Abstract
Although protective effects of the cochlea’s efferent feedback pathways have been well documented, prior work has focused on hair cell damage and cochlear threshold elevation and, correspondingly, on the high sound pressure levels (> 100 dB SPL) necessary to produce them. Here we explore the noise-induced loss of cochlear neurons that occurs with lower intensity exposures and in the absence of permanent threshold shifts. Using confocal microscopy to count synapses between hair cells and cochlear nerve fibers, and using measurement of auditory brainstem responses and otoacoustic emissions to assess cochlear pre- and post-synaptic function, we compare the damage from a weeklong exposure to moderate-level noise (84 dB SPL) in mice with varying degrees of cochlear de-efferentation induced by surgical lesion to the olivocochlear pathway. Such exposure causes minimal acute threshold shift and no chronic shifts in mice with normal efferent feedback. In de-efferented animals, there was up to 40% loss of cochlear nerve synapses and a corresponding decline in the amplitude of the auditory brainstem response. Quantitative analysis of the de-efferentation in inner vs. outer hair cell areas suggested that outer hair cell efferents are most important in minimizing this neuropathy, presumably by virtue of their sound-evoked feedback reduction of cochlear amplification. The moderate nature of this acoustic overexposure suggests that cochlear neurons are at risk even in everyday acoustic environments, and, thus, that the need for cochlear protection is plausible as a driving force in the design of this feedback pathway.
Keywords: Auditory neuropathy, olivocochlear, hair cells, cochlea, noise, acoustic injury, feedback
Introduction
Overexposure to intense sound can damage or destroy cochlear sensory cells and thereby lead to permanent elevation of cochlear thresholds (Liberman and Dodds, 1984). Given the obvious importance of threshold sensitivity to auditory function, most research on noise-induced hearing loss has focused on hair cell damage and threshold shift as the key structural and functional metrics of the effects of noise. Recent studies, however, show degeneration of up to 50% of the cochlea’s sensory neurons after noise exposures that have been adjusted in either level or duration so as to produce only transient threshold elevation and no loss of, or permanent damage to, the sensory cells (Kujawa and Liberman, 2009). This primary neuronal degeneration, which may be a type of glutamate excitotoxicity (Pujol et al., 1993; Pujol and Puel, 1999), appears within hours post-exposure as a loss of synaptic terminals on the inner hair cells (Robertson, 1983), which are normally innervated by 95% of the cochlea’s sensory fibers (Spoendlin, 1972). Death of the neuronal somata, the spiral ganglion cells, is much slower, continuing for months to years post-exposure (Liberman and Kiang, 1978; Kujawa and Liberman, 2009). This type of diffuse and subtotal neuronal loss does not elevate thresholds, but likely causes problems hearing in difficult (noisy) listening environments, a common audiological complaint (Kujawa and Liberman, 2009).
The discovery of a new metric of noise-induced cochlear damage, which has revealed deleterious effects of exposures that otherwise appear atraumatic, has led us to re-examine the role of cochlear efferent system in protecting the ear from acoustic overexposure. Although many studies have shown that eliminating activity in this sound-evoked negative-feedback loop carried by the fibers of the olivocochlear (OC) bundle increases noise-induced threshold shifts (Kujawa and Liberman, 1997), such experiments involve exposure to sound pressure levels of 100 – 120 dB SPL for durations of 1–4 hrs. Since such continuous high-level exposures are rare in the absence of man-made devices, some have questioned the biological significance of this protective effect (Kirk and Smith, 2003), suggesting it is an epiphenomenon that may be useful in the today’s noisy world, but that it cannot represent the biological advantage that has driven the evolution of this feedback system.
The present experiments were designed to ask whether efferent feedback protects cochlear neurons from moderate-level sound exposures that are more common in the natural world. As a starting point, we chose a level, 84 dB SPL, that is, for example, well below those measured in the middle of a tree-frog chorus (> 90 dB SPL (Narins, 1982)), and well below that of a bat echolocation call (~100 dB SPL (Xie and Henson, 1998)). The 84 dB level is also well within the federal guidelines for an 8-hr/day exposure for a lifetime (http://www.osha.gov). Results show that normal mice exposed for 1 wk to such a moderate-level noise, with a spectrum targeting the most sensitive portion of the hearing range, show no measureable permanent threshold shifts. However, confocal analysis of immunostained cochleas reveals loss of up to 20% of the afferent synapses on inner hair cells in some cochlear regions. Surgical removal of efferent feedback doubles the synaptic degeneration such that up to 40% of the synapses are missing 2 wks after the termination of the exposure. Comparison of lesions designed to selectively destroy either the lateral (L)OC neurons innervating the cochlear neurons or the medial (M)OC neurons innervating the outer hair cells, suggests that the protection arises predominately from the MOC pathway.
Materials and Methods
Animals and Groups
Male mice of the CBA/CaJ strain entered the experimental protocol at 6-8 wks of age and were assigned to one of four groups: 1) Control animals underwent no surgical procedure and no purposeful noise exposure; 2) Expose Only animals underwent no surgical procedure before the calibrated exposure to noise; 3) in COCB Cut animals, the crossed olivocochlear (OC) bundle was surgically transected 10 days prior to the noise exposure; and 4) in LSO Injection animals, a neurotoxin (melittin) was stereotaxically injected to target the lateral superior olive (LSO) on the right side (Le Prell et al., 2003), 1 wk prior to the noise. For each animal in the Expose Only, the COCB Cut and the LSO Lesion groups, cochlear function was assessed bilaterally via auditory brainstem responses (ABRs) and distortion product otoacoustic emissions (DPOAEs), both 4 days before and 10 days after the termination of the noise exposure. Immediately after the final cochlear function test, animals were fixed by intracardiac perfusion, and both cochleas were removed for histological processing and subsequent confocal analysis of hair cell and synaptic degeneration. Final group sizes are given in the relevant figure captions.
Cochlear Function Tests
For measuring cochlear function via ABRs and DPOAEs, animals were anesthetized with a ketamine/xylazine mixture and placed in an acoustically electrically shielded room maintained at 32° C. Acoustic stimuli were delivered through a custom EPL Acoustic System consisting of two miniature dynamic earphones used as sound sources (CUI CDMG15008-03A) and an electret condenser microphone (Knowles FG-23329-PO7) coupled to a probe tube to measure sound pressure near the eardrum (for details see http://www.masseyeandear.org/research/ent/eaton-peabody/epl-engineering-resources/epl-acoustic-system/). Digital stimulus generation and response processing were handled by digital I-O boards from National Instruments driven by custom software written in LabVIEW. For ABRs, stimuli were 5-msec tone pips (0.5 msec cos2 rise-fall) delivered in alternating polarity at 35/sec. Electrical responses were sampled via Grass needle electrodes at the vertex and pinna with a ground reference near the tail and amplified 10,000X with a 0.3 – 3 kHz passband. Responses to as many as 1024 stimuli were averaged at each sound pressure level, as level was varied in 5 dB steps from below threshold up to 80 dB SPL. ABR thresholds were defined, by visual inspection of stacked waveforms, as the lowest SPL at which the wave morphology conformed to a consistent pattern (with peak latencies increasing systematically as SPL is reduced). For DPOAEs, stimuli were two primary tones f1 and f2 (f2/f1 = 1.2), with f1 level always 10 dB above f2 level. Primaries were swept in 5 dB steps from 20 to 80 dB SPL (for f2). The DPOAE at 2f1-f2 was extracted from the ear canal sound pressure after both waveform and spectral averaging. Noise floor was defined as the average of 6 spectral points below, and 6 above, the 2f1-f2 point. Threshold was computed by interpolation as the primary level (f2) required to produce a DPOAE of 0 dB SPL.
Noise Exposure
Animals were exposed to an 8-16 kHz octave-band noise at 84 dB SPL for 1 wk in specially modified mouse cages, with a CUI Miniature Dynamic earphone (15 mm diameter) mounted at either end of the cage, near the top to prevent blockage from bedding or the mice themselves. SPLs were calibrated at the start and end of each 1-wk exposure: levels varied by < 1 dB at different points in the cage. Several cages were driven simultaneously, and 2-4 mice were housed per cage to minimize acoustic shielding from clustering. Animals had free access to food and water throughout.
Brainstem lesions and histological verification
For brainstem surgery, mice were anesthetized with a ketamine/xylazine mixture. COCB cuts were made with a microknife on the floor of the IVth ventricle after a posterior craniotomy and cerebellar elevation. For lesions of the LSO, the mouse was held in a stereotaxic apparatus with the scalp retracted. A micropipette filled with 10 mM melittin was lowered into the brain, through an opening over the right lambdoidal suture, at a position 0.49 mm caudal and 0.12 mm lateral to the bregma. At a depth of 0.69 mm, 0.2 μl of melittin was injected by a 1-μl Hamilton syringe. Brainstems were fixed in 10% formalin, cryoprotected (30% sucrose) and cut on a freezing microtome at 40 μm in the transverse plane. Sections were treated histochemically to reveal acetylcholinesterase activity (Osen and Roth, 1969).
Cochlear Processing and Immunostaining
Mice were perfused intracardially with 4% paraformaldehyde in phosphate buffer. Cochleas were decalcified, dissected into half-turns and permeabilized by freeze/thawing. The half-turns were blocked in 5% normal horse serum (NHS) with 1% Triton X-100 (TX) in PBS for 1 hr, followed by incubation for ~19 hrs at 37°C in primary antibodies diluted in 1% NHS with 1% TritonX. Antibodies always included 1) mouse (IgG1) anti-CtBP2 from BD Biosciences at 1:200 and 2) rabbit anti-VAT from Sigma at 1:1000 to allows quantification of pre-synaptic ribbons in inner hair cells and cochlear efferent terminals respectively. To quantify post-synaptic elements in the inner hair cell area, we used either 1) chicken anti-NF-H from Chemicon at 1:1000 or 2) mouse (IgG2) anti-GluA2, from Millipore at 1:2000. Primary incubations were followed by 2 sequential 60-min incubations at 37°C in species-appropriate secondary antibodies with 1% TritonX.
Cochlear Histological Analysis
Two types of information were extracted from both inner and outer hair cell areas in each cochlea: 1) counts of afferent ribbon synapses and 2) quantification of the degree of de-efferentation. Both analyses were based on high-power confocal z-stacks obtained at half-octave intervals along the cochlear spiral, i.e. at 5.6, 8.0, 11.3, 16.0, 22.6, 32.0, 45.3 and 64 kHz. To accurately identify regions of interest, cochlear lengths were obtained for each case by tracing the spiral in low-power images of the dissected epithelial whole mounts using a custom ImageJ plugin (http://www.masseyeandear.org/research/ent/eaton-peabody/epl-histology-resources/) that translates cochlear position into frequency according to the published map for the mouse (Muller et al., 2005). Confocal z-stacks were obtained at specified cochlear frequency regions with a glycerol-immersion objective (63X, N.A. 1.3) at 3.17X digital zoom on a Leica TCS SP5. Image spacing in the z plane was set to 0.25 μm, and the z-span was carefully adjusted for each stack to include all synaptic elements in all of the 8-12 hair cells from each row included in each stack, typically requiring 75-100 images per stack. Two adjacent stacks were always obtained in each cochlear region sampled.
Synaptic counts
Pre-synaptic ribbons and post-synaptic glutamate receptor patches were counted from the confocal z-stacks using the connected components tool in Amira software (Visage Imaging), which finds and displays each voxel space in an image stack containing exclusively pixel values greater than a user-set criterion. By comparing the “connected components” display to the maximum projection, the user can adjust the criterion to capture all the elements of interest; because the analysis is done in 3-D, the result accurately separates elements superimposed in z. To quantitatively assess the pairing of pre- and post-synaptic elements, we use custom software that extracts the voxel space within 1 μm around each ribbon (or receptor patch) and produces a thumbnail array of these miniature projections, that can be easily scanned to count synapses (i.e. ribbons with closely apposed receptor patches) vs. orphan ribbons or orphan receptor patches (Liberman et al., 2011). Synaptic counts were always expressed on a per hair cell basis. Hair cells in each stack were counted by increasing the image output-gain (gamma adjust): IHC nuclei stain faintly with the CtBP2 antibody, and the OHC somata are visible via their faint background label in several confocal channels, as well as by the presence of synaptic ribbons, even when the efferent terminals are missing.
Degree of de-efferentation
The degree of de-efferentation was assessed in both IHC and OHC areas from maximum projections of the VAT-immunostaining in the z-stacks. In the OHC area, the total number of VAT-positive terminals was counted in each stack and divided by the number of hair cells (~10 OHCs in each of the three rows). In the IHC area, because the terminals are smaller and more numerous, accurate counting is difficult. Thus a semi-quantitative analysis was performed with a 4 point scale: 3 = profuse, 2 = moderate, 1= sparse and 0 = none (Darrow et al., 2007).
Results
A. Olivocochlear lesions and their assessment
The olivocochlear (OC) pathway consists of two major divisions (Warr and Guinan, 1979), each of which has been implicated in the control of cochlear noise damage (Maison and Liberman, 2000; Darrow et al., 2007). The medial (M)OC pathway originates bilaterally from cell bodies in the ventral nucleus of the trapezoid body (Fig. 1A) and projects via myelinated axons to the outer hair cells (Fig. 1B), where release of acetylcholine decreases their normal contribution to cochlear mechanical amplification (for review see (Guinan, 2006). The lateral (L)OC pathway originates from cell bodies in the ipsilateral lateral superior olive (LSO, Fig. 1A) and projects via unmyelinated axons to synapse on the unmyelinated terminals of cochlear neurons (Fig. 1B), near their afferent synapses with the inner hair cells (IHCs).
Figure 1.
Schematics illustrating the brainstem origins (A) and peripheral targets (B) of the lateral olivocochlear (LOC) and medial olivocochlear (MOC) efferent pathways to the cochlea. A: Schematic cross-section through the mouse brainstem showing the lateral superior olive (LSO), where the LOC cell bodies arise, and the crossed olivocochlear bundle (COCB), made up largely of MOC fibers projecting to the opposite ear. B: Schematic cross section through the organ of Corti, showing the synaptic contacts of 1) LOC terminals and the dendrites of cochlear nerve (afferent) fibers in the inner hair cell (IHC) area and 2) MOC terminals on OHCs.
To parse the contributions of MOC vs. LOC pathways to the control of noise-induced cochlear neuropathy, we produced two kinds of brainstem lesions: 1) cutting the crossed olivocochlear bundle (COCB, Fig. 2L) or 2) destroying one LSO by stereotaxic injection (Fig. 2I). Cutting the COCB, where it passes near the dorsal surface of the brainstem at the floor of the IVth ventricle (Figs. 1A, 2J) should remove 75-80% of the MOC innervation to both ears, leaving the LOC system intact, since, in mouse, almost all the LOC projections and only 20-25% of MOC projections to each ear arise ipsilaterally (Brown, 1993), and therefore survive a midline transection. By the same logic, destroying one LSO by unilateral neurotoxin injection can selectively destroy only the LOC to the ipsilateral ear, leaving the MOC system intact bilaterally.
Figure 2.
The success of de-efferentation was assessed by analysis of the organ of Corti (A-F) and the brainstem (G-L), stained for cholinergic markers. A-F: Maximum projections of confocal z-stacks of the organ of Corti, viewed from the endolymphatic surface, and immunostained for a cholinergic marker (VAT: vesicular acetylcholine transporter) in the red channel, and a synaptic ribbon marker (CtBP2: C-terminal binding protein) in green. Panels A-C each shows ~10 adjacent IHCs, with their unstained nuclei shown by dashed circles: A is a Control; B illustrates near-complete loss of LOC terminals after a successful LSO lesion, and C shows near-complete sparing of LOC terminals after a successful COCB cut. The OHCs are outside the field of view, towards the top of the images. Panels D-F each shows ~12 OHCs from each row, from the same cochlear region and the same case shown in the paired image to the left. D is a Control, where as many as 4 MOC terminals cluster under a single OHC (arrows); E shows survival of MOC terminals after a successful LSO lesion; and F shows loss of MOC terminals after a successful COCB cut. The regular array of synaptic ribbons (arrows) shows that the OHCs are still present. Scale bar in F applies to all panels. G-K: Brainstem sections through the left (G, H) and right (J, K) superior olivary complexes, stained for acetylcholinesterase to show the cell bodies of LOC and MOC neurons 6 wks after neurotoxin injection into the right LSO in one case (G,H) or a COCB cut in another (J, K). Post-surgery survival was 6 wks in all cases. The schematic below each pair of images (G, J) summarizes which OC projections remain in each case.
In this study, we assessed the success of de-efferentation in two ways: 1) qualitatively, by analysis of brainstem sections histochemically stained for acetylcholinesterase to reveal the cholinergic somata of LOC and MOC neurons (Figs. 2G-K), and 2) quantitatively, by analysis of cochleas immunostained for another cholinergic marker (vesicular acetylcholine transporter – VAT) to quantify the distributions of MOC and LOC terminals in the OHC (Figs. 2D,E,F) and IHC (Figs. 2A,B,C) areas, respectively. When viewed as epithelial whole mounts, i.e. orthogonal to the section plane schematized in Fig. 1B, VAT-positive OC terminals in a control ear are clearly visible in both the IHC (Fig. 2A) and OHC (Fig. 2D) areas. In the OHC area, the terminals are large and discrete enough to be unambiguously counted: the number of terminals per OHC is clearly reduced by a successful COCB cut (Fig. 2F). In the IHC area, the terminals are too small to count, but a successful LOC lesion can clearly eliminate virtually all the VAT-positive puncta from the inner spiral bundle (Fig. 2B), where the LOC terminals normally intermingle with the unmyelinated dendrites of cochlear neurons near their IHC synapses, marked by the CtBP2-positive puncta (green in Fig. 2A,B,C). The semi-quantitative analysis of the LOC innervation used here was adopted from a prior study (Darrow et al., 2007): see Methods for further details.
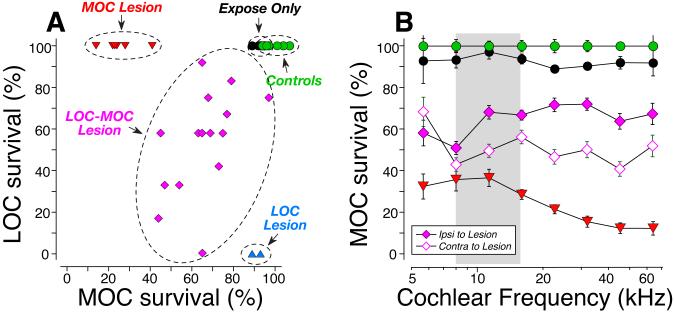
The cochlear de-efferentation analysis suggests that a successful COCB cut removes ~80% of the MOC terminals to both ears (red symbols in Fig. 3A), as predicted by the published anatomy in mouse (Brown, 1993). It also appears that the LSO injections damaged both MOC and LOC cells (purple symbols in Fig. 3A) in the majority of cases, and selectively damaged the LOC system in only two cases (blue symbols in Fig. 3A). The quantitative conclusions from the cochlear analysis were consistent with those drawn more qualitatively from the brainstem sections. For example, note the clear reduction in MOC cells adjacent to the LSO injection in Fig. 2H in comparison to the control side in Fig. 2G. Similarly, note the bilateral loss of MOC cells (and the bilateral sparing of LOC cells) in the COCB cut case (Figs. 2J,K) compared to the control side of the LSO lesion case (Fig. 2G). Following the LSO injections that also hit the MOC cell group, the loss of MOC cells was, on average, greater on the contralateral side than on the ipsilateral side (Fig. 3B), which is also consistent with the known anatomy (Fig. 1A).
Figure 3.
Quantification of MOC and LOC de-efferentation. A: In each case, two confocal z-stacks similar to those in Fig. 2 were obtained from each of eight cochlear regions, evenly spaced from apex to base of the spiral. Techniques for estimating the loss of MOC and LOC terminals in each stack are described in Methods. Values for each ear represent the average of all stacks from all cochlear regions. For Controls (n=6 ears from 3 cases), Expose Only (n=6 ears from 3 cases) and COCB cut (MOC lesion) cases (N=8 ears from 4 cases), data are also averaged across both ears. For the unilateral LSO injections, only the data for the ipsilateral ear are shown. The LSO injection cases are segregated into two groups: 2 ears (from 2 cases) with “pure” LOC lesion, and the remaining 30 ears (from 15 cases) with combined LOC/MOC lesion. Data for MOC terminals are normalized with respect to Control animals. Data for LOC terminals are normalized by comparison between the two ears of one case. B: The mean survival (±SEM) of MOC terminals, as a function of cochlear location, for each of the groups in A, with the LOC-MOC lesion group further divided to separately show the ears ipsi vs. contra to the lesion (see key).
B. Noise-induced cochlear dysfunction with and without OC feedback
To help differentiate pre- and post-synaptic dysfunction in the cochlear periphery, we measure cochlear function in two ways: DPOAEs and ABRs. DPOAEs are sounds created by electrical distortions in the normal sensory epithelium that are reverse-transduced into mechanical motion, amplified by OHC “motors” (Liberman et al., 2004), and then reverse-propagated back to the eardrum where they radiate into the ear canal as sound pressure that can be measured with a sensitive microphone. Normal DPOAEs do not require normal IHC function or a normal cochlear nerve (Takeno et al., 1994). The earliest wave of the ABR (Wave 1) represents the synchronous sound-evoked spike activity in the cochlear nerve. When response diminutions in DPOAE and ABR are matched, problems likely arise from OHC dysfunction, whereas ABR anomalies in the presence of normal DPOAEs suggest the presence of cochlear neuropathy in the absence of OHC damage (Mills, 2003; Kujawa and Liberman, 2009).
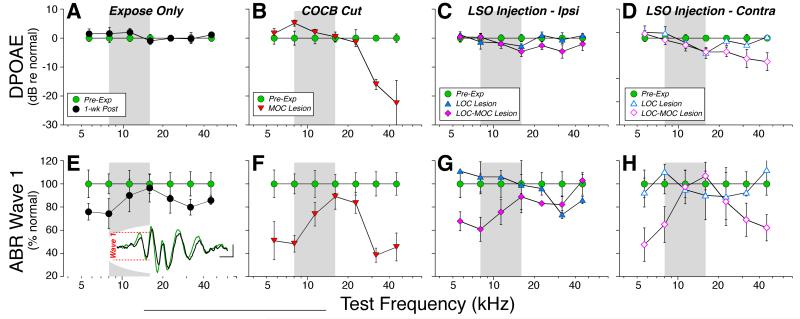
In ears with a normal OC innervation (Fig. 4A, Expose Only), exposure to the 1-wk 84 dB SPL noise produced only a small threshold shift (< 15 dB), when measured immediately (within 1hr) after removal from the noise. This shift recovered so quickly that we tracked it only with DPOAEs, which can be acquired in ~1/10 the time. When measured 1 week post-exposure, all cochlear thresholds had returned to normal, whether measured by ABRs (Fig. 4E) or DPOAEs (Fig. 4A). In contrast, in ears with a successful COCB cut, i.e. with loss of up to 80% of the MOC terminals on OHCs (Fig. 3A), there was permanent threshold shift approaching 20 dB at the high frequencies via both DPOAEs (Fig. 4B) and ABRs (Fig. 4F), suggesting the presence of minor OHC damage. Indeed, hair cell counts reveal scattered loss of OHCs in the MOC Lesion cases, but not in the Expose Only ears (Fig. 5) Among the LSO injection cases, the ipsilateral ears showed essentially complete threshold recovery, including those with selective LOC lesion (Fig. 4C,G). The persistent, high-frequency loss in the ears contralateral to LSO Injections matches the loss in the COCB Cut ears and strongly suggests that it is the crossed MOC projection that provide the protective effect against the OHC damage suggested here. Statistical analysis (by two-way ANOVA) confirmed that, of all the pairwise pre-vs. post-exposure comparisons, the only groups showing significant (P<0.05) shifts were those with MOC lesion (p = 0.002 for DPOAE and p= 0.007 for ABR) and those contralateral to the LSO injection with a combined LOC-MOC lesion (p=0.002 for DPOAE and p< 0.001 for ABR).
Figure 4.
Noise-induced threshold shifts, as seen via either DPOAE (A-D) or ABR (E-H). Animals are grouped as defined in Fig. 3. A,E: Animals exposed without prior de-efferentation surgery show small temporary threshold shifts (TTS) when measured immediately (< 1 hr) after exposure (grey circles, A only). However, 1 wk later, thresholds have recovered, as measured by either DPOAEs (A) or ABRs (E). Pre-exposure and 1-wk post-exposure measures are from 10 ears (5 cases); the 1-hr post-exposure measures are from 6 ears (3 cases). B,F: Animals exposed after successful COCB cuts show moderate permanent threshold shifts 1-wk post exposure (n=8 ears from 4 cases), as measured via DPOAEs (B) or ABRs (F). C,G and D,H: Animals exposed after successful LSO injections show moderate permanent threshold shifts 1-wk post exposure, but only in the ear contralateral to the lesion (D,H). Pre-exposure and 1-wk post-exposure measures are means from 17 animals, averaged separately by ear, and segregated into two groups (LOC only vs. LOC & MOC) as described in Fig. 3. For each panel, shifts are computed re mean thresholds for the pre-exposure data from that group. Error bars indicate ± SEM in all panels.
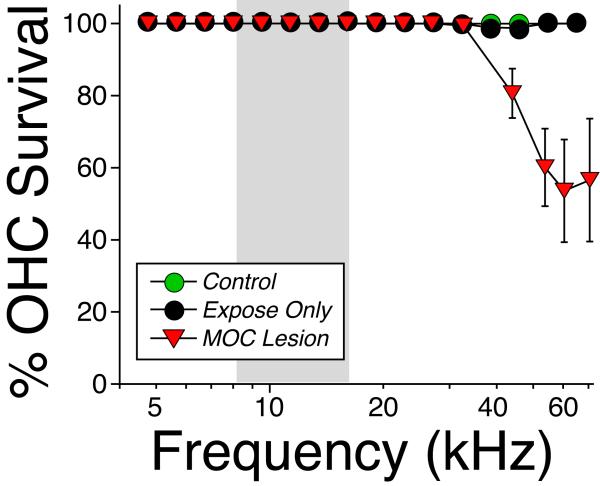
Figure 5.
Hair cell loss after noise exposure was restricted to the OHCs in the basal end of the cochlea and only in cases with loss of OHC efferent innervation. Mean OHC survival (± SEM) is plotted as a function of cochlear location for all ears of the groups from Fig. 4A,E. There was no IHC loss in any of the cases.
Prior mouse studies on cochlear effects of high-level noise (Kujawa and Liberman, 2009) noted that neither DPOAE nor ABR thresholds is sensitive to diffuse degeneration of the cochlear nerve, but suggested that such neuropathy could be revealed in the suprathreshold amplitudes of ABR wave 1 (see inset to Fig. 6E): since each synchronized neuron contributes equally to the electrical field potential, the mean decrease in ABR amplitudes should parallel the mean loss of cochlear nerve fibers, so long as function of the OHC “cochlear amplifier” is unaltered.
Figure 6.
Suprathreshold response amplitude for ABR Wave 1 (E-H) is reduced at many frequencies for which DPOAEs (A-D) recover. Experimental groups are defined in Fig. 3; group sizes are given in Fig. 4. For DPOAEs (A-D), response amplitudes are averaged for primary levels from 60 – 80 dB SPL and then normalized re the pre-exposure means for each group: negative values indicate that post-exposure amplitudes were smaller than pre-exposure. For ABRs (E-H), wave 1 amplitudes for 60 – 80 dB tone pips were averaged for each group at each test frequency and then normalized re pre-exposure means for the same group. Error bars indicate ± SEM in all panels. Inset to Panel E shows mean ABR waveforms (8 kHz, 80 dB SPL) before (green) vs. 1 wk after (black) noise exposure: Wave 1 amplitude is indicated for the pre-exposure curve.
The data in Fig. 6 (i.e. normalized suprathreshold amplitudes as a function of stimulus frequency) suggest that the 1-wk exposure causes modest neuropathy in the normal ear, which is significantly exacerbated in the absence of MOC feedback. In the normal ear (Expose Only), the recovery of DPOAE suprathreshold amplitudes (Fig. 6A) as well as threshold responses (Fig. 4A) suggests full recovery of OHC function throughout the ear’s dynamic range. In contrast, the decrease in Wave 1 amplitudes suggest a neural loss of up to 25%, especially at the apical and basal ends of the cochlea (Fig. 6E). After loss of the MOC system (COCB Cut), the decline in neural responses grows to 60%, and remains most striking in the apical and basal extremes (Fig. 6F). The diminution in OHC function at high frequencies (Fig. 4B, 5B) makes the high-frequency neural data harder to interpret, but the wave-1 amplitude decrements at low frequencies (Fig. 6F) strongly suggest primary neuropathy. The results from the LSO Injections also implicate the MOC system, since there was no wave 1 decline when the LOC projection was destroyed (Fig. 6C,G), but large declines were seen contralateral to the injection (Fig. 6D,H), where MOC degeneration is the greatest (Fig. 2I). Statistical analysis (by two-way ANOVA) confirmed that, of all the pairwise pre- vs. post-exposure comparisons of suprathreshold amplitudes in lesion ears, ABR differences were significant only in the groups with MOC lesion or combined MOC-LOC lesion.
C. Noise-induced cochlear neuropathy with vs. without OC feedback
To assess the loss of cochlear-nerve synapses, we concentrate on the IHC area, because 95% of cochlear neurons, i.e. the type-I neurons, make synaptic contact exclusively with IHCs (Spoendlin, 1972). As schematized in Fig. 7F, each type-I neuron contacts a single IHC via a single terminal bouton. At this synaptic contact, ultrastructural studies typically show one discrete patch of closely apposed pre- and post-synaptic membrane specialization and one pre-synaptic ribbon, an electron-dense body within the IHC surrounded by a halo of synaptic vesicles (Liberman, 1980).
Figure 7.
Confocal analysis shows noise-induced loss of IHC afferent synapses. A-D: Maximum projections from image-stacks of the organ of Corti immunostained for a pre-synaptic marker (CtBP2: red) and a post-synaptic marker (GluR2: green). A and C show surface views of 5-6 adjacent IHCs from the 8-kHz region in a control (A) and an exposed (B) case: positions of IHC nuclei are shown by dashed circles. In the control ear, virtually all synaptic ribbons are paired with a glutamate receptor patch (red-fill green arrows); in exposed ears, there are a few orphan ribbons (red arrows in C). B and D display each image stack in yz projection to show that unpaired ribbons are far from the IHC’s basal pole (red arrows in D). Scale bar in C applies to A, B, and D. E: To quantify juxtaposition of pre- and post-synaptic elements, custom software creates an array of thumbnails, each displaying the voxel space 1 μm around each ribbon. From such arrays, orphan ribbons (red arrows) can be distinguished from complete synapses (red-fill green arrows). F: Schematic of the IHC synaptic region oriented similarly to the yz projections (B, D). G: Mean ribbon counts (± SEMs) from 8 cochlear locations in each ear show the noise-induced loss of synapses and its exacerbation after cutting the COCB. Group sizes were: Control – 6 ears from 3 cases; Expose Only – 18 ears from 9 cases; MOC Lesion – 8 ears from 4 cases. H: Analysis of ribbon-based thumbnail arrays (as in E) shows that, outside of the most apical cochlear regions, virtually all ribbons are part of a synaptic complex, in both unexposed and exposed ears.
To quantify these afferent synapses in the confocal, we use antibodies against 1) CtBP2, a major constituent of the pre-synaptic ribbon (Khimich et al., 2005) and 2) one of the AMPA-type glutamate receptors (GluA2), expressed in the post-synaptic terminal (Matsubara et al., 1996). When viewed in the confocal in whole-mount preparations of the organ of Corti, each cochlear-nerve/IHC synapse appears as a juxtaposed pair of CtBP2/GluA2 puncta (arrows in Fig. 7A), clustered in the subnuclear zone of the IHCs (Fig. 7A,B). Counts in control (unexposed) ears show from 10 – 20 ribbons per IHC, depending on cochlear frequency/location (Fig. 7G). To accurately quantify the juxtaposition between ribbons and receptors, we use custom software (Liberman et al., 2011) that re-displays the 3-D voxel space immediately around each identified ribbon and creates a montage of thumbnails like those in Fig 6E, which is easily scanned to identify ribbon/receptor pairs (red-filled green arrows) as well as orphan ribbons (red arrows). Such analyses show that, in control ears, almost every ribbon is paired with a glutamate receptor patch (Fig. 7H). Similarly, almost every glutamate receptor patch is paired with pre-synaptic ribbon (data not shown).
In exposed ears, there are fewer ribbons per IHC (Fig. 7C,D), and orphan ribbons are slightly more common (red arrows in Fig. 7C,D, E), especially in the peri-nuclear region, where synapses are absent in unexposed ears (Fig. 7B,D). Ribbon counts in exposed ears suggest a synaptopathy throughout large parts of the cochlear spiral (Fig. 7G). This pathology is clearly exacerbated by cutting the COCB and thereby removing MOC feedback (Fig. 7G). Analysis of the juxtaposition between pre and post-synaptic elements suggests that, even in exposed ears, ribbon counts are a good proxy for synaptic counts, except in the apical-most cochlear regions, where there are numerous orphan ribbons (and ribbon counts must underestimate the degree of neuropathy).
The magnitude and cochlear distribution of noise-induced neuropathy in our experimental groups is best seen by normalizing the ribbon counts to place-matched values from unexposed controls (Fig. 8). Synaptic loss in the Expose Only group peaks at ~20% near the 8 kHz and 32 kHz regions (Fig. 8A). This pattern of synaptic loss is not related in any simple fashion to the MOC innervation density in Control ears, which peaks (at 2.8 terminals/OHC) at 16 kHz and falls monotonically to values half that at 5.6 (1.2 terminals/OHC) and 64 kHz (1.4 terminals/OHC). The loss in the COCB Cut cases shows the same pattern across the cochlear spiral, but the magnitude of the mean loss is doubled to ~40% at 8 and 32 kHz. The LSO Injection cases show patterns reminiscent of the ABR data (Fig. 6): i.e. the neuropathy is generally no worse after LOC destruction (blue triangles in Fig. 8C), but is exacerbated when the MOC is involved, especially on the side contralateral to the injection (purple diamonds in Fig. 7D), where the largest MOC denervation is seen (Fig. 3B). Removing both MOC and LOC projections, without purposeful noise exposure, causes minimal synaptopathy: an average 10% loss, when evaluated 4 wks after the surgery (data not shown).
Figure 8.
IHC synaptic-ribbon loss is maximal in ears with significant MOC lesions. Experimental groups are defined in Fig. 3A; group means (± SEMs) are shown. Ribbons were counted in 8 cochlear locations (from ~20 adjacent IHCs in each location) in each case. Data for each group are normalized with respect to age-matched, unexposed Controls (n=6 ears from 3 cases). The Expose Only group included 18 ears from 9 cases. Other group sizes are given in Fig. 3A: note that there are only two cases in the LOC Lesion group.
The analyses in Figs. 6 and 8 are based on animal groups defined using de-efferentation metrics that are averaged across the entire cochlear spiral (Fig. 3). This pan-cochlear average must introduce error if the degree of de-efferentation differs with cochlear location in each case. To minimize that error, we have also analyzed the data in a regionally discrete way by plotting, for each of the eight cochlear regions analyzed, the degree of de-efferentation vs. the degree of noise-induced neuropathy (Fig. 9). The data from two of the most highly affected regions, 8 and 45 kHz, show a clear correlation between the two values and further underscore the conclusion that the MOC system is more important in minimizing neuropathy than the LOC system.
Figure 9.
The loss of IHC synapses is correlated with the loss of MOC innervation, in both the 8 kHz region (A) and the 45 kHz region (B), i.e. the apical and basal extremes, where the neuropathy is greatest (see Fig. 8). Large symbols are for group means; small symbols are for individual animals. Error bars show ± SEMs. The percent survival of MOC terminals is computed as described for Fig. 3; the percent survival of IHC synapses is computed as described for Fig. 8. Dashed lines represent best fits to the scatterplots: slopes and y intercepts (x=0) are 1.34 and −49.68 for panel A and 1.04 and −16.18 for panel B.
Discussion
A. Primary neuronal degeneration and cochlear threshold recovery
Acoustic overexposure can damage many structures of the middle and inner ear if exposure levels are high enough and exposure durations are long enough; however, the cochlear hair cells appear to be among the most vulnerable cells in the auditory periphery (Liberman and Kiang, 1978). Within minutes after an exposure severe enough to cause permanent threshold elevations, the hair cells are swollen (Wang et al., 2002), within hours they can disappear, and, within days, the gaps they leave in the sensory epithelium (Bohne and Rabbitt, 1983) are resealed by the surrounding supporting cells of the organ of Corti. This rapid hair cell degeneration can occur, initially, without loss of any other cochlear cell type. In contrast, the time course of neuronal degeneration appears to be slower. The peripheral myelinated axons of cochlear nerve fibers begin to disappear no sooner than 1-2 weeks post-exposure, and loss of their cell bodies in the spiral ganglion is not noticeable for at least a month (Liberman and Kiang, 1978). This stark difference in degenerative time course has led to the view that hair cell loss is the primary event in the ear’s response to noise, and that neural degeneration only occurs secondarily to the loss of hair cells, presumably due to loss of neurotrophic support.
Recent work has challenged this view. Within 24 hrs after acoustic overexposure, there can be 40 – 50% loss of cochlear nerve synapses on surviving hair cells (Kujawa and Liberman, 2009). This permanent neuropathy, which appears to be a type of glutamate excitotoxicity (Pujol et al., 1993; Pujol and Puel, 1999), went unnoticed for many years because 1) the degenerating terminals are unmyelinated, and therefore impossible to see in routine light-microscopic analysis, 2) the subsequent degeneration of the (more easily visible) myelinated axons and cell bodies takes from months to years, and 3) this neuropathy can occur despite complete recovery of cochlear thresholds, as measured by either ABRs or OAEs.
How can there be 40-50% loss cochlear nerve fibers without significant threshold elevation of the ABR, which reflects the summed activity of neurons in the ascending auditory pathway, starting with the cochlear nerve? One simple hypothesis is that the noise-induced degeneration is selective for that subset of cochlear neurons (40% of the total population) that normally has high thresholds and low spontaneous rates (SRs) (Liberman, 1978). Single-fiber recordings in noise-exposed guinea pigs suggest that this is indeed the case (Lin et al., 2011a). Although ABR thresholds are unaffected, the neuropathy is revealed in the attenuation of ABR amplitudes at high stimulus levels, where low-SR fibers normally begin to contribute to the summed response.
Prior work on this type of primary neural degeneration (Kujawa and Liberman, 2009; Lin et al., 2011b) used noise exposures at high intensities (100 – 106 dB SPL) and short durations (2 hrs). The present study focused on a much lower level (84 dB SPL) and longer duration (1 wk). The results clearly show that exposures more like those in everyday environments are also neuropathic (Fig. 8A), even when the gain-control feedback via the olivocochlear efferent system is intact. Although there may be inter-species differences in vulnerability between mouse and man, if an 84 dB exposure, causing only a mild (15 dB) and transient threshold shift, can cause irreversible cochlear synaptopathy, then many common workplace and leisure exposures to noise are likely more dangerous to hearing health than current federal guidelines indicate.
B. Primary neuronal degeneration and OC-mediated protection
The medial olivocochlear (MOC) neurons constitute a sound-evoked negative feedback loop (Guinan, 2006). As sound level increases, MOC firing rate increases (Liberman and Brown, 1986). This feedback suppresses the normal contribution of outer hair cells to amplification of sound-induced motion in the sensory epithelium (Wiederhold and Kiang, 1970), which effectively decreases the intensity of the stimulus. Despite clear descriptions of the suppressive effects of MOC activation on everything from cochlear mechanics (Cooper and Guinan, 2006) to cochlear nerve response (Guinan and Stankovic, 1996), its functional significance remains controversial. Is it designed to protect the ear from acoustic injury (Rajan, 1988), to improve signal detection in a noisy environment (Winslow and Sachs, 1987), or to mediate selective attention (Scharf et al., 1994)?
The cochlear effects of activating the lateral olivocochlear (LOC) system are less well characterized, because their axons are unmyelinated and difficult to electrically stimulate in a direct fashion (Gifford and Guinan, 1987). Indirect activation, via stimulation of the inferior colliculus, suggests that the LOC system can slowly modulate the overall excitability of cochlear neurons to acoustic stimulation (Groff and Liberman, 2003). Although it has been suggested that this modulation is important in the interaural balancing of cochlear nerve activity that is necessary for accurate sound localization (Darrow et al., 2006a), other studies have suggested that the LOC feedback is also important in protecting the ear from acoustic injury (Ruel et al., 2001). One line of evidence for LOC-mediated protection is indirect: immunohistochemical evidence for dopaminergic transmission in one subgroup of LOC fibers (Darrow et al., 2006b) coupled with pharmacological evidence that cochlear perfusion of dopaminergic antagonists elicits the type of cochlear-nerve terminal swelling that is also elicited by noise (Ruel et al., 2001). A second line of evidence is the increased cochlear threshold shifts seen after selective LOC lesions, but that study did not specifically investigate synaptopathy in the IHC area (Darrow et al., 2007).
In the present study, enhanced neural degeneration was seen only in ears with significant MOC lesions, i.e. 1) bilaterally after cutting the OC bundle at the midline, which eliminates much of the MOC innervation to both ears without affecting the LOC system or 2) contralateral to focal lesions of the olivary complex which involved both LOC and MOC systems. The clear contralateral bias to the olivary lesion affects provides strong evidence that the primary effect here is due to the MOC system not the LOC, given that the LOC projection is overwhelming ipsilateral whereas the MOC projection is predominately contralateral (Guinan et al., 1983). The contralateral bias also provides strong evidence that the effect does not arise from interruption of the middle ear muscle reflex, which is also driven from nearby brainstem structures with projections that are exclusively ipsilateral (Vacher et al., 1989).
C. OC-mediated protection – epiphenomenon or design feature?
Although MOC protective effects are well documented, prior work used cochlear threshold shift as the metric of noise-induced damage (Rajan, 1988; Kujawa and Liberman, 1997), and high-level exposures (greater than ~105 dB SPL) are required to produce the permanent hair cell damage that underlies these shifts (Kujawa and Liberman, 1997). As such, MOC protection, though possibly important in our noisy, industrialized society, is arguably an epiphenomenon that could not have driven the evolutionary development of this feedback system (Kirk and Smith, 2003), which is present in all vertebrate hair cell systems (Guinan, 2006).
Here we show that olivocochlear feedback also protects the ear from noise-induced cochlear neuropathy, which can be elicited at much lower sound pressures: the 84 dB stimulus we used is a lower intensity than that which is created by vocalizations in many natural environments (e.g. (Narins, 1982; Xie and Henson, 1998)). This moderate-level, long-duration exposure was designed as a step towards the most interesting, but practically more difficult, question of whether, perhaps, cochlear neurons cannot survive years of routine use without the gain control supplied by olivocochlear feedback. Chronic self-stimulation by vocalization may present a significant damage risk to the ear without protection from efferent feedback: it is interesting in this regard that several studies have suggested that the olivocochlear reflex is activated in anticipation of vocalization (Suga and Jen, 1975; Xie and Henson, 1998).
Cochlear nerve activity is driven by an extremely active glutamatergic synapse, with spontaneous discharge rates in excess of 100 sp/sec in some neurons, and maximum sound-driven rates that exceed 400 sp/sec in many neurons, even in the steady state (Liberman, 1978). Given relatively small dynamic ranges, i.e. 20-40 dB (Schalk and Sachs, 1980; Taberner and Liberman, 2005), and relatively low thresholds (30 dB SPL even for many high-threshold fibers in the middle of the hearing range (Liberman, 1978; Taberner and Liberman, 2005)), these maximum discharge rates are elicited continuously by continuous stimuli at intensities of only 60-80 dB SPL. Such relatively moderate sound levels are routine in many natural environments.
In the context of MOC-mediated protection from cochlear neuropathy, it may not be a coincidence that the suppressive effects elicited in cochlear nerve fibers via activation of the MOC system are maximal for low-SR fibers and at moderate sound pressure levels (Guinan and Stankovic, 1996), i.e. precisely the fiber type that is most vulnerable, and at the intensity range addressed in the present study. MOC activation reduced maximum discharge rate in some low-SR fibers by more than 20% (Guinan and Stankovic, 1996). If glutamate excitotoxicity in the cochlear nerve is proportional to discharge rate, this type of MOC feedback should clearly be protective. The special vulnerability of low-SR fibers to excitotoxicity may be due, at least in part, to their low mitochondrial content (Liberman, 1980), given the known importance of mitochondria in buffering the Ca++ overload that is a key trigger for excitotoxic neural damage (Szydlowska and Tymianski, 2010).
If nerve damage is related to sustained discharge rate, a hair-cell based threshold shift might also be protective, if it reduces discharge during the exposure. Such an effect could explain why the pattern of noise-induced neuropathy (Figs. 7A,B) was complementary to that of the acute damage to OHC function (Fig. 4A). The threshold shift peak at 16 kHz (Fig. 4A) is classic for acoustic overexposure, i.e. maximum damage occurs at frequencies 1/2 octave above the noise band, because of level-dependent changes in cochlear mechanics (Cody and Johnstone, 1981). Perhaps the neuropathy was minimal at 16 kHz because the accumulating threshold shift during the exposure limited the cochlear nerve stimulation. The idea that increasing damage to one cochlear structure can decrease damage to another has been suggested in other acoustic injury studies at much higher sound pressure levels (Wang et al., 2002).
Acknowledgements
Research supported by grants from the NIDCD: R01 DC 0188 and P30 DC 05209. Flawless technical support in the preparation and immunostaining of cochlear wholemounts by Leslie Liberman is gratefully acknowledged.
References Cited
- Bohne BA, Rabbitt KD. Holes in the reticular lamina after noise exposure: implication for continuing damage in the organ of Corti. Hear Res. 1983;11:41–53. doi: 10.1016/0378-5955(83)90044-8. [DOI] [PubMed] [Google Scholar]
- Brown MC. Fiber pathways and branching patterns of biocytin-labeled olivocochlear neurons in the mouse brainstem. J Comp Neurol. 1993;337:600–613. doi: 10.1002/cne.903370406. [DOI] [PubMed] [Google Scholar]
- Cody AR, Johnstone BM. Acoustic trauma: single neuron basis for the “half-octave shift”. J Acoust Soc Am. 1981;70:707–711. doi: 10.1121/1.386906. [DOI] [PubMed] [Google Scholar]
- Cooper NP, Guinan JJ., Jr. Efferent-mediated control of basilar membrane motion. J Physiol. 2006;576:49–54. doi: 10.1113/jphysiol.2006.114991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow KN, Maison SF, Liberman MC. Cochlear efferent feedback balances interaural sensitivity. Nat Neurosci. 2006a;9:1474–1476. doi: 10.1038/nn1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow KN, Maison SF, Liberman MC. Selective removal of lateral olivocochlear efferents increases vulnerability to acute acoustic injury. J Neurophysiol. 2007;97:1775–1785. doi: 10.1152/jn.00955.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow KN, Simons EJ, Dodds L, Liberman MC. Dopaminergic innervation of the mouse inner ear: evidence for a separate cytochemical group of cochlear efferent fibers. J Comp Neurol. 2006b;498:403–414. doi: 10.1002/cne.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford ML, Guinan JJ., Jr. Effects of electrical stimulation of medial olivocochlear neurons on ipsilateral and contralateral cochlear responses. Hear Res. 1987;29:179–194. doi: 10.1016/0378-5955(87)90166-3. [DOI] [PubMed] [Google Scholar]
- Groff JA, Liberman MC. Modulation of cochlear afferent response by the lateral olivocochlear system: activation via electrical stimulation of the inferior colliculus. J Neurophysiol. 2003;90:3178–3200. doi: 10.1152/jn.00537.2003. [DOI] [PubMed] [Google Scholar]
- Guinan JJ., Jr. Olivocochlear efferents: anatomy, physiology, function, and the measurement of efferent effects in humans. Ear and hearing. 2006;27:589–607. doi: 10.1097/01.aud.0000240507.83072.e7. [DOI] [PubMed] [Google Scholar]
- Guinan JJ, Jr., Stankovic KM. Medial efferent inhibition produces the largest equivalent attenuations at moderate to high sound levels in cat auditory-nerve fibers. J Acoust Soc Am. 1996;100:1680–1690. doi: 10.1121/1.416066. [DOI] [PubMed] [Google Scholar]
- Guinan JJ, Jr., Warr WB, Norris BE. Differential olivocochlear projections from lateral versus medial zones of the superior olivary complex. J Comp Neurol. 1983;221:358–370. doi: 10.1002/cne.902210310. [DOI] [PubMed] [Google Scholar]
- Khimich D, Nouvian R, Pujol R, Tom Dieck S, Egner A, Gundelfinger ED, Moser T. Hair cell synaptic ribbons are essential for synchronous auditory signalling. Nature. 2005;434:889–894. doi: 10.1038/nature03418. [DOI] [PubMed] [Google Scholar]
- Kirk CE, Smith DW. Protection from acoustic trauma is not a primary function of the medial olivocochlear efferent system. J Assoc Res Otolaryngol. 2003;4:445–465. doi: 10.1007/s10162-002-3013-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Conditioning-related protection from acoustic injury: effects of chronic deefferentation and sham surgery. J Neurophysiol. 1997;78:3095–3106. doi: 10.1152/jn.1997.78.6.3095. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell CG, Shore SE, Hughes LF, Bledsoe SC., Jr. Disruption of lateral efferent pathways: functional changes in auditory evoked responses. J Assoc Res Otolaryngol. 2003;4:276–290. doi: 10.1007/s10162-002-3018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman LD, Wang H, Liberman MC. Opposing gradients of ribbon size and AMPA receptor expression underlie sensitivity differences among cochlear-nerve/hair-cell synapses. J Neurosci. 2011;31:801–808. doi: 10.1523/JNEUROSCI.3389-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC. Auditory-nerve response from cats raised in a low-noise chamber. J Acoust Soc Am. 1978;63:442–455. doi: 10.1121/1.381736. [DOI] [PubMed] [Google Scholar]
- Liberman MC. Morphological differences among radial afferent fibers in the cat cochlea: An electron-microscopic study of serial sections. Hear Res. 1980;3:45–63. doi: 10.1016/0378-5955(80)90007-6. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Kiang NY. Acoustic trauma in cats. Cochlear pathology and auditory-nerve activity. Acta oto-laryngologica. 1978;358:1–63. [PubMed] [Google Scholar]
- Liberman MC, Dodds LW. Single-neuron labeling and chronic cochlear pathology. III. Stereocilia damage and alterations of threshold tuning curves. Hear Res. 1984;16:55–74. doi: 10.1016/0378-5955(84)90025-x. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Brown MC. Physiology and anatomy of single olivocochlear neurons in the cat. Hear Res. 1986;24:17–36. doi: 10.1016/0378-5955(86)90003-1. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Zuo J, Guinan JJ., Jr. Otoacoustic emissions without somatic motility: can stereocilia mechanics drive the mammalian cochlea? J Acoust Soc Am. 2004;116:1649–1655. doi: 10.1121/1.1775275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Furman AC, Kujawa SG, Liberman MC. Noise-induced primary neural degneration in guinea pig: Does vulnerability depend on spontaneous discharge rate? In: Santi PA, editor. Midwinter Meeting of the Association for Research in Otolaryngology. 2011a. p. 56. [Google Scholar]
- Lin HW, Furman AC, Kujawa SG, Liberman MC. Primary neural degeneration in the Guinea pig cochlea after reversible noise-induced threshold shift. J Assoc Res Otolaryngol. 2011b;12:605–616. doi: 10.1007/s10162-011-0277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison SF, Liberman MC. Predicting vulnerability to acoustic injury with a noninvasive assay of olivocochlear reflex strength. J Neurosci. 2000;20:4701–4707. doi: 10.1523/JNEUROSCI.20-12-04701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara A, Laake JH, Davanger S, Usami S, Ottersen OP. Organization of AMPA receptor subunits at a glutamate synapse: a quantitative immunogold analysis of hair cell synapses in the rat organ of Corti. J Neurosci. 1996;16:4457–4467. doi: 10.1523/JNEUROSCI.16-14-04457.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills DM. Differential responses to acoustic damage and furosemide in auditory brainstem and otoacoustic emission measures. J Acoust Soc Am. 2003;113:914–924. doi: 10.1121/1.1535942. [DOI] [PubMed] [Google Scholar]
- Muller M, von Hunerbein K, Hoidis S, Smolders JW. A physiological place-frequency map of the cochlea in the CBA/J mouse. Hear Res. 2005;202:63–73. doi: 10.1016/j.heares.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Narins PM. Effects of Masking Noise on Evoked Calling in the Puerto Riean Coqui (Anura: Leptodactylidae) Journal of Comparative Phsyiology A. 1982;147:439–446. [Google Scholar]
- Osen KK, Roth K. Histochemical localization of cholinesterases in the cochlear nuclei of the cat, with notes on the origin of acetylcholinesterase-positive afferents and the superior olive. Brain Res. 1969;16:165–185. doi: 10.1016/0006-8993(69)90092-4. [DOI] [PubMed] [Google Scholar]
- Pujol R, Puel JL. Excitotoxicity, synaptic repair, and functional recovery in the mammalian cochlea: a review of recent findings. Ann N Y Acad Sci. 1999;884:249–254. doi: 10.1111/j.1749-6632.1999.tb08646.x. [DOI] [PubMed] [Google Scholar]
- Pujol R, Puel JL, Gervais d’Aldin C, Eybalin M. Pathophysiology of the glutamatergic synapses in the cochlea. Acta Otolaryngol. 1993;113:330–334. doi: 10.3109/00016489309135819. [DOI] [PubMed] [Google Scholar]
- Rajan R. Effect of electrical stimulation of the crossed olivocochlear bundle on temporary threshold shifts in auditory sensitivity. I. Dependence on electrical stimulation parameters. J Neurophysiol. 1988;60:549–568. doi: 10.1152/jn.1988.60.2.549. [DOI] [PubMed] [Google Scholar]
- Robertson D. Functional significance of dendritic swelling after loud sounds in the guinea pig cochlea. Hearing Res. 1983;9:263–278. doi: 10.1016/0378-5955(83)90031-x. [DOI] [PubMed] [Google Scholar]
- Ruel J, Nouvian R, Gervais d’Aldin C, Pujol R, Eybalin M, Puel JL. Dopamine inhibition of auditory nerve activity in the adult mammalian cochlea. Eur J Neurosci. 2001;14:977–986. doi: 10.1046/j.0953-816x.2001.01721.x. [DOI] [PubMed] [Google Scholar]
- Schalk TB, Sachs MB. Nonlinearities in auditory-nerve fiber responses to bandlimited noise. J Acoust Soc Am. 1980;67:903–913. doi: 10.1121/1.383970. [DOI] [PubMed] [Google Scholar]
- Scharf B, Magnan J, Collet L, Ulmer E, Chays A. On the role of the olivocochlear bundle in hearing: a case study. Hear Res. 1994;75:11–26. doi: 10.1016/0378-5955(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Spoendlin HH. Innervation densities of the cochlea. Acta Otolaryng. 1972;73:235–248. doi: 10.3109/00016487209138937. [DOI] [PubMed] [Google Scholar]
- Suga N, Jen PH. Peripheral control of acoustic signals in the auditory system of echolocating bats. J Exp Biol. 1975;62:277–311. doi: 10.1242/jeb.62.2.277. [DOI] [PubMed] [Google Scholar]
- Szydlowska K, Tymianski M. Calcium, ischemia and excitotoxicity. Cell Calcium. 2010;47:122–129. doi: 10.1016/j.ceca.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Taberner AM, Liberman MC. Response properties of single auditory nerve fibers in the mouse. J Neurophysiol. 2005;93:557–569. doi: 10.1152/jn.00574.2004. [DOI] [PubMed] [Google Scholar]
- Takeno S, Harrison RV, Ibrahim D, Wake M, Mount RJ. Cochlear function after selective inner hair cell degeneration induced by carboplatin. Hear Res. 1994;75:93–102. doi: 10.1016/0378-5955(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Vacher SR, Guinan JJ, Jr., Kobler JB. Intracellularly labeled stapedius-motoneuron cell bodies in the cat are spatially organized according to their physiologic responses. J Comp Neurol. 1989;289:401–415. doi: 10.1002/cne.902890306. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hirose K, Liberman MC. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J Assoc Res Otolaryngol. 2002;3:248–268. doi: 10.1007/s101620020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr WB, Guinan JJ., Jr. Efferent innervation of the organ of Corti: two separate systems. Brain Res. 1979;173:152–155. doi: 10.1016/0006-8993(79)91104-1. [DOI] [PubMed] [Google Scholar]
- Wiederhold ML, Kiang NY. Effects of electric stimulation of the crossed olivocochlear bundle on single auditory-nerve fibers in the cat. J Acoust Soc Am. 1970;48:950–965. doi: 10.1121/1.1912234. [DOI] [PubMed] [Google Scholar]
- Winslow RL, Sachs MB. Effect of electrical stimulation of the crossed olivocochlear bundle on auditory nerve response to tones in noise. J Neurophysiol. 1987;57:1002–1021. doi: 10.1152/jn.1987.57.4.1002. [DOI] [PubMed] [Google Scholar]
- Xie DH, Henson OW., Jr. Tonic efferent-induced cochlear damping in roosting and echolocating mustached bats. Hearing research. 1998;124:60–68. doi: 10.1016/s0378-5955(98)00122-1. [DOI] [PubMed] [Google Scholar]