Abstract
In higher organisms, innate scavenging cells maintain physiologic homeostasis by removal of the billions of apoptotic cells generated on a daily basis. Apoptotic cell removal requires efficient recognition and uptake by professional and non-professional phagocytic cells, which are governed by an array of soluble and apoptotic cell-integral signals resulting in immunologically silent clearance. While apoptosis is associated with profound suppression of adaptive and innate inflammatory immunity, we have only begun to scratch the surface in understanding how immunologic tolerance to apoptotic self manifest at either the molecular or cellular level. In the last 10 years data has emerged implicating professional phagocytes, most notably stromal macrophages and CD8α+CD103+ dendritic cells, as critical in initiation of the regulatory cascade that will ultimately lead to long-term whole animal immune tolerance. Importantly, recent work by our lab and others has shown that alterations in apoptotic cell perception by the innate immune system either by removal of critical phagocytic sentinels in secondary lymphoid organs or blockage of immunosuppressive pathways leads to pronounced inflammation with a breakdown of tolerance towards self. This challenges the paradigm that apoptotic cells are inherently immunosuppressive suggesting that apoptotic cell tolerance is a “context dependent” event.
Introduction
Apoptosis, or programmed cell death, was first coined in modern terms in 1972, translated from Greek meaning “dropping off or falling off of petals from flowers or leaves from trees” which resembled the observed condensation of the cytoplasm and nucleus in a manner which was distinct from necrotic cell death[1]. Apoptosis is indispensible for tissue remodeling during embryogenesis and is a fundamental tenet of inflammation and lymphocyte generation in higher vertebrates. However rapid removal and destruction of the cellular corpse (efferocytosis) is the critical, final component of the apoptotic pathway and when disrupted, has profound consequences for the organism leading to massive inflammation and often death. Thus it is not surprising that there is a significant amount of redundancy in the mechanisms responsible for apoptotic cell recognition and capture. Nevertheless, there are clearly dominant mechanisms which, when disrupted, lead to diseases of chronic inflammation including atherosclerosis, autoimmunity, and cancer[2].
Efficiency of efferocytosis is remarkable. It is estimated that an apoptotic cell loss of >1 billion cells/day occurs in the average adult yet it is difficult to find apoptotic cells in most tissues, including those with high cell turnover such as the thymus and secondary lymphoid organs[3]. Efferocytosis is manifest primarily by the action of “professional phagocytes” (i.e. macrophages, dendritic cells) supplemented by the many non-professional phagocytes that, in general, remove neighboring apoptotic cells during tissue remodeling[4]. However, phagocytosis and destruction alone is inadequate to drive tolerance which requires active participation from both the innate and adaptive arms of the immune system not only for establishment of immunosuppressive conditions, but for the continued maintenance of tolerance and the prevention of undesirable immunity. Moreover, uptake of apoptotic cells may play an essential role in protective adaptive immune responses in many instances including infection and cancer[5–8]. Thus, efferocytosis is an essential component of most (if not all) immune responses which, by extension, would suggest the context of apoptosis is critical in determining if the immunologic outcomes of cell death is regulation or inflammation.
Apoptosis itself is a varied process resulting from cell extrinsic or intrinsic signals and stresses leading to numerous physiologic changes in the cell including massive caspase activation, loss of mitochondrial membrane potential and exposure of phosphatidylserine to the extracellular environment which combine to irreversibly commit the cell to the apoptotic process[9]. This point of no return is ill defined, owing to the heterogeneous nature of apoptosis in general, and is likely to be revised as new information comes to light. However, a useful definition was suggested by Bratton and Henson who described two criteria: i) loss of plasma membrane integrity; ii) cell fragmentation into discrete bodies or engulfment by phagocytes or adjacent cells[10] and it is this endpoint we will use in the following essay to define immunologically relevant apoptosis. Nevertheless, the actual mechanics of apoptosis are beyond the scope of this review, which focuses on the process of apoptotic cell clearance and immunologic tolerance. In the text below, we will discuss basic mechanisms believed to drive apoptotic cell tolerance as well as the complex cell-cell and molecular interactions required for immunologic tolerance towards apoptotic self and the consequences for breakdown of tolerance towards apoptotic self.
The four steps in efferocytosis: find me, tickle me, eat me, and process (destroy) me
Phagocytic clearance of apoptotic cells involves four broadly defined steps required for recognition, uptake, and clearance (see Figure 1). While the individual mechanisms involved in each step may vary considerably depending on the cell and tissue type, the overall mechanism appears to be universal and shared between professional and non-professional phagocytes[11]. As a whole the system is powerfully efficient and tightly regulated by signals delivered via both soluble or membrane bound ligands to ensure rapid recognition and removal of cells in early apoptosis.
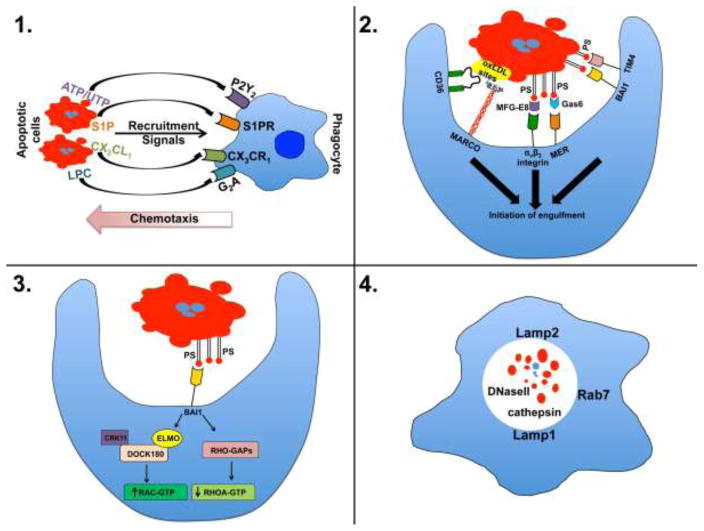
Figure 1. The four steps in efferocytosis.
1) In the “find me” stage cellular commitment to apoptosis results in the production/release of chemotactic agents that recruit and, perhaps, activate local phagocytes priming them for phagocytic uptake of the cellular corpse. 2) During the “tickle me” stage phagocytes encountering dying cells interact by a variety of receptors that can directly or indirectly (via bridging molecules) recognize exposure of native and modified forms of the membrane-integral lipid phosphatidlyserine (PS) or other exposed, modified self constitutions (the example shown here is MARCO and CD36 binding modified lipoproteins). 3) During the “eat me” stage proper ligand/receptor engagement activates downstream signal transduction activating ELMO and Rac-dependent cytoskeletal changes resulting in plasma membrane invagination and internalization of the apoptotic cell. 4) During the “process me” phase the apoptotic cell is degraded in Lamp+ lysosomes allowing for repurposing of the apoptotic material for antigen presentation and elicitation of adaptive, tolerogenic immunity. oxLDL, oxidized low density lipoprotein.
When cells initially enter apoptosis it is critical that they attract motile phagocytes to initiate the efferocytic process. In recent years a number of such diffusible chemotactic mediators have been identified including nucleotides (ATP, UTP) which are recognized by the widely expressed purinergic receptor P2Y2, the chemokine fractalkine (CX3CL1) which is the ligand for the receptor CXCR1, and the lipids lysophosphatidylcholine (LPC) and sphingosine-1-phosphate (S1P) which are ligands for the G2A receptor and S1P receptors 1 to 5 respectively[12–15]. All of the apoptotic cell find me signals identified to date are either quickly degraded (nucleotides) or are present in high concentrations in circulation (i.e. S1P and LPC) suggesting they provide local signals to recruit resident macrophages and dendritic cells, but are unlikely to attract monocytes from circulation, or even phagocytes from more distant points in the same tissue. It is interesting to note that disruption of P2Y2 increased apoptotic cell accumulation in the thymus, presumably via reduced phagocyte recruitment, but failed to lead to the severe systemic autoimmune/inflammatory disease observed when other mechanisms involved in efferocytosis are disrupted[14]. The simple explanation is redundancy in the recruitment mechanisms obviated an indispensible need for nucleotide-driven chemo-attraction. However, the increase in apoptotic cell presence in P2Y2 knock-out mice suggests this is not the case. CD14 knock-out mice show a similar defect in apoptotic cell clearance, presumably due to reduced tethering after phagocyte recruitment[16]. In spite of this, there is no apparent inflammatory defect and macrophages can generate apoptotic cell driven anti-inflammatory responses that are equivalent to wild type mice suggesting that at least under certain conditions cell clearance is not required for immune regulation.
An intriguing hypothesis suggests that the primary role of the find-me signal is priming of phagocytes rather than recruitment[17]. As several important eat-me signals produced by macrophages require activation for production (i.e. MFG-E8), such a system would serve the dual purpose of recruitment and uptake[18]. It is noteworthy that in Drosophila and C. elegans migration is often not required for phagocytosis and the apoptotic cell itself may induce uptake of the cell corpse[19]. Of course, even in this context the find-me signal is not absolutely required as apoptotic cells in circulation track rapidly to phagocytes in the spleen and liver making it unlikely that find-me signals would be sufficient to prime the phagocyte for uptake[20,21].
While find-me signals can bring phagocytes into the proximity of apoptotic cells specific recognition of the apoptotic cell is critical for effective clearance and prevention of inflammatory immunity. This recognition is based on the presence of two classes of molecules, i) those which are newly exposed on the cell surface, or ii) alteration of existing surface molecules by oxidation or sugar modification[22]. The most well-known eat-me signal is surface exposure of the membrane-integral lipid phosphatidylserine (PS), which is normally found on the luminal side of the plasma membrane[23]. Originally, it was hypothesized that a single PS receptor existed which provided universal recognition of the exposed lipid on the surface of apoptotic cells[24,25]. However, it is now evident that PS is recognized by numerous receptors that can either bind PS directly, or indirectly via a number of bridging molecules naturally present in tissues and circulation in basal and activated states[10].
Localization of PS in the plasma membrane is asymmetric with a large bias towards the lumen. The maintenance of the viable cell membrane lipid topography is energy dependent requiring ATP and aminophospholipid translocases[26]. Early after the cell enters the apoptotic program there is a rapid relocation of PS to the outer leaflet of the plasma membrane exposing the phosphate head and allowing for recognition by phagocytic cells[23,27]. This rapid accumulation of PS (>200-fold increase at 2 hours compared to viable cells)[28] is the most universally observed change in apoptotic cells and is widely considered to be one of the major indicators for removal. The reason for the asymmetric distribution is not known but PS is translocated to the apical side of the plasma membrane after activation in many cell types including B cells and neutrophils raising the possibility that PS surface compartmentalization is required for normal functions in addition to efferocytosis[29–32].
A seemingly major breakthrough came in 2000 when Fadok and colleagues published a landmark paper demonstrating a monoclonal antibody raised against TGF-β/β-glucan stimulated macrophages could block apoptotic cell uptake by macrophages in culture[24]. Moreover, a 48kDa antigen isolated from a phage display library, which was recognized by the antibody, was identified at the putative PS receptor. However, the role of the PS receptor in efferocytosis is controversial as conflicting data have suggested it plays a minimal role in phagocytosis and may rather serve a primary function in vertebrate development independent of apoptotic cell clearance[33]. Other direct binding PS receptors have been identified including members of the T-cell immunoglobulin (TIM) family and the G-protein coupled receptor family member brain angiogenesis inhibitor 1 (BAI1)[34,35]. It is surprising that disruption of either TIM4 or BAI1 does not lead to significant homeostatic defects or substantial inflammatory/autoimmune disease suggesting that direct PS recognition is not a prerequisite for the regulatory apoptotic cell response in vivo[36,37]. TIM4 has a short cytoplasmic domain that is not required for phagocytic activity and it is probable that its primary function is to act as a tethering molecule similar in function to CD14[38]. In contrast BAI1 signals via a Rac cascade initiating apoptotic cell ingestion suggesting a more active role in actin reorganization and endocytosis[35].
A variety of bridging molecules or opsonins can mediate an indirect interaction between exposed PS on apoptotic cells and phagocytes. Milk fat globule-epidermal growth factor factor 8 (MFG-E8) binds with high affinity to exposed PS enabling uptake via the integrin αvβ3[18]. In stark contrast to TIM4 disruption, deletion of MFG-E8 results in a severe autoimmune phenotype indicating a prominent role in immune regulation[39]. Similarly growth arrest-specific (Gas)6 links exposed PS to the Tyro-Axl-Mer (TAM) receptor tyrosine kinase family of receptors, and similar to MFG-E8 disruption, genetic deletion of TAMs leads to delayed apoptotic cell clearance and significant autoimmune disease[40–42]. It is not clear why disruption of some PS recognition systems yields relatively minor phenotypes while disruption of others leads to severe disease as in all cases there appears to be a defect in apoptotic cell clearance. It is possible that variegated PS receptor expression on phagocyte populations may naturally predispose some receptors to a more prominent role in regulating apoptotic cell driven inflammatory responses.
Macrophages exhibit a sensitivity threshold to PS exposure for phagocytic uptake of cells[28]. This and related observations have prompted the concept that rapid exposure of PS allows for differentiation of apoptotic cells from the viable population by phagocytes. However, viable cells with a mutant form of the scramblase TMEM16F express high levels of surface-exposed PS and are not phagocytosed by macrophages in vitro or in vivo[43]. A possible explanation is PS exposure may be a much more common occurrence than is generally considered. As stated above, PS exposure may be relatively frequent in response to cellular activation or differentiation[26]. Thus, while PS surface translocation may occur rapidly once a cell enters the apoptotic pathway, the signal delivered may not be specific enough to serve a primary decision making role in immunoregulatory mechanisms required for homeostasis. Viable cells express a number of “don’t eat me” signals that could account for the lack of uptake despite significant PS exposure. These include plasminogen activator inhibitor (PAI)-1, a serine protease inhibitor of fibrinolysis that co-localizes with calreticulin (an eat me signal recognized by CD91)[44]. Other potential don’t eat me signals include CD31 and CD47[45,46]. However, CD31 and CD47 knock out mice do not show significant homeostatic perturbations involving viable cell phagocytosis making it unlikely that don’t eat me signals alone are responsible for the prevention PS-exposed live cell destruction. Oxidized lipids, including PS, are recognized via direct and indirect mechanisms by a number of receptors highly expressed by professional phagocytes including the scavenger receptors SR-A, MARCO and CD36[47–49]. Thus, it is highly probable that while PS exposure may provide an important tether aiding in apoptotic cell clearance it is the presence of modified self that is the signal for the phagocytic engulfment of the cellular body and initiation of a regulatory response in professional phagocytes. Toda et al recently reported that apoptotic cell phagocytosis was a two-step process requiring both Tim4 recognition of PS and integrin-mediated recognition of apoptotic cell bound MFG-E8[50]. This would be consistent with a model of PS-mediated tethering and recognition of modified-self (i.e. oxidized PS) resulting in uptake and clearance.
Apoptotic cell uptake is governed by Rho GTPases including the ELMO/Doc180/Rac module that is associated with BAI1-mediated endocytosis[35]. The intracellular endocytic machinery involved in apoptotic cell uptake is widely expressed suggesting that the internal mechanism of phagocytosis will be relatively similar regardless of cell type. Thus the specificity of efferocytosis may depend on the array of apoptotic cell sensors expressed by the phagocyte[51]. Similarly the manner of uptake may depend on the phagocyte involved as it has been proposed that endocytosis occurs both by formation of a large phagosome enveloping the cell and the surrounding media and by a “zipper” mechanism with sequential ligand/receptor engagement forming a close-fitting phagosome[52,10].
Destruction of internalized apoptotic cells occurs in lysosomes. Little is known regarding the mechanism of digestion and it remains to be seen if the actual degradation pathway is distinct from that used to remove other particulate antigens. However, Henson and colleagues reported that lysosome acidification occurred more rapidly after apoptotic cell uptake compared to FcR-mediated uptake of opsonized viable cells[53]. Furthermore, Blander and Medzhitov have found that uptake of microbes is divergent from apoptotic cells as internal “sorting” occurs in the endosomal compartment whereby particles (e.g. bacteria) with strong TLR activity rapidly localize to LAMP-1+ lysosomes resulting in maturation and antigen presentation while apoptotic cells are initially excluded from lysosomes and not presented effectively, even in the presence of free LPS[54,55]. However, if apoptotic cells were directly coupled to microbial ligands, their antigens were actively presented to CD4+ T cells[55]. While this is seemingly at odds with the observations reported by Dr. Henson’s group described above[53], it should be kept in mind that lysosomal localization is probably the eventual endpoint for any endocytosed particulate antigen and antigen presentation is necessary for induction of immunologic tolerance (see below). Therefore, while there are clear differences in the internal sorting of antigens, we have yet to fully understand how this impacts specific mechanisms of immunologic regulation. Moreover, there is the strong possibility that specific uptake mechanisms involved in efferocytosis may lead to activation of both general, and apoptotic cell-specific degradation activity. Consistent with this, Peng and Elkon reported that apoptotic cells taken up by dendritic cells in MFG-E8 deficient mice show altered vesicular trafficking and defects in lysosome acidification[56]. Regardless, it is evident that rapid destruction of the internalized apoptotic cell is of paramount importance. DNaseII is an endonuclease that is most active at low pH and is responsible for DNA digestion in the lysosomal compartment[57]. Deletion of DNase II is embryonic lethal due to anemia associated with the inability of macrophages to digest expelled erythroid nuclei during fetal erythropoiesis resulting in significant production of the inflammatory cytokine interferon(IFN)-β[58]. The fetal lethality could be prevented by deletion of the IFN-βgene or usage of a conditional DNase II knock out mouse, however the animals developed severe polyarthritis, high levels of tumor necrosis factor (TNF)-α, and accumulation of DNA in macrophage lysosomes[59]. The dramatic phenotype in DNase-deficient mice provides strong evidence that destruction of internalized nucleic acids associated with apoptotic cells is critical to limit innate inflammatory potential of apoptotic material. There is conflicting data as to whether inflammation in DNaseII deficient mice is the result of innate sensing by toll like receptor (TLR)-9[60,61]. However, recently it was reported that Trex1 knock out mice developed severe autoimmune disease due to increased activity of the cytoplasmic DNA sensor STING[62]. Thus there are likely multiple innate mechanisms to detect intracellular DNA that could be activated by apoptotic cell constituents.
Immunologic Tolerance to Apoptotic Self
Efferocytosis is often referred to as an immunologically silent mechanism for the clearance of cellular corpses. Implicit in these words is the notion that, mechanistically, phagocytosis of apoptotic cells is sufficient to maintain homeostasis with out further action, however recent evidence has suggested that location of death matters for immunologic outcome. Non-professional phagocytes have limited ability to interact with the immune system, thus in this case efferocytosis may be as advertised, a silent event. Nevertheless, it is evident that apoptotic cell exposure induces rapid expression of immune suppressive cytokines in antigen presenting cells (APCs) creating an immediately suppressive microenvironment and setting the stage for selection and expansion of regulatory lymphocyte populations ultimately culminating in the generation of “infectious” tolerance[63].
Apoptotic Cell Tolerance in the Spleen
Tolerance is a complex process involving both innate and adaptive immune elements. Much of what is known regarding the impact of apoptotic cells on dendritic cells (DCs) and macrophages (MΦs) is derived from in vitro studies which in general have repeatedly demonstrated two related concepts; i) apoptotic cells induce significant production of anti-inflammatory cytokines such as transforming growth factor(TGF)-β and IL-10, and ii) exposure to apoptotic cells suppresses subsequent responsiveness to proinflammatory stimuli[64–66]. However, the in vivo picture is much more complex and it is apparent that apoptotic cells induce a mixed cytokine response which is dominated by TGF-β and IL-10 but also includes a cocktail of cytokines considered proinflammatory such as IFN-γ, IFN-α/β, IL-17a (TLM, unpublished observations), IL-6, IL-12, and TNF-α[20,67]. Moreover, when sorted splenic phagocyte populations that had eaten apoptotic thymocytes were examined, we found a cell specific pattern of cytokine induction suggestive of a division of labor in the apoptotic cell response[67].
Splenic architecture
Splenic apoptotic cell clearance in response to systemic (i.e. intravenous) cell challenge provides a useful platform to study early innate mechanisms involved in apoptotic cell tolerance. Moreover, tolerance to apoptotic cells delivered systemically (i.e. i/v) is abrogated in splenectomized mice demonstrating the primacy of the spleen in acquired tolerance towards apoptotic self[68,69]. The spleen is a remarkable organ that has a three-fold function as a secondary lymphoid organ, a scavenger of particulates in circulation, and iron recycling[70]. The splenic architecture consists of a lymphoid while pulp, which is comprised of a sheath of T cells surrounding branching arterioles (periarteriolar lymphoid sheath, PALS) with adjacent follicular B cells and a red pulp which is populated primarily by MΦs, neutrophils, and DCs. The spleen is unique in that it features a venous sinusoidal system without an endothelial cell lining which aids in the capture and destruction of aged red blood cells[70]. In mice the arterial branches end in a marginal sinus that demarcates the border between the red and white pulp. The sinus itself is lined by a layer of mucosal addressin cell-adhesion molecule (MAdCAM)-1+ cells that serve as an entry barrier to the white pulp creating a protected environment within the follicles[71–73]. On the red pulp side of the marginal sinus the MAdCAM-1+ cell layer is fenestrated allowing for free movement of material entering from circulation into the red pulp. The marginal zone (MZ) is a transitional area on the red pulp side of the marginal sinus where lymphocytes enter the spleen before migrating to the white pulp. Migration in to the white pulp is an active event that is controlled by poorly defined mechanisms although both chemokines and S1P are involved[74]. The MZ is inhabited by several specialized subsets of phagocytes and B cells which play an essential role in innate and early adaptive immune responses to a wide variety of bacterial and viral pathogens[75]. A definitive feature of the MZ is the presence of a specialized MZ macrophage population (MZMs) that constitutively expresses high levels of the type-I scavenger receptors scavenger receptor (SR)-A and macrophage receptor with collagenous structure (MARCO), and the lectin SIGNR1, a homologue of the human DC receptor DC-SIGN[20,67,21,76]. These macrophages are highly phagocytic and have the ability to capture a wide array of molecules, in particular carbohydrates and lipids[77,78]. Another specialized macrophage population in the MZ is defined by expression of the adhesion molecule siglec-1 (CD169). These cells express high levels of non-specific esterase and are differentiated by staining with the monoclonal antibody MOMA1[79]. While MZMs are found within the MZ red pulp region, MOMA1+ M s are found in more intimate association with the white pulp appearing as a continuous band one or two cells thick underneath the MAdCAM1+ cells on the follicular side of the sinus.
Marginal zone macrophages and control of apoptotic cell clearance
MZMs are critical for trapping of particulate material >500nm in the spleen, and our lab and others have shown that apoptotic cells administered i/v rapidly track to the MZ where they are phagocytosed, primarily by MZMs[20]. This initial capture is a critical event in the generation of tolerance to self as Tanaka and colleagues demonstrated delayed clearance of apoptotic cells and reduced immune tolerance to apoptotic cell-associated antigens when MZMs and MOMA1+ MΦs were absent[80]. Consistent with this, we found that MZM and MOMA1+ MΦ depletion accelerated development of systemic tolerance breakdown in mouse models of systemic lupus erythematosus and promoted inflammatory immunity towards apoptotic cell antigens[20]. An interesting observation which was consistent in both studies was the altered uptake patterns in mice lacking MZMs and MOMA1+ MΦs. Tanaka’s group reported decreased apoptotic cell phagocytosis by CD8α+ DCs with paralleled increased uptake in CD11b+ DCs[80]. We saw similar changes in DC phagocytosis of apoptotic cells (TLM, unpublished observations) and additionally found large increases in phagocytosis by CD68+ MΦs[20]. Moreover, altered uptake was associated with rapid accumulation of apoptotic material in the white pulp, a stark contrast to the normal follicular exclusion of apoptotic cells that occurs in the spleen. Remarkably, we found in the absence of MZMs, the cytokine profile elicited by apoptotic cells was reversed, with an attenuated TGF-β response and elevated proinflammatory cytokine production.
It is unresolved why the lack of MZMs resulted in the changes observed. If MZMs are a major factor in apoptotic cell clearance in the spleen then it could be argued that increased presence of free apoptotic cells led to altered uptake patterns in the remaining phagocytes, however if this was the case then the increase would be expected to be represented in all phagocytes proportionately which does not occur. MZMs are in intimate contact with MZ B cells and DC populations, which is critical for positioning within the MZ. Depletion of MZMs leads to a mobilization of MZ B cells and presumably alters DCs positioning as well (although this has not been formally demonstrated to our knowledge)[81]. Thus in a second possible mechanism, the loss of MZMs may result in DC mobilization, changing the level of exposure to apoptotic cells from circulation, the basal activation state of the DCs, or both. Stromal macrophages can also regulate apoptotic cell uptake in neighboring phagocytes by a mechanism dependent on lipoxygenase activity[82]. Hence, a third possibility is that MZMs are directly regulating the ability of other phagocytes in the MZ and red pulp to clear apoptotic material promoting development of tolerance.
Control of the macrophage response to apoptotic cells
There has been significant progress in our understanding of how phagocytes capture and engulf apoptotic cells and the end effects of apoptotic cell uptake (e.g. TGF-β production). However, mechanistically we only have a partial understanding of the conditions elicited by apoptotic cells to induce these regulatory effects. An early report by Voll et al showed that apoptotic cells induced significant production of the immunoregulatory cytokine IL-10 after LPS stimulation in monocytes with parallel decreases in TNF-α, IL-12, and IL-1β[66]. Inhibition was contact dependent and could be replicated by antibody-mediated crosslinking of the scavenger receptor CD36. Subsequent studies have confirmed this and found a host of anti-inflammatory mediators are elicited by apoptotic cell phagocytosis, most notably TGF-β and prostaglandins. However, soluble factors may not be responsible for direct regulation of phagocyte inflammatory function as Kim et al reported that apoptotic cell-mediated inhibition of IL-12 production was dependent on induction of a zinc finger transcription factor, GC-binding protein, which directly inhibited activity of the IL-12 p35 and p40 promoters independent of IL-10 or TGF-β[83].
The TAM receptors (Axl, Mer, and Tyro3) are a family of receptor tyrosine kinases possessing an extracellular domain containing immunoglobulin-related and fibronectin III domains, a single pass trans-membrane domain, and an intracellular PTK signaling domain[84]. TAMs act as receptors for two known PS-binding soluble proteins, Gas6 and protein S, and antagonize inflammatory cytokine production by STAT-1-dependent induction of suppressor of cytokine signaling (SOCS) proteins 1 and 3[41]. TAMs also inhibit TLR signaling via SOCS, and thus may be required for apoptotic cell mediated inhibition of LPS responsiveness[41]. Moreover, Sen et al reported apoptotic cell-mediated activation of Mer antagonized LPS-driven NF-κB activation by a PI3K/AKT-dependent mechanism[85]. As production of many inflammatory cytokines is dependent on NF-κB, this suggests that Mer (and TAMs in general) target inflammatory cytokine production at multiple signal transduction points. It is interesting to note that apoptotic cells produce Gas6 and thus may prime themselves for uptake by TAMs. This implies TAM activity may play an important constitutive role in acquisition of a suppressive macrophage and dendritic cell phenotype after apoptotic cell encounter, a notion supported by the severe autoimmune phenotype in mice deficient in TAM receptor expression[86].
Similarly enzymatic activity has been suggested to play a critical role in the regulatory response elicited by apoptotic cells. Arginase is an enzyme existing in two isoforms in mammals that converts L-arginine to L-ornithine and urea. Arginase activity is often associated with anti-inflammatory macrophage activity and antagonizes IFN-γ and iNOS function both in vitro and in vivo[87]. Brune and colleagues recently demonstrated that arginase (Arg) II can be induced by apoptotic cell-derived S1P in macrophages by an ERK5/CREB-dependent mechanism in vitro[88,89]. Importantly, Arg II induction was required for apoptotic cell-mediated inhibition of iNOS activity indicative of a link between Arg activity and the anti-inflammatory capacity of apoptotic cells. Analogously, we found apoptotic cells induce a significant (18-fold increase), transient burst in splenic, MZM-specific Arg II expression (TLM, unpublished observations). Based on the kinetics of induction, we surmise Arg II may play a role in initial dampening of inflammatory responses immediately after efferocytosis but is unlikely to be involved in downstream effector responses, a possibility we are currently testing.
Like arginase, indoleamine 2,3 dioxygenase (IDO) is an enzyme with two isoforms in mammals (IDO1 and IDO2), that catabolizes indole-ring containing compounds (including the essential amino acid tryptophan) to N-formyl kynurenine[90]. IDO1 is relevant in the context of apoptotic cell tolerance as it is a critical mediator of peripheral immune suppression in cancer, chronic inflammatory disease, and immune privilege[90]. Moreover, apoptotic cells can induce IDO by an IFN-γ; dependent-mechanism in vitro[91]. However, the in vivo relevance of this finding was not known so we tested the role of IDO1 in immune tolerance to apoptotic cell. We found that i/v administration of apoptotic thymocytes stimulated prominent IDO1 expression in the spleen 18–24 hours after injection, particularly in the MZ[67]. IDO1 activity is most often associated with CD8α+ and plasmacytoid DCs, but after apoptotic cell injection it was the SignR1+ MZMs which showed increased IDO1 mRNA and IDO-mediated suppressive effects were dependent on the presence of MZMs. This result was somewhat surprising as CD8α+ DCs are believed to be critically important to splenic tolerance (see below) and suggested if IDO regulates the splenic DC response to apoptotic material then it must be a trans effect. Supporting this interpretation, when IDO activity was blocked with a pharmacologic agent (D-1-methyl-tryptophan), CD8α+ DC production of TGF-β was completely lost, there was a significant induction of TNF-α and IL-12 message specifically in MZMs, and CD4+ T cell tolerance to apoptotic cell antigens was lost[67]. Thus IDO1 induction is likely a critical early step in the initiation of apoptotic cell tolerance and IDO1 activity is necessary for the regulation of apoptotic cell-induced cytokine production in MZMs and DCs.
IDO can create immune regulation by two primary mechanisms, both involving the catabolism of tryptophan. Tryptophan (Trp) is an essential amino acid that is relatively rare in the cell and micro-environment compared to other amino acids[90]. Depletion of intracellular Trp by IDO induces an amino acid withdrawal response mediated by the integrated stress response kinase general control non-repressed (GCN)2[92]. Activation of GCN2 results in a profound alteration of cellular behavior, most of which is poorly understood; however, in phagocytes GCN2 can potently suppress IL-6 production and may aid in activation of regulatory T cells[93,90]. IDO activity also reduces micro-environmental Trp levels activating GCN2 in neighboring cells which is critical for IDO-mediated inhibition of naive T cell proliferation, CD8+T cell cytotoxic activity, and activation of FoxP3+CD4+ regulatory T cells[92,94]. We found that apoptotic cell challenge induced IDO-dependent expression of C/EBP homologous protein (CHOP) 10, a downstream target of the GCN2 pathway, in DCs and MZMs[67]. Moreover, TGF- induction after apoptotic cell challenge was lost in CHOP−/− mice suggesting a direct link between IDO1, CHOP, and suppression. If confirmed, this would represent a previously unknown tolerogenic mechanism in macrophages, one that could potentially be exploited. For example, the coccidostat halofuginone was recently shown to prevent inflammatory T cell development by GCN2 activation[95]. Ten years ago we reported halofuginone could prevent autoreactivity in a mouse model of systemic autoimmune disease[96]. While the mechanism of action was not known at the time, this clearly suggests that GCN2 can profoundly affect inflammatory immunity and halofuginone may be useful in tolerogenic vaccine approaches.
IDO can also regulate immune reactions by production of Trp catabolic degradation products known collectively as kynurenines. Kynurenines promote T cell apoptosis and inhibit CD28 signal transduction[97]. Moreover, recently L-kynurenine was identified as a high affinity ligand for the aryl hydrocarbon receptor (AhR)[98]. The AhR, best known as a receptor for dioxin, is a cytosolic transcription factor that binds cyclic hydrocarbons[99]. Depending on the ligand, AhR activity has been associated with either pro or anti-inflammatory activity, irrespective it is clear AhR has a potent impact on MΦ, DC, and T cell function[99]. AhR activation by kynurenines enhances suppressive T cell development from naïve precursors and regulates immunogenicity of dendritic cells in vitro[98,100]. Moreover, AhR ligation can drive IDO expression in a positive feedback loop indicative of intricately linked biologic function[98,101]. The role of AhR in apoptotic cell tolerance has not been tested to date but apoptotic cells induce AhR activity in macrophages in vitro and in vivo (TLM, unpublished observations) suggesting the pathway may be involved in apoptotic cell regulatory mechanisms.
Splenic dendritic cells and apoptotic cell tolerance
The spleen contains a diverse population of CD11c+ DCs strategically positioned for antigen acquisition and lymphocyte interactions in the red and white pulp. Two majority splenic DC populations are differentiated by expression CD8α defining function and localization. CD8αneg DCs are found predominantly in the MZ and red pulp and express the endocytic marker 33D1[102]. In contrast CD8α+ DCs are found primarily in the white pulp, although there is a minority population in the MZ and red pulp. The DC subsets are functionally segregated as CD8αneg DCs express high levels of intracellular machinery necessary for MHC II expression and antigen loading, and as a consequence are effective simulators of CD4+ T cells[102]. Conversely, CD8α+ DCs, which also express the c-type lectin CD205, are effective at cross presentation of antigens in MHC I and are consequently potent stimulators of CD8+ T cells[103,102].
The importance of DCs in maintenance of peripheral tolerance was demonstrated by recent data showing that constitutive ablation of CD11c+ DC resulted in fatal autoimmune disease[104]. It is not wholly clear what role the various splenic DC populations play in induction of apoptotic cell tolerance but in general most studies have suggested CD8α+CD205+CD11c+ DCs are required for tolerance initiation. CD8α+ DCs express an array of receptors that could facilitate uptake of apoptotic cells including PSR,β5A integrin, CD36, scavenger receptor BI, CD14, and CD68[105]. Consistent with this, Iyoda et al demonstrated that apoptotic splenocytes are rapidly phagocytosed by CD8+CD205+ DCs in the spleen after i/v administration and this uptake results in suppression of adaptive CD8+ T cell inflammatory responses[105,106]. The population of CD8α+ DCs responsible for apoptotic cell tolerance has been further reduced recently to a minority langerin (CD103)+ DC population residing in the MZ[107]. These cells appear to be distinct from CD8α+CD103neg DCs, exhibiting a profound inability to produce IL-6 in response to TLR-ligands and increased apoptotic cell phagocytic ability. Moreover, specific deletion of the CD103+ subset by cytochrome C injection blocked apoptotic cell-induced tolerance indicative of a primary role in the tolerogenic circuit[107].
Apoptotic cell antigen-specific immune suppression has been attributed to the cross-presentation function of CD8α+ DCs, however cross presentation appears to function independent of efferocytosis suggesting that this is a general feature of this DC subset and not specifically related to apoptotic cell processing[103,108]. There is significant debate over the mechanisms of CD8α+ DC mediated apoptotic cell tolerance. A likely dominant mechanism involves induction of suppressive FoxP3+ regulatory T cells (Tregs) from the naïve T cell population. The induction of Tregs by DC8α+ DCs requires endogenous TGFβ- and low antigen availability[109]. Our lab has shown that apoptotic cell injection i/v induced prominent TGF-β production in CD8α+ DCs[67]. Moreover cellular fragments are efficient at directing antigens towards MHC II presentation pathways suggesting a mechanism of tolerance consistent with the notion of DC directed FoxP3+Treg generation[110].
Alternatively, Griffith et al have identified a novel population of TNF-related apoptosis-inducing ligand (TRAIL)+ CD8+ T cells that is induced by apoptotic cell challenge[111]. TRAIL expression is provoked in activated CD8+ T cells in the absence of CD4+ T cell help and is likely involved in activation induced cell death[112]. However apoptotic cell-induced TRAIL+CD8+ T cells, while apparently functionally “helpless”, could mediate the deletion of antigen-exposed T cells and were sufficient for at least a partial transfer of tolerance to secondary recipients[111]. In the absence of TRAIL apoptotic cells did not elicit an inflammatory immune response indicating the mechanism played a specific role in regulation of CD4 T cell response rather than overall apoptotic cell mediated immune suppression. Combined, these findings provide strong mechanistic support for the role of CD8α+ DCs in downstream tolerance as they can effectively induce multiple regulatory lymphocyte subsets.
Tingible-body macrophages
Secondary lymphoid organs including the spleen are sites of massive in situ lymphocyte expansion and contraction necessitating rapid removal of apoptotic cells that are inevitably generated as a result of immunity. Germinal centers (GCs) are areas of intense B cell proliferation that form at the interface of the B and T cell areas of the white pulp and are a significant source of apoptotic cells in the spleen. Clearance is accomplished primarily by a CD68+ MΦ population known as tingible body macrophages (TBM), a name derived from the phagocytized apoptotic cells (i.e. tingible bodies) observable in these specialized phagocytes. TBMs are also identified by the follicular dendritic cell marker (FDC) FDC-M1, which was found to be the apoptotic cell opsonin MFG-E8[113]. MFG-E8 production in the GC is FDC-derived and lymphotoxin deficient mice, which lack FDCs but retain TBMs, fail to produce MFG-E8 and exhibit defects in TBM uptake of apoptotic bodies[113]. Moreover, there is an accumulation of apoptotic cells on the surface of the TBMs in MFG-E8 deficient mice indicating that tethering is intact but the second signal needed for engulfment is absent. Thus, in the GC efferocytosis requires a cooperative mechanism whereby cells that drive the GC response (FDCs) are partially responsible for cleanup by “licensing” TBMs for apoptotic cell phagocytosis. The importance of this is clear as MFG-E8 deletion drives significant immunopathology[39]. Likewise, Mer−/− mice show defects in GC B cell clearance and develop an autoimmune pathology similar to that seen in MFG-E8−/− mice[42,114,115]. It is curious that abrogation of either MFG-E8 or Mer function results in defective TBM function in the GC as both bridging molecules bind modified PS. The redundancy is likely a default protective mechanism for living cells ensuring that multiple apoptotic cell sensors must be engaged for completion of efferocytosis.
Other cells involved in splenic apoptotic cell tolerance
While the cell populations described above likely play a dominant role in splenic apoptotic cell tolerance mounting evidence suggest other MZ cell populations may play a indispensible role in generation of long term immune apoptotic cell-mediated regulation. Given the intimate nature of the association between cells in the MZ this is not surprising, however, we are still in the early stages of understanding how cell-cell communication in the MZ contributes to the apoptotic cell immune response.
NKT cells are an unconventional T cell subset that recognizes lipids presented in the context of the MHC-like molecule CD1d[116]. Invariant (i)NTK cells express the Vα18-Jα18 T cell receptor (TCR) and may be positively selected by self lipids suggesting that while they can react vigorously with microbial lipid products, iNKT cells may be inherently self reactive which would by extension imply a role tolerogenic homeostasis[117,118]. Thus it is not surprising that iNKT cells are associated with peripheral immune tolerance and alterations in iNKT cell activity or frequency are linked with autoimmune diseases including multiple sclerosis, type 1 diabetes, systemic lupus erythematosus, and rheumatoid arthritis[119–123]. iNKT cells can promote Treg development in a DC dependent manner either directly through CD1d binding, or indirectly by extracellular ATP-induced programmed death ligand (PD-L) 1 and 2 expression, or production of regulatory cytokines including IL-2, IL-4, and IL-10, and IFN-γ[124–128]. Moreover, CD1d−/− mice show reduced Treg numbers suggesting a direct link between NKT cells and Treg mediated tolerogenic homeostasis[127].
A direct role for iNKT cells in apoptotic cell tolerance was recently described by our collaborator Mikael Karlsson who published compelling data demonstrating that mice lacking either the invariant Jα18 TCR chain or CD1d developed exaggerated responses to apoptotic cells administered i/v including induction of anti-DNA IgG auto-antibodies, increased immune complex deposition in the glomeruli, and increased splenic germinal center formation[129]. The iNKT cell response to apoptotic cells was dependent on MZ B cells as transfer of CD1dneg B cells to MZ B cell deficient mice (CD19−/−) replicated the elevated response in CD1d−/− mice while transfer of CD1d+ B cells gave a wild type response to apoptotic cell challenge[129]. MZ B cells express high levels of CD1d and although the exact positioning of self-reactive iNKT cells is the spleen is not known, based on the localization of apoptotic cells after i/v administration we can reasonably assume that the iNKT cells must be located in the MZ as well. Moreover, since CD1d was required, it is likely that cognate iNKT-CD1d mediated recognition of lipids is required for iNKT cell tolerance. A major question, yet to be resolved, is whether iNKT cells recognize apoptotic cell-specific lipids presented by MZ B cells or endogenous lipids presented in the context of activation. Brennan et al reported iNKT cells recognized self-lipids upregulated after TLR-ligand activation, contributing to the antimicrobial NKT cell response[130]. Thus it is possible that in the context of efferocytosis, activated phagocytes may increase presentation of self-lipids facilitating iNKT cell function. A related study reported MZ B cells could directly respond to apoptotic cells by a B cell receptor-dependent mechanism resulting in significant IL-10 production[131]. The MZ B cells internalized apoptotic cells directly sensing apoptotic DNA by TLR9. This is somewhat unexpected, as B cells are not considered significant apoptotic cell phagocytes. However, the MZ B cell population is a substantial source of humoral autoreactivity and natural μ immunoglobulin (IgM)[132,133]. While natural IgM directs apoptotic cells towards phagocytes for destruction, this observation indicates an additional function of self-reactivity in the MZ B cell compartment may be capture and direct induction of apoptotic cell tolerance[134,135].
MZ B cells and MZMs are in intimate contact and splenic positioning of both cell population is regulated by the scavenger receptor MARCO[81]. Thus, it is likely that the tolerogenic response of MZMs and MZ B cells is interconnected. For example, iNKT cells produce substantial amounts of IFN-γ after apoptotic cell challenge[129]. IFNs are potent IDO induction agents in multiple settings suggesting that IDO expression in MZMs may be iNKT cell derived-IFN dependent (see Figure 2). Consistent with this, GalCer (a NKT cell agonist) induces significant IDO activity and CD1d−/− mice have a defect in IDO expression in response to CpG containing DNA (Dr. Andrew Mellor, personal communication). While the role of iNKT cells in apoptotic cell induced IDO is not known, iNKT cell-derived IFN-γ driving IDO expression it would be consistent with the literature. If true, this will be an important finding providing direct mechanistic links between iNKT cells, MZ B cells, and MZMs in apoptotic cell tolerance.
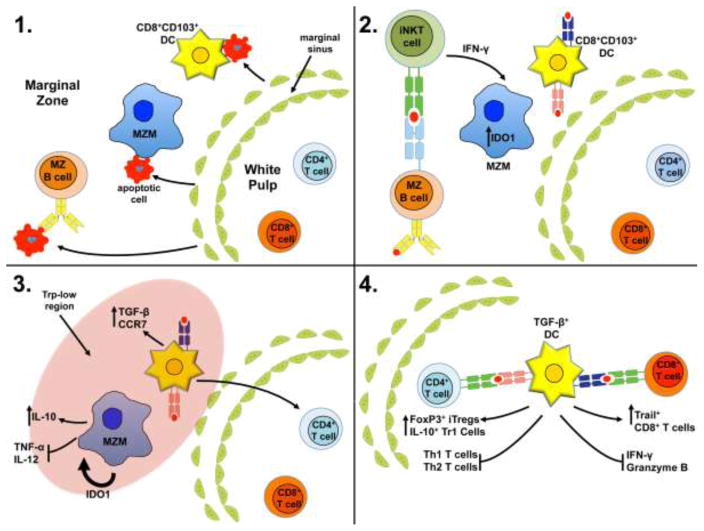
Figure 2. Hypothetical model of induced splenic tolerance to apoptotic cells.
1) Circulating apoptotic cells enter the spleen through the marginal sinus traveling towards the red pulp. The apoptotic cells are captured in the marginal zone (MZ), primarily by marginal zone macrophages (MARCO+ MZM) and resident CD8+ DCs. However, MZ B cells also capture apoptotic cells in a B cell receptor-dependent manner. 2) Activated MZ B cells present either endogenous lipids or lipids derived from captured, internalized apoptotic cells in CD1d molecules to invariant NKT cells stimulating rapid production of IFN-γ. IFN-γ stimulates the MZMs to induce intracellular expression of the immunoregulatory enzyme IDO1. 3) IDO1 acts on the MZMs in an autocrine and paracrine fashion promoting apoptotic cell-induced IL-10 production while inhibiting proinflammatory cytokine synthesis. Moreover, induction of IDO creates a zone of low tryptophan promoting stress response driven TGF-β production in CD8+CD103+ tolerogenic DCs. The tolerogenic DCs also up regulate surface expression of the chemokine receptor CCR7 promoting migration into the PALS. 4) Once in the PALS the TGF-β+CD8+CD103+ DCs present apoptotic cell antigens by both MHC I and MHC II-dependent mechanisms promoting development of FoxP3+Tregs, IL-10+Tr1 T cells (i.e. IL-10+ CD4+ T cells), and Trail+ CD8+ T cells while inhibiting inflammatory CD4+ and CD8+ T cell function, promoting T cell anergy and deletion.
Systemic tolerance in the liver
Relatively little is known about apoptotic cell-driven immune tolerance in other tissue sites. However, it is reasonable to assume that similar mechanisms will contribute to immune tolerance in tissues that have access to lymphatic drainage or the circulatory system. The liver receives most of its blood from the hepatic portal vein, which first passes the gastrointestinal tract[21]. As a consequence, the blood entering the liver contains digested material and microbial products from the large and small intestine; hence a major function of the liver is to filter out microbial contaminants such as LPS[136,137]. The liver is well adapted to this and, like the intestinal mucosa, appears to be conditioned towards a default state of tolerance with high-level suppressive cytokine production[138]. Moreover, the liver, like other filtering organs, contains significant numbers of phagocytes that serve a scavenging function including DC subsets such as plasmacytoid DCs, CD8α+ DCs, and a little understood NK1.1+ natural killer DC subset[139,140]. Unlike splenic DCs, liver DCs seemed primed to produce suppressive cytokines synthesizing high levels of IL-10 after stimulation[141,142]. Moreover, liver DCs are weak T cell stimulators compared to splenic DCs which is attributable to their tolerogenic nature[143]. The liver also contains a large population of F4/80+ macrophage-like Kupffer cells (KCs). These cells are highly phagocytic and are the major scavenger population in the liver. Furthermore, portal venous tolerance is dependent on KCs suggesting a primary role in hepatic immune suppression[144]. KCs produce IL-10 in response to LPS stimulation and express FasL and PD-L1 indicating that they likely suppress T cells responses directly via contact-dependent killing and indirectly via suppressive cytokine production[138].
A major function of the liver is the destruction of aging erythrocytes and neutrophils[145,146]. Likewise, apoptotic T cells are trapped in the liver[147,148]. Thus, like the spleen, the liver contends with a significant volume of apoptotic cells that must be cleared quietly and efficiently. Multiple cell populations are apparently involved in hepatic apoptotic cell phagocytosis including DCs, hepatocytes, KCs, and liver sinusoidal endothelial cells[149]. However, KCs appear to play a dominant role in apoptotic cell-mediated immune suppression as KC-depletion abrogates the ability of apoptotic cells to mediate liver tolerance[150]. Functional analysis demonstrated KCs rapidly phagocytose apoptotic cells from circulation and apoptotic cell contact induces IL-10 synthesis in a TGF-β-dependent manner[150]. Of particular note is the observation that maximal immune suppressive activity in the liver occurs 3–7 days after apoptotic cell administration. We have found a similar time frame is required for recipients to accept skin allografts after an i/v apoptotic cell tolerization. Zhang et al speculate that this time is required for optimal induction of suppressive cytokines[150], however it is more likely that this is the affector phase required for activation/expansion of resident regulatory T and NKT cell subsets to effect final tolerogenic mechanisms.
The liver is an organ with high endogenous arginase activity. Callery et al found that low arginine conditions enhanced KC suppressive activity promoting prostaglandin-E2 production and suppression of T cell proliferation[144]. Similarly, the liver is the major source of tryptophan 2,3 dioxygenase (TDO) activity[90]. While structurally unrelated to IDO, TDO oxidatively cleaves tryptophan in a mechanistically similar fashion[90,151]. Tumors expressing TDO were found to be potently immunosuppressive suggesting that TDO possesses immune regulatory properties similar to IDO[152,153]. Thus high arginase and TDO activity in the liver likely creates a paucity of micro-environmental arginine and tryptophan contributing to basal suppression.
Recently hepatic stellate cells (HSCs) were found to directly inhibit DC allostimulatory function via a mechanism dependent on IDO expression[154]. HSCs reside in the subendothelial space and function as a stromal APC, expressing MHCI, MHCII, and CD1d[138]. HSCs can induce regulatory T cells in an IFN-γ-dependent manner, promoting allograft tolerance[155,156]. Given the ability of IFNs to induce IDO it possible there is a mechanistic link between HSC and immunologic tolerance to apoptotic self. IDO is distinct from TDO in that it is responsive to inflammatory signals while TDO activity is primarily constitutive. Thus high basal TDO and arginase may promote general immune suppression while IDO induction in response to immune activation may play a more specific role in initiation of adaptive tolerance, a concept that warrants testing.
Immune therapy based on apoptotic cells
Autoimmune and autoinflammatory disease results from an imbalance in the regulatory/inflammatory mechanisms that define the immune response. Apoptotic cells have natural, potent immuno-tolerogenic potential making them an ideal platform for immune therapy. Moreover, by utilizing a patient’s own tissue as a source of apoptotic material, we will be able to take advantage of natural suppressive mechanisms while minimizing adverse outcomes. Thus, while still in its infancy, there are several promising branches to this approach that may pave the way for creation of personalized tolerogens and therapeutic interventions in autoimmune disease and transplantation.
Apoptotic cells in the clinic
More than 30 years ago Miller et al demonstrated that splenocytes treated with the crosslinking reagent 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (ECDI) could induce profound tolerance when delivered i/v[157]. ECDI is commonly used to link antigens to red blood cells for experimental vaccination protocols. However, when splenocytes are treated with ECDI they undergo rapid apoptosis and can stimulate Foxp3+CD4+ Tregs by a PD-L1 dependent mechanism in vivo[158]. The tolerance is mediated partially by direct antigen presentation originating from the ECDI-treated cells themselves, however the dominant mechanism is dependent on recipient splenic phagocytes, IL-10 production, and expansion of induced Tregs[159,160]. In vivo ECDI-treated cells are processed in a manner phenotypically similar to naturally occurring apoptotic cells with a rapid localization to the spleen and phagocytosis by F4/80+ macrophages inducing antigen specific anergy in effector T cells, an effect that is partially reversible by the addition of IL-2[160]. Thus in many respects induction of ECDI-treated cell tolerance appears mechanistically similar to apoptotic cell tolerance. An advantage of ECDI-based approaches it the relative ease of linking antigen to the apoptotic cells (since ECDI is itself a crosslinking agent). Thus in autoimmune diseases such as rheumatoid arthritis, type I diabetes, and multiple sclerosis (MS) where putative candidate antigens driving disease have been identified, ECDI-crosslinking of target antigen to apoptotic cells may provide an effective tolerogenic vaccine. In particular, there is extensive experimental history of ECDI treatment in mouse models of MS prompting a recent phase I clinical trial. In this trial peripheral blood mononuclear cells were collected from nine MS patients and treated with ECDI in the presence of a mixture of seven immuno-dominant peptides derived from myelin oligodendrocyte protein, myelin basic protein, and proteolipid protein and infused back into the patients. The treatment was well tolerated with no adverse reactions and high dose cell infusion succeeded in suppressing antigen-specific T cell responses (Dr. Stephen Miller, personal communication).
Extracorporeal photopheresis (ECP) was a technique originally developed as a therapy for cutaneous T cell lymphoma (CTCL). The procedure involves oral administration of photo-responsive psoralen and subsequent leukopheresis coupled with ultraviolet A (UVA) light exposure resulting in crosslinking of the cellular DNA and apoptosis[161–163]. ECP shows remarkable clinical efficacy in CTCL and has been an FDA approved first line treatment for the condition since 1988[164,165]. In addition to cutaneous cancer, ECP treatment may be beneficial in many chronic inflammatory diseases such as type 1 diabetes, Crohn’s disease, scleroderma, and nephrogenic systemic fibrosis[166–169]. Moreover, ECP improved transplant acceptance and increased survival times in patients with steroid-resistant chronic graft versus host (GvHD) disease[164,170]. There is not a satisfactory answer to explain the seeming dichotomy of this approach as a treatment for CTCL on one hand and as an immunosuppressive on the other. Mechanistically, ECP appears to function by apoptotic cell immuno-tolerance mechanisms described above. For example, in an experimental model of GvHD, ECP protected mice from pathology by inhibiting effector CD8+ T cell responses and promoting expansion of Tregs[171]. Moreover, in the absence of IL-10 ECP-mediated suppression is lost[172]. Thus, it is likely that ECP mediated tolerance will prove to more or less overlap with the mechanisms involved in natural immune tolerance to apoptotic cells. Rao et al reported ECP increased serum TGF-β in GvHD and CTCL patients; however, there was a slight decrease in overall Treg numbers likely due to ECP-treatment, which would remove lymphocytes from circulation [173]. The authors of the study speculated that increased Treg activity may have a negative impact on the lymphoma. This argument runs counter to the enormous body of literature suggesting that Tregs promote cancer establishment providing progressively potent immune suppression. However, at least one study found a positive correlation between FoxP3+ Treg accumulation in the skin and survival times in CTCL patients[174]. Thus Tregs may play a distinct role in lymphoma development as compared to solid tumors, explaining the efficacy of ECP.
Engineered tolerogenic particulate antigen approaches
Apoptotic cells posses multiple properties that can create a complex immuno-regulatory environment. However, there are inherent problems with using apoptotic cells as a source of tolerogenic vaccines, namely large numbers of viable cells must be isolated and treated ex vivo creating sourcing and logistical issues. Moreover, the long-term stability of apoptotic cell-tolerogens is unlikely necessitating fresh isolation and processing of material for each administration creating quality control issues. A potential solution is to engineer more stable antigen containing particles that mimic the properties of apoptotic cells eliciting the same natural immuno-regulatory circuits.
The IDO regulatory pathway suppresses adaptive T cell and inflammatory APC responses and promotes FoxP3+CD4+ Treg development and activation. Thus this represents an attractive potential target for immuno-modulatory therapy. In this vein we recently demonstrated that IDO induction by i/v administration of DNA complexed with the cationic polymer polyethylenimine (DNA nanoparticles, DNPs) protected mice from experimental arthritis blocking IL-6 and IL-17a production and effector T cell responses[175]. The DNPs rapidly accumulate in the MZ where they are phagocytosed by splenic DCs and MZMs (Huang et al, manuscript in preparation). DNP-mediated IDO expression is dependent on intracellular sensing of complexed DNA by a TLR9-independent mechanism resulting in type I IFN production. Moreover we have observed similar results in mouse models of antibody-mediated nephritis suggesting that DNPs are effective in creating immuno-suppressive conditions (TLM, unpublished observations). However the suppression is general, not antigen-specific; therefore, efficacy in treatment of autoimmune or autoinflammatory disease will likely be short lived. Nevertheless, polyethylenimine contains a highly cationic backbone which readily complexes with DNA and is currently considered one of the most effective non-viral methods of intracellular gene delivery in vivo[176–178]. Replacing the DNP-complexed DNA with a eukaryotic expression vector containing target antigens may serve to more fully replicate tolerance induced by apoptotic cells as it would satisfy the criteria of creating an immediately suppressive microenvironment and providing the antigens necessary for adaptive tolerance to take hold.
However, delivery of antigen in a particulate form may be sufficient to drive tolerance outright. Coupling of peptide with poly(lactide-co-glycolide) (PLG), a biodegradable nanoparticle was sufficient to drive long term T cell tolerance and protect mice from relapsing experimental encephalomyelitis (R-EAE) (Getts et al, Nature Biotechnology, in press). Uptake of the PLG nanoparticles was specific for MZMs and mediated by the scavenger receptor MARCO. This was in contrast to apoptotic cells (ECDI-treated), which did not require MACRO to suppress T cell responses. In addition, PLG nanoparticle-mediated tolerance is mainly driven by T cell anergy and is not dependent on IL-10, PD-L1 or Treg activity. However, PLG tolerance required particles approximately 500nm in size (roughly equivalent to the size of an apoptotic cell) while particles less than 100nm failed to generate a tolerogenic response, likely due to ineffective phagocytosis. A major question raised by these results is why an antigen that does not resemble an apoptotic cell could elicit a similar tolerogenic response. MZMs are most likely constantly exposed to apoptotic cells entering from circulation. Consequently, in the default state, MZMs are prone to exhibit significant regulatory activity. Antigens targeted to MZMs in a particulate manner that do not perturb basal activity may be incorporated into the tolerogenic pathway resulting in immune suppression. This may explain why IL-10 and Tregs are not mechanistically involved in PLG-mediated anergy induction. If this is the case, then the addition of further apoptotic cell characteristics (e.g. IDO induction) may enhance the tolerogenic circuit providing more enduring and effective long-term benefit.
Table 1.
Apoptotic cell processing defects associated with disease phenotypes.
| Genes Involved | Disease Associated |
|---|---|
| 1) Find-Me Signals | |
| CX3CL1 | Neurodegenerative Disease [179] |
| CX3CR1 | Atherosclerosis |
| 2) Eat-Me Signals | |
| Mer | SLE [40, 42] |
| Glomerulonephritis [181] | |
| Atherosclerosis [182] | |
| MFG-E8 | Glomerulonephritis, SLE [39, 56] |
| Neurodegenerative Disease [183, 184] | |
| GAS-6 | SLE [185] |
| C1q | SLE [186] |
| TIM4 | Mild Autoimmunity [36] |
| 3) Engulf-Me Signal | |
| ELMO1 | Type-1 Diabetes [187] |
| 4) Post-Engulfment Signals | |
| LXRα/β | SLE [188] |
| PPARδ | SLE [189] |
| DNase II | Arthritis [59] |
Perspectives.
The last two decades have seen an explosion in our understanding of how apoptotic cells influence immunity. Thus we have seen the role of apoptotic cells in the immune response grow from one of refuse requiring rapid janitorial service to an essential partner likely required for all immune responses. Not simply as a mechanism to remove unnecessary lymphocytes, but as a tool to effectively deliver antigens in a particulate form which can readily be phagocytosed by APCs. The apoptotic cell comes embedded with significant amounts of information allowing for rapid assessment and progression to either adaptive immunity (inflammation) or regulation (tolerance). With increased understanding of the role apoptotic cells have in immunity comes the hope that these mechanisms can be harnessed for clinical good. Indeed we have taken our first tentative steps in this direction and the results have been extremely encouraging. Further elucidation of the mechanisms involved in immunologic tolerance to apoptotic-self at the molecular and cellular level will enable us to fine tailor our approaches, not only to improve therapeutic tolerogenic in a general sense, but to specific needs in varying contexts.
Bibliography
- 1.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elliott MR, Ravichandran KS. Clearance of apoptotic cells: implications in health and disease. J Cell Biol. 2010;189(7):1059–1070. doi: 10.1083/jcb.201004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Platt N, Suzuki H, Kodama T, Gordon S. Apoptotic thymocyte clearance in scavenger receptor class A-deficient mice is apparently normal. J Immunol. 2000;164(9):4861–4867. doi: 10.4049/jimmunol.164.9.4861. [DOI] [PubMed] [Google Scholar]
- 4.Clancy RM, Neufing PJ, Zheng P, O’Mahony M, Nimmerjahn F, Gordon TP, Buyon JP. Impaired clearance of apoptotic cardiocytes is linked to anti-SSA/Ro and -SSB/La antibodies in the pathogenesis of congenital heart block. J Clin Invest. 2006;116(9):2413–2422. doi: 10.1172/JCI27803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188(7):1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392(6671):86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 7.Jinushi M, Nakazaki Y, Dougan M, Carrasco DR, Mihm M, Dranoff G. MFG-E8-mediated uptake of apoptotic cells by APCs links the pro- and antiinflammatory activities of GM-CSF. J Clin Invest. 2007;117(7):1902–1913. doi: 10.1172/JCI30966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eguchi M, Kikuchi Y. Binding of Salmonella-specific antibody facilitates specific T cell responses via augmentation of bacterial uptake and induction of apoptosis in macrophages. J Infect Dis. 2010;201(1):62–70. doi: 10.1086/648615. [DOI] [PubMed] [Google Scholar]
- 9.Kroemer G, El-Deiry WS, Golstein P, Peter ME, Vaux D, Vandenabeele P, Zhivotovsky B, Blagosklonny MV, Malorni W, Knight RA, Piacentini M, Nagata S, Melino G. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ. 2005;12(Suppl 2):1463–1467. doi: 10.1038/sj.cdd.4401724. [DOI] [PubMed] [Google Scholar]
- 10.Bratton DL, Henson PM. Neutrophil clearance: when the party is over, clean-up begins. Trends Immunol. 2011;32(8):350–357. doi: 10.1016/j.it.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parnaik R, Raff MC, Scholes J. Differences between the clearance of apoptotic cells by professional and non-professional phagocytes. Curr Biol. 2000;10(14):857–860. doi: 10.1016/s0960-9822(00)00598-4. [DOI] [PubMed] [Google Scholar]
- 12.Lauber K, Bohn E, Krober SM, Xiao YJ, Blumenthal SG, Lindemann RK, Marini P, Wiedig C, Zobywalski A, Baksh S, Xu Y, Autenrieth IB, Schulze-Osthoff K, Belka C, Stuhler G, Wesselborg S. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113(6):717–730. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- 13.Truman LA, Ford CA, Pasikowska M, Pound JD, Wilkinson SJ, Dumitriu IE, Melville L, Melrose LA, Ogden CA, Nibbs R, Graham G, Combadiere C, Gregory CD. CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood. 2008;112(13):5026–5036. doi: 10.1182/blood-2008-06-162404. [DOI] [PubMed] [Google Scholar]
- 14.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461(7261):282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gude DR, Alvarez SE, Paugh SW, Mitra P, Yu J, Griffiths R, Barbour SE, Milstien S, Spiegel S. Apoptosis induces expression of sphingosine kinase 1 to release sphingosine-1-phosphate as a “come-and-get-me” signal. Faseb J. 2008;22(8):2629–2638. doi: 10.1096/fj.08-107169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devitt A, Gregory CD. Measurement of apoptotic cell clearance in vitro. Methods Mol Biol. 2004;282:207–221. doi: 10.1385/1-59259-812-9:207. [DOI] [PubMed] [Google Scholar]
- 17.Ravichandran KS. Find-me and eat-me signals in apoptotic cell clearance: progress and conundrums. J Exp Med. 2010;207(9):1807–1817. doi: 10.1084/jem.20101157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417(6885):182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 19.Ziegenfuss JS, Biswas R, Avery MA, Hong K, Sheehan AE, Yeung YG, Stanley ER, Freeman MR. Draper-dependent glial phagocytic activity is mediated by Src and Syk family kinase signalling. Nature. 2008;453(7197):935–939. doi: 10.1038/nature06901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGaha TL, Chen Y, Ravishankar B, van Rooijen N, Karlsson MC. Marginal zone macrophages suppress innate and adaptive immunity to apoptotic cells in the spleen. Blood. 2011;117(20):5403–5412. doi: 10.1182/blood-2010-11-320028. [DOI] [PubMed] [Google Scholar]
- 21.Wermeling F, Karlsson MC, McGaha TL. An anatomical view on macrophages in tolerance. Autoimmun Rev. 2009;9(1):49–52. doi: 10.1016/j.autrev.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Lauber K, Blumenthal SG, Waibel M, Wesselborg S. Clearance of apoptotic cells: getting rid of the corpses. Mol Cell. 2004;14(3):277–287. doi: 10.1016/s1097-2765(04)00237-0. [DOI] [PubMed] [Google Scholar]
- 23.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148(7):2207–2216. [PubMed] [Google Scholar]
- 24.Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405(6782):85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann PR, de Cathelineau AM, Ogden CA, Leverrier Y, Bratton DL, Daleke DL, Ridley AJ, Fadok VA, Henson PM. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J Cell Biol. 2001;155(4):649–659. doi: 10.1083/jcb.200108080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balasubramanian K, Schroit AJ. Aminophospholipid asymmetry: A matter of life and death. Annu Rev Physiol. 2003;65:701–734. doi: 10.1146/annurev.physiol.65.092101.142459. [DOI] [PubMed] [Google Scholar]
- 27.Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182(5):1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borisenko GG, Matsura T, Liu SX, Tyurin VA, Jianfei J, Serinkan FB, Kagan VE. Macrophage recognition of externalized phosphatidylserine and phagocytosis of apoptotic Jurkat cells--existence of a threshold. Arch Biochem Biophys. 2003;413(1):41–52. doi: 10.1016/s0003-9861(03)00083-3. [DOI] [PubMed] [Google Scholar]
- 29.Dillon SR, Constantinescu A, Schlissel MS. Annexin V binds to positively selected B cells. J Immunol. 2001;166(1):58–71. doi: 10.4049/jimmunol.166.1.58. [DOI] [PubMed] [Google Scholar]
- 30.Dillon SR, Mancini M, Rosen A, Schlissel MS. Annexin V binds to viable B cells and colocalizes with a marker of lipid rafts upon B cell receptor activation. J Immunol. 2000;164(3):1322–1332. doi: 10.4049/jimmunol.164.3.1322. [DOI] [PubMed] [Google Scholar]
- 31.Frasch SC, Berry KZ, Fernandez-Boyanapalli R, Jin HS, Leslie C, Henson PM, Murphy RC, Bratton DL. NADPH oxidase-dependent generation of lysophosphatidylserine enhances clearance of activated and dying neutrophils via G2A. J Biol Chem. 2008;283(48):33736–33749. doi: 10.1074/jbc.M807047200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frasch SC, Henson PM, Nagaosa K, Fessler MB, Borregaard N, Bratton DL. Phospholipid flip-flop and phospholipid scramblase 1 (PLSCR1) co-localize to uropod rafts in formylated Met-Leu-Phe-stimulated neutrophils. J Biol Chem. 2004;279(17):17625–17633. doi: 10.1074/jbc.M313414200. [DOI] [PubMed] [Google Scholar]
- 33.Wolf A, Schmitz C, Bottger A. Changing story of the receptor for phosphatidylserine-dependent clearance of apoptotic cells. EMBO Rep. 2007;8(5):465–469. doi: 10.1038/sj.embor.7400956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450(7168):435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 35.Park D, Tosello-Trampont AC, Elliott MR, Lu M, Haney LB, Ma Z, Klibanov AL, Mandell JW, Ravichandran KS. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450(7168):430–434. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez-Manzanet R, Sanjuan MA, Wu HY, Quintana FJ, Xiao S, Anderson AC, Weiner HL, Green DR, Kuchroo VK. T and B cell hyperactivity and autoimmunity associated with niche-specific defects in apoptotic body clearance in TIM-4-deficient mice. Proc Natl Acad Sci U S A. 2010;107(19):8706–8711. doi: 10.1073/pnas.0910359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong K, Valdez PA, Tan C, Yeh S, Hongo JA, Ouyang W. Phosphatidylserine receptor Tim-4 is essential for the maintenance of the homeostatic state of resident peritoneal macrophages. Proc Natl Acad Sci U S A. 2010;107(19):8712–8717. doi: 10.1073/pnas.0910929107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park D, Hochreiter-Hufford A, Ravichandran KS. The phosphatidylserine receptor TIM-4 does not mediate direct signaling. Curr Biol. 2009;19(4):346–351. doi: 10.1016/j.cub.2009.01.042. [DOI] [PubMed] [Google Scholar]
- 39.Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, Nagata S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304(5674):1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 40.Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411(6834):207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 41.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131(6):1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 42.Cohen PL, Caricchio R, Abraham V, Camenisch TD, Jennette JC, Roubey RA, Earp HS, Matsushima G, Reap EA. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J Exp Med. 2002;196(1):135–140. doi: 10.1084/jem.20012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Segawa K, Suzuki J, Nagata S. Constitutive exposure of phosphatidylserine on viable cells. Proc Natl Acad Sci U S A. 2011;108(48):19246–19251. doi: 10.1073/pnas.1114799108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park YJ, Liu G, Lorne EF, Zhao X, Wang J, Tsuruta Y, Zmijewski J, Abraham E. PAI-1 inhibits neutrophil efferocytosis. Proc Natl Acad Sci U S A. 2008;105(33):11784–11789. doi: 10.1073/pnas.0801394105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg PA, Michalak M, Henson PM. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123(2):321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 46.Brown S, Heinisch I, Ross E, Shaw K, Buckley CD, Savill J. Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature. 2002;418(6894):200–203. doi: 10.1038/nature00811. [DOI] [PubMed] [Google Scholar]
- 47.Elomaa O, Kangas M, Sahlberg C, Tuukkanen J, Sormunen R, Liakka A, Thesleff I, Kraal G, Tryggvason K. Cloning of a novel bacteria-binding receptor structurally related to scavenger receptors and expressed in a subset of macrophages. Cell. 1995;80(4):603–609. doi: 10.1016/0092-8674(95)90514-6. [DOI] [PubMed] [Google Scholar]
- 48.Wermeling F, Chen Y, Pikkarainen T, Scheynius A, Winqvist O, Izui S, Ravetch JV, Tryggvason K, Karlsson MC. Class A scavenger receptors regulate tolerance against apoptotic cells, and autoantibodies against these receptors are predictive of systemic lupus. J Exp Med. 2007;204(10):2259–2265. doi: 10.1084/jem.20070600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boullier A, Friedman P, Harkewicz R, Hartvigsen K, Green SR, Almazan F, Dennis EA, Steinberg D, Witztum JL, Quehenberger O. Phosphocholine as a pattern recognition ligand for CD36. J Lipid Res. 2005;46(5):969–976. doi: 10.1194/jlr.M400496-JLR200. [DOI] [PubMed] [Google Scholar]
- 50.Toda S, Hanayama R, Nagata S. Two-step engulfment of apoptotic cells. Mol Cell Biol. 2012;32(1):118–125. doi: 10.1128/MCB.05993-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gumienny TL, Brugnera E, Tosello-Trampont AC, Kinchen JM, Haney LB, Nishiwaki K, Walk SF, Nemergut ME, Macara IG, Francis R, Schedl T, Qin Y, Van Aelst L, Hengartner MO, Ravichandran KS. CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell. 2001;107(1):27–41. doi: 10.1016/s0092-8674(01)00520-7. [DOI] [PubMed] [Google Scholar]
- 52.Krysko DV, Denecker G, Festjens N, Gabriels S, Parthoens E, D’Herde K, Vandenabeele P. Macrophages use different internalization mechanisms to clear apoptotic and necrotic cells. Cell Death Differ. 2006;13(12):2011–2022. doi: 10.1038/sj.cdd.4401900. [DOI] [PubMed] [Google Scholar]
- 53.Erwig LP, McPhilips KA, Wynes MW, Ivetic A, Ridley AJ, Henson PM. Differential regulation of phagosome maturation in macrophages and dendritic cells mediated by Rho GTPases and ezrin-radixin-moesin (ERM) proteins. Proc Natl Acad Sci U S A. 2006;103(34):12825–12830. doi: 10.1073/pnas.0605331103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science. 2004;304(5673):1014–1018. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 55.Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440(7085):808–812. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 56.Peng Y, Elkon KB. Autoimmunity in MFG-E8-deficient mice is associated with altered trafficking and enhanced cross-presentation of apoptotic cell antigens. J Clin Invest. 2011;121(6):2221–2241. doi: 10.1172/JCI43254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Evans CJ, Aguilera RJ. DNase II: genes, enzymes and function. Gene. 2003;322:1–15. doi: 10.1016/j.gene.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 58.Kawane K, Fukuyama H, Kondoh G, Takeda J, Ohsawa Y, Uchiyama Y, Nagata S. Requirement of DNase II for definitive erythropoiesis in the mouse fetal liver. Science. 2001;292(5521):1546–1549. doi: 10.1126/science.292.5521.1546. [DOI] [PubMed] [Google Scholar]
- 59.Kawane K, Ohtani M, Miwa K, Kizawa T, Kanbara Y, Yoshioka Y, Yoshikawa H, Nagata S. Chronic polyarthritis caused by mammalian DNA that escapes from degradation in macrophages. Nature. 2006;443(7114):998–1002. doi: 10.1038/nature05245. [DOI] [PubMed] [Google Scholar]
- 60.Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, Oyabu J, Murakawa T, Nakayama H, Nishida K, Akira S, Yamamoto A, Komuro I, Otsu K. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485(7397):251–255. doi: 10.1038/nature10992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okabe Y, Kawane K, Akira S, Taniguchi T, Nagata S. Toll-like receptor-independent gene induction program activated by mammalian DNA escaped from apoptotic DNA degradation. J Exp Med. 2005;202(10):1333–1339. doi: 10.1084/jem.20051654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gall A, Treuting P, Elkon KB, Loo YM, Gale M, Jr, Barber GN, Stetson DB. Autoimmunity initiates in nonhematopoietic cells and progresses via lymphocytes in an interferon-dependent autoimmune disease. Immunity. 2012;36(1):120–131. doi: 10.1016/j.immuni.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Waldmann H. Tolerance can be infectious. Nat Immunol. 2008;9(9):1001–1003. doi: 10.1038/ni0908-1001. [DOI] [PubMed] [Google Scholar]
- 64.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101(4):890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest. 2002;109(1):41–50. doi: 10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390(6658):350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 67.Ravishankar B, Liu H, Shinde R, Chandler P, Baban B, Tanaka M, Munn DH, Mellor AL, Karlsson MC, McGaha TL. Tolerance to apoptotic cells is regulated by indoleamine 2,3-dioxygenase. Proc Natl Acad Sci U S A. 2012;109(10):3909–3914. doi: 10.1073/pnas.1117736109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Griffith TS, Yu X, Herndon JM, Green DR, Ferguson TA. CD95-induced apoptosis of lymphocytes in an immune privileged site induces immunological tolerance. Immunity. 1996;5(1):7–16. doi: 10.1016/s1074-7613(00)80305-2. [DOI] [PubMed] [Google Scholar]
- 69.Ferguson TA, Herndon J, Elzey B, Griffith TS, Schoenberger S, Green DR. Uptake of apoptotic antigen-coupled cells by lymphoid dendritic cells and cross-priming of CD8(+) T cells produce active immune unresponsiveness. J Immunol. 2002;168(11):5589–5595. doi: 10.4049/jimmunol.168.11.5589. [DOI] [PubMed] [Google Scholar]
- 70.Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5(8):606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- 71.Kraal G, Schornagel K, Streeter PR, Holzmann B, Butcher EC. Expression of the mucosal vascular addressin, MAdCAM-1, on sinus-lining cells in the spleen. Am J Pathol. 1995;147(3):763–771. [PMC free article] [PubMed] [Google Scholar]
- 72.Tanaka H, Hataba Y, Saito S, Fukushima O, Miyasaka M. Phenotypic characteristics and significance of reticular meshwork surrounding splenic white pulp of mice. J Electron Microsc (Tokyo) 1996;45(5):407–416. doi: 10.1093/oxfordjournals.jmicro.a023459. [DOI] [PubMed] [Google Scholar]
- 73.Girkontaite I, Sakk V, Wagner M, Borggrefe T, Tedford K, Chun J, Fischer KD. The sphingosine-1-phosphate (S1P) lysophospholipid receptor S1P3 regulates MAdCAM-1+ endothelial cells in splenic marginal sinus organization. J Exp Med. 2004;200(11):1491–1501. doi: 10.1084/jem.20041483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol. 2005;23:127–159. doi: 10.1146/annurev.immunol.23.021704.115628. [DOI] [PubMed] [Google Scholar]
- 75.Kraal G, Mebius R. New insights into the cell biology of the marginal zone of the spleen. Int Rev Cytol. 2006;250:175–215. doi: 10.1016/S0074-7696(06)50005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Geijtenbeek TB, Groot PC, Nolte MA, van Vliet SJ, Gangaram-Panday ST, van Duijnhoven GC, Kraal G, van Oosterhout AJ, van Kooyk Y. Marginal zone macrophages express a murine homologue of DC-SIGN that captures blood-borne antigens in vivo. Blood. 2002;100(8):2908–2916. doi: 10.1182/blood-2002-04-1044. [DOI] [PubMed] [Google Scholar]
- 77.Hughes DA, Fraser IP, Gordon S. Murine macrophage scavenger receptor: in vivo expression and function as receptor for macrophage adhesion in lymphoid and non-lymphoid organs. Eur J Immunol. 1995;25(2):466–473. doi: 10.1002/eji.1830250224. [DOI] [PubMed] [Google Scholar]
- 78.Kraal G, van der Laan LJ, Elomaa O, Tryggvason K. The macrophage receptor MARCO. Microbes Infect. 2000;2(3):313–316. doi: 10.1016/s1286-4579(00)00296-3. [DOI] [PubMed] [Google Scholar]
- 79.Kraal G, Janse M. Marginal metallophilic cells of the mouse spleen identified by a monoclonal antibody. Immunology. 1986;58(4):665–669. [PMC free article] [PubMed] [Google Scholar]
- 80.Miyake Y, Asano K, Kaise H, Uemura M, Nakayama M, Tanaka M. Critical role of macrophages in the marginal zone in the suppression of immune responses to apoptotic cell-associated antigens. J Clin Invest. 2007;117(8):2268–2278. doi: 10.1172/JCI31990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karlsson MC, Guinamard R, Bolland S, Sankala M, Steinman RM, Ravetch JV. Macrophages control the retention and trafficking of B lymphocytes in the splenic marginal zone. J Exp Med. 2003;198(2):333–340. doi: 10.1084/jem.20030684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Uderhardt S, Herrmann M, Oskolkova OV, Aschermann S, Bicker W, Ipseiz N, Sarter K, Frey B, Rothe T, Voll R, Nimmerjahn F, Bochkov VN, Schett G, Kronke G. 12/15-lipoxygenase orchestrates the clearance of apoptotic cells and maintains immunologic tolerance. Immunity. 2012;36(5):834–846. doi: 10.1016/j.immuni.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 83.Kim S, Elkon KB, Ma X. Transcriptional suppression of interleukin-12 gene expression following phagocytosis of apoptotic cells. Immunity. 2004;21(5):643–653. doi: 10.1016/j.immuni.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 84.Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8(5):327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sen P, Wallet MA, Yi Z, Huang Y, Henderson M, Mathews CE, Earp HS, Matsushima G, Baldwin AS, Jr, Tisch RM. Apoptotic cells induce Mer tyrosine kinase-dependent blockade of NF-kappaB activation in dendritic cells. Blood. 2007;109(2):653–660. doi: 10.1182/blood-2006-04-017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lu Q, Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001;293(5528):306–311. doi: 10.1126/science.1061663. [DOI] [PubMed] [Google Scholar]
- 87.Popovic PJ, Zeh HJ, 3rd, Ochoa JB. Arginine and immunity. J Nutr. 2007;137(6 Suppl 2):1681S–1686S. doi: 10.1093/jn/137.6.1681S. [DOI] [PubMed] [Google Scholar]
- 88.Johann AM, Barra V, Kuhn AM, Weigert A, von Knethen A, Brune B. Apoptotic cells induce arginase II in macrophages, thereby attenuating NO production. Faseb J. 2007;21(11):2704–2712. doi: 10.1096/fj.06-7815com. [DOI] [PubMed] [Google Scholar]
- 89.Barra V, Kuhn AM, von Knethen A, Weigert A, Brune B. Apoptotic cell-derived factors induce arginase II expression in murine macrophages by activating ERK5/CREB. Cell Mol Life Sci. 2011;68(10):1815–1827. doi: 10.1007/s00018-010-0537-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McGaha TL, Huang L, Lemos H, Metz R, Mautino M, Prendergast GC, Mellor AL. Amino acid catabolism: a pivotal regulator of innate and adaptive immunity. Immunol Rev. 2012;249(1):135–157. doi: 10.1111/j.1600-065X.2012.01149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Williams CA, Harry RA, McLeod JD. Apoptotic cells induce dendritic cell-mediated suppression via interferon-gamma-induced IDO. Immunology. 2008;124(1):89–101. doi: 10.1111/j.1365-2567.2007.02743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, Mellor AL. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22(5):633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 93.Sharma MD, Hou DY, Liu Y, Koni PA, Metz R, Chandler P, Mellor AL, He Y, Munn DH. Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes. Blood. 2009;113(24):6102–6111. doi: 10.1182/blood-2008-12-195354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, Azuma M, Blazar BR, Mellor AL, Munn DH. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117(9):2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sundrud MS, Koralov SB, Feuerer M, Calado DP, Kozhaya AE, Rhule-Smith A, Lefebvre RE, Unutmaz D, Mazitschek R, Waldner H, Whitman M, Keller T, Rao A. Halofuginone inhibits TH17 cell differentiation by activating the amino acid starvation response. Science. 2009;324(5932):1334–1338. doi: 10.1126/science.1172638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McGaha T, Kodera T, Phelps R, Spiera H, Pines M, Bona C. Effect of halofuginone on the development of tight skin (TSK) syndrome. Autoimmunity. 2002;35(4):277–282. doi: 10.1080/0891693021000001235. [DOI] [PubMed] [Google Scholar]
- 97.Hayashi T, Mo JH, Gong X, Rossetto C, Jang A, Beck L, Elliott GI, Kufareva I, Abagyan R, Broide DH, Lee J, Raz E. 3-Hydroxyanthranilic acid inhibits PDK1 activation and suppresses experimental asthma by inducing T cell apoptosis. Proc Natl Acad Sci U S A. 2007;104(47):18619–18624. doi: 10.1073/pnas.0709261104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nguyen NT, Kimura A, Nakahama T, Chinen I, Masuda K, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci U S A. 2010;107(46):19961–19966. doi: 10.1073/pnas.1014465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Esser C, Rannug A, Stockinger B. The aryl hydrocarbon receptor in immunity. Trends Immunol. 2009;30(9):447–454. doi: 10.1016/j.it.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 100.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185(6):3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vogel CF, Goth SR, Dong B, Pessah IN, Matsumura F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun. 2008;375(3):331–335. doi: 10.1016/j.bbrc.2008.07.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, Steinman RM, Nussenzweig MC. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315(5808):107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 103.den Haan JM, Lehar SM, Bevan MJ. CD8(+) but not CD8(−) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192(12):1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ohnmacht C, Pullner A, King SB, Drexler I, Meier S, Brocker T, Voehringer D. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med. 2009;206(3):549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Iyoda T, Shimoyama S, Liu K, Omatsu Y, Akiyama Y, Maeda Y, Takahara K, Steinman RM, Inaba K. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J Exp Med. 2002;195(10):1289–1302. doi: 10.1084/jem.20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu K, Iyoda T, Saternus M, Kimura Y, Inaba K, Steinman RM. Immune tolerance after delivery of dying cells to dendritic cells in situ. J Exp Med. 2002;196(8):1091–1097. doi: 10.1084/jem.20021215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Qiu CH, Miyake Y, Kaise H, Kitamura H, Ohara O, Tanaka M. Novel subset of CD8{alpha}+ dendritic cells localized in the marginal zone is responsible for tolerance to cell-associated antigens. J Immunol. 2009;182(7):4127–4136. doi: 10.4049/jimmunol.0803364. [DOI] [PubMed] [Google Scholar]
- 108.Pooley JL, Heath WR, Shortman K. Cutting edge: intravenous soluble antigen is presented to CD4 T cells by CD8− dendritic cells, but cross-presented to CD8 T cells by CD8+ dendritic cells. J Immunol. 2001;166(9):5327–5330. doi: 10.4049/jimmunol.166.9.5327. [DOI] [PubMed] [Google Scholar]
- 109.Yamazaki S, Dudziak D, Heidkamp GF, Fiorese C, Bonito AJ, Inaba K, Nussenzweig MC, Steinman RM. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J Immunol. 2008;181(10):6923–6933. doi: 10.4049/jimmunol.181.10.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Inaba K, Turley S, Yamaide F, Iyoda T, Mahnke K, Inaba M, Pack M, Subklewe M, Sauter B, Sheff D, Albert M, Bhardwaj N, Mellman I, Steinman RM. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J Exp Med. 1998;188(11):2163–2173. doi: 10.1084/jem.188.11.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Griffith TS, Kazama H, VanOosten RL, Earle JK, Jr, Herndon JM, Green DR, Ferguson TA. Apoptotic cells induce tolerance by generating helpless CD8+ T cells that produce TRAIL. J Immunol. 2007;178(5):2679–2687. doi: 10.4049/jimmunol.178.5.2679. [DOI] [PubMed] [Google Scholar]
- 112.Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434(7029):88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 113.Kranich J, Krautler NJ, Heinen E, Polymenidou M, Bridel C, Schildknecht A, Huber C, Kosco-Vilbois MH, Zinkernagel R, Miele G, Aguzzi A. Follicular dendritic cells control engulfment of apoptotic bodies by secreting Mfge8. J Exp Med. 2008;205(6):1293–1302. doi: 10.1084/jem.20071019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rahman ZS, Shao WH, Khan TN, Zhen Y, Cohen PL. Impaired apoptotic cell clearance in the germinal center by Mer-deficient tingible body macrophages leads to enhanced antibody-forming cell and germinal center responses. J Immunol. 2010;185(10):5859–5868. doi: 10.4049/jimmunol.1001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shao WH, Zhen Y, Eisenberg RA, Cohen PL. The Mer receptor tyrosine kinase is expressed on discrete macrophage subpopulations and mainly uses Gas6 as its ligand for uptake of apoptotic cells. Clin Immunol. 2009;133(1):138–144. doi: 10.1016/j.clim.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 117.Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 118.Facciotti F, Ramanjaneyulu GS, Lepore M, Sansano S, Cavallari M, Kistowska M, Forss-Petter S, Ni G, Colone A, Singhal A, Berger J, Xia C, Mori L, De Libero G. Peroxisome-derived lipids are self antigens that stimulate invariant natural killer T cells in the thymus. Nat Immunol. 2012;13(5):474–480. doi: 10.1038/ni.2245. [DOI] [PubMed] [Google Scholar]
- 119.Esteban LM, Tsoutsman T, Jordan MA, Roach D, Poulton LD, Brooks A, Naidenko OV, Sidobre S, Godfrey DI, Baxter AG. Genetic control of NKT cell numbers maps to major diabetes and lupus loci. J Immunol. 2003;171(6):2873–2878. doi: 10.4049/jimmunol.171.6.2873. [DOI] [PubMed] [Google Scholar]
- 120.Wither J, Cai YC, Lim S, McKenzie T, Roslin N, Claudio JO, Cooper GS, Hudson TJ, Paterson AD, Greenwood CM, Gladman D, Pope J, Pineau CA, Smith CD, Hanly JG, Peschken C, Boire G, Fortin PR. Reduced proportions of natural killer T cells are present in the relatives of lupus patients and are associated with autoimmunity. Arthritis Res Ther. 2008;10(5):R108. doi: 10.1186/ar2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu L, Van Kaer L. Natural killer T cells and autoimmune disease. Curr Mol Med. 2009;9(1):4–14. doi: 10.2174/156652409787314534. [DOI] [PubMed] [Google Scholar]
- 122.Yang JQ, Wen X, Liu H, Folayan G, Dong X, Zhou M, Van Kaer L, Singh RR. Examining the role of CD1d and natural killer T cells in the development of nephritis in a genetically susceptible lupus model. Arthritis Rheum. 2007;56(4):1219–1233. doi: 10.1002/art.22490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Caielli S, Sorini C, Falcone M. The dangerous liaison between iNKT cells and dendritic cells: does it prevent or promote autoimmune diseases? Autoimmunity. 2011;44(1):11–22. doi: 10.3109/08916931003782130. [DOI] [PubMed] [Google Scholar]
- 124.Roelofs-Haarhuis K, Wu X, Gleichmann E. Oral tolerance to nickel requires CD4+ invariant NKT cells for the infectious spread of tolerance and the induction of specific regulatory T cells. J Immunol. 2004;173(2):1043–1050. doi: 10.4049/jimmunol.173.2.1043. [DOI] [PubMed] [Google Scholar]
- 125.Caielli S, Conforti-Andreoni C, Di Pietro C, Usuelli V, Badami E, Malosio ML, Falcone M. On/off TLR signaling decides proinflammatory or tolerogenic dendritic cell maturation upon CD1d-mediated interaction with invariant NKT cells. J Immunol. 2010;185(12):7317–7329. doi: 10.4049/jimmunol.1000400. [DOI] [PubMed] [Google Scholar]
- 126.Pillai AB, George TI, Dutt S, Strober S. Host natural killer T cells induce an interleukin-4-dependent expansion of donor CD4+CD25+Foxp3+ T regulatory cells that protects against graft-versus-host disease. Blood. 2009;113(18):4458–4467. doi: 10.1182/blood-2008-06-165506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hua J, Liang S, Ma X, Webb TJ, Potter JP, Li Z. The interaction between regulatory T cells and NKT cells in the liver: a CD1d bridge links innate and adaptive immunity. PLoS One. 2011;6(11):e27038. doi: 10.1371/journal.pone.0027038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hegde S, Lockridge JL, Becker YA, Ma S, Kenney SC, Gumperz JE. Human NKT cells direct the differentiation of myeloid APCs that regulate T cell responses via expression of programmed cell death ligands. J Autoimmun. 2011;37(1):28–38. doi: 10.1016/j.jaut.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wermeling F, Lind SM, Jordo ED, Cardell SL, Karlsson MC. Invariant NKT cells limit activation of autoreactive CD1d-positive B cells. J Exp Med. 2010;207(5):943–952. doi: 10.1084/jem.20091314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Brennan PJ, Tatituri RV, Brigl M, Kim EY, Tuli A, Sanderson JP, Gadola SD, Hsu FF, Besra GS, Brenner MB. Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nat Immunol. 2011;12(12):1202–1211. doi: 10.1038/ni.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Miles K, Heaney J, Sibinska Z, Salter D, Savill J, Gray D, Gray M. A tolerogenic role for Toll-like receptor 9 is revealed by B-cell interaction with DNA complexes expressed on apoptotic cells. Proc Natl Acad Sci U S A. 2012;109(3):887–892. doi: 10.1073/pnas.1109173109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Qian Y, Wang H, Clarke SH. Impaired clearance of apoptotic cells induces the activation of autoreactive anti-Sm marginal zone and B-1 B cells. J Immunol. 2004;172(1):625–635. doi: 10.4049/jimmunol.172.1.625. [DOI] [PubMed] [Google Scholar]
- 133.Ehrenstein MR, Notley CA. The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol. 2010;10(11):778–786. doi: 10.1038/nri2849. [DOI] [PubMed] [Google Scholar]
- 134.Quartier P, Potter PK, Ehrenstein MR, Walport MJ, Botto M. Predominant role of IgM-dependent activation of the classical pathway in the clearance of dying cells by murine bone marrow-derived macrophages in vitro. Eur J Immunol. 2005;35(1):252–260. doi: 10.1002/eji.200425497. [DOI] [PubMed] [Google Scholar]
- 135.Ogden CA, Kowalewski R, Peng Y, Montenegro V, Elkon KB. IGM is required for efficient complement mediated phagocytosis of apoptotic cells in vivo. Autoimmunity. 2005;38(4):259–264. doi: 10.1080/08916930500124452. [DOI] [PubMed] [Google Scholar]
- 136.Lumsden AB, Henderson JM, Kutner MH. Endotoxin levels measured by a chromogenic assay in portal, hepatic and peripheral venous blood in patients with cirrhosis. Hepatology. 1988;8(2):232–236. doi: 10.1002/hep.1840080207. [DOI] [PubMed] [Google Scholar]
- 137.Catala M, Anton A, Portoles MT. Characterization of the simultaneous binding of Escherichia coli endotoxin to Kupffer and endothelial liver cells by flow cytometry. Cytometry. 1999;36(2):123–130. doi: 10.1002/(sici)1097-0320(19990601)36:2<123::aid-cyto6>3.3.co;2-p. [DOI] [PubMed] [Google Scholar]
- 138.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 139.O’Connell PJ, Morelli AE, Logar AJ, Thomson AW. Phenotypic and functional characterization of mouse hepatic CD8 alpha+ lymphoid-related dendritic cells. J Immunol. 2000;165(2):795–803. doi: 10.4049/jimmunol.165.2.795. [DOI] [PubMed] [Google Scholar]
- 140.Pillarisetty VG, Katz SC, Bleier JI, Shah AB, Dematteo RP. Natural killer dendritic cells have both antigen presenting and lytic function and in response to CpG produce IFN-gamma via autocrine IL-12. J Immunol. 2005;174(5):2612–2618. doi: 10.4049/jimmunol.174.5.2612. [DOI] [PubMed] [Google Scholar]
- 141.Abe M, Tokita D, Raimondi G, Thomson AW. Endotoxin modulates the capacity of CpG-activated liver myeloid DC to direct Th1-type responses. Eur J Immunol. 2006;36(9):2483–2493. doi: 10.1002/eji.200535767. [DOI] [PubMed] [Google Scholar]
- 142.Averill L, Lee WM, Karandikar NJ. Differential dysfunction in dendritic cell subsets during chronic HCV infection. Clin Immunol. 2007;123(1):40–49. doi: 10.1016/j.clim.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.De Creus A, Abe M, Lau AH, Hackstein H, Raimondi G, Thomson AW. Low TLR4 expression by liver dendritic cells correlates with reduced capacity to activate allogeneic T cells in response to endotoxin. J Immunol. 2005;174(4):2037–2045. doi: 10.4049/jimmunol.174.4.2037. [DOI] [PubMed] [Google Scholar]
- 144.Callery MP, Mangino MJ, Flye MW. Arginine-specific suppression of mixed lymphocyte culture reactivity by Kupffer cells--a basis of portal venous tolerance. Transplantation. 1991;51(5):1076–1080. doi: 10.1097/00007890-199105000-00028. [DOI] [PubMed] [Google Scholar]
- 145.Terpstra V, van Berkel TJ. Scavenger receptors on liver Kupffer cells mediate the in vivo uptake of oxidatively damaged red blood cells in mice. Blood. 2000;95(6):2157–2163. [PubMed] [Google Scholar]
- 146.Shi J, Gilbert GE, Kokubo Y, Ohashi T. Role of the liver in regulating numbers of circulating neutrophils. Blood. 2001;98(4):1226–1230. doi: 10.1182/blood.v98.4.1226. [DOI] [PubMed] [Google Scholar]
- 147.Crispe IN, Dao T, Klugewitz K, Mehal WZ, Metz DP. The liver as a site of T-cell apoptosis: graveyard, or killing field? Immunol Rev. 2000;174:47–62. doi: 10.1034/j.1600-0528.2002.017412.x. [DOI] [PubMed] [Google Scholar]
- 148.John B, Crispe IN. Passive and active mechanisms trap activated CD8+ T cells in the liver. J Immunol. 2004;172(9):5222–5229. doi: 10.4049/jimmunol.172.9.5222. [DOI] [PubMed] [Google Scholar]
- 149.Wang Y, Gao Y, Yuan X, Xia W, Luo Y, Sun E, Chen ZK. The liver mediates apoptotic cell-induced immune regulation. Scand J Immunol. 2008;68(3):297–305. doi: 10.1111/j.1365-3083.2008.02141.x. [DOI] [PubMed] [Google Scholar]
- 150.Zhang M, Xu S, Han Y, Cao X. Apoptotic cells attenuate fulminant hepatitis by priming Kupffer cells to produce interleukin-10 through membrane-bound TGF-beta. Hepatology. 2011;53(1):306–316. doi: 10.1002/hep.24029. [DOI] [PubMed] [Google Scholar]
- 151.Forouhar F, Anderson JL, Mowat CG, Vorobiev SM, Hussain A, Abashidze M, Bruckmann C, Thackray SJ, Seetharaman J, Tucker T, Xiao R, Ma LC, Zhao L, Acton TB, Montelione GT, Chapman SK, Tong L. Molecular insights into substrate recognition and catalysis by tryptophan 2,3-dioxygenase. Proc Natl Acad Sci U S A. 2007;104(2):473–478. doi: 10.1073/pnas.0610007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, Jugold M, Guillemin GJ, Miller CL, Lutz C, Radlwimmer B, Lehmann I, von Deimling A, Wick W, Platten M. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478(7368):197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 153.Pilotte L, Larrieu P, Stroobant V, Colau D, Dolusic E, Frederick R, De Plaen E, Uyttenhove C, Wouters J, Masereel B, Van den Eynde BJ. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc Natl Acad Sci U S A. 2012;109(7):2497–2502. doi: 10.1073/pnas.1113873109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sumpter TL, Dangi A, Matta BM, Huang C, Stolz DB, Vodovotz Y, Thomson AW, Gandhi CR. Hepatic Stellate Cells Undermine the Allostimulatory Function of Liver Myeloid Dendritic Cells via STAT3-Dependent Induction of IDO. J Immunol. 2012;189(8):3848–3858. doi: 10.4049/jimmunol.1200819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yang HR, Chou HS, Gu X, Wang L, Brown KE, Fung JJ, Lu L, Qian S. Mechanistic insights into immunomodulation by hepatic stellate cells in mice: a critical role of interferon-gamma signaling. Hepatology. 2009;50(6):1981–1991. doi: 10.1002/hep.23202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dangi A, Sumpter TL, Kimura S, Stolz DB, Murase N, Raimondi G, Vodovotz Y, Huang C, Thomson AW, Gandhi CR. Selective expansion of allogeneic regulatory T cells by hepatic stellate cells: role of endotoxin and implications for allograft tolerance. J Immunol. 2012;188(8):3667–3677. doi: 10.4049/jimmunol.1102460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Miller SD, Wetzig RP, Claman HN. The induction of cell-mediated immunity and tolerance with protein antigens coupled to syngeneic lymphoid cells. J Exp Med. 1979;149(3):758–773. doi: 10.1084/jem.149.3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Luo X, Pothoven KL, McCarthy D, DeGutes M, Martin A, Getts DR, Xia G, He J, Zhang X, Kaufman DB, Miller SD. ECDI-fixed allogeneic splenocytes induce donor-specific tolerance for long-term survival of islet transplants via two distinct mechanisms. Proc Natl Acad Sci U S A. 2008;105(38):14527–14532. doi: 10.1073/pnas.0805204105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Turley DM, Miller SD. Peripheral tolerance induction using ethylenecarbodiimide-fixed APCs uses both direct and indirect mechanisms of antigen presentation for prevention of experimental autoimmune encephalomyelitis. J Immunol. 2007;178(4):2212–2220. doi: 10.4049/jimmunol.178.4.2212. [DOI] [PubMed] [Google Scholar]
- 160.Getts DR, Turley DM, Smith CE, Harp CT, McCarthy D, Feeney EM, Getts MT, Martin AJ, Luo X, Terry RL, King NJ, Miller SD. Tolerance induced by apoptotic antigen-coupled leukocytes is induced by PD-L1+ and IL-10-producing splenic macrophages and maintained by T regulatory cells. J Immunol. 2011;187(5):2405–2417. doi: 10.4049/jimmunol.1004175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Song PS, Tapley KJ., Jr Photochemistry and photobiology of psoralens. Photochem Photobiol. 1979;29(6):1177–1197. doi: 10.1111/j.1751-1097.1979.tb07838.x. [DOI] [PubMed] [Google Scholar]
- 162.Edelson R, Berger C, Gasparro F, Jegasothy B, Heald P, Wintroub B, Vonderheid E, Knobler R, Wolff K, Plewig G, et al. Treatment of cutaneous T-cell lymphoma by extracorporeal photochemotherapy. Preliminary results. N Engl J Med. 1987;316(6):297–303. doi: 10.1056/NEJM198702053160603. [DOI] [PubMed] [Google Scholar]
- 163.Peritt D. Potential mechanisms of photopheresis in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006;12(1 Suppl 2):7–12. doi: 10.1016/j.bbmt.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 164.Sanford KW, Balogun RA. Extracorporeal photopheresis: clinical use so far. J Clin Apher. 2012;27(3):126–131. doi: 10.1002/jca.21217. [DOI] [PubMed] [Google Scholar]
- 165.Scarisbrick JJ, Taylor P, Holtick U, Makar Y, Douglas K, Berlin G, Juvonen E, Marshall S. U.K. consensus statement on the use of extracorporeal photopheresis for treatment of cutaneous T-cell lymphoma and chronic graft-versus-host disease. Br J Dermatol. 2008;158(4):659–678. doi: 10.1111/j.1365-2133.2007.08415.x. [DOI] [PubMed] [Google Scholar]
- 166.Jonson CO, Pihl M, Nyholm C, Cilio CM, Ludvigsson J, Faresjo M. Regulatory T cell-associated activity in photopheresis-induced immune tolerance in recent onset type 1 diabetes children. Clin Exp Immunol. 2008;153(2):174–181. doi: 10.1111/j.1365-2249.2008.03625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Reinisch W, Nahavandi H, Santella R, Zhang Y, Gasche C, Moser G, Waldhor T, Gangl A, Vogelsang H, Knobler R. Extracorporeal photochemotherapy in patients with steroid-dependent Crohn’s disease: a prospective pilot study. Aliment Pharmacol Ther. 2001;15(9):1313–1322. doi: 10.1046/j.1365-2036.2001.01054.x. [DOI] [PubMed] [Google Scholar]
- 168.Knobler RM, French LE, Kim Y, Bisaccia E, Graninger W, Nahavandi H, Strobl FJ, Keystone E, Mehlmauer M, Rook AH, Braverman I. A randomized, double-blind, placebo-controlled trial of photopheresis in systemic sclerosis. J Am Acad Dermatol. 2006;54(5):793–799. doi: 10.1016/j.jaad.2005.11.1091. [DOI] [PubMed] [Google Scholar]
- 169.Mathur K, Morris S, Deighan C, Green R, Douglas KW. Extracorporeal photopheresis improves nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis: three case reports and review of literature. J Clin Apher. 2008;23(4):144–150. doi: 10.1002/jca.20170. [DOI] [PubMed] [Google Scholar]
- 170.Ward DM. Extracorporeal photopheresis: how, when, and why. J Clin Apher. 2011;26(5):276–285. doi: 10.1002/jca.20300. [DOI] [PubMed] [Google Scholar]
- 171.Gatza E, Rogers CE, Clouthier SG, Lowler KP, Tawara I, Liu C, Reddy P, Ferrara JL. Extracorporeal photopheresis reverses experimental graft-versus-host disease through regulatory T cells. Blood. 2008;112(4):1515–1521. doi: 10.1182/blood-2007-11-125542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Maeda A, Schwarz A, Bullinger A, Morita A, Peritt D, Schwarz T. Experimental extracorporeal photopheresis inhibits the sensitization and effector phases of contact hypersensitivity via two mechanisms: generation of IL-10 and induction of regulatory T cells. J Immunol. 2008;181(9):5956–5962. doi: 10.4049/jimmunol.181.9.5956. [DOI] [PubMed] [Google Scholar]
- 173.Rao V, Saunes M, Jorstad S, Moen T. Cutaneous T cell lymphoma and graft-versus-host disease: a comparison of in vivo effects of extracorporeal photochemotherapy on Foxp3+ regulatory T cells. Clin Immunol. 2009;133(3):303–313. doi: 10.1016/j.clim.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 174.Gjerdrum LM, Woetmann A, Odum N, Burton CM, Rossen K, Skovgaard GL, Ryder LP, Ralfkiaer E. FOXP3+ regulatory T cells in cutaneous T-cell lymphomas: association with disease stage and survival. Leukemia. 2007;21(12):2512–2518. doi: 10.1038/sj.leu.2404913. [DOI] [PubMed] [Google Scholar]
- 175.Huang L, Lemos HP, Li L, Li M, Chandler PR, Baban B, McGaha TL, Ravishankar B, Lee JR, Munn DH, Mellor AL. Engineering DNA nanoparticles as immunomodulatory reagents that activate regulatory T cells. J Immunol. 2012;188(10):4913–4920. doi: 10.4049/jimmunol.1103668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhong D, Jiao Y, Zhang Y, Zhang W, Li N, Zuo Q, Wang Q, Xue W, Liu Z. Effects of the gene carrier polyethyleneimines on structure and function of blood components. Biomaterials. 2012 doi: 10.1016/j.biomaterials.2012.09.060. [DOI] [PubMed] [Google Scholar]
- 177.Nimesh S. Polyethylenimine as a promising vector for targeted siRNA delivery. Curr Clin Pharmacol. 2012;7(2):121–130. doi: 10.2174/157488412800228857. [DOI] [PubMed] [Google Scholar]
- 178.Tiera MJ, Shi Q, Winnik FM, Fernandes JC. Polycation-based gene therapy: current knowledge and new perspectives. Curr Gene Ther. 2011;11(4):288–306. doi: 10.2174/156652311796150408. [DOI] [PubMed] [Google Scholar]
- 179.Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9(7):917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 180.Combadiere C, Potteaux S, Gao JL, Esposito B, Casanova S, Lee EJ, Debre P, Tedgui A, Murphy PM, Mallat Z. Decreased atherosclerotic lesion formation in CX3CR1/apolipoprotein E double knockout mice. Circulation. 2003;107(7):1009–1016. doi: 10.1161/01.cir.0000057548.68243.42. [DOI] [PubMed] [Google Scholar]
- 181.Shao WH, Zhen Y, Rosenbaum J, Eisenberg RA, McGaha TL, Birkenbach M, Cohen PL. A protective role of Mer receptor tyrosine kinase in nephrotoxic serum-induced nephritis. Clin Immunol. 2010;136(2):236–244. doi: 10.1016/j.clim.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Thorp E, Cui D, Schrijvers DM, Kuriakose G, Tabas I. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe−/− mice. Arterioscler Thromb Vasc Biol. 2008;28(8):1421–1428. doi: 10.1161/ATVBAHA.108.167197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nandrot EF, Anand M, Almeida D, Atabai K, Sheppard D, Finnemann SC. Essential role for MFG-E8 as ligand for alphavbeta5 integrin in diurnal retinal phagocytosis. Proc Natl Acad Sci U S A. 2007;104(29):12005–12010. doi: 10.1073/pnas.0704756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Fuller AD, Van Eldik LJ. MFG-E8 regulates microglial phagocytosis of apoptotic neurons. J Neuroimmune Pharmacol. 2008;3(4):246–256. doi: 10.1007/s11481-008-9118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ekman C, Jonsen A, Sturfelt G, Bengtsson AA, Dahlback B. Plasma concentrations of Gas6 and sAxl correlate with disease activity in systemic lupus erythematosus. Rheumatology (Oxford) 2011;50(6):1064–1069. doi: 10.1093/rheumatology/keq459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Botto M, Dell’Agnola C, Bygrave AE, Thompson EM, Cook HT, Petry F, Loos M, Pandolfi PP, Walport MJ. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19(1):56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 187.Shimazaki A, Kawamura Y, Kanazawa A, Sekine A, Saito S, Tsunoda T, Koya D, Babazono T, Tanaka Y, Matsuda M, Kawai K, Iiizumi T, Imanishi M, Shinosaki T, Yanagimoto T, Ikeda M, Omachi S, Kashiwagi A, Kaku K, Iwamoto Y, Kawamori R, Kikkawa R, Nakajima M, Nakamura Y, Maeda S. Genetic variations in the gene encoding ELMO1 are associated with susceptibility to diabetic nephropathy. Diabetes. 2005;54(4):1171–1178. doi: 10.2337/diabetes.54.4.1171. [DOI] [PubMed] [Google Scholar]
- 188.NAG, Bensinger SJ, Hong C, Beceiro S, Bradley MN, Zelcer N, Deniz J, Ramirez C, Diaz M, Gallardo G, de Galarreta CR, Salazar J, Lopez F, Edwards P, Parks J, Andujar M, Tontonoz P, Castrillo A. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31(2):245–258. doi: 10.1016/j.immuni.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mukundan L, Odegaard JI, Morel CR, Heredia JE, Mwangi JW, Ricardo-Gonzalez RR, Goh YP, Eagle AR, Dunn SE, Awakuni JU, Nguyen KD, Steinman L, Michie SA, Chawla A. PPAR-delta senses and orchestrates clearance of apoptotic cells to promote tolerance. Nat Med. 2009;15(11):1266–1272. doi: 10.1038/nm.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]