphine (M-cond., 10 mg/kg) (B) or a sub-threshold morphine dose at 0.5 mg/kg (C), or injected in home cage without conditioning with the same two doses of morphine (M-inj., B,C). CeA tissues were collected 2 h after morphine conditioning as shown in A (n = 6/group). * p < 0.05, ** p < 0.01.
Figure 4.
Overexpression of CeA GluA1 subunits facilitates associative learning of morphine reward. A, Behaviors of CPP induced by multiple conditioning sessions with saline or a low dose of morphine (0.5 mg/kg, s.c.) (n = 6 rats/group). CPP tests were conducted after each number of sessions and after the completion of all conditioning sessions (postcond., postconditioning). * p < 0.05 (compared to the pretest in the same group). B, CPP behaviors after similar conditioning sessions with saline (n = 6) or morphine (0.5 mg/kg) in rats injected into CeA with AAV-GluA1 (n = 9) or AAV-GFP (n = 6). * p < 0.05 (compared to the pretest in the same group); # p < 0.05 (compared to the Morphine/AAV-GFP group). C, CPP behaviors in CeA AAV-GluA1- or AAV-GFP-injected rats conditioned with multiple sessions of morphine at 3 mg/kg, followed by daily conditioning sessions of extinction training with saline only (n = 9 rats/group). * p < 0.05 (compared to the pretest in the same group); # p < 0.05 (compared to the AAV-GFP group); ** p < 0.01; n.s., not significant. D, CPP behaviors in normal rats (n = 8/group) induced by conditioning with morphine at 3 mg/kg and 10 mg/kg, followed by daily sessions of extinction training. * p < 0.05 (compared to the pretest in the same group); # p < 0.05 (compared to the 3 mg/kg group).
GluA1 overexpression facilitates associative learning of morphine reward
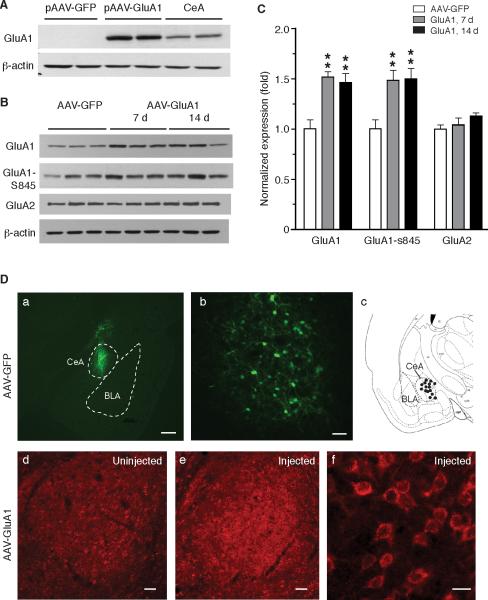
To determine this GluA1 role, we overexpressed GluA1 in CeA by using an adeno-associated viral (AAV) vector. Lysate of CHO cells transiently transfected by a constructed vector (pAAV-GluA1) showed a strong immunoreactive band of GluA1 (Fig. 3A). After bilateral microinjection of the vector packaged in AAV2 virus (AAV-GluA1, 1 μl, 5×109 GC) into CeA, we found a 1.5 fold increase in GluA1 expression in CeA tissues collected 7 and 14 d post-injection when compared to CeA tissues of control rats similarly injected with AAV-GFP virus (7 d: t = 5.056, p < 0.01; 14 d: t = 3.741, p < 0.01, Fig. 3B,C). Phosphorylated GluA1-Ser845 subunits, an activated form required for membrane trafficking of AMPA receptors (Choi et al., 2011), were also increased in CeA of AAV-GluA1-injected rats. The AAV-GluA1 virus did not alter the expression of GluA2 subunits in CeA (Fig. 3B,C), indicating a selective overexpression of GluA1 by the virus. Supporting the GluA1 overexpression induced by CeA microinjection of the AAV-GluA1 viruses (Fig. 3D a–c), CeA neurons showed clearly stronger signals of immunoreactive GluA1 in the AAV-GluA1-injected side of CeA than those on the uninjected side (Fig. 3D d–f).
Figure 3.
Adeno-associated virus (AAV) vector-mediated overexpression of GluA1 subunits in CeA. A, Validation of AAV plasmid carrying the cDNA encoding rat GluA1(flip) in cultured CHO cells. Control cells were transfected with AAV-GFP plasmid and samples from CeA tissues were shown as positive control. B,C, Western blot data (B) and summarized results (C) of GluA1, phosphorylated GluA1 at S845 and GluA2 proteins in CeA tissues collected from rats (n = 6/group) 7 or 14 days after bilateral intra-CeA infusion of 1 μl AAV-GluA1 virus or AAV-GFP virus. D, Representative micrographs of immunostaining and illustration after CeA microinjection, showing AAV-mediated eGFP expression within the defined boundary of CeA in low magnification (a, scale bar = 500 μM) and high magnification (b, scale bar = 50 μM), a cartoon depicting successful injection sites (black dots) within the CeA (c), and CeA GluA1 expression from a rat in an uninjected side (d, scale bar = 50 μM) and in the other side injected with AAV-GluA1 in low (e, scale bar = 50 μM) and high magnification (f, scale bar = 20 μM). CeA preparations were prepared at post-injection day 10. BLA, basolateral amygdala. ** p < 0.01.
We then used these rats with viral overexpression of CeA GluA1 to examine the CPP acquisition phase (from pretest to the first significant CPP) during which CeA-involved learning and consolidation of context–reward association (associative learning) is proposed to occur for the CPP behavior. Conditioned with morphine at 0.5 mg/kg (s.c.), naïve rats required a minimum of 3 conditioning sessions to acquire significant CPP, with more conditioning sessions inducing similar CPP in magnitude (Fig. 4A, two-way ANOVA: conditioning, F(6, 84) = 3.81, p = 0.002; morphine, F(1, 14) = 6.47, p = 0.023). Rats injected with AAV-GFP in the CeA displayed a similar time course of CPP acquisition to that of naïve rats, requiring at least 3 conditioning sessions for CPP induction (Fig. 4B). In contrast, in rats with CeA injection of AAV-GluA1, a singe conditioning session with the same morphine dose (0.5 mg/kg, s.c.) was sufficient to induce significant CPP (Fig. 4B). Moreover, the CPP magnitude in these rats further increased with each additional conditioning session until after 4 sessions, and was significantly larger after each session and at the post-conditioning test than those in AAV-GFP-injected rats (Fig. 4B, two-way ANOVA: conditioning, F(6, 78) = 23.57, p = 0.0001; GluA1, F(1, 13) = 13.06, p = 0.003). Saline conditioning of naïve or AAV-GluA1-injected rats did not induce CPP after these conditioning sessions. These results support the notion that the enhanced effect of morphine in CPP acquisition of morphine reward is mediated, at least partially, by increased CeA GluA1 function that facilitates the learning and consolidation of morphine reward in CPP behavior.
To further determine this facilitating effect of GluA1 on the associative learning, we examined the behavior of CPP extinction induced by extinction training, a new form of associative learning that is required to reduce CPP and drug-seeking behavior (Sutton et al., 2003). Conditioning with a higher dose of morphine (3 mg/kg) induced CPP after a single session in both groups of AAV-GFP-injected control rats and AAV-GluA1-injected rats, but stronger CPP was induced in the AAV-GluA1 group after each conditioning session than controls during CPP acquisition (Fig. 4C). During CPP extinction induced by daily sessions of conditioning with saline only, a single session of extinction training significantly reduced the CPP in the AAV-GluA1 group so that the remaining CPP was no longer different from that in the AAV-GFP group. After that, the CPP in both groups extinguished at a similar rate following additional training sessions, with the same number of extinction sessions required to reach complete extinction in both groups (Fig. 4C, two-way ANOVA: conditioning, F(5, 70) = 18.47, p = 0.0001; GluA1, F(1, 14) = 10.28, p = 0.006). To exclude the possibility that CPP of a higher magnitude would naturally extinguish faster, we did similar extinction experiments in two groups of naïve rats with statistically different magnitudes of CPP behaviors induced by two different doses of morphine (3 mg/kg and 10 mg/kg). As shown in Figure 4D, whereas the CPP of lower magnitude was completed extinguished after 5 sessions, the CPP of higher magnitude extinguished much slower, with significant CPP behavior remaining after 9 training sessions (two-way ANOVA: extinction training, F(6, 84) = 38.02, p = 0.0001; morphine dose, F(1, 14) = 7.31, p = 0.017). Thus, as the extinction training engages a separate form of associative learning without involvement of a morphine effect, these findings further support the notion that upregulation of CeA GluA1 function facilitates associative learning in CPP behavior.
GluA1 downregulation inhibits associative learning of morphine reward
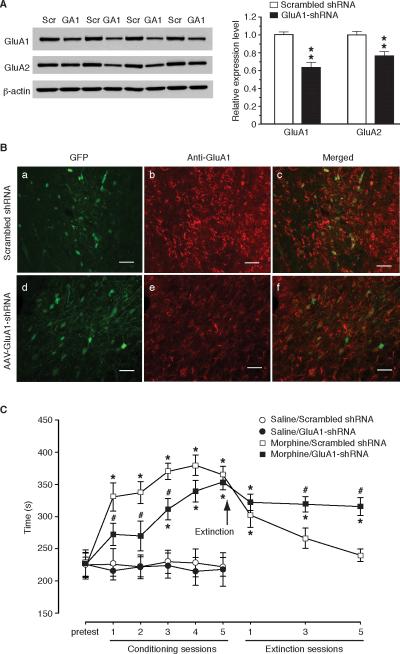
To further validate the learning-facilitating effect of CeA GluA1, we used an AAV-GluA1-shRNA vector to downregulate the expression of GluA1 in CeA. Western blotting analysis showed that, 10 days after bilateral infusion of 1 μl AAV-GluA1-shRNA-GFP vector into CeA, the level of synaptosomal GluA1 protein was decreased by about 36% in CeA tissues when compared to that from rats injected with AAV-scrambled shRNA vector (t = 5.552, p < 0.01, Fig. 5A). Consistent with reports of several previous studies (Zamanillo et al., 1999; Mead and Stephens, 2003a, b), the expression level of synaptosomal GluA2 protein also was decreased to a less extent in GluA1-shRNA-injected rats for unknown mechanisms (t = 3.727, p < 0.01, Fig. 5A). This GluA1-shRNA-mediated GluA1 downregulation was confirmed by immunohistochemical staining of GFP-positive CeA neurons for GluA1 immunoreactivity (Fig. 5B).
Figure 5.
AAV-GluA1-shRNA-mediated downregulation of CeA GluA1 subunits inhibits associative learning of morphine reward. A, Western blotting analysis of CeA synaptosomal GluA1 (GA1) and GluA2 proteins in rats (n = 6/group) with bilateral CeA injection of AAV-GluA1-shRNA or AAV-scrambled (Scr) shRNA. CeA tissues were collected at post-injection day 10. ** p < 0.01. B, Representative micrographs of GluA1 immunoreactivity and GFP expression in CeA neurons from a rat after CeA injection of AAV-scrambled shRNA (a–c) or AAV-GluA1-shRNA (d–f). Scale bars = 50 μM. C, CPP behaviors in rats with bilateral CeA injection of AAV-scrambled shRNA or AAV-GluA1-shRNA after multiple conditioning sessions with saline (n = 6 rats/group) or morphine (3 mg/kg, n = 10 rats/group), followed by daily conditioning sessions of extinction training. * p < 0.05 (compared to the pretest in the same group); # p < 0.05 (compared to the morphine/scrambled shRNA group).
In AAV-scrambled shRNA-injected control rats, one conditioning session with morphine at 3 mg/kg (s.c.) induced CPP, with more morphine-conditioning sessions producing slightly stronger CPP (Fig. 5C), similar to the CPP acquisition by the same morphine dose in AAV-GFP-injected control rats (Fig. 4C). However, in rats with CeA injection of AAV-GluA1-shRNA, similar morphine conditioning failed to induce CPP even after two sessions and at least three sessions were required to induce significant CPP, which was significantly smaller in magnitude than that in the control group (Fig. 5C). Of note is that the GluA1-shRNA-injected rats was able to acquire CPP that was comparable in magnitude to that of control rats after 5 conditioning sessions, making it unlikely that the delay in CPP acquisition was due to a reduced pharmacological effect of morphine. Consistent evidence was shown in analysis of CPP extinction. The CPP in scrambled shRNA-injected control rats diminished to almost complete extinction after 5 sessions of extinction training, whereas the CPP in GluA1-shRNA-injected rats largely persisted with most CPP remaining after such 5 training sessions (Fig. 5C, two-way ANOVA: conditioning, F(5, 90) = 20.43, p < 0.001; GluA1-shRNA, F(1, 18) = 8.38, p = 0.0096; extinction training, F(3, 54) = 17.25, p < 0.0001; extinction/GluA1-shRNA, F(1, 18) = 7.16, p = 0.015). These results indicate that downregulation of CeA GluA1, together with some downregulation of GluA2, inhibits associative learning in a drug-free context of reward-related behavior, providing further support for the facilitating effect of CeA GluA1 on associative learning of opioid reward.
GluA1-overpressing neurons exhibit properties of GluA2-lacking AMPA receptors
To identify synaptic changes responsible for the GluA1 overexpression-mediated acceleration of CPP acquisition, we obtained CeA slices from rats after single-side CeA injection of AAV vectors and performed whole-cell voltage-clamp recordings in infected, GFP-labeled neurons in CeA of the injected side and control CeA neurons in the other uninjected side (Fig. 6A). In those GFP-positive, likely AAV-GluA1-transfected neurons, we found a significant increase in the ratio of AMPA EPSC over NMDA EPSC when compared to the ratio in control neurons (one-way ANOVA: F(2, 33) = 4.909, p < 0.05; post hoc Tukey's test: p < 0.05 for both comparisons, Fig. 6B,C). Compared to controls, no significant change in the ratio was observed in AAV-GluA1-shRNA-infected neurons. Next, we analyzed the rectification property of AMPA EPSCs, a unique feature of GluA2-lacking, Ca++-permeable AMPA receptors (Isaac et al., 2007). The current-voltage relationship of AMPA EPSCs in GluA1-overexpressing cells showed significant inward rectification (one-way ANOVA: F(2, 34) = 5.629, p < 0.01; post hoc Tukey's test: control vs. GluA1-GFP, p < 0.05; GluA1-GFP vs. GluA1-shRNA, p < 0.05, Fig. 6D). Furthermore, blocking GluA2-lacking AMPA receptors by bath application of 1-naphthyl acetyl spermine (NASPM, 100 μM) significantly decreased the EPSC amplitude only in GluA1-overexpressing cells (one-way ANOVA: F(2, 22) = 8.365, p < 0.01; post hoc Tukey's test: p < 0.01 for both comparisons, Fig. 6E). In comparison with controls, no statistically significant change was found either in the rectification of AMPA EPSCs or in the NASPM effect in AAV-GluA1-shRNA-infected neurons, perhaps reflecting the viral downregulation of both GluA1 and GluA2 shown in Figure 5A. In addition, the paired-pulse ratio (PPR), a commonly used synaptic measure for changes in the probability of presynaptic transmitter release (Zucker and Regehr, 2002), remained unaltered across the three groups of neurons (one-way ANOVA: F(2, 31) = 0.1652, p = 0.8485, Fig. 6F), suggesting that the change in GluA1 subunit expression primarily affected the properties of postsynaptic AMPA receptors rather than presynaptic glutamate release in these CeA neurons.
Figure 6.
GluA1 overexpression increases synaptic properties of GluA2-lacking AMPA receptors in CeA neurons. A, Illustration of stimulation (S) placement in BLA, and recording (R) of a viral vector-transfected neuron and EPSC in a CeA slice. A transfected cell was identified by the GFP fluorescence (top middle) and recorded in whole-cell configuration (top right) with a typical postsynaptic current (PSC) and excitatory postsynaptic current (EPSC) in the presence of picrotoxin (50 μM) (bottom right). B, Representative AMPA EPSCs and NMDA EPSCs in a control cell and in transfected cells from animals after single-side CeA injection of the mixture (GluA1-GFP) of AAV-GluA1 and AAV-GFP vectors (3:1) or AAV-GluA1-shRNA-GFP vector. C, Summarized results of the AMPA/NMDA EPSC ratio in the three indicated cell groups. Numbers in columns are cell numbers in each group. D, EPSCs at holding potentials of +40 mV and -70 mV in the presence of the NMDA receptor antagonist D-AP5 (50 μM) (top) and the calculated rectification index of EPSCs (bottom) in the three cell groups. E, EPSCs (top) and summarized results (bottom) before and during application of the GluA2-lacking AMPA receptor antagonist NASPM (100 μM) in the indicated cell groups. F, Evoked EPSC pairs (50 ms apart, top) and summarized data of paired-pulse ratios (bottom) in the three cell groups. * p < 0.05, ** p < 0.01.
GluA2-lacking AMPA receptor is important for morphine-induced CPP
We then determined whether CeA GluA2-lacking AMPA receptors were critical for the acquired CPP behavior induced by morphine. We found that CPP in rats conditioned with morphine (10 mg/kg, 4 sessions) was significantly attenuated by bilateral intra-CeA infusion of NASPM (40 μg, each side) (morphine group: t = 6.517, p < 0.01; morphine plus NASPM group: t = 4.804, p < 0.01; two-way ANOVA: F(1,14) = 9.39, p < 0.01, Fig. 7A). CeA infusion of NASPM alone did not change preference behavior in naïve rats (t = 0.8285, p = 0.4451). To further determine the behavioral significance of CeA AMPA receptors, we substituted morphine with AMPA to directly activate the AMPA receptors in the conditioning procedures. Rats similarly conditioned in 4 sessions with AMPA (100 ng in 0.5 μl, each side) bilaterally infused into CeA displayed significant CPP (AMPA group: t = 7.069, p < 0.01; AMPA plus NASMP group: t = 4.990, p < 0.01), and similar to morphine-induced CPP, the AMPA-induced CPP also was largely inhibited by CeA pre-infusion of NASPM (40 μg, each side, 30 min before AMPA infusion) (two-way ANOVA: F(1,14) = 9.78, p < 0.01, Fig. 7B). Thus, it appears that activation of CeA AMPA receptors is rewarding, sufficient to induce CPP behavior, and the GluA2-lacking AMPA receptor is important for this rewarding effect, consistent with its role in facilitating the acquisition of reward-related behavior.
Figure 7.
GluA2-lacking AMPA receptors in CeA are involved in acquired behavior of opioid reward. A, Behaviors of CPP in rats conditioned in 4 sessions with PBS as vehicle, morphine (10 mg/kg) without or with bilateral pre-CeA infusion (15 min before) of NASPM (40 μg, each side), or vehicle with CeA pre-infusion of NASPM. ** p < 0.01 (compared to the pretest); ## p < 0.01 (compared between the indicated two groups) (n = 8 rats in each group). B, CPP behaviors in similar groups of rats with morphine substituted by AMPA (100 ng, each side) infused similarly into CeA in the conditioning procedure. ** p < 0.01 (compared to the pretest); ## p < 0.01 (compared between the indicated two groups). N = 8 rats in each group.
GluA2 knockdown facilitates associative learning of morphine reward
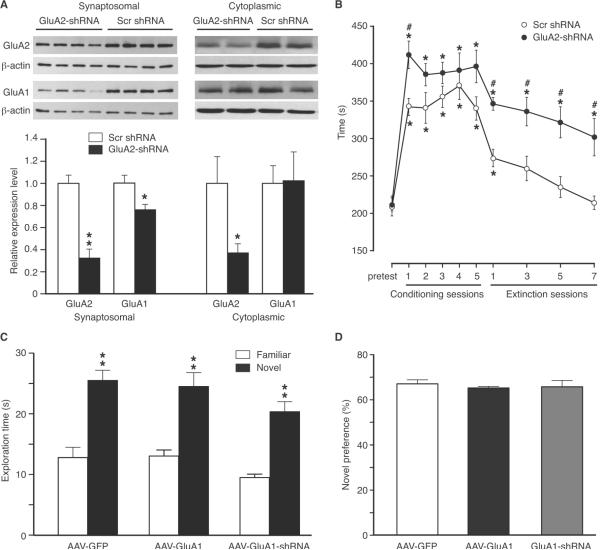
To determine the role of CeA GluA2 subunits in the behavior of opioid reward, we constructed AAV-GluA2-shRNA vectors and infused the virus into CeA to knockdown GluA2 expression. Bilateral infusion of AAV-GluA2-shRNA vectors significantly decreased the GluA2 level by 68% and 63% in CeA synaptosomal (t = 6.066, p < 0.01) and cytoplasmic (t = 3.164, p < 0.05) protein preparations, respectively, when compared to those from animals treated with AAV-scrambled shRNA (Fig. 8A). In contrast, cytoplasmic GluA1 level was not altered by the AAV-GluA2-shRNA vector, while synaptosomal GluA1 was moderately reduced (t = 2.762, p < 0.05), a finding consistent with a previous report (Mead and Stephens, 2003b). Behaviorally, control rats treated with CeA infusion of scrambled GluA2-shRNA displayed CPP after one session and the following 4 sessions of conditioning with morphine (3 mg/kg, s.c.); however, rats treated similarly with AAV-GluA2-shRNA showed consistently stronger CPP than control rats (two-way ANOVA: F(1.16) = 6.88, p = 0.0185, Fig. 8B), an effect similar to that of GluA1 overexpression (Fig. 4C). But unlike the learning-facilitating effect of GluA1 overexpression on both CPP acquisition and CPP extinction, GluA2 knockdown failed to accelerate CPP extinction (Fig. 8B), with CPP in both groups declined at a similar rate during extinction training, as in the rat groups treated with two different morphine doses (Fig. 4D).
Figure 8.
Downregulation of CeA GluA2 facilitates CPP but does not change CPP extinction. A, Representative Western blots (top) and summarized data (bottom) of synaptosomal and cytoplasmic GluA2 and GluA1 proteins in the CeA from rats (n = 4/group) treated with CeA infusion of AAV-scrambled (scr) shRNA or AAV-GluA2-shRNA. B, CPP behaviors in rats with bilateral CeA injection of AAV-scrambled shRNA (n = 8) or AAV-GluA2-shRNA (n = 10) after multiple conditioning sessions with morphine (3 mg/kg, s.c.), followed by daily conditioning sessions of extinction training with saline only. * p < 0.05 (compared to the pretest in the same group); # p < 0.05 (compared to the scrambled shRNA group). C,D, Exploration times (C) and novel preference (D) in the novel object recognition test in rats (n = 6 each group) after CeA infusion of AAV-GFP, AAV-GluA1 or AAV-GluA1-shRNA vectors.
CeA GluA1 is not involved in learning of novel objects
Finally, we conducted the novel object recognition test to determine whether CeA GluA1 was involved in the learning process of novel objects and related recognition memory. Behaviors of object exploration were compared among three groups of rats with CeA infusion of AAV-GFP for control, AAV-GluA1 for GluA1 overexpression, or AAV-GluA1-shRNA for GluA1 knockdown. Rats in all three groups preferred exploring the novel object to the familiar object (AAV-GFP: t = 5.316, p < 0.01; AAV-GluA1: t = 4.576, p < 0.01; AAV-GluA1-shRNA: t = 6.158, p < 0.01, Fig. 8C). The exploration times in the AAV-GluA1-shRNA group slightly decreased when compared to the other two groups, but the difference was not statistically significant (one-way ANOVA: F(2, 15) = 2.058, p = 0.1622, Fig. 8C). When the novel preference was compared, there was no significant difference among the three groups (one-way ANOVA: F(2, 15) = 0.2349, p = 0.7935, Fig. 8D). This suggests that upregulation or downregulation of CeA GluA1 does not have a generalized effect on object learning and recognition memory.
Discussion
In the present study, we have provided biochemical, electrophysiological and behavioral data to suggest that increased expression and function of GluA2-lacking AMPA receptors in CeA likely facilitates the learning and consolidation of environmental context–reward association, an important process in the development of drug-seeking behavior as well as in relapse of such behaviors in opioid addiction.
The amygdala is known for regulating and coordinating emotional behaviors in response to various persistent environmental stimuli and emotional events generally promote learning and memory via amygdala functions (Baxter and Murray, 2002; Everitt et al., 2003; Maren, 2005; Knapska et al., 2007; Murray, 2007; Roozendaal et al., 2009; Tye et al., 2011). The vast majority of previous studies have been focused on amygdala regulation of negative emotional behaviors represented by fear responses (Kalin et al., 2004; Maren, 2005; Knapska et al., 2007; Ehrlich et al., 2009; Tye et al., 2011). Recent evidence increasingly suggests that the amygdala including CeA is part of the brain's reward circuitry and is important also in regulation of positive emotions represented by reward-related responses (Baxter and Murray, 2002; See et al., 2003). The role of amygdala including CeA in behaviors of opioid reward has been shown in a number of studies and the receptor and signaling systems involved include NMDA receptors, cannabinoid CB1 receptors, dopamine receptors, neurokinin-1 receptors, ERK and PKMzeta (Gadd et al., 2003; Zarrindast et al., 2003; Rezayof et al., 2007; Li et al., 2008; Bishop et al., 2011; He et al., 2011; Li et al., 2011; Rezayof et al., 2011). However, local injection of morphine into the lateral nucleus of the amygdala did not induce CPP (Olmstead and Franklin, 1997).
Our data from the current study indicate that reward conditioning with morphine increases expression and synaptic strength of GluA2-lacking AMPA receptors in CeA neurons, which promotes the associative learning of opioid reward in positive emotional behaviors. This is consistent with a recent study showing that in vivo optogenetic stimulation of the glutamatergic pathway from BLA to nucleus accumbens (NAc) promotes motivated response of reward-seeking behaviors (Stuber et al., 2011). Given the extensive glutamatergic inputs from BLA neurons onto CeA neurons (Pitkanen et al., 1997; Swanson and Petrovich, 1998), the adaptive increase in GluA1 expression and in the synaptic strength of GluA2-lacking AMPA receptors in CeA could be driven by persistent stimulating activity of the BLA–CeA glutamatergic pathway and operates as part of the mechanisms underlying motivated reward-seeking behaviors through facilitated reward learning.
This CeA GluA1 function of learning facilitation is in agreement with a general rewarding effect of GluA1 subunits and AMPA receptors found in other reward-related brain areas. For instance, repeated exposure to morphine or cocaine increases GluA1 expression in VTA and hippocampal neurons, and GluA1 upregulation in VTA enhances the rewarding effect of morphine or motivation for cocaine self-administration (Fitzgerald et al., 1996; Carlezon et al., 1997; Billa et al., 2010; Choi et al., 2011). Activation of CeA AMPA receptors by local infusion of AMPA increases extracellular dopamine level in several brain regions including prefrontal cortex and NAc (Stalnaker and Berridge, 2003). Our data suggest that CeA GluA1 is important in the learning and acquisition of reward-seeking behavior, as evidenced by increased expression of synaptic GluA1 during the proposed period of reward learning and consolidation. It is interesting to note that the synaptic GluA1 protein returned to pre-morphine level after the acquisition and establishment of CPP behavior while synaptic GluA2-lacking AMPA receptors were still required partially for the established CPP behavior. This is consistent with previous reports of no change in GluA1 expression in VTA and NAc of rats with established, cocaine-induced CPP (Carlezon and Nestler, 2002; Plant et al., 2006; Hu et al., 2007; Bachtell and Self, 2008; Matsuo et al., 2008; Edwards et al., 2009; Choi et al., 2011), and might reflect an intense increase in synaptic GluA1 through new GluA1 synthesis and synaptic insertion required for synaptic facilitation only during associative learning of reward, with synaptic GluA1 returning to a more moderate and sustainable level after acquisition. This notion of CeA GluA1 more important for associative learning and acquisition of reward is in line with the general concept that CeA primarily is not involved in storage of emotional memory (Maren, 2005; Knapska et al., 2007; Vlachos et al., 2009). As supported by our electrophysiological data, upregulation of GluA1 expression may change the subunit composition of membrane AMPA receptors and switch GluA2-containing AMPA receptors to GluA2-lacking AMPA receptors with enhanced synaptic strength in response to glutamatergic input activities (Bowers et al., 2010).
Extinction of addictive behaviors in animals involves a type of learning that is thought to form a new memory, which suppresses behavioral response to a previously learned stimulus (Bouton, 2002; See et al., 2003). Since no drug was involved in extinction training, it permitted the current study to determine CeA GluA1 role in an alternative form of associative learning without the influence of the pharmacological effect of morphine. Our data of CPP extinction support the effect of CeA GluA1-mediated learning facilitation rather than a sensitized pharmacological effect of morphine per se. This role of CeA GluA1 is in line with previous studies demonstrating a generally consistent extinction-promoting effect of GluA1 subunits in cocaine-seeking behavior in other brain regions of reward (Sutton et al., 2003; Engblom et al., 2008). Of note is the report that deletion of AMPA receptor binding protein Narp, which is highly expressed in the amygdala, blocks extinction of morphine-induced CPP (Crombag et al., 2009). Interestingly, mice lacking GluA1 can learn to associate a light/tone stimulus with food reward, but show inhibited ability to associate the cue with a new behavior of reward (Mead and Stephens, 2003a). In addition, our results of the novel object recognition test indicate that CeA GluA1, while important in associative learning of opioid reward, may not be involved in learning of novel objects and related recognition memory.
Our data showed that shRNA downregulation of GluA1 or GluA2 also significantly decreased GluA2 or GluA1, respectively, in CeA synaptosomal preparations. This is unlikely caused by the off-target effect of the shRNA sequence because the shRNA sequence does not have potential target sequences of the other subunit in rat mRNA by blast analysis. In fact, this associated change in the expression of GluA1 and GluA2 has been reported in previous studies, which showed that deletion of either of GluA1 or GluA2 would disrupt and decrease the expression of the other subunit in BLA and hippocampus (Zamanillo et al., 1999; Mead and Stephens, 2003a, b). However, overexpression of GluA1 did not increase the GluA2 level in our study, nor was it reported in previous studies (Carlezon et al., 1997; Shi et al., 1999; Mack et al., 2001; Sutton et al., 2003; Rumpel et al., 2005; Schmitt et al., 2005; Matsuo et al., 2008). Thus, it appears that, whereas GluA2-lacking AMPA receptors could be upregulated independently of GluA2 subunits in the synapse, a certain level of GluA1 and GluA2 subunits may be required to maintain the synaptic function of GluA1/2 AMPA receptors. Although the molecular mechanism for interactive assembly and trafficking of GluA1 and GluA2 heterotetramers is still unclear, it is consistent with the notion that, of GluA1 and GluA2 subunits, lack of one type inhibits membrane insertion of the other and their co-assembly on synapses (Zamanillo et al., 1999). The shared facilitating effect on CPP acquisition but differential effects on CPP extinction by GluA1 overexpression and GluA2 knockdown may indicate that GluA1 is more actively and predominantly involved in the associative learning than GluA2. As knockdown of one subunit type also decreases the other, this off-target effect should be cautiously considered in data interpretation of GluA1 and GluA2 roles.
Given the direct glutamatergic projections from BLA to CeA and predominant glutamatergic neurons in BLA (Sah et al., 2003), it would be intriguing to investigate whether GluA1 in BLA neurons has a similar or differential role in associative learning for behaviors of opioid reward. Due to potential spillover of the injected viral vector into BLA, such a role of BLA GluA1 in the behavioral effects we observed cannot be excluded.
In summary, the present study suggests that increased expression of GluA1 subunits, and consequently upregulation of GluA2-lacking AMPA receptors, in CeA neurons may facilitate learning and consolidation of context-drug reward association, resulting in accelerated acquisition of behavior of opioid reward and its inhibition by new conditioning stimuli. In a clinical perspective, this may imply an important role of CeA GluA1 subunits in promoting early development of drug-seeking behaviors leading to drug addiction and in alleviating drug-seeking behaviors after drug withdrawal.
Acknowledgements
This work was supported by the National Institute on Drug Abuse grants DA023069 and DA027541.
Footnotes
Conflict of interest
The authors declare no financial conflict of interest.
References
- Bachtell RK, Self DW. Renewed cocaine exposure produces transient alterations in nucleus accumbens AMPA receptor-mediated behavior. J Neurosci. 2008;28:12808–12814. doi: 10.1523/JNEUROSCI.2060-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtell RK, Choi KH, Simmons DL, Falcon E, Monteggia LM, Neve RL, Self DW. Role of GluR1 expression in nucleus accumbens neurons in cocaine sensitization and cocaine-seeking behavior. Eur J Neurosci. 2008;27:2229–2240. doi: 10.1111/j.1460-9568.2008.06199.x. [DOI] [PubMed] [Google Scholar]
- Balland B, Lachamp P, Strube C, Kessler JP, Tell F. Glutamatergic synapses in the rat nucleus tractus solitarii develop by direct insertion of calcium-impermeable AMPA receptors and without activation of NMDA receptors. J Physiol. 2006;574:245–261. doi: 10.1113/jphysiol.2006.108738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc. 2006;1:1306–1311. doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- Bie B, Zhu W, Pan ZZ. Rewarding morphine-induced synaptic function of delta-opioid receptors on central glutamate synapses. J Pharmacol Exp Ther. 2009;329:290–296. doi: 10.1124/jpet.108.148908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billa SK, Liu J, Bjorklund NL, Sinha N, Fu Y, Shinnick-Gallagher P, Moron JA. Increased insertion of glutamate receptor 2-lacking alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors at hippocampal synapses upon repeated morphine administration. Mol Pharmacol. 2010;77:874–883. doi: 10.1124/mol.109.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SF, Lauzon NM, Bechard M, Gholizadeh S, Laviolette SR. NMDA receptor hypofunction in the prelimbic cortex increases sensitivity to the rewarding properties of opiates via dopaminergic and amygdalar substrates. Cerebr Cort. 2011;21:68–80. doi: 10.1093/cercor/bhq060. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bowers MS, Chen BT, Bonci A. AMPA receptor synaptic plasticity induced by psychostimulants: the past, present, and therapeutic future. Neuron. 2010;67:11–24. doi: 10.1016/j.neuron.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr., Nestler EJ. Elevated levels of GluR1 in the midbrain: a trigger for sensitization to drugs of abuse? Trends Neurosci. 2002;25:610–615. doi: 10.1016/s0166-2236(02)02289-0. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr., Boundy VA, Haile CN, Lane SB, Kalb RG, Neve RL, Nestler EJ. Sensitization to morphine induced by viral-mediated gene transfer. Science. 1997;277:812–814. doi: 10.1126/science.277.5327.812. [DOI] [PubMed] [Google Scholar]
- Choi KH, Edwards S, Graham DL, Larson EB, Whisler KN, Simmons D, Friedman AK, Walsh JJ, Rahman Z, Monteggia LM, Eisch AJ, Neve RL, Nestler EJ, Han MH, Self DW. Reinforcement-related regulation of AMPA glutamate receptor subunits in the ventral tegmental area enhances motivation for cocaine. J Neurosci. 2011;31:7927–7937. doi: 10.1523/JNEUROSCI.6014-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Dickson M, Dinenna M, Johnson AW, Perin MS, Holland PC, Baraban JM, Reti IM. Narp deletion blocks extinction of morphine place preference conditioning. Neuropsychopharmacology. 2009;34:857–866. doi: 10.1038/npp.2008.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Graham DL, Whisler KN, Self DW. Phosphorylation of GluR1, ERK, and CREB during spontaneous withdrawal from chronic heroin self-administration. Synapse. 2009;63:224–235. doi: 10.1002/syn.20601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Engblom D, Bilbao A, Sanchis-Segura C, Dahan L, Perreau-Lenz S, Balland B, Parkitna JR, Lujan R, Halbout B, Mameli M, Parlato R, Sprengel R, Luscher C, Schutz G, Spanagel R. Glutamate receptors on dopamine neurons control the persistence of cocaine seeking. Neuron. 2008;59:497–508. doi: 10.1016/j.neuron.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW. Appetitive behavior: impact of amygdala-dependent mechanisms of emotional learning. Ann N Y Acad Sci. 2003;985:233–250. [PubMed] [Google Scholar]
- Fitzgerald LW, Ortiz J, Hamedani AG, Nestler EJ. Drugs of abuse and stress increase the expression of GluR1 and NMDAR1 glutamate receptor subunits in the rat ventral tegmental area: common adaptations among cross-sensitizing agents. J Neurosci. 1996;16:274–282. doi: 10.1523/JNEUROSCI.16-01-00274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadd CA, Murtra P, De Felipe C, Hunt SP. Neurokinin-1 receptor-expressing neurons in the amygdala modulate morphine reward and anxiety behaviors in the mouse. J Neurosci. 2003;23:8271–8280. doi: 10.1523/JNEUROSCI.23-23-08271.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- He YY, Xue YX, Wang JS, Fang Q, Liu JF, Xue LF, Lu L. PKMzeta maintains drug reward and aversion memory in the basolateral amygdala and extinction memory in the infralimbic cortex. Neuropsychopharmacology. 2011;36:1972–1981. doi: 10.1038/npp.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Real E, Takamiya K, Kang MG, Ledoux J, Huganir RL, Malinow R. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 2007;131:160–173. doi: 10.1016/j.cell.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Ann Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Witkin BM, Zangen A, Wise RA. Rewarding effects of AMPA administration into the supramammillary or posterior hypothalamic nuclei but not the ventral tegmental area. J Neurosci. 2004;24:5758–5765. doi: 10.1523/JNEUROSCI.5367-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac JT, Ashby MC, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54:859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Knapska E, Radwanska K, Werka T, Kaczmarek L. Functional internal complexity of amygdala: focus on gene activity mapping after behavioral training and drugs of abuse. Physiol Rev. 2007;87:1113–1173. doi: 10.1152/physrev.00037.2006. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Wang XS, Dai RP, Zhang JY, Zhou XF, Hao W, Li CQ. The activation of NMDA receptor-ERK pathway in the central amygdala is required for the expression of morphine-conditioned place preference in the rat. Neurotox Res. 2011;20:362–371. doi: 10.1007/s12640-011-9250-2. [DOI] [PubMed] [Google Scholar]
- Li YQ, Li FQ, Wang XY, Wu P, Zhao M, Xu CM, Shaham Y, Lu L. Central amygdala extracellular signal-regulated kinase signaling pathway is critical to incubation of opiate craving. J Neurosci. 2008;28:13248–13257. doi: 10.1523/JNEUROSCI.3027-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack V, Burnashev N, Kaiser KM, Rozov A, Jensen V, Hvalby O, Seeburg PH, Sakmann B, Sprengel R. Conditional restoration of hippocampal synaptic potentiation in Glur-A-deficient mice. Science. 2001;292:2501–2504. doi: 10.1126/science.1059365. [DOI] [PubMed] [Google Scholar]
- Maren S. Synaptic mechanisms of associative memory in the amygdala. Neuron. 2005;47:783–786. doi: 10.1016/j.neuron.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Matsuo N, Reijmers L, Mayford M. Spine-type-specific recruitment of newly synthesized AMPA receptors with learning. Science. 2008;319:1104–1107. doi: 10.1126/science.1149967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- Mead AN, Stephens DN. Selective disruption of stimulus-reward learning in glutamate receptor gria1 knock-out mice. J Neurosci. 2003a;23:1041–1048. doi: 10.1523/JNEUROSCI.23-03-01041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead AN, Stephens DN. Involvement of AMPA receptor GluR2 subunits in stimulus-reward learning: evidence from glutamate receptor gria2 knock-out mice. J Neurosci. 2003b;23:9500–9507. doi: 10.1523/JNEUROSCI.23-29-09500.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA. The amygdala, reward and emotion. Trends Cogn Sci. 2007;11:489–497. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Olmstead MC, Franklin KB. The development of a conditioned place preference to morphine: effects of microinjections into various CNS sites. Behav Neurosci. 1997;111:1324–1334. doi: 10.1037//0735-7044.111.6.1324. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2nd Edition Academic press; Sydney: 1986. [Google Scholar]
- Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Plant K, Pelkey KA, Bortolotto ZA, Morita D, Terashima A, McBain CJ, Collingridge GL, Isaac JT. Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat Neurosci. 2006;9:602–604. doi: 10.1038/nn1678. [DOI] [PubMed] [Google Scholar]
- Rezayof A, Golhasani-Keshtan F, Haeri-Rohani A, Zarrindast MR. Morphine-induced place preference: involvement of the central amygdala NMDA receptors. Br Res. 2007;1133:34–41. doi: 10.1016/j.brainres.2006.11.049. [DOI] [PubMed] [Google Scholar]
- Rezayof A, Sardari M, Zarrindast MR, Nayer-Nouri T. Functional interaction between morphine and central amygdala cannabinoid CB1 receptors in the acquisition and expression of conditioned place preference. Behav Br Res. 2011;220:1–8. doi: 10.1016/j.bbr.2011.01.023. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Ersche KD, Everitt BJ. Drug addiction and the memory systems of the brain. Ann N Y Acad Sci. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Schmitt WB, Sprengel R, Mack V, Draft RW, Seeburg PH, Deacon RM, Rawlins JN, Bannerman DM. Restoration of spatial working memory by genetic rescue of GluR-A-deficient mice. Nat Neurosci. 2005;8:270–272. doi: 10.1038/nn1412. [DOI] [PubMed] [Google Scholar]
- See RE, Fuchs RA, Ledford CC, McLaughlin J. Drug addiction, relapse, and the amygdala. Ann N Y Acad Sci. 2003;985:294–307. doi: 10.1111/j.1749-6632.2003.tb07089.x. [DOI] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Sommer B, Keinanen K, Verdoorn TA, Wisden W, Burnashev N, Herb A, Kohler M, Takagi T, Sakmann B, Seeburg PH. Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS. Science. 1990;249:1580–1585. doi: 10.1126/science.1699275. [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Berridge CW. AMPA receptor stimulation within the central nucleus of the amygdala elicits a differential activation of central dopaminergic systems. Neuropsychopharmacology. 2003;28:1923–1934. doi: 10.1038/sj.npp.1300268. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Schmidt EF, Choi KH, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL, Self DW. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421:70–75. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Vlachos A, Korkotian E, Schonfeld E, Copanaki E, Deller T, Segal M. Synaptopodin regulates plasticity of dendritic spines in hippocampal neurons. J Neurosci. 2009;29:1017–1033. doi: 10.1523/JNEUROSCI.5528-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanillo D, Sprengel R, Hvalby O, Jensen V, Burnashev N, Rozov A, Kaiser KM, Koster HJ, Borchardt T, Worley P, Lubke J, Frotscher M, Kelly PH, Sommer B, Andersen P, Seeburg PH, Sakmann B. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science. 1999;284:1805–1811. doi: 10.1126/science.284.5421.1805. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Rezayof A, Sahraei H, Haeri-Rohani A, Rassouli Y. Involvement of dopamine D1 receptors of the central amygdala on the acquisition and expression of morphine-induced place preference in rat. Br Res. 2003;965:212–221. doi: 10.1016/s0006-8993(02)04201-4. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Pan ZZ. Synaptic mechanism for functional synergism between delta-and mu-opioid receptors. J Neurosci. 2010;30:4735–4745. doi: 10.1523/JNEUROSCI.5968-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Cai Y, Zou F, Bie B, Pan ZZ. Epigenetic suppression of GAD65 expression mediates persistent pain. Nat Med. 2011;17:1448–1455. doi: 10.1038/nm.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Bie B, Pan ZZ. Involvement of non-NMDA glutamate receptors in central amygdala in synaptic actions of ethanol and ethanol-induced reward behavior. J Neurosci. 2007;27:289–298. doi: 10.1523/JNEUROSCI.3912-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Ann Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]