Abstract
Signaled active avoidance (AA) paradigms train subjects to prevent an aversive outcome by performing a learned behavior during the presentation of a conditioned cue. This complex form of conditioning involves Pavlovian and instrumental components, which produce competing behavioral responses that must be reconciled for the subject to successfully avoid an aversive stimulus. In signaled AA paradigm for rat, we tested the hypothesis that the instrumental component of AA training recruits infralimbic prefrontal cortex (ilPFC) to inhibit central amygdala (CeA) mediated Pavlovian reactions. Pre-training lesions of ilPFC increased conditioned freezing while causing a corresponding decrease in avoidance; lesions of CeA produced opposite effects, reducing freezing and facilitating avoidance behavior. Pharmacological inactivation experiments demonstrated that ilPFC is relevant to both acquisition and expression phases of AA learning. Inactivation experiments also revealed that AA produces an ilPFC-mediated diminution of Pavlovian reactions that extends beyond the training context, even when the conditioned stimulus is presented in an environment that does not allow the avoidance response. Finally, injection of a protein synthesis inhibitor into either ilPFC or CeA impaired or facilitated AA, respectively, showing that avoidance training produces two opposing memory traces in these regions. These data support a model in which AA learning recruits ilPFC to inhibit CeA-mediated defense behaviors, leading to a robust suppression of freezing that generalizes across environments. Thus, ilPFC functions as an inhibitory interface, allowing instrumental control over an aversive outcome to attenuate the expression of freezing and other reactions to conditioned threat.
Introduction
The acquisition of signaled active avoidance (AA) behavior depends on two sequential forms of learning (Mowrer and Lamoreuax, 1946). Initially, subjects undergo Pavlovian threat conditioning, in which a previously neutral conditioned stimulus (CS) comes to predict an aversive unconditioned stimulus (US; Pavlov, 1927; Rescorla and Wagner, 1972). In subsequent trials, subjects acquire an instrumental avoidance contingency, in which a behavior performed during the CS prevents US delivery (Cain and LeDoux, 2008; Mowrer and Lamoreuax, 1946). In a typical experiment, a rat is placed in a divided chamber and trained to shuttle between compartments during an auditory CS to avoid a foot shock US.
Intriguingly, these two forms of conditioning yield opposing behavioral endpoints, and early trials of AA training are characterized by a conflict between mutually exclusive responses to the C S. While Pavlovian information is essential for signaled AA behavior, it produces freezing and other conditioned reactions that obstruct avoidance. As the instrumental contingency is acquired, subjects achieve a robust suppression of these reactive behaviors (Cain and LeDoux, 2010; Solomon and Wynne, 1954), which remain attenuated even when the CS is presented in environments that do not allow subjects to perform the avoidance response (Kamin et al, 1963). Thus, by gaining control over US delivery, subjects successfully inhibit the expression of deeply instated behavioral reactions that normally prevail in situations of expected threat (Blanchard et al, 2005; Fanselow and Lester, 1988; LeDoux, 2012).
This study explores the neural substrates by which competing CS-evoked responses are resolved over the course of signaled AA training. Varying lines of evidence implicate the central nucleus of the amygdala (CeA) in the production of the conditioned freezing responses (Ciocchi et al, 2010; Haubensak et al, 2010; Wilensky et al, 2006) that directly opposes AA (Choi et al, 2010; Lazaro-Munoz et al, 2010). In contrast, stimulation of the infralimbic prefrontal cortex (ilPFC) blunts the excitatory response of brainstem-projecting CeA neurons (Likhtik et al, 2005; Quirk et al, 2003) crucial for the expression of freezing (Ciocchi et al, 2010; Duvarci et al, 2011). Complementary studies suggest a role for ilPFC in forms of learning that attenuate the freezing response (Morgan and LeDoux, 1995; Quirk et al, 2000; Santini et al, 2004; Sierra-Mercado et al, 2011), while other experiments demonstrate the involvement of ilPFC in the detection of aversive behavioral contingencies (Amat et al, 2005; Amat et al, 2006). As such, we hypothesize that the conflict between Pavlovian and instrumental processes is resolved by ilPFC, which exerts feed-forward inhibition on CeA to suppress the conditioned reactions that oppose avoidance. The experiments described below use lesion and pharmacological techniques to assess the role of ilPFC and CeA in a signaled AA paradigm previously established in this laboratory. Results suggest that ilPFC is a key substrate by which behavioral control can alter the expression of CeA-mediated defense reactions.
Materials and Methods
Animals
Subjects were 166 naïve male Sprague-Dawley rats (Hilltop Laboratories) weighing 250–300g at the time of arrival. Rats were individually housed in plastic tubs with ad libitum access to food and water, and kept on a 12h light/dark cycle (lights on at 8AM). All procedures were approved by the NYU University Animal Welfare Committee.
Apparatus
Signaled active avoidance apparatus
Signaled active avoidance training occurred in 6 identical Plexiglas and metal rectangular shuttle boxes (50.8 × 25.4 × 30.5cm, LWH) separated into two equal compartments by a metal divider placed halfway along the length of the chamber (Coulbourn Instruments). A passage in the divider (8 × 9cm, WH) allowed animals to move freely between compartments. The floor was comprised of conductive stainless steel bars. Two speakers mounted on opposite walls of the chamber delivered a 5kHz, 70db tone CS; a .7mA footshock US was administered via the floor by a scrambled shocker. The chamber was lit by two .5W light bulbs, one in each compartment. The shuttle box was housed within a lager sound-attenuating cubicle.
Shuttling (movement from one compartment to the other) was monitored by two infrared arrays, each comprised of 5 emitter-detector pairs and located on either side of the metal divider. Sessions were also recorded on DVD by a pair of black and white infrared cameras, one in each compartment.
CS test apparatus
Two distinct contexts were used to test the animals’ response to the CS outside the avoidance-training environment. These contexts were created in 8 identical square chambers (26×28×20cm, LWH; Coulbourn Instruments) made of clear Plexiglas and metal with a floor of stainless steel bars. In one context, a black Plexiglas square was placed over the floor bars and the chamber was scented with peppermint. In the other, the floor bars were left exposed, a pattern of black and white stripes was placed behind the two clear Plexiglas walls and the chamber was scented with lavender. In both contexts a tone CS identical to the one used in the avoidance environment was delivered from a wall-mounted speaker. Sessions were recorded using a black and white infrared cameras anchored to the ceiling. Each chamber was housed within a sound-attenuating cubicle.
Surgery
Electrolytic lesions
Animals were anesthetized with a cocktail of ketamine (75 mg/kg) and xylazine (10 mg/kg) and mounted into a steroetaxic frame. The skull was exposed and bilateral boreholes were made. A stainless steel electrode, insulated except for a .5 mm exposed tip, was lowered into the target brain regions to produce a lesion. Coordinates for ilPFC were: AP + 2.5, ML ± 0.6, DV −5.2; 1 mA of current was passed for 10 sec. Coordinates for plPFC were: AP +3.2, ML ±0.6, DV −4.0; 1 mA of current was passed for 10 sec. Coordinates for CeA lesion were: AP −1.8, −2.3, −2.8; ML ±3.8, ±4.0, ±4.4; DV −8.4; .5mA of current was passed for 10 sec at all drop points. For sham lesions, the electrode was lowered into place, but no current was passed. Animals were allowed to recover for 14 days before the initiation of behavioral training.
ilPFC and CeA cannula implantation
Animals were anesthetized with a cocktail of ketamine/xylazine (dose as above), mounted into the stereotaxis and implanted with two 22-gauge chronic guide cannula aimed at ilPFC or CeA. Coordinates for ilPFC were: AP +2.8, ML ±3.1, DV −3.8; cannula angled at 30° (as in Sierra Mercado et al., 2010). Coordinates for CeA were: AP −2.3, ML ±4.0, DV −8.2. A stainless steel obdurator was used to keep the cannula patent prior to injection. Animals were allowed 14 days recovery before the initiation of behavioral training.
Drugs and Intracranial Infusions
Muscimol
The GABAA agonist muscimol was used to inactivate ilPFC. Muscimol was dissolved in aCSF and adjusted to pH 7.4. The dose utilized was .02µg/.2µL/side for all inactivation experiments. Animals were gently restrained and fitted with internal cannulae attached to a 1µL Hamilton syringe with PE 20 tubing. An infusion pump administered the injection at a rate of .1µL/min for 2min; the internal cannulae were left in place for another minute to allow for diffusion. All muscimol injections began 20min before behavioral testing.
Anisomycin
The protein synthesis inhibitor anisomycin was used to impair consolidation in the ilPFC and CeA. Anisomycin was slowly dissolved in aCSF using HCl; once sufficiently suspended in vehicle, the pH was adjusted to 7.4 using NaOH. The dose utilized was 12.5µg/.2µL/side. Infusion techniques were identical to those used for muscimol, except that all anisomycin injections began 20min after the cessation of behavioral testing.
Behavioral Procedures
Signaled active avoidance training
On the day prior to the initiation of training, all animals were habituated to the shuttle box for 1 hr; shuttling (movement from one sub-compartment to the other) was recorded as a measure of baseline locomotor activity. 24 hr later the first of 5 consecutive daily avoidance-training sessions began. Each session started with a 5 min acclimation period in which no stimuli were presented. The 1st trial of the 1st session was a Pavlovian trial – a 15 sec tone CS preceded a 1 sec foot shock US regardless of whether the animal performed the avoidance response (shuttling) during CS presentation. This allowed all animals to acquire the Pavlovian contingency at the same point in training. All subsequent trials were avoidance trials. The CS lasted a maximum of 15 sec and was followed immediately by a US that lasted a maximum of 15 sec. If the animal shuttled during CS presentation, the tone terminated immediately and the US was not delivered; this was scored as an avoidance response. If the animal shuttled during US presentation, the shock immediately ceased and this was scored as an escape response. Each session was comprised of 30 avoidance CSs with a varying inter-trial interval that averaged 120 sec.
Stimulus delivery and data collection was fully automated by a PC running GraphicState software (Coulbourn Instruments). Freezing was scored offline by a trained rater blinded to group, and was defined as immobility with the exception of respiratory movement. For freezing during signaled avoidance training, every 5th avoidance CS starting with the 1st was scored and then averaged to generate a representative sample for a given session. On the 1st day, freezing to the initial Pavlovian CS was scored as a baseline, but not included in the average for that day.
c-Fos test
This experiment involved three groups, including standard ilPFC and sham lesion groups, as well as an sham lesion group and was placed in the avoidance apparatus for the same period as the other groups, but received no conditioning. This last group served as a control for baseline levels of c-Fos activity evoked by handling, the apparatus, etc. Animals were habituated to the shuttlebox before receiving 2 normal sessions of avoidance training. The 3rd session involved 15 CSs with no USs, in order to test the effects of the CS on CeA c-Fos immunoreactivity without the potential confound of shock. 90 min after the cessation of this session, animals were deeply anesthetized and perfused.
CS test
In this experiment, animals received only 3 consecutive daily sessions of signaled avoidance, which is the normative time to asymptote in the avoidance paradigm employed here. On the day prior to the initiation of avoidance training, animals were habituated to the chamber for 1 hr, then to one of two alternate contexts for 1 hr. After the 1st avoidance session, animals were placed back in the alternate context to which they were habituated on the preceding day, allowed to acclimate for 5 min and then played a 2 min CS. On the following day, after the 2nd avoidance training session, animals were habituated for 1 hr to the alternate context that they did not previously experience. Finally, following the third and final avoidance session, animals were infused with either muscimol or vehicle into ilPFC 20 min prior to being placed in the context to which they had been habituated the day before. After 5 min of acclimation, animals were exposed to a 2 min CS. Subjects experienced the two alternate contexts in a counterbalanced order.
Freezing was scored offline, as described above. Defecation was used as a corroborating measure of conditioned fear; animals were removed from the chamber immediately following both CS test sessions, and the number of boluses was counted by the experimenter.
Shock-free probe session
Animals were habituated to the shuttle box and trained for 2 sessions of avoidance prior to a 3rd and final session in which no US was delivered. Either muscimol or vehicle was infused into the ilPFC 20 minutes prior to this shock-free session, which consisted of thirty unreinforced CSs that were inactivated by shuttling. Animals received no shock at any point; the trial was otherwise identical to a normal AA session.
Perfusion and Histology
Cannula/lesion placement
At the end of each behavioral experiment, animals were deeply anesthetized and transcardially perfused with 10% buffered formalin. Brains were removed and sliced into 40 µm sections using a cryostat. Sections were then stained with cressyl violet and examined under a microscope. A camera lucida was used to demarcate the extent of lesions and to determine cannula placement.
c-Fos immunochemistry
90 minutes following the end of behavioral testing in the c-Fos experiment, animals were deeply anesthetized and transcardially perfused with 4% paraformaldehyde. Brains were cut into 40 µm sections on a freezing microtome; sections containing ilPFC were stained with cressyl violet to determine the extent of lesion, while sections containing CeA were processed for c-Fos. Free-floating sections were washed with phosphate-buffered saline (PBS; 0.01M, pH 7.6 at room temperature) between the different treatments and stained using the ABC method. Sections were incubated for 1 h in 1% bovine serum albumin (Sigma, St. Louis, CA) to block nonspecific binding, and then incubated for 24 h in the c-Fos primary antibody—1:10,000 c-Fos (polyclonal rabbit anti-c-Fos, Santa Cruz Biotechnology, Santa-Cruz, CA) + 0.2% Triton X (Sigma, St. Louis, MO) + 1% BSA. Sections were incubated for 30 min in the secondary antibody Anti Rabbit IgG (Elite-Anti Rabbit Vector Kit, Vector Labs, Burlingame, CA), and for 30 min in the avidin–biotin–horseradish-peroxidase complex (Elite-Anti Rabbit Vector Kit, Vector labs). Staining was visualized using the chromogen Very Intense Purple (Vector Labs) + 0.01% H2O2 (Vector Labs). Sections were then dehydrated and cover-slipped.
c-Fos-positive cells were recorded by visual inspection in a light microscope. The CeA-containing section quantified from each animal was most similar to Bregma −2.28. At 4× magnification, the right CeA was framed in the center 4 squares of a 10×10 grid. Magnification was then increased to 20X for quantification. c-Fos counts were made by two trained raters blinded to experimental condition. The average of these two counts was used as the score for each animal.
Statistical Analysis
Avoidance and freezing data derived from lesion, muscimol and anisomycin experiments involving a multi-session learning curve were analyzed using a two-way mixed design ANOVA with a between subjects factor of group (lesion or drug, depending on the experiment) and a within subjects factor of session. When appropriate, Bonferroni post-hoc tests were used for multiple comparisons of group differences across individual sessions. For the CS-test experiment, two-way ANOVA was followed by two-tailed t-test for post-hoc analysis, because these data only required a single comparison. c-Fos data were analyzed with a one-way ANOVA, followed by Bonferroni post-hoc tests comparing lesion to sham and lesion to box control groups. Two-tailed t-tests were also used to analyze data from experiments that required the comparison of two means only, such as the shock-free probe experiment and behavioral data from the c-Fos experiment.
Results
Electrolytic Lesions and Signaled Active Avoidance
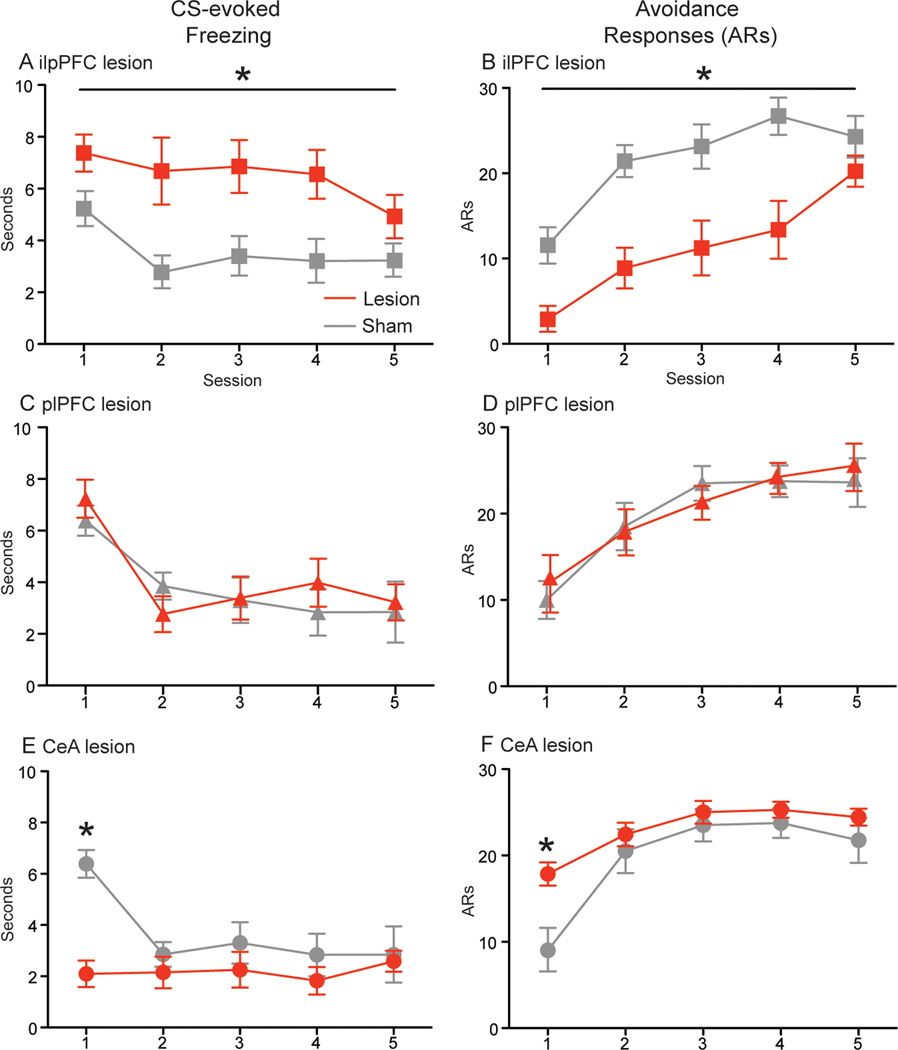
An initial series of experiments utilized electrolytic lesions to assess the role of the prefrontal cortex and amygdala in the transition from conditioned freezing to active avoidance (greatest and least extent of all lesions displayed in Fig. 1). In the first of these, rats were prepared with electrolytic ilPFC lesions (n=8) or sham lesions (n=8) prior to 5 daily sessions of active avoidance training. A two-way ANOVA with a between subjects factor of LESION and a within subjects factor of SESSION was performed on CS-evoked freezing data collected during active avoidance training. This analysis revealed a main effect for LESION [F(1,14)= 14.78, p=.002], indicating that ilPFC lesion increased conditioned freezing across sessions (Fig. 2A). An identical analysis was performed on avoidance responses from the same animals. ANOVA revealed a main effect for LESION [F(1,14)= 17.32, p=.001], indicating that ilPFC lesion reduced the number of avoidance responses across sessions (Fig. 2B). Thus, ilPFC suppresses Pavlovian defense reactions, thereby facilitating the acquisition of the avoidance response.
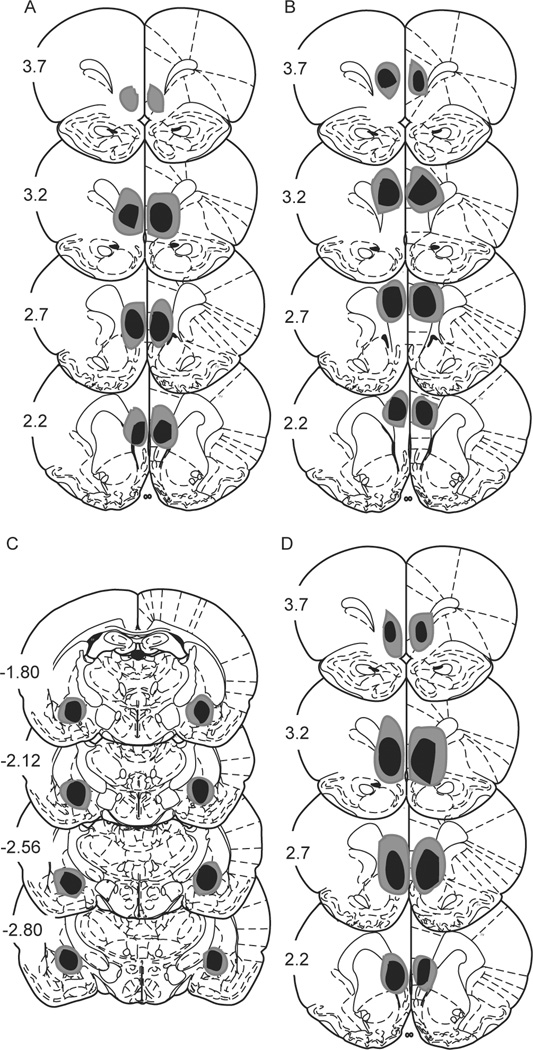
Fig. 1.
Lesion placements. Shaded areas represent the greatest (gray) and least (black) extent of electrolytic lesions; numbers reflect distance from Bregma in mm. Lesions A–C occurred prior to 5 days of signaled active avoidance training, lesion D occurred prior to 2 days of signaled active avoidance followed by a 15 CS test for c-Fos. A) infralimbic prefrontal cortex lesions, B) prelimbic prefrontal cortex lesions, C) central nucleus of the amygdala lesions, D) infralimbic prefrontal cortex lesions.
Fig. 2.
Effects of pre-training electrolytic lesions on mean (±SEM) freezing (left column) and mean (±SEM) avoidance responses (right column). A–B) ilPFC lesion (n=8) and sham (n=8), C–D) plPFC lesion (n=8) and sham (n=8), E–F) CeA lesion (n=7) and sham (n=7). *indicates p<.05.
Because some ilPFC lesions extended dorsally into the ventral portions of the adjacent prelimbic prefrontal cortex (plPFC), a control experiment targeting plPFC was performed. ANOVA revealed no differences between lesion (n=8) and sham (n=8) groups for either freezing or avoidance (Figs. 2C and 2D). It seems that plPFC lesions have no effect on avoidance training when conducted at a pre-training time point.
In order to examine the role of an important substrate of CS-evoked freezing, animals were prepared with electrolytic CeA lesions (n=7) or sham lesions (n=7) prior to active avoidance training. CS-evoked freezing from lesion and sham groups was analyzed using a two-way mixed design ANOVA with a between subjects factor of LESION and a within subjects factor of SESSION. This analysis revealed a LESION X SESSION interaction [F(4, 48)=6.19, p<.001], and Bonferroni post-hoc tests confirmed a significant decrease in freezing by the lesion group during the 1st session (p<.01; Fig. 2E). An identical ANOVA was performed on avoidance responses from the same animals. This revealed a LESION X SESSION interaction [F(4, 48)=3.89, p=.008], which Bonferroni post-hoc tests confirmed was caused by a significant increase in avoidance responses among lesion animals during the 1st session (p<.01; Fig. 2F). These data are commensurate with a wide body of evidence implicating CeA in Pavlovian threat conditioning (Ciocchi et al, 2010; Haubensak et al, 2010; Wilensky et al, 2006), and indicate that CeA underpins the coditioned reactions that oppose active avoidance behavior.
To control for the possible locomotor effects of ilPFC and CeA lesions, subjects’ shuttling behavior was recorded during habituation to the avoidance apparatus. ilPFC lesion animals shuttled a mean 69.5 (±4.5) times, and their corresponding sham controls shuttled a mean 75.9 (±3.9) times during the 1hr habituation session; two-tailed t-test found no difference between lesion and sham. CeA lesion animals shuttled a mean 91.4 (±9.3) times, while their corresponding shams shuttled a mean 101 (±9.9) times during habituation; two-tailed t-test found no difference between lesion and sham. These data suggest that lesions of ilPFC and CeA do not disrupt basic locomotion.
Taken together, these lesion data suggest opposite roles for ilPFC and CeA in the acquisition of signaled active avoidance, with ilPFC facilitating the avoidance response by attenuating conditioned freezing, and CeA opposing avoidance by driving the expression of Pavlovian defense reactions.
To examine whether the behavioral effects of ilPFC lesion are associated with changes in CeA activity, animals were prepared with ilPFC and sham lesions and given two normal sessions of signaled active avoidance training before a test session comprised of 15 CSs with no USs. Following this 15 CS test session, animals were sacrificed and processed for c-Fos in CeA. c-fos counts from ilPFC lesion (n=8) and sham lesion (n=8) groups were analyzed along with an additional sham lesion group (n=8) that received no conditioning and served as a box control. Separate two-tailed t-tests revealed that animals with ilPFC lesions showed higher levels of CS-evoked freezing (p=.021, Fig. 3A) and made fewer avoidance responses (p=.032, Fig. 3B) during the 15 CS test session. A one-way ANOVA performed on CeA c-Fos counts from ilPFC lesion, sham lesion, and box control groups revealed a significant main effect [F(2, 21)=4.772, p=.020] (Fig. 3C); Bonferroni post-hoc tests confirmed a significant increase in the ilPFC lesion group relative to shams (p<.05), as well as a significant increase in the ilPFC lesion group relative to box controls (p<.05). These data demonstrate that the effects of ilPFC lesion are associated with enhanced activity in CeA, implying that ilPFC inhibits freezing and facilitates avoidance by suppressing activity in a key substrate of Pavlovian defensive behavior.
Fig. 3.
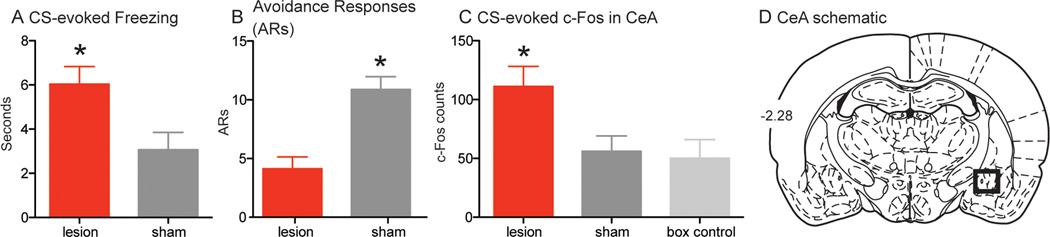
Effects of ilPFC lesion on behavior and CeA c-Fos activity during a 15 CS test session. A) Mean (± SEM) freezing, B) Mean (± SEM) avoidance responses, C) Mean (± SEM) c-Fos counts in CeA, D) Schematic of CeA sections sampled for c-Fos, number reflects distance from Bregma in mm.
Pharmacological Inactivation of ilPFC and Signaled Active Avoidance
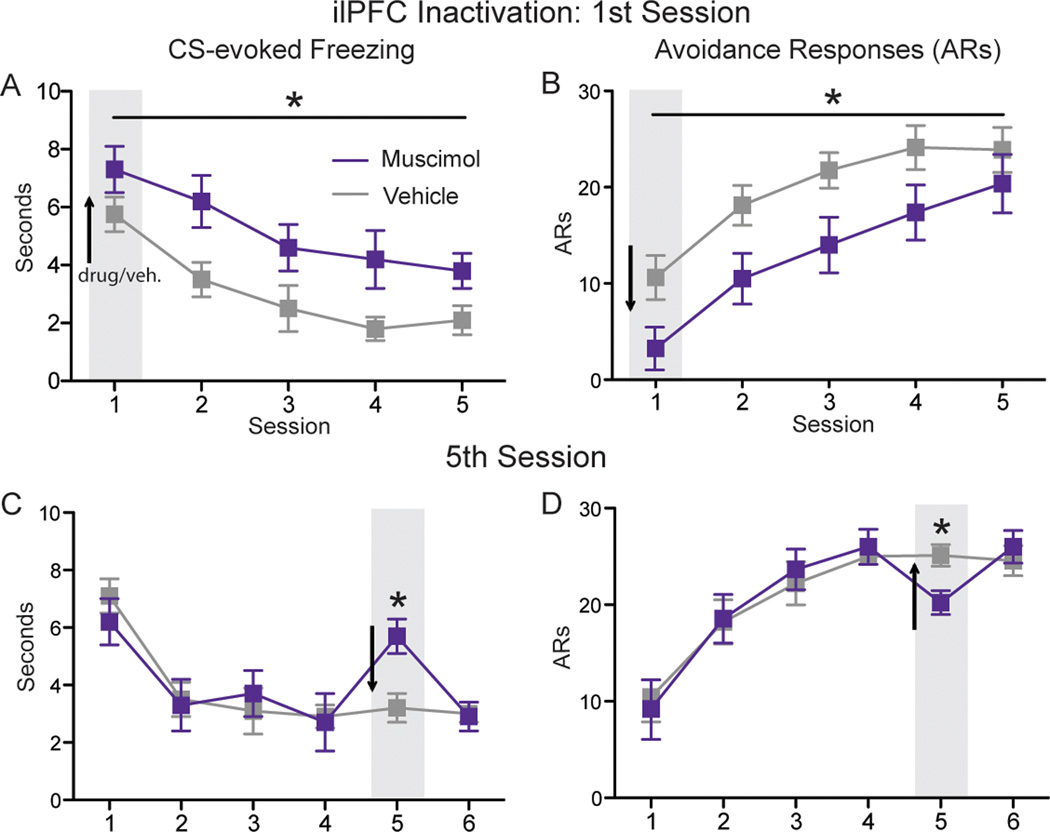
Though spatially discrete, lesions lack temporal precision, and electrolytic lesions destroy fibers of passage. To control for these issues, as well as to examine the role of ilPFC during the acquisition and expression phases of the active avoidance learning curve, two temporary inactivation experiments were conducted. Inactivation of ilPFC was achieved by the intracranial microinjection of the GABAA agonist muscimol. In the first experiment, muscimol (n=8) or vehicle (n=8) was infused before the first session of avoidance training in order to assess the role of ilPFC in acquisition (cannula placement displayed in Fig. 4A). CS-evoked freezing from muscimol and vehicle groups was analyzed using a two-way ANOVA with a between subjects factor of DRUG and a within subjects factor of SESSION. This analysis revealed a main effect for DRUG [F(1,14)=10.28, p<.001], indicating that ilPFC muscimol caused a significant increase in freezing across 5 daily sessions of training (Fig. 5A). An identical ANOVA was performed on avoidance responses from the same animals. This analysis revealed a main effect for DRUG [F(1, 14)= 7.26, p=.017], indicating that ilPFC muscimol caused a significant decrease in avoidance responses across training (Fig. 5B). These data suggest that a functioning ilPFC is required during the acquisition phase of active avoidance learning in order for animals to effectively suppress freezing and achieve normal levels of avoidance behavior in subsequent sessions.
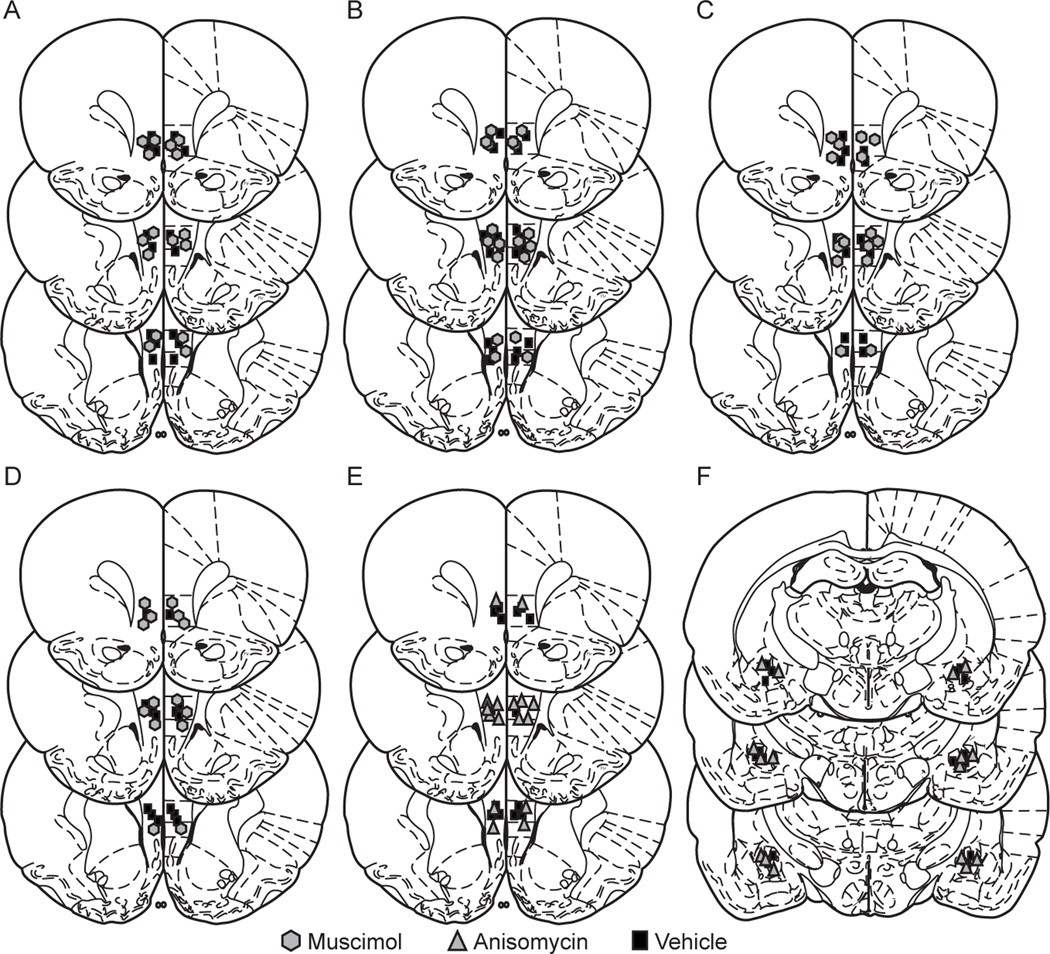
Fig. 4.
Cannula placements for all intracranial microinjection experiments: numbers reflect distance from Bregma in mm. A) cannula placement for ilPFC inactivation prior to the 1st session of AA training, B) cannula placement for ilPFC inactivation prior to the 5th session of active avoidance training, C) cannula placement for ilPFC inactivation prior to CS-test in an alternate context, D) cannula placement for ilPFC inactivation prior to a shock-free probe session, E) cannula placement for ilPFC protein synthesis inhibition following the 1st session of active avoidance training, F) cannula placement for CeA protein synthesis inhibition following the 1st session of active avoidance training.
Fig. 5.
The effects of ilPFC inactivation on freezing (left column) and avoidance responses (right column), light gray bars and black arrows indicate the session before which muscimol or vehicle was injected into ilPFC. A) Mean (±SEM) freezing and B) mean (±SEM) avoidance responses when ilPFC was infused with muscimol (n=8) or vehicle (n=8) prior to the 1st session of training. C) Mean (±SEM) freezing and D) mean (±SEM) avoidance responses when ilPFC was infused with muscimol (n=9) or vehicle (n=9) prior to the 5th session of training. *indicates p<.05.
To examine the role of ilPFC during the expression phase of training, muscimol (n=9) or vehicle (n=9) was infused prior to the 5th session of active avoidance (cannula placement displayed in Fig. 4B). Animals were given an additional day of training to assess the lingering effects of muscimol treatment. Normal acquisition occurred over the first 4 days of training. CS-evoked freezing from the final 3 sessions of active avoidance (sessions 4–6) was analyzed using a two-way ANOVA with a between subjects factor of DRUG and a within subjects factor of SESSION. This analysis revealed a DRUG X SESSION interaction [F(2,32)=4.29, p=.044], which Bonferroni post-hoc tests confirmed was driven by a significant increase in freezing among the muscimol group during the 5th session of training (p<.01; Fig. 5C). An identical ANOVA was performed on avoidance responses from the same animals. This analysis revealed a DRUG X SESSION interaction [F(2, 32)= 8.37, p=.001], which Bonferroni post-hoc tests confirmed was driven by a significant decrease in the lesion group relative to sham controls (p<.01; Fig. 5D). Though ilPFC remains relevant to active avoidance during the expression phase of behavior, these results indicate that the effect of inactivation is transient once the response has been fully acquired.
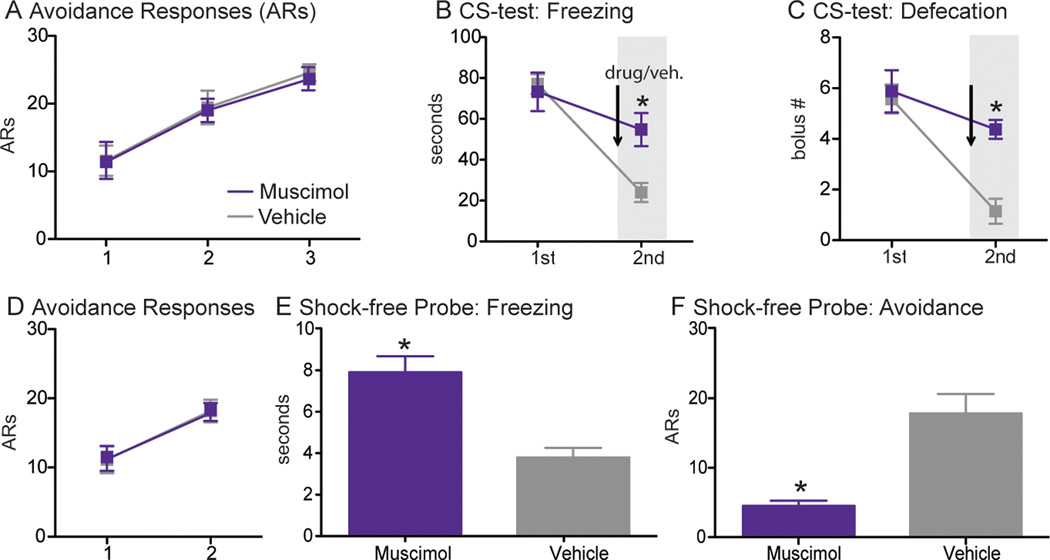
Previous work suggests that active avoidance training attenuates the expression of CS-evoked defense reactions across environments, even in contexts that do not allow the avoidance response (Kamin et al, 1963). To confirm and extend this intriguing result, the effect of ilPFC inactivation on conditioned reactions was examined when the CS was presented in an alternate context, in which shuttling was not possible (cannula placement displayed in Fig. 4C). Animals were given 3 daily sessions of avoidance training (Fig 6A); a CS-test was conducted after both the 1st and 3rd sessions in two unique environments. Muscimol (n=8) or vehicle (n=8) was infused into ilPFC prior to the 2nd and final CS-test. Freezing data from the CS-tests were analyzed using a two-way ANOVA with a between subjects factor of DRUG and a within subjects factor of TEST. This analysis revealed a DRUG X TEST interaction [F(1, 14)=17.22, p=.001], which two-tailed t-test confirmed was driven by an increase in freezing in the muscimol group during the 2nd CS-test (p<.01; Fig. 6B).
Fig. 6.
The effects of ilPFC inactivation on a CS-test and a shock-free probe session. A) Mean (±SEM) avoidance responses during 3 sessions of active avoidance training; animals were exposed to the CS in an alternate environment following the 1st and 3rd sessions. B) Mean (±SEM) CS-evoked freezing during 2 CS-tests; the light gray bar and black arrow indicate that muscimol (n=8) or vehicle (n=8) was injected into ilPFC prior to the 2nd test. C) Mean (±SEM) defecation (number of boluses) from the same subjects during 2 CS-tests. D) Mean (±SEM) avoidance responses during 2 sessions of AA training; this was followed by a shock-free probe session prior to which muscimol (n=7) or vehicle (n=7) was injected into ilPFC. E) Mean (±SEM) avoidance responses and F) mean (±SEM) CS-evoked freezing during the shock-free probe. * indicates p<.05.
As a corroborating measure, defecation during the CS-test was also measured. These data were analyzed with a two-way ANOVA identical to the one used to analyze freezing. This revealed a DRUG X TEST interaction [F(1, 14)=19.73, p<.001], which two-tailed t-test confirmed was driven by a significant increase in the muscimol group relative to vehicle controls during the 2nd session (p<.01; Fig. 6C). Thus, as active avoidance training progresses, the expression of Pavlovian reactions is strongly attenuated by ilPFC even when the animal encounters the CS in environments that do not permit avoidance behavior.
Data from the acquisition and expression experiments suggest that ilPFC inactivation increased the animal’s latency to escape the US. ilPFC muscimol injection prior to the 1st session of active avoidance training yielded an average escape latency of 1.63 (±.12) sec, compared to .82 (±.35) sec in vehicle injected controls; two-tailed t-test revealed that muscimol-treated subjects took longer to shuttle during the US (p<.001; data not shown graphically). A statistically significant increase was also observed when muscimol was injected into ilPFC prior to the 5th session, with an average escape latency of 1.82 (±.32) in drug-treated animals and .93 (±.27) in vehicle-treated controls; two-tailed t-test confirmed increased escape latencies in ilPFC inactivated subjects (p=.008; data not shown graphically). As this escape effect is caused by a manipulation that increases freezing and decreases avoidance, these data open the possibility that prolonged contact with foot shock disrupts active avoidance behavior. To control for this, the effects of ilPFC inactivation were tested on a US-free probe session conducted after two normal sessions of active avoidance training (cannula placement displayed in Fig. 4D). Mean CS-evoked freezing during this session was analyzed with a two-tailed t-test, which revealed a significant increase between the muscimol (n=7) and control (n=7) groups (p<.001; Fig. 6E). Two-tailed t-test performed on avoidance responses from the same animals revealed a significant decrease among the muscimol group (p<.001; Fig. 6F). Thus, ilPFC inactivation increases freezing and decreases avoidance even when the US is not present, suggesting that prolonged US exposure cannot explain our results.
Protein Synthesis Inhibition and Signaled Active Avoidance
To ascertain whether ilPFC and CeA are important storage sites for the different types of information acquired during active avoidance training, two separate experiments were performed in which the protein synthesis inhibitor anisomycin was injected into either ilPFC or CeA following the 1st avoidance session (ilPFC and CeA cannula placements displayed in Figs. 4E and 4F, respectively). For ilPFC injections, CS-evoked freezing data from anisomycin (n=8) and vehicle (n=8) groups were analyzed using a two-way ANOVA with a between subjects factor of DRUG and a within subjects factor of SESSION. This analysis revealed a DRUG X SESSION interaction [F(4, 56)=2.56, p=.046], which Bonferroni post-hoc tests confirmed was driven by a significant increase in freezing by the anisomycin group relative to controls on the 2nd day of training (p<.01; Fig. 7A).
Fig. 7.
The effects of local protein synthesis inhibition on signaled active avoidance training; light gray bars and black arrows indicate that anisomycin or vehicle was injected into ilPFC or CeA folowing the 1st session. A) Mean (±SEM) freezing and B) mean (±SEM) avoidance responses when anisomycin (n=8) or vehicle (n=8) was infused into ilPFC after the 1st session. C) Mean (±SEM) freezing D) mean (±SEM) avoidance responses when anisomycin (n=8) or vehicle (n=8) was injected into CeA following the 1st session. * indicates p<.05.
An identical ANOVA was performed on avoidance data from the same animals. This analysis revealed a DRUG X SESSION interaction [F(4,56)=2.67, p=.039], which Bonferroni post-hoc tests confirmed was driven by a significant decrease in the anisomycin group relative to vehicle controls during the 2nd session of training (p<.01; Fig. 7B). Thus, protein synthesis inhibition in ilPFC following the 1st session of avoidance prevented the animal from effectively suppressing Pavlovian reactions in the subsequent session.
Opposite results were obtained when anisomycin was injected into CeA following the 1st session of active avoidance training. Avoidance data from anisomycin (n=8) and vehicle (n=8) groups were analyzed using a two-way ANOVA with a between subjects factor of DRUG and a within subjects factor of SESSION. This analysis revealed a DRUG X SESSION interaction [F(4, 56)=2.55, p=.048], which Bonferroni post-hoc tests confirmed was driven by a significant increase in the anisomycin group relative to controls on the 2nd day of training (p<.01; Fig 7D), even though an identical ANOVA revealed no differences for CS-evoked freezing at any point (Fig 7C). Thus, plasticity in CeA opposes avoidance even when levels of conditioned freezing are relatively low. Together, these data suggest that ilPFC and CeA encode contrasting and dissociable memory traces during avoidance learning.
Discussion
This study confirms that signaled active avoidance (AA) training leads to a robust suppression of Pavlovian threat reactions, in particular conditioned freezing. Lesion data demonstrate opposing roles for the infralimbic prefrontal cortex (ilPFC) and central nucleus of the amygdala (CeA) in AA learning, with ilPFC facilitating avoidance by inhibiting both CeA activity and the CS-evoked freezing it governs. Pharmacological inactivation experiments establish a role for ilPFC during both acquisition and expression phases of the AA learning curve, though the effect of inactivation only persists into subsequent drug-free sessions when administered during acquisition. Inactivation results also demonstrate that signaled AA produces an ilPFC-mediated reduction in CS-evoked freezing not only in the avoidance context, but also in alternate environments in which training did not occur. Finally, injection of a protein synthesis inhibitor into either ilPFC or CeA demonstrates that AA training creates two competing memory traces in these regions, facilitating or opposing the avoidance response, respectively. These data support a hypothetical model in which the instrumental avoidance contingency recruits ilPFC to exert feed-forward inhibition on CeA, inhibiting those Pavlovian threat reactions that occlude active avoidance behavior.
Though ilPFC can attenuate CeA activity and the reactive behaviors it governs (Likhtik et al, 2005; Quirk et al, 2003; Milad et al; 2002), this must occur via an intervening mechanism in order for excitatory cortical inputs to inhibit amygdalar outputs. One candidate for the intermediary between ilPFC and CeA is the GABAergic intercalated cell masses (ICM) of the amygdala. Anatomical studies demonstrate that ilPFC projects to ICM (Pinard et al, 2012; Pinto and Sesack, 2008; Sesack et al, 1989), while anatomical and physiological data suggest that ICM innervate and inhibit CeA (Amir et al, 2011; Marowsky et al, 2005). Disinhibition of ilPFC causes immediate early gene induction in ICM (Berretta et al, 2005), and robust spiking occurs in ICM when ilPFC is electrically stimulated (Amir et al, 2011). Thus, ICM seem well situated to translate cortical excitation into the inhibition of CeA activity, thus attenuating the expression of Pavlovian reactions and facilitating AA. This hypothetical arrangement is consistent with prevalent models of amygdala function (LeDoux, 2000; Maren & Quirk, 2004), insofar as reduced CeA output causes a decrease in specific behavioral endpoints, while sparing the predictive relationship between CS and US necessary for successful avoidance learning.
Our results suggest that ilPFC underpins an inhibitory form of behavioral learning that occurs during the acquisition of signaled AA. Post-training anisomycin injections in ilPFC obstruct avoidance and enhance the expression of freezing in subsequent drug-free sessions, suggesting that ilPFC encodes a memory that directly opposes the expression of CeA-mediated conditioned behavior. Extinction also involves the learned inhibition of Pavlovian reactions (Herry et al, 2010; Myers and Davis, 2002) and depends on ilPFC to suppress CeA activity (Quirk et al, 2000; Santini et al, 2004; Sierra-Mercado et al, 2011). In spite of similar substrates and behavioral endpoints, extinction and the suppression of freezing in AA are likely based on different types of information processing. Extinction learning occurs via unreinforced CS presentation, while the predictive relationship between CS and US must remain constant through the acquisition of AA for the cue to trigger the aversively motivated avoidance response. Instead, it seems more likely that acquired control over US delivery creates an inhibitory memory, which feeds back onto conditioned freezing. Thus, the instrumental response has a synergistic relationship with the suppression of Pavlovian reactions, with successful avoidance increasing the degree to which freezing is inhibited, and vice versa. By moving through this upward spiral, the subject transitions from a state dominated by innate defensive behaviors to one in which instrumental action replaces CS-evoked reactions. Thus, ilPFC both responds to and facilitates the subject’s degree of behavioral control by reducing the expression of those responses that obstruct AA.
We believe that our data implicate the instrumental avoidance contingency as a key trigger for the inhibition of Pavlovian reactions in AA training – however, there are other possible explanations for the diminution of conditioned freezing. Bolles (1970) proposed that freezing is a maladaptive in the AA paradigm and is thus punished, effectively unmasking the avoidance response; other researchers argue that freezing is not amenable to punishment (Fanselow, 1994). It is also possible that the escape contingency plays an important role, and that the ability to deactivate the shock by shuttling feeds back onto the expression of CS-evoked fear. While our results demonstrate that ilPFC inactivation increases freezing and reduces avoidance even in a shock-free session, it is conceivable that the acquisition of instrumental escape does contribute to the formation of an inhibitory memory in ilPFC.
These data demonstrate that signaled AA training produces a broad reduction in defensive reactions evoked by the CS, even when presented in environments that do not permit the avoidance response. This result emphasizes the degree to which instrumental control over the US can alter the animal’s response to cues that predict it. The literature on coping behavior in humans and animals describes a dichotomy between “proactive” and “reactive” responses to stressful stimuli (Koolhaas et al, 1999). Proactive coping is characterized by behavioral strategies designed to influence or control an individual’s environment, whereas reactive coping involves more traditional “fear” or “anxiety” responses, such as withdrawal or immobility (Henry and Stephens, 1977; Koolhaas et al, 1999). Thus, by teaching subjects to prevent an aversive outcome with an instrumental behavior, signaled AA training causes a robust and pervasive transition from a reactive to a proactive coping style. As reactive coping is associated with deleterious health outcomes (Koolhaas et al, 1999), this research highlights ilPFC, as well as its putative primate homologue the subgenual cingulate (Wallis, 2012), as an important target for therapies designed to facilitate adaptive coping strategies.
In summary, the Pavlovian and instrumental components of AA training produce competing behavioral outputs, which the subject resolves by attenuating the expression of conditioned reactions that obstruct the avoidance response. Data presented here suggest that this process depends on ilPFC, which selectively inhibits the expression of CeA-mediated Pavlovian threat reactions in a manner that preserves the representation of the CS-US relationship. Although freezing is one of the rodent’s primary defenses against imminent danger, it serves to increase the animal’s contact with the aversive US in the AA paradigm. The acquisition of behavioral control over US delivery creates an inhibitory memory that effectively suppresses conditioned freezing, allowing instrumental behavior to predominate. Thus, ilPFC is part of an adaptive circuit that allows learned information to guide behavior when innate responses are ultimately detrimental.
Acknowledgements
This project was supported by Grant Number F32MH094061 awarded to JMM by the National Institute of Mental Health, as well as by Grant Number R01DA029053 awarded to JEL by the National Institute on Drug Abuse and by Grant Number R01MH038774 awarded to JEL by the National Institute of Mental Health. We acknowledge Claudia Farb for her important contributions to our c-Fos experiment, as well as Christopher Gonzalez for his assistance in conducting these studies.
Footnotes
The authors declare no competing financial interests.
References
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavioral and dorsal raphe nucleus. Nat. Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Amat J, Paul E, Zarza C, Watkins LR, Maier SF. Previous experience with behavioral control over stress blocks the behavioral and dorsal raphe nucleus activating effects of uncontrollable stress: role of ventral medial prefrontal cortex. J. Neurosci. 2006;26:13264–13272. doi: 10.1523/JNEUROSCI.3630-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir A, Amano T, Pare D. Physiological identification and infralimbic responsiveness of rat intercalated amygdala neurons. J. Neurophysiol. 2011;105:3054–3066. doi: 10.1152/jn.00136.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta S, Pantazopoulos H, Caldera M, Pare D. Infralimbic cortex activation increases c-Fos expression in intercalated neurons of the amygdala. Neuroscience. 2005;132:943–953. doi: 10.1016/j.neuroscience.2005.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ, Griebel G. Defensive responses to predator threat in the rat and mouse. Curr. Protoc. Neurosci. 2005 doi: 10.1002/0471142301.ns0819s30. Chapter 8, Unit 8.19. [DOI] [PubMed] [Google Scholar]
- Bolles RC. Species-specific defensive reactions and avoidance learning. Psychol. Rev. 1970;77:32–48. [Google Scholar]
- Cain CK, LeDoux JE. Brain mechanisms of Pavlovian and instrumental aversive conditioning. In: Blanchard RJ, Blanchard DC, Griebel G, Nutt DJ, editors. Handbook of Anxiety and Fear. New York: Elsevier; 2008. [Google Scholar]
- Choi JS, Cain CK, LeDoux JE. The role of the amygdala in the expression of auditory signaled two-way active avoidance. Learn. Mem. 2010;17:139–147. doi: 10.1101/lm.1676610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Muller C, Luthi A. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- Da Costa Gomez TM, Behbehani MM. An electrophysiological characterization of the projection from the central nucleus of the amygdala to the periaqueductal gray of the rat: the role of opioid receptors. Brain Research. 1995;689:21–31. doi: 10.1016/0006-8993(95)00525-u. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Popa D Pare D. Central amygdala activity during fear conditioning. J. Neurosci. 2011;31:289–294. doi: 10.1523/JNEUROSCI.4985-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Lester LS. A functional behavioristic approach to aversively motivated behavior: predatory imminence as a determinant of the topography of defensive behavior. In: Bolles RC, Beecher MD, editors. Evolution and learning. Hillsdale, England: Lawrence Erlbaum Associates; 1988. pp. 185–212. [Google Scholar]
- Fanselow MS. Neural organization of the defensive behavior system for fear. Psychon. Bul. Rev. 1994;1:429–438. doi: 10.3758/BF03210947. [DOI] [PubMed] [Google Scholar]
- Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R. Orbitomedial prefrontal cortical projections to distinct longitudinal columns of the periaqueductal gray in the rat. J. Comp. Neurol. 2000;422:556–578. doi: 10.1002/1096-9861(20000710)422:4<556::aid-cne6>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R, Biag J, Dong H-W, Deisseroth K, Callaway EM, Fanselow MS, Luthi A, Anderson DJ. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature. 2010;468:270–276. doi: 10.1038/nature09553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JP, Stephens PM. Stress, health and the social environment: a sociobiological approach to medicine. Berlin: Springer; 1977. [Google Scholar]
- Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Luthi A. Neuronal circuits of fear extinction. Eur. J. Neurosci. 2010;31:599–612. doi: 10.1111/j.1460-9568.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- Kamin LJ, Brimer CJ, Black AH. Conditioned suppression as a monitor of fear of the CS in the course of avoidance training. J. Comp. Physiol. Psych. 1963;56:497–501. doi: 10.1037/h0047966. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Korte SM, DeBoer SF, Van Der Vegt BJ, Van Reenan CG, Hopster H, De Jong IC, Ruis MAW, Blokhuis HJ. Coping styles in animals: current status in behavior and stress-physiology. Neurosci. Biobehav. Rev. 1999;23:925–935. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- Lazaro-Munoz G, LeDoux JE, Cain CK. Sidman instrumental avoidance initially depends on lateral and basal amygdala and is constrained by central amygdala-mediated Pavlovian processes. Biol. Psychiatry. 2010;15:1120–1127. doi: 10.1016/j.biopsych.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J. Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J. Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Rethinking the emotional brain. Neuron. 2012;73:653–676. doi: 10.1016/j.neuron.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Pelletier JG, Paz R, Pare D. Prefrontal control of the amygdala. J. Neurosci. 2005;25:7429–7437. doi: 10.1523/JNEUROSCI.2314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signaling of fear memory. Nat. Rev. Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Marowsky A, Yanagawa Y, Obata K, Vogt KE. A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron. 2005;48:1025–1037. doi: 10.1016/j.neuron.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav. Neurosci. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- Mowrer OH, Lamoreaux RR. Fear as an intervening variable in avoidance conditinoning. J. Comp. Psychol. 1946;39:29–50. doi: 10.1037/h0060150. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflexes. London: Routledge and Kegan Paul; 1927. [Google Scholar]
- Pinard CR, Macagni F, McDonald AJ. Medial prefrontal cortical innervation of the intercalated nuclear region of the amygdala. 2012;205:112–124. doi: 10.1016/j.neuroscience.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A, Sesack SR. Ultrastructural analysis of prefrontal cortical inputs to the rat amygdala: spatial relationships to presumed dopamine axons and D1 and D2 receptors. 2008;213:159–175. doi: 10.1007/s00429-008-0180-6. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of the prefrontal cortex decreases the responsiveness of central amygdala output neurons. J. Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J. Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of classical conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical Conditioning II Current Research and Theory. New York: Appleton-Century-Crofts; 1972. [Google Scholar]
- Santini E, Muller RU, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J. Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucogglutinin. J. Comp. Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2010;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon RL, Wynne LC. Traumatic avoidance learning: the principles of anxiety conservation and partial irreversibility. Psychol. Rev. 1954;61:353–385. doi: 10.1037/h0054540. 1954. [DOI] [PubMed] [Google Scholar]
- Viviani D, Charlet A, ven der Burg E, Robinet C, Nurni N, Abatis M, Magara F, Stoop R. Oxytocin gates fear responses through distinct outputs from the central amygdala. Science. 2011;333:104–107. doi: 10.1126/science.1201043. [DOI] [PubMed] [Google Scholar]
- Wallis JD. Cross-species studies of orbitofrontal cortex and value-based decision-making. Nat. Neurosci. 2012;15:13–19. doi: 10.1038/nn.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schaffe GE, Kristense MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation and expression of Pavlovain fear conditioning. J. Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]