Abstract
Gap junction (GJ) channels composed of Connexin36 (Cx36) are widely expressed in the mammalian CNS and form electrical synapses between neurons. Here we described a novel modulatory mechanism of Cx36 GJ channels that is dependent on intracellular free magnesium ([Mg2+]i). We examined junctional conductance (gj) and its dependence on transjunctional voltage (Vj) at different [Mg2+]i in cultures of HeLa or N2A cells expressing Cx36. We found that Cx36 GJs are partially inhibited at resting [Mg2+]i, thus, gj can be augmented or reduced by lowering or increasing [Mg2+]i, respectively. Similar changes in gj and Vj-gating were observed using MgATP or K2ATP in pipette solutions, which increases or decreases [Mg2+]i, respectively. Changes in phosphorylation of Cx36 or in [Ca2+]i were not involved in the observed Mg2+-dependent modulation of gj. Magnesium ions permeate the channel and transjunctional asymmetry in [Mg2+]i resulted in asymmetric Vj-gating. The gj of GJs formed of Cxs 26, 32, 43, 45 and 47 was also reduced by increasing [Mg2+]i, but was not increased by lowering [Mg2+]i; single channel conductance did not change. We showed that [Mg2+]i affects both open probability and the number of functional channels, likely through binding in the channel lumen. Finally, we showed that Cx36-containing electrical synapses between neurons of the trigeminal mesencephalic nucleus in rat brain slices are similarly affected by changes in [Mg2+]i. Thus, this novel modulatory mechanism could underlie changes in neuronal synchronization under conditions in which ATP levels, and consequently [Mg2+]i, are modified.
Introduction
Electrical synapses are specialized junctions between neurons formed by clusters of gap junction (GJ) channels that allow direct intercellular communication of electrotonic potential, signaling molecules and metabolites. Each GJ channel is formed by two apposed hemichannels (aHCs), each of which is formed by six connexin (Cx) subunits. Neurons in the adult brain, as well as insulin-secreting β-cells in the pancreas, express Cx36 (Condorelli et al., 1998; Sohl et al., 1998; Serre-Beinier et al., 2000). GJs composed of a single Cx isoform generally show a maximum junctional conductance (gj) at transjunctional voltage (Vj) equal zero and a symmetric gj decrease with increasing Vj of either polarity. The change in gj is attributed to the presence of two Vj-sensitive gates in each aHC, the ‘fast’ and the ‘slow’ gates (Bukauskas and Weingart, 1994). Cx36-containing electrical synapses are expressed in many regions of the mammalian CNS, such as the trigeminal mesencephalic (MesV) nucleus, inferior olive, thalamus, hippocampus, cortex and retina (Connors and Long, 2004), and are thought to promote neuronal synchronization and coordinated activity of various neuronal networks (Bennett and Zukin, 2004).
Normally, the intracellular concentration of free magnesium ([Mg2+]i) is ~10 times lower than total magnesium (Grubbs, 2002); most of the Mg2+ is bound to ATP, and changes in cytosolic ATP concentration ([ATP]i) produce opposite changes in [Mg2+]i (Luthi et al., 1999). Under physiological conditions, neuronal [ATP]i increases during glucose and lactate exposure (Ainscow et al., 2002), and when neuronal activity is reduced during sleep (Dworak et al., 2010). Conversely, ATP levels are reduced by increased neuronal activity during wake periods and hyperactivity (Dworak et al., 2010). Pathological conditions, such as hypoxia, ischemia, and seizures, produce long-lasting depletion of [ATP]i and elevated [Mg2+]i (Murphy et al., 1989; Headrick and Willis, 1991; Helpern et al., 1993). In contrast, traumatic brain injury results in a ~50% reduction in [Mg2+]i for several days (Cernak et al., 1995; Heath and Vink, 1996; Suzuki et al., 1997). Resting brain [Mg2+]i is also reduced in patients with neurological diseases, such as Parkinson’s (Barbiroli et al., 1999) and Alzheimer’s (Andrasi et al., 2000); or increased in patients with schizophrenia (Hinsberger et al., 1997). Taken together these findings suggest that changes in neuronal [Mg2+]i during physiological as well as pathological conditions can be sufficient to modulate electrical synapses.
Here we show that Cx36 GJs are inhibited by resting [Mg2+]i (~1 mM) and that gj can be augmented or reduced by lowering or increasing [Mg2+]i, respectively. We find that intracellular ATP is critical for the Mg2+-dependent modulation of Cx36 GJs and propose that Mg2+ is directly involved in channel gating by interacting with a sensorial domain located in the channel lumen. Our results indicate that Mg2+ occupancy of Cx36 GJ channels induces a reduction in open probability by increasing sensitivity to Vj-induced closure of fast gates, and stabilization of a closed conformation of slow gates. Finally, we show that Cx36-containing electrical synapses between MesV neurons respond similarly to changes in [Mg2+]i, indicating that this novel Mg2+-dependent modulatory mechanism of Cx36 GJs is relevant for the modification of neuronal electrical transmission.
Materials and Methods
Cell lines and culture conditions
Experiments were performed in HeLa (Human cervical carcinoma cells, ATCC CCL2) or N2A (mouse neuroblastoma cells, CCL-131) cells transfected with Cx26, Cx32, Cx36, Cx43, Cx45 or Cx47 wild type or fused with colour variants of green fluorescent proteins (EGFP or CFP) attached to the C-terminus. We also used Novikoff cells expressing endogenous Cx43. Cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 8% fetal calf serum, 100 μg/ml streptomycin and 100 units/ml penicillin, and maintained at 37°C in humidified air with 5% CO2. Vectors for transfection and cell lines stably expressing the Cxs used were developed in collaboration with the laboratories of Dr. K. Willecke (Cx36 and Cx47) and Dr. D.W. Laird (Cx43). More details on these issues are published elsewhere (Bukauskas et al., 2000; Teubner et al., 2000; Teubner et al., 2001). Phosphomimetic mutants of Cx36 were introduced into wild-type Cx36 (Al-Ubaidi et al., 2000) at Ser110 and Ser293 using the Quickchange Multi Site-directed mutagenesis kit (Agilent, Cedar Creek, TX). Mutants were subcloned into pEGFP-N1 (Clontech, Mountain View, CA) and transfected into HeLa cells using Lipofectamine 2000 (Invitrogen, Eugene, OR).
In vitro electrophysiological measurements
Experiments were performed in a modified Krebs-Ringer solution containing (in mM): 140 NaCl, 4 KCl, 2 CaCl2, 1 MgCl2, 2 CsCl, 1 BaCl2, 5 glucose, 2 pyruvate, 5 HEPES (pH 7.4). Recording pipettes (3–5 MΩ) were filled with standard pipette solution containing (in mM): 140 KCl, 10 NaAsp, 1 MgCl2, 0.26 CaCl2, 2 EGTA, 5 HEPES (pH 7.2). To study the effect of [Mg2+]i, from 0.01 mM to 10 mM, we used pipette solutions containing different concentrations of MgCl2 (Table 1) and applied the web-based Maxchelator software to calculate free ionic concentrations (www.stanford.edu/~cpatton/webmaxcS.htm). To study the effect of divalents other than Mg2+ we used pipette solutions containing (in mM): 140 KCl, 10 NaAsp, 5 HEPES (pH=7.2), with or without 2 mM XCl2 of X divalent. For simultaneous electrophysiological and fluorescence recordings, cells were grown on glass coverslips and transferred to an experimental chamber mounted on the stage of an inverted microscope (Olympus IX70) equipped with a fluorescence imaging system. Cells were perfused with modified Krebs-Ringer solution at room temperature. Junctional conductance (gj) was measured using a dual whole-cell voltage clamp system. Briefly, each cell of a pair was voltage clamped independently with a separate patch clamp amplifier (EPC-8; HEKA). By stepping the voltage in cell-1 (V1) and keeping the voltage in cell-2 (V2) constant we generate a transjunctional voltage (Vj=ΔV1), and the corresponding junctional current (Ij) was measured as the negative of the current change in cell-2, Ij = −ΔI2; Ij has the same polarity as the voltage step in cell-1. Thus, gj was obtained from the equation gj= Ij/Vj. Signals were acquired and analysed using custom-made software (Trexler et al., 1999) and an A/D converter from Axon Instruments.
Table 1.
Composition of pipette solutions used in this study.
Concentrations of free Mg2+ and Ca2+ were calculated using Maxchelator software (see methods). In addition to the components indicated, each solution contained (in mM): 10 NaAsp, 5 TEA, 5 HEPES. Differences in osmolarity were compensated for with different concentrations of KCl, and solutions were titrated to pH = 7.2 with KOH.
| Solution | Free [Mg2+] (mM) | MgCl2 (mM) | Free [Ca2+] (nM) | CaCl2 (mM) | EGTA (mM) | EDTA (mM) | BAPTA (mM) | KCl (mM) |
|---|---|---|---|---|---|---|---|---|
| I | 0.01 | 0.14 | 25 | 0.89 | 5 | 0.2 | 2 | 121.2 |
| II | 0.1 | 0.13 | 25 | 0.86 | 5 | 0 | 2 | 121 |
| III | 1 | 1.26 | 25 | 0.83 | 5 | 0 | 2 | 119.3 |
| IV | 5 | 6.1 | 25 | 0.72 | 5 | 0 | 2 | 112.2 |
| V | 10 | 12 | 25 | 0.62 | 5 | 0 | 2 | 103.5 |
| VI | 10 | 12 | 0 | 0 | 5 | 0 | 10 | 100.4 |
Brain-slice preparation and ex vivo electrophysiological measurements
A minimum number of animals were sacrificed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals, and according to the Institutional Animal Care and Use Committee of Albert Einstein College of Medicine. Transverse brain stem slices (250-μm thick) were prepared from male or female Sprague-Dawley rats (age: P14–P18). Slices were obtained using a vibratome (DTK Microslicer) and placed in cold sucrose solution containing (in mM): 248 sucrose, 2.69 KCl, 1.25 KH2PO4, 26 NaHCO3, 10 Glucose, 2 CaCl2, and 2 MgSO4. The slices were then transferred to an incubation chamber filled with sucrose solution at room temperature and incubated for 60 min. The sucrose solution was slowly replaced by physiological solution containing (in mM); 124 NaCl, 2.69 KCl, 1.25 KH2PO4, 26 NaHCO3, 10 Glucose, 2 CaCl2, and 2 MgSO4. Sections were kept at room temperature in the physiological solution until they were transferred into the recording chamber. The recording chamber, mounted on an upright microscope stage (Nikon Eclipse E600), was continuously perfused with physiological solution (1–1.5 ml/min) at room temperature. Whole cell patch recordings were performed under visual control using infrared differential interference contrast optics (IR-DIC). MesV neurons were identified on the basis of their location, large spherical somata and characteristic electrophysiological properties in response to both depolarizing and hyperpolarizing current pulses (Curti et al., 2012). Recording pipettes (6–12 MΩ) were filled with intracellular solution containing (in mM): 140 K-gluconate, 3 MgCl2, 0.2 EGTA, 10 HEPES (pH 7.2). Free Mg2+ was adjusted to 0.01 mM by adding 4 mM EDTA, or to 5 mM by adding 2 mM MgCl2. Simultaneous recordings were made using a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA), acquired and analyzed using Igor software (Wave Metrics, Portland OR).
Estimates of the junctional conductance between MesV neurons (Gj) were calculated following (Bennett, 1966) and (Parker et al., 2009):
where Rinput1 and Rinput2 represent the input resistance of cell-1 and cell-2, respectively, and Rtransfer represents the transfer resistance between coupled cells. The Rtransfer is defined as the amplitude of the voltage response measured in cell-1 or cell-2, divided by the amplitude of the current step injected into cell-2 or cell-1, respectively. To calculate Gj in current clamp configuration, hyperpolarizing current pulses (−300 pA) of 200 – 400 ms duration were injected into one cell and the resulting voltage deflections were measured in both cells. A total of 5 to 20 single responses were averaged in order to improve the signal to noise ratio, and for each coupled pair the mean Gj was calculated as the average from the values in both directions. In a linear system, Rtransfer is equal in both directions. These estimates assume a simple two-neuron model with passive membrane properties coupled directly by a single domain isopotential on each side. Voltage dependence of Gj is assumed to be negligible, and additional pathways via coupled dendrites or adjacent coupled neurons are excluded. These assumptions are reasonable for Cx36 GJs (Srinivas et al., 1999; Teubner et al., 2000; Moreno et al., 2005) and anatomy of MesV neurons (Curti et al., 2012).
Fluorescence imaging and magnesium transfer studies
Fluorescence signals were acquired using an ORCA digital camera (Hamamatsu Corp., Bridgewater, NJ) with UltraVIEW software for image acquisition and analysis (Perkin Elmer Life Sciences, Boston, MA). For magnesium transfer studies, the tetrapotassium salt of the magnesium fluorescent probe (50 μM), Mag-Fluo-4 (Invitrogen, Eugene, OR), was introduced into cell-1 of a pair through a patch pipette with modified standard pipette solution without MgCl2. Pipette solution for cell-2 was modified with addition of 9 mM MgCl2. Typically, breaking into cell-1 was followed by an increased fluorescence intensity in cell-1 (FI1). After reaching steady-state in cell-1, the patch in cell-2 was opened and the changes in FI1 and gj were measured. The rate of FI1 changes (AU/min) was calculated as the difference between FI1 at the moment of breaking into cell-2 and FI1 after 2 min.
Data analysis
The analysis and statistics were performed using SigmaPlot software. Averaged data are reported as the means ± SEM. Means for each group were compared using unpaired student’s t-test.
Results
Intracellular magnesium-dependent modulation of junctional conductance and voltage–gating of Cx36 GJ channels
In order to understand the influence of Mg2+ on Cx36-mediated electrical coupling, we studied changes in gj and gj–Vj dependence at different [Mg2+]i in pairs of HeLa cells expressing Cx36 wild-type, and HeLa or N2A cells expressing Cx36 tagged with EGFP (Cx36-EGFP) using dual whole-cell patch clamp. We changed [Mg2+]i, using pipette solutions containing from 0.01 to 10 mM free Mg2+ ([Mg2+]p) with compositions (Table 1) determined using the Maxchelator program (see Methods). The pipette solutions also contained 5 mM EGTA and 2 mM BAPTA to buffer free Ca2+ concentration at 25 nM. Basal gj was measured with small amplitude Vj ramps (+20 to −20 mV; 1.3 s in duration), and gj–Vj dependence was measured with high amplitude Vj ramps (from 0 to −100 mV; 50 s in duration). When both pipettes contained [Mg2+]p = 0.01 mM, gj of Cx36 GJs increased after patch opening (Fig. 1A), and became less Vj sensitive than in control condition with [Mg2+]p = 1 mM (Fig. 1B). In contrast, [Mg2+]p = 5 mM in both pipettes led to a reduction in gj (Fig. 1C) and increased Vj-sensitivity compared to control (Fig. 1D). In both cases the gj reached steady-state within ~20 min. A steady-state gj–[Mg2+]p curve normalized to initial gj values (Fig. 1E) shows a half maximal effective concentration (EC50) of 0.45 mM. All gj Vj relations shown in Fig. 1B and 1D were fitted using a stochastic 16-state model (S16SM) of voltage gating (Paulauskas et al., 2012) containing in series two Vj-sensitive gates for each aHC, a fast and a slow gate (Bukauskas and Weingart, 1994).
Figure 1.
Mg2+-dependent modulation of gj and Vj-gating in HeLa cells expressing Cx36-EGFP GJs. A and C, Lower panels show dynamics of gj (normalized to initial gj value) during repeated Vj ramps (+20 to − 20 mV and 1.3 s in duration; top traces) at [Mg2+]p equal 0.01 (A) and 5 mM (C). B and D, gj–Vj dependence (normalized to initial gj value at Vj=0) obtained during 50 s long Vj ramps (from 0 to −100 mV; middle traces) derived from data shown in (A) and (C), respectively; the numbers on the gj–Vj plots correspond to numbers on gj traces in (A) and (C). Fitted curves shown in color were obtained using the S16SM. Grey lines show fitted curves obtained from control gj–Vj plots ([Mg2+]p = 1 mM). E, Concentration-response relation of gj (normalized to initial gj value) as a function of [Mg2+]p; EC50 ≈ 0.45 mM. Averaged data are shown in colored circles. Data of individual experiments are shown in grey triangles. Roman numerals correspond to different solutions shown in Table 1. F, Averaged gj – Vj dependencies (normalized to gj value at Vj = 0) obtained at different [Mg2+]p (curves in black) were fitted using a S16SM (gj–Vj plots in colors). Roman numerals correspond to different Mg2+ concentrations shown in (E). Parameters obtained after fitting are shown in Table 2. G–I, Open probabilities of fast and slow gates in α and β aHCs (PoF,α, PoS,α and PoF,β, PoS,β, respectively) depending on Vj were calculated using parameters obtained in F for [Mg2+]p equal 0.01 (G), 1 (H) and 10 mM (I).
In the S16SM, fast gates have an open state with conductance (γF,open) and a “closed” or residual state with conductance (γF,res > 0), and slow gates have an open state with conductance (γS,open) and a closed state with zero conductance (γS,closed = 0). Therefore, the channel can occupy one of the 16 possible states made by the combination of 4 states of the fast gates with 4 states of the slow gates. For simplicity we assume that γF,open = γS,open, and that conductance of the fully open channel γopen = γF,open/4 = γS,open/4. The behavior of each gate is characterized by Boltzmann equilibrium constants between open and residual/closed states, for fast (KF,o↔res = e AF (−Π·VF−VF,o)) or slow (KS,o↔c = eAS(−Π·VS−VS,o)) gates, where A (AF and AS) characterize the maximal steepness of changes in open probability (PoF and PoS) as a function of voltage across the gate (VF and VS), Vo (VF,o and VS,o) is the voltage across the gate at which its probability to be in the open state is 0.5, and Π is a gating polarity (+1 or − 1). Thus, the S16SM allowed us to estimate gating parameters characterizing sensitivity to Vj for each gate and the number of operational/functional channels (NF). In addition, the S16SM uses an exponential function to describe rectification over voltage (e.g., γF,res = γF,res,0 e−VF / RF,res, where γF,res,0 is γF,res at VF = 0), therefore allowing to estimate rectification coefficients for the conductive states of fast (RF,open and RF,res) and slow (RS,open) gates. Vj across the GJ channel is the sum of voltages across all gates, Vj =VF,α+VS,α+VF,β+VS,β, where α and β stand for the two aHCs. Closing one gate changes the voltage across the other three gates in series, and this affects the probability of the state’s changing over a discreet time interval. The residual conductance of the GJ channel (γres) typically is ~1/5th of γopen (Bukauskas and Verselis, 2004), which is measured when one of fast gates is in the closed/residual state and three other gates in series are in the open state (1/γres=1/γF,res+1/γF,open+2/γS,open).
Values of A and Vo for fast and slow gates estimated during the fitting process allowed calculation of open probabilities for each gate in α and β aHCs (PoF,α, PoS,α, PoF,β, and PoS,β) and the probability of a GJ channel to reside in a fully open state (Po; four gates in the open state) as a function of Vj. Fitting of averaged experimental gj–Vj dependence normalized to gj values at Vj = 0 (black lines in Fig. 1F; we assumed symmetry of the gj–Vj relation around Vj = 0; fitted curves are in colors) revealed that with reduction of [Mg2+]p, VF,o and VS,o increased while AF and AS remained relatively constant (Table 2). Vo and A for both fast and slow gates, and γF,res were set as free parameters during the fitting process (Table 2). Fig. 1G-I shows open probability of each gate as functions of Vj derived from gj–Vj plots obtained at different [Mg2+]p and shown in 1F. At [Mg2+]p = 0.01 mM (Fig. 1G) all gates remain open (Po close to unity) over the entire Vj range. At [Mg2+]p = 1 mM (Fig. 1H), PoF,α and PoF,β showed enhanced sensitivity to Vj with a value of 0.8 at Vj = 0, and PoS,α and PoS,β remain close to unity at Vj = 0. Therefore, on average only 64% of functional GJ channels have all four gates in the open state at [Mg2+]p = 1 mM and Vj = 0. At [Mg2+]p = 10 mM (Fig. 1I), PoF,α and PoF,β show even higher sensitivity to Vj corresponding the shift of PoF,α and PoF,β plots along the Vj axis, but PoS,α and PoS,β did not change very much. As a result, Po at Vj = 0 was reduced to ~0.36. Thus, Po at [Mg2+]i = 10 mM was ~2.8 fold less than Po at 0.01 mM, and changes in PoF,α and PoF,β caused by changes in Vos account for ~80% of the changes in gj as a function of [Mg2+]i. The remaining ~20% can be explained by changes in NF, where channels may enter into a Mg2+-occupied (long-lived) closed conformation of the slow gate.
Table 2.
Parameters of voltage-gating estimated with S16SM.
Parameters were obtained from the fitting of gj–Vj data shown in Figures 1F and 3H using the stochastic 16-state model of GJ channels (S16SM).
| Fast gate | Slow gate | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Figure (Cx) | Solution | AF (mV−1) | VF,o (mV) | γF,open (pS) | γF,res (pS) | RF,res (mV) | AS (mV−1) | VS,o (mV) | γS,open (pS) | γS,closed (pS) |
| 1F (Cx36) | I | 0.041 | 122 | 24 | 0.6 | - | 0.100 | 154 | 24 | 0 |
| II | 0.031 | 87 | 24 | 0.7 | - | 0.062 | 158 | 24 | 0 | |
| III | 0.037 | 41 | 24 | 0.5 | - | 0.085 | 77 | 24 | 0 | |
| V | 0.045 | 12 | 24 | 0.6 | - | 0.084 | 68 | 24 | 0 | |
| 3H (Cx47) | I | 0.30 | 40 | 220 | 11.2 | 296 | 0.99 | 131 | 220 | 0 |
| III | 0.20 | 34 | 220 | 7.0 | 322 | 0.10 | 124 | 220 | 0 | |
| IV | 0.14 | 27 | 220 | 6.2 | 272 | 0.09 | 103 | 220 | 0 | |
| V | 0.16 | 21 | 220 | 6.2 | 210 | 0.11 | 86 | 220 | 0 | |
To test whether the decrease in coupling under high [Mg2+]i depends on changes in intracellular free Ca2+ concentration ([Ca2+]i), we enhanced the buffering capacity of the pipette solution by increasing BAPTA to 10 mM (Table 1; Solution VI), which reduced [Ca2+]p close to zero. We did not find significant differences in gj decay under high [Mg2+]i conditions (10 mM) with normal/control (25 nM) or close to zero [Ca2+]p (Fig. 2A). HeLa cells expressing Cx36 and Cx36-EGFP exhibited similar Mg2+-dependent modulation of gj (Fig. 2B–C). However, neuroblastoma cells expressing Cx36-EGFP (N2A-Cx36-EGFP) showed higher increase in gj than HeLa cells expressing Cx36-EGFP when exposed to 0.01 mM [Mg2+]p (Fig. 2B). In summary, Cx36 GJs exhibited a “run-up” or “run-down” in gj when [Mg2+]p in both pipette solutions was lower or higher than ~1.3 mM, respectively, and we assume that 1.3 mM is the resting [Mg2+]i in HeLa cells under our experimental conditions (Fig. 1E). Changes in [Mg2+]i have a marked effect on VF,o, which affects mainly Po, and to a small degree NF.
Figure 2.
Differences in [Mg2+]i-dependent modulation of gj in HeLa or N2A cells expressing Cxs 26, 32, 36, 43, 45 or 47; changes in [Ca2+]i are not involved in [Mg2+]i-dependent modulation of Cx36. All data represent mean gj (normalized to initial gj value). A, Normalized gj measured in HeLa Cx36-EGFP cell pairs using pipette solutions containing 10 mM free Mg2+ and 2 mM BAPTA (free Ca2+ = 25 nM) or 10 mM BAPTA (free Ca2+ ≈ 0). B–C, Normalized gj measured using pipette solutions containing 0.01 (B) or 5 mM of free Mg2+ (C) in HeLa Cx36WT, HeLa Cx36-EGFP or N2A Cx36-EGFP cell pairs. D–E, Normalized gj measured using pipette solutions containing 0.01 (D) or 5 mM (E) of free Mg2+ in HeLa cells expressing Cxs 26, 32, 36, 43, 45 or 47. Numbers of cell pairs are indicated within columns; *p < 0.05, ns: non-significant p values.
Junctional conductance of GJ channels formed of Cxs 26, 32, 43, 45 or 47 is also reduced by increase in intracellular magnesium
To compare Cx36 to other Cxs from different phylogenetic groups with respect to the effects of [Mg2+]i, we studied Mg2+-dependent modulation of gj in HeLa cells expressing: Cx43 (α-group); Cx26 and Cx32 (β-group); and Cx45 and Cx47 (γ-group). In contrast to Cx36 (δ-group), [Mg2+]p = 0.01 mM did not increase gj above initial values in all other tested Cxs (Fig. 2D). However, [Mg2+]p = 5 mM reduced gj in all tested Cxs (Fig. 2E). To compare Cx36 to other Cxs expressed in the CNS with respect to gj–Vj dependence at different [Mg2+]i, we chose Cx43, Cx45, and Cx47 which are expressed in astrocytes, neurons and oligodendrocytes, respectively. Similar to the effect on Cx36, high [Mg2+]p increased sensitivity to Vj in Cx43 and Cx47 expressing cells (Fig. 3A–B and 4E–F); increased Vj-sensitivity was not observed in Cx45 expressing cells (Fig. 3C–D). Increase in [Mg2+]p to 10 mM decreased gj for Cx47 (normalized to initial values) to 0.08 (Fig. 3G). Steady-state gj–Vj relationships (normalized to gj at Vj = 0) at different [Mg2+]p for Cx47 (black lines in Fig. 3H) were fitted using the S16SM (colored lines in Fig. 3H); all parameters are shown in Table 2. Elevation in [Mg2+]i moderately increased AF and the maximal steepness of gj vs Vj changes, and markedly decreased VF,o, as is reflected in narrowing of the (flat topped) bell shaped gj–Vj plot (Fig. 3F and H). However, PoF and PoS at Vj = 0 remained constant and close to unity for all [Mg2+]p, as reflected in the flat top of the gj–Vj relation (Fig. 3I–J). Therefore, all changes in gj observed at Vj = 0 under different [Mg2+]i are due to a reduction in NF and not to changes in parameters of Vj-gating. In contrast to Cx36, gj of Cxs 26, 32, 43, 45 and 47 GJs showed spontaneous “run down” even at [Mg2+]p= 0.01 mM (Fig. 2D). Therefore all data in the gj [Mg2+] dependence for Cx47 are below unity (Fig. 3G). This decay in gj of Cx47 was prevented by adding 3 mM K2ATP to solutions with low [Mg2+]p (not shown), indicating that stability of these GJs depends on ATP as observed for GJs formed between cardiomyocytes (Sugiura et al., 1990; Verrecchia et al., 1999).
Figure 3.
[Mg2+]i-dependent modulation of gj and Vj-gating of GJs formed by other Cxs expressed in CNS does not affect single channel conductance. A,C,E, Changes of Ij in response to repeated 50 s long Vj ramps from 0 to − 100 mV and intermediate small amplitude steps (−15 mV) using [Mg2+]p = 5 mM in Novikoff (A) HeLa Cx45 (C) and HeLa Cx47-EGFP (E) cell pairs. B,D,F, First (purple) and last (cyan) gj–Vj relations (normalized to initial gj value at Vj=0) obtained from experiments shown in (A,C,E), respectively. Last gj–Vj relation normalized to gj value at Vj = 0 (grey) is also shown for comparison. G, Concentration-response relation of gj (normalized to initial gj value) as a function of [Mg2+]p for Cx47; EC50 ≈ 2.8 mM. Averaged data are shown in colored circles; roman numerals correspond to different pipette solutions shown in Table 1. H, Averaged gj–Vj dependence (normalized to gj at Vj=0) obtained at different [Mg2+]p for Cx47; roman numerals correspond to different [Mg2+] shown in (G) and Table 1. Experimental gj–Vj plots shown in black were fitted using the S16SM; calculated values of gating parameters are shown in Table 2, and the best fitting gj–Vj plots are shown in colors which correspond to colors of circles in (G). I–J, Open probabilities of fast and slow gates in α and β aHCs (PoF,α, PoS,α and PoF,β, PoS,β, respectively) depending on Vj were calculated using parameters obtained in (H) for [Mg2+]p equal 0.01 (I) and 10 mM (J). K, Ij record of Cx43-CFP GJs obtained at Vj = −80 mV. L, Histogram from data in (K) shows a series of peaks separated by ~96.5 ± 8 pS. M, Single channel conductance for Cx47-EGFP obtained in response to a Vj step of −52 mV. The histogram shows peaks for the closed state, substate (γs = 11.2 ± 2.3 pS) and open state (γo = 49 ± 5.6 pS). The arrow-head indicates a slow transition from the closed state to open state, and the arrow indicates a fast transition from the open state to the substate. N, Ij trace from a HeLa Cx47-EGFP cell pair in response to 1.5 s long Vj ramps from 90 to −90 mV using [Mg2+]p = 5 mM after 32, 37 and 42 min of recording.
Figure 4.
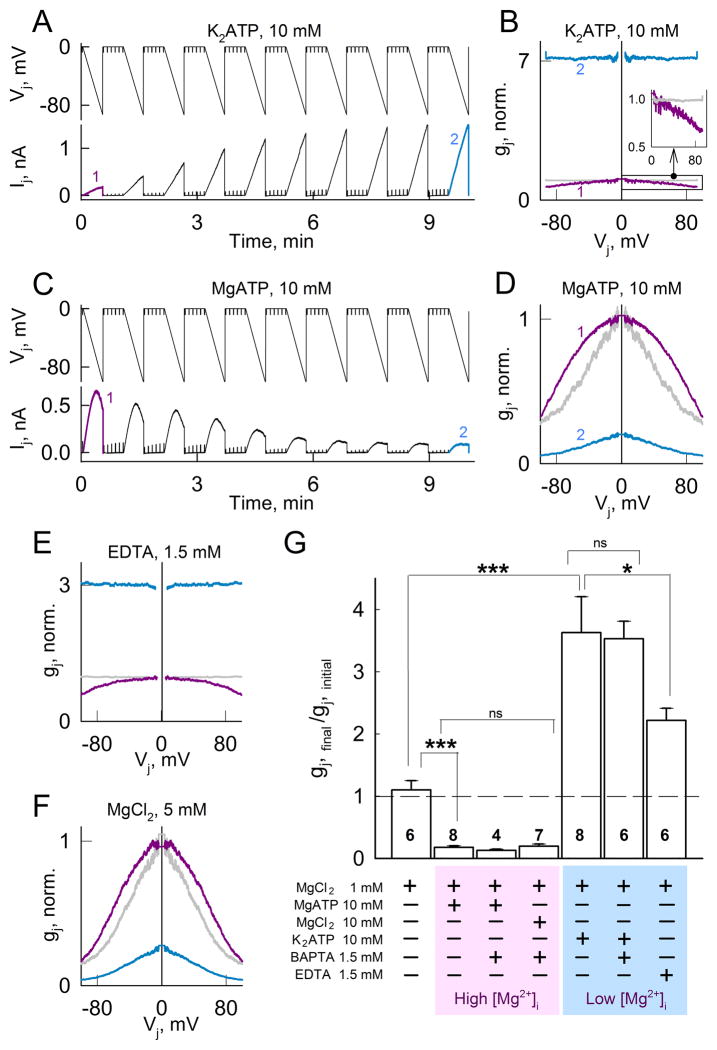
Intracellular K2ATP and MgATP have opposite effects on gj and Vj-gating in HeLa cells expressing Cx36-EGFP GJ channels. A and C, Ijs recorded during repeated 35 s long Vj ramps from 0 to −100 mV. Brief voltage steps of −10 mV were used to measure gj in between Vj ramps. Pipette solutions contained 10 mM K2ATP (A) or MgATP (C). B and D, First (purple) and last (cyan) gj–Vj relations (normalized to initial gj value at Vj=0) measurements obtained from experiments shown in (A) and (C), respectively. E and F, First (purple) and last (cyan) gj–Vj relations (normalized to initial gj at Vj = 0) obtained using the same Vj protocol as in (A). Pipette solutions contained 1.5 mM EDTA (E) or 5 mM MgCl2 (F). In all gj–Vj plots we assumed that the gj–Vj relation for Vj > 0 was the mirror image of that for Vj < 0, i.e. symmetrical around Vj = 0, and the last gj–Vj plots normalized to gj at Vj = 0 (grey) are also shown for comparison. G, Changes in normalized gj for the different compositions of the pipette solutions shown at the bottom. Values were obtained from the ratios of the steady-state final gj to the initial gj. Numbers of independent experiments are shown in the histogram bars; *p < 0.05; ***p < 0.001; ns = non-significant p values.
Single channel conductance of Cx43 and Cx47 GJs is not affected by high intracellular magnesium
Previous studies of Cx36 GJs have shown that γopen is very small compared to those of GJs formed of other Cxs (Srinivas et al., 1999; Teubner et al., 2000; Moreno et al., 2005), making the study of changes in γopen under different [Mg2+]i impracticable. Thus, we tested whether γopen of GJ channels formed by Cxs with relatively high γopen (Cx43 and Cx47) were modified by 5 mM [Mg2+]p (Table 1; solution IV). Experiments were performed in HeLa cells expressing Cx43-CFP or Cx47-EGFP. The γopen of Cx43 and 47 values under high [Mg2+]p did not differ significantly from those previously reported under control conditions (Moreno et al., 1994; Bukauskas et al., 2000; Teubner et al., 2001). We found that, at [Mg2+]p = 5 mM, γopen of Cx43 and Cx47 GJ channels was 96.5 ± 8 pS (Fig. 3K–L), and 49 ± 5.6 pS (Fig. 3M), respectively. In the experiment shown in Fig. 3K, initial gj at time 0 was ~40 nS, corresponding to ~400 open Cx43 GJ channels. High [Mg2+]p reduced gj and ~36 min later no more than three GJ channels were open simultaneously. Similarly, in the experiment shown in Fig. 3N, initial gj was ~6 nS, corresponding to ~120 open Cx47 GJ channels. High [Mg2+]p reduced gj, and after 32, 37 and 42 min, maximum numbers of channels open were 7, 3 and 1, respectively. In summary, reduction in conductance at Vj 0 of Cx43 and Cx47 GJs by [Mg2+]p = 5 mM results from a reduction of NF without changes in γopen.
Intracellular ATP-dependent modulation of junctional conductance and voltage-gating of Cx36 GJs
Adding K2ATP to the pipette solution, which reduces free [Mg2+]p, or MgATP, which increases free [Mg2+]p (Luthi et al., 1999), replicated results obtained with low or high free [Mg2+]p, respectively (solutions in Table 1) (Fig. 1). Indeed, adding 10 mM of K2ATP to pipette solution with MgCl2 = 1 mM (see Methods) increased gj (Fig. 4A and G) and decreased its sensitivity to Vj (Fig. 4B). Similar results were obtained when [Mg2+]p was reduced by adding the Mg2+ chelator EDTA (Fig. 4E). In contrast, addition of 10 mM MgATP, which increases free Mg2+ (Luthi et al., 1999), decreased gj (Fig. 4C and G) and increased its sensitivity to Vj (Fig. 4D). Similar results were obtained by adding 5 mM MgCl2 (Fig. 4F). These results show that low free Mg2+ conditions obtained by adding K2ATP, or high free Mg2+ conditions obtained by adding MgATP have a marked effect on gj. Addition of 1.5 mM BAPTA to solutions with K2ATP or MgATP to minimize changes in [Ca2+]p showed no differences (Fig. 4G). In summary, we attribute the effect of ATP on gj of Cx36 GJs mainly to its capacity to modify [Mg2+]i.
Magnesium ions permeate Cx36 and Cx47 GJ channels
To determine whether Mg2+ permeates GJ channels and discard the hypothesis of a physical occlusion of the channel pore, we used a cell-impermeant fluorescent Mg2+ indicator (Mag-Fluo-4 or MF4) in HeLa Cx36-EGFP or Cx47-EGFP cell pairs. First, we opened the patch in cell-1 (arrow in Fig. 5A–B) with a pipette containing 50 μM MF4 and nominally zero [Mg2+]p. Fluorescence intensity in cell-1 (FI1), increased to a plateau, presumably corresponding to a low [Mg2+]i due to loss into pipette-1 and basal fluorescence of MF4. Then, we opened the patch in cell-2 (arrowhead in Fig. 5A–B) connecting it to a pipette containing 10 mM MgCl2 and measured FI1 and gj (upper panels) determined by applying repeated small amplitude Vj ramps (as shown in Fig. 1A). FI1 rapidly increased indicating flux of Mg2+ from cell-2 to cell-1. When the GJ blocker octanol was applied, gj and FI1 decreased due to the closure of GJs between cell-1 and 2 and diffusion of Mg2+ into pipette-1 (Fig. 5A–B). For different pair of cells, the rate of increase of FI1 (measured in arbitrary units (AU) for the first two minutes after breaking into cell-2) was proportional to gj for both Cx36 and Cx47 and about half as fast per unit gj for Cx36 as for Cx47 (Fig. 5C). The same flux data were plotted as a function of the number of open channels obtained by dividing gj by γopen of Cx36 (although we could not reliably measure γopen for Cx36, we used the published value of 6 pS (Moreno et al., 2005)) and Cx47 (γopen = 55 pS (Teubner et al., 2001)); for these values of γopen the per channel flux for Cx36 was ~1/20th of that for Cx47 (Fig. 5D). In summary, Cx36 and Cx47 GJ channels are permeable to Mg2+, and the Mg2+ flux per nS gj for Cx47 was about twice that for Cx36. For comparison, the ratio of γopen’s of Cx36 and Cx47, which are presumably K+ dominated, is ~1/10.
Figure 5.
Permeation of Cx36 or Cx47 GJ channels by Mg2+ ions. A–B, Mag-fluo-4 (MF4) fluorescence intensity measured in cell-1 (FI1) of HeLa Cx36-EGFP (A) and HeLa Cx47-EGFP (B) cell pairs increased after opening the patch in cell-1 (arrow). After FI1 reached a plateau (normalized to this value), pipette-2 was opened (arrowhead) and FI1 again increased. Pipette-1 contained 50 μM MF4 and zero MgCl2, and pipette-2 contained 10 mM MgCl2 (top diagram). The gj measurements were started after patch opening in cell-2 (top panel). FI1 and gj decreased after bath application of the GJ blocker, octanol (1 mM); decrease in FI1 is ascribable to loss of Mg2+ into pipette-1. C–D, Rates of FI1 changes in HeLa Cx36-EGFP (grey; n = 12) and HeLa Cx47-EGFP (black; n = 15) cell pairs measured after patch opening in cell-2 and plotted over gj (C) or the calculated number of open channels (D). Grey and black lines are linear regressions for Cx36-EGFP (R2 =0.71) and Cx47-EGFP (R2 =0.9) data, respectively. AU = arbitrary units.
Transjunctional asymmetry of free magnesium ions results in asymmetric voltage gating of Cx36 GJs
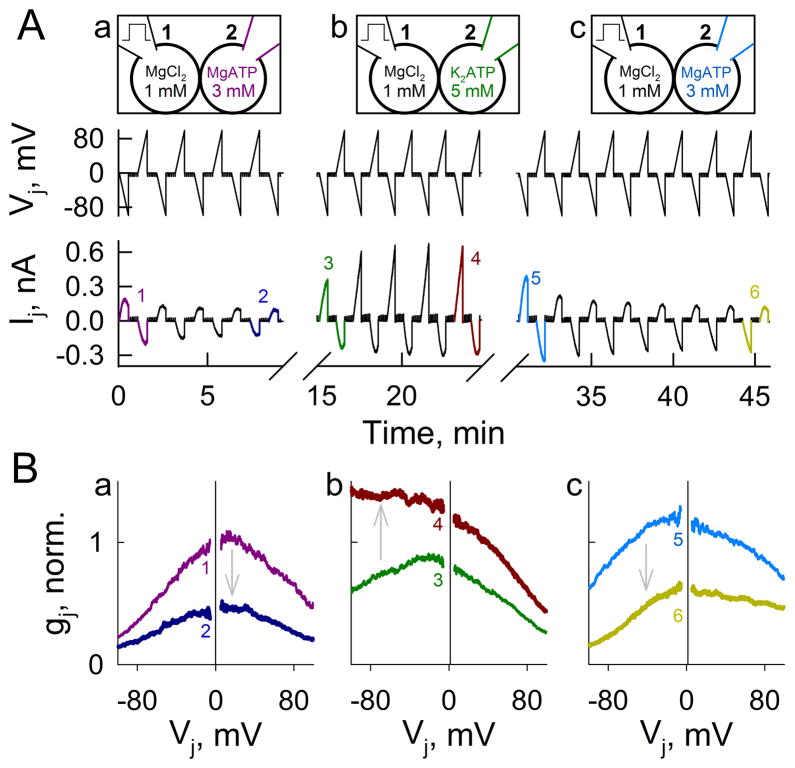
Since Mg2+ ions permeate Cx36 GJ channels, Vj applied to GJs with differing Mg2+ concentration on the two sides will alter the Mg2+ distribution within the channel through ionophoresis. Relative positivity in the cell with lower [Mg2+]i will decrease the Mg2+ occupancy of the channel. If the sensorial domain for Mg2+ is located within the channel lumen, relative positive Vj on the lower [Mg2+]i side should decrease Vj gating sensitivity of fast gates and increase PoF. The opposite changes should occur with relative negativity in the cell with lower [Mg2+]i. In homotypic Cx36 GJs, with a gradient of [Mg2+]p (0.01 mM in pipette-1 and 5 mM in pipette-2), relative positive Vj on the lower [Mg2+] side increased gj, while relative negative Vj on the lower [Mg2+] side decreased gj (Fig. 6A–B). The changes in gj were independent to which pipette the voltage was applied (Fig. D–E). Decrease in gj during relative negativity on the lower [Mg2+] side implies that the electric field increases Mg2+ occupancy of Cx36 channels, while increase in gj during relative positivity on the same side implies that the field decreases Mg2+ occupancy. Thus, transjunctional asymmetry of [Mg2+]i resulted in an asymmetric gj–Vj relationship, probably involving sensitivity to both Vj and Mg2+. Asymmetric gj–Vj dependence could also be achieved by having MgATP or K2ATP in one pipette to increase or reduce [Mg2+]i, respectively, on one side of the junction (Fig. 6C and F).
Figure 6.
Transjunctional asymmetry of [Mg2+]i causes asymmetric Vj gating of homotypic Cx36-EGFP GJs. A and D, Transjunctional asymmetry in [Mg2+]i (see diagrams at the top in (B) and (E) for free Mg2+ concentration in pipette solutions and stimulation site) caused asymmetry in Vj-gating with decrease in gj for relative negativity on the low [Mg2+] side. Vj steps (±80 mV) of opposite polarities produced opposite effect on gj. Small amplitude repeated Vj ramps (±20 mV, same as in Fig. 1A) were used to measured gj between Vj steps. B and E, gj–Vj relations (normalized to gj value at Vj=0) measured by applying long (60 s) Vj ramps from 0 to +100 and −100 mV. Relative positivity on the high [Mg2+] side decreased gj. C and F, Asymmetric concentration of MgATP (C; top diagram) or K2ATP (F; top diagram) was associated with asymmetry of gj–Vj dependence (normalized to gj value at Vj=0).
To test whether Vj–gating asymmetry caused by a [Mg2+]i gradient is reversible, we performed an experiment in which pipette-2 containing relatively high [Mg2+]p (MgATP, 3 mM) was replaced by a pipette containing low [Mg2+]p (K2ATP, 5 mM), and then replaced again by a pipette containing the original solution, high [Mg2+]p (MgATP, 3 mM) (Fig. 7A). Pipette-1 contained standard pipette solution (MgCl2=1 mM) throughout the experiment. During the first 8 min, gj decreased developing a gj–Vj asymmetry with somewhat higher sensitivity to Vj at negativity on the side with standard pipette solution (Fig. 7Ba). After the first exchange of pipette-2, gj increased and Vj gating asymmetry reversed very quickly (Fig. 7Bb). After the second exchange of pipette-2, gj decreased and Vj gating asymmetry reversed again very quickly (Fig. 7Bc). In summary, Vj has a strong effect on the Mg2+-dependent modulation, suggesting a presence within the channel lumen of a sensorial domain for Mg2+.
Figure 7.
Fast reversal of asymmetric gj–Vj dependence by reversal of transjunctional gradient of [Mg2+]i. A, Changes in Ij during consecutive 35 s long Vj ramps from 0 to −100 mV and from 0 to 100 mV. Initially, cell-1 was loaded with a control/standard pipette solution (MgCl2, 1 mM) and cell-2 contained MgATP (Aa). From ~9 to 14 min after onset, pipette-2 was carefully detached and replaced with a pipette containing K2ATP (Ab) reversing the Mg2+ gradient. From ~25 to 30 min after onset, pipette-2 was replaced with a pipette containing MgATP (Ac) reversing the Mg2+ gradient once again. B, gj–Vj plots (normalized to initial gj value at Vj=0) from ramp pairs in (A) designated with numbers 1 and 2 (Ba), 3 and 4 (Bb), and 5 and 6 (Bc).
The capability of Vj steps of alternating polarity to induce closures and openings of Cx36 GJ channels under a transjunctional concentration gradient of Mg2+ in tens of seconds (Fig. 6A and D) and reversal of gj–Vj asymmetry by exchange of pipette solutions (Fig. 7) indicate that the Mg2+-dependent effect on gj is reversible. It is most likely that gj changes are caused by direct Mg2+ effects on channel function rather than through posttranslational modifications (no ATP in pipette solutions) or insertion/removal of channels from the junctional plaque. Furthermore, if Cx36 has a cytoplasmic Mg2+ binding site(s), then the Mg2+-dependent closure of one aHC exposed to high [Mg2+]i would result in Po close to zero. We did not observe a comparable reduction in gj at Vj = 0 between experiments with symmetric ([Mg2+]p = 5 mM in both pipettes) or asymmetric ([Mg2+]p = 5 mM in pipette-1; 0.01 mM in pipette-2) Mg2+ conditions, suggesting that Mg2+ sensorial domain is not located in the cytoplasmic side of the channel. Together, these results indicate that the effect of Mg2+ is completely reversible and suggest that the site(s) of action of Mg2+ are in the channel pore-lining residues where Vjs affect the ionic occupancy of the channel through ionophoresis, and possibly binding affinity.
High intracellular magnesium stabilizes a closed conformation of Cx36 GJ channels
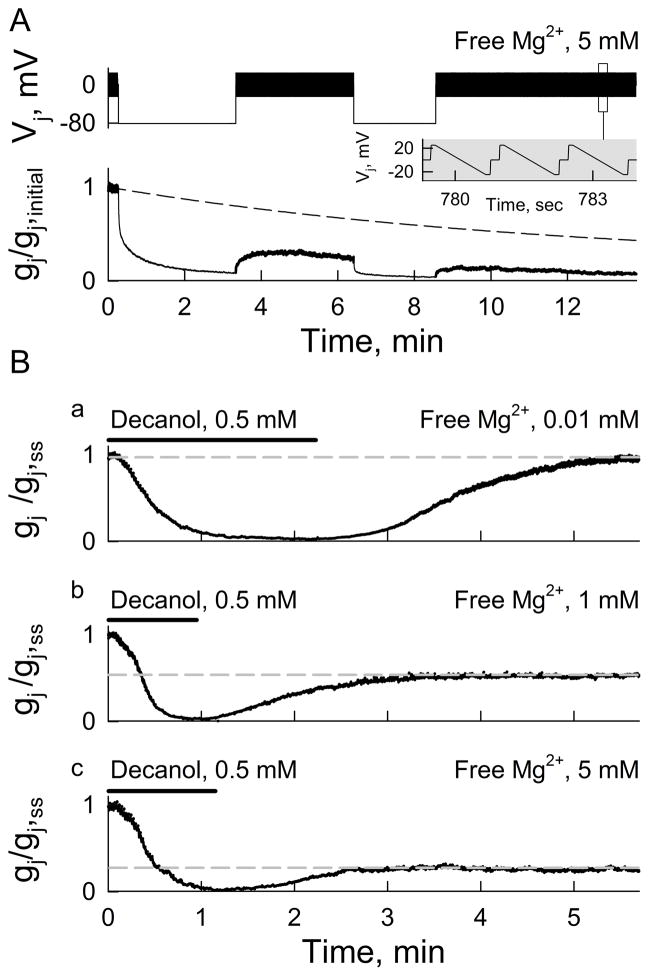
To test whether [Mg2+]i affects the recovery of gj after closing Cx36 GJ channels by Vj-gating, we examined changes in gj after applying Vj steps in symmetrical [Mg2+]p = 5 mM (Fig. 8A). Vj-gating at high Mg2+ induced a fast reduction in gj followed by a slow continuous decay. The recovery of gj after Vj steps showed fast and slow components (Fig. 8A), presumably due to the opening of fast and slow gates, respectively. However, the recovery of gj did not reach values of an averaged curve of gj decay obtained in the absence of Vj steps (Fig. 8A; dashed line). These results indicate that increasing Vj-gating and the Mg2+ occupancy by Vj-dependent ionophoresis in Cx36 GJ channels increased the speed of the Mg2+-dependent reduction in gj. Moreover, at [Mg2+]p = 5 mM the values of gj obtained using Vj steps to accelerate the decay were smaller than those obtained at the same [Mg2+]p without using Vj steps, suggesting that high Mg2+ could stabilize a closed conformation, possibly of slow gates. We were not able to assess gj recovery after Vj-gating in low [Mg2+]p due to the lack of Vj-sensitivity (Fig. 1).
Figure 8.
Recovery of gj after decrease induced by Vj- or chemical gating depends on [Mg2+]i. A, Lower panel shows dynamics of gj recovery (normalized to initial gj value) after Vj steps (−80 mV; top panel) at [Mg2+]p = 5 mM. Small amplitude repeated Vj ramps (±21 mV, 1.3 s; inset) were used to measure gj before and after Vj steps. Dashed line represents averaged decay in gj in the absence of Vj steps using pipette solutions with [Mg2+]p = 5 mM. B, Dynamics of gj recovery normalized to steady-state gj value (gj,ss) before decanol (0.5 mM) application to induce uncoupling at [Mg2+]p equal 0.01 mM (Ba), 1 mM (Bb) and 5 mM (Bc). Small amplitude repeated Vj ramps (same as in A) were used to measure gj. Grey dashed lines show levels of gj recovery during washout from decanol.
To close Cx36 GJ channels at Vj = 0, we used the chemical uncoupler decanol (0.5 mM), and examined gj recovery during washout at different [Mg2+]p (Fig. 8B). These experiments were normalized to gj values after reaching the steady-state (gj,ss) at each [Mg2+]p (~20 min after opening patches), and time zero was adjusted to the beginning of decanol perfusion. Decanol was applied until gj neared zero and then washed out with normal bath solution. Full recovery of gj was reached only at [Mg2+]p = 0.01 mM (Fig. 8Ba). Recovery of gj was ~50 % at [Mg2+]p = 1 mM (Fig. 8Bb) and ~25% at [Mg2+]p = 5 mM (Fig. 8Bc). A possible explanation for these results is that binding of Mg2+ in the Cx36 GJ channels stabilizes a closed conformation, explaining the low recovery of gj after closing the channels with Vj-gating or chemical-gating under high [Mg2+]p.
A phosphomimetic mutant of Cx36 shows magnesium-dependent modulation of junctional conductance similar to Cx36 wild type
Function of Cx36 GJs strongly depends on phosphorylation of two intracellular serine residues, which are phosphorylated by CaMKII, PKG and PKA (Mitropoulou and Bruzzone, 2003; Ouyang et al., 2005; Patel et al., 2006; Alev et al., 2008; Kothmann et al., 2009). Furthermore, phosphatases are highly dependent on [Mg2+]i (Merlevede et al., 1984). Therefore, we tested whether changes in phosphorylation of Cx36 might be involved in the observed Mg2+-dependent changes of gj using mutants of Cx36 in which serines 110 and 293 were replaced by aspartates, resembling negatively charged phosphoserine residues. These “phosphomimetic” mutants are locked in a pseudo phosphorylated state that cannot be dephosphorylated, thus allowing study of Mg2+-dependent modulation of gj independently of phosphorylation or dephosphorylation at these sites. We found that combined point mutations, S110D and S293D, exhibited slightly more increase in gj at low [Mg2+]i and slightly less decay of gj at high [Mg2+]i. However, in neither case were the values significantly different from those measured in Cx36. In summary, these phosphomimetic mutants behave similarly to the wild-type Cx36, suggesting that changes in phosphorylation of at least these two serine residues are not involved in the Mg2+-dependent modulation of gj.
Modulation of junctional conductance by different divalent cations in Cx36 GJ channels
To rule out a possible nonspecific effect of surface charge screening and test the specificity of the effects of Mg2+ ions on Cx36, we examined gj using pipette solutions containing divalent cations from alkaline earth metals (Ca2+ or Ba2+) or transition metals (Mn2+, Cd2+ or Zn2+). Because EGTA and BAPTA are not good buffers for all these divalents (Patton et al., 2004), we prepared solutions without EGTA or BAPTA and compared results with a control solution of nominally zero divalents (see Methods). The control solution increased gj to ~2.5 fold of initial gj (Fig. 9). All divalents at a concentration of 2 mM decreased gj (Fig. 9A–B), but with different times to reach 5% of initial gj (Fig. 9A and C), indicating that nonspecific screening of charges in the membrane surface and/or cytoplasmic side of the Cx36 protein does not play a major role in this inhibition. Because 2 mM Zn2+ produced the fastest inhibition, we used solutions with 0.2 mM Zn2+ to compare the degree of inhibition and time to reach the steady-state. Interestingly, 0.2 mM Zn2+, like 2 mM Zn2+, almost completely blocked gj (Fig. 9A–B), but took five times longer to reach 5% of initial gj (Fig. 9A and C). In summary, all examined divalent cations strongly inhibited conductance of Cx36 GJs but with different kinetics. These results suggest that divalent cations act through a relatively nonspecific negatively charged binding site in Cx36 rather than through surface charge screening.
Figure 9.
Divalent cations decrease gj of Cx36 GJs. A, Dynamics of gj (normalized to initial gj value) changes for different divalent cations. B, Average gj (normalized to initial gj values) from experiments using pipette solution containing: nominally zero divalents (n = 5); 2 mM free Mg2+ (n = 3), Ca2+ (n = 5), Ba2+ (n = 5), Mn2+ (n = 6), Cd2+ (n = 4) and Zn2+ (n = 3); 0.2 mM free Zn2+ (n = 4). C, Average time for gj to undergo 95% of the change from initial to virtual steady-state conductance after beginning dual whole-cell voltage clamp.
Intracellular magnesium-dependent modulation of junctional conductance in neurons of the MesV nucleus
To test whether native electrical synapses expressing Cx36 are sensitive to changes in [Mg2+]i, we examined changes in the strength of electrical coupling by measuring junctional conductance (Gj) between pairs of MesV neurons at low or high [Mg2+]i. The MesV nucleus is formed by the somata of primary afferents originating in jaw-closing muscles whose cell bodies are located within the CNS rather than peripheral ganglia (Nagy et al., 1986). These large somata (Fig 10A) are electrically coupled through Cx36-containing somato-somatic GJs (Curti et al., 2012). We recorded from pairs of MesV under current clamp configuration, and Gj was indirectly estimated using interleaved hyperpolarizing current steps on each cell (see Methods) (Fig. 10B). When recording with a solution containing [Mg2+]p = 0.01 mM in both pipettes, we observed a progressive increase in Gj following patch opening (Fig. 10C). In contrast, solutions containing [Mg2+]p = 5 mM produced a progressive reduction of Gj (Fig. 10C). Averaged Gj (normalized to initial values) showed that low [Mg2+]i led to a 18 ±4 % increase in Gj after ~10 minutes of patch opening, whereas high [Mg2+]i produced a 21 ±3 % reduction (Figs. 10D). Longer lasting (~30 min) experiments with 5 mM [Mg2+]i showed further reductions in Gj (more than 30%; not shown). Thus, native Cx36-containing electrical synapses exhibited similar sensitivity to [Mg2+]i, suggesting that this mechanism could operate in vivo.
Figure 10.
Mg2+-dependent modulation of Gj in pairs of MesV neurons. A, IR-DIC image of a pair of electrically coupled MesV neurons during dual whole-cell patch clamp. B, Simultaneous current clamp recordings from a pair of electrically coupled MesV neurons; arrows indicate the direction of the spread of electrotonic potential. Voltage traces were recorded from cell-1 and cell-2 (V1 and V2, respectively) during 300 ms hyperpolarizing current steps of −300 pA injected either in cell-1 or in cell-2 (I1 and I2, respectively). C, Time course of changes in mean Gj (normalized to initial values) at [Mg2+]p equal 0.01 (grey) and 5 mM (black). Each point represents an average from 5 independent experiments. D, Mean percentage changes of Gj from initial values after 12 min of patch openings with [Mg2+]p equal 0.01 (grey) and 5 mM (black). Numbers of cell pairs are indicated within columns; *p < 0.05.
Discussion
Although effects of divalent cations on GJ channels have long been recognized (Loewenstein, 1967; Deleze and Loewenstein, 1976; Spray et al., 1982; Noma and Tsuboi, 1987; Vera et al., 1996; Matsuda et al., 2010) the mechanisms by which Mg2+ modulates Cx36 GJs have not been studied. Here we demonstrate that control/resting levels of [Mg2+]i (~1 mM) maintain Cx36 GJ channels expressed in heterologous systems and neurons of the MesV nucleus partially inhibited and that cell-cell coupling is modulated by changes in [Mg2+]i. We showed that the Mg2+-dependent modulation of gj is caused by changes of Po and NF (Fig. 1G–I). In Cx36, the Boltzmann parameters AF and AS remained relatively constant during changes in [Mg2+]i (Table 2), indicating that changes in Po at Vj = 0 were mainly due to changes in VF,o, and that the gating charge of the Vj sensor was not modified by changes in [Mg2+]i. gj approached zero at [Mg2+]p = ~10 mM, while maximal values of gj in HeLa cells were reached by decreasing [Mg2+]p to 0.01 mM or less (Fig. 1E). Similar effects on gj were achieved by adding to the pipette solution K2ATP or MgATP (Fig. 4), which reduced or increased [Mg2+]i, respectively. These data suggest that ATP affects gj mainly through its capacity to modulate free [Mg2+]i (Luthi et al., 1999). In our studies [Ca2+]i was strongly buffered at low values (~25 nM) with BAPTA and EGTA indicating that Mg2+, not Ca2+, ions were involved in the observed changes in gj.
Our data show that high [Mg2+]i also reduces gj of GJ channels formed of Cxs 26, 32, 43, 45 and 47 (Fig. 2E). However, we did not observe an increase in gj for these Cxs at [Mg2+]i = 0.01 mM (Fig. 2D). Thus, Cx36 was the only Cx examined that exhibited a substantial inhibition of GJ channels at control/resting levels of [Mg2+]i. This suggests that changes in [Mg2+]i under physiological or pathological conditions can modulate preferentially Cx36-mediated cell-cell coupling. The γopen of Cx43 and Cx47 did not change under enhanced [Mg2+]p (Fig. 3L–M) demonstrating that Mg2+–mediated decrease in gj for these Cxs was not due to reduction in γopen. GJs formed of both Cx36 and Cx47 are permeable to Mg2+ (Fig. 5); thus, Vj may modify the Mg2+ occupancy of the channel by ionophoresis and also by changing the on and off rates of Mg2+ binding. We conclude that Mg2+ acts inside the Cx36 channel lumen based on the following facts: 1) Mg2+ permeates the channel (Fig. 5); 2) transjunctional gradients of [Mg2+]i caused asymmetric gj–Vj dependence (Figs. 6 and 7); 3) the averaged gj,ss at Vj = 0 observed using transjunctional asymmetric [Mg2+]i (5 mM in pipette-1; 0.01 mM in pipette-2) is higher than averaged gj,ss obtained under symmetric high [Mg2+]i (5 mM). Mg2+-dependent changes in gj were too fast to be explained by an insertion or removal of channels during Vjs of different polarities (Fig. 6) and after changes in [Mg2+]i (Fig. 7). Furthermore, we did not detect changes in the size or fluorescence intensity of Cx36-EGFP junctional plaques (not shown).
The Mg2+ occupancy of Cx36 GJ channels reduces PoF (Fig. 1), by favoring transitions of fast gates into a closed state, and also appears to stabilize a long-lived closed conformation of slow gates (Fig. 8), which in the S16SM is reflected in a reduction of NF. Low [Mg2+]i conditions allowed for a full recovery of gj from uncoupling induced by chemical-gating (Fig. 8B). However, in the presence of high [Mg2+]i the recovery of gj from uncoupling induced by chemical-or Vj-gating was markedly reduced (Fig. 8A–B). These results suggest that Mg2+stabilizes the closed conformation of the slow gate mediated by chemical uncouplers or Vj. The relative slow kinetics of the Mg2+-dependent changes in gj at Vj = 0 (Fig. 1; ~20 min to reach steady state) could be the combined results of a preferential binding of Mg2+ to a closed state (Po = 0.64 at [Mg2+]p = 1 mM and Vj = 0) and a low rate of unbinding (off rate) leading to the stabilization of a closed channel conformation. In addition, Cx36 GJ channels may require coordinated binding of Mg2+ to more than one binding site to stabilize the closed channel conformation. The effect of Mg2+ on the reduction of NF is reversible (Fig. 7), and channels can become operational/functional again in low [Mg2+]i; which suggests a stochastic release of Mg2+ from binding site(s). Mg2+-dependent modulation of gj was not affected by phosphomimetic mutations of Cx36 at residues, S110 and S293. We conclude that changes in phosphorylation at those positions are not necessary for the observed Mg2+-dependent modulation of gj; however, we do not rule out the possibility that phosphorylation of Cx36 could modify coupling at different [Mg2+]i. In addition, the fact that asymmetry in gj–Vj dependence can be reversed in the same cell pair by exchanging pipettes with different [Mg2+]p (Fig. 7), and openings or closures can be consecutively induced by Vj steps of different polarity over relative short times using pipette solutions without ATP (Fig. 6A and D), strongly suggest that ATP-dependent posttranslational modifications, such as phosphorylation, are not involved in the Mg2+-dependent modulation of Cx36 GJ channels. The inhibition of Cx36 GJ channels could also be induced by divalent cations other than Mg2+, and each divalent exhibited a different kinetics of gj decay (Fig. 9). These differences suggest that nonspecific screening of surface charges in the Cx36 protein or membrane phospholipids is unlikely to be a primary mechanism of Mg2+-dependent changes in gj. Importantly, Cx36 GJ channels showed high sensitivity to [Zn2+]i, which plays an important role in CNS and pancreatic β-cells (Frederickson et al., 2000; Slepchenko and Li, 2012).
Finally, Cx36-containing electrical synapses between MesV neurons showed similar, although smaller, Mg2+-dependent changes in cell-to-cell coupling compared to those observed in HeLa and N2A cells (Fig. 10C), suggesting that this mechanism could operate in the brain. The differences in the magnitude of the Mg2+ effect could be explained by differences between MesV neurons and heterologous expression systems, such as intrinsic buffer capacity, resting levels of [Mg2+]i, Cx36 levels of expression, and the presence of another Cx (based on residual coupling between MesV neurons in Cx36 knockout mice (Curti et al., 2012)). Nonetheless, electrical synapses between MesV neurons showed a clear and significant bidirectional modulation in Gj (~±20%) dependent on [Mg2+]i (Fig. 10D), which is consistent with results obtained in HeLa and N2A cells. Furthermore, the magnitude of changes in MesV neurons is likely to be of physiological relevance, since long-term depression producing an equivalent reduction in Gj between neurons of the thalamic reticular nucleus has been suggested to produce changes in neuronal synchronization (Landisman and Connors, 2005).
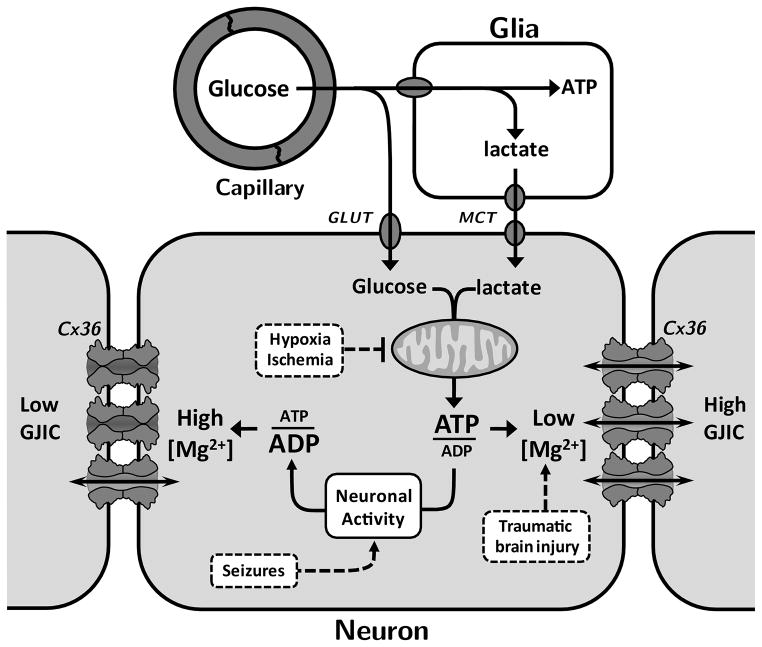
In conclusion, the function of Cx36 GJ channels is strongly modulated by changes in [Mg2+]i. Effects of intracellular ATP on Cx36 GJs result, at least in part, from modulation of [Mg2+]i. We showed that: 1) a substantial fraction of Cx36 GJ channels are inhibited even at resting levels of [Mg2+]i (~1 mM); 2) Cx36 GJ channels are permeable to Mg2+ ions, which can enter the channel and interact with pore-lining residues; 3) the Cx36 GJ channel lumen contains a sensorial domain for divalent cations that upon binding induces a reduction in Po and stabilization of a closed channel conformation. Thus, physiological conditions where cytosolic ATP decreases, e.g., increased neuronal activity during prolonged waking periods, or pathological conditions such as hypoxia, ischemia or seizures, which lead to increased [Mg2+]i, might reduce neuronal synchronization via reduction in the strength of electrical synapses formed of Cx36 (Fig 11). On the other hand, physiological conditions where cytosolic ATP increases, e.g., reduced neuronal activity during sleep period or pathological conditions such as traumatic brain injury might induce an increase in Cx36-mediated gap junctional intercellular communication (GJIC) and neuronal synchronization (Fig. 11). In addition, increased synchronization of inhibitory neurons could lead to a reduction in synchronous activity of excitatory neurons. Modulation in the rates of binding or unbinding of Mg2+ through modification of the binding site(s) by physiological stimuli, such as posttranslational modifications, lipophilic neuromodulators, and changes in pHi, could be a source for fast control of Cx36-mediated GJIC and synchronization through electrical synapses.
Figure 11.
Diagram illustrating relation between ATP/ADP ratio and [Mg2+]i and the effect on Cx36 GJ channels. During sleep and reduced neuronal activity, ATP/ADP ratio is increased by mitochondrial metabolism of glucose and lactate coming from capillaries and surrounding glia, respectively. During wake period and enhanced neuronal activity, ATP/ADP ratio is decreased. Changes in ATP have a direct effect on [Mg2+]i leading to changes in Cx36-dependent gap junctional intercellular communication (GJIC). Pathological conditions, such as hypoxia, ischemia and seizures may result in decreased GJIC, while traumatic brain injury may lead to increased GJIC. GLUT, glucose transporter; MCT, monocarboxylate transporter.
Acknowledgments
We thank Dr. Vytautas K. Verselis and Dr. Thaddeus A. Bargiello for helpful comments and discussions, and Angele Bukauskiene for excellent technical assistance. Nicolás Palacios-Prado is a Howard Hughes Medical Institute International Student Research Fellow. This work was supported by the National Institute of Health grants: EY 12857 to J.O.; DC 011099 and R21NS 055726 to A.E.P.; NS 55363 to M.V.L.B; and R01NS 072238 and R01HL 084464 to F.F.B.
Footnotes
Conflict of interests: The authors declare no competing financial interests.
References
- Ainscow EK, Mirshamsi S, Tang T, Ashford ML, Rutter GA. Dynamic imaging of free cytosolic ATP concentration during fuel sensing by rat hypothalamic neurones: evidence for ATP-independent control of ATP-sensitive K(+) channels. J Physiol. 2002;544:429–445. doi: 10.1113/jphysiol.2002.022434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ubaidi MR, White TW, Ripps H, Poras I, Avner P, Gomes D, Bruzzone R. Functional properties, developmental regulation, and chromosomal localization of murine connexin36, a gap-junctional protein expressed preferentially in retina and brain. J Neurosci Res. 2000;59:813–826. doi: 10.1002/(SICI)1097-4547(20000315)59:6<813::AID-JNR14>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Alev C, Urschel S, Sonntag S, Zoidl G, Fort AG, Hoher T, Matsubara M, Willecke K, Spray DC, Dermietzel R. The neuronal connexin36 interacts with and is phosphorylated by CaMKII in a way similar to CaMKII interaction with glutamate receptors. Proc Natl Acad Sci USA. 2008;105:20964–20969. doi: 10.1073/pnas.0805408105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrasi E, Igaz S, Molnar Z, Mako S. Disturbances of magnesium concentrations in various brain areas in Alzheimer’s disease. Magnes Res. 2000;13:189–196. [PubMed] [Google Scholar]
- Barbiroli B, Martinelli P, Patuelli A, Lodi R, Iotti S, Cortelli P, Montagna P. Phosphorus magnetic resonance spectroscopy in multiple system atrophy and Parkinson’s disease. Mov Disord. 1999;14:430–435. doi: 10.1002/1531-8257(199905)14:3<430::aid-mds1007>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Bennett MV, Zukin RS. Electrical coupling and neuronal synchronization in the Mammalian brain. Neuron. 2004;41:495–511. doi: 10.1016/s0896-6273(04)00043-1. [DOI] [PubMed] [Google Scholar]
- Bennett MVL. Physiology of electrotonic junctions. Ann NY Acad Sci. 1966;37:509–539. doi: 10.1111/j.1749-6632.1966.tb50178.x. [DOI] [PubMed] [Google Scholar]
- Bukauskas FF, Weingart R. Voltage-dependent gating of single gap junction channels in an insect cell line. Biophys J. 1994;67:613–625. doi: 10.1016/S0006-3495(94)80521-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukauskas FF, Verselis VK. Gap junction channel gating. Biochim Biophys Acta. 2004;1662:42–60. doi: 10.1016/j.bbamem.2004.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukauskas FF, Jordan K, Bukauskiene A, Bennett MV, Lampe PD, Laird DW, Verselis VK. Clustering of connexin 43-enhanced green fluorescent protein gap junction channels and functional coupling in living cells. Proc Natl Acad Sci USA. 2000;97:2556–2561. doi: 10.1073/pnas.050588497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernak I, Radosevic P, Malicevic Z, Savic J. Experimental magnesium depletion in adult rabbits caused by blast overpressure. Magnes Res. 1995;8:249–259. [PubMed] [Google Scholar]
- Condorelli DF, Parenti R, Spinella F, Salinaro AT, Belluardo N, Cardile V, Cicirata F. Cloning of a new gap junction gene (Cx36) highly expressed in mammalian brain neurons. Eur J Neurosci. 1998;10:1202–1208. doi: 10.1046/j.1460-9568.1998.00163.x. [DOI] [PubMed] [Google Scholar]
- Connors BW, Long MA. Electrical synapses in the mammalian brain. Annu Rev Neurosci. 2004;27:393–418. doi: 10.1146/annurev.neuro.26.041002.131128. [DOI] [PubMed] [Google Scholar]
- Curti S, Hoge G, Nagy JI, Pereda AE. Synergy between electrical coupling and membrane properties promotes strong synchronization of neurons of the mesencephalic trigeminal nucleus. J Neurosci. 2012;32:4341–4359. doi: 10.1523/JNEUROSCI.6216-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleze J, Loewenstein WR. Permeability of a cell junction during intracellular injection of divalent cations. J Membr Biol. 1976;28:71–86. doi: 10.1007/BF01869691. [DOI] [PubMed] [Google Scholar]
- Dworak M, McCarley RW, Kim T, Kalinchuk AV, Basheer R. Sleep and brain energy levels: ATP changes during sleep. J Neurosci. 2010;30:9007–9016. doi: 10.1523/JNEUROSCI.1423-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederickson CJ, Suh SW, Silva D, Thompson RB. Importance of zinc in the central nervous system: the zinc-containing neuron. J Nutr. 2000;130:1471S–1483S. doi: 10.1093/jn/130.5.1471S. [DOI] [PubMed] [Google Scholar]
- Grubbs RD. Intracellular magnesium and magnesium buffering. Biometals. 2002;15:251–259. doi: 10.1023/a:1016026831789. [DOI] [PubMed] [Google Scholar]
- Headrick JP, Willis RJ. Cytosolic free magnesium in stimulated, hypoxic, and underperfused rat heart. J Mol Cell Cardiol. 1991;23:991–999. doi: 10.1016/0022-2828(91)91635-5. [DOI] [PubMed] [Google Scholar]
- Heath DL, Vink R. Traumatic brain axonal injury produces sustained decline in intracellular free magnesium concentration. Brain Res. 1996;738:150–153. doi: 10.1016/0006-8993(96)00957-2. [DOI] [PubMed] [Google Scholar]
- Helpern JA, Vande Linde AM, Welch KM, Levine SR, Schultz LR, Ordidge RJ, Halvorson HR, Hugg JW. Acute elevation and recovery of intracellular [Mg2+] following human focal cerebral ischemia. Neurology. 1993;43:1577–1581. doi: 10.1212/wnl.43.8.1577. [DOI] [PubMed] [Google Scholar]
- Hinsberger AD, Williamson PC, Carr TJ, Stanley JA, Drost DJ, Densmore M, MacFabe GC, Montemurro DG. Magnetic resonance imaging volumetric and phosphorus 31 magnetic resonance spectroscopy measurements in schizophrenia. J Psychiatry Neurosci. 1997;22:111–117. [PMC free article] [PubMed] [Google Scholar]
- Kothmann WW, Massey SC, O’Brien J. Dopamine-stimulated dephosphorylation of connexin 36 mediates AII amacrine cell uncoupling. J Neurosci. 2009;29:14903–14911. doi: 10.1523/JNEUROSCI.3436-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landisman CE, Connors BW. Long-term modulation of electrical synapses in the mammalian thalamus. Science. 2005;310:1809–1813. doi: 10.1126/science.1114655. [DOI] [PubMed] [Google Scholar]
- Loewenstein WR. Cell surface membranes in close contact. Role of calcium and magnesium ions. J Colloid Interface Sci. 1967;25:34–46. doi: 10.1016/0021-9797(67)90007-0. [DOI] [PubMed] [Google Scholar]
- Luthi D, Gunzel D, McGuigan JA. Mg-ATP binding: its modification by spermine, the relevance to cytosolic Mg2+ buffering, changes in the intracellular ionized Mg2+ concentration and the estimation of Mg2+ by 31P-NMR. Exp Physiol. 1999;84:231–252. [PubMed] [Google Scholar]
- Matsuda H, Kurata Y, Oka C, Matsuoka S, Noma A. Magnesium gating of cardiac gap junction channels. Prog Biophys Mol Biol. 2010;103:102–110. doi: 10.1016/j.pbiomolbio.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Merlevede W, Vandenheede JR, Goris J, Yang SD. Regulation of ATP-Mg-dependent protein phosphatase. Curr Top Cell Regul. 1984;23:177–215. [PubMed] [Google Scholar]
- Mitropoulou G, Bruzzone R. Modulation of perch connexin35 hemi-channels by cyclic AMP requires a protein kinase A phosphorylation site. J Neurosci Res. 2003;72:147–157. doi: 10.1002/jnr.10572. [DOI] [PubMed] [Google Scholar]
- Moreno AP, Saez JC, Fishman GI, Spray DC. Human connexin43 gap junction channels. Regulation of unitary conductances by phosphorylation. Circ Res. 1994;74:1050–1057. doi: 10.1161/01.res.74.6.1050. [DOI] [PubMed] [Google Scholar]
- Moreno AP, Berthoud VM, Perez-Palacios G, Perez-Armendariz EM. Biophysical evidence that connexin-36 forms functional gap junction channels between pancreatic mouse beta-cells. Am J Physiol Endocrinol Metab. 2005;288:E948–956. doi: 10.1152/ajpendo.00216.2004. [DOI] [PubMed] [Google Scholar]
- Murphy E, Steenbergen C, Levy LA, Raju B, London RE. Cytosolic free magnesium levels in ischemic rat heart. J Biol Chem. 1989;264:5622–5627. [PubMed] [Google Scholar]
- Nagy JI, Buss M, Daddona PE. On the innervation of trigeminal mesencephalic primary afferent neurons by adenosine deaminase-containing projections from the hypothalamus in the rat. Neuroscience. 1986;17:141–156. doi: 10.1016/0306-4522(86)90232-0. [DOI] [PubMed] [Google Scholar]
- Noma A, Tsuboi N. Dependence of junctional conductance on proton, calcium and magnesium ions in cardiac paired cells of guinea-pig. J Physiol. 1987;382:193–211. doi: 10.1113/jphysiol.1987.sp016363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang X, Winbow VM, Patel LS, Burr GS, Mitchell CK, O’Brien J. Protein kinase A mediates regulation of gap junctions containing connexin35 through a complex pathway. Brain Res Mol Brain Res. 2005;135:1–11. doi: 10.1016/j.molbrainres.2004.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker PR, Cruikshank SJ, Connors BW. Stability of electrical coupling despite massive developmental changes of intrinsic neuronal physiology. J Neurosci. 2009;29:9761–9770. doi: 10.1523/JNEUROSCI.4568-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel LS, Mitchell CK, Dubinsky WP, O’Brien J. Regulation of gap junction coupling through the neuronal connexin Cx35 by nitric oxide and cGMP. Cell Commun Adhes. 2006;13:41–54. doi: 10.1080/15419060600631474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton C, Thompson S, Epel D. Some precautions in using chelators to buffer metals in biological solutions. Cell Calcium. 2004;35:427–431. doi: 10.1016/j.ceca.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Paulauskas N, Pranevicius H, Mockus J, Bukauskas FF. A stochastic 16-state model of voltage-gating of gap junction channels enclosing fast and slow gates. Biophys J. 2012;102:2471–2480. doi: 10.1016/j.bpj.2012.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serre-Beinier V, Le Gurun S, Belluardo N, Trovato-Salinaro A, Charollais A, Haefliger JA, Condorelli DF, Meda P. Cx36 preferentially connects beta-cells within pancreatic islets. Diabetes. 2000;49:727–734. doi: 10.2337/diabetes.49.5.727. [DOI] [PubMed] [Google Scholar]
- Slepchenko KG, Li YV. Rising intracellular zinc by membrane depolarization and glucose in insulin-secreting clonal HIT-T15 beta cells. Exp Diabetes Res. 2012;2012:190309. doi: 10.1155/2012/190309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohl G, Degen J, Teubner B, Willecke K. The murine gap junction gene connexin36 is highly expressed in mouse retina and regulated during brain development. FEBS Lett. 1998;428:27–31. doi: 10.1016/s0014-5793(98)00479-7. [DOI] [PubMed] [Google Scholar]
- Spray DC, Stern JH, Harris AL, Bennett MV. Gap junctional conductance: comparison of sensitivities to H and Ca ions. Proc Natl Acad Sci USA. 1982;79:441–445. doi: 10.1073/pnas.79.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas M, Rozental R, Kojima T, Dermietzel R, Mehler M, Condorelli DF, Kessler JA, Spray DC. Functional properties of channels formed by the neuronal gap junction protein connexin36. J Neurosci. 1999;19:9848–9855. doi: 10.1523/JNEUROSCI.19-22-09848.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura H, Toyama J, Tsuboi N, Kamiya K, Kodama I. ATP directly affects junctional conductance between paired ventricular myocytes isolated from guinea pig heart. Circ Res. 1990;66:1095–1102. doi: 10.1161/01.res.66.4.1095. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Nishina M, Endo M, Matsushita K, Tetsuka M, Shima K, Okuyama S. Decrease in cerebral free magnesium concentration following closed head injury and effects of VA-045 in rats. Gen Pharmacol. 1997;28:119–121. doi: 10.1016/s0306-3623(96)00148-6. [DOI] [PubMed] [Google Scholar]
- Teubner B, Odermatt B, Guldenagel M, Sohl G, Degen J, Bukauskas F, Kronengold J, Verselis VK, Jung YT, Kozak CA, Schilling K, Willecke K. Functional expression of the new gap junction gene connexin47 transcribed in mouse brain and spinal cord neurons. J Neurosci. 2001;21:1117–1126. doi: 10.1523/JNEUROSCI.21-04-01117.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teubner B, Degen J, Sohl G, Guldenagel M, Bukauskas FF, Trexler EB, Verselis VK, De Zeeuw CI, Lee CG, Kozak CA, Petrasch-Parwez E, Dermietzel R, Willecke K. Functional expression of the murine connexin 36 gene coding for a neuron-specific gap junctional protein. J Membr Biol. 2000;176:249–262. doi: 10.1007/s00232001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trexler EB, Bukauskas FF, Bennett MVL, Bargiello TA, Verselis VK. Rapid and direct effects of pH on connexins revealed by the connexin46 hemichannel preparation. J Gen Physiol. 1999;113:721–742. doi: 10.1085/jgp.113.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera B, Sanchez-Abarca LI, Bolanos JP, Medina JM. Inhibition of astrocyte gap junctional communication by ATP depletion is reversed by calcium sequestration. FEBS Lett. 1996;392:225–228. doi: 10.1016/0014-5793(96)00794-6. [DOI] [PubMed] [Google Scholar]
- Verrecchia F, Duthe F, Duval S, Duchatelle I, Sarrouilhe D, Herve JC. ATP counteracts the rundown of gap junctional channels of rat ventricular myocytes by promoting protein phosphorylation. J Physiol. 1999;516:447–459. doi: 10.1111/j.1469-7793.1999.0447v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]