Abstract
Stress, which involves a heightened arousal and excitability, triggers important adaptive responses to maintain homeostasis and prepare a response. In the current studies, we administered a psychological stressor of 2 h acute restraint on rats, and found that 24 h after the cessation of the restraint session, there was a significant decrease in VTA DA neuron population activity and a significant attenuation in amphetamine-induced locomotor activity. Systemic or intra-BLA administration of the β-noradrenergic receptor antagonist, propranolol, reversed the decrease, suggesting that the delayed attenuation of DA neuron firing following a stressor depends on a noradrenaline-mediated mechanism. This alteration in DA activity may adaptively prepare the individual to avoid the stressor, or in the extreme, may be a factor that contributes to pathological changes in behavior or physiological responses.
Introduction
Stress is defined as any threat, either real or perceived, to the homeostasis and well-being of an organism (Herman and Cullinan, 1997; Sawchenko et al., 2000; Morilak et al., 2005; Joels and Baram, 2009) and which may cause the subject to initiate appropriate responses to adapt to the changing environment (de Kloet et al., 2005; McEwen, 2007; Ulrich-Lai and Herman, 2009). The temporal profiles of stress responses span from milliseconds to days (Joels and Baram, 2009). The rapid actions provoke vigilance and alertness of the situation for an optimal strategy to face the challenge, while the late actions promote the sustained and adaptive components of stress responses. Thus, how fast a stress response can be evoked and how long it lasts is critical to the physiological homeostasis and adaptation of the individual.
Previously, we found that 2 h acute restraint significantly increased ventral tegmental area (VTA) dopaminergic (DA) system responsivity when tested soon after the stressful event (Valenti et al., 2011). However, given that the effects of stressors change over time, it was not known whether this effect was maintained or was a transitional period preceding subsequent alterations, such as those that occur in the opponent-process model (Solomon and Corbit, 1974; Koob et al., 1997). Secondly, given the known involvement of the noradrenergic system in stress responses (Morilak et al., 2005), how the noradrenergic system impacted the stress-induced changes in VTA DA neuron activity were of interest both with respect to mechanisms and potential therapeutic interventions. Noradrenergic neurons in the locus coeruleus (LC) are activated by stressful stimuli (Rasmussen et al., 1986; Aston-Jones et al., 1991; Morilak et al., 2005). The LC projects diffusely onto many forebrain nuclei (Jones and Moore, 1977) that are major targets relevant to stress, including basloateral nuclei of the amygdala (BLA; composed of the lateral, basal, and accessory basal nuclei) (LeDoux, 2000), which provides feedback to the LC via reciprocal projections (Van Bockstaele et al., 2001; Buffalari and Grace, 2007) and potentially modulates the VTA via the nucleus accumbens (NAc) (Sesack and Grace, 2010) and other structures (Conde et al., 1995; McDonald et al., 1996; Pitkanen et al., 2000; Herry et al., 2008). The BLA is well known for its involvement in aversive (stressful) learning and is highly modulated by norepinephrine (NE) (McGaugh, 2004; Roozendaal et al., 2006; Tully and Bolshakov, 2010; Debiec et al., 2011; Johnson et al., 2011), placing it as a strong candidate in mediating the interaction between the noradrenergic and dopaminergic systems.
Using the combined techniques of electrophysiological recordings, behavioral testing, pharmacological manipulations, and immunohistochemical approaches, we examined the delayed response of the VTA DA neurons to 2 h acute restraint and the modulatory role of the noradrenergic system in this response. We hypothesize that the immediate increase of the VTA DA neuron population activity and amphetamine-induced locomotor activity transition over time to a different, opposite homeostatic state that may be related to NE actions within the BLA.
Materials and Methods
Subjects and materials
A total of 212 male Sprague Dawley rats (300-400g; Harlan Laboratories) were used in this study; 72 rats were used for electrophysiological recordings, 128 rats for behavioral testing, and 12 rats for immunohistochemistry. Rats were housed for at least 5 d in pairs in a temperature (22°C)- and humidity (47%)-controlled facility upon arrival, with lights maintained on a 12 h light/dark cycle (lights on at 7:00 A.M.) and food and water available ad libitum. Handling of all animals, surgery, and experiment protocols were in accordance with the guidelines outlined in the United States Public Health Service Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
Stress paradigms
A psychological stressor of acute restraint was used on awake rats. Rats were restrained in Plexiglas cylinders (internal diameter of 6.2 cm, length adjusted accordingly to rat size; IITC life science) for a 2 h session between 9:00 A.M. to 12:00 P.M., across all studies. Control animals were placed in the holding cage for an equivalent 2 h period. Our protocol states that any rats showing excessive distress in terms of extended vocalizations or struggling should be removed from the study; however, none of the rats fit the criteria for exclusion. For immunohistochemical studies, rats were restrained for a 30 min session, which has been shown effective at inducing cfos expression in regions of interests (Goebel et al., 2009).
Acute surgery and electrophysiological recordings from anesthetized rats
Single-unit extracellular recordings were performed on anesthetized rats between 9:00 A.M. to 5:00 P.M. (Valenti et al., 2011). Rats were anesthetized with 8% chloral hydrate (400 mg/kg, i.p.) and placed in a stereotaxic apparatus (David Kopf Instruments); core body temperature of 37°C was maintained by a temperature-controlled heating pad (FST). Supplemental injections were administered as needed. The skull overlying the recording region in the VTA [from bregma: anteroposterior (AP), −5.3 mm; mediolateral (ML), −0.6 mm] was removed, and the dura was resected. Single-barrel electrodes (2 mm outer diameter Omegadot filament glass; World Precision Instruments) were constructed using a vertical microelectrode puller (PE-2; Narishige), and the recording barrel filled with 2% Pontamine sky blue in 2 M NaCl with in situ impedance of 4-10 MΩ (measured at 1kHz). Glass electrodes were lowered into the VTA [from brain surface: dorsoventral (DV), −6.5 to −9.0 mm] using a hydraulic microdrive (David Kopf Instruments, model 640). Signals from the recording electrode were amplified by a headstage before being fed into a window discriminator/amplifier (1000 gain, 50-16k Hz bandpass; Fintronics Inc.), then into an audio monitor (Grass Instruments, model AM8), and displayed on an oscilloscope (Tektronix) for real-time monitoring. Data were collected using a data acquisition board interface, monitored on-line, and analyzed off-line using computer software, Powerlab (AD instruments).
DA neurons were identified with open filter setting (high pass, 50 Hz; low pass, 16k Hz) and distinguished from other VTA neurons based on their unique long-duration waveform, slow irregular firing rate, and other well-established criteria (Grace and Bunney, 1983; Grace et al., 2007; Ungless and Grace, 2012). Three parameters of VTA DA neuron activity were calculated. (1) Population activity was assessed by passing the electrode through the VTA in a predetermined grid pattern of six to nine tracks separated by 200 μm; all spontaneously active DA neurons encountered per electrode track were counted. After isolating a DA neuron, 3 min of activity was recorded to determine the (2) firing rate and (3) percentage burst firing. Percentage burst firing was quantified by examining the proportion of action potentials occurring in bursts, with bursts defined as the occurrence of two spikes with an interspike interval (ISI) of <80 ms indicating the initiation of a burst, and two spikes with an ISI >160 ms signaling burst termination (Grace and Bunney, 1984).
Locomotor response to psychostimulants
The locomotor response to psychostimulants was assessed as described previously (Lodge and Grace, 2008). Rats were housed with a reverse 12 h light/dark cycle (lights on 7:00 P.M.) for at least 1 week before behavioral experiments. On testing days (9:00 A.M. to 5:00 P.M.), rats were acclimatized to an open-field arena (Coulbourn Instruments) where spontaneous locomotor activity in the X-Y plane was monitored for 30 min by beam breaks and recorded with TruScan software (Coulbourn Instruments). Rats were then administered either a challenge dose of amphetamine (0.5 mg/kg, i.p.) or saline (control animals, i.p.), and locomotor activity was recorded for an additional 90 min (Lodge and Grace, 2007). The order of testing was counterbalanced across groups. It should be noted that locomotor activity was examined during the active part of the diurnal cycle, whereas DA recordings were performed during the inactive part of the cycle. Importantly, recent data have demonstrated that DA neurons do not display differences in firing across the diurnal cycle (Luo et al., 2008).
Survival surgery and drug administration
The β-noradrenergic receptor antagonist, propranolol (Sigma), was administered either systemically or intra-BLA. For systemic administration, drugs were dissolved in sterile saline to a final concentration of 5 mg/ml. Rats were given propranolol (5 mg/kg, i.p.) or saline as the control. For intra-BLA administration, rats were implanted bilaterally with 26 gauge stainless-steel guide cannulae (Plastic One) targeting the BLA [from the bregma: AP, −2.9 mm; ML, +5.0 mm; DV, −7.6 mm] under isoflurane inhalation anesthesia (Halocarbon Laboratories; 1-3% in oxygen). Four additional anchor screws (FST) were mounted and the headstage was fixed in place with dental cement. Rats were then administered postoperative analgesic (2 mg/kg ketoprofen, s.c. and 10% Tylenol Children’s Syrup added to rat chow). At least 7 d of recovery were allowed before drug administration. 33 gauge injectors protruding 1.0 mm past the end of the implanted guide cannulae were inserted and the rats received infusions of propranolol (0.5 μg per side) or saline as the control. All drugs were delivered in 0.2 μl of sterile saline (0.1 μl/min), and another minute was allowed for diffusion of the drug before the internal injectors were removed. The dosages used have been proven effective in earlier studies (Salmon and Stanford, 1989; Roozendaal et al., 2006).
Histology
At the cessation of the electrophysiology experiments, the recording site was marked via electrophoretic ejection (Kation Scientific, model BAB-501) of Pontamine sky blue dye from the tip of the recording electrode (-20 μA constant current for 40 min). Rats were killed by an overdose of anesthetic (chloral hydrate, additional 400 mg/kg, i.p.) or CO2. All rats were decapitated, and their brains were removed, fixed for at least 48 h (8% paraformaldehyde in PBS), and cryoprotected (25% sucrose in PBS) until saturated. Brains were sectioned (60 μm coronal sections), mounted onto gelatin-chrome alum-coated slides and stained with cresyl violet for histochemical verification of electrode and/or cannula sites.
Immunohistochemistry
Rats were anesthetized by isoflurane inhalation (Halocarbon Laboratories; 1-3% in oxygen) and oriented in a Kopf stereotaxic instrument in the flat skull position. A small hole (~1-2 mm diameter) was opened in the skull to expose the cortical surface overlying the BLA [from bregma: AP, −2.9 mm; ML, −5.0 mm; DV, −8.6 mm] tracer delivery target site. A pulled glass micropipette (approximately 20 μm outer tip diameter) was attached to the arm of the stereotaxic apparatus. Using negative pressure, the micropipette was backfilled with a 1% solution of the retrograde tracer of fluorogold (FG; Fluorochrome) diluted in 0.1 M cacodylic acid, and then a microwire connected to a current source (Stoelting) was lowered into the tracer solution. FG was unilaterally delivered by iontophoresis into the BLA using a 7 s pulsed positive current of 5 μA for a duration of 5 min. Iontophoretic parameters were determined through pilot studies and were found to produce discrete, localized tracer delivery sites with minimal tracer diffusion into adjacent regions. The micropipette tip was left in place for 1 min after iontophoresis, and then was withdrawn. The skin over the skull was closed with stainless steel clips and rats were injected postoperatively with analgesic (2 mg/kg ketoprofen, s.c.). Rats were returned to their home cage after regaining consciousness and full mobility.
After a 10–14 day post-iontophoresis survival time, the rats were subjected to the restraint procedures. In order to capture the time window of maximal cfos expression 90 min after the manipulation (Knapska and Maren, 2009), the restraint protocol was modified with half of the rats restrained in Plexiglas cylinders for a 30 min session, returned to their home cage and left undisturbed for 1 h, while the other half remained in their home cage throughout the time interval. All rats were then deeply anesthetized with sodium pentobarbital (Nembutal, 100 mg/kg, i.p.) and perfused transcardially with 100 ml saline followed by 500 ml of fixative (4% paraformaldehyde in 0.1M PBS). Brains were post fixed overnight at 4 °C and cryoprotected in 20% sucrose solution for 24-72 h. Brains were sectioned coronally (35 μm) using a freezing microtome. Sections were collected sequentially into 6 adjacent sets and were stored in cryopreservant at −20 °C for later immunohistochemical processing.
One tissue section series (containing sections spaced by 210 μm) from each rat was used for dual immunoperoxidase localization of nuclear cfos protein and cytoplasmic neural tracer, FG. Tissue sections were removed from storage and rinsed in buffer (0.1 M sodium phosphate, pH=7.4) for 1 h prior to immunohistochemical procedures. Immunoperoxidase localization of cfos protein followed previously established protocols (Bienkowski and Rinaman, 2011). Tissue sections were incubated overnight in buffer containing 0.3% Triton-X100, 1% normal donkey serum, 1% bovine serumalbumin (BSA), and a rabbit polyclonal anti-cfos antibody (1:50,000, Dr. Philip Larsen, Denmark). After rinsing with buffer, sections were incubated in biotinylated donkey anti-rabbit IgG (1:500; Jackson Immunochemicals) and Vectastatin ABC Elite reagents (Vector Laboratories) followed by a solution of nickel sulfate and diaminobenzidine (DAB) with hydrogen peroxide to produce a blue-black reaction product within the nucleus of cfos positive neurons. Sections were then rinsed in buffer and incubated overnight in buffer containing 0.3% Triton-X100, 1% normal donkey serum, and rabbit anti-FG (1:30,000; Chemicon International) to localize neural tracer. After rinsing, sections were incubated in biotinylated secondary donkey anti-rabbit IgG (1:500) and Vectastatin ABC Elite reagents followed by a non-intensified DAB-hydrogen peroxide reaction to produce brown immunoprecipitate localizing the neural tracer delivery site and retrogradely-labeled neurons. Immunostained tissue sections were rinsed in buffer, mounted onto Superfrost Plus microscope slides (Fisher Scientific), allowed to dry overnight, dehydrated and defatted in graded ethanols and xylene, and coverslipped using Cytoseal 60 (VWR).
Dual-immunoperoxidase labeled tissue sections from each rat were analyzed using a light microscope to determine the number and proportion of retrogradely-labeled BLA afferents activated to express cfos after restraint. Criteria for counting tracer positive neurons included the presence of brown cytoplasmic immunoreactivity and a visible nucleus. Tracer-positive neurons in which the nucleus contained blue-black cfos immunoreactivity were considered double-labeled, regardless of cfos labeling intensity. Tracer-positive neurons were quantified in sections spaced by 210 μm through the locus coeruleus (LC) [from bregma: AP, ~ −9.5 mm to −10.1 mm], comprising 2-3 tissue sections (spaced by 210 μm) per rat.
Data analysis and statistics
Electrophysiological analysis of single-unit neuron activities was performed using computer softwares, Powerlab (AD instruments) and Nex (NEX Technologies). Locomotor behavior was recorded using TruScan software (Coulbourn Instruments). For immunohistochemical data, the total number of single labeled (tracer only) and double-labeled (tracer + cfos) neurons in LC was counted. The percentage of activated neurons was calculated as (tracer + cfos)/[(tracer only) + (tracer + cfos)]×100. All data are represented as the mean ± SEM unless otherwise stated and were submitted to analysis of variance (ANOVA) or t-test. Post hoc comparisons in the form of Neuman-Keuls tests were performed after a significance of p < 0.05. All statistics were calculated using SPSS (IBM).
Results
VTA DA neurons
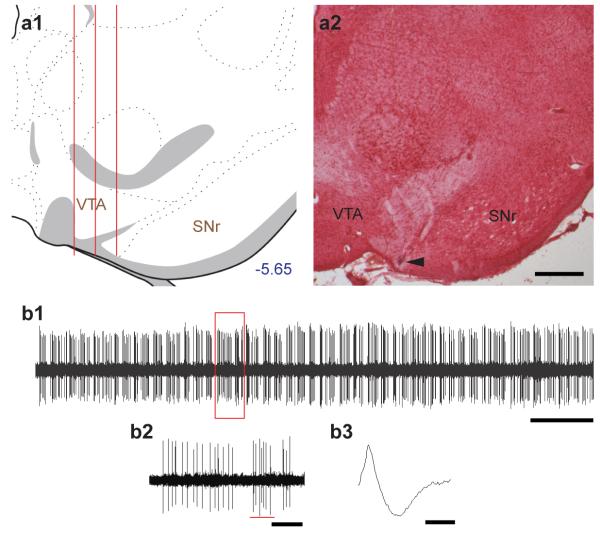
VTA DA activity was assessed by passing the electrode through the VTA in a predetermined grid pattern of six (3 × 2) to nine (3 × 3) tracks separated by 200 μm (Valenti et al., 2011) (Fig. 1a1 and 1a2). Population activity (number of spontaneously firing DA neurons per electrode track), mean firing rate, and percent burst firing (i.e., the proportion of action potentials occurring in bursts, with burst onset defined as the occurrence of two spikes with an interspike interval (ISI) of <80 ms, and termination defined as two spikes with an ISI >160 ms (Grace and Bunney, 1984)) were calculated (Fig. 1b1 and 1b2). DA neurons (Fig. 1b3) were identified with open filter setting (high pass, 50 Hz; low pass, 16k Hz) and distinguished from other VTA neurons based on their unique long-duration waveform (> 2.5 ms), slow irregular firing rate (< 5 Hz), and other well-established criteria (Grace and Bunney, 1983; Grace et al., 2007). By employing this standard set of criteria, we were able to accurately identify the large majority of DA neurons reported in this study (Ungless and Grace, 2012).
Figure 1.
Evaluation of VTA DA neuron activity states. (a1) DA neurons were sampled with predetermined grid pattern of six (3 × 2) to nine (3 × 3) tracks separated by 200 μm. (a2) A representative Pontamine sky blue deposit. Arrowhead indicates the final location of the electrode. Scale bar, 500 μm. (b1) A segment of spontaneous VTA DA activity. Scale bar, 10 s. (b2) A closer view of DA activity. Underlying red line indicates a burst event. Scale bar, 1s. (b3) A representative waveform of a DA neuron. Scale bar, 1 ms.
A significant decrease in VTA DA neuron population activity and attenuated amphetamine-induced locomotor activity is present 24 h after acute restraint stress
Previously, we found that 2 h of acute restraint significantly increased VTA DA neuron population activity and amphetamine-induced locomotor activity when tested immediately after the stressful event (Valenti et al., 2011). In this study, we further explored whether this enhancement is transient or long-lasting by testing these responses 24 h after the acute restraint.
The subjects were 58 male Sprague Dawley rats: 18 rats were used for DA electrophysiological recordings and 40 rats for behavioral testing. For electrophysiological recordings, rats were assigned into one of the two groups: one that was restrained for a 2 h session (Res, n = 10), and a control group that was held in the holding cage for the equivalent amount of time without restraint (NoRes, n = 8).
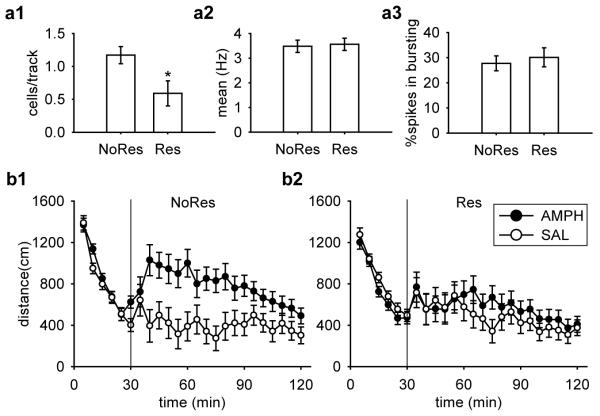
In contrast to the increased DA neuron population activity observed soon after restraint, we found a significant decrease in population activity 24 h after the 2 h acute restraint by approximately 50% (Fig. 2a1; [t(16) = −2.43, p = 0.03]). A total of 53 DA neurons were recorded in Res group and 83 DA neurons in NoRes group. No differences in the average firing rate (Fig. 2a2; [t(134) = 0.22, p = 0.83]) or percent of spikes in bursts (Fig. 2a3; [t(134) = 0.51, p = 0.61]) was observed.
Figure 2.
There was a significant decrease in VTA DA neuron population activity and an attenuated amphetamine-induced locomotor response measured 24 h after acute restraint. (a1) DA neuron population activity in Restraint rats (Res; n = 10) was significantly lower than No-Restraint (NoRes; n = 8) controls (*, p < 0.05), with no significant difference between (a2) mean firing rates or (a3) bursting activity. DA neurons recorded: Res (n = 53), NoRes (n = 83). Amphetamine (AMPH) induced significantly higher locomotor activity in NoRes rats (b1), but not in Res rats (b2). (p < 0.05). Group sizes: Res-SAL (n = 10), Res-AMPH (n = 10), NoRes-SAL (n = 9), and NoRes-AMPH (n = 10).
Multiple studies have shown that the level of DA neuron population activity correlates with the behavioral responses to amphetamine (Lodge and Grace, 2007, 2008; Valenti et al., 2011; Cifelli and Grace, 2012). In order to provide additional data to support the finding of a delayed decrease in DA system activity, we examined whether such a decrease corresponded to a decreased behavioral response to amphetamine.
The same procedure was used for Res and NoRes for the locomotor behavioral testing. One NoRes-SAL rat was lost due to computer error, yielding the final group sizes: Res-SAL (n = 10), Res-AMPH (n = 10), NoRes-SAL (n = 9), and NoRes-AMPH (n = 10).
We found that following administration of 0.5 mg/kg amphetamine, only rats in the NoRes group displayed significantly higher levels of locomotor activity compared to their saline controls (Fig. 2b). For the 30 min acclimation, there was an equivalent decrease in locomotor activity across sampling blocks (5 min bin) in all groups [Restraint × Drug × Sampling Block, F(5,175) < 1]. For the 90 min post-injection testing, an ANOVA revealed a significant main effect of Drug [F(1,35) = 6.62, p = 0.01], and a significant three-way interaction among Restraint, Drug, and Sampling Block[F(17, 595) = 1.67, p = 0.045]. Planned comparisons suggested that there was no significant difference between saline controls (Res-SAL and NoRes-SAL, p = 0.55). The locomotor activity was only significantly higher after administering amphetamine in NoRes rats compared to their saline controls (Fig. 2b1; NoRes-AMPH and NoRes-SAL, p = 0.006), but not in Res rats (Fig. 2b2; Res-AMPH and Res-SAL, p = 0.51). The effect was most robust at the onset of amphetamine administration, which wore off at later time points. Planned comparisons demonstrated significantly higher locomotor activity in NoRes-AMPH rats compared to Res-AMPH rats between post-injection sampling blocks of 10 to 20 min (ps < 0.05).
Pre-restraint systemic administration of the β-noradrenergic receptor antagonist, propranolol, blocked the decrease in VTA DA neuron population activity and prevented the attenuation of amphetamine-induced locomotor activity observed 24 h after restraint
Given that stressful events are known to lead to increased release of noradrenaline (Morilak et al., 2005; Roozendaal et al., 2009), we tested whether pre-restraint blockade of noradrenaline with systemic administration of the β-noradrenergic receptor antagonist, propranolol, prevented the decrease in VTA DA neuron population activity 24 h after acute restraint.
The subjects were 41 male Sprague-Dawley rats: 17 rats were used for DA electrophysiological recordings and 24 rats for behavioral testing. For electrophysiological recordings, all rats were restrained for a 2 h session. Half of the rats were administered propranolol systemically pre-restraint (PRO, n = 8; 5 mg/kg, i.p.), and the other half with saline as the control (SAL, n = 9; i.p.).
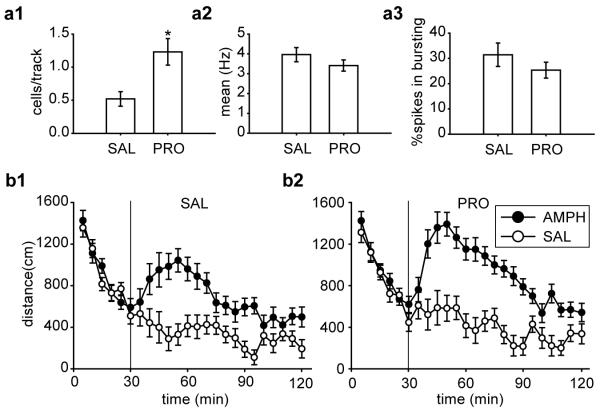
We found that pre-restraint blockade of the noradrenaline β receptors blocked the delayed decrease in VTA DA neuron population activity, which was significantly higher by 130% in animals pre-treated with propranolol compared to their saline controls (Fig. 3a1). There was a significant difference between SAL and PRO [t(15) = −3.15, p = 0.007]. Indeed, in PRO treated rats, DA neuron population activity was nearly identical to that of NoRes control rats in the first experiment (95% confidence interval from 0.87 to 1.48). A total of 42 DA neurons were recorded in the SAL group and 78 DA neurons in the PRO group. Propranolol administration did not affect the average firing rate (Fig. 3a2; [t(118) = 1.17, p = 0.24]) or percent burst firing (Fig. 3a3; [t(118) = 1.11, p = 0.27]). In contrast, in naïve rats that did not receive the restraint stress, there was no difference between propranolol and saline treated animals with respect to DA neuron population activity [SAL, 1.02 ± 0.12 (n = 6); PRO, 1.04 ± 0.30 (n = 6); t(10) = 0.06, p = 0.96], average firing rate [SAL, 4.25 ± 0.34 (n = 55); PRO, 4.72 ± 0.29 (n = 56); t(109) = 1.07, p = 0.29], or percent bursting firing [SAL, 31.67 ± 3.99 (n = 55); PRO, 31.45 ± 3.93 (n = 56); t(109) = 0.04, p = 0.97]. Therefore, the effect of propranolol was not due to nonspecific action of noradrenergic signaling independent of the restraint stress.
Figure 3.
Pre-restraint systemic administration of the β-noradrenergic receptor antagonist, propranolol, blocked the decrease in VTA DA neuron population activity and prevented the attenuation of amphetamine-induced locomotor activity measured 24 h after restraint. For all rats restrained for a 2 h session, propranolol pre-treatment (PRO; n = 8) significantly increased (a1) the DA neuron population activity than saline (SAL; n = 9) control (*, p < 0.05), with no significant difference between (a2) mean firing rates or (a3) bursting activity. DA neurons recorded: SAL (n = 42), PRO (n = 78). AMPH induced higher locomotor activity regardless pre-treatment (b1, SAL; b2, PRO), with significantly higher locomotor activity produced in rats pre-treated with PRO. (all ps < 0.05). Group sizes: SAL-SAL (n = 6), SAL-AMPH (n = 5), PRO-SAL (n = 5), and PRO-AMPH (n = 6).
Given that pre-restraint systemic propranolol administration blocked the decrease in DA neuron population activity 24 h after restraint, we examined whether this treatment also prevented the decreased behavioral response to amphetamine. All rats were restrained for a 2 h session, and the same procedure was used for PRO and SAL for the locomotor behavioral testing. One PRO-SAL rat was lost to computer error, and one SAL-AMPH rat failed to acclimate to the open arena during the first 30 min, yielding the final group sizes: SAL-SAL (n = 6), SAL-AMPH (n = 5), PRO-SAL (n = 5), and PRO-AMPH (n = 6).
We found that propranolol pre-treated rats showed a more robust increase in amphetamine-induced locomotor activity compared to saline pre-treated controls (Fig. 3b) when tested 24 h after the cessation of the acute restraint. For the 30 min acclimation, there was an equivalent decrease in locomotor activity across sampling blocks (5 min bin) in all groups [Treatment × Drug × Sampling Block, F(5,90) < 1]. For the 90 min post-injection testing, an ANOVA revealed a significant main effect of Treatment [F(1,18) = 6.12, p = 0.024], a significant main effect of Drug [F(1,18) = 50.04, p < 0.001], and a significant two-way interaction between Drug and Sampling Block [F(17,306) = 5.04, p < 0.001]. Planned comparisons suggested that there was no significant difference between saline controls regardless of pre-restraint treatment (SAL-SAL and PRO-SAL, p = 0.41). Unlike in the first experiment in which 2h acute restraint completely blocked the response to amphetamine (Fig. 2b2), in this study amphetamine induced an increased locomotor activity compared to their saline controls regardless of pre-restraint treatment (Fig. 3b1, SAL-SAL and SAL-AMPH, p = 0.001; Fig. 3b2, PRO-SAL and PRO-AMPH, p < 0.001). However, following amphetamine administration, there was a significantly higher locomotor activity in propranolol pre-treated rats compared to saline pre-treated rats (increase by 34%; Fig. 3b1 and 3b2, PRO-AMPH and SAL-AMPH, p = 0.016).
Post-restraint systemic administration of the β-noradrenergic receptor antagonist, propranolol, blocked the decrease in VTA DA neuron population activity and prevented the attenuation of amphetamine-induced locomotor activity 24 h following restraint
Stress-induced monoaminergic release generally occurs within minutes after the onset of the stressor and seldom outlasts the duration of stressor exposure (Joels and Baram, 2009). In the previous experiment, pre-restraint blockade of noradrenergic β receptors prevented the decrease in VTA DA neuron population activity and the attenuated response to amphetamine 24 h after restraint. However, to be an effective treatment of a stress-induced depression, a manipulation should be effective when administered after the stressor. Thus, we examined whether blockade of noradrenaline immediately after the release from a 2 h acute restraint session is sufficient and effective in blocking the attenuation of the DA system observed 24 h after restraint.
The subjects were 50 male Sprague Dawley rats: 18 rats were used for DA electrophysiological recordings and 32 rats for behavioral testing. For electrophysiological recordings, all rats were restrained for a 2 h session. Eight of the rats were administered propranolol systemically post-restraint (PRO, n = 8; 5 mg/kg, i.p.), and the others with saline as the control (SAL, n = 10; i.p.).
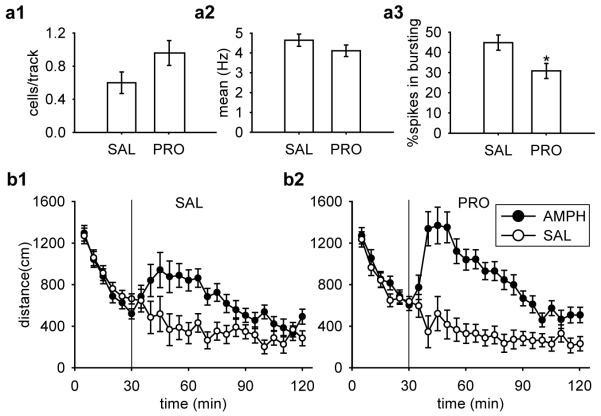
Similar to that observed with pre-restraint blockade of noradrenaline, the population activity in propranolol post-treated rats was increased by 60% and restored to values that are within a 95% confidence interval (C.I.) of NoRes control rats in the first study (Fig. 4a1). A total of 52 DA neurons were recorded in the SAL group and 56 DA neurons in the PRO group. Propranolol administration did not affect the average firing rate (Fig. 4a2; [t(106) = 1.26, p = 0.21]) but significantly decreased the percentage of spikes in bursts (Fig. 4a3; [t(106) = 2.64, p = 0.01]).
Figure 4.
Post-restraint systemic administration of the β-noradrenergic receptor antagonist, propranolol, blocked the decrease in VTA DA neuron population activity and prevented the attenuation of amphetamine-induced locomotor activity observed 24 h after restraint. For all rats restrained for a 2 h session, propranolol post-treatment (PRO; n = 8) restored (a1) the DA neuron population activity to that of non-restrained controls, with no significant difference between (a2) mean firing rates and (a3) a significant decrease in bursting activity (*, p < 0.05). DA neurons recorded: SAL (n = 52), PRO (n = 56). AMPH induced higher locomotor activity regardless post-treatment (b1, SAL; b2, PRO), with significantly higher locomotor activity for rats pre-treated with PRO. (all ps < 0.05). Group sizes: SAL-SAL (n = 8), SAL-AMPH (n = 8), PRO-SAL (n = 8), and PRO-AMPH (n = 7).
All rats were restrained for a 2 h session, and the same procedure was used for PRO and SAL for the locomotor behavioral testing. Injection error occurred in one of the propranolol treated rats leading to unknown dosage injected and thus this rat was excluded from data analysis, yielding the final group sizes: SAL-SAL (n = 8), SAL-AMPH (n = 8), PRO-SAL (n = 8), and PRO-AMPH (n = 7).
We found that rats treated with propranolol after restraint showed a more robust increase in amphetamine-induced locomotor activity compared to saline post-treated controls 24 h after the cessation of the acute restraint (Fig. 4b). For the 30 min acclimation, there was an equivalent decrease in locomotor activity across sampling blocks (5 min bin) in all groups [Treatment × Drug × Sampling Block, F(5,135) < 1]. For the 90 min post-injection testing, an ANOVA revealed a significant main effect of Drug [F(1,27) = 41.62, p < 0.001], and a significant two-way interaction between Drug and Sampling Block [F(17,459) = 6.62, p < 0.001]. Planned comparisons suggested that there was no significant difference between saline controls regardless of post-restraint treatment (SAL-SAL and PRO-SAL, p = 0.67). Similar to the results obtained with propranolol pre-treatment, in this study amphetamine induced an increased locomotor activity compared to their saline controls regardless of post-restraint treatment (Fig. 4b1, SAL-SAL and SAL-AMPH, p = 0.003; Fig. 4b2, PRO-SAL and PRO-AMPH, p < 0.001). However, following amphetamine administration, there was a significantly higher locomotor activity in propranolol post-treated rats compared to saline post-treated rats (increase by 32%; Fig. 4b1 and 4b2, PRO-AMPH and SAL-AMPH, p = 0.031).
Acute restraint activated LC neurons projecting to the BLA
The previous two experiments demonstrated that systemic propranolol administration pre- and post-restraint effectively blocked the stress-induced decrease in DA system activity 24 h following restraint. Given that the noradrenergic neurons in the LC are activated by stressful events (Rasmussen et al., 1986; Aston-Jones et al., 1991; Morilak et al., 2005; Goebel et al., 2009), we first established whether LC neurons that project directly from the LC to the BLA are activated by acute restraint.
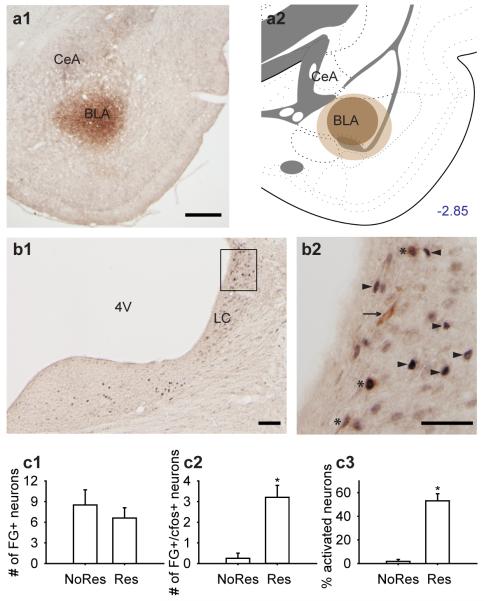
The subjects were 12 male Sprague-Dawley rats. Fluorogold retrograde tracer was injected into the BLA (targeting the basal nucleus) and half of the rats were subjected to a 30 min restraint session. cfos activation was examined 30 minutes after restraint, although 2h of restraint were used in the electrophysiological study. This was necessary in order to examine maximal cfos activation, which is transient in nature (maximal at 90 min), but should nonetheless reveal which systems are activated during this exposure. Three rats were excluded because the tracer missed the target of the BLA, yielding the final group sizes: Res (n = 5) and NoRes (n = 4).
A representative tracer delivery site is shown in Fig. 5a1, and an illustration of the largest and smallest sizes of tracer spread shown in Fig. 5a2. Tracers were discrete and localized to the delivery sites with minimal diffusion into adjacent regions. Retrogradely-labeled BLA afferents were found in the LC (Fig. 5b1 and 5b2). The number of retrogradely-labeled LC neurons was equivalent in both Res and NoRes groups (Fig. 5c1). There was no significant difference between Res and NoRes in tracer-positive neurons [(tracer only) + (tracer + cfos); t(7) = −0.74, p = 0.483]. However, among the tracer-positive neurons in the LC, more neurons were activated by acute restraint as indicated by positive cfos immunoreactivity (Fig. 5c2 and 5c3). There was a significant difference between dual-labeled neurons (tracer + cfos) between Res and NoRes [Fig. 5c2; t(7) = 4.23, p = 0.004]. Acute restraint activated a significantly higher percentage of the LC neurons that project directly onto the BLA [Fig. 5c3; t(7) = 7.49, p < 0.001].
Figure 5.
Acute restraint activated LC neurons projecting to the BLA. (a1) A representative tracer delivery site of FG into the BLA. Scale bar, 500 μm. (a2) Illustration showing the largest (light brown) and smallest (dark brown) spread sizes of the tracer at the delivery site. (b1) Retrogradely-labeled BLA afferents were found in the LC. Scale bar, 100 μm. (b2) A magnified view of LC. Arrowhead, cfos only. Arrow, tracer only. *, tracer+cfos. Scale bar, 50 μm. (c1) The number of retrogradely-labeled LC neurons [(tracer only) + (tracer+cfos)] were equivalent across restraint (Res; n = 5) and control (NoRes; n = 4) conditions. (c2) The number of dual-labeled neurons was significantly higher for Restraint rats than No-Restrant controls (*, p < 0.05). (c3) Acute restraint activated a significantly higher percentage of the LC neurons that directly project to the BLA (*, p < 0.05).
Pre-restraint intra-BLA administration of the β-noradrenergic receptor antagonist, propranolol, blocked the decrease in VTA DA neuron population activity and prevented the attenuation of amphetamine-induced locomotor activity 24 h following restraint
From the experiments above, we showed that systemic administration of propranolol prevented the attenuation of DA system activity 24 hours after restraint. Moreover, the direct projection from the LC onto the BLA is highly activated after the acute restraint session, placing the BLA as a strong candidate for mediating the LC NE effects on VTA DA neuron activity. In this study, we examined whether pre-restraint blockade of β-noradrenergic receptors in the BLA is sufficient and effective at blocking the decrease in VTA DA neuron population activity and preventing the attenuation of amephetamine-induced locomotor activity 24 h following restraint stress.
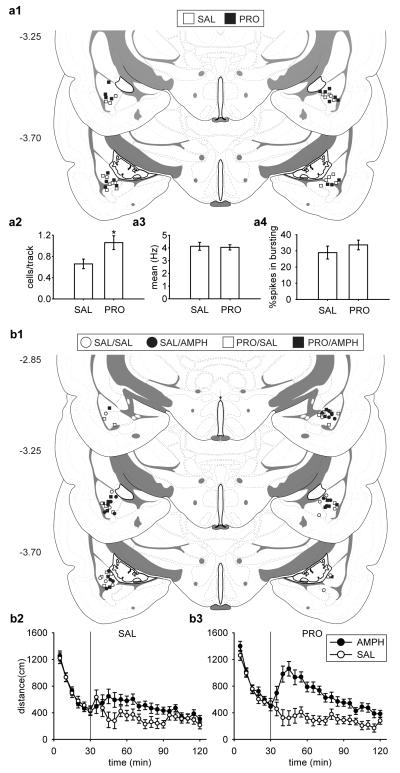
The subjects were 51 male Sprague Dawley rats bilaterally implanted with guiding cannulae targeting the BLA: 19 rats were used for DA electrophysiological recordings and 32 rats for behavioral testing. For electrophysiological recordings, all rats were restrained for a 2 h session. Half of the rats were administered intra-BLA propranolol pre-restraint (PRO, n = 9; 0.5 μg/0.2 μl per side), and the other half with saline as the control (SAL, n = 10; 0.2 μl per side).
The placements of injection sites from all rats included in this study are shown in Fig. 6a1. Given the small volume of injection compared to other relevant studies (Roozendaal et al., 2008; Zimmerman and Maren, 2010), the spread of the infused drug is estimated to remain well within the confines of the BLA. We found that, as with systemic administration, DA neuron population activity was significantly higher in animals pre-treated with propranolol compared to their saline controls by 62% and restored to values that are within a 95% confidence interval (C.I.) of NoRes control rats in the first study (Fig. 6a2). There was a significant difference between SAL and PRO [t(17) = −2.65, p = 0.017]. A total of 59 DA neurons were recorded in SAL group and 86 DA neurons in PRO group. Propranolol administration did not affect the average firing rate (Fig. 6a3; [t(143) = 0.22, p = 0.823]) or percentage of spikes in bursts (Fig. 6a4; [t(143) = −0.96, p = 0.337]).
Figure 6.
Pre-restraint intra-BLA administration of the β-noradrenergic receptor antagonist, propranolol, blocked the decrease in VTA DA neuron population activity and reversed the attenuation of amphetamine-induced locomotor activity measured 24 h after restraint. (a1, b1) Cannulae placements of all rats included in this study. For all rats restrained for a 2 h session, propranolol pre-treatment (PRO; n = 9) significantly increased (a2) the DA neuron population activity than saline (SAL; n = 10) control (*, p < 0.05), with no significant difference between (a3) mean firing rates or (a4) bursting activity. DA neurons recorded: SAL (n = 59), PRO (n = 86). AMPH induced higher locomotor activity regardless pre-treatment (b2, SAL; b3, PRO), with significantly higher locomotor activity in rats pre-treated with PRO. (all ps < 0.05). Group sizes: SAL-SAL (n = 7), SAL-AMPH (n = 8), PRO-SAL (n = 8), and PRO-AMPH (n = 8).
We next examined whether the intra-BLA propranolol administration pre-restraint also normalized behavioral response to amphetamine. All rats were restrained for a 2 h session, and the same procedure was used for PRO and SAL for the locomotor behavioral testing. The cap of one rat in SAL-SAL group fell off and was thus excluded from data analysis, yielding the final group sizes: SAL-SAL (n = 7), SAL-AMPH (n = 8), PRO-SAL (n = 8), and PRO-AMPH (n = 8).
The placements of injection sites from all rats included in this study are shown in Fig. 6b1. We found that, compared to saline pre-treated controls, propranolol pre-treated rats showed a more robust increase in amphetamine-induced locomotor activity 24 h after the cessation of the acute restraint (Fig. 6b). For the 30 min acclimation, there was an equivalent decrease in locomotor activity across sampling blocks (5 min bin) in all groups [Treatment × Drug × Sampling Block, F(5,135) < 1]. For the 90 min post-injection testing, an ANOVA revealed a significant main effect of Drug [F(1,27) = 26.8, p < 0.001], a significant two-way interaction between Treatment and Drug [F(1,27) = 4.62, p = 0.041], and a significant two-way interaction between Drug and Sampling Block [F(17,459) = 4.28, p < 0.001]. Planned comparisons suggested that there was no significant difference between saline controls regardless of pre-restraint treatment (SAL-SAL and PRO-SAL, p = 0.699). Similar to the results of the earlier systemic propranolol treatment experiments, in this study amphetamine induced an increase in locomotor activity compared to their saline controls regardless of intra-BLA pre-treatment (Fig. 6b2, SAL-SAL and SAL-AMPH, p = 0.045; Fig. 6b3, PRO-SAL and PRO-AMPH, p < 0.001). However, following amphetamine administration, there was a significantly higher locomotor activity in propranolol pre-treated rats compared to saline pre-treated rats (increased by 39%; Fig. 6b2 and 6b3, PRO-AMPH and SAL-AMPH, p = 0.012).
Discussion
In the current studies, correlated electrophysiological recordings, behavioral testing, pharmacological manipulations, and immunohistochemical approaches were used to assess the delayed response of VTA DA activity to acute restraint and how it is modulated by the noradrenergic system. Our results revealed that 24 h after the cessation of the 2 h restraint session, there was a significant decrease in VTA DA neuron population activity and a significant attenuation of amphetamine-induced locomotor activity. Moreover, systemic or intra-BLA blockade of the stress-induced increase in noradrenaline reversed the decrease in DA function. These data suggest that the noradrenergic and the dopaminergic systems interact to generate the appropriate responses when responding to a stressful event.
DA neurons exhibit burst firing when the behaving animals encounter a behaviorally salient stimulus, such as one predicting reward (Schultz, 1998). In order for burst firing to take place, the DA neuron must be in a spontaneously firing condition (Floresco et al., 2003; Sesack and Grace, 2010). Thus, the proportion of DA neurons firing spontaneously, the population activity, determines the amplitude of the DA phasic response, thus serving as an amplification factor of the salience signal (Lodge and Grace, 2006). Immediately following the acute restraint, there was a significant increase in VTA DA neuron population activity and amphetamine-induced locomotor activity (Valenti et al., 2011). Such a stress-induced increase is proposed to mimic the situation where an animal needs to attend to the salient stimulus in the environment and prepare for an appropriate goal-directed behavior. It has been suggested that acute restraint-induced changes in the DA system are persistent (Pacchioni et al., 2007). However, we found that 24 h after the acute restraint, there was a significant decrease in VTA DA neuron population activity, as well as a decreased behavioral response to amphetamine, which resembled those encountered in animals exposed to a chronic stressor (Moore et al., 2001). Whether this decrease is adaptive or maladaptive is open to question; however, one result of a suppressed state following a stressor would be to avoid the condition that gave rise to this stress. A context-linked decrease in DA neuron activity would be expected to attenuate a desire to be re-introduced into the stressor environment. Thus, an immediate increase in DA activity could facilitate escape, and a delayed attenuation could prevent a second occurrence of the stressor. In many ways, this is functionally analogous to the opponent process theory proposed to occur during drug exposure (Solomon and Corbit, 1974; Koob et al., 1997), in which the initial heightened response of the DA system generates an adaptive, opposing response. It is essential to follow the temporal changes in DA neuron population activity to further understand whether the decrease 24 h after the cessation of the acute restraint causes the animal to adaptively avoid a stressor, or alternately, is a contributing factor that leads to pathological changes in behavior or physiological responses (Cabib and Puglisi-Allegra, 1996). Although the ability of restraint to attenuate amphetamine-induced locomotion was robust across procedures, there was some inconsistency in the magnitude of the effect produced across experiments; i.e., the amphetamine response on the restraint animals is diminished in Figures 3 & 4 but is nearly abolished in Figures 2 & 6. The source of this variance at this point is unclear, and whether it was a factor related to the surgery required for cannula implantation or the stress of the systemic injection would need to be evaluated using identical testing conditions across age- and condition-matched subjects.
Stressful stimuli potently activated the noradrenergic neurons in the LC (Rasmussen et al., 1986; Aston-Jones et al., 1991; Morilak et al., 2005). In this study we demonstrated that pre- and post-restraint blockade of noradrenaline with the β-noradrenergic receptor antagonist, propranolol, successfully reversed the stress-induced decrease in VTA DA neuron population activity and attenuation in amphetamine-induced locomotor activity. The fact that post-restraint administration is effective suggests that the potentially negative response to a previously experienced stressor can be disrupted. However, propranolol given to naïve animals did not increase VTA DA neuron population activity, suggesting that the β-receptor blocker is effective only when the NE levels are elevated. Thus, to effectively prevent the negative response from developing into sustained pathological long-term consequences, the intervention would have to be done in a timely manner, which is particularly critical and could be a caveat in terms of clinical application (Grissom and Bhatnagar, 2011). The effect of restraint-activated noradrenaline on VTA neurons appears to be meditated through the BLA. The LC and the BLA are reciprocally connected (Van Bockstaele et al., 2001; Buffalari and Grace, 2007), and LC neurons that project directly onto the BLA are highly activated after acute restraint. Moreover, local BLA administration of propranolol blocked the changes in VTA DA neuron population activity and the attenuated behavioral response to amphetamine. Earlier studies have demonstrated that the BLA and its modulation by norepinephrine is critically involved in the consolidation of a stressful memory (McGaugh, 2004; Roozendaal et al., 2009). Therefore, it is possible that local blockade of β-noradrenergic receptors in the BLA prevented the consolidation of the restraint experience, and thus allowed the VTA to behave normally. It is worth pointing out that different subtypes of β-receptors in the BLA exert opposing effects: β3-decreases, while β1-/β2-increase, local excitability (Silberman et al., 2010). Since propranolol acts primarily on β1-/β2-receptors, it is unlikely that this effect was due to a disruption of β3-mediated actions.
There are several potential pathways through which the BLA can modulate VTA DA activity; those of particular relevance to stress include projections to the PFC, the HIPP, and the NAc (Sesack and Grace, 2010). The BLA is reciprocally connected with the PFC and the HIPP, whereas the PFC and the HIPP are also reciprocally connected (Thierry et al., 2000). All three structures have direct excitatory projections to the NAc (Thierry et al., 2000; Sesack and Grace, 2010), which then directly, and indirectly through the ventral pallidum (VP), project onto the VTA in an inhibitory manner. In addition, the amygdala projects to the rostromedial tegmental nucleus (Lavezzi and Zahm, 2011) and the VP (Maslowski-Cobuzzi and Napier, 1994), both of which can inhibit the VTA. Therefore, the amygdala can influence VTA activity via a complex network that includes areas that can provide highly integrated information to this region. As the VTA receives integrative information from a diffused neural network, it raises the question of whether the single structure of the BLA is responsible for all of the stress-induced changes in VTA DA neuronal activity. Following the acute restraint, the immediate increase in VTA DA neuron population activity and behavioral response to amphetamine is reversed by inactivating the HIPP (Valenti et al., 2011); however, completely blocking HIPP activity does not decrease DA neuron population activity. In contrast, the decrease in VTA DA neuron population activity and behavioral response to amphetamine 24 h after restraint is reversed by blocking β-noradrenergic receptors in the BLA. Therefore, the HIPP and the BLA appear to exert equivalent but opposite modulatory effects on VTA DA neuron firing and the behavioral response to amphetamine.
In conclusion, the DA system is potently regulated by the integration of distinct contextual, emotional, and behaviorally salient stimuli. Moreover, the output of the DA neurons provides a critical feedback to these systems, especially the NAc, that regulate goal-directed behaviors. The balance of such multiple structures enables an organism to adapt to its environment. Conversely, when taken to an extreme, disruption of this balance can lead to maladaptive responses. By understanding the dynamics of these systems and their interactions, the ability to treat the dysregulation aroused by pathological stressors may be realized.
Acknowledgements
This work was supported by United States Public Health Service Grants DA15408 (A. A. G.). We thank Niki MacMurdo, Dr. Linda Rinaman, and Vicki Maldovan, for their valuable advice and technical assistance.
Footnotes
Conflict of interest:
The authors declare no competing financial interests.
References
- Aston-Jones G, Chiang C, Alexinsky T. Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Prog Brain Res. 1991;88:501–520. doi: 10.1016/s0079-6123(08)63830-3. [DOI] [PubMed] [Google Scholar]
- Bienkowski MS, Rinaman L. Immune challenge activates neural inputs to the ventrolateral bed nucleus of the stria terminalis. Physiol Behav. 2011;104:257–265. doi: 10.1016/j.physbeh.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, Grace AA. Noradrenergic modulation of basolateral amygdala neuronal activity: opposing influences of alpha-2 and beta receptor activation. J Neurosci. 2007;27:12358–12366. doi: 10.1523/JNEUROSCI.2007-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib S, Puglisi-Allegra S. Stress, depression and the mesolimbic dopamine system. Psychopharmacology (Berl) 1996;128:331–342. doi: 10.1007/s002130050142. [DOI] [PubMed] [Google Scholar]
- Cifelli P, Grace AA. Pilocarpine-induced temporal lobe epilepsy in the rat is associated with increased dopamine neuron activity. Int J Neuropsychopharmacol. 2012;15:957–964. doi: 10.1017/S1461145711001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde F, Maire-Lepoivre E, Audinat E, Crepel F. Afferent connections of the medial frontal cortex of the rat. II. Cortical and subcortical afferents. J Comp Neurol. 1995;352:567–593. doi: 10.1002/cne.903520407. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Debiec J, Bush DE, LeDoux JE. Noradrenergic enhancement of reconsolidation in the amygdala impairs extinction of conditioned fear in rats--a possible mechanism for the persistence of traumatic memories in PTSD. Depress Anxiety. 2011;28:186–193. doi: 10.1002/da.20803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Goebel M, Stengel A, Wang L, Tache Y. Restraint stress activates nesfatin-1-immunoreactive brain nuclei in rats. Brain Res. 2009;1300:114–124. doi: 10.1016/j.brainres.2009.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--1. Identification and characterization. Neuroscience. 1983;10:301–315. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984;4:2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Grissom NM, Bhatnagar S. The basolateral amygdala regulates adaptation to stress via beta-adrenergic receptor-mediated reductions in phosphorylated extracellular signal-regulated kinase. Neuroscience. 2011;178:108–122. doi: 10.1016/j.neuroscience.2010.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Joels M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LR, Hou M, Prager EM, Ledoux JE. Regulation of the Fear Network by Mediators of Stress: Norepinephrine Alters the Balance between Cortical and Subcortical Afferent Excitation of the Lateral Amygdala. Front Behav Neurosci. 2011;5:23. doi: 10.3389/fnbeh.2011.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BE, Moore RY. Ascending projections of the locus coeruleus in the rat. II. Autoradiographic study. Brain Res. 1977;127:25–53. [PubMed] [Google Scholar]
- Knapska E, Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn Mem. 2009;16:486–493. doi: 10.1101/lm.1463909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Caine SB, Parsons L, Markou A, Weiss F. Opponent process model and psychostimulant addiction. Pharmacol Biochem Behav. 1997;57:513–521. doi: 10.1016/s0091-3057(96)00438-8. [DOI] [PubMed] [Google Scholar]
- Lavezzi HN, Zahm DS. The mesopontine rostromedial tegmental nucleus: an integrative modulator of the reward system. Basal Ganglia. 2011;1:191–200. doi: 10.1016/j.baga.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion Circuits in the Brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology. 2006;31:1356–1361. doi: 10.1038/sj.npp.1300963. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27:11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Amphetamine activation of hippocampal drive of mesolimbic dopamine neurons: a mechanism of behavioral sensitization. J Neurosci. 2008;28:7876–7882. doi: 10.1523/JNEUROSCI.1582-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo AH, Georges FE, Aston-Jones GS. Novel neurons in ventral tegmental area fire selectively during the active phase of the diurnal cycle. Eur J Neurosci. 2008;27:408–422. doi: 10.1111/j.1460-9568.2007.05985.x. [DOI] [PubMed] [Google Scholar]
- Maslowski-Cobuzzi RJ, Napier TC. Activation of dopaminergic neurons modulates ventral pallidal responses evoked by amygdala stimulation. Neuroscience. 1994;62:1103–1119. doi: 10.1016/0306-4522(94)90347-6. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Moore H, Rose HJ, Grace AA. Chronic cold stress reduces the spontaneous activity of ventral tegmental dopamine neurons. Neuropsychopharmacology. 2001;24:410–419. doi: 10.1016/S0893-133X(00)00188-3. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO. Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1214–1224. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Pacchioni AM, Cador M, Bregonzio C, Cancela LM. A glutamate-dopamine interaction in the persistent enhanced response to amphetamine in nucleus accumbens core but not shell following a single restraint stress. Neuropsychopharmacology. 2007;32:682–692. doi: 10.1038/sj.npp.1301080. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann N Y Acad Sci. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Morilak DA, Jacobs BL. Single unit activity of locus coeruleus neurons in the freely moving cat. I. During naturalistic behaviors and in response to simple and complex stimuli. Brain Res. 1986;371:324–334. doi: 10.1016/0006-8993(86)90370-7. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, Van der Zee EA, McGaugh JL. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proc Natl Acad Sci U S A. 2006;103:6741–6746. doi: 10.1073/pnas.0601874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Castello NA, Vedana G, Barsegyan A, McGaugh JL. Noradrenergic activation of the basolateral amygdala modulates consolidation of object recognition memory. Neurobiol Learn Mem. 2008;90:576–579. doi: 10.1016/j.nlm.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon P, Stanford SC. Beta-adrenoceptor binding correlates with behaviour of rats in the open field. Psychopharmacology (Berl) 1989;98:412–416. doi: 10.1007/BF00451697. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Li HY, Ericsson A. Circuits and mechanisms governing hypothalamic responses to stress: a tale of two paradigms. Prog Brain Res. 2000;122:61–78. doi: 10.1016/s0079-6123(08)62131-7. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Ariwodola OJ, Chappell AM, Yorgason JT, Weiner JL. Lateral paracapsular GABAergic synapses in the basolateral amygdala contribute to the anxiolytic effects of β3 adrenoceptor activation. Neuropsychopharmacology. 2010;35:1886–96. doi: 10.1038/npp.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychol Rev. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Thierry AM, Gioanni Y, Degenetais E, Glowinski J. Hippocampo-prefrontal cortex pathway: anatomical and electrophysiological characteristics. Hippocampus. 2000;10:411–419. doi: 10.1002/1098-1063(2000)10:4<411::AID-HIPO7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Tully K, Bolshakov VY. Emotional enhancement of memory: how norepinephrine enables synaptic plasticity. Mol Brain. 2010;3:15. doi: 10.1186/1756-6606-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Grace AA. Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci. 2012;27:27. doi: 10.1016/j.tins.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti O, Lodge DJ, Grace AA. Aversive stimuli alter ventral tegmental area dopamine neuron activity via a common action in the ventral hippocampus. J Neurosci. 2011;31:4280–4289. doi: 10.1523/JNEUROSCI.5310-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Bajic D, Proudfit H, Valentino RJ. Topographic architecture of stress-related pathways targeting the noradrenergic locus coeruleus. Physiol Behav. 2001;73:273–283. doi: 10.1016/s0031-9384(01)00448-6. [DOI] [PubMed] [Google Scholar]
- Zimmerman JM, Maren S. NMDA receptor antagonism in the basolateral but not central amygdala blocks the extinction of Pavlovian fear conditioning in rats. Eur J Neurosci. 2010;31:1664–1670. doi: 10.1111/j.1460-9568.2010.07223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]