Abstract
Various pathological conditions are associated with changes in multiple protein biomarkers, and these changes can be assessed using xMAP™ beads and the Luminex™ platform. Although this platform is most commonly utilized in the analysis of biomarkers in serum, plasma, or urine, it is often desirable to measure these analytes in tissues (e.g., biopsy specimens). We have developed a simple, rapid method of tissue isolation in which excised tissues are permeabilized in RNAlater ™ at 4°C overnight, followed by Luminex™ analysis for inflammatory biomarkers (cytokines). This method was compared with flash-freezing in both mouse liver and human debrided wound specimens, and may be of utility in other clinical settings.
Keywords: Luminex™ cytokine, chemokine, biomarker, mouse, human
INTRODUCTION
The increasing focus on biomarkers of disease progression or therapeutic efficacy has led to the broad application of various proteomic techniques to clinical samples (1–3). One technique that has been utilized widely has been that of multiplexed, bead-based flow cytometry (i.e., Luminex, Luminex, Austin, TX) (4). This technology is now in routine experimental use for analysis of cytokines in various biofluids. However, there are many situations in which it would be desirable to assess cytokines or other biomarkers in excised tissues, especially in clinical studies (e.g., (5)) and perhaps at some point in a diagnostic fashion. One factor limiting the broad utility of this methodology is the ease of sample handling in the clinical environment, where snap-freezing or other procedures designed to minimize protein degradation may be difficult to accomplish owing to lack of equipment or personnel.
Herein, we describe a simple method for Luminex™- compatible, rapid, and convenient tissue preparation. The method relies on a commercially available tissue preparation medium (RNAlater, Ambion, Austin, TX), which is an aqueous, nontoxic tissue storage reagent that rapidly permeates tissues. The main use of this reagent is to stabilize and protect cellular RNA. Significantly, the recommended method for utilizing RNAlater™ involves initial storage for 24 hr at 4°C, followed by later freezing at −20 or −80°C (6). Thus, this storage medium would appear to be ideal for use in clinical studies or in settings where access to liquid nitrogen or other flash-freezing equipment is limited.
MATERIALS AND METHODS
Tissue sources
Mouse tissues were obtained as follows. Following approval of the proposed animal studies by the University of Pittsburgh Institutional Animal Care and Use Committee, C57Bl/6 mice were either untreated (n=4) or subjected to acute inflammation through the bolus injection of 3 mg/kg bacterial lipopolysaccharide (LPS) for 2 hr (n=4). Human tissues were obtained as follows. All studies were carried out following approval by the University of Pittsburgh Institutional Review Board. Discarded wound debridement tissues were obtained from foot wounds characterized by excessive scars (n=2) or exhibiting nonspecific granulation tissue (n=2).
Tissue preservation protocols
Mouse samples were preserved as follows. Immediately after cardiac puncture blood collection, mouse liver was collected by either flashfreezing in liquid nitrogen or placed in RNAlater™ solution for tissue preservation, as follows. From one mouse, a small portion of liver was quickly placed in a microcentrifuge tube containing 0.5–1ml of RNAlater™ solution. From the same mouse, another small portion of liver was placed in a labeled whirl pack bag and submerged in liquid nitrogen. The liquid nitrogen frozen liver sample was then removed from liquid nitrogen, and placed at −80°C for long-term storage. Owing to sample limitations, human debrided wound tissue was only preserved in RNAlater™ as follows. Tissues were ≤0.5 cm™ in order to allow the RNAlater™ solution to completely permeate into the tissue. Tissues were stored overnight at 4°C, then at −80°C for long-term storage.
Sample processing protocol
Approximately 50 mg of the tissue was transferred to a 2.0 ml microcentrifuge tube containing 1 ml of 1× BioSource tissue extraction reagent (San Diego, CA) (Catalog Number FNN0071) supplemented with 10 μl of 100mM phenylmethanesulfonyl fluoride in ethanol as a protease inhibitor. The tissue was homogenized for 15–30 sec until the sample was in a consistent solution. The sample was placed on ice, if processing multiple samples, and was then centrifuged at 4°C for 10 min at 10,000×g. After centrifugation, the supernatant was collected and placed in a new microcentrifuge tube, placed on ice, and assayed for protein content using the biocinchoninic acid (BCA) protein assay (Pierce, Rockford, IL) using the manufacturer’s protocol. Depending on tissue type, a 1:5 or 1:10 dilution may be necessary before addition of samples to the BCA assay.
Analysis of cytokines
Mouse cytokines were detected using a Luminex™100 IS apparatus using the BioSource International Mouse 20-plex® Luminex™ beadset. Human cytokines were detected using the Human 25-plex® Luminex™ beadset (BioSource).
Statistical analysis
Cytokine levels are presented as mean±SEM. Differences between the levels of a give cytokine measured in flash-frozen tissue vs. that cytokine assessed in RNAlater™-preserved tissue were assessed by Student’s t-test analysis using SigmaStat™ software (SPSS, Chicago, IL).
RESULTS
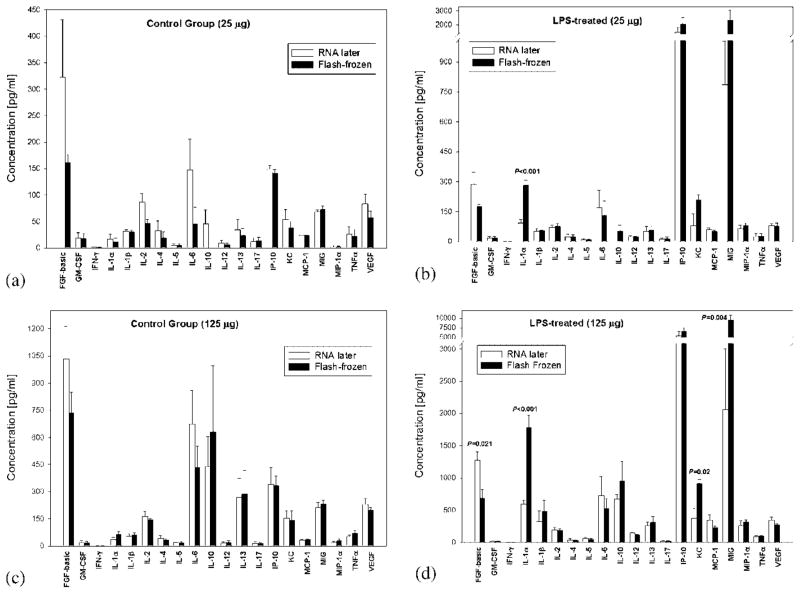
Our primary goals in this study were (1) to determine whether inflammatory cytokines and chemokines could be detected using Luminex™ technology in human biopsy tissues and (2) to determine whether tissue preservation in RNAlater™ could be comparable to snap-freezing as a Luminex™-compatible tissue preservation technique. We sought to address the latter point first. In order to do so, we reasoned that snapfreezing and RNAlater™ must be compared in a setting in which elevation of cytokines is known. The mouse liver is a primary site of inflammation in the setting of experimental endotoxemia and sepsis (7) and, therefore, we chose mouse liver as our benchmark tissue. Significantly, Luminex™ methodology has been used previously to assess cytokines in 25 μg of flash-frozen mouse liver [5]. Accordingly, C57Bl/6 mice were either untreated (n=4) or subjected to acute inflammation through the bolus injection of 3 mg/kg bacterial LPS for 2hr (n=4). Figure 1 shows the levels of inflammatory cytokines detected in mouse livers preserved either by snap-freezing or in RNAlater™ following the manufacturer’s protocol. Figure 1 shows the levels of cytokines in control livers (A) and LPS-treated livers (B), respectively, with 25 μg of total liver protein assayed. In some cases (e.g., basic fibroblast growth factor (FGF)), the experimental variability in cytokine/chemokine levels was high. However, only interleukin- 1α (IL-1α) differed in a statistically significant fashion when comparing the levels of cytokines from livers preserved using RNAlater™ vs. flash-freezing (92.4±17 vs. 281.8±26.7; P<0.001). If 125 μg of protein assayed, several cytokines and chemokines exhibited statistically significant differences between preservation methods when examining livers of endotoxemic mice (comparing RNAlater™ vs. flash-freezing, respectively), as seen in Figure 1D (basic FGF: 1,275.4±129.3 vs. 684.8±139; P<0.021; IL-1α: 593±58.5 vs. 1,777±191.5; P<0.001; keratinocytederived chemokine: 370.7±160.2 vs. 912±63.9; P<0.02; macrophage-inducible gene: 2,053±950.7 vs. 9,517±1,350; P<0.004). Interestingly, no statistically significant differences were observed between preservation methods in livers obtained from nonendotoxemic (control) mice (Fig. 1C). As these differences seen when assaying 125 μg of liver protein did not follow any specific trend (e.g., higher levels in flash-frozen samples), it is not clear if they were because of the isolation method or other assay conditions.
Fig. 1.
Luminex™ cytokine assays in mouse liver: Comparison of flash-freezing vs. RNAlater™. C57Bl/6 mice were either untreated (Panels A and C; n=4) or subjected to acute inflammation through the bolus injection of 3 mg/kg bacterial lipopolysaccharide (LPS) for 2 hr (Panels B and D; n=4). Following euthanasia, the livers were obtained and portions were preserved using either flash-freezing (black bars) or RNAlater™ (white bars). Either 25 (Panels A and B) or 125 (Panels C and D) μg of each sample was assayed for inflammatory cytokines and chemokines using the BioSource International Mouse 20-plex® Luminex™ beadset. Statistically significant differences between flash-freezing and RNAlater™ as a preservation method are depicted by asterisks and specific P values (all by Student’s t-test).
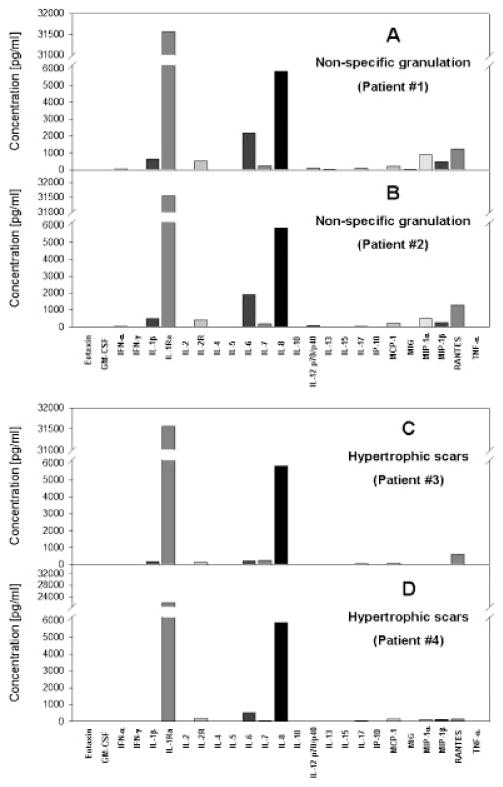
As mentioned above, the primary goal of our studies was to determine whether inflammatory cytokines and chemokines could be detected in human tissues using the RNAlater™ method. Wound healing is a process that involves inflammation, and this inflammatory response is aberrant in various syndromes characterized by aberrant skin healing (8,9). To date, no diagnostic assay based on routine analysis of inflammatory cytokines in debrided wound tissue has been described. Accordingly, we obtained discarded wound debridement tissues from foot wounds characterized by excessive scars (n=2) or exhibiting nonspecific granulation tissue (n=2; all studies were carried out following approval by the University of Pittsburgh Institutional Review Board). The debrided wound tissue was preserved in RNAlater ™ as in Figure 1, and 25 μg was assayed for cytokines using the Human 25-plex® Luminex™ beadset (BioSource). These studies suggest that debrided wound tissue is very high in IL-1 receptor antagonist as well as IL-8, with elevated IL-6 distinguishing between the granulation tissue and the hypertrophic scar (Fig. 2).
Fig. 2.
Luminex™ cytokine assays in human debrided wound tissue: Comparison of granulation tissue vs. hypertrophic scar. Discarded wound debridement tissues were obtained from patients exhibiting nonspecific granulation tissue (Panels A and B, each representing a single patient’s sample) or suffering from hypertrophic scars (Panels C and D, each representing a single patient’s sample). The samples were preserved immediately in RNAlater™, and 25 μg was assayed for cytokines using the Human 25-plex® Luminex™ beadset (BioSource).
DISCUSSION
These studies suggest that tissue preservation using RNAlater™ is a rapid, convenient, and Luminex™- compatible preservation method. To our knowledge, this is the first report of the analysis of human wound tissue cytokines ex vivo using the Luminex™ system. Nonetheless, the purpose of this study was not to establish a diagnostic assay for conditions involving aberrant wound healing, but rather to demonstrate the general utility of this simple method that could be used in future studies. These studies must be extended to other settings in diverse clinical tissue samples before this technique can be considered fully equivalent to flash-freezing in all respects.
A central, practical limitation when attempting to translate a research technique into potential diagnostic use relates to the ease of sample preservation. In today’s extremely complex clinical environment, it is often impractical to demand the routine snap-freezing of tissues in order to preserve tissues properly for analysis of labile proteins such as cytokines. Yet, cytokines are increasingly being examined as possible biomarkers of disease (7,10,11). The Luminex™ platform is rapidly emerging as the platform of choice for cytokine analysis because of its ability to measure many cytokines in a small volume of sample (4). Although the typical use of this technology is in the analysis of cytokines in biofluids such as serum, plasma, and urine, there is growing interest in the detection of cytokines in tissues using Luminex™. Thus, we sought to find and test a simple, Luminex™-compatible method of tissue preservation that would be suitable for a clinical environment.
We compared snap-freezing, the benchmark method for tissue preservation, with preservation in RNAlater™, as the latter has been suggested to help preserve proteins as well as RNA (6). To do so, we utilized a well-established experimental protocol for inducing inflammation (endotoxemia in mice) in order to minimize variability. We found that there was essentially no difference between snap-freezing and RNAlater™ when assaying 25 μg of liver tissue. One notable exception was IL-1α, which was higher in the snap-frozen tissues. This result may suggest that this cytokine is more labile than the others detected in the Luminex™ panel, though earlier studies on stability of cytokines in blood suggested that IL-1α is intermediate in stability as compared with other cytokines (e.g., IL-6) (12). We found that assaying a higher amount of whole tissue (125 μg) resulted in greater differences between flash-freezing and RNAlater™, though these differences did not show a consistent trend for higher recovery for one preservation method vs. another. We therefore suggest that any laboratory planning on utilizing this method should determine the recovery of its cytokine(s) of interest in tissues preserved in RNAlater™ vs. flashfreezing and also determine the maximal amount of protein that can be utilized in the assay.
Our primary goal was to demonstrate that cytokines could be detected using Luminex™ methodology in human tissues preserved using RNAlater™. We show for the first time that human debrided wound tissue expresses a variety of inflammatory cytokines, supporting numerous studies that implicate inflammation in the wound healing response (both normal and aberrant) (8,9). Substantial additional work, however, is necessary in order to demonstrate a diagnostic utility for these cytokine patterns in human wound healing. However, this study will hopefully reduce the effort necessary to do so in future studies.
In conclusion, we suggest that our method will be of utility in both clinical and research settings. However, further work in additional clinical tissue samples is necessary.
Acknowledgments
Grant sponsor: National Institutes of Health; Grant numbers: P50- GM-53789–09; R01-HL-76157–02; Grant sponsors: National Institute on Disability and Rehabilitation Research; Grant number: H133E070024; Grant sponsors: Commonwealth of Pennsylvania; Pittsburgh Lifesciences Greenhouse; Pittsburgh Tissue Engineering Initiative.
Abbreviations
- FGF
fibroblast growth factor
- IL
interleukin
- IP-10
interferon-γ-inducible protein, 10 kDa
- KC
keratinocyte-derived chemokine
- LPS
bacterial lipopolysaccharide
- MIG
macrophageinducible gene
References
- 1.Moseley FL, Bicknell KA, Marber MS, Brooks G. The use of proteomics to identify novel therapeutic targets for the treatment of disease. J Pharm Pharmacol. 2007;59:609–628. doi: 10.1211/jpp.59.5.0001. [DOI] [PubMed] [Google Scholar]
- 2.Lv LL, Liu BC. High-throughput antibody microarrays for quantitative proteomic analysis. Expert Rev Proteomics. 2007;4:505–513. doi: 10.1586/14789450.4.4.505. [DOI] [PubMed] [Google Scholar]
- 3.Cho WC. Proteomics technologies and challenges. Genomics proteomics Bioinformatics. 2007;5:77–85. doi: 10.1016/S1672-0229(07)60018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vignali DA. Multiplexed particle-based flow cytometric assays. J Immunol Methods. 2000;243:243–255. doi: 10.1016/s0022-1759(00)00238-6. [DOI] [PubMed] [Google Scholar]
- 5.Olle EW, Ren X, McClintock SD, et al. Matrix metalloproteinase- 9 is an important factor in hepatic regeneration after partial hepatectomy in mice. Hepatology. 2006;44:540–549. doi: 10.1002/hep.21314. [DOI] [PubMed] [Google Scholar]
- 6.RNAlater® Tissue Collection. RNA Stabilization Solution; Austin, TX: Ambion, Inc; 2007. [Google Scholar]
- 7.Jarrar D, Chaudry IH, Wang P. Organ dysfunction following hemorrhage and sepsis: Mechanisms and therapeutic approaches (review) Int J Mol Med. 1999;4:575–583. doi: 10.3892/ijmm.4.6.575. [DOI] [PubMed] [Google Scholar]
- 8.Hart J. Inflammation. 1: Its role in the healing of acute wounds. J Wound Care. 2002;11:205–209. doi: 10.12968/jowc.2002.11.6.26411. [DOI] [PubMed] [Google Scholar]
- 9.Hart J. Inflammation. 2: Its role in the healing of chronic wounds. J Wound Care. 2002;11:245–249. doi: 10.12968/jowc.2002.11.7.26416. [DOI] [PubMed] [Google Scholar]
- 10.Lauta VM. A review of the cytokine network in multiple myeloma: Diagnostic, prognostic, and therapeutic implications. Cancer. 2003;97:2440–2452. doi: 10.1002/cncr.11072. [DOI] [PubMed] [Google Scholar]
- 11.Blake GJ. Inflammatory biomarkers of the patient with myocardial insufficiency. Curr Opin Crit Care. 2003;9:369–374. doi: 10.1097/00075198-200310000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Thavasu PW, Longhurst S, Joel SP, Slevin ML, Balkwill FR. Measuring cytokine levels in blood. Importance of anticoagulants, processing, and storage conditions. J Immunol Methods. 1992;153:115–124. doi: 10.1016/0022-1759(92)90313-i. [DOI] [PubMed] [Google Scholar]