Abstract
Objectives
We assessed intergenerational transmission of smoking in mother-child dyads.
Methods
We identified classes of youth smoking trajectories using mixture latent trajectory analyses with data from the Children and Young Adults of the National Longitudinal Survey of Youth (n=6349). We regressed class membership on prenatal and postnatal exposure to maternal smoking, including social and behavioral variables, to control for selection.
Results
Youth smoking trajectories entailed early-onset persistent smoking, early-onset experimental discontinued smoking, late-onset persistent smoking, and nonsmoking. The likelihood of early onset versus late onset and early onset versus nonsmoking were significantly higher among youths exposed prenatally and postnatally versus either postnatally alone or unexposed. Controlling for selection, the increased likelihood of early onset versus nonsmoking remained significant for each exposure group versus unexposed, as did early onset versus late onset and late onset versus nonsmoking for youths exposed prenatally and postnatally versus unexposed. Experimental smoking was notable among youths whose mothers smoked but quit before the child’s birth.
Conclusions
Both physiological and social role-modeling mechanisms of intergenerational transmission are evident. Prioritization of tobacco control for pregnant women, mothers, and youths remains a critical, interrelated objective.
Women who smoke during pregnancy are more likely to have offspring who become adolescent smokers.1–7 Studies link mother’s smoking during pregnancy with youths’ earlier smoking initiation,3,7–9 greater persistence in regular smoking,3,7 and stronger nicotine dependency.6,8,10,11
Hypothesized physiological pathways for mother-to-child transmission of smoking are reviewed elsewhere12–14 and may include inherited susceptibility to addiction alone or in combination with in utero neurodevelopmental exposure and scarring that activates nicotine susceptibility. Furthermore, because few women who smoke during pregnancy quit after delivery15,16 higher rates of smoking among offspring may reflect role modeling of maternal smoking behavior. Notably, parental smoking is hypothesized to demonstrate pro-smoking norms and solidify pro-smoking attitudes.17,18
Studies considering both smoking during pregnancy and subsequent maternal smoking outcomes have sought to distinguish between these proposed social and physiological transmission pathways.1–4,6,7,9,19 Similarly, studies controlling for family sociodemographic factors1,2,4,5,7,8,10,11,19,20 or maternal propensity for health or risk taking1,2,9,10 have sought to further distinguish direct physiological or social transmission from selection. Studies considering children’s cognitive and behavioral outcomes have shown that selection by maternal social and behavioral precursors to smoking during pregnancy strongly biases findings on smoking during pregnancy21,22; however, it remains unclear whether this is also the case for youth smoking. Some studies2,3,5,6,19 have observed that smoking during pregnancy operates independently of subsequent maternal smoking. A few have found that smoking during pregnancy is only independently associated in select analyses (e.g., for initiation but not frequency or number of cigarettes6,9 or only among females7,20). Several have found that smoking during pregnancy does not operate independently of subsequent maternal smoking behavior,1,4 and the remaining studies do not address postnatal maternal smoking.8,9,11
We explored whether these inconsistencies in findings supporting social or physiological mechanisms for intergenerational transmission can be accounted for by more comprehensively examining maternal and child smoking behavior. Previous work has established the advantages of statistical models for youth smoking trajectories that capture initiation, experimentation, cessation, or continued use.23–28 Studies focusing on parental smoking concurrent with youth smoking suggest that postnatal exposures may differentially predispose youths for specific smoking trajectories.24,26–28 Only 3 known studies have considered whether smoking during pregnancy influences youth smoking progression, and these have shown greater likelihood of early regular use3,11 and telescoping to dependence.8 However, limitations of sample selectivity and measurement and modeling of maternal and youth smoking outcomes restrict the generalizability and scope of these findings.29 To specifically address these limitations and more comprehensively assess hypothesized intergenerational transmission pathways, we used US population–representative data, latent variable techniques, and a rich set of data on maternal and youth smoking and social and behavioral selection factors. We characterized trajectories of youth smoking from adolescence through young adulthood and considered exposure to various maternal smoking patterns from prebirth to the child’s early adolescence.
METHODS
We study intergenerational transmission of smoking using data from a large population-representative survey of US mother-child dyads and by employing mixture latent trajectory analyses and multinomial logistic regression models.
Data
Data were from the Children and Young Adults of the National Longitudinal Survey of Youth 1979 cohort (NLSY79-CYA), a public use panel survey of all offspring of women in a population-representative cohort (NLSY79) commissioned by the US Bureau of Labor Statistics.30 The NLSY79-CYA used a biennial, cohort-sequential design in which all children born to NLSY79 women by 1986 as well as all subsequent children born after 1986 have been followed. The NLSY79-CYA thus includes multiple birth cohorts and children per mother. We selected respondents aged 14 to 25 years observed at any of the biennial surveys between 1994 and 2006 (i.e., birth cohorts 1970–1992). The NLSY79-CYA yearly completion rates range from 83.0% to 88.4%.31 By 2006, 6643 youths aged 14 years and older were eligible for the NLSY79-CYA and had been located for at least1 interview between 1994 and 2006. From this sample, 6349 youths responded to questions about cigarette smoking at least once.
The youth smoking trajectory is characterized by using latent class analysis with a set of dichotomous reports of cigarette smoking in the past 30 days constructed for each respondent from biennial survey assessments via computer-assisted personal interviewing. Because the set of smoking in the past 30 days for each respondent is truncated (with the earliest possible report at 14 years), we validated trajectories using a retrospective assessment of the respondent’s report of the age they first smoked cigarettes and most recent report of ever smoked cigarettes from the biennial computer-assisted personal interview.
We constructed several variables to describe maternal smoking patterns before, during, and after the pregnancy and birth of the respondent. We created mother ever smoked daily as a dichotomous indicator for any maternal report of daily smoking in the NLSY79 substance use history supplements taken in 1992, 1994, and 1998. We created mother smoked during pregnancy as a categorical indicator for mother’s reported cigarette consumption (did not smoke, < 1 pack/day, or a combination of 1–2 packs/day and 2 or more packs/day) from the NLSY79 birth history taken within 1 year of birth for this study’s sample. Because of notable item nonresponse (n=1792), we used an identical retrospective question in the 2004 NLSY79-CYA to confirm reliability across the 2 assessments and to fill nonresponses. We created mother ever smoked daily by smoked during pregnancy to distinguish between respondents whose mother never smoked daily or during pregnancy, smoked daily but not during pregnancy, smoked daily and smoked during pregnancy less than 1 pack per day, and smoked daily and smoked during pregnancy 1 or more packs per day. We excluded from the sample respondents whose mother never smoked daily but smoked less than 1 pack per day during pregnancy (n=32). We created mother’s smoking history to distinguish the full pattern of prepregnancy, prenatal, and postnatal exposures. This variable extends the previous composite variable by addressing the timing of initiation and cessation of daily smoking (reported and updated in the 3 NLSY79 substance use supplements) in relationship to the youth’s date of birth. The 6 exposure categories are depicted in Figure 1: never smoked daily or during pregnancy (45.2%); quit daily before birth of child and did not smoke during pregnancy (7.4%); did not smoke during pregnancy but relapsed to daily smoking (10.0%); did not smoke during pregnancy, relapsed, but then quit daily smoking (6.6%); smoked any cigarettes during pregnancy and smoked daily but quit after birth (6.7%); and smoked any cigarettes during pregnancy and smoked daily after birth (24.2%).
FIGURE 1. Distribution of prepregnancy, prenatal, and postnatal maternal smoking patterns experienced by US youths: NLSY79-CYA, 1994–2006.
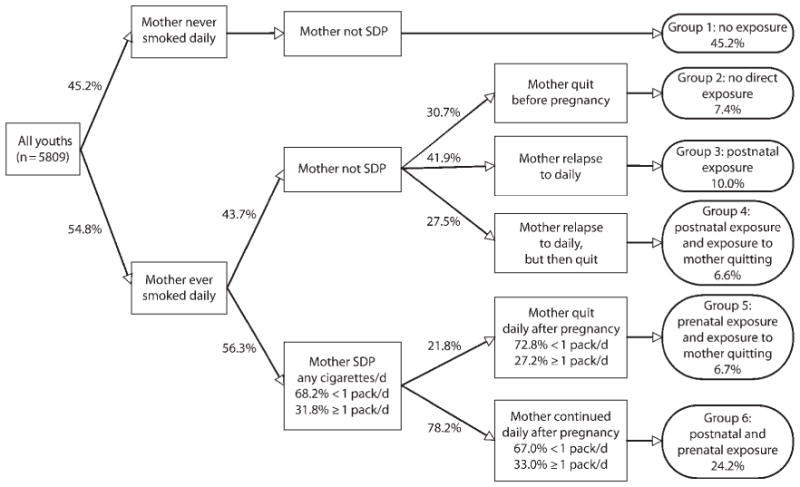
Note. SDP = smoked during pregnancy. Data on prenatal and postnatal exposure to maternal smoking is appended to the NLSY79-CYA study sample using reports to a collection of cigarette consumption questions answered by the mothers in the NLSY79 birth history and smoking supplements 1979–2006. Weighted proportions adjust for clustering within families. The sample entails all youths responding to questions about cigarette smoking when aged 14–25 y during the period 1994–2006 in the NLSY-CYA (n = 6349), excluding respondents with missing data on mother’s daily smoking or smoking during pregnancy (n = 508) and respondents whose mother’s smoked during pregnancy any cigarettes/d but never smoked daily (n = 32).
The variables we used to control for sample design and selection into maternal smoking exposure groups entail youth sociodemographics (age at baseline, age at first smoking assessment, gender, and race/ethnicity), maternal sociodemographics (age at child’s birth, and educational attainment and marital status when the child was aged 14 years), and maternal proclivity to health or risk behavior (breastfed, prenatal care, and a score of the mother’s endorsement in 1980 of 12 adolescent delinquency behaviors from the NLSY79-modified Self-Reported Delinquency Interview30,32). Summary statistics and item nonresponse are available in Appendix A (available as a supplement to the online version of this article at http://www.ajph.org).
Statistical Procedures
We first characterized youth smoking trajectories for youths aged 14 to 25 years using mixture latent trajectory analysis (LTA)33 to identify those with similar age patterns of smoking in the past 30 days. LTA uses statistical evidence rather than a priori assumptions to characterize trajectories.33,34 By using the multiple assessments of smoking in the past 30 days over the biennial surveys (1994–2006) to describe the smoking trajectory rather than 1 or more retrospective measures of a respondent’s smoking history, our analysis also had the advantages of a time-sampling approach with reduced measurement error for the trajectory of smoking entries and exits.35
Our LTA statistical model entailed parameters for class membership probabilities and class-specific variable endorsement probabilities for smoking at each age and thus did not impose any functional form on the age trajectory of smoking in the past 30 days within each of the smoking trajectory classes. Consequently, the model accommodated dynamic age patterns of entry, exit, and even relapse in smoking in the past 30 days. We used full information maximum likelihood estimation (which estimates model parameters in the presence of missing data36) to appropriately model the biennial and cohort structure of the sample smoking in the past 30 days response patterns whereby 35% of the sample was surveyed more than 4 times, 23% 3 times, 23% twice, and 19% once (n=6349). We assessed model fit using the Akaike information criterion and sample size–adjusted Bayes information criterion.37 We estimated respondents’ posterior probability of membership for each smoking trajectory class and assigned them the smoking trajectory class with the highest posterior probability.33
We then considered the relationship between youth smoking trajectories and maternal smoking patterns. The sample entails no missing data on the maternal smoking pattern or variables used to control for selection (n=5027). We first assessed the bivariate relationship between maternal and youth smoking using descriptive statistics and unadjusted multinomial logistic regression models (Table 1). Then, we reported the adjusted odds ratios (AORs) for how prenatal and postnatal exposure to maternal smoking patterns influenced the odds of each youth smoking trajectory, adjusting for the variables we used to control for selection (Table 2). We tested gender differences in the relationship between youth and maternal smoking reported in Tables 1 and 2 using interaction terms. We found no statistically significant gender differences (findings not shown).
TABLE 1.
Distribution and Respective Odds of US Youth Smoking Trajectories by Prenatal and Postnatal Exposure to Maternal Smoking: NLSY79-CYA, 1994–2006
| Characteristics | Youth Smoking Trajectory-Weighted Frequencies
|
Smoking Trajectories UOR (95% CI)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Early Onset, % (No.) | Early Experiment, % (No.) | Late Onset, % (No.) | Nonsmoker, % (No.) | Total, % (No.) | Early Onset vs Nonsmoker, UOR (95% CI) | Early Experiment vs Nonsmoker, UOR (95% CI) | Late Onset vs Nonsmoker, UOR (95% CI) | Early Onset vs Late Onset, UOR (95% CI) | Early Experiment vs Late Onset, UOR (95% CI) | |
| Total sample | 14.7 (633) | 2.7 (127) | 18.8 (958) | 63.7 (3309) | 100.0 (5027) | |||||
| Mother ever smoked | ||||||||||
| Never daily (Ref) | 8.7 (202) | 1.8 (48) | 15.1 (385) | 74.4 (1854) | 100.0 (2489) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Ever daily | 19.7 (431) | 3.5 (79) | 21.9 (573) | 54.9 (14 155) | 100.0 (2538) | 3.06*** (2.42, 3.87) | 2.66*** (1.66, 4.25) | 1.96*** (1.62, 2.38) | 1.56** (1.20, 2.02) | 1.35 (0.84, 2.13) |
| Mother smoked during pregnancy | ||||||||||
| Never (Ref) | 11.1 (366) | 2.4 (83) | 17.1 (650) | 69.5 (2572) | 100.0 (3671) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Any cigarettes/d | 23.0 (267) | 3.6 (44) | 22.8 (308) | 50.6 (737) | 100.0 (1356) | 2.85*** (2.27, 3.57) | 2.09** (1.32, 3.31) | 1.83*** (1.50, 2.23) | 1.56** (1.21, 2.00) | 1.14 (0.72, 1.82) |
| < 1 pack/d | 21.1 (175) | 3.8 (33) | 22.7 (223) | 52.5 (556) | 100.0 (987) | 2.52*** (1.96, 3.24) | 2.11** (1.28, 3.49) | 1.75*** (1.41, 2.19) | 1.44* (1.08, 1.91) | 1.20 (0.73, 1.00) |
| ≥1 pack/d | 27.2 (92) | 3.2 (11) | 23.1 (85) | 46.5 (181) | 100.0 (369) | 3.66*** (2.64, 5.07) | 2.02 (0.90, 4.52) | 2.02*** (1.44, 2.83) | 1.81** (1.23, 2.67) | 1.00 (0.44, 2.30) |
| Mother ever smoked by smoked during pregnancy | ||||||||||
| Never daily | 8.7 (202) | 1.8 (48) | 15.1 (385) | 74.4 (1854) | 100.0 (2489) | 0.46*** (0.34, 0.61) | 0.42** (0.24, 0.74) | 0.59*** (0.47, 0.74) | 0.77 (0.56, 1.06) | 0.72 (0.41, 1.27) |
| Ever daily, but did not smoke during pregnancy (Ref) | 15.5 (164) | 3.4 (35) | 20.8 (265) | 60.2 (718) | 100.0 (1182) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Ever daily and smoked during pregnancy any cigarettes/d | 23.0 (267) | 3.6 (44) | 22.8 (308) | 50.6 (737) | 100.0 (1356) | 1.76*** (1.33, 2.32) | 1.25 (0.72, 2.15) | 1.30* (1.03, 1.65) | 1.35 (0.99, 1.84) | 0.96 (0.55, 1.66) |
| Mother ever smoked by smoked during pregnancy | ||||||||||
| Ever daily and smoked during pregnancy < 1 pack/d | 21.1 (175) | 3.8 (33) | 22.7 (223) | 52.5 (556) | 100.0 (987) | 1.59** (1.16, 2.10) | 1.26 (0.71, 2.25) | 1.25 (0.97, 1.61) | 1.25 (0.89, 1.74) | 1.01 (0.56, 1.82) |
| Ever daily and smoked during pregnancy ≥1 packs/d | 27.2 (92) | 3.2 (11) | 23.1 (85) | 46.5 (181) | 100.0 (369) | 2.26*** (1.57, 3.26) | 1.21 (0.51, 2.84) | 1.44* (1.00, 2.06) | 1.58* (1.03, 2.41) | 0.84 (0.35, 2.03) |
| Mother’s smoking history | ||||||||||
| Group 1: never daily (Ref) | 8.7 (202) | 1.8 (48) | 15.1 (385) | 74.4 (1854) | 100.0 (2489) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Group 2: quit daily before pregnancy | 12.0 (33) | 3.3 (9) | 10.6 (37) | 74.2 (224) | 100.0 (303) | 1.37 (0.85, 2.22) | 1.83 (0.73, 4.57) | 0.70 (0.45, 1.00) | 1.96* (1.11, 3.48) | 2.62* (1.04, 6.56) |
| Group 3: did not smoke during pregnancy, then relapse to daily | 18.3 (83) | 4.5 (17) | 24.2 (133) | 53.1 (284) | 100.0 (517) | 2.92*** (2.05, 4.17) | 3.49*** (1.80, 6.77) | 2.24*** (1.60, 2.93) | 1.31 (0.88, 1.94) | 1.56 (0.70, 3.07) |
| Group 4: did not smoke during pregnancy, relapsed, then quit daily | 15.5 (48) | 2.1 (9) | 27.0 (95) | 55.4 (210) | 100.0 (362) | 2.38*** (1.49, 3.80) | 1.55 (0.60, 3.97) | 2.40*** (1.72, 3.34) | 0.99 (0.61, 1.61) | 0.65 (0.24, 1.71) |
| Group 5: smoked during pregnancy any cigarettes/d, quit daily after pregnancy | 17.6 (42) | 3.9 (8) | 26.2 (70) | 52.3 (174) | 100.0 (294) | 2.87*** (1.78, 4.61) | 3.07* (1.22, 7.71) | 2.47*** (1.71, 3.56) | 1.16 (0.69, 1.97) | 1.25 (0.51, 3.03) |
| Group 6: smoked during pregnancy any cigarettes/d, continued daily after pregnancy | 24.5 (225) | 3.5 (36) | 21.8 (238) | 50.2 (563) | 100.0 (1062) | 4.16*** (3.18, 5.45) | 2.90*** (1.66, 5.06) | 2.14*** (1.68, 2.72) | 1.95*** (1.44, 2.63) | 1.36 (0.77, 2.30) |
Note. CI = confidence interval; NLSY79-CYA = Children and Young Adults of the National Longitudinal Survey of Youth 1979; UOR = unadjusted odds ratio. Youth smoking trajectories were characterized as early-onset, early-experiment, late-onset smoker or nonsmoker on the basis of a latent trajectory analysis fit to data on repeated assessments of smoking in the past 30 d by respondents aged 14–25 y observed between 1994–2006 in the NLSY79-CYA. UORs of smoking trajectories relative to the early-experiment smoking trajectory group are not shown because statistical inference is limited by sample power for this group. Frequencies 95% CIs, and tests of statistical significance have been adjusted for survey weights and clustering within families.
P < .05;
P < .01;
P < .001.
TABLE 2.
Relationship Between Youth Smoking Trajectories and Prenatal and Postnatal Exposure to Maternal Smoking, Adjusting for Social and Behavioral Selection Factors: NLSY79-CYA, 1994–2006
| Characteristics | Smoking Trajectories
|
||||
|---|---|---|---|---|---|
| Early Onset vs Nonsmoker, AOR (95% CI) | Early Experiment vs Nonsmoker, AOR (95% CI) | Late Onset vs Nonsmoker, AOR (95% CI) | Early Onset vs Late Onset, AOR (95% CI) | Experiment vs Late Onset, AOR (95% CI) | |
| Mother’s smoking history | |||||
| Group 1: never smoked daily (Ref) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Group 2: quit daily before pregnancy | 1.62 (0.93, 2.66) | 3.01* (1.21, 7.50) | 1.06 (0.67, 1.68) | 1.53 (0.85, 2.74) | 2.84* (1.11, 7.30) |
| Group 3: did not smoke during pregnancy, then relapsed to daily | 2.07*** (1.44, 2.99) | 2.46* (1.23, 4.91) | 1.66** (1.23, 2.25) | 1.24 (0.83, 1.86) | 1.48 (0.72, 3.02) |
| Group 4: did not smoke during pregnancy, relapsed, then quit daily | 1.86* (1.14, 3.03) | 1.08 (0.40, 2.88) | 1.83** (1.27, 2.62) | 1.02 (0.61, 1.70) | 0.59 (0.22, 1.61) |
| Group 5: smoked during pregnancy any cigarettes/d, quit daily after pregnancy | 2.12** (1.29, 3.48) | 2.45 (0.93, 6.43) | 1.97** (1.31, 2.95) | 1.08 (0.62, 1.87) | 1.25 (0.50, 3.13) |
| Group 6: smoked during pregnancy any cigarettes/d, continued daily after pregnancy | 2.75*** (2.03, 3.73) | 2.12* (1.14, 3.94) | 1.66*** (1.28, 2.16) | 1.65** (1.18, 2.31) | 1.28 (0.68, 2.38) |
| Youth sociodemographics | |||||
| Age at baseline | 0.82*** (0.74, 0.92) | 0.86 (0.73, 1.023) | 0.95 (0.87, 1.03) | 0.87* (0.78, 0.97) | 0.91 (0.77, 1.08) |
| Gender | |||||
| Female (Ref) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Male | 1.21 (0.98, 1.50) | 0.56** (0.37, 0.87) | 1.45*** (1.21, 1.73) | 0.84 (0.15, 0.66) | 0.39*** (0.25, 0.61) |
| Race/ethnicity | |||||
| Non-Hispanic White (Ref) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Hispanic | 0.66** (0.49, 0.89) | 0.93 (0.52, 1.68) | 0.88 (0.68, 1.14) | 0.75 (0.55, 1.03) | 1.06 (0.58, 1.94) |
| Black | 0.23*** (0.17, 0.31) | 0.19*** (0.10, 0.36) | 0.57*** (0.45, 0.73) | 0.40*** (0.30, 0.55) | 0.33** (0.17, 0.63) |
| Maternal sociodemographics | |||||
| Age at birth | 0.86*** (0.83, 0.88) | 0.75*** (0.70, 0.81) | 0.83*** (0.81, 0.86) | 1.03 (0.10, 1.07) | 0.90** (0.84, 0.97) |
| Education when child was aged 14 y | |||||
| High school (Ref) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| < high school | 1.21 (0.88, 1.66) | 1.00 (0.52, 1.91) | 0.91 (0.68, 1.22) | 1.33 (0.96, 1.84) | 1.10 (0.58, 2.09) |
| > high school | 0.92 (0.70, 1.19) | 0.90 (0.51, 1.57) | 0.80 (0.64, 0.10) | 1.14 (0.85, 1.54) | 1.12 (0.64, 1.96) |
| Marital status when child was aged 14 y | |||||
| Married with father in household (Ref) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Never married | 1.65** (1.15, 2.36) | 1.36 (0.55, 3.38) | 1.45* (1.05, 2.01) | 1.14 (0.54, 1.70) | 0.94 (0.37, 2.38) |
| Formerly married | 1.48** (1.15, 1.91) | 1.16 (0.70, 1.92) | 1.31* (1.04, 1.64) | 1.14 (0.86, 1.49) | 0.89 (0.54, 1.48) |
| Maternal health/risk behavior | |||||
| Breastfed child | |||||
| No (Ref) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 0.79 (0.62, 1.02) | 1.15 (0.56, 1.85) | 1.00 (0.81, 1.24) | 0.79 (0.60, 1.04) | 1.15 (0.71, 1.86) |
| Obtained prenatal care | |||||
| No (Ref) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 0.69 (0.24, 1.94) | 0.33 (0.09, 1.24) | 0.57 (0.26, 1.25) | 1.20 (0.39, 3.65) | 0.57 (0.14, 2.38) |
| Adolescent delinquency behavior score | 1.10* (1.02, 1.18) | 0.88 (0.14, 1.04) | 0.95 (0.89, 1.02) | 1.15*** (1.06, 1.24) | 0.92 (0.78, 1.10) |
Note. AOR = adjusted odds ratio; CI = confidence interval; NLSY79-CYA = Children and Young Adults of the National Longitudinal Survey of Youth 1979. Youth smoking trajectories are characterized as early onset, early experiment, late onset or nonsmoker on the basis of a latent trajectory analysis fit to data on repeated assessments of smoking in the past 30 d by respondents aged 14–25 y observed between 1994 and 2006 in the NLSY79-CYA. AORs for smoking trajectories relative to the experiment smoking trajectory group are not shown because statistical inference limited by sample power for this group. CIs and tests of statistical significance have been adjusted for survey weights and clustering within families.
P < .05;
P < .01;
P < .001.
All analyses used weights and corrected standard errors38 to address the complex sampling structure of the NLSY79-CYA and inclusion of siblings. We implemented LTA using Mplus 5.21 (Muthén and Muthén, Los Angeles, CA) and the multinomial logistic regression models using Stata/MP 11 (StataCorp LP, College Station, TX).
RESULTS
We characterized trajectories of youth smoking from adolescence through young adulthood (Figure 2) and considered exposure to various maternal smoking patterns from prebirth to the child’s early adolescence (Figure 1) in unadjusted models and in models with controls for social and behavioral selection factors.
FIGURE 2. Rates of smoking in the past 30 days among US youths by predicted youth smoking trajectory group: NLSY79-CYA, 1994–2006.
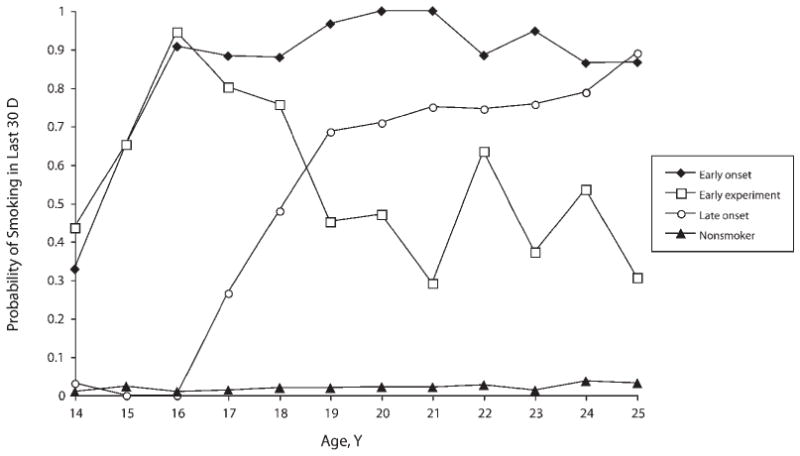
Note. Youth smoking trajectories are characterized as early onset, early experiment, late onset, or nonsmoker on the basis of a latent trajectory analysis fit to data on repeated assessments of smoking in the past 30 d by respondents aged 14–25 y observed between 1994 and 2006 in the NLSY79-CYA.
Youth Smoking Trajectory
We characterized US youth smoking trajectories for youths aged 14 to 25 years using LTA and the set of repeated assessments of smoking in the past 30 days. We fit models with 1 to 5 latent classes, with each class representing a different smoking trajectory. Goodness of fit statistics indicated that 4 classes best fit the data. We used the LTA parameters for the likelihood of smoking in the past 30 days at each age (Figure 2; Appendix B, available as a supplement to the online version of this article at http://www.ajph.org) to describe and label the 4 latent smoking trajectory classes as early-onset, early-experiment, and late-onset smoker and nonsmoker. We validated these descriptions and labels by calculating the median age first smoked cigarettes and proportion ever smoked for each class.
Early-onset smokers (14.7% prevalence) had rates of smoking in the past 30 days that increased rapidly from approximately 30% to 90% between the ages of 14 and 16 years and remained high at each subsequent age through young adulthood (86.6% at 25 years). The age trajectory of smoking in the past 30 days suggests early initiation of smoking followed by continued use. This suggestion is validated by the fact that all respondents had smoked and initiated smoking at a median age of 12 years.
Early-experiment smokers (2.7% prevalence) had a similar age pattern of smoking in the past 30 days between the ages of 14 and 16 years, but then the rates of smoking in the past 30 days dropped back to approximately 30% by 21 years and remained at an average of approximately 35% through 25 years. This age trajectory suggests early initiation of smoking followed by quitting during the transition to adulthood. This suggestion is validated by the fact that all respondents had smoked and initiated smoking at the mean age of 12 years, which is not statistically significantly different from that for early-onset smokers (P=.112).
Late-onset smokers (18.8% prevalence) reported essentially no smoking in the past 30 days before age 16 years but then had dramatically increasing rates (climbing from essentially zero to 68.5% from 16–19 years), with continued increases to 25 years, when 89.0% reported smoking in the past 30 days. Their age trajectory of smoking in the past 30 days suggests later initiation of smoking than early-onset or early-experiment smokers followed by continued use. This is validated by the fact that all respondents had smoked and initiated smoking at the mean age of14 years, which is significantly later than both early-onset (P≤.001) and early-experiment (P≤.001) smokers.
Nonsmokers (63.7% prevalence) reported very low to essentially no smoking in the past 30 days at every age. The average rate of smoking in the past 30 days for those aged 14 to 25 years was 2.0%. Although the validation showed that about half the group had smoked at least 1 cigarette in their lives (53.2%), the smoking in the past 30 days’ pattern revealed that this smoking occurred before their first assessment of smoking in the past 30 days, and that when aged 14 to 25 years they had become nonsmokers.
Maternal to Child Transmission of Smoking
We considered the intergenerational relationships between maternal and youth smoking by first evaluating bivariate relationships between the smoking trajectories of youths and the smoking patterns of their mothers.
Table 1 shows the proportion of youths in each smoking trajectory by the mother’s smoking pattern and bivariate odds ratios (ORs) contrasting each youth smoking trajectory with respect to the other (with the exception of contrasts between early-experiment smoking and both early-onset and late-onset smoking, for which no bivariate ORs were statistically significant at P<.05).
Youths whose mothers ever smoked daily and those whose mothers smoked any cigarettes during pregnancy were more likely to develop all 3 smoking trajectories instead of becoming nonsmokers. For both maternal smoking outcomes, youths had about 3 times the odds of early-onset smoking, more than 2 times the odds of early-experiment smoking, and nearly 2 times the odds of late-onset smoking versus non-smoking (Table 1). For both outcomes, they also had 1.56 times higher odds of early-onset than late-onset smoking. Because of the similarities in findings for exposure to ever daily and any smoked during pregnancy compared with no exposure, we contrasted the risk of any smoked during pregnancy against ever smoked daily. The odds of early-onset (OR=1.76) and late-onset (OR=1.30) smoking versus nonsmoking remained statistically significant.
Table 1 also shows that among youths exposed to any smoking during pregnancy, the odds of early-onset and late-onset smoking versus nonsmoking as well as early-onset versus late-onset smoking are higher if the mother smoked more cigarettes during pregnancy. Across all the youth smoking trajectories, however, the increased odds of greater exposure to maternal cigarette consumption was statistically significantly higher only for early-onset smoking versus nonsmoking (P=.036). Youths have (3.66/2.52)=45% higher odds of early-onset smoking versus nonsmoking if their mother smoked more than 1 pack per day versus less than 1 pack per day.
We next evaluated the relationship between the detailed maternal smoking trajectory variable and the relative odds of youth smoking trajectories. Similar to findings for ever smoked by smoked during pregnancy, we observed higher odds for earlier, nonexperimental smoking among youths with higher pack per day smoking during pregnancy exposure. However, none of these differences between 1 or more packs per day smoked during pregnancy versus less than 1 pack per day smoked during pregnancy were statistically significant (findings not shown). Thus, in Tables 1 and 2 the maternal smoking history variable combines less than 1 and 1 or more packs per day smoked during pregnancy to assess any smoked during pregnancy exposure.
The Table 1 unadjusted ORs show that with the exception of youths whose mothers quit before pregnancy, all maternal smoking patterns significantly increased the likelihood that youths would become early-onset, early-experiment, or late-onset smokers compared with nonsmoking. The AORs in Table 2 assess these intergenerational smoking relationships, controlling for social and behavioral characteristics that have been previously shown to predict smoking during pregnancy.21,22 Consistent with potential selection, mother’s propensity for risk taking, marital status, educational attainment, age at the child’s birth, and race/ethnicity all significantly predict the likelihood of 1 or more of the youth smoking trajectories, as do the child’s age at baseline and gender. Despite this evidence for selection, the maternal smoking pattern remained an important predictor of differences in youth smoking trajectories, albeit with reduced odds and statistical significance (Table 2).
Youths exposed prenatally to maternal smoking are the most likely to smoke. We distinguished between 2 groups of these youths: those whose mothers never reported quitting after any smoking during pregnancy and those whose mothers did report quitting after any smoking during pregnancy. Both groups have significantly higher risks of being early-onset and late-onset smokers versus nonsmokers compared with youths whose mothers never smoked. However, only youths whose mothers continued to smoke also had higher odds of early-onset versus late-onset smoking (OR=1.65). Additionally, the odds of early-onset smoking versus nonsmoking was larger for youths whose mothers did not quit smoking after smoking during pregnancy (OR=2.75) than for those whose did (OR= 2.12), albeit not statistically significantly so. In supplementary analyses contrasting maternal quitting among those exposed to at least 1 pack per day of smoking during pregnancy, the lower probability of early-onset smoking than nonsmoking among those whose mothers quit versus those who continued is statistically significantly lower (P=.044).
We also distinguished between 2 groups of youths exposed to postnatal maternal smoking but not smoking during pregnancy. In 1 group, the mothers relapsed to smoking after the child’s birth. In the other, the mother relapsed to smoking but then subsequently quit during the child’s youth or early adolescence. Both groups were significantly more likely to be early-onset smokers (OR=2.07 and 1.86, respectively) or late-onset smokers (OR=1.66 and 1.83, respectively) than were nonsmokers, but only those whose mother did not quit were also significantly more likely to be early-experiment smokers than nonsmokers (OR=2.46).
Finally, we considered youths whose mothers smoked daily but did so before the child was born. These youths had the highest statistically significant odds of being early-experiment smokers—about 3 times the odds of early-experiment smoking versus both non-smoking (OR=3.01) and late-onset smoking (OR=2.84)—as did youths whose mothers never smoked. These findings should be interpreted with caution, however, because the sample size was low for early experimenters whose mothers smoked before their birth.
DISCUSSION
This study makes several contributions to the literature on the intergenerational transmission of smoking. First, we have contributed to the growing literature that uses latent trajectory models to differentiate longitudinal patterns of entry and exit or progression of youth cigarette smoking.23–26,28,39–45 Only 2 of these have used a US representative sample of youths.39,40 Ours is the first study, to our knowledge, to extend these findings to a US representative sample of mother-child dyads. We observed a late-onset (19% prevalence) and an early-onset (15%) group for which average age of initiation varied but the progression to a regular pattern of smoking in the past 30 days did not, an early-experiment group (3%) that began early but predominantly quit smoking in the past 30 days by the time they were aged 20 years, and a nonsmoker group (64%) with essentially no smoking in the past 30 days when aged between 14 and 25 years. These smoking trajectories are consistent with the characteristics and prevalence of youth smoking groups identified previously.23–26,28,39–45
Second, we characterized patterns of maternal daily smoking before and after the child’s birth in relationship to any cigarette smoking during pregnancy to assess the relative contribution of exposure to maternal smoking prenatally and postnatally. Previous studies have taken a first step toward distinguishing between negative social role modeling (via concurrent maternal smoking) and physiological or genetic transmission (via smoking during pregnancy) by assessing their independent effects on youth smoking.2–7,9,19 Our findings build on that work by showing that youths exposed to maternal smoking both prenatally and postnatally or postnatally alone not only were more likely than were youths unexposed to maternal smoking to become persistent smokers by young adulthood (early-onset or late-onset smokers vs non-smokers) but were also more likely to become early-onset than late-onset smokers. Furthermore, consistent with previous findings on the independence of transmission through smoking during pregnancy and subsequent maternal smoking,2,3,5,6,19 those exposed to maternal smoking prenatally and postnatally were significantly more likely to become early-onset and late-onset smokers than were those exposed postnatally alone. Our findings are new in demonstrating that continued exposure to maternal smoking from the prenatal to postnatal period intensifies the likelihood that youths progress to regular smoking by 25 years, becoming early-onset or late-onset smokers.
Our assessment of a detailed maternal smoking variable allowed us to extend previous findings on independent effects of smoking during pregnancy and concurrent maternal smoking2,3,5,6,19 by evaluating whether subsequent exposure to positive social role models (via maternal cessation) tempers the physiological or genetic transmission indicated by smoking during pregnancy. We found that all youths exposed to smoking during pregnancy were significantly more likely to become early-onset smokers and late-onset smokers than were nonsmokers; however, only for youths whose mothers continued smoking after smoking during pregnancy was early-onset smoking significantly more likely than late-onset smoking. Furthermore, although the lower odds of early-onset smoking associated with mother’s cessation did not reach statistical significance in the sample of all youths exposed to smoking during pregnancy, supplementary analyses with youths exposed to at least 1 pack per day did find significantly lower odds of early-onset smoking. Taken together, these findings are suggestive, but not unequivocal, evidence that maternal role modeling of antismoking behavior during the ages when youths begin late-onset smoking may temper risks associated with prenatal exposure.
The more detailed maternal smoking trajectories have also allowed us to evaluate dimensions of intergenerational pathways operating without exposure to smoking during pregnancy that have not been previously described. Notably, we found that youths whose mothers had smoked before their birth but who were not directly exposed prenatally or postnatally were more likely than any other exposure group to become early-experiment smokers. The sample of mothers and children in this group is insufficient to allow further consideration of the factors that differentiate them from those who progress to regular smoking, but it may be a promising area for future research.
Finally, we observed that when intergenerational transmission is studied using a smoking trajectory outcome for youths rather than a static, cross-section of this trajectory, there is no evidence for gender differences in the relationship between smoking during pregnancy and youth smoking. Although 1 study has observed gender differences in the effect of smoking during pregnancy,20 others have failed to find statistically significant differences7 or have observed that differences are small.3
Limitations
Although this is, to our knowledge, the first study to consider maternal and youth trajectories of smoking using population-representative data and latent classification techniques, several limitations warrant discussion. First, all smoking measures use self-reported data. Despite this limitation, self-report has been shown to provide a reasonable estimate of actual smoking,46,47 and the NLSY79-CYA used computer-assisted personal interviewing to reduce reporting bias. Furthermore, the primary source of maternal smoking during pregnancy data comes from reports within 1 year of the child’s birth, addressing a limitation of long recall times discussed elsewhere.2,48 Second, we were unable to assess the role of the father’s smoking and exposure to secondhand smoke in the household, which may exacerbate the genetic, physiological, and social mechanisms for intergenerational transmission. That said, previous studies have found maternal effects are stronger than paternal effects.20,49,50
Third, the sample we used for this study was large, but the low prevalence of the early-experiment group (n=127) has limited statistical power in analyses including this group. Furthermore, because the data on smoking are reported biennially our trajectories could not capture short-term fluctuations in cigarette behavior but rather described overarching patterns of youth smoking across adolescence and young adulthood. Finally, as the result of the cohort structure of the NLSY79-CYA sample the richest portion of data covered individuals aged 14 to 16 years. Thus, our findings may be more representative of smoking patterns in adolescence and less representative of smoking patterns in early adulthood than have been described in previous population-representative studies.39,40
Conclusions
Reduction of cigarette smoking among youths and pregnant women are 2 top public health prevention efforts in tobacco control.51 Despite progress toward Healthy People 2010 tobacco control objectives, targets for both of these groups remain unfulfilled.52–54 Our findings strengthen the evidence for intergenerational transmission of smoking phenotypes,55 supporting both physiological and genetic transmission through smoking during pregnancy as well as social role modeling of maternal smoking. They underscore the importance of prevention and intervention not only in pregnancy but also subsequent to birth, when relapse is common.15,16 Furthermore, they highlight the importance of halting or reversing youth trajectories in which smoking becomes highly prevalent by young adult childbearing ages.
Supplementary Material
Acknowledgments
The research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R03 grant 060673: Intergenerational Determinants of Youth Smoking Trajectories, to principal investigator M. M. W.).
We would like to thank Michael Pollard, Peter Brownell, and one anonymous reviewer for their comments on an earlier draft of this article.
Footnotes
Reprints can be ordered at http://www.ajph.org by clicking the “Reprints/Eprints” link.
Contributors
M. M. Weden drafted the initial article. M. M. Weden and J. N. V. Miles jointly originated the project and carried out the data preparation and analysis and approved the final article.
Human Participant Protection
This was an analysis carried out on publicly available data and institutional review was not required.
References
- 1.Cornelius MD, Leech SL, Goldschmidt L, Day NL. Is prenatal tobacco exposure a risk factor for early adolescent smoking? A follow-up study. Neurotoxicol Teratol. 2005;27(4):667–676. doi: 10.1016/j.ntt.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 2.O’Callaghan FV, O’Callaghan M, Najman JM, Williams GM, Bor W, Alati R. Prediction of adolescent smoking from family and social risk factors at 5 years, and maternal smoking in pregnancy and at 5 and 14 years. Addiction. 2006;101(2):282–290. doi: 10.1111/j.1360-0443.2006.01323.x. [DOI] [PubMed] [Google Scholar]
- 3.Al Mamun A, O’Callaghan FV, Alati R, et al. Does maternal smoking during pregnancy predict the smoking patterns of young adult offspring? A birth cohort study. Tob Control. 2006;15(6):452–457. doi: 10.1136/tc.2006.016790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts KH, Munafo MR, Rodriguez D, et al. Longitudinal analysis of the effect of prenatal nicotine exposure on subsequent smoking behavior of offspring. Nicotine Tob Res. 2005;7(5):801–808. doi: 10.1080/14622200500262840. [DOI] [PubMed] [Google Scholar]
- 5.Lawlor DA, O’Callaghan MJ, Mamun AA, Williams GM, Bor W, Najman JM. Early life predictors of adolescent smoking: findings from the Mater-University study of pregnancy and its outcomes. Paediatr Perinat Epidemiol. 2005;19(5):377–387. doi: 10.1111/j.1365-3016.2005.00674.x. [DOI] [PubMed] [Google Scholar]
- 6.Lieb R, Schreier A, Pfister H, Wittchen HU. Maternal smoking and smoking in adolescents: a prospective community study of adolescents and their mothers. Eur Addict Res. 2003;9(3):120–130. doi: 10.1159/000070980. [DOI] [PubMed] [Google Scholar]
- 7.Griesler PC, Kandel DB, Davies M. Maternal smoking in pregnancy, child behavior problems, and adolescent smoking. J Res Adolesc. 1998;8(2):159–185. [Google Scholar]
- 8.Oncken C, McKee S, Krishnan-Sarin S, O’Malley S, Mazure C. Gender effects of reported in utero tobacco exposure on smoking initiation, progression and nicotine dependence in adult offspring. Nicotine Tob Res. 2004;6(5):829–833. doi: 10.1080/1462220042000282555. [DOI] [PubMed] [Google Scholar]
- 9.Porath AJ, Fried PA. Effects of prenatal cigarette and marijuana exposure on drug use among offspring. Neurotoxicol Teratol. 2005;27(2):267–277. doi: 10.1016/j.ntt.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 10.O’Callaghan FV, Al Mamun A, O’Callaghan M, et al. Maternal smoking during pregnancy predicts nicotine disorder (dependence or withdrawal) in young adults–a birth cohort study. Aust N Z J Public Health. 2009;33(4):371–377. doi: 10.1111/j.1753-6405.2009.00410.x. [DOI] [PubMed] [Google Scholar]
- 11.Buka SL, Shenassa ED, Niaura R. Elevated risk of tobacco dependence among offspring of mothers who smoked during pregnancy: a 30-year prospective study. Am J Psychiatry. 2003;160(11):1978–1984. doi: 10.1176/appi.ajp.160.11.1978. [DOI] [PubMed] [Google Scholar]
- 12.Niaura R, Bock B, Lloyd EE, Brown R, Lipsitt LP, Buka S. Maternal transmission of nicotine dependence: psychiatric, neurocognitive and prenatal factors. Am J Addict. 2001;10(1):16–29. doi: 10.1080/105504901750160420. [DOI] [PubMed] [Google Scholar]
- 13.Hellstrom-Lindahl E, Nordberg A. Smoking during pregnancy: a way to transfer the addiction to the next generation? Respiration. 2002;69(4):289–293. doi: 10.1159/000063261. [DOI] [PubMed] [Google Scholar]
- 14.Shea AK, Steiner M. Cigarette smoking during pregnancy. Nicotine Tob Res. 2008;10(2):267–278. doi: 10.1080/14622200701825908. [DOI] [PubMed] [Google Scholar]
- 15.Floyd RL, Rimer BK, Giovino GA, Mullen PD, Sullivan SE. A review of smoking in pregnancy–effects on pregnancy outcomes and cessation efforts. Annu Rev Public Health. 1993;14:379–411. doi: 10.1146/annurev.pu.14.050193.002115. [DOI] [PubMed] [Google Scholar]
- 16.US Department of Health and Human Services. Women and Smoking: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention; 2001. [Google Scholar]
- 17.Chassin L, Presson CC, Rose JS, Sherman SJ. Maternal socialization of adolescent smoking: intergenerational transmission of smoking-related beliefs. Psychol Addict Behav. 1998;12(3):206–216. doi: 10.1037//0012-1649.34.6.1189. [DOI] [PubMed] [Google Scholar]
- 18.Darling N, Cumsille P. Theory, measurement, and methods in the study of family influences on adolescent smoking. Addiction. 2003;98:21–36. doi: 10.1046/j.1360-0443.98.s1.3.x. [DOI] [PubMed] [Google Scholar]
- 19.Cornelius MD, Leech SL, Goldschmidt L, Day NL. Prenatal tobacco exposure: is it a risk factor for early tobacco experimentation? Nicotine Tob Res. 2000;2(1):45–52. doi: 10.1080/14622200050011295. [DOI] [PubMed] [Google Scholar]
- 20.Kandel DB, Wu P, Davies M. Maternal smoking during pregnancy and smoking by adolescent daughters. Am J Public Health. 1994;84(9):1407–1413. doi: 10.2105/ajph.84.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sen B, Swaminathan S. Maternal prenatal substance use and behavior problems among children in the US. J Ment Health Policy. 2007;10(4):189–206. [PubMed] [Google Scholar]
- 22.D’Onofrio BM, Van Hulle CA, Waldman ID, et al. Smoking during pregnancy and offspring externalizing problems: an exploration of genetic and environmental confounds. Dev Psychopathol. 2008;20(1):139–164. doi: 10.1017/S0954579408000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chassin L, Presson CC, Pitts SC, Sherman SJ. The natural history of cigarette smoking from adolescence to adulthood in a Midwestern community sample: multiple trajectories and their psychosocial correlates. Health Psychol. 2000;19(3):223–231. [PubMed] [Google Scholar]
- 24.Orlando M, Tucker JS, Ellickson PL, Klein DJ. Developmental trajectories of cigarette smoking and their correlates from early adolescence to young adulthood. J Consult Clin Psychol. 2004;72(3):400–410. doi: 10.1037/0022-006X.72.3.400. [DOI] [PubMed] [Google Scholar]
- 25.Soldz S, Cui XJ. Pathways through adolescent smoking: a 7-year longitudinal grouping analysis. Health Psychol. 2002;21(5):495–504. [PubMed] [Google Scholar]
- 26.Abroms L, Simons-Morton B, Haynie DL, Chen RS. Psychosocial predictors of smoking trajectories during middle and high school. Addiction. 2005;100(6):852–861. doi: 10.1111/j.1360-0443.2005.01090.x. [DOI] [PubMed] [Google Scholar]
- 27.Brook JS, Pahl K, Ning YM. Peer and parental influences on longitudinal trajectories of smoking among African Americans and Puerto Ricans. Nicotine Tob Res. 2006;8(5):639–651. doi: 10.1080/14622200600789627. [DOI] [PubMed] [Google Scholar]
- 28.Karp I, O’Loughlin J, Paradis G, Hanley J, Difranza J. Smoking trajectories of adolescent novice smokers in a longitudinal study of tobacco. Ann Epidemiol. 2005;15(6):445–452. doi: 10.1016/j.annepidem.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Avenevoli S, Merikangas KR. Familial influences on adolescent smoking. Addiction. 2003;98(suppl 1):1–20. doi: 10.1046/j.1360-0443.98.s1.2.x. [DOI] [PubMed] [Google Scholar]
- 30.Center for Human Resource Research. National Longitudinal Survey of Youth 1979 Cohort (NLSY79) User’s Guide. Columbus: Ohio State University; [Accessed January 26, 2011]. Available at: http://www.nlsinfo.org/nlsy79/docs/79html/tableofcontents.html. [Google Scholar]
- 31.Center for Human Resource Research. NLSY79 Child & Young Adult Data Users Guide. Columbus: Ohio State University; 2006. [Accessed January 26, 2011]. Available at: http://www.nlsinfo.org/pub/usersvc/Child-Young-Adult/2006ChildYA-DataUsersGuide.pdf. [Google Scholar]
- 32.Rowe DC, Gulley BL. Sibling effects on substance use and delinquency. Criminology. 1992;30(2):217–234. [Google Scholar]
- 33.Muthén B. Second generation structural equation modeling with a combination of categorical and continuous latent variables. In: Collins LM, Sayer AG, editors. New Methods for the Analysis of Change. Washington, DC: American Psychological Association; 2001. pp. 291–322. [Google Scholar]
- 34.Collins LM, Lanza SM. Latent Class and Latent Transition Analysis: With Applications in the Social, Behavioral, and Health Sciences. Hoboken, NJ: Wiley; 2009. [Google Scholar]
- 35.Thomson C, Holmberg M, Baer DM. A brief report on a comparison of time-sampling procedures. J Appl Behav Anal. 1974;7(4):623–626. doi: 10.1901/jaba.1974.7-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allison P. Missing Data. Thousand Oaks, CA: Sage; 2009. [Google Scholar]
- 37.Muthén LK, Muthén BO. Mplus User’s Guide. 5. Los Angeles, CA: Muthén and Muthén; 2008. [Google Scholar]
- 38.StataCorp. Stata Programming Reference Manual: Release 11. College Station, TX: 2009. [Google Scholar]
- 39.Pollard MS, Tucker JS, Green HD, Kennedy D, Go MH. Friendship networks and trajectories of adolescent tobacco use. Addict Behav. 2010;35(7):678–685. doi: 10.1016/j.addbeh.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Costello DM, Dierker LC, Jones BL, Rose JS. Trajectories of smoking from adolescence to early adulthood and their psychosocial risk factors. Health Psychol. 2008;27(6):811–818. doi: 10.1037/0278-6133.27.6.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bernat DH, Erickson DJ, Widorne R, Perry CL, Forster JL. Adolescent smoking trajectories: results from a population-based cohort study. J Adolesc Health. 2008;43(4):334–340. doi: 10.1016/j.jadohealth.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lessov-Schlaggar CN, Hops H, Brigham J, et al. Adolescent smoking trajectories and nicotine dependence. Nicotine Tob Res. 2008;10(2):341–351. doi: 10.1080/14622200701838257. [DOI] [PubMed] [Google Scholar]
- 43.Riggs NR, Chou CP, Li CY, Pentz MA. Adolescent to emerging adulthood smoking trajectories: when do smoking trajectories diverge, and do they predict early adulthood nicotine dependence? Nicotine Tob Res. 2007;9(11):1147–1154. doi: 10.1080/14622200701648359. [DOI] [PubMed] [Google Scholar]
- 44.Audrain-McGovern J, Rodriguez D, Tercyak KP, Cuevas J, Rodgers K, Patterson F. Identifying and characterizing adolescent smoking trajectories. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2023–2034. [PubMed] [Google Scholar]
- 45.Colder CR, Mehta P, Balanda K, et al. Identifying trajectories of adolescent smoking: an application of latent growth mixture modeling. Health Psychol. 2001;20(2):127–135. doi: 10.1037//0278-6133.20.2.127. [DOI] [PubMed] [Google Scholar]
- 46.Dolcini MM, Adler NE, Lee P, Bauman KE. An assessment of the validity of adolescent self-reported smoking using three biological indicators. Nicotine Tob Res. 2003;5(4):473–483. [PubMed] [Google Scholar]
- 47.Chan D. Why ask me? Are self-report data really that bad? In: Lance CE, Vandenberg RJ, editors. Statistical and Methodological Myths and Urban Legends: Doctrine, Verity and Fable in Organizational and Social Sciences. New York, NY: Routledge; 2008. pp. 309–336. [Google Scholar]
- 48.Kandel DB, Udry JR. Prenatal effects of maternal smoking on daughters’ smoking: nicotine or testosterone exposure? Am J Public Health. 1999;89(9):1377–1383. doi: 10.2105/ajph.89.9.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Distefan JM, Gilpin EA, Choi WS, Pierce JP. Parental influences predict adolescent smoking in the United States, 1989–1993. J Adolesc Health. 1998;22(6):466–474. doi: 10.1016/s1054-139x(98)00013-5. [DOI] [PubMed] [Google Scholar]
- 50.Griffin KW, Botvin GJ, Doyle MM, Diaz T, Epstein JA. A six-year follow-up study of determinants of heavy cigarette smoking among high-school seniors. J Behav Med. 1999;22(3):271–284. doi: 10.1023/a:1018772524258. [DOI] [PubMed] [Google Scholar]
- 51.US Department of Health and Human Services. Healthy People 2010: Understanding and Improving Health. 2. Washington, DC: US Government Printing Office; 2000. [Google Scholar]
- 52.Tong VT, Jones JR, Dietz PM, D’Angelo D, Bombard JM. Trends in smoking before, during, and after pregnancy–Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 31 sites, 2000–2005. MMWR Surveill Summ. 2009;58(4):1–29. [PubMed] [Google Scholar]
- 53.Suellentrop K, Morrow B, Williams L, D’Angelo D. Monitoring progress toward achieving Maternal and Infant Healthy People 2010 objectives–19 states, Pregnancy Risk Assessment Monitoring System (PRAMS), 2000–2003. MMWR Surveill Summ. 2006;55(9):1–11. [PubMed] [Google Scholar]
- 54.US Dept of Health and Human Services. Healthy People 2010 Midcourse Review. Washington, DC: 2006. [Accessed January 26, 2011]. Available at: http://www.healthypeople.gov/2010/data/midcourse/html/default.htm. [Google Scholar]
- 55.Chassin L, Presson C, Seo DC, et al. Multiple trajectories of cigarette smoking and the intergenerational transmission of smoking: a multigenerational, longitudinal study of a Midwestern community sample. Health Psychol. 2008;27(6):819–828. doi: 10.1037/0278-6133.27.6.819. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


