Itch sensation is transmitted from the skin to the spinal cord by small diameter, unmyelinated Cfibers and thinly myelinated A-δ fibers (Ringkamp et al., 2011; Schmelz, 2010). In human skin, C-fibers mediating pruritus are either mechanically-insensitive, histamine-sensitive nerves or mechanicallysensitive, polymodal nociceptors which are unresponsive or only weakly responsive to histamine (Ringkamp et al., 2011; Schmelz, 2010). Additionally, data combined from human and non-human primate experiments, identified mechanically-sensitive Aδ fibers that contribute to histamine-induced and non-histamine, cowhage spicule-induced itch (Ringkamp et al., 2011). Histologic markers for itchsensing nerves in human skin include the capsaicin receptor, transient receptor potential vanilloid 1 (TRPV1) (Ringkamp et al., 2011; Schmelz, 2010), as well as the vasoactive peptides calcitonin gene related peptide (CGRP) or substance P (SP) (Davidson and Giesler, 2010). However, these histologic markers alone cannot differentiate A-δ and C fibers that perceive itch from those that sense pain. In rodent models, gastrin releasing peptide receptor (GRPR), expressed in the dorsal horn lamina I, mediates a CNS itch-specific pathway (Sun and Chen, 2007; Sun et al., 2009). In mice, gastrin releasing peptide (GRP), the ligand for GRPR, is expressed in both the skin and the dorsal root ganglion by peptidergic (ie, containing CGRP and SP) nerves that express TRPV1 (Fleming et al., 2012; Lagerstrom et al., 2010; Liu et al., 2009; Sun and Chen, 2007; Tominaga et al., 2009). Although GRP is not likely to be the principal excitatory neurotransmitter activating GRPR-expressing spinal cord dorsal horn neurons, GRP-expressing neurons still may mediate itch-specific signals from the skin to the spinal cord in rodents (Akiyama et al., 2012; Lagerstrom et al., 2010). Accordingly, MrgprA3 expression defines a subset of mouse primary cutaneous sensory neurons that mediate itch, and 93% of MrgprA3-positive neurons co-expressed GRP (Han et al., 2012; Liu et al., 2009). Given the putative role of GRP-expressing nerves in mediating itch in these animal models, we sought to determine whether non-diseased human skin contains GRP-expressing nerves that show histologic features of primary afferent pruriceptors.
Disease-free human skin from 17 patients (9 females, mean age 66.9 y.o. +/- 14.7; 8 males 61.2 y.o. +/- 9; Table S1), was used for fluorescence immunohistochemistry on 80 μm thick sections (see supplemental methods). GRP(+) nerves (confirmed by co-staining with PGP9.5) were identified in all patients and from all sites (Tables 1, S1). GRP(+) nerves were primarily located in the papillary dermis as free nerve endings terminating at the dermoepidermal junction (DEJ) (Figure 1), and in this anatomic skin compartment, comprised between ~5 and 15% of all PGP9.5(+) staining (Table S1). GRP(+) nerves were not identified within the epidermis of human skin but were identified tightly associated with some appendageal structures, including hair follicles and eccrine glands (Figure S1). Determining regional variation in GRP nerve density has relevance to future studies comparing diseased to non-diseased skin. After categorizing the samples into three anatomic locations (scalp/head/neck, trunk, and extremities), we found only modest site differences in the % of PGP9.5 nerve length that is GRP(+) at the DEJ (Table 1), indicating near uniform anatomic distribution of GRP(+) nerves in human papillary dermis.
Table 1.
| Human Papillary Dermal Nerve Staining | Head/Neck/Scalp | Trunk | Extremities |
|---|---|---|---|
| % of PGP9.5(+) nerves also GRP(+)1 | 7.7, 3.8 – 12.12 | 7.8, 4.3 – 10.9 | 9.8, 7.6 – 16.62 |
| % of PGP9.5(+) nerves also CGRP(+)1 | 8.4, 4.6 – 17.33 | 20.8, 13.1 – 30.43a | 17.9, 10.3 – 30.33b |
| % of GRP(+) nerves also CGRP(+)1 | 36.3, 6.9 – 70.24 | 81.9, 42.6 – 97.34a | 67.2, 35.8 – 86.64b |
| % of CGRP(+) nerves also GRP(+)1 | 20.4, 5.9 – 44.5 | 36.6, 22.5 – 40.1 | 35.7, 21.3 – 63.2 |
| % of PGP9.5(+) nerves also SubP(+)1 | 8.8, 4.2 – 14.7 | 5.1, 2.2 – 10.5 | 8.1, 7.2 – 15.2 |
| % of GRP(+) nerves also SubP(+)1 | 82.6, 37.3 – 100 | 57.7, 0.0 – 100 | 100, 74.2 – 100 |
| % of SubP(+) nerves also GRP(+)1 | 69.3, 46.6 – 100 | 42.5, 0.0 – 89.2 | 100, 88.3 – 100 |
| % GRP(+) nerves that also C-fibers1 | 48.1, 20.1 – 73.6 | 27.5, 11.3 – 48.4 | 12.9, 0 – 47.8 |
| % of GRP(+) nerves also Aδ-fibers1 | 51.9, 26.4 – 79.9 | 72.5, 51.6 – 88.9 | 87.1, 52.2 - 100 |
| % of GRP(+) C-fiber nerves also SP(+)1 | 74.8, 40.6 - 100 | 52, 1 - 100 | 100, 17.3 - 100 |
| % of GRP(+) Aδ-fibers nerves also SP(+)1 | 100, 59.3- 100 | 100, 95.2 - 100 | 100, 100 - 100 |
median, 25% - 75% quartiles.
p=0.0184, alpha 0.05
p=0.005 ; p=0.0037
p=0.0058, p=0.0294
Figure 1.
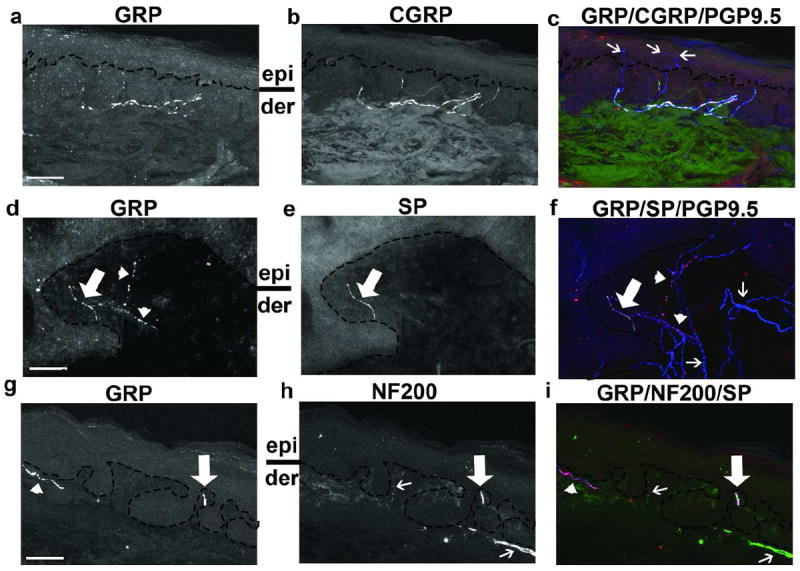
Characterization of GRP-expressing nerves in the DEJ/papillary dermis in human skin. Immunohistochemical triple-staining showing (a,d,g) GRP, (b) CGRP, and (c) overlay of GRP (red), CGRP (green), and PGP9.5 (blue), (e) SP, (f) overlay of GRP (red), SP (green), and PGP9.5 (blue), (h) NF200, and (i) overlay of GRP (red), NF200 (green), and SP (blue). (c), white pseudocolor indicates GRP(+)/CGRP(+)/PGP9.5(+) staining. (c), thin arrows highlight GRP(-)/CGRP(-)/PGP9.5(+) intraepidermal nerve fibers. (d-f), thick arrows indicate GRP(+)/SP(+)/PGP9.5(+) staining; (d,f), arrowheads highlight GRP(+)/SP(-)/PGP9.5(+) staining [pink pseudocolor, (f)]. (f) thin arrows highlight GRP(-)/SP(-)/PGP9.5(+) nerves. (g,i), arrowhead indicates GRP(+)/NF200(-)/SP(+) staining [pink pseudocolor, (i)]. (h,i), thin arrow highlights GRP(-)/NF200(+)/SP(-) staining. (g-i), thick arrows indicate GRP(+)/NF200(+)/SP(+) staining [white pseudocolor, (i)]. (a,d,h), epi, epidermis; der, dermis. Scale bars: 50 μm.
Itch-sensing fibers are thought to be peptidergic, expressing SP and CGRP (Davidson and Giesler, 2010). We identified GRP-expression in both CGRP(+) and CGRP(-) (Figures 1 and S1, Table S2) and SP(+) and SP(-) nerves (Figure 1, Table S3) at the DEJ. The majority of papillary dermal GRP(+) nerves were SP(+) (median 98.2, 25%-75% quartiles, 32.8%-100%), while these GRP(+) nerves were significantly less likely to be CGRP(+) (median 50.9, 25%-75% quartiles, 18.8%-80%; p=0038). Similarly, GRP(+) neurons comprised a significantly larger proportion of SP(+) nerves at the DEJ (median 86.4%, 25%-75% quartiles, 40.0%-100%) than of CGRP(+) nerves (median, 28.0%, 25%-75% quartiles, 8.1%-43.3%; p<0.0001). Finally, this GRP antibody co-labeled with a peptidergic nerve fiber population in mouse skin (Figure S2, Table S4), similar to that previously reported (Fleming et al., 2012; Lagerstrom et al., 2010; Liu et al., 2009; Sun and Chen, 2007; Tominaga et al., 2009).
As both Aδ- and C-fibers mediate itch, we next examined if GRP(+) fibers at the DEJ belonged to the Aδ-fiber (NF200+) and/or the C-fiber (NF200-) populations of nerves (see methods). Combining the 17 subjects, GRP(+) nerves comprised 38.1% (median, 25%-75% quartiles, 8.7%-60.0%) of C-fibers and 66.3% (median, 25%-75% quartiles, 41.0%-91.3%) of Aδ fibers (Figure 1, Table 1), with this difference being significant (ChiSquare 7.63, DF1, Prob>ChiSq 0.0057). The majority of GRP(+) C-fibers and GRP(+) Aδ-fibers co-expressed SP (median 88.4%, 25%-75% quartiles, 34.3%-100%; median 100%, 25%-75% quartiles, 95.9%-100%, respectively) (Figure 1).
Our identification, in human skin, of GRP-expressing C- and A-δ fibers that co-express either SP or CGRP, makes these neurons candidate primary afferent pruriceptors. Moreover, putative broad GRPR expression in human skin, including keratinocytes, makes possible additional functions for this nerve population (Staniek et al., 1996). As for SP and CGRP nerves, GRP-expressing nerves comprised a minority of the nerve plexus in human papillary dermis and predominantly terminated at the DEJ without intraepidermal extension. Itch-specific signaling has been known to reside superficially in human skin (Magerl, 1996). However, whether human intraepidermal nerve fibers (IENFs) and/or papillary dermal nerves signal itch is not clear. Pain-sensing pathways, thought to reside deeper in the dermis, may block, at the level of the spinal cord, a co-activated superficial itch signal (Davidson and Giesler, 2010; Ross, 2011; Schmelz, 2010). This superficial itch signal in humans may reside preferentially at the DEJ, and not in IENFs, given the paradoxical loss of IENFS in some chronic pruritic conditions (Maddison et al., 2008; Oaklander and Rissmiller, 2002). In mice, molecular silencing of a population of TRPV1(+) primary nociceptive sensory neurons unmasked a chronic cutaneous scratching phenotype, possibly mediated by residual signaling from GRP(+) itch-sensing primary sensory neurons (Lagerstrom et al., 2010; Liu et al., 2010). Extending this work to humans, the location of GRP(+) nerves makes this population a prime candidate to selectively signal itch from the DEJ in some skin diseases where pain-only sensing nerves, such as in the epidermis, are preferentially lost—a hypothesis we are now actively testing.
Supplementary Material
Acknowledgments
This study was supported in part by CTSA grant UL1-TR000157 from the NIH.
Abbreviations
- GRP
Gastrin-releasing peptide
- SP
Substance P
- GRP
Calcitonin Gene Related Peptide
- NF200
Neurofilament 200
- PGP9.5
Protein gene product 9.5
- TRPV1
Transient Receptor Potential Vanilloid 1
- DEJ
Dermoepidermal Junction
Footnotes
Conflict of Interest:
The authors state no conflict of interest.
References
- Akiyama T, Tominaga M, Davoodi A, Nagamine M, Blansit K, Horwitz A, et al. Roles for substance P and gastrin releasing peptide as neurotransmitters released by primary afferent pruriceptors. Journal of neurophysiology. 2012 doi: 10.1152/jn.00539.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Giesler GJ. The multiple pathways for itch and their interactions with pain. Trends in neurosciences. 2010;33:550–558. doi: 10.1016/j.tins.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming MS, Ramos D, Han SB, Zhao J, Son YJ, Luo W. The majority of dorsal spinal cord gastrin releasing peptide is synthesized locally whereas neuromedin B is highly expressed in pain- and itch-sensing somatosensory neurons. Molecular pain. 2012;8:52. doi: 10.1186/1744-8069-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, et al. A subpopulation of nociceptors specifically linked to itch. Nature neuroscience. 2012;16:174–182. doi: 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerstrom MC, Rogoz K, Abrahamsen B, Persson E, Reinius B, Nordenankar K, et al. VGLUT2-dependent sensory neurons in the TRPV1 population regulate pain and itch. Neuron. 2010;68:529–542. doi: 10.1016/j.neuron.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Abdel Samad O, Zhang L, Duan B, Tong Q, Lopes C, et al. VGLUT2-dependent glutamate release from nociceptors is required to sense pain and suppress itch. Neuron. 2010;68:543–556. doi: 10.1016/j.neuron.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison B, Namazi MR, Samuel LS, Sanchez J, Pichardo R, Stocks J, et al. Unexpected diminished innervation of epidermis and dermoepidermal junction in lichen amyloidosus. The British journal of dermatology. 2008;159:403–406. doi: 10.1111/j.1365-2133.2008.08685.x. [DOI] [PubMed] [Google Scholar]
- Magerl W. Neural Mechanisms of Itch Sensation. Technical Corner from IASP Newsletter 1996 [Google Scholar]
- Oaklander AL, Rissmiller JG. Postherpetic neuralgia after shingles: an under-recognized cause of chronic vulvar pain. Obstetrics and gynecology. 2002;99:625–628. doi: 10.1016/s0029-7844(01)01663-5. [DOI] [PubMed] [Google Scholar]
- Ringkamp M, Schepers RJ, Shimada SG, Johanek LM, Hartke TV, Borzan J, et al. A role for nociceptive, myelinated nerve fibers in itch sensation. J Neurosci. 2011;31:14841–14849. doi: 10.1523/JNEUROSCI.3005-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SE. Pain and itch: insights into the neural circuits of aversive somatosensation in health and disease. Current opinion in neurobiology. 2011;21:880–887. doi: 10.1016/j.conb.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Schmelz M. Itch and pain. Neuroscience and biobehavioral reviews. 2010;34:171–176. doi: 10.1016/j.neubiorev.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Staniek V, Misery L, Peguet-Navarro J, Sabido O, Cuber JC, Dezutter-Dambuyant C, et al. Expression of gastrin-releasing peptide receptor in human skin. Acta dermato-venereologica. 1996;76:282–286. doi: 10.2340/0001555576282286. [DOI] [PubMed] [Google Scholar]
- Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science (New York, NY) 2009;325:1531–1534. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Ogawa H, Takamori K. Histological characterization of cutaneous nerve fibers containing gastrin-releasing peptide in NC/Nga mice: an atopic dermatitis model. The Journal of investigative dermatology. 2009;129:2901–2905. doi: 10.1038/jid.2009.188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


