Abstract
Background
Serum γ-glutamyltranspeptidase (GGT), a marker of fatty liver disease (FLD), predicts mortality in young adults. However, the association between serum GGT and mortality in older adults is unclear.
Objectives
To examine if elevated serum GGT predicts all-cause, cardiovascular (CVD), and liver mortality in community-dwelling older adults.
Design and setting
A prospective cohort study including 2364 participants (mean-age 70 yr, BMI-24.5 kg/m2, 54% women) from the Rancho Bernardo Study who attended a research visit in 1984–87 when multiple metabolic covariates were ascertained including serum GGT. They were followed for a mean (± standard deviation) of 13.7 (±6.2) years.
Measurement
Multivariable-adjusted Cox-proportional hazards analyses were conducted to examine the association between elevated serum GGT (>51 U/L in men and > 33 U/L in women) and all-cause, CVD, and liver mortality.
Results
In these older men and women, cumulative mortality was 56.2% (n=1329) with CVD and liver mortality accounting for 49.4% and 2.3% of all deaths, respectively, over 32,387 person-years of follow-up. In multivariate analyses (adjusted for age, sex, alcohol use, body-mass-index, total cholesterol, HDL cholesterol, serum triglyceride, smoking status, systolic blood pressure, diabetes mellitus, serum interleukin-6, and c-reactive protein), serum GGT elevation was significantly associated with all-cause (HR, 1.55, 95% CI, 1.21–1.98), CVD (HR, 1.51, 95% CI, 1.04–2.17), and liver mortality (HR, 9.10, 95% CI, 3.42–24.26).
Conclusions
In community-dwelling older adults, serum GGT is an independent predictor of all-cause, CVD, and liver mortality.
Keywords: NAFLD, GGT, and death
Introduction
Serum gamma-glutamyltranspeptidase (GGT) is a blood test routinely used in clinical practice to evaluate individuals for fatty liver disease (1). It is elevated in both alcohol and obesity-associated fatty liver (1). Although serum GGT is predominantly secreted by the liver, it is present in all epithelial cells of the body where it plays an important role in glutathione metabolism. Cellular GGT is responsible for metabolizing extracellular reduced glutathione and replenishing intracellular glutathione by assimilation and reutilization of precursor amino acids (2). A rise in GGT is associated with a parallel increase in reactive oxygen species that induce cellular injury and lead to glutathione depletion especially in the presence of iron, which may potentiate the effect of free radical damage to cells (2, 3). Therefore, serum GGT is a marker of oxidative stress (4). This mechanism has been thought to be responsible for its association with atherosclerosis, and cardiovascular disease (CVD) (4, 5). It has been also shown that active GGT enzyme is present in atherosclerotic plaques and might contribute to its progression (6). We recently showed that serum GGT and metabolic syndrome traits have significant genetic covariance that may explain the genetic link between CVD and fatty liver disease (7)
Serum GGT concentrations are associated with prevalent and incident cardiovascular disease (CVD) (8, 9). Recent studies show that serum GGT predicts CVD mortality in young adults (8, 10–12). A 12 year follow-up study by Ruhl and Everhart using the 1988–94 NHANES dataset (mean age 44 years) demonstrated that elevated serum GGT (>51 U/L in men and > 33 U/L in women) was an independent predictor of all-cause and liver mortality, but not CVD mortality in multivariate analyses (13). However, age-adjusted analyses showed an association between log transformed serum GGT (continuous variable) and CVD mortality.
A nested case-control study derived from the Minnesota Heart Study showed that GGT was a robust predictor of CVD mortality only in younger adults, but not among participants who were aged 70 years or more (14). Ghouri and colleagues conducted a systematic review of published data on GGT and cardiovascular risk and reported that the strength of association between serum GGT and cardiovascular mortality was not significant in older adults (15). Therefore, it is unclear if serum GGT predicts all-cause, CVD, and liver mortality in older adults in a community-dwelling cohort.
We hypothesized that: 1) Elevated serum GGT is associated with an increased risk of CVD, liver, and all-cause mortality in older men and women; 2) The association between elevated serum GGT and mortality is independent of metabolic risk factors. In order to test our hypotheses, we conducted a prospective study to examine the association between elevated serum GGT levels and all-cause, CVD, and liver mortality in a well-characterized, community-dwelling, population-based cohort of older men and women residing within Southern California in the United States.
Methods
Ethics Statement
This study was approved by the UCSD IRB and conducted according to the principles expressed in the Declaration of Helsinki. A written informed consent was obtained from all participants.
Setting and Design
We conducted a prospective study in older community-dwelling participants of the Rancho Bernardo Study (RBS), who attended a clinical research examination between 1984 and 1987. RBS is a well-characterized cohort that was established in 1972 when 82% of the residents of Rancho Bernardo, a southern California town, were recruited. The details of the cohort, selection criteria, and purpose of the RBS have been published previously (16–18). Derivation of the study cohort: Of the 2466 participants, aged 30 years or more, who attended examination in the l984–87 clinic visit, we excluded 36 who did not have serum GGT data, and 66 who had missing data on alcohol use, weight and height, lipids, fasting plasma glucose, smoking, and/or the presence or absence of a history of diabetes, leaving 2364 participants for these analyses (19).
Exposure: Serum GGT
Based on results from fasting morning serum samples(collected in the period between 1984 and 1987), participants were classified into two GGT groups: elevated serum GGT and normal serum GGT at baseline visit. Elevated serum GGT was defined as >51 U/L in men and > 33 U/L in women. These categories were defined a priori and are consistent with the largest previously published study of a representative sample of the United States (13). Serum GGT was measured in the UCSD clinical laboratory using the colorimetric method during the research visit.
Follow-up
Rancho Bernardo participants have been followed annually for vital status with follow-up available until December 2005 (an average of 13.7 (± 6.2 [standard deviation]) years of follow-up). The study protocol was approved by the institutional review board of the University of California, San Diego and a written informed consent was obtained from all the participants.
Co-variates
Height and weight were measured by a trained investigator during the research visit and BMI was calculated using weight in kg and divided by square of height in meters (Kg/M2). Two morning blood pressure readings were measured in seated resting participants by a specially trained technician using a mercury sphygmomanometer, according to the Hypertension Detection and Follow-up Program protocol (20). Plasma glucose was measured using the glucose-oxidase method. Fasting plasma total cholesterol, high-density lipoprotein (HDL), and triglyceride levels were measured using enzymatic methods in a laboratory certified by the Centers for Disease Control (21). Participants with an average systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 or receiving medications for the treatment of hypertension were classified as hypertensive. Diabetes mellitus was defined as a fasting plasma glucose ≥ 7 mmol/L (126 mg/dl) or treatment with either insulin or an oral hypoglycemic medication. Alcohol use was ascertained by a questionnaire and has been previously validated by levels of HDL (22). Alcohol use was self-reported to a trained interviewer who asked about frequency, amount, and type of alcohol. Participants were asked questions regarding frequency, type, and quantity of alcoholic beverages consumed in the past week. For the present analysis, alcohol (beer, wine, or spirits) was quantified as the number of alcoholic drinks consumed in a day with one drink equivalent to 10 grams of alcohol. Participants who reported no alcohol use in the last year were classified as non-drinkers. Smoking was stratified into: current smokers, past smokers, and never smokers. Current smokers, or anyone who smoked 10 cigarettes or more in the last year, were classified as a smoker. At the same visit, fasting blood samples (serum) were frozen at −70°C. IL-6 was measured in previously unthawed serum using a high sensitivity (0.094 pg/ml) commercial Elisa (Quantikine HS, human IL-6 immunoassay; R&D Systems, Minneapolis, MN). CRP was measured using a high sensitivity clinical laboratory assay using a previously unthawed serum.
Outcomes
Outcomes included all-cause, CVD, and liver mortality until December 2005.
Assessment of outcome
Vital statistics are known for 96 percent of participants. Death certificates were obtained for 90 percent of decedents and classified for underlying cause of death by a certified nosologist using the International Classification of Diseases (ICD), Ninth Revision. CVD deaths included codes 390–459 and deaths from all encompassed codes 0–999. Liver deaths included underlying or associated cause of death by codes 70.2–70.9, 155, 275.0–275.1, and 571–573. This is consistent with prior studies because the number of deaths reported due to liver disease on death certificates were small (n=13) and using underlying cause of death alone may grossly underestimate liver mortality (13, 23). Mortality follow-up was performed until December 31, 2005.
Statistical Analysis
Baseline characteristics were compared by serum GGT status using a t-test for continuous variables and a chi-square test for categorical variables. Cox-proportional hazards regression analysis was conducted to examine the hazards ratio (an estimate of relative risk) of mortality (all-cause, CVD, and liver) associated with elevated serum GGT vs. normal GGT at baseline. The conditions of proportional hazards analysis were met and confirmed by relatively constant risk ratio through examination of −log (−log) plots of survival versus time.
Hierarchical adjustment was conducted and the following models were examined: age-sex adjusted, and multi-variable models adjusted for age-sex-alcohol-BMI-total cholesterol-HDL cholesterol-serum triglyceride-smoking-systolic blood pressure-diabetes mellitus, IL-6 and CRP. These co-variates were chosen because they have been shown to be associated with elevated GGT in previously published studies (7, 13, 24).
Additionally, we analyzed the effect of serum GGT as a continuous variable (GGT was log transformed to fulfill the conditions of normality for these analyses) and conducted Cox-proportional hazards regression analysis to examine the hazards ratio (an estimate of relative risk) of mortality (all-cause, CVD and liver) associated with 1 log increase in serum GGT.
To examine whether the GGT mortality associations existed within normal GGT range, participants were classified into quartiles of GGT (quartile 4 with highest GGT and quartile 1 with lowest GGT). Age-sex adjusted Cox-proportional hazard rates and 95% CI were calculated and p-value for trend was used to examine the association of GGT quartile (dose) on mortality.
A two-sided p-value < 0.05 was considered statistically significant. SAS version 9.1 (SAS Institute, Cary NC) was used for all analyses.
Results
Baseline data
The prevalence of elevated GGT was 6.7% (n=158) in this cohort. Median GGT was 9 IU/L with an interquartile range of 8 IU/L. Table 1. shows the baseline characteristics of the participants classified by elevated versus normal GGT. Individuals with elevated GGT were more likely to be obese, hypertensive with higher systolic blood pressure, dyslipidemic, and diabetic. Normal GGT levels were more common in non-smokers and non-drinkers of alcohol.
Table 1.
Baseline characteristics of participants based upon elevated versus normal gamma-glutamyltranspeptidase levels in the Rancho Bernardo Study cohort
| N | TOTAL SAMPLE | NORMAL GGT (N = 2206) | ELEVATE D GGT (N = 158) | P-VAL UE | |
|---|---|---|---|---|---|
| Age, years (SD) | 2364 | 69.7 (10.5) | 69.7 (10.6) | 68.6 (9.3) | .14 |
| Women, n (%) | 2364 | 1320 (55.8) | 1224 (55.5) | 96 (60.8) | .20 |
| Body mass index (kg/m2) | |||||
| Mean, SD | 2323 | 24.9 (3.7) | 24.9 (3.6) | 26.0 (4.4) | <.001 |
| 18–<25, n (%) | 1255 (54.0) | 1187 (54.8) | 68 (43.9) | <.002 | |
| 25–29, n (%) | 865 (37.2) | 802 (37.0) | 63 (40.6) | ||
| 30 +, n (%) | 203 (8.7) | 179 (8.2) | 24 (15.5) | ||
| Alcohol, n (%) | 2364 | ||||
| None | 869 (36.8) | 816 (37.0) | 53 (33.5) | <.02 | |
| <= median drinks/day | 739 (31.3) | 700 (31.7) | 39 (24.7) | ||
| > median drinks/day | 756 (32.0) | 690 (31.3) | 66 (41.8) | ||
| SBP (SD) | 2362 | 138.9 (21.9) | 138.6 (22.0) | 144.2 (19.9) | <.002 |
| Hypertension, n (%) | 2364 | 1741 (73.7) | 1602 (72.6) | 139 (88.0) | < .0001 |
| Lipid Levels (SD) | |||||
| Total cholesterol | 2364 | 219.9 (40.0) | 218.86 (39.4) | 234.4 (44.6) | < .0001 |
| HDL | 2364 | 61.7 (18.7) | 61.8 (18.5) | 61.5 (21.6) | .87 |
| Triglyceride, median (IQR) | 2364 | 100.0 (76.0) | 97.0 (74.0) | 132.0 (96.0) | < .0001 |
| Total/HDL ratio | 2364 | 3.9 (1.3) | 3.8 (1.3) | 4.1 (1.4) | <.004 |
| FPG gm/dl (SD) | 2364 | 100.5 (20.1) | 99.9 (18.6) | 109.0 (34.1) | <.001 |
| Diabetes, n (%) | 2364 | 339 (14.3) | 298 (13.5) | 41 (25.9) | < .0001 |
| Current smoker, n (%) | 2364 | 289 (12.2) | 268 (12.2) | 21 (13.3) | .67 |
| CRP, median (IQR) | 1800 | 1.7 (3.0) | 1.7 (2.8) | 3.0 (6.0) | < .0001 |
| IL-6, median (IQR) | 1847 | 2.3 (2.2) | 2.2 (2.1) | 3.0 (2.8) | .0001 |
For variables whose median and IQR are shown, p-value corresponds to Wilcoxon two-sample test, difference between all other continuous variables assessed by t-test, chi square used for categorical variables.
Abbreviations: SD; standard deviation, SBP; systolic blood pressure, HDL; high density lipoprotein, FPG; fasting plasma glucose, CRP; c-reactive protein, IL-6; interleukin-6.
Elevated serum GGT and mortality
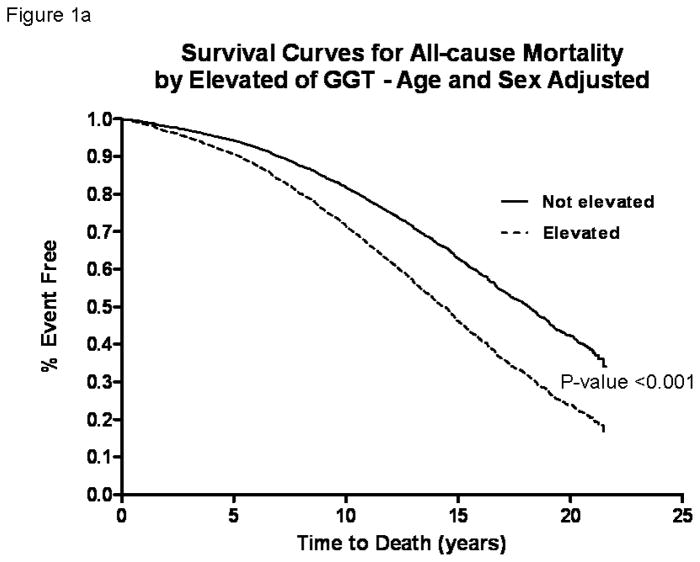
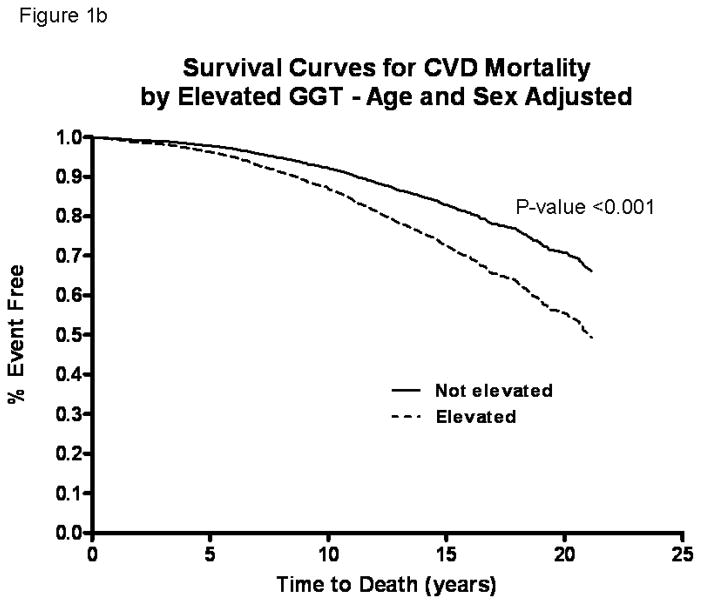
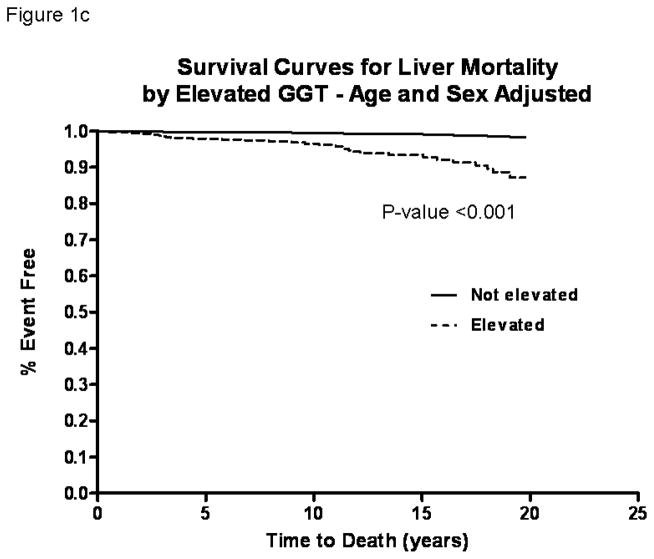
In these older adults (mean age 70 years at baseline, age range between 30 and 93), the death rate during the study period was 56.2% (n=1329). CVD deaths accounted for 49.4% (n=657) and liver deaths accounted for 2.3% (n=31) of all deaths over 32,387 person-years of follow-up. Age-sex-adjusted Cox-proportional hazard models showed that the risk of all-cause (figure 1a), CVD (figure 1b), and liver (figure 1c) mortality was significantly increased in participants with elevated serum GGT compared to those with normal GGT by HR 1.67 (95% CI, 1.35–2.06), 1.71 (95% CI, 1.26–2.32), and 9.05 (95% CI, 4.04–20.27) respectively (table 2).
Figure 1.
Survival curves comparing participants with elevated serum gamma-glutamyltranspeptidase (GGT) versus normal GGT at baseline who were followed for upto 21-years in the Rancho Bernardo Study for all-cause (a), cardiovascular (b) and liver (c) mortality
Table 2.
Hazard ratios for all-cause, cardiovascular and liver mortality comparing elevated (>51 U/L in men and >30 U/L in women) gamma-glutamyltranspeptidase (GGT) versus normal GGT
| N | NORMAL GGT | ELEVATED GGT HR (95% CI) | P VALUE | |
|---|---|---|---|---|
| All-cause Mortality | ||||
| Age-sex adjusted | 2364 | Referent | 1.67 (1.35–2.06) | <.0001 |
| Multivariate#-adjusted | 1790 | Referent | 1.55 (1.21–1.98) | .0005 |
| CVD Mortality | ||||
| Age-sex adjusted | 2364 | Referent | 1.71 (1.26–2.32) | .0006 |
| Multivariate-IL-6 adjusted | 1790 | Referent | 1.51 (1.04–2.17) | .0289 |
| Liver Disease Mortality | ||||
| Age-sex adjusted | 2364 | Referent | 9.05 (4.04–20.27) | <.0001 |
| Multivariate#-adjusted | 1790 | Referent | 9.10 (3.42–24.26) | <.0001 |
HR; hazards ratio, CI; confidence interval
Multivariate-model includes age-sex-alcohol-BMI-Total cholesterol-HDL cholesterol-serum triglyceride-smoking-systolic blood pressure-diabetes mellitus, IL-6 and CRP.
A two-tailed p-value of less than 0.05 was considered statistically significant
Results remained consistent even after multi-variable-adjustment by controlling for age, sex, alcohol, BMI, total cholesterol, HDL cholesterol, serum triglyceride, smoking, systolic blood pressure, diabetes mellitus, IL-6 and CRP. The multi-variable adjusted hazards of all-cause, CVD, and liver mortality were 1.55 (95% CI, 1.21–1.98), 1.51 (95% CI, 1.04–2.17), and 9.10 (95% CI, 3.42–24.26) respectively (table 2)
Serum GGT (log transformed) and mortality
When serum GGT (log transformed) was analyzed as a continuous variable in age-sex-adjusted analyses, the hazards of all-cause, CVD, and liver mortality with one standard deviation increase in log GGT were 1.28 (95% CI, 1.18–1.40), 1.34 (95% CI, 1.19–1.51), and 2.41 (95% CI, 1.61–3.59), respectively (table 3). Findings remained consistent and statistically significant in multi-variable analyses, with hazards of all-cause, CVD, and liver mortality of 1.21 (95% CI, 1.09–1.34), 1.28 (95% CI, 1.10–1.49), and 2.52 (95% CI, 1.52–4.17) respectively (table 3). On multivariate analysis, age at recruitment (p<0.0001), male gender (p<0.0001), BMI (0.02), systolic blood pressure (p<0.0001), and current smoking (p<0.0001) were also independent predictors of overall mortality.
Table 3.
The hazards associated with one log increment in serum gamma-glutamyltranspeptidase and all-cause, cardiovascular and liver-related mortality in older adults
| N | LN GGT HR (95% CI) | P VALUE | |
|---|---|---|---|
| All-cause Mortality | |||
| Age-sex adjusted | 2364 | 1.28 (1.18–1.40) | <.0001 |
| Multivariate#-adjusted | 1790 | 1.21 (1.09–1.34) | .0004 |
| CVD Mortality | |||
| Age-sex adjusted | 2364 | 1.34 (1.19–1.51) | <.0001 |
| Multivariate-IL-6 adjusted | 1790 | 1.28 (1.10–1.49) | .0017 |
| Liver Disease Mortality | |||
| Age-sex adjusted | 2364 | 2.41 (1.61–3.59) | <.0001 |
| Multivariate#-adjusted | 1790 | 2.52 (1.52–4.17) | .0003 |
LN; natural log transformed, HR; hazards ratio, CI; confidence interval
Multivariate-model includes age-sex-alcohol-BMI-Total cholesterol-HDL cholesterol-serum triglyceride-smoking-systolic blood pressure-diabetes mellitus, IL-6 and CRP.
A two-tailed p-value of less than 0.05 was considered statistically significant
Assessment of interaction between serum GGT and age
In order to examine whether there was any interaction between serum GGT and age in predicting all-cause, CVD and liver mortality we performed Wald test for interaction. The Wald test for interaction was not significant for an interaction between serum GGT and age for any of the outcomes including all-cause (p-value=0.17), CVD (p-value = 0.37) and liver mortality (p-value = 0.39).
Serum GGT quartiles and mortality
We also examined the association between serum GGT within normal range (divided into quartiles 1 to 4; quartile 4 with highest GGT, and quartile 1 with lowest GGT as referent group) and all-cause, CVD, and liver mortality. Age-sex adjusted Cox-proportional hazard models showed that the risk of all-cause mortality in quartile 4 (serum GGT: >15 U/L), quartile 3 (serum GGT: 10–14U/L), and quartile 2 (serum GGT: 7–9U/L) as compared to quartile 1 (serum GGT: 6 or less) were 1.43 (95% CI, 1.22–1.67), 1.22 (95% CI, 1.05–1.42), and 0.99 (95% CI, 0.85–1.15) respectively (p-value for trend <0.0001). Results remained consistent for CVD mortality. The hazards of CVD mortality in quartiles 4, 3, and 2 as compared to quartile 1 were 1.46 (95% CI, 1.17–1.83), 1.27 (95% CI, 1.02–1.58), and 0.98 (95% CI, 0.79–1.21) respectively (p-value for trend <0.0001). Hazard rate of liver mortality did not change significantly. The hazards of liver mortality in quartiles 4, 3, and 2 as compared to quartile 1 were 0.33 (95% CI, 0.09–1.24), 0.64 (95% CI, 0.21–1.98), and 1.91 (95% CI, 0.77–4.72) respectively (p-value for trend <0.096).
Discussion
Main findings
The main findings of this study include: 1) Elevated serum GGT is an independent and robust predictor of all-cause, CVD, and liver mortality in older adults in this Southern California community-dwelling cohort. 2) Serum GGT, even within normal range, is predictive of CVD and all-cause mortality and may have a role as a prognostic marker.
Relevance with previously published literature
Several groups have shown that serum GGT level is related to surrogate markers of coronary atherosclerosis, like arterial stiffness and coronary artery calcium (25, 26) and cross-sectionally and longitudinally predicts CVD independent of alcohol intake (9, 27). There is emerging data on the association between serum GGT and CVD mortality from large population-based studies with the majority of studies favoring the association between serum GGT and all-cause and CVD mortality in both diabetic and non-diabetic population (10–12, 28–31). Moreover, GGT was found to be independently associated with the complexity of coronary lesions in patients with preexisting coronary artery disease (32). Ruttmann and colleagues reported that serum GGT is an independent predictor of CVD mortality over 17 years of follow-up in the Vorarlberg Health Monitoring and Promotion Program Study (mean age, 42 years) conducted in Austrian men and women (10). Furthermore, Strasak and colleagues showed that a longitudinal increase in GGT predicts CVD mortality in this Austrian population-based cohort (11). Lee et al reported that serum GGT predicts CVD and all-cause mortality in 3451 Framingham study participants (mean age 44) over 19 years of follow-up (12). Wannamethee et al. conducted a study in 6997 British men aged 40–59 years with no known heart disease or diabetes drawn from general practices of 24 towns and reported that serum GGT was an independent predictor of CVD mortality (33). Ruhl and Everhart analyzed the NHANES (1988–94) data to examine the longitudinal association between elevated serum GGT (>51 U/L in men and > 33 U/L in women) and all-cause mortality over a 12 year follow-up (until December, 2000) in US (mean age 44 years) adults (13). In this study, the association between log transformed serum GGT and CVD mortality was significant in age-adjusted analyses but did not remain statistically significant after adjustment for potential confounding variables in multi-variable analyses (13). They reported that elevated serum GGT predicts all-cause and liver mortality, but not CVD mortality in multivariable analyses (13). In summary, the studies referred to in this paper strongly suggest that serum GGT is predictive of incident CVD and all-cause mortality in young and middle-aged adults (5). However, these studies provide unclear evidence whether serum GGT in older adults increases all-cause and CVD mortality (13–15).
Association of age with serum GGT and mortality
Lee and colleagues conducted a nested case-control study derived from the Minnesota Heart Survey with 5–12 years of follow-up and reported that serum GGT was a robust predictor of mortality due to CVD in younger individuals, but did not predict mortality in individuals aged 70 years or more (14). In addition, it was unclear whether serum GGT (within normal range and/or elevated serum GGT) predicts mortality in older adults in a community-dwelling cohort in the United States. This study provides new data that serum GGT both within and above the normal range predicts all-cause and liver mortality upto 21 year follow-up in older adults (median age of 72 years) residing in a suburban community in the United States.
Mechanism
Previous studies have suggested that individuals with fatty liver have increased CVD mortality (34–37). We have recently shown that GGT shares genetic co-determination with NAFLD risk factors such as insulin resistance, uric acid, dyslipidemia, and hypertension (7). Kozakova et al recently demonstrated that elevated GGT, as a component of the fatty liver index, is an independent determinant of early atherosclerotic plaques (38). Haring and colleagues showed that presence of hepatic steatosis is predictive of increased mortality associated with serum GGT (39). It is plausible that serum GGT is an intermediate phenotype that is linked to metabolic syndrome traits resulting in excess mortality via several inter-linked metabolic pathways (5, 12 ). However, we speculate that there are hitherto unknown underlying mechanisms that may be responsible for the association between fatty liver (elevated serum GGT) and mortality. These mechanisms may be independent of co-variates that were adjusted for in the multi-variate analyses in this study and beyond the scope of this epidemiologic study (40). We believe that serum GGT is an intermediate phenotype rather than causal in its association with mortality. However, it may have value as a prognostic marker of occult fatty liver and long term risk of mortality (5).
Limitations and strengths of the study
We acknowledge several limitations to this study. The RBS cohort is mainly Caucasian and therefore these findings may not be generalizable to other bio-geographic ethnic groups. However, the homogeneity of our population is also a strength of our study that improves the internal validity of our findings. The results are likely generalizable to other older white men and women across the Western World. Individuals with other forms of chronic liver disease were not identified due to lack of availability of anti-HCV and HBsAg. Based upon NHANES data, the prevalence of anti-HCV and HBsAg seropositivity in suburban White men and women in this age group is < 0.5% (41). Therefore, it is unlikely to bias the results of our study. Additionally, misclassification of these individuals in the highest category would bias the results towards the null. Liver biopsies could not be performed to evaluate for liver disease in this population-based study because it is not feasible. Other limitations common to our study and prior studies on this topic include: 1) single baseline measure of exposure; 2) alcohol use ascertained by self-report. However, alcohol use has not been associated with increased mortality in the Rancho Bernardo Study. Therefore, we believe that association between serum GGT and mortality is independent of alcohol use, as seen in other studies (12). A major strength of this study is that the median age of participants at baseline was 72 years. This is considerably higher than previously published studies. Therefore, these results are generalizable to the older White suburban US population, and may have strong public health significance. Our findings suggest that serum GGT is associated with excess mortality independent of metabolic syndrome risk factors in older adults.
Conclusions
We conducted a prospective cohort study in a community-dwelling cohort of older men and women who were followed for up to 21 years (with a mean of 13.7 years of follow-up) and found that serum GGT is an independent predictor, after adjusting for age, sex, alcohol, BMI, total cholesterol, HDL cholesterol, serum triglyceride, smoking, systolic blood pressure, diabetes mellitus, IL-6 and CRP, of all-cause, CVD, and liver mortality. The association between serum GGT and increased mortality is independent of metabolic syndrome traits and alcohol use. We believe that GGT is not a cause of increased mortality. More likely, it is an intermediate phenotype or a marker for other processes for which better understanding is needed. Future studies are needed to find the shared genetic and environmental mechanistic links between serum GGT, metabolic syndrome traits, and excess mortality.
Acknowledgments
The authors would like to thank Dr. James E. Everhart (NIDDK/NIH) for his critical review of the manuscript and insightful comments.
Funding Support: This work is supported in part by the American Gastroenterological Association (AGA) Foundation – Sucampo – ASP Designated Research Award in Geriatric Gastroenterology and by a T. Franklin Williams Scholarship Award; Funding provided by: Atlantic Philanthropies, Inc, the John A. Hartford Foundation, the Association of Specialty Professors, and the American Gastroenterological Association and K23 DK090303 to Rohit Loomba, MD, MHSc. This research was funded in part with the support of the UCSD Digestive Diseases Research Development Center, U.S. PHS grant #DK080506. This work was supported by the National Institutes of Health/National Institute on Aging grants AG07181 and AG028507, and the National Institute of Diabetes and Digestive and Kidney Diseases, grant DK31801.
Role of Funding Agencies: Funding agencies did not have any role in the design and conduct of the study, collection, management, analysis or interpretation of the data; preparation, review, or approval of the manuscript.
Abbreviations
- GGT
γ-glutamyltransferase
- BMI
body mass index
- NAFLD
nonalcoholic fatty liver disease
Footnotes
Author contribution:
Study concept and design: Rohit Loomba
Acquisition of data: Elizabeth Barrett-Connor and Ricki Bettencourt
Analysis and interpretation of data: Rohit Loomba, Ricki Bettencourt, David Brenner, and Elizabeth Barrett-Connor
Drafting of the manuscript: Rohit Loomba and Elizabeth Barrett-Connor
Critical revision of the manuscript for important intellectual content: Rohit Loomba, Elizabeth Barrett-Connor, Benjamin Cohen, Iliana Doycheva, Christina Wassel and David Brenner
Statistical analysis: Ricki Bettencourt Obtained funding: Elizabeth Barrett-Connor and Rohit Loomba
Technical or material support and study supervision: Elizabeth Barrett-Connor
There is no conflict of interest.
Conflict of interest: No conflicts of interest exist
References
- 1.Whitfield JB. Gamma glutamyltransferase. Crit Rev Clin Lab Sci. 2001;38:263–355. doi: 10.1080/20014091084227. [DOI] [PubMed] [Google Scholar]
- 2.Glass GA, Stark AA. Promotion of glutathione-gamma-glutamyltranspeptidase-dependent lipid peroxidation by copper and ceruloplasmin: the requirement for iron and the effects of antioxidants and antioxidant enzymes. Environ Mol Mutagen. 1997;29:73–80. doi: 10.1002/(sici)1098-2280(1997)29:1<73::aid-em10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 3.Ravuri C, Svineng G, Pankiv S, Huseby NE. Endogenous production of reactive oxygen species by the NADPH oxidase complexes is a determinant of γ-glutamyltransferase expression. Free Radic Res. 2011;45:600–10. doi: 10.3109/10715762.2011.564164. [DOI] [PubMed] [Google Scholar]
- 4.Mason JE, Starke RD, Van Kirk JE. Gamma-glutamyltransferase: a novel cardiovascular risk biomarker. Prev Cardiol. 2010;13:36–41. doi: 10.1111/j.1751-7141.2009.00054.x. [DOI] [PubMed] [Google Scholar]
- 5.Grundy SM. Gamma-glutamyltransferase: another biomarker for metabolic syndrome and cardiovascular risk. Arterioscler Thromb Vasc Biol. 2007;27:4–7. doi: 10.1161/01.ATV.0000253905.13219.4b. [DOI] [PubMed] [Google Scholar]
- 6.Franzini M, Corti A, Martinelli B, Del Corso A, Emdin M, Parenti GF, et al. Gamma-glutamyltransferase activity in human atherosclerotic plaques- biochemical similarities with circulating enzyme. Atherosclerosis. 2009;202:119–27. doi: 10.1016/j.atherosclerosis.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 7.Loomba R, Rao F, Zhang L, Khandrika S, Ziegler MG, Brenner DA, O’Connor DT. Genetic covariance between gamma-glutamyltranspeptidase and fatty liver risk factors: role of beta2-adrenergic receptor genetic variation in twins. Gastroenterology. 2010;139:836–845. 845, e831. doi: 10.1053/j.gastro.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee DH, Silventoinen K, Hu G, Jacobs DR, Jr, Jousilahti P, Sundvall J, Tuomilehto J. Serum gamma-glutamyltransferase predicts non-fatal myocardial infarction and fatal coronary heart disease among 28,838 middle-aged men and women. Eur Heart J. 2006;27:2170–6. doi: 10.1093/eurheartj/ehl086. [DOI] [PubMed] [Google Scholar]
- 9.Fraser A, Harris R, Sattar N, Ebrahim S, Smith GD, Lawlor DA. Gamma-glutamyltransferase is associated with incident vascular events independently of alcohol intake: analysis of the British Women’s Heart and Health Study and Meta-Analysis. Arterioscler Thromb Vasc Biol. 2007;27:2729–2735. doi: 10.1161/ATVBAHA.107.152298. [DOI] [PubMed] [Google Scholar]
- 10.Ruttmann E, Brant LJ, Concin H, Diem G, Rapp K, Ulmer H. Gamma-glutamyltransferase as a risk factor for cardiovascular disease mortality: an epidemiological investigation in a cohort of 163,944 Austrian adults. Circulation. 2005;112:2130–2137. doi: 10.1161/CIRCULATIONAHA.105.552547. [DOI] [PubMed] [Google Scholar]
- 11.Strasak AM, Kelleher CC, Klenk J, Brant LJ, Ruttmann E, Rapp K, Concin H, et al. Longitudinal change in serum gamma-glutamyltransferase and cardiovascular disease mortality: a prospective population-based study in 76,113 Austrian adults. Arterioscler Thromb Vasc Biol. 2008;28:1857–1865. doi: 10.1161/ATVBAHA.108.170597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DS, Evans JC, Robins SJ, Wilson PW, Albano I, Fox CS, Wang TJ, et al. Gamma glutamyltransferase and metabolic syndrome, cardiovascular disease, and mortality risk: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2007;27:127–133. doi: 10.1161/01.ATV.0000251993.20372.40. [DOI] [PubMed] [Google Scholar]
- 13.Ruhl CE, Everhart JE. Elevated serum alanine aminotransferase and gamma-glutamyltransferase and mortality in the United States population. Gastroenterology. 2009;136:477–485. e411. doi: 10.1053/j.gastro.2008.10.052. [DOI] [PubMed] [Google Scholar]
- 14.Lee DH, Buijsse B, Steffen L, Holtzman J, Luepker R, Jacobs DR., Jr Association between serum gamma-glutamyltransferase and cardiovascular mortality varies by age: the Minnesota Heart Survey. Eur J Cardiovasc Prev Rehabil. 2009;16:16–20. doi: 10.1097/HJR.0b013e32830aba5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghouri N, Preiss D, Sattar N. Liver enzymes, nonalcoholic fatty liver disease, and incident cardiovascular disease: A narrative review and clinical perspective of prospective data. Hepatology. 2010;52:1156–1161. doi: 10.1002/hep.23789. [DOI] [PubMed] [Google Scholar]
- 16.Barrett-Connor E, Khaw K. Family history of heart attack as an independent predictor of death due to cardiovascular disease. Circulation. 1984;69:1065–1069. doi: 10.1161/01.cir.69.6.1065. [DOI] [PubMed] [Google Scholar]
- 17.Barrett-Connor EL, Cohn BA, Wingard DL, Edelstein SL. Why is diabetes a stronger risc factor for fatal ischemic heart diasease in women than in men? The Rancho Bernardo Study. JAMA. 1991;265:627–31. [PubMed] [Google Scholar]
- 18.Barrett-Connor E, Wingard DL, Criqui MH. Postmenopausal estrogen use and heart disease risk factors in the 1980s. Rancho Bernardo, Calif, revisited. JAMA. 1989;261:2095–2100. [PubMed] [Google Scholar]
- 19.Loomba R, Bettencourt R, Barrett-Connor E. Synergistic association between alcohol intake and body mass index with serum alanine and aspartate aminotransferase levels in older adults: the Rancho Bernardo Study. Aliment Pharmacol Ther. 2009;30:1137–1149. doi: 10.1111/j.1365-2036.2009.04141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The hypertension detection and follow-up program: Hypertension detection and follow-up program cooperative group. Prev Med. 1976;5:207–215. doi: 10.1016/0091-7435(76)90039-6. [DOI] [PubMed] [Google Scholar]
- 21.Lipid and Lipoprotein Analysis. 2. Vol. 1. US Government Printing Office, US Dept of Health, Education, and Welfare; Washington, DC: 1974. Lipid Research Clinics Program Manual of Laboratory Operations. NIH publication No. 75–628. [Google Scholar]
- 22.Barrett-Connor E, Suarez L. A community study of alcohol and other factors associated with the distribution of high density lipoprotein cholesterol in older vs.younger men. Am J Epidemiol. 1982;115:888–893. doi: 10.1093/oxfordjournals.aje.a113376. [DOI] [PubMed] [Google Scholar]
- 23.Everhart JE, Ruhl CE. Burden of Digestive Diseases in the United States Part III: Liver, Biliary Tract, and Pancreas. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 24.Lim JS, Kim YJ, Chun BY, Yang JH, Lee DH, Kam S. The association between serum GGT level within normal range and risk factors of cardiovascular diseases. J Prev Med Public Health. 2005;38:101–106. [PubMed] [Google Scholar]
- 25.Park JS, Kang SA, Yoo JS, Ahn CW, Cha BS, Kim KR, Lee HC. Association between γ-glutamyltransferase, adiponectin and arterial stiffness. J Atheroscler Thromb. 2012;19:90–7. doi: 10.5551/jat.9779. [DOI] [PubMed] [Google Scholar]
- 26.Atar AI, Yilmaz OC, Akin K, Selcoki Y, Er O, Eryonucu B. Association between gamma-glutamyltrasferase and coronary artery calcification. Int J Cardiol. 2012 doi: 10.1016/j.ijcard.2012.03.157. Ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Emdin M, Passino C, Michelassi C, Donato L, Pompella A, Paolicchi A. Additive prognostic value of gamma-glutamyltransferase in coronary artery disease. Int J Cardiol. 2008 doi: 10.1016/j.ijcard.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 28.Kazemi-Shirazi L, Endler G, Winkler S, Schickbauer T, Wagner O, Marsik C. Gamma glutamyltransferase and long-term survival: is it just the liver? Clin Chem. 2007;53:940–6. doi: 10.1373/clinchem.2006.081620. [DOI] [PubMed] [Google Scholar]
- 29.Monami M, Balzi D, Lamanna C, Melani C, Cocca C, Lotti E, Fedeli A, Masotti G, et al. Prognostic value of serum liver enzymes levels with type 2 diabetic patients. Diabetes Metab Res Rev. 2007;23:625–30. doi: 10.1002/dmrr.744. [DOI] [PubMed] [Google Scholar]
- 30.Kengne AP, Czernichow S, Stamatakis E, Hamer M, Batty GD. Gamma-glutamyltransferase and risk of cardiovascular disease mortality in people with and without diabetes: Pooling of three British Health Surveys. J Hepatol. 2012 doi: 10.1016/j.jhep.2012.06.034. Ahead of print. [DOI] [PubMed] [Google Scholar]
- 31.Sluik D, Beulens JW, Weikert C, van Dieren S, Spijkerman AM, van der ADL, Fritsche A, Joost HG, et al. Gamma-glutamyltrasferase, cardiovascular disease and mortality in individuals with diabetes mellitus. Diabetes Metab Res Rev. 2012;28:284–8. doi: 10.1002/dmrr.2261. [DOI] [PubMed] [Google Scholar]
- 32.Aksakal E, Tanboga IH, Kurt M, Kaygin MA, Kaya A, Isik T, Ekinci M, Sevimli S, Acikel M. The relation of serum gamma-glutamyltransferase levels with coronary lesion complexity and long-term outcome in patients with stable coronary artery disease. Atherosclerosis. 2012;221:596–601. doi: 10.1016/j.atherosclerosis.2012.01.044. [DOI] [PubMed] [Google Scholar]
- 33.Wannamethee SG, Lennon L, Shaper AG. The value of gamma-glutamyltransferase in cardiovascular risk prediction in men without diagnosed cardiovascular disease or diabetes. Atherosclerosis. 2008;201:168–75. doi: 10.1016/j.atherosclerosis.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 34.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 35.Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, Younossi ZM. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7:234–238. doi: 10.1016/j.cgh.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–73. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 37.Söderberg C, Stal P, Askling J, Glaumann H, Lindberg G, Marmur J, Hultcrantz R. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595–602. doi: 10.1002/hep.23314. [DOI] [PubMed] [Google Scholar]
- 38.Kozakova M, Palombo C, Eng MP, Dekker J, Flyvbjerg A, Mitrakou A, Gastaldelli A, Ferrannini E RISC Investigators. Fatty liver index, gamma-glutamyltransferase, and early carotid plaques. Hepatology. 2012;55:1406–15. doi: 10.1002/hep.25555. [DOI] [PubMed] [Google Scholar]
- 39.Haring R, Wallaschofski H, Nauck M, Dorr M, Baumeister SE, Volzke H. Ultrasonographic hepatic steatosis increases prediction of mortality risk from elevated serum gamma-glutamyltranspeptidase levels. Hepatology. 2009;50:1403–1411. doi: 10.1002/hep.23135. [DOI] [PubMed] [Google Scholar]
- 40.Emdin M, Pompella A, Paolicchi A. Gamma-glutamyltransferase, atherosclerosis, and cardiovascular disease: triggering oxidative stress within the plaque. Circulation. 2005;112:2078–2080. doi: 10.1161/CIRCULATIONAHA.105.571919. [DOI] [PubMed] [Google Scholar]
- 41.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]