Abstract
Purpose
TNFerade biologic is a novel means of delivering tumor necrosis factor alpha to tumor cells by gene transfer. We herein report final results of the largest randomized phase III trial performed to date among patients with locally advanced pancreatic cancer (LAPC) and the first to test gene transfer against this malignancy.
Patients and Methods
In all, 304 patients were randomly assigned 2:1 to standard of care plus TNFerade (SOC + TNFerade) versus standard of care alone (SOC). SOC consisted of 50.4 Gy in 28 fractions with concurrent fluorouracil (200 mg/m2 per day continuous infusion). TNFerade was injected intratumorally before the first fraction of radiotherapy each week at a dose of 4 × 1011 particle units by using either a percutaneous transabdominal or an endoscopic ultrasound approach. Four weeks after chemoradiotherapy, patients began gemcitabine (1,000 mg/m2 intravenously) with or without erlotinib (100 to 150 mg per day orally) until progression or toxicity.
Results
The analysis included 187 patients randomly assigned to SOC + TNFerade and 90 to SOC by using a modified intention-to-treat approach. Median follow-up was 9.1 months (range, 0.1 to 50.5 months). Median survival was 10.0 months for patients in both the SOC + TNFerade and SOC arms (hazard ratio [HR], 0.90; 95% CI, 0.66 to 1.22; P = .26). Median progression-free survival (PFS) was 6.8 months for SOC + TNFerade versus 7.0 months for SOC (HR, 0.96; 95% CI, 0.69 to 1.32; P = .51). Among patients treated on the SOC + TNFerade arm, multivariate analysis showed that TNFerade injection by an endoscopic ultrasound-guided transgastric/transduodenal approach rather than a percutaneous transabdominal approach was a risk factor for inferior PFS (HR, 2.08; 95% CI, 1.06 to 4.06; P = .032). The patients in the SOC + TNFerade arm experienced more grade 1 to 2 fever and chills than those in the SOC arm (P < .001) but both arms had similar rates of grade 3 to 4 toxicities (all P > .05).
Conclusion
SOC + TNFerade is safe but not effective for prolonging survival in patients with LAPC.
INTRODUCTION
Pancreatic cancer is among the most lethal malignancies in the Western world, as attested to by a mortality that closely rivals its incidence.1 Approximately 30% to 40% of patients present with unresectable, locally advanced pancreatic cancer (LAPC),2,3 for which acceptable treatment options include chemoradiotherapy (CRT) or chemotherapy alone.4–9 CRT typically results in tumor stability, with only a small subset of patients (10% to 15%) exhibiting an objective response.10 Five-year survival for patients with LAPC remains dismal at less than 2%.11 Novel treatments and methods of enhancing current therapeutic modalities are needed.
Tumor necrosis factor alpha is a potent inflammatory cytokine with substantial anticancer activity.12–19 Multiple animal studies and clinical trials have shown that tumor necrosis factor alpha (TNF-α) is effective against solid tumors, but the treatment has ultimately failed because of severe systemic toxicity consisting of hypotension and shock-like symptoms.20–29 TNFerade biologic (GenVec, Gaithersburg, MD) represents a novel means of selectively delivering TNF-α to tumor cells by gene transfer through intratumoral vector injection. TNFerade (AdGVEGR.TNF.11D) is a second generation E1-, E4-, and partial E3-deleted, replication-deficient adenovirus serotype 5 vector containing TNF-α cDNA ligated downstream from the early growth response protein 1 (Egr-1) promoter.30 Egr-1 is induced by ionizing radiation,31 thus allowing for spatial and temporal constraint of TNF-α production to the radiation field17,30,32 and markedly attenuating systemic toxicity in preclinical studies.33,34 Furthermore, spatiotemporal joining of irradiation and TNF-α production exploits documented synergy between these modalities.21,33,35,36
Early-phase clinical trials show encouraging evidence for local efficacy of TNFerade against multiple tumor types,37,38 although TNFerade has not been observed to have any systemic anticancer activity. The predominant toxicities accompanying TNFerade administration in these trials were fever and chills. A phase I/II dose-escalation study in 50 patients with LAPC showed that TNFerade at doses of 4 × 109 to 4 × 1011 particle units (PU) weekly with fluorouracil-based CRT was well-tolerated.39 Furthermore, data were consistent with a dose-dependent increase in stabilization of treated tumors. On the basis of these results, a multicenter, randomized phase III trial of TNFerade in conjunction with CRT was conducted to assess efficacy and safety for LAPC.
PATIENTS AND METHODS
Patients
The study population consisted of patients with biopsy-confirmed, unresectable LAPC. Unresectable disease was defined by extension to the superior mesenteric artery and/or celiac axis with no fat plane separating the tumor and these arterial structures or obstruction of the superior mesenteric-portal vein confluence. Eligibility criteria included age ≥18 years, Karnofsky performance status (KPS) ≥ 70%,40 life expectancy more than 3 months, and adequate hepatic, hematologic, immune, and renal function. Patients with technically resectable tumors (T1–T3) were also eligible if they were deemed unresectable because of medical comorbidities or refusal of surgery.
Exclusion criteria included evidence of metastatic disease, previous pancreatic cancer therapy, previous target field irradiation, clinically significant ascites, bulky celiac adenopathy (≥2.5 cm), or nonadenocarcinoma histology. It must be noted, however, that patients with unknown metastatic disease status (Mx) because of inability to obtain a contrast chest computed tomography (CT) scan or because of some other impediment to completing radiologic evaluation were allowed to enroll.
All patients provided written informed consent before enrollment. The study was approved by each center’s institutional review board or ethics committee and complied with provisions of the Good Clinical Practice guidelines and Declaration of Helsinki, as well as with Food and Drug Administration regulations.
Study Design
This open-label, randomized, controlled phase III trial was conducted at 39 sites throughout the continental United States. Eligible patients were randomly assigned 2:1 to the maximum-tolerated dose of TNFerade (4 × 1011 PU) plus standard-of-care therapy (SOC + TNFerade) versus standard-of-care therapy alone (SOC). Randomization was stratified by center and KPS (≥ 80% or < 80%). SOC consisted of continuous infusion fluorouracil and concurrent radiotherapy, followed by gemcitabine or gemcitabine plus erlotinib maintenance therapy at investigator discretion. TNFerade was administered by intratumoral injection by using a CT/ultrasound-guided percutaneous transabdominal approach (PTA) or an endoscopic ultrasound-guided (EUS) transgastric/transduodenal approach before radiotherapy on day 1 of each of the first 5 weeks of CRT. CRT took place on days 1 through 5 of each week. Radiotherapy consisted of 45 Gy delivered in 25 fractions of 1.80 Gy followed by a boost to the tumor plus a 1-cm margin consisting of 5.40 Gy in three fractions of 1.80 Gy. Radiotherapy was delivered by using three-dimensional conformal or intensity-modulated techniques. Concurrent fluorouracil was started before radiotherapy on day 1 of each week and administered by continuous infusion (200 mg/m2 per day) until conclusion of radiotherapy each week. Four weeks following CRT, patients were allowed to begin maintenance gemcitabine (1,000 mg/m2 intravenously on days 1, 8, and 15 of 4-week cycles) with/without erlotinib (100 to 150 mg per day orally). Maintenance therapy was continued until radiographically documented disease progression or unacceptable toxicity occurred. Excessive morbidity and mortality attributable to TNFerade was monitored by an independent data and safety monitoring board. Crossover between study arms was not permitted.
Outcomes and Assessments
Overall survival was measured from date of random assignment until date of death. Predefined secondary outcomes included progression-free survival (PFS), tumor response rates, and surgical downstaging rates. An independent blinded central reading laboratory reviewed CT scans and magnetic resonance imaging (MRI) scans to assess for progression and tumor response according to Response Evaluation Criteria in Solid Tumors (RECIST).41 Safety was assessed by using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0 (NCI CTCAE v3.0).
Statistical Analysis
The primary outcome was overall survival. A sample size of 299 patients (199 randomly assigned to SOC + TNFerade, 100 to SOC) was required to achieve 85% power to detect an absolute difference in overall survival rate at 12 months of 20% between the two treatment regimens, based on a two-sided χ2 test at a significance level of 0.025. The study planned to recruit 330 patients to account for10% dropout. With this sample size, there would be 80% power to determine a hazard ratio (HR) of 0.667, assuming approximately 3.5 years of enrollment and 1 year of follow-up. Three interim analyses were planned, the first to assess for futility was based on response rate at 3 months, and the second and third interim analyses to test for superiority and futility were conducted after one third (92 deaths) and two thirds (184 deaths) of the total events required, respectively. The trial was discontinued following the third interim analysis on the basis of futility.
Analyses of primary and secondary efficacy end points of overall survival and PFS were based on a modified intention-to-treat population that included all randomly assigned patients who received at least one study treatment and considered allocation of patients to treatment groups as randomly assigned. Stratified log-rank tests and multivariate Cox regression were used to compare survival and progression between treatment groups. Multivariate Cox models included the following factors: treatment group, age, sex, KPS, cancer antigen 19-9 (CA19-9), T-stage, N-stage, M-stage, prior cancer history, prior cancer treatment, and study sites. Toxicities, adverse events (AEs), and compliance were compared by using Fisher’s exact test. All tests were two-sided, and P values were not adjusted for multiple comparisons. Statistical analysis was performed with SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
Patients
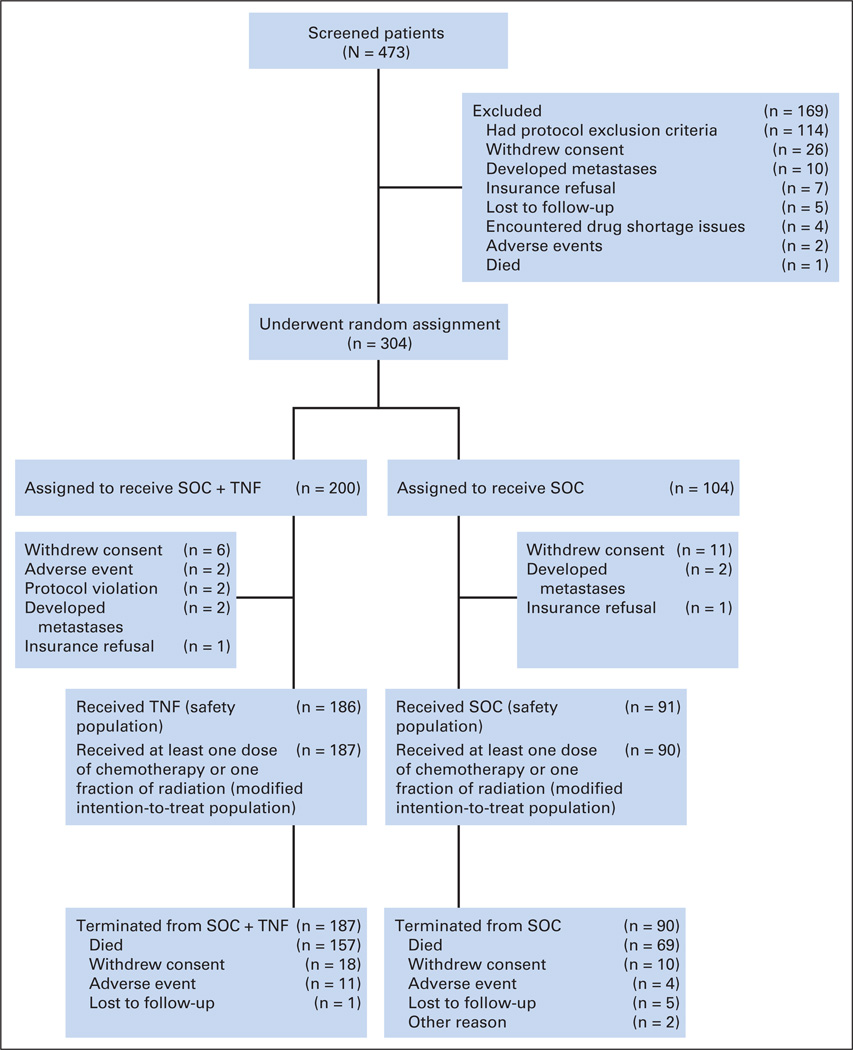
From April 5, 2005, to March 30, 2010, 473 patients were screened. Of these, 304 were randomly assigned as depicted in Figure 1 to yield 187 patients in the SOC + TNFerade arm and 90 in the SOC arm who were included in the modified intention-to-treat analysis. Median follow-up was 9.1 months (range, 0.1 to 50.5 months) for all patients and 8.1 months for patients still living at study termination. Of 39 participating institutions, 11 (28%) enrolled ≥ 10 patients. There were no significant differences in demographic or baseline disease characteristics between groups (Table 1).
Fig 1.
CONSORT diagram; enrollment and outcomes. SOC, standard of care; TNF, TNFerade.
Table 1.
Baseline Characteristics of the Intention-to-Treat Population
| Total | FU/RT (SOC) |
FU/RT + TNFerade |
|||||
|---|---|---|---|---|---|---|---|
| Characteristic | No. | % | No. | % | No. | % | P |
| No. of patients | 277 | 90 | 187 | ||||
| Age, years | .85 | ||||||
| Mean | 63.5 | 63.3 | 63.5 | ||||
| SD | 11.0 | 10.7 | 11.2 | ||||
| Median | 64.4 | 64.8 | 64.3 | ||||
| Range | 28.7–85.6 | 28.7–84.8 | 31.0–85.6 | ||||
| Sex | .30 | ||||||
| Male | 160 | 57.8 | 48 | 53.3 | 112 | 59.9 | |
| Female | 117 | 42.2 | 42 | 46.7 | 75 | 40.1 | |
| Race | .72 | ||||||
| White | 212 | 76.5 | 72 | 80 | 140 | 74.9 | |
| African American | 34 | 12.3 | 11 | 12.2 | 23 | 12.3 | |
| Hispanic | 14 | 5.1 | 3 | 3.3 | 11 | 5.9 | |
| Other | 17 | 6.1 | 4 | 4.5 | 13 | 6.9 | |
| Prior cancer history | .34 | ||||||
| Yes | 35 | 12.6 | 14 | 15.6 | 21 | 11.2 | |
| No | 242 | 87.4 | 76 | 84.4 | 166 | 88.8 | |
| Prior cancer treatment | .31 | ||||||
| Yes | 31 | 11.2 | 13 | 14.4 | 18 | 9.6 | |
| No | 246 | 88.8 | 77 | 85.6 | 169 | 90.4 | |
| KPS, % | .41 | ||||||
| 90 to 100 | 187 | 68.0 | 64 | 71.9 | 123 | 66.1 | |
| < 90 | 88 | 32.0 | 25 | 28.1 | 63 | 33.9 | |
| 80 to 100 | 264 | 96.0 | 87 | 97.8 | 177 | 95.2 | .51 |
| < 80 | 11 | 4.0 | 2 | 2.2 | 9 | 4.8 | |
| T stage | .42 | ||||||
| 1 | 1 | 0.36 | 1 | 1.1 | 0 | 0 | |
| 2 | 17 | 6.1 | 5 | 5.6 | 12 | 6.4 | |
| 3 | 68 | 24.6 | 25 | 27.8 | 43 | 23.0 | |
| 4 | 191 | 68.9 | 59 | 65.6 | 132 | 70.6 | |
| N stage | .097 | ||||||
| N0 | 116 | 41.9 | 37 | 41.1 | 79 | 42.3 | |
| N1 | 106 | 38.3 | 41 | 45.6 | 65 | 34.8 | |
| Nx | 55 | 19.9 | 12 | 13.3 | 43 | 23.0 | |
| M stage | .75 | ||||||
| M0 | 266 | 96.0 | 86 | 95.6 | 180 | 96.3 | |
| Mx | 11 | 4.0 | 4 | 4.4 | 7 | 3.7 | |
| CA19-9 | |||||||
| ≥ 1,000 | 72 | 26.3 | 19 | 21.4 | 53 | 28.6 | .24 |
| < 1,000 | 202 | 73.7 | 70 | 78.6 | 132 | 71.4 | |
Abbreviations: CA19-9, cancer antigen 19-9; FU, fluorouracil; KPS, Karnofsky performance status; RT, radiotherapy; SD, standard deviation; SOC, standard of care.
Efficacy
Overall survival
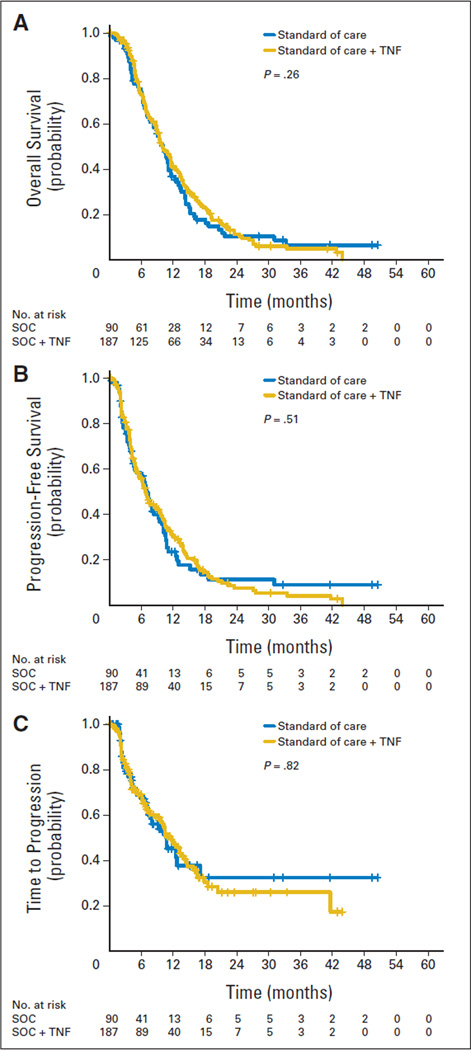
Median survival stratified by site and KPS (≥ 80% and < 80%) was similar for SOC + TNFerade and SOC (10.0 v 10.0 months; HR, 0.90; 95% CI, 0.66 to 1.22; P = .26; Table 2; Fig 2A). In the SOC + TNFerade arm, 16 patients (8.6%) died within 3 months following CRT as opposed to 12 patients (13.3%) in the SOC arm.
Table 2.
Summary of Efficacy Measures by Intention-to-Treat Group
| SOC + TNFerade (n = 187) | SOC (n = 90) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | No. | % | 95% CI | No. | % | 95% CI | HR* | 95% CI | P* |
| Overall survival, months† | 0.90 | 0.66 to 1.22 | .26 | ||||||
| Median | 10.0 | 8.8 to 11.6 | 10.0 | 7.6 to 11.2 | |||||
| 12-month survival rate | 41.0 | 33.5 to 48.3 | 36.7 | 26.3 to 47.1 | |||||
| 18-month survival rate | 23.1 | 16.9 to 29.9 | 17.7 | 10.1 to 27.0 | |||||
| 24-month survival rate | 11.3 | 0.07 to 17.1 | 10.3 | 0.05 to 18.6 | |||||
| Progression-free survival, months | 0.96 | 0.69 to 1.32 | .51 | ||||||
| Median | 6.8 | 5.5 to 8.8 | 7.0 | 4.6 to 9.2 | |||||
| Time to radiologic progression, months | 1.07 | 0.71 to 1.62 | .82 | ||||||
| Median | 11.6 | 9.6 to 14.1 | 10.8 | 7.3 to 17.2 | |||||
| Level of radiologic response‡ | 97 | 50 | .74 | ||||||
| Complete | 0 | 0 | |||||||
| Partial | 8 | 8.2 | 6 | 12.0 | |||||
| Stable disease | 72 | 74.2 | 37 | 74.0 | |||||
| Progressive disease | 17 | 17.5 | 7 | 14.0 | |||||
Abbreviations: HR, hazard ratio; SOC, standard of care.
According to the protocol, the comparison of the treatment groups should be stratified by site and Karnofsky performance status (KPS; ≥ 80% and < 80%). A log-rank test was used to compare the two treatments stratified by site and KPS, and the HR was estimated from a Cox regression model adjusting for site and KPS status.
Median and 12-month, 18-month, and 24-month survival rates were estimated by using the Kaplan-Meier method.
Radiologic response was evaluated for a subgroup of 147 patients (97 from the SOC + TNFerade arm and 50 from the SOC arm) by an independent central reading laboratory that was blinded to treatment assignment.
Fig 2.
Kaplan-Meier curves depicting (A) overall survival, (B) progression-free survival, and (C) time to radiologic progression by modified intention-to-treat groups. P values given in each panel represent the significance level obtained when groups were compared by univariate analysis. SOC, standard of care; TNF, TNFerade.
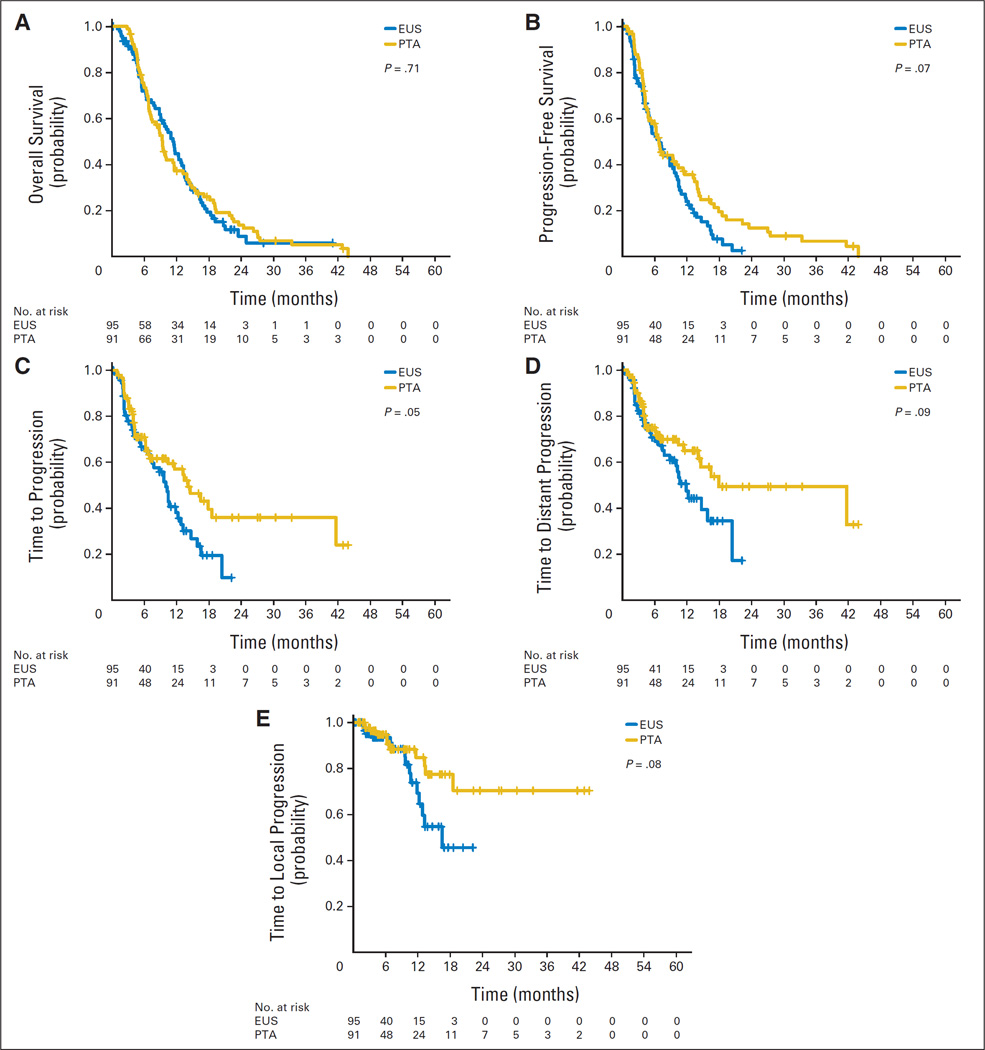
Multivariate analysis identified five baseline characteristics prognostic for survival: age, M stage (M0 v Mx), prior cancer history, prior cancer treatment, and baseline plasma CA19-9 (Appendix Table A1, online only). When adjusted for demographic and clinical factors, treatment with TNFerade remained a nondeterminant of overall survival (HR, 0.85; 95% CI, 0.61 to 1.19; P = .34). Among patients treated with SOC + TNFerade, no difference in median survival was observed for TNFerade delivery by PTA compared with EUS (9.4 v 11.5 months, respectively; HR, 1.06; 95% CI, 0.77 to 1.47; P = .71; Table 3; Fig 3A). Eighteen patients (10%) in the SOC + TNFerade arm went on to have successful surgical resection versus 10 patients (11%) in the SOC arm (P = .68) with margin-negative resections occurring in 78% and 60% of patients, respectively (P = .40).
Table 3.
Summary of Efficacy Measures Among Patients in the SOC + TNFerade Arm by TNFerade Delivery Method
| EUS (n = 95) | PTA (n = 91) | ||||||
|---|---|---|---|---|---|---|---|
| Outcome (months)* | Median | 95% CI | Median | 95% CI | HR† | 95% CI | P† |
| Overall survival | 11.5 | 9.1 to 13.3 | 9.4 | 7.3 to 11.6 | 1.06 | 0.77 to 1.47 | .71 |
| Progression-free survival | 6.8 | 5.0 to 9.6 | 6.8 | 4.8 to 10.4 | 1.37 | 0.97 to 1.92 | .07 |
| Time to radiologic progression | 10.0 | 7.2 to 12.3 | 14.1 | 7.0 to 41.6 | 1.52 | 1.00 to 2.33 | .05 |
| Time to distant metastasis | 11.9 | 8.8 to 20.4 | 17.9 | 14.1 to N/A | 1.52 | 0.93 to 2.47 | .09 |
| Time to local progression | 16.5 | 11.8 to N/A | N/A | 18.5 to N/A | 1.99 | 0.92 to 4.32 | .08 |
Abbreviations: EUS, endoscopic ultrasound guided (transgastric/transduodenal approach); HR, hazard ratio; N/A, not achieved; PTA, percutaneous transabdominal approach; SOC, standard of care.
Median survival and times to progression were estimated by using the Kaplan-Meier method.
HR and corresponding P value were computed on the basis of a univariate Cox regression model.
Fig 3.
Kaplan-Meier curves depicting (A) overall survival, (B) progression-free survival, (C) time to any radiologic progression, (D) time to distant radiologic progression, and (E) time to local radiologic progression by TNFerade delivery method among patients treated on the standard of care + TNFerade arm. P values given in each panel represent the significance level obtained when groups were compared by univariate analysis. EUS, endoscopic ultrasound guided (transgastric/transduodenal approach); PTA, percutaneous transabdominal approach.
PFS
Median PFS was similar between SOC + TNFerade and SOC arms (6.8 v 7.0 months, respectively; HR, 0.96; 95% CI, 0.69 to 1.32; P = .51; Fig 2B). Among patients treated on the SOC + TNFerade arm, multivariate analysis showed TNFerade injection by EUS rather than PTA to be a risk factor for inferior PFS (HR, 2.08; 95% CI, 1.06 to 4.06; P = .032; Fig 3B) after adjusting for age, sex, KPS, CA19-9, T stage, N stage, M stage, prior cancer history, prior cancer treatment, and study sites.
Time to radiologic progression
Median time to any radiologic progression was no different between SOC + TNFerade and SOC arms (11.6 v 10.8 months; HR, 1.07; 95% CI, 0.71 to 1.62; P = .82; Fig 2C). Within 3 months of CRT initiation, 47 patients (25.1%) in the SOC + TNFerade arm and 27 patients (30.0%) in the SOC arm developed metastatic disease.
Among patients treated with SOC + TNFerade, median time to progression was shorter for TNFerade administration by EUS compared with PTA (10.0 v 14.1 months; HR, 1.52; 95% CI, 1.00 to 2.33; P = .05; Table 3; Fig 3C). Multivariate analysis confirmed injection by EUS versus PTA as a risk factor for decreased time to progression (HR, 2.46; 95% CI, 1.15 to 5.28; P = .02) after adjusting for patient characteristics and clinical factors. Univariate analyses showed trends toward decreased time to distant progression (HR, 1.52; 95% CI, 0.93 to 2.47; P = .09) and local progression (HR, 1.99; 95% CI, 0.92 to 4.32; P = .08) for TNFerade administration via EUS (Table 3; Figs 3D and 3E).
Radiologic response rates
Tumor response was assessed by independent radiologic review for 147 patients (97 [51.9%] of 187 patients in the SOC + TNFerade arm and 50 [55.6%] of 90 patients in the SOC arm). There was no difference in response rates within this subset of patients (Table 2). Independent radiologic review was not completed for the remaining randomly assigned patients because of discontinuation of the trial for futility after planned interim analysis.
Treatment Compliance
In the SOC + TNFerade arm, 82.4% completed combined TNFerade and CRT, whereas 81.1% in the SOC arm completed CRT (P = .87). Of patients treated with SOC + TNFerade, 51.3% received TNFerade by EUS and 48.7% received TNFerade by PTA. EUS resulted in successful dose delivery in 450 (96.8%) of 465 attempts, and PTA resulted in successful dose delivery in 428 (98.2%) of 436 attempts (P = .21). Of patients who received TNFerade by EUS, 91.7% completed treatment with the prescribed five doses of TNFerade versus 91.2% of those who received TNFerade by PTA (P = .99).
At least 1 day of radiation treatment was missed by 121 patients (64.7%) in the SOC + TNFerade arm versus 56 patients (62.2%) in the SOC arm (P = .69; Appendix Table A2, online only). Only a minority of patients missed days of radiation treatment because of adverse events (27 [22.3%] of 121 in the SOC + TNFerade arm and seven [12.5%] of 56 in the SOC arm; P = .15), with the majority of missed days resulting from radiation facility closures because of holidays, equipment failure, or maintenance. All patients considered to have completed CRT made up all missed treatments on non-weekdays or at the end of therapy.
Gemcitabine administration was similar, with 68.4% versus 70.0% receiving maintenance therapy (P = .89) consisting of 11.0 ± 9.2 doses in the SOC + TNFerade arm and 12.5 ± 10.7 doses in the SOC arm on average (P = .34). In the SOC + TNFerade arm, 21.9% received erlotinib versus 15.6% in the SOC arm (P = .26), and mean duration of therapy was 2.6 ± 2.8 versus 1.8 ± 2.3 months, respectively (P = .43). Average erlotinib dose was 105 ± 43 mg for the SOC + TNFerade arm and 104 ± 31 mg for the SOC arm (P = .91).
Safety and Toxicity
The overall incidence of definite or probable treatment-related grade 2 to 4 AEs was 75.9% for the SOC + TNFerade arm and 65.6% for the SOC arm (P = .08). A breakdown of AE by category (gastrointestinal, hematologic, nongastrointestinal/nonhematologic) and highest grade experienced per patient is provided in Table 4, along with the most commonly occurring toxicities within each category. AEs reported for patients receiving TNFerade were predominantly grade 1 to 2 in severity and either gastrointestinal or constitutional. Grade 1 to 2 pyrexia, chills, rigors, and sweats occurred at higher frequency for the patients in the SOC + TNFerade arm than for those in the SOC arm (81.7% v 14.3%; P < .001), but the rate of these constitutional toxicities at the grade 3 to 4 level was not significantly different between arms (3.2% v 1.1%; P = .43). All grade 3 to 4 AEs are summarized in Appendix Table A3 (online only) and occurred at similar frequencies in the SOC + TNFerade arm versus the SOC arm (all P > .05).
Table 4.
Categorization of Adverse Events by Toxicity Type and Highest Grade Experienced Per Patient
| SOC + TNFerade |
SOC | ||||
|---|---|---|---|---|---|
| Toxicity Type | No. | % | No. | % | P* |
| GI† | |||||
| Grade 2 | 52 | 28.0 | 16 | 17.6 | .07 |
| Grade 3 | 31 | 16.7 | 10 | 11.0 | .28 |
| Grade 4 | 3 | 1.6 | 0 | 0.0 | .55 |
| Hematologicठ| |||||
| Grade 2 | 20 | 10.8 | 12 | 13.2 | .55 |
| Grade 3 | 50 | 26.9 | 23 | 25.3 | .89 |
| Grade 4 | 10 | 5.4 | 9 | 9.9 | .21 |
| Non-GI/nonhematologic¶ | |||||
| Grade 2 | 41 | 22.0 | 16 | 17.6 | .43 |
| Grade 3 | 20 | 10.8 | 5 | 5.5 | .18 |
| Grade 4 | 2 | 1.2 | 2 | 2.2 | .60 |
Abbreviation: SOC, standard of care.
P values are computed by using Fisher’s exact test.
In descending order of frequency, the most commonly occurring GI toxicities were nausea/vomiting, abdominal pain, and anorexia in the SOC + TNFerade arm versus nausea/vomiting, diarrhea, and anorexia in the SOC arm.
In both arms, the majority of hematologic toxicities (> 85%) took place during gemcitabine-based maintenance therapy following chemoradiotherapy.
In descending order of frequency, the most commonly occurring hematologic toxicities were neutropenia, thrombocytopenia, anemia, and lymphopenia in both the SOC + TNFerade and SOC arms.
In descending order of frequency, the most commonly occurring non-GI/ nonhematologic toxicities were fatigue, chills/rigors/sweats, pyrexia, and dehydration in the SOC + TNFerade arm versus fatigue, dehydration, dermatitis, and hypokalemia in the SOC arm.
More patients receiving SOC + TNFerade experienced grade 2 to 4 AEs related to CRT (50.8% v 37.8% for SOC; P = .05; Appendix Table A2). Grade 3 to 4 laboratory abnormalities occurred at similar rates (72.7% for the SOC + TNFerade arm v 67.8% for the SOC arm; P = .40), with the majority in both groups occurring during gemcitabine maintenance therapy. Rates of grade 2 to 4 treatment-related toxicity were similar for EUS and PTA administration of TNFerade (P = .80).
There was a trend toward greater overall incidence of serious adverse events (SAEs) from any cause in the SOC + TNFerade arm (80.2%) compared with the SOC arm (70.0%; P = .07). The majority of SAEs in both arms were due to disease progression with only 25.7% of SOC + TNFerade patients and 16.7% of SOC patients experiencing treatment-related SAEs (P = .13). SAEs qualifying as thrombotic events occurred at similar rates between arms (9.6% for SOC + TNFerade v 11.9% for SOC; P = .68).
DISCUSSION
To the best of our knowledge, this is the largest prospective study conducted to date in patients with LAPC and the first randomized trial to examine the efficacy of gene transfer therapy in pancreatic cancer. It is unclear why adding TNFerade to SOC failed to improve survival. Given the potency exhibited by TNFerade in preclinical studies,17,29,32–35 we find it difficult to attribute the lack of survival benefit solely to unsuccessful tumor cell killing, although we cannot rule out this possibility. The following are alternative explanations: (1) delivery of TNFerade was ineffective, (2) the radiation-inducible promoter was not successfully activated, or (3) the majority of patients succumbed to metastatic disease and therefore more aggressive local therapy had minimal influence on the natural history of the disease. Herein, we attempt to evaluate each possibility in succession.
Pancreatic tumors have been previously described as exceedingly fibrous, hypoxic masses,42–44 the composition of which may impede delivery of sufficient TNFerade throughout the tumor entirety to have a significant effect on tumor response. During TNFerade injections, difficulty penetrating the tumor capsule was noted. It is, therefore, quite possible that large regions of tumor were not exposed to TNFerade. Problematic penetration of the tumor capsule has also been described during pancreatic biopsies and fiducial implantation.45–48 This rationale may also explain why PTA injection produced favorable disease progression outcomes compared with EUS. Considering the difficulty of achieving homogeneous dispersion of TNFerade throughout large, fibrotic pancreatic tumors, it is possible that greater variability existing in EUS operator skill across the 39 participating institutions compared with the more straightforward PTA technique may have resulted in reduced efficacy.
It does appear that the radiation-inducible promoter was activated during radiotherapy, as demonstrated by increased pyrexia and flu-like systemic symptoms in the SOC + TNFerade arm. Although more common for patients in the SOC + TNFerade arm, the vast majority of these constitutional symptoms were grade 1 to 2 in severity and were substantially reduced compared with those described in previous studies of tumor necrosis factor alpha.22–28
Many patients in our study (33.7% in the SOC + TNFerade arm and 43.3% in the SOC arm) either developed metastases or died during the first 3 months following initiation of CRT. These findings are consistent with other studies in which 20% to 30% of patients with LAPC developed metastatic disease soon after chemotherapy or CRT.49,50 Therefore, several centers now treat LAPC with 2 to 4 months of chemotherapy and proceed with CRT if there is no evidence of systemic progression. This approach may protect against metastatic disease and “select” patients likely to benefit from aggressive local therapy.
We did not observe any difference in local response with the addition of TNFerade. One challenge of treating LAPC is determining whether CRT was effective. For example, following CRT, most locally advanced tumors are stable on CT imaging and, consequently, are not surgically explored. In the case of borderline resectable disease, however, tumors are often explored as long as there is no local or distant progression following CRT. Interestingly, several of these patients, up to 15% in some series, are found to have complete pathologic responses despite manifesting no change on CT imaging.51,52 Furthermore, a majority undergo margin- and node-negative resections, suggesting that some tumors respond to therapy without clear radiographic evidence.53,54 In this study, only 10% of patients underwent resection, so definitive conclusions cannot be drawn. MRI and/or positron emission tomography/CT imaging following CRT may better predict tumor response and enhance determination of optimal surgical candidates.55,56
It is encouraging that compliance was similar between arms despite the aggressive treatment protocol. This finding demonstrates that weekly EUS or PTA injection of TNFerade or future drugs in combination with CRT is feasible. In aggregate, grade 2 to 4 toxicity was greater for patients in the SOC + TNFerade arm. However, the increased toxicity did not preclude completion of treatment, suggesting that conditional expression of TNF-α under the Egr-1 promoter adequately limited systemic toxicity. This represents a substantial improvement from previous studies of TNF-α (eg, under a constitutive promoter) in which grade 3 to 4 toxicity related to TNF-α was dose-limiting or exceedingly high.20–29
Implications for Future Treatment
For patients with LAPC, weekly EUS or PTA injection in combination with CRT is feasible. Integrating a conditional promoter into the therapeutic gene transfer vector is a viable method of limiting severe systemic toxicity. Future studies requiring intratumoral drug injection may benefit from consideration of techniques to achieve better pancreatic tumor penetration and drug distribution given the fibrous, hypoxic nature of these tumors. In addition, future studies testing aggressive local therapies should consider enrolling patients with borderline resectable tumors, which are generally smaller and more likely to be explored despite minimal radiographic change. Since tumor response by CT alone is limited following CRT, the utility of other methods, such as positron emission tomography/CT and MRI, should be investigated.
In conclusion, TNFerade administered in the fashion tested here was shown to be safe, but not effective in prolonging survival in patients with unresectable LAPC. Given the high incidence of subsequent metastatic disease in patients with LAPC, patients should receive up-front aggressive chemotherapy to select patients who are more likely to benefit from local therapy.
Acknowledgments
Supported by GenVec.
Appendix
Table A1.
Multivariable Analysis Comparing Survival Between SOC + TNFerade and SOC Treatment Arms Performed by Using Cox Proportional Hazards Modeling
| Variables | HR | 95% CI | P |
|---|---|---|---|
| Treatment | .34 | ||
| SOC + TNFerade v SOC | 0.85 | 0.61 to 1.19 | |
| Age | 1.02 | 1.00 to 1.03 | .05 |
| Sex | .20 | ||
| Female v male | 1.24 | 0.89 to 1.73 | |
| KPS, % | .23 | ||
| < 90 v ≥ 90 | 1.26 | 0.86 to 1.86 | |
| CA19-9 | .002 | ||
| ≥ 1,000 v < 1,000 | 1.76 | 1.23 to 2.51 | |
| T stage | .12 | ||
| T4 v T1/T2/T3 | 1.35 | 0.93 to 1.97 | |
| N stage | .52 | ||
| N1 v N0 | 1.10 | 0.78 to 1.56 | |
| Nx v N0 | 1.31 | 0.81 to 2.13 | |
| M stage | .02 | ||
| M0 v Mx | 3.26 | 1.21 to 8.83 | |
| Prior cancer history | .005 | ||
| Yes v no | 6.03 | 1.72 to 21.1 | |
| Prior cancer treatment | .001 | ||
| Yes v no | 0.11 | 0.03 to 0.41 | |
| Study sites | .93 | ||
NOTE. The model included the baseline variables of age, sex, Karnofsky performance status (KPS; < 90% v ≥ 90%), T stage, N stage, M stage, cancer antigen 19-9 (CA19-9; ≥ 1,000 v < 1,000), prior cancer history, prior cancer treatment, and study site.
Abbreviations: HR, hazard ratio; SOC, standard of care.
Table A2.
Summary of Compliance With CRT and AEs Broken Down by Attributed Cause
| SOC + TNFerade (n = 186) |
SOC (n = 90) | ||||
|---|---|---|---|---|---|
| Outcome | No. | % | No. | % | P* |
| Compliance with CRT, No. of patients who missed | |||||
| At least 1 day | 121 | 65 | 56 | 62 | .69 |
| Exactly 1 day | 62 | 33 | 38 | 42 | .14 |
| Exactly 2 days | 20 | 11 | 17 | 19 | .09 |
| Exactly 3 days | 9 | 5 | 3 | 3 | .76 |
| Exactly 4 days | 7 | 4 | 4 | 4 | .75 |
| 5 or more days | 23 | 12 | 5 | 6 | .09 |
| No. of patients who missed day(s) because of an AE due to CRT | 27 | 15 | 7 | 8 | .15 |
| Grade 2 to 4 AE† | 95 | 51 | 34 | 38 | .05 |
| Grade 2 AE | 72 | 39 | 27 | 30 | .18 |
| Grade 3 AE | 47 | 25 | 15 | 17 | .13 |
| Grade 4 AE | 7 | 4 | 3 | 3 | .99 |
| No. of patients with grade 2 to 4 AE due to any element of treatment | 142 | 76 | 59 | 66 | .08 |
| TNFerade only | 19 | 10 | N/A | N/A | |
| CRT only | 30 | 16 | 16 | 18 | .73 |
| Gemcitabine only | 32 | 17 | 24 | 26 | .08 |
| Study procedure only | 7 | 4 | 0 | 0 | .10 |
| More than one element of treatment | 54 | 29 | 19 | 21 | .19 |
| No. of patients with grade 3 or 4 laboratory abnormality | 136 | 73 | 61 | 68 | .40 |
NOTE. Adverse events (AEs) were considered to be due to an element of treatment if rated as definitely or probably due to that element. The majority of missed treatment days were the result of radiation facility closures because of holidays, equipment failure, or maintenance, although only a minority of patients missed days of radiation treatment due to AEs. All patients who were considered to have completed chemoradiotherapy (CRT) made up any missed treatment days on non-weekdays or at the end of the course of therapy.
Abbreviations: N/A, not applicable; SOC, standard of care.
P values were computed by using Fisher’s exact test.
Number (and percentages) of grades 2, 3, and 4 AEs due to CRT do not total number with grade 2 to 4 AEs due to CRT (and 100%) because some patients experienced more than one grade of AE due to CRT.
Table A3.
Incidence of Grade 3 to 4 Treatment-Related Adverse Events Ordered by Frequency of Occurrence Among Patients on the SOC + TNFerade Arm
| SOC + TNFerade (n = 186) | SOC (n = 91) | |||||||
|---|---|---|---|---|---|---|---|---|
| Grade 3 | Grade 4 | Grade 3 | Grade 4 | |||||
| Toxicity Type | No. | % | No. | % | No. | % | No. | % |
| Lymphopenia | 26 | 13.98 | 8 | 4.30 | 9 | 9.89 | 2 | 2.20 |
| Abdominal pain/chest pain/back pain | 23 | 12.37 | 3 | 1.61 | 8 | 8.79 | 0 | 0 |
| Hypokalemia/hyperkalemia | 20 | 10.75 | 0 | 0 | 5 | 5.49 | 0 | 0 |
| Vomiting | 16 | 8.60 | 1 | 0.54 | 6 | 6.59 | 0 | 0 |
| Hyponatremia | 15 | 8.06 | 0 | 0 | 4 | 4.40 | 0 | 0 |
| Nausea | 13 | 6.99 | 0 | 0 | 3 | 3.30 | 0 | 0 |
| Bilirubin | 12 | 6.45 | 1 | 0.54 | 0 | 0 | 2 | 2.20 |
| Infection/bacteremia/sepsis | 12 | 6.45 | 1 | 0.54 | 3 | 3.30 | 0 | 0 |
| Anorexia | 11 | 5.91 | 0 | 0 | 3 | 3.30 | 0 | 0 |
| Biliary obstruction/cholangitis/jaundice | 11 | 5.91 | 6 | 3.23 | 8 | 8.79 | 0 | 0 |
| Dehydration | 11 | 5.91 | 0 | 0 | 0 | 0 | 1 | 1.10 |
| Anemia | 10 | 5.38 | 0 | 0 | 1 | 1.10 | 0 | 0 |
| Platelets/coagulopathy | 10 | 5.38 | 0 | 0 | 2 | 2.20 | 0 | 0 |
| Confusion/dizziness | 9 | 4.84 | 1 | 0.54 | 3 | 3.30 | 0 | 0 |
| Fatigue | 7 | 3.76 | 1 | 0.54 | 1 | 1.10 | 0 | 0 |
| GI obstruction/bleeding/abscess/hernia | 7 | 3.76 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hyperglycemia | 6 | 3.23 | 1 | 0.54 | 6 | 6.59 | 1 | 1.10 |
| Diarrhea/increased bowel movements | 5 | 2.69 | 0 | 0 | 4 | 4.40 | 0 | 0 |
| Hypophosphatemia | 5 | 2.69 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypoproteinemia/hyperproteinemia | 5 | 2.69 | 0 | 0 | 0 | 0 | 1 | 1.10 |
| Thrombosis/embolism | 5 | 2.69 | 3 | 1.61 | 1 | 1.10 | 1 | 1.10 |
| ALT | 4 | 2.15 | 0 | 0 | 0 | 0 | 0 | 0 |
| AST | 4 | 2.15 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fever | 4 | 2.15 | 1 | 0.54 | 1 | 1.10 | 0 | 0 |
| Liver function test/hepatic steatosis/diabetes | 4 | 2.15 | 2 | 1.08 | 1 | 1.10 | 0 | 0 |
| Fracture/injury/joint damage | 3 | 1.61 | 0 | 0 | 0 | 0 | 0 | 0 |
| Muscle weakness | 3 | 1.61 | 1 | 0.54 | 1 | 1.10 | 0 | 0 |
| Neutropenia | 3 | 1.61 | 0 | 0 | 1 | 1.10 | 0 | 0 |
| Renal complications | 3 | 1.61 | 0 | 0 | 1 | 1.10 | 0 | 0 |
| Weight loss | 3 | 1.61 | 0 | 0 | 1 | 1.10 | 0 | 0 |
| Alkaline phosphatase | 2 | 1.08 | 0 | 0 | 3 | 3.30 | 0 | 0 |
| Chills/sweats | 2 | 1.08 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypotension/hypertension | 2 | 1.08 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leukopenia | 2 | 1.08 | 0 | 0 | 1 | 1.10 | 1 | 1.10 |
| Rash/pruritus | 2 | 1.08 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ascites | 1 | 0.54 | 0 | 0 | 2 | 2.20 | 0 | 0 |
| Infarction/hematoma/hemorrhage | 1 | 0.54 | 0 | 0 | 0 | 0 | 1 | 1.10 |
| Shortness of breath | 1 | 0.54 | 2 | 1.08 | 0 | 0 | 0 | 0 |
NOTE. Statistical comparison between standard of care (SOC) + TNFerade and SOC arms by Fisher’s exact test yielded no significant differences in grade 3 or 4 toxicity based on a two-sided α level of .05.
Footnotes
Presented as a poster at the 48th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 1–5, 2012.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author’s immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO’s conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Kenneth J. Chang, GenVec (C) Stock Ownership: None Honoraria: None Research Funding: Kenneth J. Chang, GenVec Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Joseph M. Herman, Marcia I. Canto, Daniel A. Laheru
Administrative support: Hongyan Cai
Collection and assembly of data: Joseph M. Herman, Kenneth J. Chang, Gretchen E. Taylor, Ross C. Donehower, Hongyan Cai, Dionne T. Savage, Marcia I. Canto, Jason Klapman, Tony Reid, Raj J. Shah, Sarah E. Hoffe, Alexander Rosemurgy, Daniel A. Laheru
Data analysis and interpretation: Joseph M. Herman, Aaron T. Wild, Hao Wang, Phuoc T. Tran, Timothy M. Pawlik, Mark A. Ziegler, Christopher L. Wolfgang, Daniel A. Laheru
Manuscript writing: All authors
Final approval of manuscript: All authors
Clinical trial information: NCT00051467.
Contributor Information
Joseph M. Herman, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine
Aaron T. Wild, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine
Hao Wang, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine.
Phuoc T. Tran, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine
Kenneth J. Chang, H.H. Chao Comprehensive Digestive Disease Center, University of California Irvine Medical Center, Orange
Gretchen E. Taylor, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine
Ross C. Donehower, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine
Timothy M. Pawlik, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine
Mark A. Ziegler, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine
Hongyan Cai, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine.
Dionne T. Savage, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine
Marcia I. Canto, Johns Hopkins University School of Medicine, Baltimore, MD
Jason Klapman, H. Lee Moffitt Cancer Center & Research Institute, Tampa, FL.
Tony Reid, Rebecca and John Moores Cancer Center, University of California San Diego School of Medicine, La Jolla, CA.
Raj J. Shah, University of Colorado School of Medicine, Aurora, CO
Sarah E. Hoffe, H. Lee Moffitt Cancer Center & Research Institute, Tampa, FL
Alexander Rosemurgy, H. Lee Moffitt Cancer Center & Research Institute, Tampa, FL.
Christopher L. Wolfgang, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine
Daniel A. Laheru, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Sener SF, Fremgen A, Menck HR, et al. Pancreatic cancer: A report of treatment and survival trends for 100,313 patients diagnosed from 1985–1995, using the National Cancer Database. J Am Coll Surg. 1999;189:1–7. doi: 10.1016/s1072-7515(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 3.Tempero MA, Arnoletti JP, Behrman S, et al. Pancreatic adenocarcinoma. J Natl Compr Canc Netw. 2012;10:703–713. doi: 10.6004/jnccn.2012.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moertel CG, Frytak S, Hahn RG, et al. Therapy of locally unresectable pancreatic carcinoma: A randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil—The Gastrointestinal Tumor Study Group. Cancer. 1981;48:1705–1710. doi: 10.1002/1097-0142(19811015)48:8<1705::aid-cncr2820480803>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 5.Gastrointestinal Tumor Study Group. Treatment of locally unresectable carcinoma of the pancreas: Comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone—Gastrointestinal Tumor Study Group. J Natl Cancer Inst. 1988;80:751–755. [PubMed] [Google Scholar]
- 6.Chauffert B, Mornex F, Bonnetain F, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer: Definitive results of the 2000–01 FFCD/SFRO study. Ann Oncol. 2008;19:1592–1599. doi: 10.1093/annonc/mdn281. [DOI] [PubMed] [Google Scholar]
- 7.Klaassen DJ, MacIntyre JM, Catton GE, et al. Treatment of locally unresectable cancer of the stomach and pancreas: A randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil—an Eastern Cooperative Oncology Group study. J Clin Oncol. 1985;3:373–378. doi: 10.1200/JCO.1985.3.3.373. [DOI] [PubMed] [Google Scholar]
- 8.Loehrer PJ, Powell ME, Cardenes HR, et al. A randomized phase III study of gemcitabine in combination with radiation therapy versus gemcitabine alone in patients with localized, unresectable pancreatic cancer: E4201. J Clin Oncol. 2008;26:214s. doi: 10.1097/COC.0b013e3181e9c103. (suppl; abstr 4506) [DOI] [PubMed] [Google Scholar]
- 9.Loehrer PJ, Sr, Feng Y, Cardenes H, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: An Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29:4105–4112. doi: 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudra S, Narang AK, Pawlik TM, et al. Evaluation of predictive variables in locally advanced pancreatic adenocarcinoma patients receiving definitive chemoradiation. Pract Radiat Oncol. 2011;2:77–85. doi: 10.1016/j.prro.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 12.Mocellin S, Rossi CR, Pilati P, et al. Tumor necrosis factor, cancer and anticancer therapy. Cytokine Growth Factor Rev. 2005;16:35–53. doi: 10.1016/j.cytogfr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 13.MacGill RS, Davis TA, Macko J, et al. Local gene delivery of tumor necrosis factor alpha can impact primary tumor growth and metastases through a host-mediated response. Clin Exp Metastasis. 2007;24:521–531. doi: 10.1007/s10585-007-9089-3. [DOI] [PubMed] [Google Scholar]
- 14.Naredi PL, Lindnér PG, Holmberg SB, et al. The effects of tumour necrosis factor alpha on the vascular bed and blood flow in an experimental rat hepatoma. Int J Cancer. 1993;54:645–649. doi: 10.1002/ijc.2910540420. [DOI] [PubMed] [Google Scholar]
- 15.Mauceri HJ, Beckett MA, Liang H, et al. Translational strategies exploiting TNF-alpha that sensitize tumors to radiation therapy. Cancer Gene Ther. 2009;16:373–381. doi: 10.1038/cgt.2008.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimura K, Gelmann EP. Tumor necrosis factor-alpha and Fas activate complementary Fasassociated death domain-dependent pathways that enhance apoptosis induced by gamma-irradiation. J Biol Chem. 2000;275:8610–8617. doi: 10.1074/jbc.275.12.8610. [DOI] [PubMed] [Google Scholar]
- 17.Seung LP, Mauceri HJ, Beckett MA, et al. Genetic radiotherapy overcomes tumor resistance to cytotoxic agents. Cancer Res. 1995;55:5561–5565. [PubMed] [Google Scholar]
- 18.Hallahan DE, Spriggs DR, Beckett MA, et al. Increased tumor necrosis factor alpha mRNA after cellular exposure to ionizing radiation. Proc Natl Acad Sci U S A. 1989;86:10104–10107. doi: 10.1073/pnas.86.24.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lejeune FJ, Liénard D, Matter M, et al. Efficiency of recombinant human TNF in human cancer therapy. Cancer Immun. 2006;6:6. [PubMed] [Google Scholar]
- 20.Hieber U, Heim ME. Tumor necrosis factor for the treatment of malignancies. Oncology. 1994;51:142–153. doi: 10.1159/000227329. [DOI] [PubMed] [Google Scholar]
- 21.Chapman PB, Lester TJ, Casper ES, et al. Clinical pharmacology of recombinant human tumor necrosis factor in patients with advanced cancer. J Clin Oncol. 1987;5:1942–1951. doi: 10.1200/JCO.1987.5.12.1942. [DOI] [PubMed] [Google Scholar]
- 22.Feinberg B, Kurzrock R, Talpaz M, et al. A phase I trial of intravenously-administered recombinant tumor necrosis factor-alpha in cancer patients. J Clin Oncol. 1988;6:1328–1334. doi: 10.1200/JCO.1988.6.8.1328. [DOI] [PubMed] [Google Scholar]
- 23.Spriggs DR, Sherman ML, Michie H, et al. Recombinant human tumor necrosis factor administered as a 24-hour intravenous infusion: A phase I and pharmacologic study. J Natl Cancer Inst. 1988;80:1039–1044. doi: 10.1093/jnci/80.13.1039. [DOI] [PubMed] [Google Scholar]
- 24.Lienard D, Ewalenko P, Delmotte JJ, et al. High-dose recombinant tumor necrosis factor alpha in combination with interferon gamma and melphalan in isolation perfusion of the limbs for melanoma and sarcoma. J Clin Oncol. 1992;10:52–60. doi: 10.1200/JCO.1992.10.1.52. [DOI] [PubMed] [Google Scholar]
- 25.Thom AK, Alexander HR, Andrich MP, et al. Cytokine levels and systemic toxicity in patients undergoing isolated limb perfusion with high-dose tumor necrosis factor, interferon gamma, and melphalan. J Clin Oncol. 1995;13:264–273. doi: 10.1200/JCO.1995.13.1.264. [DOI] [PubMed] [Google Scholar]
- 26.Fraker DL, Alexander HR, Andrich M, et al. Treatment of patients with melanoma of the extremity using hyperthermic isolated limb perfusion with melphalan, tumor necrosis factor, and interferon gamma: Results of a tumor necrosis factor dose-escalation study. J Clin Oncol. 1996;14:479–489. doi: 10.1200/JCO.1996.14.2.479. [DOI] [PubMed] [Google Scholar]
- 27.Alexander HR, Jr, Bartlett DL, Libutti SK, et al. Isolated hepatic perfusion with tumor necrosis factor and melphalan for unresectable cancers confined to the liver. J Clin Oncol. 1998;16:1479–1489. doi: 10.1200/JCO.1998.16.4.1479. [DOI] [PubMed] [Google Scholar]
- 28.Alexander HR, Libutti SK, Bartlett DL, et al. A phase I–II study of isolated hepatic perfusion using melphalan with or without tumor necrosis factor for patients with ocular melanoma metastatic to liver. Clin Cancer Res. 2000;6:3062–3070. [PubMed] [Google Scholar]
- 29.Marr RA, Hitt M, Muller WJ, et al. Tumour therapy in mice using adenovirus vectors expressing human TNFa. Int J Oncol. 1998;12:509–515. doi: 10.3892/ijo.12.3.509. [DOI] [PubMed] [Google Scholar]
- 30.Hallahan DE, Mauceri HJ, Seung LP, et al. Spatial and temporal control of gene therapy using ionizing radiation. Nat Med. 1995;1:786–791. doi: 10.1038/nm0895-786. [DOI] [PubMed] [Google Scholar]
- 31.Datta R, Rubin E, Sukhatme V, et al. Ionizing radiation activates transcription of the EGR1 gene via CArG elements. Proc Natl Acad Sci U S A. 1992;89:10149–10153. doi: 10.1073/pnas.89.21.10149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mauceri HJ, Hanna NN, Wayne JD, et al. Tumor necrosis factor alpha (TNF-alpha) gene therapy targeted by ionizing radiation selectively damages tumor vasculature. Cancer Res. 1996;56:4311–4314. [PubMed] [Google Scholar]
- 33.Staba MJ, Mauceri HJ, Kufe DW, et al. Adenoviral TNF-alpha gene therapy and radiation damage tumor vasculature in a human malignant glioma xenograft. Gene Ther. 1998;5:293–300. doi: 10.1038/sj.gt.3300594. [DOI] [PubMed] [Google Scholar]
- 34.Chung TD, Mauceri HJ, Hallahan DE, et al. Tumor necrosis factor-alpha-based gene therapy enhances radiation cytotoxicity in human prostate cancer. Cancer Gene Ther. 1998;5:344–349. [PubMed] [Google Scholar]
- 35.Manome Y, Kunieda T, Wen PY, et al. Transgene expression in malignant glioma using a replication-defective adenoviral vector containing the Egr-1 promoter: Activation by ionizing radiation or uptake of radioactive iododeoxyuridine. Hum Gene Ther. 1998;9:1409–1417. doi: 10.1089/hum.1998.9.10-1409. [DOI] [PubMed] [Google Scholar]
- 36.Gupta VK, Park JO, Jaskowiak NT, et al. Combined gene therapy and ionizing radiation is a novel approach to treat human esophageal adenocarcinoma. Ann Surg Oncol. 2002;9:500–504. doi: 10.1007/BF02557275. [DOI] [PubMed] [Google Scholar]
- 37.Senzer N, Mani S, Rosemurgy A, et al. TNFerade biologic, an adenovector with a radiation-inducible promoter, carrying the human tumor necrosis factor alpha gene: A phase I study in patients with solid tumors. J Clin Oncol. 2004;22:592–601. doi: 10.1200/JCO.2004.01.227. [DOI] [PubMed] [Google Scholar]
- 38.Mundt AJ, Vijayakumar S, Nemunaitis J, et al. A Phase I trial of TNFerade biologic in patients with soft tissue sarcoma in the extremities. Clin Cancer Res. 2004;10:5747–5753. doi: 10.1158/1078-0432.CCR-04-0296. [DOI] [PubMed] [Google Scholar]
- 39.Hecht JR, Farrell JJ, Senzer N, et al. EUS or percutaneously guided intratumoral TNFerade biologic with 5-fluorouracil and radiotherapy for first-line treatment of locally advanced pancreatic cancer: A phase I/II study. Gastrointest Endosc. 2012;75:332–338. doi: 10.1016/j.gie.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hutchinson TA, Boyd NF, Feinstein AR, et al. Scientific problems in clinical scales, as demonstrated in the Karnofsky index of performance status. J Chronic Dis. 1979;32:661–666. doi: 10.1016/0021-9681(79)90096-1. [DOI] [PubMed] [Google Scholar]
- 41.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 42.Hata H, Mori H, Matsumoto S, et al. Fibrous stroma and vascularity of pancreatic carcinoma: Correlation with enhancement patterns on CT. Abdom Imaging. 2010;35:172–180. doi: 10.1007/s00261-008-9460-0. [DOI] [PubMed] [Google Scholar]
- 43.Johnson PT, Outwater EK. Pancreatic carcinoma versus chronic pancreatitis: Dynamic MR imaging. Radiology. 1999;212:213–218. doi: 10.1148/radiology.212.1.r99jl16213. [DOI] [PubMed] [Google Scholar]
- 44.Adsay NV, Bandyopadhyay S, Basturk O, et al. Chronic pancreatitis or pancreatic ductal adenocarcinoma? Semin Diagn Pathol. 2004;21:268–276. doi: 10.1053/j.semdp.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Sakamoto H, Kitano M, Komaki T, et al. Prospective comparative study of the EUS guided 25-gauge FNA needle with the 19-gauge Trucut needle and 22-gauge FNA needle in patients with solid pancreatic masses. J Gastroenterol Hepatol. 2009;24:384–390. doi: 10.1111/j.1440-1746.2008.05636.x. [DOI] [PubMed] [Google Scholar]
- 46.Fabbri C, Polifemo AM, Luigiano C, et al. Endoscopic ultrasound-guided fine needle aspiration with 22- and 25-gauge needles in solid pancreatic masses: A prospective comparative study with randomisation of needle sequence. Dig Liver Dis. 2011;43:647–652. doi: 10.1016/j.dld.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 47.Sanders MK, Moser AJ, Khalid A, et al. EUS-guided fiducial placement for stereotactic body radiotherapy in locally advanced and recurrent pancreatic cancer. Gastrointest Endosc. 2010;71:1178–1184. doi: 10.1016/j.gie.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 48.Hovdenak N, Lees WR, Pereira J, et al. Ultrasound-guided percutaneous fine-needle aspiration cytology in pancreatic cancer. Br Med J (Clin Res Ed) 1982;285:1183–1184. doi: 10.1136/bmj.285.6349.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fischer R, Breidert M, Keck T, et al. Early recurrence of pancreatic cancer after resection and during adjuvant chemotherapy. Saudi J Gastroenterol. 2012;18:118–121. doi: 10.4103/1319-3767.93815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haeno H, Gonen M, Davis MB, et al. Computational modeling of pancreatic cancer reveals kinetics of metastasis suggesting optimum treatment strategies. Cell. 2012;148:362–375. doi: 10.1016/j.cell.2011.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barugola G, Partelli S, Crippa S, et al. Outcomes after resection of locally advanced or borderline resectable pancreatic cancer after neoadjuvant therapy. Am J Surg. 2012;203:132–139. doi: 10.1016/j.amjsurg.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 52.Stokes JB, Nolan NJ, Stelow EB, et al. Preoperative capecitabine and concurrent radiation for borderline resectable pancreatic cancer. Ann Surg Oncol. 2011;18:619–627. doi: 10.1245/s10434-010-1456-7. [DOI] [PubMed] [Google Scholar]
- 53.Gillen S, Schuster T, Meyer Zum Büschen-felde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: A systematic review and metaanalysis of response and resection percentages. PLoS Med. 2010;7:e1000267. doi: 10.1371/journal.pmed.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McClaine RJ, Lowy AM, Sussman JJ, et al. Neoadjuvant therapy may lead to successful surgical resection and improved survival in patients with borderline resectable pancreatic cancer. HPB (Oxford) 2010;12:73–79. doi: 10.1111/j.1477-2574.2009.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wahl RL, Jacene H, Kasamon Y, et al. From RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(suppl 1):122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Serrano OK, Chaudhry MA, Leach SD. The role of PET scanning in pancreatic cancer. Adv Surg. 2010;44:313–325. doi: 10.1016/j.yasu.2010.05.007. [DOI] [PubMed] [Google Scholar]