Abstract
One of the primary limitations of cell therapy for myocardial infarction is the low survival of transplanted cells, with a loss of up to 80% of cells within 3 days of delivery. The aims of this study were to investigate the distribution of nutrients and oxygen in infarcted myocardium and to quantify how macromolecular transport properties might affect cell survival. Transmural myocardial infarction was created by controlled cryoablation in pigs. At 30 days post-infarction, oxygen and metabolite levels were measured in peripheral skeletal muscle, normal myocardium, infarct border zone, and infarct interior. The diffusion coefficients of fluorescein or FITC-labeled dextran (0.3–70 kD) were measured in these tissues using fluorescence recovery after photobleaching. Vascular density was measured via endogenous alkaline phosphatase staining. To examine the influence of these infarct conditions on cells used therapeutically in vivo, skeletal myoblast survival and differentiation were studied in vitro under the oxygen and glucose concentrations measured in the infarct tissue. Glucose and oxygen concentrations, as well as vascular density were significantly reduced in infarct when compared to uninjured myocardium and infarct borderzone, although the degree of decrease differed. The diffusivity of molecules smaller than 40 kD was significantly higher in infarct center and border zone as compared to uninjured heart. Skeletal myoblast differentiation and survival were decreased stepwise from control to hypoxia, starvation, and ischemia conditions. Although oxygen, glucose, and vascular density were significantly reduced in infarcted myocardium, the rate of macromolecular diffusion was significantly increased, suggesting that diffusive transport may not be inhibited in infarct tissue, and thus supply of nutrients to transplanted cells may be possible. In vitro studies mimicking infarct conditions suggest that increasing nutrients available to transplanted cells may significantly increase their ability to survive in infarct.
Keywords: Myocardial infarction, microdialysis, stem cell therapy, diffusion, infarct
Introduction
Although improvements in cardiovascular and surgical therapies have reduced the mortality of acute cardiovascular events dramatically, patients who survive myocardial infarction remain at high risk for heart failure. Over 500,000 new cases of heart failure develop in the United States each year, with a mortality rate of 20% within one year and 85% within 12 years of diagnosis [1]. Cell transplantation has emerged as an experimental therapy for heart failure with the potential for preventing the progression from infarction to heart failure [2]. Although cell transplantation has proven somewhat effective at improving cardiac function clinically [3,4,5,6], the effects of these therapies are limited, potentially due to the low retention and survival of cells in an infarct [4,7]. In fact, more than 80% of cells may die within 3–4 days of implantation [8,9].
Limited cellular survival may be due to a number of factors, including the inflammatory response to cells expanded in vitro, the physical strain on cells during injection, and the ischemic milieu into which the cells are transplanted [4,7,10,11]. Preliminary studies suggest that greater than 85% of cells survive harvest and delivery, suggesting that local factors in the ischemic microenvironment may be a major contributor to poor survival. In support of this, several investigators have achieved varying levels of success at improving the survival of transplanted cells by preconditioning cells prior to transplantation [12], administering drugs [13], utilizing gene therapy to promote vascularization [14,15], or by altering the infarct environment via laser or gene-induced angiogenesis [16,17,18]. However, further understanding of the infarct environment may improve survival of implanted cells. Although some data exist on the perfusion of infarcts [19,20], and a number of studies have examined oxygen and glucose levels during ischemia or ischemia-reperfusion injury [21] little to no data exist regarding either the distribution or transport properties of macromolecules, metabolites, or oxygen in mature infarct scar.
To quantify the concentration of oxygen, glucose, and lactate in chronically infarcted myocardium and compare those values to levels found in uninjured heart tissue, we used techniques previously established for measurements of nutrient and oxygen levels in tumor biology [22,23,24]. Furthermore, we used fluorescence recovery after photobleaching (FRAP) to measure the diffusion properties of molecules of increasing size (0.3–70 kD) in infarcted and uninjured heart. After obtaining accurate measurements of diffusion properties and the level of oxygen and nutrients in infarct, we examined how skeletal myoblasts transplanted into environments with similar properties respond over time. We then maintained C2C12 myoblasts in these conditions and examined cell growth, death, and differentiation potential over two weeks.
Methods
Surgical procedure
All experiments were conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee at Duke University. Yorkshire pigs (N=10) were premedicated with telazol (5mg/kg) and maintained on 1–5% isoflurane in room air. A left antero-lateral thoracotomy was made in the fifth intercostal space, and the pericardium was incised laterally to expose the distal left anterior descending (LAD) coronary artery. A 3-cm cryoprobe (Frigitronics, Cooper Surgical, Trumbull, CT), cooled to −170°C by liquid nitrogen, was applied to the surface of the left ventricle just proximal to the last diagonal branch of the LAD for 4 minutes to obtain a reproducible, 80% to transmural infarct (Figure 1).
Figure 1.
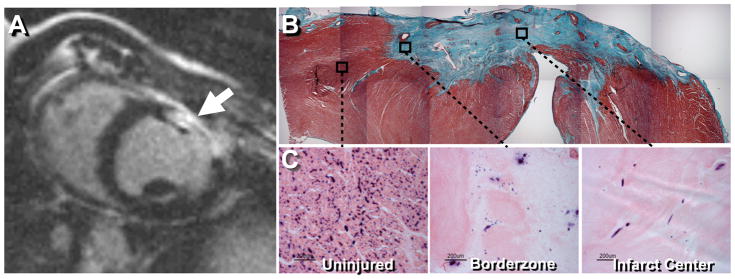
(A) Contrast-enhanced MRI confirming the presence of infarcted myocardium (arrow) at 3 weeks post cryoinjury. (B) Masson’s Trichrome stain of 30 day old myocardial infarct site showing full transmural infarct. (C) Endogenous alkaline phosphatase staining, with eosin counterstain, showing endothelial cells within uninjured myocardium, infarct borderzone, and infarct center.
Magnetic Resonance Imaging (MRI)
At approximately 3 weeks post infarct, contrast-enhanced MRI (ceMRI) was used to confirm the presence of infarcted myocardium (Figure 1A), using a 1.5T scanner (Siemens, Sonata, Erlangen, Germany) with an IR-FLASH pulse sequence [25]. Short-axis views were obtained from 1 cm below the level of the mitral valve every 1 cm throughout the left ventricle at 5 min after contrast administration (gadopentetate dimeglumine, 0.15 mmol/kg bodyweight). A 14 x 28 cm flexible surface coil was used, and with an in-plane image resolution of 1 x 1 mm and slice thickness of 5 mm.
Oxygen tension and nutrient levels in vivo
In vivo analysis of infarct scar was performed 30±3 days post injury to assure measurements were performed on healed infarct scar in a time frame similar to that used clinically for stem cell delivery [26].
Oxygen levels
An OxyLite E system (Oxford Optronix, Oxford, UK) was used to measure local (3–5 mm3) oxygen concentration uninjured myocardium, infarct border zone (defined as interior to within ~0.5 cm of the infarct edge), center infarct, and at a reference site in peripheral skeletal muscle. Optical probes were inserted parallel to the myocardial surface, at a depth of ~4 mm, into the myocardium, borderzone, and infarct center. After a 10 minute equilibration, oxygen concentrations were recorded once every 10 minutes for one hour. pO2 was measured in skeletal muscle as a control.
Metabolite levels
Glucose and lactate levels in infarct, borderzone, uninjured myocardium and peripheral skeletal muscle were examined using microdialysis under low flow rate conditions. Microdialysis probes, with a 20 kD molecular weight cutoff (CMA Microdialysis, Solna, Sweden) were inserted into uninjured myocardium, borderzone, interior infarct, and peripheral skeletal muscle at ~4 mm depth. After an equilibration time of approximately 20 minutes to allow for steady state to be reached, six sequential 10 minute samples were taken at a saline flow rate of 2μl/min. A freely diffusible molecule, urea, was used as a recovery marker so that the measured recovery could be used to calculate true extracellular concentrations of the nutrients measured even if true equilibrium conditions were not achieved in the measured tissues [27]. Corrections were made using the equation:
| (Eq. 1) |
where [X]tissue is the concentration of a given analyte in the tissue, [X]meas is the concentration of analyte obtained from measurement of the microdialysate taken from that tissue, and [Urea]blood is the concentration of urea in a sample of blood taken during collection of the microdialysate sample.
Histology
Hearts were removed, rinsed in saline, and placed in 30% sucrose solution in PBS that was changed twice over 48 hours prior. Frozen sections (5 μm) were made from tissue blocks containing the infarct and stained with hematoxylin-eosin (H&E) and Masson’s Trichrome. In a subset of hearts (n=3), additional sections were stained for alkaline phosphatase for quantification of vascularity [28].
Fluorescence Recovery After Photobleaching (FRAP)
FRAP experiments were performed at room temperature in Dulbecco’s phosphate buffered saline on a laser scanning confocal microscope (LSM510, Carl Zeiss, Thornwood, NY). Fresh tissue samples were incubated at 4°C overnight in solutions containing fluorescein or fluorescein-labeled dextrans of 3, 10, 40, and 70 kD. FRAP experiments were performed as previously described [29]. Briefly, photobleaching was performed at 488 nm emission using 100% laser power from a 15.0 mW argon laser. Imaging was performed using the same laser at 1% power and an emission wavelength of 505 nm. Following an initial background image, an area was photobleached. A series of images was then recorded to track the recovery of fluorescence into the area. All images were acquired with a 20x objective, which yielded a 512x512 pixel image with a resolution of 0.9 μm/pixel. Image analysis was performed as described previously [29] using the method of Axelrod et al. [30].
In vitro Survival Studies
C2C12 mouse myoblasts (ATCC) were plated under normal growth conditions (20% oxygen with media containing 1000mg/L glucose DMEM, 10% HS, and 0.5% gentamicin) and grown to ~70% confluence. Plates were washed once with PBS and shifted to control, hypoxia (0.5% oxygen), low glucose (100mg/L glucose), or simulated infarct/ischemia (low glucose + hypoxia) conditions. Cells were differentiated in DMEM (with or without glucose as the condition dictated) with 0.5% gentamicin.
Media were replaced daily and harvested media was examined for dead cells using a Live/Dead kit (Invitrogen, Carlsbad, CA) and for lactate dehydrogenase (LDH) levels (CytoTox96 Assay, Promega, Madison WI). Plates were photographed daily, and cell confluence and myotube number per high powered field (HPF, 100X) were quantified.
Data analysis
Data were compared using ANOVA with Fisher’s post hoc test for comparisons among groups (α=0.05).
Results
Infarct model
Myocardial infarct scars were consistently 80% of the wall depth to transmural, with reduced cellularity and microvascular density, as determined by H&E and alkaline phosphatase staining (Figure 1B and C). The number of alkaline phosphatase positive cells decreased significantly from 313±38 cells/mm2 in uninjured myocardium to 27±7 cells/mm2 in borderzone and 11±1 cells/mm2 in the interior of porcine cryoinfarct at one month (mean±SEM, p<0.05).
Oxygen and analyte measurements
Oxygen and nutrient levels were measured in myocardial infarct at 1 month using oxygen optodes and microdialysis probes placed within the infarcted myocardium, with the location verified in histologic sections by disruption in the midst of scar (Figure 2A). Oxygen levels in infarct were decreased ~90% in comparison to skeletal muscle or uninjured heart (p<0.01, Figure 2B). Glucose levels, as measured by microdialysis, were significantly reduced (p<0.05) in infarct when compared to skeletal muscle, uninjured heart, borderzone, or plasma (Figure 2C). Lactate levels in infarct interior were not significantly different from to those in skeletal muscle, uninjured heart, borderzone, or plasma (Figure 2D).
Figure 2.
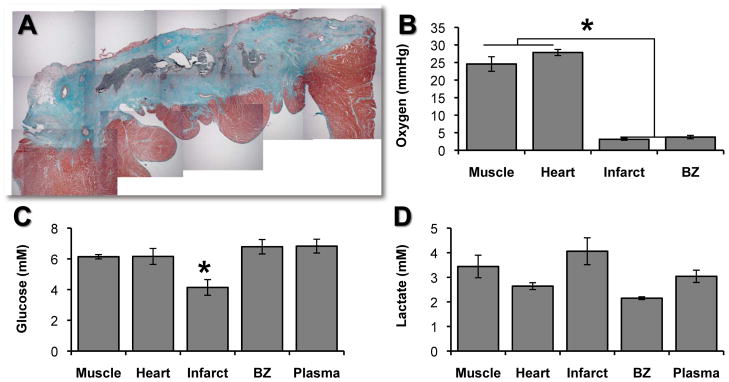
(A) Masson’s Trichrome stain of myocardial infarct showing location of microdialysis and oxygen probes(marked by reverse perfusion (microdialysis) or injection (oxygen probe) of 0.1% Evans blue dye). (B) Oxygen concentration is significantly lower in myocardial infarct scar than in skeletal muscle, uninjured heart, or borderzone (*p<0.05). (C) Glucose concentration in myocardial infarct scar was significantly lower than in the borderzone, plasma, or uninjured heart (*p<0.05). (D) Lactate concentrations measured in skeletal muscle, uninjured heart, myocardial infarct scar, infarct borderzone, and blood plasma varied widely in individual measurements within groups; there were no significant differences between groups. All bars are mean + s.e.m.
Diffusion Properties
Diffusion coefficients for molecules up to 10 kD was significantly increased (p<0.05), with a trend continuing up to 40 kD (p<0.1, Figure 3). The characteristic diffusion time (τ) of 0.3 kD molecules was approximately 40X larger in infarct than in uninjured heart. For larger molecules (40 kD), the τ in infarct was 60X that of uninjured heart tissue.
Figure 3.
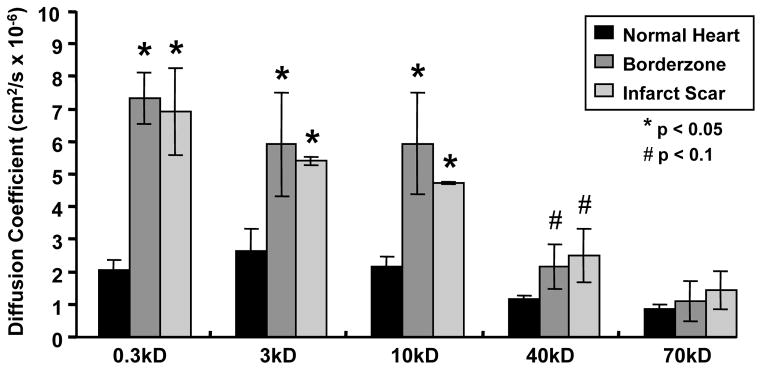
Mean diffusion coefficients of 0.3 kD and 10 kD molecules were significantly increased in infarct center and borderzone when compared to uninjured myocardium (*p<0.05, †p<0.1). Mean calculated diffusion coefficients at 40 kD and 70 kD tended to increase in infarct center and borderzone relative to uninjured heart, but the results are not statistically significant. All bars are mean + s.e.m.
In Vitro Studies
Hypoxia
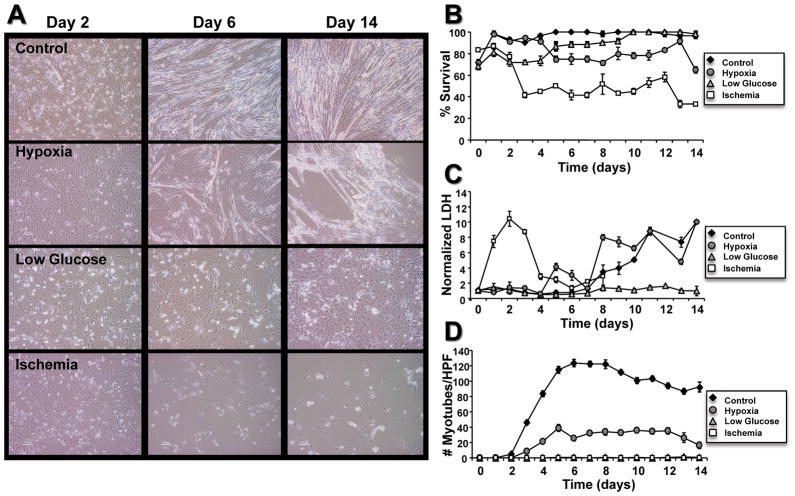
Under hypoxic conditions (0.5% oxygen), proliferation confluence and differentiation capacity of myoblasts was significantly reduced. Beginning at day 5 of differentiation, confluence of myoblasts was significantly reduced (p<0.05 vs. control, Figure 4A); however, the number of dead cells under hypoxia was similar to control throughout the experiment (Figure 4B). LDH secretion increased significantly from baseline beginning at day 6 and was similar to control (Figure 4C). These results are consistent with reduced cell growth rather than increased cell death under 0.5% oxygen. Supporting this is the finding that myotube formation began by day 3 of differentiation, with maximal myotube formation (41 myotubes/HPF) occurring by day 5 (p<0.05 vs. control). Myotube number remained between 20 and 40 myotubes/HPF (~25% control), (Figure 4D) throughout the experiment.
Figure 4.
(A) Micrographs show varying degrees of differentiation of C2C12 myoblasts under control, hypoxia, low glucose, or ischemia conditions.. Myotubes are evident in both control and hypoxia groups by day 6. (B) Survival of myoblasts over time under control, hypoxia, or low glucose was greater than under ischemia conditions. (C) Lactate dehydrogenase (LDH) levels released into media by cells under experimental conditions (normalized to Day 0 levels). LDH levels of cells under ischemia conditions followed closely with the peak in cell death. LDH levels in hypoxia and control conditions rose only when the cultures were primarily composed of myotubes. (D) Count of fully differentiated myoblasts under control, hypoxia, starvation, or ischemia conditions over 14 days. Myoblasts were evident only after differentiation under control or ischemia conditions.
Low Glucose Conditions
Low glucose “starvation” conditions significantly reduced both cell number and differentiation during the first week of the experiment (p<0.05 vs. control), but by day 10, the remaining myoblasts had proliferated to near confluence, similar to control cells (Figure 4A). Cell death was similar to control as well, with maximal cell death occurring on day 1 (Figure 4B). LDH levels remained minimal throughout the experiment under starvation conditions, with no significant changes from initial conditions (Figure 4C). Few myotubes were formed under starvation conditions, with a single multinucleated cell per HPF observed in some fields and none in most fields beginning on day 5 of differentiation (p<0.01 vs. control and hypoxia, Figure 4D). Thus, although confluence was high, and growth factors low the typical differentiation conditions differentiation did not occur.
Simulated infarct conditions
The combination of low glucose and hypoxia, simulating conditions seen in infarct, significantly reduced C2C12 number and myotube formation. From day 4 of differentiation to the end of the experiment, confluence ranged from 30–50% (Figure 4A, p<0.05 vs. control). Under ischemic conditions, myoblast death was maximal on day 2 of differentiation and was more than two times the cell death seen under any other condition (Figure 4B, p<0.05). Furthermore, LDH levels were 10X greater than control, or any other condition, by day 2 of differentiation and declined thereafter, due to reduced cell numbers (Figure 4C, p<0.05). No myotubes were visible on any plate at any time of the experiment (p<0.01 vs. control and hypoxia) (Figure 4D).
Discussion
The findings of this study show that glucose and oxygen levels are significantly reduced in infarcted myocardium, likely due to limited vascularity and reduced perfusion. While these data are consistent with previous reports [31,32] based on the assumption that infarct is hypoxic and has reduced blood flow, this study provides direct quantitative measures of oxygen and nutrients in a mature porcine infarct as a model system for the injection of cells for cardiac therapies [3,5,6,33,34,35].
The myocardial infarct scar is hypoxic within several hundred microns of the scar border. Glucose levels, however, are reduced more gradually (over millimeters), with nutrient levels in borderzone similar to those measured in uninjured myocardial tissue. The diffusion coefficient of the infarct area and borderzone was increased in comparison to uninjured heart tissue for molecules ranging in size up to 40 kD. Molecules in this size range include glucose (0.18 kD), insulin (5 kD), and VEGF (42 kD). The increased diffusivity of the infarct and borderzone are likely due to the reduced cellularity of the infarct area [36] that allows relatively unimpeded diffusion of small molecules.
Although the diffusion coefficients in the infarct were relatively high, the concentrations of glucose and oxygen in this area remained low. Specifically, oxygen levels in infarct were approximately 10% those in the uninjured heart, while glucose levels were nearly 2/3 those in the infarct (Figure 2). The disparate magnitude of the effect of infarct on the concentrations of these two solutes suggests that the balance between molecular transport and cellular metabolism in this region differentially altered for different solutes. Low oxygen levels and vascular density have been seen in other infarct models such as the rat. For example, in a rat model of infarct using ligation of the left descending coronary artery, Wang et al. observed a large drop in the number of vessels (~82%), consistent with the ~96% drop in the number of alkaline phosphatase stained cells in the present study [37,38]. In both models, these changes were associated with reduced oxygen levels, suggesting that different models of cardiac infarct exhibit many of the same characteristics of tissue damage. This combination of low vascular density with high diffusivity, however, suggests that relatively minor increases in vascular density could increase the availability of oxygen, nutrients, and proteins in the borderzone and infarct dramatically. Since diffusion in the infarct scar is high, restoring vascular density similar to that seen in uninjured heart may not be necessary in the short term to allow survival of transplanted cells. This is an important consideration, as reestablishing vessel density in infarct similar to that in uninjured heart may be challenging [39]. Furthermore, the fact that the heart tissue is being constantly exposed to cyclic loading may further compound transport issues. Studies in muscle have shown that diffusion of large, but not small molecules is inhibited by large compressive strains [40,41]. Thus alterations in the diffusive and mechanical properties, as may occur in an infarct, could have a significant effect on physiologic relationships that exists between tissue strain and molecular transport.
To examine how these conditions affect cell physiology, we examined the survival, proliferation, and differentiation of myoblasts under hypoxia, glucose starvation, and simulated ischemia. Myoblast differentiation potential was reduced in a stepwise fashion when shifted from control conditions to hypoxia (3-fold reduction), starvation (up to 100-fold reduction), and ischemia (no myotube formation). In hypoxic and starvation conditions, this loss of potential occurred despite the ability of cells to survive and reach the confluence necessary for differentiation. Other cell types have shown varying results with oxygen and glucose deprivation. For example, bovine aortic and pulmonary endothelial cells showed reduced proliferation under hypoxic conditions but not under glucose starvation, even in the presence of reduced (~3%) oxygen [42]. In the current study, myoblast growth rate was reduced more significantly by glucose starvation than hypoxia.
In the absence of oxygen, cells produce ATP under the lactate pathway, which generates an order of magnitude less energy than oxidative phosphorylation [43]. This suggests that if glucose is plentiful – a much easier prospect than increasing oxygen availability in infarct – cells could have enough ATP energy not only to survive but to differentiate under hypoxic conditions. This approach is supported by the somewhat reduced, but still potent, ability of C2C12 cells to form myotubes under hypoxia. However, at reduced glucose levels, cells appear to lack the energy to initiate differentiation, which may reflect an attempt to minimize metabolic demands. This metabolic switch from pro-differentiation to pro-survival may allow myoblasts to survive better under stressed conditions.
In summary, this study shows that oxygen levels are reduced in infarcted myocardium to a much greater extent than is glucose. Concomitant with scarring is a decrease in vascular density that appears reflected in the lower concentration of nutrients and oxygen in infarct scar; however, diffusivity within the infarct scar was significantly higher than uninjured heart tissue. These data suggested that even mild increases in vascular density or small decreases of metabolic demand by the injected cells could vastly improve the availability of small molecules, such as glucose and oxygen, throughout the infarct scar milieu. Our cell data show that increased glucose to transplanted cells, either through increased vascular density or perhaps through the use of injected glucose containing scaffolds, might provide the energy necessary to significantly increase survival of transplanted cells. A further understanding of the relationship between the supply, transport, and demand of nutrients, oxygen, and growth factors within the infarct environment could allow maximizing the viability of cells delivered as a therapeutic for myocardial infarction.
Acknowledgments
Supported in part by NIH Grants HL63346, HL63703, AG15768, AR48182, AR50245, and AR48852.
References
- 1.AHA. Heart Disease and Stroke Statistics - 2006 Update. 2006 http://circ.ahajournals.org/cgi/content/short/113/116/e185.
- 2.Clifford DM, Fisher SA, Brunskill SJ, et al. Long-term effects of autologous bone marrow stem cell treatment in acute myocardial infarction: factors that may influence outcomes. PLoS One. 2012;7:e37373. doi: 10.1371/journal.pone.0037373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koh GY, Klug MG, Soonpaa MH, et al. Differentiation and long-term survival of C2C12 myoblast grafts in heart. J Clin Invest. 1993;92:1548–1554. doi: 10.1172/JCI116734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menasche P. Myoblast-based cell transplantation. Heart Fail Rev. 2003;8:221–227. doi: 10.1023/a:1024705214292. [DOI] [PubMed] [Google Scholar]
- 5.Schachinger V, Assmus B, Honold J, et al. Normalization of coronary blood flow in the infarct-related artery after intracoronary progenitor cell therapy: intracoronary Doppler substudy of the TOPCARE-AMI trial. Clin Res Cardiol. 2006;95:13–22. doi: 10.1007/s00392-006-0314-x. [DOI] [PubMed] [Google Scholar]
- 6.Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 7.Menasche P. Skeletal muscle satellite cell transplantation. Cardiovasc Res. 2003;58:351–357. doi: 10.1016/s0008-6363(02)00769-1. [DOI] [PubMed] [Google Scholar]
- 8.Shake JG, Gruber PJ, Baumgartner WA, et al. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Annals Of Thoracic Surgery. 2002;73:1919–1925. doi: 10.1016/s0003-4975(02)03517-8. discussion 1926. [DOI] [PubMed] [Google Scholar]
- 9.Toma C, Pittenger MF, Cahill KS, et al. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 10.Ott HC, Davis BH, Taylor DA. Cell therapy for heart failure--muscle, bone marrow, blood, and cardiac-derived stem cells. Semin Thorac Cardiovasc Surg. 2005;17:348–360. doi: 10.1053/j.semtcvs.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Chiong M, Wang ZV, Pedrozo Z, et al. Cardiomyocyte death: mechanisms and translational implications. Cell Death Dis. 2011;2:e244. doi: 10.1038/cddis.2011.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki K, Smolenski RT, Jayakumar J, et al. Heat shock treatment enhances graft cell survival in skeletal myoblast transplantation to the heart. Circulation. 2000;102:III216–221. doi: 10.1161/01.cir.102.suppl_3.iii-216. [DOI] [PubMed] [Google Scholar]
- 13.Miyagawa S, Sawa Y, Taketani S, et al. Myocardial regeneration therapy for heart failure: hepatocyte growth factor enhances the effect of cellular cardiomyoplasty. Circulation. 2002;105:2556–2561. doi: 10.1161/01.cir.0000016722.37138.f2. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki K, Murtuza B, Smolenski RT, et al. Cell transplantation for the treatment of acute myocardial infarction using vascular endothelial growth factor-expressing skeletal myoblasts. Circulation. 2001;104:I207–212. doi: 10.1161/hc37t1.094524. [DOI] [PubMed] [Google Scholar]
- 15.Mangi AA, Noiseux N, Kong D, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki K, Brand NJ, Allen S, et al. Overexpression of connexin 43 in skeletal myoblasts: Relevance to cell transplantation to the heart. J Thorac Cardiovasc Surg. 2001;122:759–766. doi: 10.1067/mtc.2001.116210. [DOI] [PubMed] [Google Scholar]
- 17.Li W, Tanaka K, Chiba Y, et al. Role of MMPs and plasminogen activators in angiogenesis after transmyocardial laser revascularization in dogs. Am J Physiol Heart Circ Physiol. 2003;284:H23–30. doi: 10.1152/ajpheart.00240.2002. [DOI] [PubMed] [Google Scholar]
- 18.Khurana R, Martin JF, Zachary I. Gene therapy for cardiovascular disease: a case for cautious optimism. Hypertension. 2001;38:1210–1216. doi: 10.1161/hy1101.099483. [DOI] [PubMed] [Google Scholar]
- 19.Kowallik P, Schulz R, Guth BD, et al. Measurement of regional myocardial blood flow with multiple colored microspheres. Circulation. 1991;83:974–982. doi: 10.1161/01.cir.83.3.974. [DOI] [PubMed] [Google Scholar]
- 20.Klein C, Nekolla SG, Bengel FM, et al. Assessment of myocardial viability with contrast-enhanced magnetic resonance imaging: comparison with positron emission tomography. Circulation. 2002;105:162–167. doi: 10.1161/hc0202.102123. [DOI] [PubMed] [Google Scholar]
- 21.Stevenson WG, Weiss JN, Wiener I, et al. Slow conduction in the infarct scar: relevance to the occurrence, detection, and ablation of ventricular reentry circuits resulting from myocardial infarction. Am Heart J. 1989;117:452–467. doi: 10.1016/0002-8703(89)90792-8. [DOI] [PubMed] [Google Scholar]
- 22.Braun RD, Lanzen JL, Snyder SA, et al. Comparison of tumor and normal tissue oxygen tension measurements using OxyLite or microelectrodes in rodents. Am J Physiol Heart Circ Physiol. 2001;280:H2533–2544. doi: 10.1152/ajpheart.2001.280.6.H2533. [DOI] [PubMed] [Google Scholar]
- 23.Blackwell KL, Kirkpatrick JP, Snyder SA, et al. Human recombinant erythropoietin significantly improves tumor oxygenation independent of its effects on hemoglobin. Cancer Res. 2003;63:6162–6165. [PubMed] [Google Scholar]
- 24.Wodnicka M, Guarino RD, Hemperly JJ, et al. Novel fluorescent technology platform for high throughput cytotoxicity and proliferation assays. J Biomol Screen. 2000;5:141–152. doi: 10.1177/108705710000500306. [DOI] [PubMed] [Google Scholar]
- 25.Schlosser T, Hunold P, Herborn CU, et al. Myocardial infarct: depiction with contrast-enhanced MR imaging--comparison of gadopentetate and gadobenate. Radiology. 2005;236:1041–1046. doi: 10.1148/radiol.2363040220. [DOI] [PubMed] [Google Scholar]
- 26.Willerson JT, Perin EC, Ellis SG, et al. Intramyocardial injection of autologous bone marrow mononuclear cells for patients with chronic ischemic heart disease and left ventricular dysfunction (First Mononuclear Cells injected in the US [FOCUS]): Rationale and design. Am Heart J. 2010;160:215–223. doi: 10.1016/j.ahj.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ettinger SN, Poellmann CC, Wisniewski NA, et al. Urea as a recovery marker for quantitative assessment of tumor interstitial solutes with microdialysis. Cancer Res. 2001;61:7964–7970. [PubMed] [Google Scholar]
- 28.Grim M, Carlson BM. Alkaline phosphatase and dipeptidylpeptidase IV staining of tissue components of skeletal muscle: a comparative study. J Histochem Cytochem. 1990;38:1907–1912. doi: 10.1177/38.12.1701462. [DOI] [PubMed] [Google Scholar]
- 29.Leddy HA, Guilak F. Site-specific molecular diffusion in articular cartilage measured using fluorescence recovery after photobleaching. Ann Biomed Eng. 2003;31:753–760. doi: 10.1114/1.1581879. [DOI] [PubMed] [Google Scholar]
- 30.Axelrod D, Koppel DE, Schlessinger J, et al. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976;16:1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bishop SP, White FC, Bloor CM. Regional myocardial blood flow during acute myocardial infarction in the conscious dog. Circ Res. 1976;38:429–438. doi: 10.1161/01.res.38.5.429. [DOI] [PubMed] [Google Scholar]
- 32.Laddis T, Manning WJ, Danias PG. Cardiac MRI for assessment of myocardial perfusion: current status and future perspectives. J Nucl Cardiol. 2001;8:207–214. doi: 10.1067/mnc.2001.112539. [DOI] [PubMed] [Google Scholar]
- 33.Assmus B, Fischer-Rasokat U, Honold J, et al. Transcoronary transplantation of functionally competent BMCs is associated with a decrease in natriuretic peptide serum levels and improved survival of patients with chronic postinfarction heart failure: results of the TOPCARE-CHD Registry. Circ Res. 2007;100:1234–1241. doi: 10.1161/01.RES.0000264508.47717.6b. [DOI] [PubMed] [Google Scholar]
- 34.Meyer GP, Wollert KC, Lotz J, et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months’ follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113:1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 35.Schachinger V, Assmus B, Britten MB, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI Trial. J Am Coll Cardiol. 2004;44:1690–1699. doi: 10.1016/j.jacc.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 36.Taylor DA, Atkins BZ, Hungspreugs P, et al. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nature Medicine. 1998;4:929–933. doi: 10.1038/nm0898-929. [DOI] [PubMed] [Google Scholar]
- 37.Wang B, Ansari R, Sun Y, et al. The scar neovasculature after myocardial infarction in rats. Am J Physiol Heart Circ Physiol. 2005;289:H108–113. doi: 10.1152/ajpheart.00001.2005. [DOI] [PubMed] [Google Scholar]
- 38.Wang B, Scott RC, Pattillo CB, et al. Microvascular transport model predicts oxygenation changes in the infarcted heart after treatment. Am J Physiol Heart Circ Physiol. 2007;293:H3732–3739. doi: 10.1152/ajpheart.00735.2007. [DOI] [PubMed] [Google Scholar]
- 39.Angelini P, Markwald RR. Stem cell treatment of the heart: a review of its current status on the brink of clinical experimentation. Tex Heart Inst J. 2005;32:479–488. [PMC free article] [PubMed] [Google Scholar]
- 40.Gefen A, Cornelissen LH, Gawlitta D, et al. The free diffusion of macromolecules in tissue-engineered skeletal muscle subjected to large compression strains. J Biomech. 2008;41:845–853. doi: 10.1016/j.jbiomech.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 41.van Nierop BJ, Stekelenburg A, Loerakker S, et al. Diffusion of water in skeletal muscle tissue is not influenced by compression in a rat model of deep tissue injury. J Biomech. 2010;43:570–575. doi: 10.1016/j.jbiomech.2009.07.043. [DOI] [PubMed] [Google Scholar]
- 42.Tucci M, Hammerman SI, Furfaro S, et al. Distinct effect of hypoxia on endothelial cell proliferation and cycling. Am J Physiol. 1997;272:C1700–1708. doi: 10.1152/ajpcell.1997.272.5.C1700. [DOI] [PubMed] [Google Scholar]
- 43.Lodish HF. Molecular cell biology. 5. W.H. Freeman and Company; New York: 2003. [Google Scholar]