Abstract
Although alcoholism is a worldwide problem resulting in millions of deaths, only a small percentage of alcohol users become addicted. Notably, the specific neural substrates responsible for individual differences in vulnerability to alcohol addiction are not known. In these studies, we used rodent models to study behavioral and synaptic correlates related to individual differences in the development of ethanol locomotor sensitization, a form of drug-dependent behavioral plasticity associated with addiction vulnerability. Male Swiss mice were treated daily with saline or 1.8 g/kg ethanol for 21 days. Locomotor activity tests were performed once a week for 15 min immediately after saline or ethanol injections. After at least eleven days of withdrawal, cohorts of saline and ethanol-treated mice were used to characterize the relationships between locomotor sensitization, ethanol drinking, and glutamatergic synaptic transmission in the nucleus accumbens. Ethanol-treated mice that expressed locomotor behavioral sensitization to ethanol drank significantly more ethanol than saline-treated subjects and ethanol-treated animals resilient to this form of behavioral plasticity. Moreover, ethanolsensitized mice also had reduced accumbal NMDA receptor function and expression, as well as deficits in NMDA receptor-dependent long term depression in the nucleus accumbens core after a protracted withdrawal. These findings suggest that disruption of accumbal core NMDA receptor-dependent plasticity may represent a synaptic correlate associated with ethanol-induced locomotor sensitization and increased propensity to consume ethanol.
Keywords: Long-Term Depression, Nucleus Accumbens, Behavioral sensitization, Ethanol, Voluntary drinking, Glutamate receptors
Introduction
Drug addiction is a pathology related to compulsive drug seeking and ingestion despite negative consequences (Robinson and Berridge, 1993; Vanderschuren and Kalivas, 2000; Hyman et al., 2006). There is a growing consensus that addiction is a disorder of neuroplasticity promoted by a strong association between drugs of abuse and their associated stimuli. Addicts have difficulty changing their focus, perseverate on their abused drugs and struggle to learn new associations. Indeed, drugs of abuse disrupt both long-term depression (LTD) and long-term potentiation of synaptic transmission in the mesolimbic system (Luscher and Malenka, 2011; Mameli and Luscher, 2011; McCool, 2011). Of note, exposure to psychostimulants can disrupt NMDA receptor-dependent LTD in the nucleus accumbens (NAc) (Thomas et al., 2001; Martin et al., 2006; Mao et al., 2009; Kasanetz et al., 2010). Importantly, evidence suggests that disruption of accumbal LTD may represent a synaptic correlate of addiction vulnerability, as it persists in rats that develop behavioral hallmarks of cocaine addiction but not in rats resilient to this “addictive” phenotype (Kasanetz et al., 2010). Drugs of abuse induce addiction in only a subset of users. Addiction is thus not simply a product of the neurobiological effects of drugs, but rather the consequence of drug exposure interacting with genetic and environmental backgrounds (Piazza and Le Moal, 1996; Deroche-Gamonet et al., 2004; Swendsen and Le Moal, 2011).
Ethanol is one of the most widely used drugs in the world and its global burden of disease is immense, with an estimated 3-4% of deaths attributed to alcohol consumption (Rehm et al., 2009; Spanagel et al., 2010). Despite these statistics, little is known about the neurobiological mechanisms contributing to individual differences in susceptibility to alcoholism. Marked heterogeneity in behavioral responsivity to ethanol has been demonstrated in animals (Bell et al., 2006; Fidler et al., 2011; Melon and Boehm, 2011). Locomotor sensitization, a d r u g-dependent behavioral adaptation defined as a progressive increase in psychomotor stimulant response, has been suggested as a behavioral marker for alcohol preference and/or abuse liability in animals (Grahame et al., 2000; Lessov et al., 2001) and humans (Newlin and Thomson, 1999). Our previous studies have identified individual differences in the development of ethanol locomotor behavioral sensitization in outbred Swiss Albino mice: while a subgroup of ethanol-treated mice show robust sensitization, others receiving identical treatment fail to show this behavioral adaptation (Souza-Formigoni et al., 1999; Abrahao et al., 2011). Since variations in sensitization may reflect individual differences in addiction vulnerability, we sought to identify behavioral and neurobiological correlates associated with vulnerability and resilience to ethanol sensitization. Previous data has indicated that ethanol sensitized and non-sensitized mice may have differences in NMDA receptor activity (Abrahao and Souza-Formigoni, 2012). Interestingly, as observed with psychostimulants, chronic ethanol exposure has also been shown to disrupt NMDA receptor-mediated LTD in the NAc (Jeanes et al., 2011). However, whether this addiction-associated form of synaptic plasticity may contribute to individual differences in vulnerability to alcoholism is not known. We therefore integrated behavioral, electrophysiological and biochemical techniques to test the hypothesis that enduring alterations in NAc glutamatergic receptor function and NMDA receptor-dependent plasticity may be associated with individual differences in ethanol-mediated locomotor sensitization and, consequently, addiction vulnerability.
Materials and Methods
Locomotor response to ethanol
Adult male Swiss Webster mice (Charles River Laboratories, Wilmington, MA) (55-62 days old), an outbred strain of mice, were group housed (4-5 mice per cage) in a temperature-controlled colony room (22 °C ± 1 °C) with lights on between 07:00 AM and 07:00 PM (except where indicated) with food and water ad libitum. All animal procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee. Locomotor activity tests were performed using Tru Scan activity systems (Coulbourn Instruments, Whitehall, PA). We first conducted an ethanol dose-response curve to select an appropriate ethanol dose for the behavioral sensitization experiments. On the first day, a drug free locomotor activity test session (15 min) was performed to assess baseline horizontal locomotor activity. One day later, mice were given intraperitoneal (i.p.) injections of either saline (0.9% w/V NaCl) or ethanol (1.5, 1.8, 2.0, 2.2, 2.5 or 2.8 g/kg, 15% w/V) using a between-subject design, and immediately placed in the locomotor activity arena where horizontal locomotor activity was monitored for 15 min. There was an overall effect of ethanol treatment (p < 0.05) and post-hoc analysis revealed a significant stimulant effect of 2.2 g/kg and 2.5 g/kg ethanol relative to saline administration (one-way ANOVA: F6,53 = 6.43, P < 0.001, data not shown). Chronic treatments and the classification of locomotor behavioral sensitization were conducted as previously described (Souza-Formigoni et al., 1999; Abrahao et al., 2009). In order to assess baseline horizontal locomotor activity, all animals were initially tested in one drug free session (15 min). One day later, mice received daily treatment (21 days) of either saline or 1.8 g/kg of ethanol i.p. (this dose was chosen from the ethanol dose-response curve because it was on the ascending part of the inverted U locomotor activity-response - curve typically observed following ethanol treatment - and it was below the peak stimulant effect). Locomotor activity was recorded for 15 min immediately after injections on treatment day 1, 7, 14 and 21. All procedures were carried out between 10:00 AM and 04:00 PM. Based on their locomotor response on Day 21, ethanol-treated mice were sorted and classified as “sensitized” mice (activity scores in the upper 33% of the distribution) and “non-sensitized” mice (activity scores in the lower 33% of the distribution). Subjects with a locomotor response in the intermediate range were not used in further studies. Since we could only conduct electrophysiological studies on one animal per day, mice were treated and classified in cohorts of 24 animals: 18 ethanol treated (which resulted in 6 sensitized, 6 non-sensitized and 6 intermediate mice) and 6 saline treated. This procedure was repeated a total of four times. For the following measures the experimenter was blind to the treatment group of the subjects.
EtOH self-administration
We used a modified “Drinking in the Dark” protocol (Finn et al., 2007) to assess voluntary ethanol intake in saline and ethanol-treated mice. Three days after the end of the chronic treatment regimen, mice were individually housed and the light-dark cycle was shifted five hours (lights on between 02:00 AM and 02:00 PM). Eight days later, animals were given forced access to a 10% ethanol (v/v) solution for three consecutive days (i.e. 10% ethanol as the only solution available). Then, 9h after the 3 days forced exposure to ethanol, voluntary ethanol intake and preference were assessed using a limited access-two bottle choice procedure (water available ad libitum, ethanol available for two hours a day, three hours into the dark cycle). Mice had access to water and 10% ethanol (v/v) during the first week. Then, after two days of withdrawal, mice had access to water and 20% ethanol (v/v) during the second week. There were no statistically significant group differences in ethanol intake during the 3 days of forced ethanol drinking or 10% ethanol / water two-bottle choice week, nor were there any significant group differences in water intake or ethanol preference.
Electrophysiology
Mouse coronal nucleus accumbens (NAc) slices (400 μm) were prepared, as previously described (Crowder et al., 2002), using a Leica VT1000S vibratome (Leica Microsystems Inc., Buffalo Grove, IL). Subjects were chronically treated with saline for 21 days or with ethanol and then classified as sensitized or non-sensitized (see above). Thirteen to twenty days following the 21 day treatment regimen, mice were anesthetized with halothane, decapitated and the brain was quickly isolated and immersed in ice-cold artificial cerebrospinal fluid (ACSF) [in mM: 126 NaCl, 3 KCl, 1.5 MgCl2, 2.4 CaCl2, 1.2 NaH2PO4, 11 Glucose and 26 NaHCO3, pH 7.4 (saturated with 95%O2 / 5%CO2). Brain slices were allowed to recover for at least for 1.5h in ACSF at room temperature before recording. Slices were maintained at room temperature until transferred to a submersion-type recording chamber. Whole-cell patch recordings of NAc neurons were performed using micropipettes (8-13 MΩ) made from 1.5 mm borosilicate grass (Sutter Instrument, Novato, CA). Recording pipettes were filled with the following internal solution (in mM): 117 Cs-methanesulphonate, 15 CsCl, 8 NaCl, 10 tetraethylammonium-Cl (TEA), 0.2 EGTA , 10 4-(2-Hydroxyethyl) piperazine-1-ethanesulfonic acid (HEPES), 2 ATP, 0.3 GTP and QX-314 [N-(2,6-dimethylphenylcarbamoylmethyl) triethylammonium chloride], pH 7.25 (290-295 mOsm). Recordings were obtained using an AxoClamp 2B amplifier (Molecular Devices, Sunnyvale, CA), digitized (Molecular Devices), and analyzed using pClamp 9.0 software (Molecular Devices). Evoked EPSCs were generated by stimulating the region just dorsal to the anterior commissure using a concentric bipolar electrode (FHC, Bowdoinham, ME) and a stimulus isolation unit (AMPI, Israel). Access resistance was frequently checked using a -10 mV voltage step. Only cells with a stable access resistance of less than 25 MΩ were used in the experimental analyses. In all experiments, GABAA IPSCs were blocked using bicuculine methiodide (20 μM, Sigma-Aldrich, St. Louis, MO). NAc neurons were voltage-clamped at -90 mV and evoked EPSCs (0.05 Hz) were recorded for at least 10 min to ensure the stability of baseline recordings. We then measured the paired-pulse ratio (PPR) of EPSCs (25, 50 and 250 ms inter-pulse interval) and collected six minutes of spontaneous EPSCs (sEPSCs) to assess possible pre- and postsynaptic alterations associated with behavioral sensitization.
AMPA/NMDA ratios were then determined using a similar method previously described (Myme et al., 2003). Briefly, we assessed the time course of AMPA EPSCs by stimulating while the cell was held at -90 mV. Then the slice was stimulated while the cell was voltage-clamped at +40 mV after two 1 minute voltage steps to -40 mV and +10 mV. Holding the cell at +40 mV provided sufficient depolarization and driving force to reveal the NMDA receptor component of evoked EPSCs. Averages of 10 EPSCs evoked at -90 mV and +40 mV were then used to determine the AMPA/NMDA ratio. To validate the AMPA/NMDA ratio, the relative contribution of AMPARs and NMDARs to evoked EPSCs was assessed. The peak AMPA response was assessed during a 2 msec window, 14 msec after the stimulus artifact, corresponding to the peak AMPA response recorded at -90 mV. The peak NMDA response was recorded during a 10 msec window, 100 msec after the stimulus artifact. Bath application of 20 μM DNQX significantly decreased the AMPA receptor current by greater than 60% while having no effect on the peak response recorded during the “NMDA window”. Subsequent application of 50 μM APV completed abolished the remaining currents in both windows (data not shown). This experiment confirms that the peak current recorded at +40 mV during the “AMPA window” was mediated predominantly by activation of AMPA receptors and the peak current recorded during the “NMDA window” was mediated solely by NMDA receptor activation. IAMPA at +40 mV/INMDA at +40 mV was taken as the AMPA/NMDA ratio. It is, however, important to note that this determination of the AMPA/NMDA ratio may be influenced by ethanol-mediated changes in glutamate receptor kinetics.
Nucleus accumbens medium spiny neurons express a form of low frequency, NMDA receptor-dependent long-term depression (LTD). Indeed, it has already been demonstrated that NMDAR-antagonist pretreatment can attenuate the induction of LTD in NAc neurons (Kombian and Malenka, 1994; Kasanetz et al., 2010). To assess the effect of behavioral sensitization on NMDAR-dependent LTD, we recorded from other NAc core and shell neurons from each of the three treatment groups. After a 10 minute baseline at -90 mV, LTD was induced using a pairing protocol, as described previously (Martin et al., 2006): 3 × 5Hz for 3 minutes, paired with a depolarization to -50 mV, 5 minute inter-train interval. LTD was quantified as the % decrease in the maximal EPSC amplitude recorded 15-30 minutes after LTD induction relative to baseline EPSCs amplitude. Considering that similar protocols can also induce NMDA receptor independent forms of LTD in the NAc (Robbe et al., 2002), we also recorded from NAc core neurons in slices from the three treatment groups in the presence of the NMDA receptor antagonist APV (50 μM) using the same LTD protocol described above. Besides, we recorded LTD in NAc core neurons in mice exposure to one administration of saline or ethanol.
It should be noted that we did not perform specific experiments to confirm the identity of the neurons studied. However, 95% of neurons in NAc are medium spiny neurons and we only recorded from neurons with an initial resting membrane potential ≤ -70 mV.
Western Blots
NAc tissue (core and shell combined) was isolated from brain slices prepared using a protocol identical to that described for electrophysiological studies. Lysis buffer (50 mM Tris pH 7.4, 0.5% sodium dodecyl sulfate, 1 mM EDTA pH 8), and protease inhibitors for mammalian tissue (Sigma, St. Louis, MO)) was added at 12 uL/mg tissue, and tissue was disrupted by brief sonication and incubated at 4°C on a rotisserie mixer for 2 hours. Protein yield was quantified using a BCA assay (Thermo Scientific, Rockford, IL). Twenty micrograms of total protein was loaded per column onto 4-20% Criterion TGX precast polyacrylamide gels (Bio-Rad, Hercules, CA), separated, and transferred to a nitrocellulose membrane (Hybond N; Amersham, Piscataway, NJ). The membrane was blocked with Tris buffered saline (TBS)-T (150 mM NaCl, 5.2 mM Na2HPO4, 1.7 mM KH2PO4, 0.05% Tween-20) containing 5% nonfat dry milk (NFM). Subsequently, blots were incubated overnight at 4°C in TBS-T/0.5% NFM containing a rabbit polyclonal or mouse monoclonal primary antibody (Millipore, Temecula, CA) that recognized NMDAR1 subunit: mouse anti-GluN1, 1:500 dilution (Cat#MAB1586); rabbit anti- GluN2A, 1:3,000 (Cat#AB1555); mouse anti- GluN2B, 1:5,000 (Cat#MAB5778); or rabbit anti-GluA2/3, 0.3 μg/ml dilution (Cat#AB1506). Following extensive washing with TBS-T, the blots were exposed to peroxidase-labeled goat anti-rabbit or goat anti-mouse secondary antibody (Sigma) at a 1:3000 dilution in TBS-T/0.5% NFM for one hour at room temperature with agitation. Detection of bound secondary antibody was performed using Super Signal West Dura Extended Duration Substrate enhanced chemiluminescence (Thermo Scientific). To normalize expression between experiments, the blots were probed with mouse monoclonal antibody directed against three housekeeping proteins (β-actin, β-tubulin and VDAC - voltage-dependent anion-selective channel; a genomically-encoded mitochondrial protein - 1:50,000 to 1:100,000 dilutions (Millipore), followed by peroxidase-labeled goat anti-mouse secondary antibody, 1:10,000 dilution (Sigma)). Band intensity was visualized and quantified using the Chemi-Doc XRS and Quantity One Analysis software (Bio-Rad). To examine surface expression of specific NMDA receptor subunits, we used the membrane-impermeable cross-linker bis(sulfosuccinimidyl) suberate (BS3; Thermo Fisher Scientific) as described previously (Grosshans et al., 2002) with slight modifications. Slices from individual saline, non-sensitized and sensitized mice were allowed to stabilize for 1 h after preparation and subsequently transferred to ACSF 1 mg/ml BS3 and allowed to incubate for 1 h at 4°C. We then rinsed slices three times with ACSF containing 20 mM Tris, pH 7.4. NAc tissue samples were dissected and frozen until tissue was used for Western blot analysis. Western blot methods were the same as described above, except that 20 μg of total protein was loaded onto precast 8 to 16% SDS-polyacrylamide gels. BS3-insensitive intracellular protein was compared with total protein from the samples not incubated with BS3 collected in parallel to calculate the percentage of protein found on the surface for each animal.
Statistics
Locomotor activity recorded during the chronic treatment test days and ethanol drinking during two bottle choice protocol were analyzed by a repeated-measures analysis of variance (ANOVA) with group (saline, sensitized and non-sensitized mice) as the independent factor. The acute locomotor response to ethanol challenge was analyzed by one-way ANOVA with group (saline, low and high responders) as the independent factor. For the electrophysiological studies, data were analyzed by one-way ANOVA using group (saline, sensitized and non-sensitized mice / saline, low and high responders) as the independent factor. For the Western blot experiments, total and percentages of “cell surface” receptors expression from individual animals were averaged within the treatment groups and compared using one-way ANOVA. In all studies, Newman-Keuls tests for multiple comparisons were used for post hoc analyses when the ANOVA detected a significant effect. The level of significance was set at P < 0.05 in all analyses.
Results
EtOH sensitization increases ethanol drinking
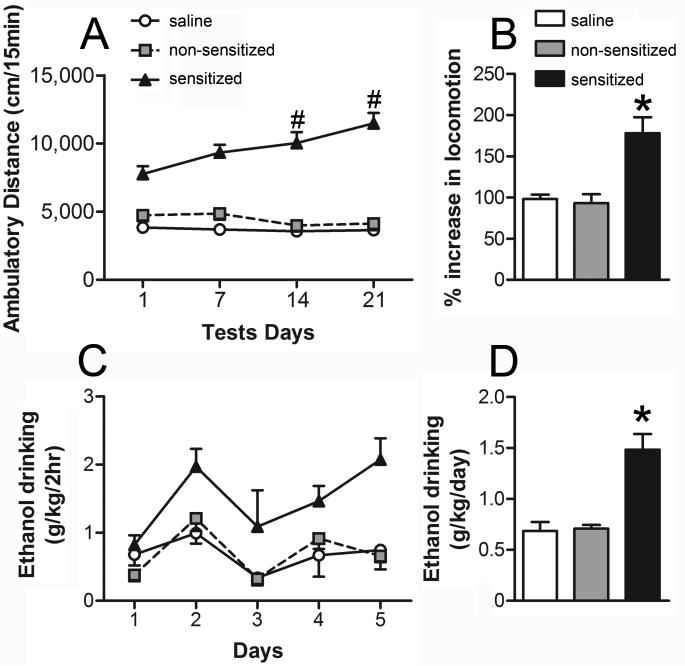
To induce locomotor sensitization, male Swiss Albino mice received daily injections of 1.8 g/kg ethanol or saline for 21 days. Two distinct behavioral profiles were observed following this treatment regimen (Fig. 1A): some mice developed robust sensitization after the ethanol treatments (sensitized mice); while other subjects, receiving the same ethanol injections, showed no significant increase in locomotor activity (non-sensitized mice), displaying a locomotor response similar to that of saline-treated controls. The repeated measure ANOVA revealed significant effects of the group (F2,79 = 112.38, P < 0.001), test (F3,237 = 7.90, P < 0.001) and group-test interaction factors (F6,237 = 11.53, P < 0.001). The Newman-Keuls post hoc test detected that the sensitized group displayed a progressive increase in locomotor activity levels, exhibiting higher ambulatory distance than their initial levels in the previous test (P < 0.05, Fig. 1A). In addition, the sensitized group showed higher activity than the saline and non-sensitized groups in all tests performed (P < 0.05). There were no significant differences between non-sensitized and saline groups. The percent increase in locomotion was higher in the sensitized mice than in the other groups as revealed by one-way ANOVA (F2,79 = 8.04, P < 0.001; Fig. 1B).
Figure 1. Development of behavioral sensitization to ethanol is associated with increased voluntary ethanol consumption.
Mice were treated daily with ethanol (1.8 g/kg. i.p., n = 54) or saline (n = 28) for 21 consecutive days. A, ambulatory distance during 15 minute tests immediately following injections on Day 1, 7, 14, and 21. Mice in the upper and lower tertile on Day 21 were classified as sensitized (n = 27) or non-sensitized (n = 27) respectively. Sensitized mice displayed a progressive increase in activity across days (#, P < 0.05, sensitized in test 3 or 4 vs. sensitized in test 1). B, % increase in locomotor response on Day 21, relative to Day 1. Sensitized mice displayed higher locomotor activity than the other two groups (*, P < 0.05). C, daily ethanol intake for the three treatment groups during a modified “Drinking in the Dark” procedure (2 hr. two-bottle choice; 20% ethanol v/v and water). Sensitized mice drank significantly more ethanol than non-sensitized and saline subjects (P < 0.05). D, average ethanol intake for the three treatment groups (*, P < 0.05, sens vs. nsens and saline).
Recently, it has been demonstrated that previous ethanol exposure can lead to an increase in ethanol self-administration (a behavioral marker of addiction vulnerability) (Fidler et al., 2011). To determine if there was any association between the development of behavioral sensitization and ethanol drinking in Swiss Albino mice, we next examined voluntary ethanol consumption in saline, non-sensitized and sensitized mice. Ethanol drinking was assessed 14 days after the locomotor sensitization protocol. During the week that mice had free choice between water and 20% ethanol, sensitized mice drank significantly more ethanol than non-sensitized mice and notably, no differences in ethanol intake were observed between non-sensitized mice and saline groups (Fig. 1C). The repeated measure ANOVA detected a significant effect of group (F2,13 = 15.40, P < 0.001) and drinking day (F4,52 = 5.76, P < 0.001), but not the group-drinking day interaction (F8,52 = 1.30). The post hoc test for the group factor revealed that sensitized mice drank more ethanol than the other two groups (Fig. 1D; P < 0.05).
EtOH sensitization induces decreased NMDA function and expression
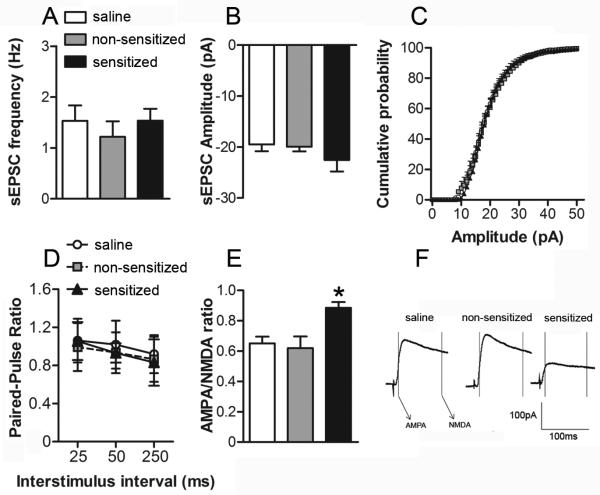
As noted earlier, synaptic alterations in the NAc are thought to play a central role in the etiology of addiction (Ikemoto, 2010). Since NAc glutamatergic synapses may be particularly sensitive to chronic exposure to drugs of abuse (Vanderschuren and Kalivas, 2000), we next sought to determine if measures of NAc glutamate receptor function and expression were associated with individual differences in ethanol locomotor sensitization. Cohorts of saline, non-sensitized and sensitized mice were euthanized 11 – 20 days after the sensitization procedure and NAc slices were prepared for whole cell patch clamp recordings from NAc core neurons. We first examined spontaneous AMPA receptor-mediated EPSCs (sEPSCs) to assess whether presynaptic release probability (sEPSC frequency) was associated with sensitization to ethanol. We found no difference in the frequency of sEPSCs among the groups (one-way ANOVA: F2,23 = 0.28; Fig. 2A). We also examined paired-pulse ratios (PPRs) of evoked AMPA receptor-mediated EPSCs. No significant group differences in PPR were observed (repeated measure ANOVA: group F2,39 = 0.70; stimulus interval F2,78 = 9.57, P < 0.05; group-stimulus interval interaction F4,78 = 0.50; Fig. 2D). These results suggest that locomotor sensitization to ethanol is not associated with presynaptic alterations in glutamatergic synaptic transmission. We next investigated whether variability in ethanol sensitization was associated with alterations in postsynaptic glutamate receptor function in the NAc core. The analyses of sEPSCs revealed no group differences in the amplitude of sEPSCs (one-way ANOVA: F2,23 = 1.00; Fig. 2B), providing initial evidence that direct alterations in AMPA receptor function may not be associated with behavioral sensitization to ethanol. There were also no statistically significant differences in the average cumulative probability distributions of sEPSC amplitudes among the treatment groups (assessed by K-S tests) (Fig. 2C). We did, however, observe a significant increase in the AMPA/NMDA ratio in cells from ethanol sensitized mice compared to the non-sensitized and saline groups (one-way ANOVA: F2,18 = 6.71, P < 0.05; Fig. 2E-F). Since there were no significant group differences in any pre- (PPR and sEPSC frequency) or postsynaptic measures of AMPA receptor activity (sEPSC amplitude), we inferred that ethanol sensitized mice may have an impairment in NAc core NMDA receptor function.
Figure 2. The development of ethanol behavioral sensitization results in decreased NAc core NMDA receptor function.
NAc core neurons were voltage-clamped at A-D, -90 mV or E-F, +40mV and evoked EPSCs (0.05 Hz) were recorded for at least 10 min to ensure the stability of baseline recordings. In all experiments, GABAA IPSCs were pharmacologically blocked using bicuculine methiodide (20 μM, Sigma). A, average frequency and B, amplitude of spontaneous EPSCs (sEPSCs) collected during six minute epochs at -90 mV from cells in slices from saline (n = 12 cells in 8 mice), non-sensitized (n = 6 cells in 6 mice) and sensitized (n = 8 cells in 8 mice) groups. C, Cumulative probability distributions of sEPSC amplitude obtained in recordings from saline, non-sensitized and sensitized groups of mice. D, paired-pulse ratio (PPR) of evoked EPSCs (25, 50 and 250 ms inter-pulse interval) at -90mV from saline (n = 15 cells in 9 mice), non-sensitized (n = 12 cells in 9 mice) and sensitized (n = 12 cells in 9 mice) groups. E, averages of 10 consecutive EPSCs evoked at +40mV for AMPA/NMDA ratios for saline, nsens and sens groups. Peak AMPA receptor-mediated current was measured at -90mV (during a 2ms window, 13ms after spike onset) and NMDA receptor-gated currents were measured in a 10ms window, 100ms after spike onset. F, AMPA/NMDA ratios in cells recorded from saline (n = 8 cells in 8 mice), non-sensitized (n = 6 cells in 6 mice) and sensitized mice (n = 7 cells in 6 mice). IAMPA at +40mV/INMDA at +40mv was taken as the AMPA/NMDA ratio. (*, P < 0.05, sens vs. non-sensitized and saline).
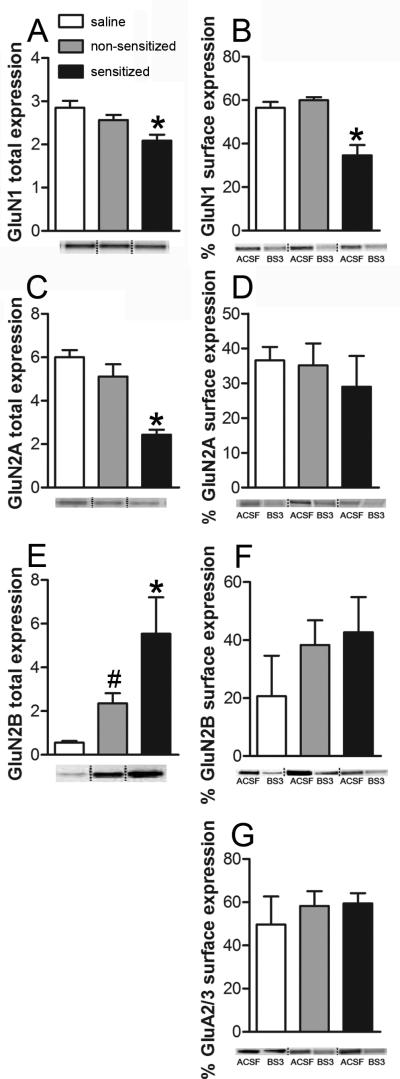
To further test this hypothesis, we also examined the expression of glutamate receptor subunits, using NAc tissue isolated from subjects in each of the three groups. Sensitized mice had a significant decrease in NMDA GluN1 subunit expression relative to non-sensitized or control subjects (one-way ANOVA: F2,15 = 7.33, P < 0.05; Fig. 3A). We also found a significant decrease in NMDA GluN2A subunit expression (one-way ANOVA: F2,15 = 21.04, P < 0.001; Fig. 3C) and a significant increase of NMDA GluN2B subunit expression in the sensitized mice (one-way ANOVA: F2,15 = 6.38, P < 0.05; Fig. 3D). We next used the membrane-impermeable cross-linker bis (sulfosuccinimidyl) suberate (BS3) as described previously (Diaz et al., 2011), to examine surface expression of NMDA receptor subunit proteins. Sensitized mice also had significantly lower surface expression of NMDA GluN1 subunit protein relative to the other two groups (one-way ANOVA: F2,15 = 17.66, P < 0.001; Fig. 3B). No differences in total or surface expression of the GluN1 subunit were observed between the non-sensitized and saline mice. Importantly, no group differences in surface expression were found for either the NMDA GluN2A (one-way ANOVA: F2,15 = 0.36; Fig. 3D) or GluN2B (one-way ANOVA: F2,15 = 0.91; Fig. 3F). Considering that GluN1 is the constitutive subunit of NMDA receptors, these data, along with the electrophysiological results, provides further evidence that behavioral sensitization to ethanol is associated with a selective disruption of NAc NMDA receptor function and expression. It is important to note that no group differences in the surface expression of AMPA GluA2/3 subunits were observed (one-way ANOVA: F2,15 = 0.36; Fig. 3G).
Figure 3. The development of ethanol behavioral sensitization results in decreased NAc NMDA receptor surface expression.
Two weeks after the end of ethanol or saline treatment, NAc tissue was isolated from brain slices prepared using a protocol identical to that described for electrophysiological studies. The slices were divided and incubated in either ACSF (total expression) or BS3-ACSF (internal expression). Graphs on the left are representative of total expression in arbitrary unit. Graphs on the right are representative of % surface expression. A, average total accumbal GluN1 expression in saline (n = 6), non-sensitized (n = 6) and sensitized (n = 6) tissue isolates (*, P < 0.05, sens vs. non-sensitized and saline). B, average percent GluN1 subunit surface expression in NAc tissue isolates from saline (n = 6), non-sensitized (n = 6) and sensitized mice (n = 6) (*, P < 0.05, sens vs. nsens and saline). C, average total accumbal GluN2A expression in saline (n = 6), non-sensitized (n = 6) and sensitized (n = 6) tissue isolates (*, P < 0.05, sens vs. nsens and saline). D, average percent GluN2A subunit surface expression in NAc tissue isolates from saline (n = 6), non-sensitized (n = 6) and sensitized mice (n = 6). E, average total accumbal GluN2B expression in saline (n = 6), nsens (n = 6) and sensitized (n = 6) tissue isolates (*, P < 0.05, sens vs. nsens and saline; #, P < 0.05, nsens vs. saline). F, average percent GluN2B subunit surface expression in NAc tissue isolates from saline (n = 6), non-sensitized (n = 6) and sensitized mice (n = 6). G, average percent GluA2/3 subunit surface expression in NAc tissue isolates from saline (n = 6), non-sensitized (n = 6) and sensitized mice (n = 6).
Ethanol sensitization is associated with impairment in NMDA receptor-dependent LTD in NAc core
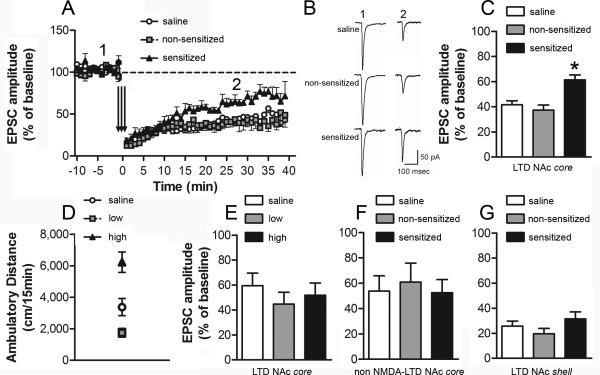
Given that the synapses made by cortical afferents onto medium spiny neurons can express NMDA receptor-dependent LTD (Thomas et al., 2000), and may be an important substrate predicting of drug seeking (Martin et al., 2006), we predicted that the expression of this form of synaptic plasticity would be reduced in ethanol sensitized mice. Robust LTD was observed in saline-treated mice and non-sensitized groups. However, the magnitude of LTD in the NAc core was significantly lower in the sensitized group (one-way ANOVA: F2,19 = 10.99, P < 0.001; Fig. 4A-C). It is important to note that low frequency stimulation protocols can also induce NMDA receptor-independent forms of LTD in the NAc (e.g. endocannabinoid dependent LTD; Robbe et al., 2002). To determine if the ethanol locomotor sensitization-associated deficit in LTD was dependent on NMDA receptor activation, we repeated the above experiment in the presence of the NMDA receptor antagonist, APV. Significant LTD was observed in slices from all three groups; however no difference in the magnitude of NMDA receptor-independent LTD were observed among saline, non-sensitized and sensitized animals (one-way ANOVA: F2,17 = 0.12; Fig. 4F). Finally, since the NAc core and shell are thought to play important, albeit distinct, roles in the pathophysiology of alcohol addiction, we also examined the effect of the sensitization protocol on LTD in NAc shell neurons. In contrast to our findings in the core, no differences among the treatment groups were noted in the induction or expression of LTD in NAc shell recordings (one-way ANOVA: F2,15 = 1.39; Fig. 4G).
Figure 4. Impairment of NAc core NMDA-dependent LTD in ethanol sensitized mice.
NAc neurons were voltage-clamped at -90 mV. LTD was induced using a pairing protocol: 3 × 5Hz, 3 minutes, paired with a depolarization to -50 mV with a 5 minute inter-train interval. EPSC amplitude (mean ± s.e.m) was measured as the maximal response 15-30 min post LTD induction. A, time-course of NAc core LTD in cells recorded from saline (n = 9 cells in 7 mice), non-sensitized (n = 7 cells in 6 mice) and sensitized (n = 6 cells in 6 mice) groups. B, representative EPSCs before (1) and after (2) the NAc core LTD protocol (A) for the three treatment groups. C, % EPSC amplitude after NAc core LTD induction (*, P < 0.05, sensitized vs. non-sensitized and saline). The magnitude of LTD was significantly lower in cells recorded from ethanol sens mice, compared to cells from nsens and saline. D, ambulatory distance during a 15 minute test immediately following i.p. administration of saline (n = 6) or ethanol 1.8 g/kg. Based on their locomotor response to ethanol, ethanol-treated mice were classified as ‘low’ (lower tertile) (n = 6) or ‘high’ (upper tertile) (n = 6) responders (*, P < 0.05, high vs. low and saline). E, % EPSC amplitude after NAc core LTD induction. No difference in the magnitude of LTD was observed in cells recorded from high ethanol responders, low ethanol responders or saline pre-treated mice. F, % EPSC amplitude after NAc core NMDA-independent LTD induction (recording in the presence of the NMDA receptor antagonist APV, 50 μM). No difference in the magnitude of NMDA-independent LTD was observed in cells recorded from saline (n = 7), ethanol non-sensitized (n = 7) and sensitized (n = 6) groups. G, % EPSC amplitude after NAc shell LTD induction. No difference in the magnitude of LTD was observed in cells recorded from saline (n = 5), non-sensitized (n = 5) and sensitized (n = 8) groups.
In some cohorts, we detected a significant difference in the initial locomotor stimulant effect of ethanol (Treatment Day 1) between mice that were eventually classified as non-sensitized and sensitized. This difference correlates with the observed individual variability in the development of locomotor sensitization to ethanol. To determine if the synaptic alterations associated with behavioral sensitization were dependent on the initial acute effect of ethanol on locomotion, we conducted a separate experiment to see if there were any differences in the expression of LTD in animals classified as “low” and “high” responders following a single acute ethanol challenge. Twenty four hours after a 15 minute baseline assessment of horizontal locomotor activity, mice received a single injection of saline or 1.8 g/kg ethanol (i.p.) and were immediately placed in the locomotor activity boxes for 15 minutes. Based on their locomotor response on the acute test day, ethanol-treated mice were classified as “high responders” (activity scores in the upper 33% of the distribution) or “low responders” (activity scores in the lower 33% of the distribution) (Fig. 4D). The intermediate group was discarded. Assessment of LTD in NAc core neurons in saline, low- and high responders was conducted 11-20 days after the acute locomotor activity test, as described above. No differences in the magnitude of LTD were observed among the three treatment groups (one-way ANOVA: F2,24 = 0.60; Fig. 4E). These data demonstrate that the disruption of NMDA receptor-dependent LTD in NAc core, observed in “sensitized” mice, was not related to a difference in initial locomotor response to ethanol. Rather, reduced LTD was only observed following the development of the ethanol-associated behavioral plasticity.
Discussion
Behavioral sensitization is an animal model that has been frequently used to study adaptive changes associated with the transition from controlled drug use to abusive use (Sanchis-Segura and Spanagel, 2006). Although the specific role of behavioral sensitization in the addiction process remains unclear, this form of behavioral plasticity involves some of the same neural circuits, neurotransmitters and receptors as other animal models of addiction (Steketee and Kalivas, 2011). As such, there is much interest in elucidating the neural substrates that contribute to the development of this form of behavioral plasticity. A number of prior studies have demonstrated large individual differences in the development of locomotor sensitization to ethanol in outbred Swiss Webster mice (Souza-Formigoni et al., 1999; Quadros et al., 2002; Abrahao et al., 2011). Since these differences are not due to alterations in ethanol metabolism (Quadros et al., 2005), this model may prove useful in identifying neural correlates of vulnerability and resilience to ethanol-induced locomotor sensitization. Indeed, in this study, we confirmed in multiple cohorts of Swiss Webster mice, that a standard ethanol sensitization treatment regimen consistently generates groups of mice that display a robust increase in ethanol-stimulated locomotor activity, as well as other groups of mice that are resilient to this form of behavioral plasticity (nsens).
A number of other elegant studies have demonstrated how individual differences in responsivity to drugs of abuse can be used to identify behavioral and neurobiological correlates of addiction vulnerability, particularly with respect to psychostimulants (Gulley et al., 2003; Deroche-Gamonet et al., 2004; Kasanetz et al., 2010). We therefore sought to take advantage of the robust individual differences in locomotor sensitization following repeated ethanol treatments to examine the relationship between ethanol sensitization, voluntary ethanol drinking behavior and glutamatergic synaptic function and plasticity after a protracted withdrawal. Mice that developed robust locomotor sensitization to ethanol voluntarily consumed more ethanol than saline-treated controls or ethanol-treated mice resilient to locomotor sensitization. Ethanol-sensitized mice also exhibited significant decreases in accumbal NMDA receptor expression and function relative to non-sensitized and control mice and ethanol sensitization was also associated with a significant impairment in NMDA receptor-dependent LTD in the core, but not the shell, of the NAc. Taken together, these studies suggest that deficits in NAc core NMDA receptor signaling and glutamatergic plasticity contribute to individual differences in the development of locomotor behavioral sensitization to ethanol.
Chronic ethanol exposure and the development of locomotor sensitization can lead to significant increases in voluntary ethanol drinking (Lessov et al., 2001; Becker and Lopez, 2004; Fidler et al., 2011; Carrara-Nascimento et al., 2012). Increased ethanol consumption, like behavioral sensitization, may represent an important behavioral marker of addiction vulnerability. Our data reveal that ethanol-sensitized mice consume more ethanol than control subjects or ethanol-treated mice that failed to develop this form of behavioral plasticity. These findings demonstrate that the development of locomotor sensitization, and not solely the chronic ethanol treatment regimen, is associated with increased ethanol drinking behavior.
NMDA receptors are known to play an important role in ethanol dependence, withdrawal, craving and relapse (Trujillo and Akil, 1995; Krystal et al., 2003). Acute ethanol treatment inhibits NMDA receptor function (Lovinger et al., 1989; Simson et al., 1991). In contrast, chronic ethanol exposure has been shown to result in an increased density of NMDARs and a facilitation of receptor function in several brain regions (Nagy, 2008), including the dorsal striatum (Wang et al., 2010) and NAc (Quadros et al., 2002; Zhou et al., 2007). It is important to note that most prior studies have examined NMDA receptor expression or function in very early stages of withdrawal, between 2 to 24 hours after the last ethanol exposure (Quadros et al., 2002; Zhou et al., 2007; Nagy, 2008). Surprisingly, our data suggest that, following a prolonged withdrawal period (11-20 days), measures of NMDA receptor expression and function in the NAc are significantly reduced in ethanol-sensitized mice, relative to non-sensitized and control subjects. Whole tissue and surface expression of the functionally obligatory GluN1 subunit of the glutamatergic NMDA receptor were significantly lower in NAc tissue isolated from sensitized mice relative to the other two groups, with no effect of treatment group on GluN2A, GluN2B and AMPA-GluA2/3 surface expression. Moreover, locomotor sensitization to ethanol was not associated with any differences in pre- or postsynaptic measures of AMPA receptor-mediated sEPSC, but AMPA/NMDA ratios were larger in recordings from sensitized mice. Although we were not able to obtain a direct measure of NMDA receptor function in these studies, the most parsimonious interpretation of these biochemical and electrophysiological data is that the development of ethanol behavioral sensitization is associated with a reduction in NAc NMDA receptor function.
Since these sensitization-related synaptic alterations were observed during a time period that was also associated with increased ethanol drinking, these data suggest that a downregulation of NAc NMDA receptor activity may represent a previously unrecognized synaptic correlate associated with increased vulnerability to alcohol addiction. In fact, heterozygous deletion of the GluN1 subunit in mice is associated with increased ethanol consumption (Du et al., 2012). A recent finding also showed reduced expression of GluN1 subunit in medial prefrontal cortex tissue three days after a chronic ethanol exposure regimen (Holmes et al., 2012). Thus, in contrast to the increase in NMDA receptor signaling associated with early withdrawal, prolonged withdrawal after chronic ethanol exposure may be associated with decreases in NMDA receptor activity and expression.
In vitro studies have previously shown that ethanol can disrupt different kinds of long term plasticity (LTD and LTP) in the NAc (Jeanes et al., 2011; Mishra et al., 2012). Deficits in NAc shell LTD were also observed in rats following a chronic ethanol vapor inhalation regimen (Jeanes et al., 2011), and many studies have reported disruptions of accumbal synaptic plasticity following locomotor behavioral sensitization or self-administration protocols with psychostimulants (Thomas et al., 2001; Martin et al., 2006; Mao et al., 2009). It is important to note that, for psychostimulants, there are significant differences in the impairment of accumbal synaptic plasticity when the recordings are made during an early or protracted withdrawal or after an acute drug challenge (for review see Madsen et al., 2012). Here we showed that, after a protracted withdrawal, ethanol-sensitized mice exhibited a significant impairment of NMDA-dependent LTD expression in the NAc core, but not in shell. Notably, in our study, significant impairment of NAc core NMDA-dependent LTD was only observed in sensitized mice and not in ethanol-treated mice that failed to develop behavioral adaptation to chronic ethanol exposure. Importantly, individual differences in locomotor activity observed after a single ethanol treatment were not associated with any significant differences in LTD expression. Therefore, the impairment of LTD that we observed seems to be associated with the development of ethanol sensitization and not with any initial differences in locomotor responsivity to ethanol between sensitized and non-sensitized mice. Impairment of accumbal LTD may represent a form of neuroadaptation associated not only with the chronic effects of abused drugs but also with a behavioral phenotype related to an increased vulnerability to addiction. Interestingly, this specificity has recently been demonstrated in a rodent model of cocaine addiction (Kasanetz et al., 2010). Although cocaine exposure initially disrupted LTD in the NAc core of all rats that self-administered cocaine, only animals that developed an “addicted” phenotype failed to recover the ability to express this form of synaptic plasticity (Kasanetz et al., 2010).
Low frequency stimulation protocols can also induce NMDA receptor-independent forms of LTD, such as endocannabinoid dependent LTD in striatal neurons (Kombian and Malenka, 1994; Robbe et al., 2002). Indeed, we observed robust LTD in the NAc core in the presence of the NMDA receptor antagonist, APV. However, under these recording conditions, no ethanol sensitization-associated impairment of LTD was observed. This finding, along with our other biochemical and electrophysiolgical data, supports our hypothesis that locomotor sensitization to ethanol is associated with a functional deficit in NAc core NMDA receptor function.
In summary, our data provide further evidence that the development of locomotor sensitization to ethanol may represent a behavioral correlate for increased vulnerability to alcohol addiction. Mice that developed this form of behavioral plasticity consumed more ethanol than non-sensitized mice, despite the fact that both groups received an identical ethanol treatment regimen prior to the voluntary drinking assay. Further, the development of ethanol locomotor behavioral sensitization was associated with a significant decrease in NAc NMDA receptor expression and function, as well as impaired NMDA receptor-mediated LTD - one form of synaptic plasticity that controls the balance of neuronal activity necessary to adapt behavior to an ever-changing environment. These synaptic alterations may contribute to the perseveration on the drug of abuse and the difficulty in learning new associations, which are key hallmarks of addiction. A better understanding of the molecular substrates responsible for this decrease in neuroplasticity could unravel new targets for the development of more effective therapies for drug and ethanol dependence.
Acknowledgements
This research was funded by National Institutes of Health grants: AA 21099, AA 17531, AA 10422, AA 14445 and by Brazilian agencies: Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior (CAPES): 0321-10-9, Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) 2008/01819-5 e Associação Fundo de Incentivo à Pesquisa (AFIP).
References
- Abrahao KP, Souza-Formigoni ML. Behavioral sensitization to ethanol results in cross-sensitization to MK-801 but not to NMDA administered intra-accumbens. Behav Brain Res. 2012;235:218–224. doi: 10.1016/j.bbr.2012.07.034. [DOI] [PubMed] [Google Scholar]
- Abrahao KP, Quadros IM, Souza-Formigoni ML. Individual differences to repeated ethanol administration may predict locomotor response to other drugs, and vice versa. Behav Brain Res. 2009;197:404–410. doi: 10.1016/j.bbr.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Abrahao KP, Quadros IM, Souza-Formigoni ML. Nucleus accumbens dopamine D1 receptors regulate the expression of ethanol-induced behavioural sensitization. Int J Neuropsychopharmacol. 2011;14:175–185. doi: 10.1017/S1461145710000441. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28:1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006;11:270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Carrara-Nascimento PF, Olive MF, Camarini R. Ethanol pre-exposure during adolescence or adulthood increases ethanol intake but ethanol-induced conditioned place preference is enhanced only when pre-exposure occurs in adolescence. Dev Psychobiol. 2012 doi: 10.1002/dev.21089. [DOI] [PubMed] [Google Scholar]
- Crowder TL, Ariwodola OJ, Weiner JL. Ethanol antagonizes kainate receptor-mediated inhibition of evoked GABA(A) inhibitory postsynaptic currents in the rat hippocampal CA1 region. J Pharmacol Exp Ther. 2002;303:937–944. doi: 10.1124/jpet.102.038471. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Diaz MR, Christian DT, Anderson NJ, McCool BA. Chronic ethanol and withdrawal differentially modulate lateral/basolateral amygdala paracapsular and local GABAergic synapses. J Pharmacol Exp Ther. 2011;337:162–170. doi: 10.1124/jpet.110.177121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Elberger AJ, Matthews DB, Hamre KM. Heterozygous deletion of NR1 subunit of the NMDA receptor alters ethanol-related behaviors and regional expression of NR2 subunits in the brain. Neurotoxicol Teratol. 2012;34:177–186. doi: 10.1016/j.ntt.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler TL, Dion AM, Powers MS, Ramirez JJ, Mulgrew JA, Smitasin PJ, Crane AT, Cunningham CL. Intragastric self-infusion of ethanol in high- and low-drinking mouse genotypes after passive ethanol exposure. Genes Brain Behav. 2011;10:264–275. doi: 10.1111/j.1601-183X.2010.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Snelling C, Fretwell AM, Tanchuck MA, Underwood L, Cole M, Crabbe JC, Roberts AJ. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12-41). Alcohol Clin Exp Res. 2007;31:939–949. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Rodd-Henricks K, Li TK, Lumeng L. Ethanol locomotor sensitization, but not tolerance correlates with selection for alcohol preference in high- and low-alcohol preferring mice. Psychopharmacology (Berl) 2000;151:252–260. doi: 10.1007/s002130000388. [DOI] [PubMed] [Google Scholar]
- Grosshans DR, Clayton DA, Coultrap SJ, Browning MD. Analysis of glutamate receptor surface expression in acute hippocampal slices. Sci STKE. 20022002:l8. doi: 10.1126/stke.2002.137.pl8. [DOI] [PubMed] [Google Scholar]
- Gulley JM, Hoover BR, Larson GA, Zahniser NR. Individual differences in cocaine-induced locomotor activity in rats: behavioral characteristics, cocaine pharmacokinetics, and the dopamine transporter. Neuropsychopharmacology. 2003;28:2089–2101. doi: 10.1038/sj.npp.1300279. [DOI] [PubMed] [Google Scholar]
- Holmes A, Fitzgerald PJ, MacPherson KP, DeBrouse L, Colacicco G, Flynn SM, Masneuf S, Pleil KE, Li C, Marcinkiewcz CA, Kash TL, Gunduz-Cinar O, Camp M. Chronic alcohol remodels prefrontal neurons and disrupts NMDAR-mediated fear extinction encoding. Nat Neurosci. 2012;15:1359–1361. doi: 10.1038/nn.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Brain reward circuitry beyond the mesolimbic dopamine system: a neurobiological theory. Neurosci Biobehav Rev. 2010;35:129–150. doi: 10.1016/j.neubiorev.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanes ZM, Buske TR, Morrisett RA. In vivo chronic intermittent ethanol exposure reverses the polarity of synaptic plasticity in the nucleus accumbens shell. J Pharmacol Exp Ther. 2011;336:155–164. doi: 10.1124/jpet.110.171009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasanetz F, Deroche-Gamonet V, Berson N, Balado E, Lafourcade M, Manzoni O, Piazza PV. Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science. 2010;328:1709–1712. doi: 10.1126/science.1187801. [DOI] [PubMed] [Google Scholar]
- Kombian SB, Malenka RC. Simultaneous LTP of non-NMDA- and LTD of NMDA-receptor-mediated responses in the nucleus accumbens. Nature. 1994;368:242–246. doi: 10.1038/368242a0. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Mason G, Trevisan L, D'Souza DC. N-methyl-D-aspartate glutamate receptors and alcoholism: reward, dependence, treatment, and vulnerability. Pharmacol Ther. 2003;99:79–94. doi: 10.1016/s0163-7258(03)00054-8. [DOI] [PubMed] [Google Scholar]
- Lessov CN, Palmer AA, Quick EA, Phillips TJ. Voluntary ethanol drinking in C57BL/6J and DBA/2J mice before and after sensitization to the locomotor stimulant effects of ethanol. Psychopharmacology (Berl) 2001;155:91–99. doi: 10.1007/s002130100699. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen HB, Brown RM, Lawrence AJ. Neuroplasticity in addiction: cellular and transcriptional perspectives. Front Mol Neurosci. 2012;5:99. doi: 10.3389/fnmol.2012.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Luscher C. Synaptic plasticity and addiction: Learning mechanisms gone awry. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.01.036. [DOI] [PubMed] [Google Scholar]
- Mao LM, Wang W, Chu XP, Zhang GC, Liu XY, Yang YJ, Haines M, Papasian CJ, Fibuch EE, Buch S, Chen JG, Wang JQ. Stability of surface NMDA receptors controls synaptic and behavioral adaptations to amphetamine. Nat Neurosci. 2009;12:602–610. doi: 10.1038/nn.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Chen BT, Hopf FW, Bowers MS, Bonci A. Cocaine self-administration selectively abolishes LTD in the core of the nucleus accumbens. Nat Neurosci. 2006;9:868–869. doi: 10.1038/nn1713. [DOI] [PubMed] [Google Scholar]
- McCool BA. Ethanol modulation of synaptic plasticity. Neuropharmacology. 2011;61:1097–1108. doi: 10.1016/j.neuropharm.2010.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melon LC, Boehm SL., 2nd Role of Genotype in the Development of Locomotor Sensitization to Alcohol in Adult and Adolescent Mice: Comparison of the DBA/2J and C57BL/6J Inbred Mouse Strains. Alcohol Clin Exp Res. 2011;35:1351–1360. doi: 10.1111/j.1530-0277.2011.01471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra D, Zhang X, Chergui K. Ethanol Disrupts the Mechanisms of Induction of Long-Term Potentiation in the Mouse Nucleus Accumbens. Alcohol Clin Exp Res. 2012 doi: 10.1111/j.1530-0277.2012.01824.x. [DOI] [PubMed] [Google Scholar]
- Myme CI, Sugino K, Turrigiano GG, Nelson SB. The NMDA-to-AMPA ratio at synapses onto layer 2/3 pyramidal neurons is conserved across prefrontal and visual cortices. J Neurophysiol. 2003;90:771–779. doi: 10.1152/jn.00070.2003. [DOI] [PubMed] [Google Scholar]
- Nagy J. Alcohol related changes in regulation of NMDA receptor functions. Curr Neuropharmacol. 2008;6:39–54. doi: 10.2174/157015908783769662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. Chronic tolerance and sensitization to alcohol in sons of alcoholics: II. Replication and reanalysis. Exp Clin Psychopharmacol. 1999;7:234–243. doi: 10.1037//1064-1297.7.3.234. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal ML. Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol. 1996;36:359–378. doi: 10.1146/annurev.pa.36.040196.002043. [DOI] [PubMed] [Google Scholar]
- Quadros IM, Nobrega JN, Hipolide DC, Souza-Formigoni ML. Increased brain dopamine D4-like binding after chronic ethanol is not associated with behavioral sensitization in mice. Alcohol. 2005;37:99–104. doi: 10.1016/j.alcohol.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Quadros IM, Hipolide DC, Frussa-Filho R, De Lucca EM, Nobrega JN, Souza-Formigoni ML. Resistance to ethanol sensitization is associated with increased NMDA receptor binding in specific brain areas. Eur J Pharmacol. 2002;442:55–61. doi: 10.1016/s0014-2999(02)01503-0. [DOI] [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci U S A. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Simson PE, Criswell HE, Johnson KB, Hicks RE, Breese GR. Ethanol inhibits NMDA-evoked electrophysiological activity in vivo. J Pharmacol Exp Ther. 1991;257:225–231. [PubMed] [Google Scholar]
- Souza-Formigoni ML, De Lucca EM, Hipolide DC, Enns SC, Oliveira MG, Nobrega JN. Sensitization to ethanol's stimulant effect is associated with region-specific increases in brain D2 receptor binding. Psychopharmacology (Berl) 1999;146:262–267. doi: 10.1007/s002130051115. [DOI] [PubMed] [Google Scholar]
- Spanagel R, et al. An integrated genome research network for studying the genetics of alcohol addiction. Addict Biol. 2010;15:369–379. doi: 10.1111/j.1369-1600.2010.00276.x. [DOI] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW. Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev. 2011;63:348–365. doi: 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swendsen J, Le Moal M. Individual vulnerability to addiction. Ann N Y Acad Sci. 2011;1216:73–85. doi: 10.1111/j.1749-6632.2010.05894.x. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Malenka RC, Bonci A. Modulation of long-term depression by dopamine in the mesolimbic system. J Neurosci. 2000;20:5581–5586. doi: 10.1523/JNEUROSCI.20-15-05581.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- Trujillo KA, Akil H. Excitatory amino acids and drugs of abuse: a role for N-methyl-D-aspartate receptors in drug tolerance, sensitization and physical dependence. Drug Alcohol Depend. 1995;38:139–154. doi: 10.1016/0376-8716(95)01119-j. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Wang J, Lanfranco MF, Gibb SL, Yowell QV, Carnicella S, Ron D. Long-lasting adaptations of the NR2B-containing NMDA receptors in the dorsomedial striatum play a crucial role in alcohol consumption and relapse. J Neurosci. 2010;30:10187–10198. doi: 10.1523/JNEUROSCI.2268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FC, Anthony B, Dunn KW, Lindquist WB, Xu ZC, Deng P. Chronic alcohol drinking alters neuronal dendritic spines in the brain reward center nucleus accumbens. Brain Res. 2007;1134:148–161. doi: 10.1016/j.brainres.2006.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]