Abstract
Background
Understanding structure-function relationships in the temporomandibular joint (TMJ) disc is a critical first step toward creating functional tissue replacements for the large population of patients suffering from TMJ disc disorders. While many of these relationships have been identified for the collagenous fraction of the disc, this same understanding is lacking for the next most abundant extracellular matrix component, sulfated glycosaminoglycans (GAGs). Though GAGs are known to play a major role in maintaining compressive integrity in GAG-rich tissues such as articular cartilage, their role in fibrocartilaginous tissues in which GAGs are much less abundant is not clearly defined. Therefore, this study investigates the contribution of GAGs to the regional viscoelastic compressive properties of the temporomandibular joint (TMJ) disc.
Method of Approach
Chondroitinase ABC (C-ABC) was used to deplete GAGs in five different disc regions, and the time course for >95% GAG removal was defined. The compressive properties of GAG depleted regional specimens were then compared to non-treated controls using an unconfined compression stress-relaxation test. Additionally, treated and non-treated specimens were assayed biochemically and histologically to confirm GAG removal.
Results
Compared to untreated controls, the only regions affected by GAG removal in terms of biomechanical properties were in the intermediate zone, the most GAG-rich portion of the disc. Without GAGs, all intermediate zone regions showed decreased tissue viscosity, and the intermediate zone lateral region also showed a 12.5% decrease in modulus of relaxation. However, in the anterior and posterior band regions no change in compressive properties was observed following GAG depletion, though these regions showed the highest compressive properties overall.
Conclusions
Although GAGs are not the major extracellular matrix molecule of the TMJ disc, they are responsible for some of the viscoelastic compressive properties of the tissue. Furthermore, the mechanical role of sulfated GAGs in the disc varies regionally in the tissue, and GAG abundance does not always correlate with higher compressive properties. Overall, this study found that sulfated GAGs are important to TMJ disc mechanics in the intermediate zone, an important finding for establishing design characteristics for future tissue engineering efforts.
Keywords: Temporomandibular Joint, Disc, Glycosaminoglycans, Stress Relaxation, Chondroitinase ABC
Introduction
The fibrocartilaginous temporomandibular joint (TMJ) disc resides between the mandibular condyle and temporal bone, aiding in the complex rotational and translational motions of the jaw. The disc’s most important roles during mastication include shock absorption and load distribution [1]. However, as much as 25% of individuals in the United States (about 80% of whom are young women) have temporomandibular disorders (TMDs) [2, 3]. The severity of TMDs vary from discomforting to debilitating and are commonly manifested through inhibited range of jaw motion, anterior disc displacement, and degeneration of the TMJ disc itself [4]. As the causes of TMD are unknown, current clinical therapy focuses mainly on pain reduction and tissue resection. It is clear, however, that the TMJ disc plays an important role in the temporomandibular joint, and its resection eventually leads to degeneration of the joint as a whole [5]. Tissue engineering efforts may address the need for replacement discs in cases where total discectomy is necessary, but before a functional TMJ disc can be engineered the tissue’s structure-function relationships must be better understood.
Currently, TMJ disc structure-function relationships are described only in terms of its most abundant biochemical element, collagen. At ~80-90% of the dry weight [6, 7], collagen is the major structural component of the disc, and its anisotropy and contributions to the tensile properties of the tissue have been thoroughly investigated. Collagen fibers form a ring-like alignment around the periphery of the TMJ disc, with a strong anteroposterior alignment through the intermediate zone [8-10]. Correspondingly, the tensile strength and stiffness of specimens tested in the anteroposterior direction are an order of magnitude greater than those in the mediolateral direction [11-13]. The compressive stiffness of the disc is at least an order of magnitude less than the tensile stiffness [14], but still appears to be related to collagen. The posterior band of the disc possesses both the highest collagen content, and also the greatest compressive moduli [15].
While the contribution of collagen to the mechanical integrity of the disc is readily measured due to its abundance, the contributions of other biochemical components of the disc to its functional properties are not as clear. Approximately 1% of the dry weight of the disc is attributed to sulfated glycosaminoglycans (GAGs), with dermatan and chondroitin sulfates being the most prevalent GAGs [10, 16]. Regionally, GAGs are found in the greatest quantities in the lateral region of the intermediate zone [7, 16, 17]. In other cartilages, such as hyaline cartilage, GAGs are highly abundant and are mainly associated with the tissue’s compressive properties [18]. Despite having the most GAG, however, the lateral region of the disc does not correspond to the highest compressive or tensile moduli [15]. It is unclear, therefore, how sulfated GAGs contribute to the mechanical properties of the disc.
To better understand the mechanical contributions of sulfated GAGs to TMJ disc properties, this study tests the viscoelastic compressive properties of the tissue with and without sulfated GAGs. GAG removal is achieved through the application of chondroitinase ABC (C-ABC) which is a GAG-cleaving enzyme that selectively cleaves chondroitin and dermatan sulfate side chains, and to a lesser degree hyaluronic acid [19]. As the GAGs within the TMJ disc are overwhelmingly chondroitin and dermatan sulfate [10], this is an optimal enzyme for testing GAG depletion. C-ABC has been used extensively to investigate the contribution of sulfated GAGs to the compressive properties of musculoskeletal tissues including articular cartilage (abundant GAG) [18, 20, 21], nucleus pulposus (low GAG) [22, 23], and ligament (scarce GAG) [24]. In articular cartilage, sulfated GAG removal resulted in a marked decrease in the tissue’s Young’s modulus [21], whereas similar treatment on ligaments increased tissue permeability but did not affect the modulus [24]. Given these findings, it was hypothesized that GAG depletion in the TMJ disc would have minimal impact on the tissue’s compressive moduli, similar to ligament. Furthermore, as GAG content varies amongst disc regions, it was hypothesized that the GAG contribution will vary regionally within the disc.
Materials and Methods
Specimen Procurement
Pig heads were obtained from a local abattoir (Yosemite Meat Company, Modesto, CA). TMJ discs were harvested from the left joint of seven female pigs of age 6-9 months. TMJ discs were carefully excised free from attachments and washed in 0.01 M phosphate-buffered saline (PBS). Hyaline articular cartilage served as a benchmark control for this investigation. Full-thickness cartilage was harvested from the tibial plateau of five 1-week-old male calves (Research 87, Boston, MA) and washed in PBS. After washing all specimens were wrapped in gauze soaked with PBS containing protease inhibitors (2mM EDTA, 150 mM sodium chloride, 5 mM benzamidine hydrochloride, 10 mM N-ethylmaleimide, and 1 mM phenylmethylsulfonyl fluoride) and then frozen at −20°C until testing.
Glycosaminoglycan Depletion
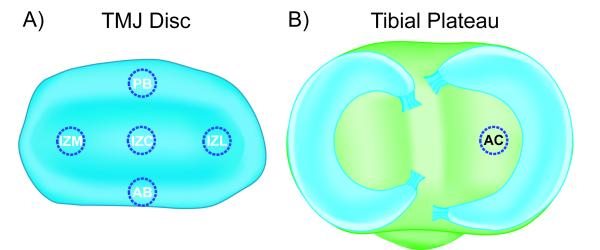
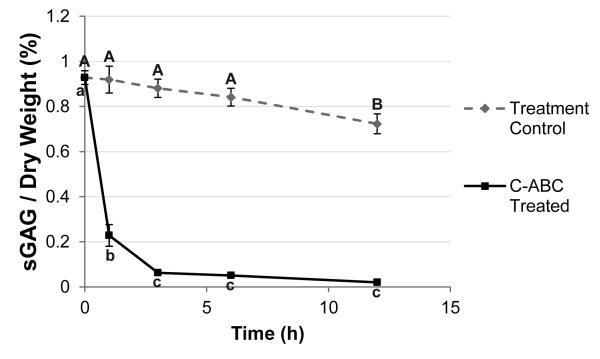
TMJ discs and hyaline cartilage samples were thawed in PBS at 4°C. A 3 mm dermal punch (Miltex, York, PA) was used to remove samples from 5 regions of the TMJ disc, spanning both the mediolateral and anteroposterior directions (Fig. 1A). Specifically, the five regions of the disc tested were: posterior band (PB), anterior band (AB), intermediate zone medial (IZM), intermediate zone central (IZC), and intermediate zone lateral (IZL). For the hyaline cartilage benchmark samples, 3 mm punches were taken from the center of the tibial plateau as shown in Fig. 1B. Depletion of sulfated GAGs was carried out with C-ABC at 1 U/mL in Tris-buffered saline (TBS) containing 300 mM sodium acetate and 0.05% bovine serum albumin (all from Sigma, St. Louis, Missouri). Treatment controls were incubated in exactly the same manner, except the buffer contained no C-ABC. GAG removal from the TMJ disc was examined using treatment durations from 1 to 12 hrs at 37°C, and a graph depicting GAG depletion over time for the most GAG-rich region of the TMJ disc (IZL) is shown in Fig. 2. Specimens that underwent mechanical testing were incubated for 3 hrs with C-ABC, or in a control buffer. This time point was chosen because it proved to be the earliest time point to provide >90% removal of sulfated GAGs while showing no effect on the GAG content of the treatment controls (Fig. 2).
Figure 1.
Investigated regions of the TMJ disc and tibial cartilage. Because of the heterogeneous nature of the TMJ disc, the contribution of GAGs to the compressive properties of the tissue was tested regionally. (A) 3 mm discs were harvested from five regions of the disc: the posterior band (PB), anterior band (AB), intermediate zone medial (IZM), intermediate zone central (IZC), and intermediate zone lateral (IZL). Hyaline articular cartilage (AC) from the tibial plateau was as a benchmark control in this investigation. (B) A 3 mm disc has harvested from the center of the tibial plateau on the medial side of the joint. (not drawn to scale)
Figure 2.
Time course of GAG removal from the TMJ disc. Samples from the IZL region of the disc were treated with C-ABC or a control buffer for up to 12 h and their final sulfated GAG content was measured. A 1-way ANOVA comparing time points within the treatment control and C-ABC treated groups is shown. Time points not connected by the same letter are statistically different from each other. C-ABC treatment produced a rapid decrease in the GAG content of the disc, which equilibrated after 3 h of treatment. A decrease in GAG content was also seen in the treatment control group, but was only statistically significant after 12 h. A 3 h incubation was chosen for all subsequent testing. Data is presented as mean ± SD.
Stress-Relaxation Compressive Testing
Prior to sulfated GAG depletion, the superior and inferior surfaces of compression samples were delicately removed with a cryotome blade until the surfaces were parallel. The final sample thicknesses ranged from 0.8 – 1.6 mm. Following C-ABC treatment, the final dimensions of the each sample were measured using digital calipers. The stress-relaxation compressive testing procedure for this investigation was similar to that used in previous studies [14, 15, 25]. Compression testing was performed in an unconfined compression chamber fitted onto a materials testing machine (Instron 5565, Canton, MA). Specimens were placed on the lower platen of a PBS-filled bath, and a rigid upper platen with a 19 mm diameter was used to apply unconfined compression. A 0.02 N tare load was applied to the sample and the platen-to-platen separation was taken as the initial specimen height from which strains were based. Samples were preconditioned with 5% strain for 15 cycles, and then a step strain of 20% was applied and held for 10 minutes. For TMJ disc samples, 7 samples per group were used for compressive testing, while 5 hyaline cartilage samples were used.
Both continuum [26-28] and viscoelastic [13, 14, 25, 29-31] models have been used to model TMJ disc compressive mechanics. To facilitate comparisons with the majority of prior reports, viscoelastic compressive properties were calculated by fitting equation 1 below, based on a Kelvin solid model, to the stress-relaxation curves [14].
| (1) |
Specimen height (z) and time of strain event (ti) were determined a priori. Deformation (u), time (t), and stress (σ) were recorded during testing. Relaxation modulus (Er), relaxation time constant (τε), and creep time constant (τσ) could be approximated from model fits, then converted into relaxation modulus, instantaneous modulus, and coefficient of viscosity equivalents.
Biochemical Analysis
Samples were frozen overnight at -20°C and then lyophilized for 48 hrs. Dry weights were recorded and then the samples were digested in papain, 125 μg/mL papain (Sigma) in 50 mmol phosphate buffer (pH 6.5) containing 2 mmol N-acetyl cysteine, for 18 hrs at 60°C. After digestion, sulfated GAG content was measured using the Blyscan Sulfated GAG Assay kit, a 1,9-dimethyl-methylene blue colorimetric assay (Accurate Chemical and Scientific Corp., Westbury, NY). Total collagen content of the samples was measured using a hydroxyproline colorimetric assay [7]. 5 samples per group were used for all biochemical analysis.
Histology
Control and C-ABC treated samples were cryoembedded in histoprep (Fisher Scientific, Pittsburgh, PA) and cryosectioned at 12 μm. To examine sulfated GAG distribution in the samples, sections were fixed with 10% phosphate buffered formalin, stained with Safranin-O, and counterstained with hematoxylin. 2 samples per group were used for histological analysis.
Statistical Analysis
TMJ disc samples were analyzed using a 2-way analysis of variance (ANOVA) to compare the two overall factors of disc region and C-ABC treatment. A Tukey’s HSD post hoc test was used where necessary. Additionally, treated and untreated samples within a single region of the disc were compared directly using a student’s t-test (Table 1 and Appendix Table 2). Treated and untreated hyaline cartilage samples were also compared with a student’s t-test. Since biochemical and biomechanical analysis was performed on the same samples, pairwise correlations were run on these samples using JMP statistical software (SAS, Cary, NC). A significance level of α = 0.05 was used for all statistical analysis.
Table 1.
Biomechanical and biochemical properties of GAG depleted TMJ disc samples. Data is presented as mean ± SD.
| TMJ Disc |
Hyaline Cartilage |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PB | AB | IZM | IZC | IZL | AC | ||||||||
| Instantaneous | Control | 1.49 ± 0.47 | 0.93 ± 0.38 | 0.39 ± 0.10 | 0.36 ± 0.16 | 0.27 ± 0.13 | 4.31 ± 0.62 | ||||||
| Modulus (MPa) | C-ABC | 1.48 ± 0.38 | 0.86 ± 0.56 | 0.38 ± 0.19 | 0.37 ± 0.09 | 0.27 ± 0.15 | 3.72 ± 0.16 | ||||||
|
| |||||||||||||
| Relaxation | Control | 30.0 ± 4.4 | 22.7 ± 2.2 | 25.0 ± 2.5 | 23.6 ± 3.8 | 24.1 ± 1.5 | ⎤ * ⎦ |
591 ± 56 | ⎤ * ⎦ |
||||
| Modulus (kPa) | C-ABC | 29.4 ± 3.5 | 22.3 ± 2.4 | 21.8 ± 2.9 | 22.1 ± 1.7 | 21.1 ± 1.6 | 295 ± 67 | ||||||
|
| |||||||||||||
| Coefficient of | Control | 4.84 ± 1.71 | 3.08 ± 0.66 | 2.42 ± 0.44 | ⎤ * ⎦ |
2.52 ± 0.53 | ⎤ * ⎦ |
2.69 ± 0.56 | ⎤ * ⎦ |
10.71 ± 2.36 | ⎤ * ⎦ |
||
| Viscosity (MPa s) | C-ABC | 4.72 ± 1.38 | 3.01 ± 0.83 | 1.70 ± 0.28 | 1.69 ± 0.41 | 1.80 ± 0.44 | 4.99 ± 1.39 | ||||||
|
| |||||||||||||
| sGAG / Dry | Control | 0.494 ± 0.121 | ⎤ * ⎦ |
0.692 ± 0.070 | ⎤ * ⎦ |
0.880 ± 0.151 | ⎤ * ⎦ |
0.758 ± 0.177 | ⎤ * ⎦ |
0.927 ± 0.249 | ⎤ * ⎦ |
19.2 ± 2.1 | ⎤ * ⎦ |
| Weight (%) | C-ABC | 0.016 ± 0.011 | 0.014 ± 0.012 | 0.035 ± 0.008 | 0.020 ± 0.010 | 0.032 ± 0.008 | 10.5 ± 1.7 | ||||||
|
| |||||||||||||
| Collagen / Dry | Control | 88.3 ± 3.8 | 85.9 ± 5.9 | 81.5 ± 5.3 | 85.0 ± 4.8 | 78.4 ± 4.3 | 70.4 ± 3.3 | ⎤ * ⎦ |
|||||
| Weight (%) | C-ABC | 87.6 ± 3.3 | 85.1 ± 5.1 | 81.6 ± 5.1 | 84.0 ± 3.5 | 80.6 ± 4.9 | 60.9 ± 3.7 | ||||||
|
| |||||||||||||
| Water | Control | 68.7 ± 2.8 | 71.0 ± 2.4 | 69.6 ± 2.0 | 70.4 ± 2.4 | 71.7 ± 3.2 | 79.9 ± 1.2 | ⎤ * ⎦ |
|||||
| Content (%) | C-ABC | 67.1 ± 3.0 | 70.4 ± 2.3 | 69.1 ± 1.8 | 70.0 ± 1.9 | 70.1 ± 1.4 | 75.8 ± 0.9 | ||||||
indicates a statically significant difference between C-ABC treated and control samples for a given region (analyzed using a t-test with α = 0.05).
Results
Compressive Properties
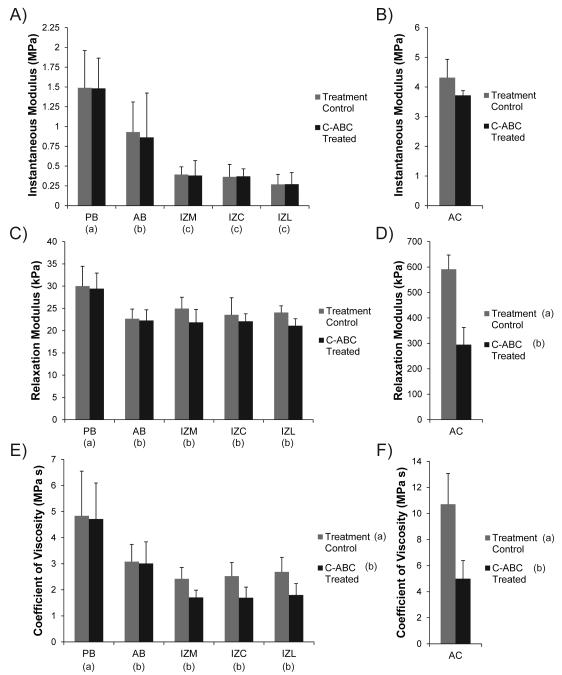
The Kelvin solid viscoelastic model was able to fit all stress-relaxation curves with a high degree of accuracy (R2 > 0.90). Experimental results are shown in Fig. 3 and the raw data can be found in Table 1. Regional variations in all compressive properties of the TMJ disc were consistent with prior reports using the same testing modality [14, 15, 25].
Figure 3.
Viscoelastic compressive properties of GAG depleted TMJ disc and hyaline cartilage samples. (A) GAG removal with C-ABC did not produce a significant difference in the instantaneous modulus of the TMJ disc. (B) C-ABC treatment also had no effect on the instantaneous modulus of the tibial cartilage, although it did trend lower. (C) Overall, C-ABC treatment did not produce a change in the relaxation modulus of TMJ disc samples. (D) GAG depletion in articular cartilage produced a ~50% decrease in the tissue’s relaxation modulus. (E) GAG depletion produced an overall decrease in the coefficient of viscosity for TMJ disc samples. All regions of the intermediate zone (IZM, IZC, IZL) displayed a ~30% decrease in viscosity. (F) C-ABC treatment of hyaline cartilage led to a ~50% decrease in the coefficient of viscosity. Data is presented as mean ± SD. Letters in brackets represent the results of a 2-way ANOVA for TMJ disc specimens and a student’s t-test for hyaline cartilage specimens. Groups not connected by the same letter are statistically different. (Note: TMJ disc regions and hyaline cartilage are plotted different scales to enhance readability)
Instantaneous Modulus
While C-ABC treatment was able to deplete sulfated GAGs from the TMJ disc, it had no effect on the instantaneous modulus of the tissue (Fig. 3A). Regionally, the bands of the disc, PB and AB, were stiffer than the intermediate zone with moduli of 1.49 and 0.93 MPa respectfully (p<0.0001). Similar to the TMJ disc, C-ABC treatment of hyaline cartilage also produced no statistically significant difference in instantaneous modulus, although it did appear to trend lower with treatment (Fig. 3B). The instantaneous modulus of hyaline cartilage was at least twice that of all regions of the disc, with a mean value of 4.31 MPa.
Relaxation Modulus
An overall comparison using a 2-way ANOVA showed no significant change in the relaxation modulus of the disc following C-ABC treatment (Fig. 3C). Regional variations were seen, with PB possessing the highest relaxation modulus at 30.0 kPa (p<0.0001). Additional analysis of treated and untreated samples within a single region (t-test) indicated that GAG depletion did produce a statistically significant decrease in IZL (p<0.02, Table 1). The overall decrease in relaxation modulus of IZL was ~12.5% from 24.1 to 21.1 kPa. In contrast to the disc, C-ABC treatment of hyaline cartilage produced a more dramatic decrease in relaxation modulus (Fig. 3D). Treatment resulted in a 50% drop in modulus from 591 to 295 kPa (p<0.0001). Overall, the relaxation modulus of the TMJ disc was >10 times less than hyaline cartilage.
Coefficient of Viscosity
Compared to the moduli, C-ABC treatment produced considerably more change in the coefficient of viscosity. A 2-way ANOVA indicated an overall decrease in viscosity across the TMJ disc due to GAG depletion (p<0.04, Fig. 3E). Specifically, C-ABC treatment produced a drop in viscosity across the entire intermediate zone (IZM, IZC, IZL) by ~30%. Viscosity of the AB and PB were not affected by treatment, although PB did possess the overall highest coefficient of viscosity at 4.84 MPa s (p<0.0001). Similar to the relaxation modulus, C-ABC treatment of hyaline cartilage produced a ~50% drop in its coefficient of viscosity from 10.71 to 4.99 MPa s (p<0.0001, Fig. 3F). The coefficient of viscosity for hyaline cartilage was found to be at least twice that of the TMJ disc.
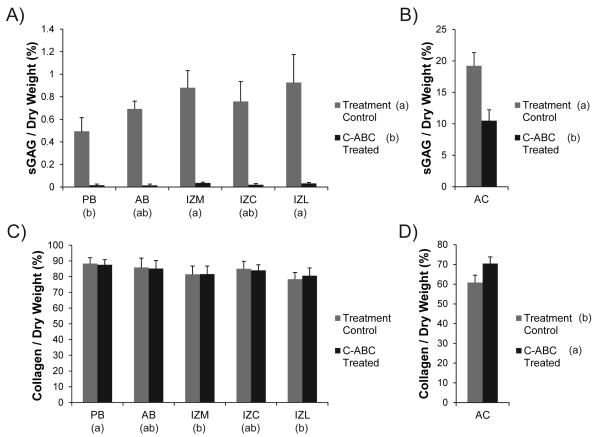
Biochemical Content
The biochemical composition of GAG depleted and control samples are displayed in Fig. 4. The corresponding raw data can be found normalized to dry weight in Table 1 and normalized to wet weight in Appendix Table 2. Regional variations in the biochemical composition of TMJ disc samples were consistent with prior reports utilizing the same assays [7, 10, 15].
Figure 4.
Sulfated GAG and collagen content of C-ABC treated samples. (A) C-ABC treatment produced extensive GAG depletion (≥96%) in all regions of the TMJ disc. (B) Using the same treatment, GAG depletion in tibial cartilage was only about 50%. (C) C-ABC treatment produced no change in collagen per dry weight within the TMJ disc. (D) Treatment of hyaline cartilage with C-ABC did increase the collagen per dry weight (~10%). All data is presented as mean ± SD. Letters in brackets represent the results of a 2-way ANOVA for the TMJ disc samples and a student’s t-test for the articular cartilage samples. Groups not connected by the same letter are statistically different. (Note: TMJ disc regions and hyaline cartilage are plotted on different scales to enhance readability)
In all regions of the disc, C-ABC treatment was able to remove ≥ 96% of the sulfated GAG content (p<0.0001, Fig. 4A). For example, the GAG content of IZL decreased from 0.93% to 0.032%. Regional GAG variation in TMJ disc samples showed that the IZL and IZM groups contained more GAG per dry weight than the PB (p<0.002). The same C-ABC treatment when applied to hyaline cartilage did not produce the same magnitude of GAG depletion. Treatment reduced the sulfated GAG / dry weight by ~50% in cartilage samples from 19.2% to 10.5% (p<0.0001, Fig. 4B). Comparing the treatment control samples, the native TMJ disc has approximately 20 times less sulfated GAG per dry weight than hyaline cartilage.
Since it is the major structural component in the TMJ disc, the collagen content of all samples was measured. C-ABC treatment of the disc had no effect on the collagen per dry weight (Fig. 4C). Regionally, the PB contained the greatest collagen content at 88.3% (p<0.005). In contrast to the disc, C-ABC treatment of hyaline cartilage did alter the collagen per dry weight, increasing it by approximately 10%, from 60.9% to 70.3% (p<0.005, Fig. 4D). This increase in collagen content is likely due to the fact that ~10% of the previous dry weight of the tissue had been removed due to GAG depletion. Overall, the native TMJ disc contained approximately 20% more collagen per dry weight than hyaline cartilage.
The water content of the specimens was also measured, as it is tends to correlate with sulfated GAG content in cartilages [18]. For TMJ disc samples, water content ranged from 67% to 71% and C-ABC treatment did not produce any significant changes in these values (Table 1). GAG depletion of hyaline cartilage did alter the tissue’s water content, reducing it from 79.9% to 75.8% (p<0.001). Comparing the two cartilages, native articular cartilage contained approximately 10% more water than the TMJ disc.
Structure-Composition Correlations
Running biomechanical and biochemical analysis on the same samples allowed pairwise correlations to be run between these function and composition parameters. In TMJ disc samples, sulfated GAG content was seen to have a significant negative correlation with both the instantaneous (p<0.01) and relaxation (p<0.03)) moduli. Interestingly, collagen content was found to have significant positive correlation with the instantaneous modulus (p<0.01) in disc samples. In contrast, sulfated GAG content was found to correlate positively with both the relaxation modulus (p<0.005) and coefficient of viscosity (p<0.003) in hyaline cartilage. No significant correlations were observed between collagen content and compressive properties in the articular cartilage samples.
Histology
Safranin-O staining of the TMJ disc clearly demonstrates the low levels of sulfated in this tissue (Fig. 5). In the control samples, positive red-orange staining can only be seen localized directly around some of the cells, with little to no staining in the bulk matrix. Decreased staining in C-ABC treated samples is visible in some regions, although difficult to detect. Inter-regional variations on the other hand are not detectible due to the low overall staining intensity. In contrast to the disc, hyaline cartilage clearly demonstrates positive Safranin-O staining throughout the entire matrix, with enhanced staining localized to the lacunae surrounding the cells. C-ABC treatment of cartilage samples produced a clear depletion of sulfated GAG starting at the edge and proceeding toward the center of the tissue. Additionally, there is a decrease in staining surrounding the cells in the center of the treated cartilage.
Figure 5.
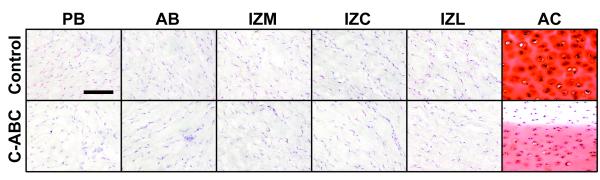
Safranin-O staining of C-ABC treated specimens to examine the distribution of sulfated GAGs. Some positive staining (red color) can be found in TMJ disc samples immediately surrounding cells, but very low overall staining was observed, and no staining was detected in the matrix. In contrast, intense positive staining can be seen throughout the matrix of articular cartilage. C-ABC treatment produced a small but visible difference in some TMJ disc samples. Treatment of hyaline cartage produced a very obvious depletion of GAGs starting at the edge of the tissue and proceeding toward the center. (Scale bar = 100 μm)
Discussion
Prior studies on TMJ disc mechanics indicate that the mechanical properties of the TMJ disc are likely more dependent on its abundant collagen fibers than its proteoglycans, but all of the structure-function relationships within the disc have not been fully investigated. To our knowledge, this study is the first to illuminate the contributions of sulfated GAGs in the TMJ disc to the tissue’s compressive properties. Although GAGs only make up about 1% of the total dry weight of the TMJ disc, the present results indicate that they are still able to impart tissue viscosity and compressive properties in the intermediate zone. Other disc regions, however, did not show an appreciable change in compressive properties following GAG depletion. This variation in contributions to compressive properties highlight complex relationships TMJ disc GAGs have with the tissue’s mechanics, and show that GAGs in the TMJ disc may have a different role than in other cartilages.
The regional variation in GAG contribution to disc compressive properties highlighted the highly heterogeneous nature of this tissue. Overall, there was a significant inverse correlation between GAG content and compressive stiffness, with the bands having the highest compressive moduli. At the same time, as the GAG content of the regions increased, so did the contribution of that GAG to the tissue’s compressive properties. For example, in the intermediate zone of the disc where GAGs are most abundant (0.76%-0.93% per dry weight), C-ABC treatment resulted in a marked decrease in tissue viscosity. Additionally, GAG removal in the most GAG-rich region of the intermediate zone, the IZL (0.93% per dry weight), also caused a decrease in modulus of relaxation. Due to the range of proteoglycan concentrations in the TMJ disc, this study was able to measure the effects of GAG abundance on compressive mechanics. Below a certain range (<0.69% concentration) GAG removal had no effect on viscoelastic compressive properties, but as GAG abundance increased (>0.74%), so did its contribution to tissue viscosity. This regional variation in sulfated GAG content, as well as GAG contribution to compressive properties, will be an important but challenging consideration for future tissue engineering efforts.
The results of the present study may provide some insight into the role of GAGs in the complex deformation patters of the TMJ disc during normal loading. Finite element and kinematic observations have indicated that the TMJ disc experiences considerable tensile, compressive, and shear loads during normal mastication and that these loads are variably distributed [32, 33]. Notably, tensile forces are most prevalent in the medial and central portions of the intermediate zone [34, 35], while the highest compressive loads are experienced by the posterior band [34, 36, 37]. The increased tissue viscosity of the intermediate zone attributed to its GAG content may allow this region of the disc to resist fluid flow out of the matrix and better withstand the tensile forces generated by the disc as it is forced against the mandibular condyle. In the posterior band, GAG content was lowest but tissue viscosity remained high, indicating that the highly organized collagen network may be the tissue’s best defense against the high compressive loads it experiences. Thus, GAG content may have an effect on the in vivo deformation patterns of some, but not all, of the TMJ disc regions.
A macroscopic view of the results presented here reveals that GAGs contribute very differently to the compressive properties of the disc than they do to hyaline articular cartilage. Prior reports show that removal of sulfated GAGs from articular cartilage decreased compressive aggregate moduli and increased permeability due to less inhibition to water leaving the tissue [18, 20, 21]. Similarly in this study, removal of ~50% of sulfated GAGs from tibial cartilage resulted in a matched decrease of ~50% in the modulus of relaxation and coefficient of viscosity. This proportional change in compressive properties due to GAG depletion was not seen in the TMJ disc. The stiffest regions of the disc under compressive loading, the PB and AB, showed no change in viscoelastic compressive properties following GAG depletion. Even in the regions of the intermediate zone which showed a response to the C-ABC treatment, the magnitudes of the changes were far smaller than for hyaline cartilage. A 97% reduction in sulfated GAG content of IZL resulted in only a 12.5% decrease in relaxation modulus and a 30% reduction in viscosity. Therefore, these results illustrate that removal of sulfated GAGs from the TMJ disc does not produce an equivalent change in viscoelastic compressive properties as was seen in hyaline cartilage.
Although the hyaline cartilage control generally responded to C-ABC treatment as expected, it produced one unanticipated finding. While the modulus of relaxation and the coefficient of viscosity decreased in an equivalent manner to the GAG depletion, the instantaneous modulus of the tissue did not decrease. Prior studies reporting a drop in compressive modulus after GAG depletion of cartilage have all reported their findings in terms of an aggregate or Young’s modulus [20, 21, 24]. Henninger et al. (2010) measured reduced peak stresses following GAG depletion of femoral cartilage, but their strain rate (0.01%/s) was significantly slower than that used in this study (10%/s). These data suggest that sulfated GAGs play a vital role in the modulus of the tissue at equilibrium, but it appears that they may play less of a role under instantaneous loading. Thus, instead of the GAGs dictating the instantaneous modulus, it may be the inertia of the pressurized interstitial fluid that is the key contributor.
There are several potential reasons why the GAGs of the TMJ disc provide a different contribution to the tissue compressive properties than the GAGs of articular cartilage. First, the sulfated GAG content of hyaline cartilage is ~20 times greater than that of the TMJ disc on a per dry weight basis. Given these differences, it follows that when tibial cartilage loses 50% of its GAGs there is a much more dramatic change in tissue composition than when the disc loses an equivalent percentage of GAGs. In addition to the quantity of GAGs, there are also important differences in the proteoglycans with which the GAGs are associated. In articular cartilage, the GAGs are primarily associated with the proteoglycan aggrecan which has numerous sulfated GAG side chains. The multitude of GAG chains provide the large negative charge that prevents water movement from the tissue [38]. In contrast, the proteoglycans in the TMJ disc are mainly decorin and biglycan [16], which only contain one and two GAG chains, respectively. Though the side chains of decorin and biglycan do not contribute a large negative charge, they alter the matrix by controlling collagen fibril diameter and organization [39-41]. Therefore, the low overall GAG content of the TMJ disc may be providing more of an indirect contribution to compressive properties by shepherding collagen molecules to form a highly organized matrix able to withstand physiologic forces. This is likely to be particularly important for the posterior band of the disc which has higher collagen content and fewer GAGs than the intermediate zone, but still retains greater compressive stiffness. Indeed, in this study a significant positive correlation was seen between collagen content of the disc and the tissue’s compressive moduli. The different types of GAGs resident in the TMJ disc may therefore play a variety of developmental and mechanical roles that contribute to the ultrastructure and biomechanical properties of the mature tissue.
In addition to illuminating the contribution of GAGs to the functional properties of the TMJ disc, the three compressive properties reported in this study can help determine the functionality of tissue engineered constructs in the future. The instantaneous modulus gives a measure of the tissue’s ability to withstand the instantaneous loading the TMJ experiences during biting or chewing. The modulus of relaxation is also an important parameter to consider, as it indicates how the disc supports sustained loading such as that experienced during jaw clenching. Finally, the tissue’s viscosity relates to the rate at which the tissue deforms and recovers and is particularly important during cyclic loading of the joint. All three of these parameters are important physiologically, and can be useful quantitative tools to determine how close tissue engineered replacements are to recapitulating essential TMJ disc properties.
This investigation provides an important insight into the role of sulfated GAGs in providing mechanical integrity to the TMJ disc, and indicates that this contribution is highly region-specific. In the outer bands of the disc, GAGs do not provide a direct contribution to compressive stiffness, but in the intermediate zone they do play a direct role in the tissue’s viscoelastic compressive properties. More investigations are needed to understand how GAGs and their associated proteoglycans can indirectly influence TMJ disc mechanics by interacting with collagen. The present research is, however, a promising advance toward understanding the role of GAGs in the disc and provides valuable design parameters for tissue engineering of a disc replacement.
Acknowledgments
The authors would like to acknowledge funding from the National Institute of Dental and Craniofacial Research via grants R01 DE015038 and R01 DE019666.
Appendix.
Table 2.
Biochemical properties of GAG depleted TMJ disc samples normalized to wet weight. Data is presented as mean ± SD.
| TMJ Disc |
Hyaline
Cartilage |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PB | AB | IZM | IZC | IZL | AC | ||||||||
| sGAG/Wet | Control | 0.154 ± 0.037 | ⎤ * ⎦ |
0.210 ± 0.019 | ⎤ * ⎦ |
0.253 ± 0.046 | ⎤ * ⎦ |
0.224 ± 0.052 | ⎤ * ⎦ |
0.262 ± 0.060 | ⎤ * ⎦ |
5.19 ± 0.45 | ⎤ * ⎦ |
| Weight (%) | C-ABC | 0.005 ± 0.004 | 0.004 ± 0.002 | 0.011 ± 0.003 | 0.006 ± 0.003 | 0.009 ± 0.002 | 2.51 ± 0.39 | ||||||
| Collagen/Wet | Control | 27.2 ± 2.2 | 24.2 ± 1.7 | 24.8 ± 1.6 | 25.2 ± 1.4 | 22.4 ± 1.7 | 16.9 ± 0.5 | ⎤ * ⎦ |
|||||
| Weight (%) | C-ABC | 29.1 ± 2.4 | 25.2 ± 1.8 | 25.2 ± 1.6 | 25.1 ± 2.1 | 23.8 ± 1.5 | 14.5 ± 0.1 | ||||||
indicates a statically significant difference between C-ABC treated and control samples for a given region (analyzed using a t-test with α = 0.05).
References
- [1].Gillbe GV. The function of the disc of the temporomandibular joint. J Prosthet Dent. 1975;33(2):196–204. doi: 10.1016/s0022-3913(75)80110-7. [DOI] [PubMed] [Google Scholar]
- [2].Solberg WK, Woo MW, Houston JB. Prevalence of mandibular dysfunction in young adults. J Am Dent Assoc. 1979;98(1):25–34. doi: 10.14219/jada.archive.1979.0008. [DOI] [PubMed] [Google Scholar]
- [3].Wilkes CH. Internal derangements of the temporomandibular joint. Pathological variations. Arch Otolaryngol Head Neck Surg. 1989;115(4):469–477. doi: 10.1001/archotol.1989.01860280067019. [DOI] [PubMed] [Google Scholar]
- [4].Farrar WB, McCarty WL., Jr. The TMJ dilemma. J Ala Dent Assoc. 1979;63(1):19–26. [PubMed] [Google Scholar]
- [5].Tanaka E, Detamore MS, Mercuri LG. Degenerative disorders of the temporomandibular joint: etiology, diagnosis, and treatment. J Dent Res. 2008;87(4):296–307. doi: 10.1177/154405910808700406. [DOI] [PubMed] [Google Scholar]
- [6].Nakano T, Scott PG. A quantitative chemical study of glycosaminoglycans in the articular disc of the bovine temporomandibular joint. Arch Oral Biol. 1989;34(9):749–757. doi: 10.1016/0003-9969(89)90082-4. [DOI] [PubMed] [Google Scholar]
- [7].Almarza AJ, Bean AC, Baggett LS, Athanasiou KA. Biochemical analysis of the porcine temporomandibular joint disc. Br J Oral Maxillofac Surg. 2006;44(2):124–128. doi: 10.1016/j.bjoms.2005.05.002. [DOI] [PubMed] [Google Scholar]
- [8].Shengyi T, Xu Y. Biomechanical properties and collagen fiber orientation of TMJ discs in dogs: Part 1. Gross anatomy and collagen fiber orientation of the discs. J Craniomandib Disord. 1991;5(1):28–34. [PubMed] [Google Scholar]
- [9].Scapino RP, Canham PB, Finlay HM, Mills DK. The behaviour of collagen fibres in stress relaxation and stress distribution in the jaw-joint disc of rabbits. Arch Oral Biol. 1996;41(11):1039–1052. doi: 10.1016/s0003-9969(96)00079-9. [DOI] [PubMed] [Google Scholar]
- [10].Detamore MS, Orfanos JG, Almarza AJ, French MM, Wong ME, Athanasiou KA. Quantitative analysis and comparative regional investigation of the extracellular matrix of the porcine temporomandibular joint disc. Matrix Biol. 2005;24(1):45–57. doi: 10.1016/j.matbio.2004.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Detamore MS, Athanasiou KA. Tensile properties of the porcine temporomandibular joint disc. J Biomech Eng. 2003;125(4):558–565. doi: 10.1115/1.1589778. [DOI] [PubMed] [Google Scholar]
- [12].Beatty MW, Bruno MJ, Iwasaki LR, Nickel JC. Strain rate dependent orthotropic properties of pristine and impulsively loaded porcine temporomandibular joint disk. J Biomed Mater Res. 2001;57(1):25–34. doi: 10.1002/1097-4636(200110)57:1<25::aid-jbm1137>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- [13].Tanne K, Tanaka E, Sakuda M. The elastic modulus of the temporomandibular joint disc from adult dogs. J Dent Res. 1991;70(12):1545–1548. doi: 10.1177/00220345910700121401. [DOI] [PubMed] [Google Scholar]
- [14].Allen KD, Athanasiou KA. Viscoelastic characterization of the porcine temporomandibular joint disc under unconfined compression. J Biomech. 2006;39(2):312–322. doi: 10.1016/j.jbiomech.2004.11.012. [DOI] [PubMed] [Google Scholar]
- [15].Kalpakci KN, Willard VP, Wong ME, Athanasiou KA. An interspecies comparison of the temporomandibular joint disc. J Dent Res. 2011;90(2):193–198. doi: 10.1177/0022034510381501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mizoguchi I, Scott PG, Dodd CM, Rahemtulla F, Sasano Y, Kuwabara M, Satoh S, Saitoh S, Hatakeyama Y, Kagayama M, Mitani H. An immunohistochemical study of the localization of biglycan, decorin and large chondroitin-sulphate proteoglycan in adult rat temporomandibular joint disc. Arch Oral Biol. 1998;43(11):889–898. doi: 10.1016/s0003-9969(98)00038-7. [DOI] [PubMed] [Google Scholar]
- [17].Nakano T, Scott PG. Changes in the chemical composition of the bovine temporomandibular joint disc with age. Arch Oral Biol. 1996;41(8-9):845–853. doi: 10.1016/s0003-9969(96)00040-4. [DOI] [PubMed] [Google Scholar]
- [18].Basalo IM, Mauck RL, Kelly TA, Nicoll SB, Chen FH, Hung CT, Ateshian GA. Cartilage interstitial fluid load support in unconfined compression following enzymatic digestion. J Biomech Eng. 2004;126(6):779–786. doi: 10.1115/1.1824123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hamai A, Hashimoto N, Mochizuki H, Kato F, Makiguchi Y, Horie K, Suzuki S. Two distinct chondroitin sulfate ABC lyases. An endoeliminase yielding tetrasaccharides and an exoeliminase preferentially acting on oligosaccharides. J Biol Chem. 1997;272(14):9123–9130. doi: 10.1074/jbc.272.14.9123. [DOI] [PubMed] [Google Scholar]
- [20].Katta J, Stapleton T, Ingham E, Jin ZM, Fisher J. The effect of glycosaminoglycan depletion on the friction and deformation of articular cartilage. Proc Inst Mech Eng H. 2008;222(1):1–11. doi: 10.1243/09544119JEIM325. [DOI] [PubMed] [Google Scholar]
- [21].Korhonen RK, Laasanen MS, Toyras J, Lappalainen R, Helminen HJ, Jurvelin JS. Fibril reinforced poroelastic model predicts specifically mechanical behavior of normal, proteoglycan depleted and collagen degraded articular cartilage. J Biomech. 2003;36(9):1373–1379. doi: 10.1016/s0021-9290(03)00069-1. [DOI] [PubMed] [Google Scholar]
- [22].Yerramalli CS, Chou AI, Miller GJ, Nicoll SB, Chin KR, Elliott DM. The effect of nucleus pulposus crosslinking and glycosaminoglycan degradation on disc mechanical function. Biomech Model Mechanobiol. 2007;6(1-2):13–20. doi: 10.1007/s10237-006-0043-0. [DOI] [PubMed] [Google Scholar]
- [23].Boxberger JI, Orlansky AS, Sen S, Elliott DM. Reduced nucleus pulposus glycosaminoglycan content alters intervertebral disc dynamic viscoelastic mechanics. J Biomech. 2009;42(12):1941–1946. doi: 10.1016/j.jbiomech.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Henninger HB, Underwood CJ, Ateshian GA, Weiss JA. Effect of sulfated glycosaminoglycan digestion on the transverse permeability of medial collateral ligament. J Biomech. 2010;43(13):2567–2573. doi: 10.1016/j.jbiomech.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Allen KD, Athanasiou KA. A surface-regional and freeze-thaw characterization of the porcine temporomandibular joint disc. Ann Biomed Eng. 2005;33(7):951–962. doi: 10.1007/s10439-005-3872-6. [DOI] [PubMed] [Google Scholar]
- [26].Fontenot MG. The Viscoelasticity of Human Temporomandibular-Joint Disks. American Journal of Physical Anthropology. 1985;66(2):168–169. [Google Scholar]
- [27].Kim KW, Wong ME, Helfrick JF, Thomas JB, Athanasiou KA. Biomechanical tissue characterization of the superior joint space of the porcine temporomandibular joint. Ann Biomed Eng. 2003;31(8):924–930. doi: 10.1114/1.1591190. [DOI] [PubMed] [Google Scholar]
- [28].Kuo J, Zhang L, Bacro T, Yao H. The region-dependent biphasic viscoelastic properties of human temporomandibular joint discs under confined compression. J Biomech. 2010;43(7):1316–1321. doi: 10.1016/j.jbiomech.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].del Pozo R, Tanaka E, Tanaka M, Okazaki M, Tanne K. The regional difference of viscoelastic property of bovine temporomandibular joint disc in compressive stress-relaxation. Med Eng Phys. 2002;24(3):165–171. doi: 10.1016/s1350-4533(01)00127-8. [DOI] [PubMed] [Google Scholar]
- [30].Tanaka E, Hirose M, Yamano E, Dalla-Bona DA, Fujita R, Tanaka M, van Eijden T, Tanne K. Age-associated changes in viscoelastic properties of the bovine temporomandibular joint disc. Eur J Oral Sci. 2006;114(1):70–73. doi: 10.1111/j.1600-0722.2006.00265.x. [DOI] [PubMed] [Google Scholar]
- [31].Tanaka E, Tanaka M, Miyawaki Y, Tanne K. Viscoelastic properties of canine temporomandibular joint disc in compressive load-relaxation. Arch Oral Biol. 1999;44(12):1021–1026. doi: 10.1016/s0003-9969(99)00097-7. [DOI] [PubMed] [Google Scholar]
- [32].Beek M, Koolstra JH, van Ruijven LJ, van Eijden TM. Three-dimensional finite element analysis of the human temporomandibular joint disc. J Biomech. 2000;33(3):307–316. doi: 10.1016/s0021-9290(99)00168-2. [DOI] [PubMed] [Google Scholar]
- [33].Donzelli PS, Gallo LM, Spilker RL, Palla S. Biphasic finite element simulation of the TMJ disc from in vivo kinematic and geometric measurements. J Biomech. 2004;37(11):1787–1791. doi: 10.1016/j.jbiomech.2004.01.029. [DOI] [PubMed] [Google Scholar]
- [34].Chen J, Akyuz U, Xu L, Pidaparti RM. Stress analysis of the human temporomandibular joint. Med Eng Phys. 1998;20(8):565–572. doi: 10.1016/s1350-4533(98)00070-8. [DOI] [PubMed] [Google Scholar]
- [35].Herring SW, Liu ZJ. Loading of the temporomandibular joint: anatomical and in vivo evidence from the bones. Cells Tissues Organs. 2001;169(3):193–200. doi: 10.1159/000047882. [DOI] [PubMed] [Google Scholar]
- [36].Tanaka E, Sasaki A, Tahmina K, Yamaguchi K, Mori Y, Tanne K. Mechanical properties of human articular disk and its influence on TMJ loading studied with the finite element method. J Oral Rehabil. 2001;28(3):273–279. [PubMed] [Google Scholar]
- [37].Tanaka E, Rodrigo DP, Miyawaki Y, Lee K, Yamaguchi K, Tanne K. Stress distribution in the temporomandibular joint affected by anterior disc displacement: a three-dimensional analytic approach with the finite-element method. J Oral Rehabil. 2000;27(9):754–759. doi: 10.1046/j.1365-2842.2000.00597.x. [DOI] [PubMed] [Google Scholar]
- [38].Swartz MA, Fleury ME. Interstitial flow and its effects in soft tissues. Annu Rev Biomed Eng. 2007;9:229–256. doi: 10.1146/annurev.bioeng.9.060906.151850. [DOI] [PubMed] [Google Scholar]
- [39].Ruhland C, Schonherr E, Robenek H, Hansen U, Iozzo RV, Bruckner P, Seidler DG. The glycosaminoglycan chain of decorin plays an important role in collagen fibril formation at the early stages of fibrillogenesis. FEBS J. 2007;274(16):4246–4255. doi: 10.1111/j.1742-4658.2007.05951.x. [DOI] [PubMed] [Google Scholar]
- [40].Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136(3):729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Corsi A, Xu T, Chen XD, Boyde A, Liang J, Mankani M, Sommer B, Iozzo RV, Eichstetter I, Robey PG, Bianco P, Young MF. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers-Danlos-like changes in bone and other connective tissues. J Bone Miner Res. 2002;17(7):1180–1189. doi: 10.1359/jbmr.2002.17.7.1180. [DOI] [PubMed] [Google Scholar]