Abstract
Background
Anemia is associated with decreased functional capacity, reduced quality of life, and worsened outcomes among patients with heart failure (HF) due to reduced left ventricular ejection fraction (HFREF). We sought to evaluate the independent effect of anemia on clinical outcomes among those with HFREF.
Methods
The HF-ACTION trial was a prospective, randomized trial of exercise therapy versus usual care in 2331 patients with HFREF. Patients with New York Heart Association class 2–4 HF and left ventricular ejection fractions of ≤ 35% were recruited. Hemoglobin (Hb) was measured up to one year prior to entry and was stratified by quintile. Anemia was defined as baseline Hb < 13 g/dl and <12 g/dl in men and women, respectively. Hb was assessed in two models; a global prediction model that had been previously developed and a modified model including variables associated with anemia and the studied outcomes.
Results
Hemoglobin was available at baseline in 1763 subjects (76% of total study population); their median age was 59.0 years, 73% were male, and 62% were Caucasian. The prevalence of anemia was 515/1763 (29%). Older age, female gender, African American race, diabetes, hypertension, and lower estimated glomerular filtration rates were all more frequent in lower Hb quintiles. Over a median follow-up of 30 months, the primary outcome of all-cause mortality or all-cause hospitalization occurred in 78% of those with anemia and 64% in those without, p<0.001. The secondary outcomes of all-cause mortality alone, cardiovascular mortality or cardiovascular hospitalization, and cardiovascular mortality or HF hospitalization occurred in 23 % vs 15%, 67% vs 54%, and 44 vs 29%, respectively, p<0.001. Heart failure hospitalizations occurred in 36% vs 22%, and urgent outpatient visits for HF exacerbations occurred in 67% and 55%, respectively, p<0.001. For the global model, there was an association observed for anemia and all-cause mortality or hospitalization, adjusted hazard ratio (HR)=1.15, 95% CI 1.01–1.32, p=0.04, but other outcomes were not significant at p<0.05. In the modified model, the adjusted HR for anemia and the primary outcome of all-cause mortality or all-cause hospitalization was 1.25, 95% CI 1.10–1.42, p<0.001. There were independent associations between anemia and all-cause death, HR=1.11, 95% CI 0.87–1.42, p=0.38; cardiovascular death or cardiovascular hospitalization, HR=1.16, 95% CI 1.01–1.33, p=0.035; and cardiovascular death and heart failure hospitalization, HR=1.27, 95% CI 1.06–1.51, p=0.008.
Conclusions
Anemia modestly is associated with increased rates of death, hospitalization, and HF exacerbation in patients with chronic HFREF. After adjusting for other important covariates, anemia is independently associated with an excess hazard for all-cause mortality and all-cause hospitalization. Anemia is also associated with combinations of cardiovascular death and cardiovascular/heart failure hospitalizations as composite endpoints.
Keywords: heart failure, chronic kidney disease, anemia, glomerular filtration rate, renal insufficiency, hospitalization, mortality
Both heart failure (HF) and chronic kidney disease (CKD) are increasing in prevalence as the population ages, and both conditions have been associated with anemia of chronic disease.1 2 Common risk factors for both CKD and HF include obesity, metabolic syndrome, diabetes, and hypertension, some of which are commonly linked with the development of anemia.3 4 5 6 Previous HF studies have consistently reported an association between reduced estimated glomerular filtration rates (eGFR), anemia, elevated levels of B-type natriuretic peptide, and increased HF mortality. 7 8 9 Anemia, even in the absence of renal disease, has also been associated with adverse clinical outcomes; however the impact of HF, anemia, and CKD, alone or in combination on mortality, adverse events, and hospitalization rates, remains of clinical interest.10 It is not certain whether the adverse effect of anemia on clinical outcomes is independent of its impact on other comorbid conditions. Of note, anemia in HF may be dynamic; when hemoglobin (Hb) rises, there have been favorable changes in left ventricular mass and improved symptoms.11 We sought to evaluate the impact of baseline anemia on the composite outcome of all-cause death and all-cause hospitalization and various cardiovascular and HF outcomes among patients with chronic systolic HF participating in the HF-ACTION trial.
Methods
Setting
Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) was a prospective, randomized trial of aerobic exercise training in patients who were medically stable, with New York Heart Association (NYHA) classes II to IV HF and measured left ventricular ejection fraction ≤ 35%. Recruitment criteria and methods for HF-ACTION have been reported elsewhere.12 13 Major exclusion criteria were comorbidities that may have precluded moderate-to-vigorous intensity exercise (e.g. peritoneal or hemodialysis) and major cardiovascular events within the last six months. The trial was designed to evaluate the composite primary end point of all-cause mortality and all-cause hospitalization over a median follow-up of 30 months in patients who were optimally medically managed and underwent medically supervised and home-based exercise training as compared with controls who received usual care.13 Primary and secondary endpoints were adjudicated by a clinical endpoints committee. Once a subject incurred one hospitalization for heart failure, subsequent hospitalizations were not reviewed by the end-point committee and were classified according to the indication by the principal investigator.
Study Sample
A total of 2331 subjects were recruited from April, 2003, through February, 2007, at 82 sites within the United States, Canada, and France; however, the study sample was reduced because 568 subjects had no measured Hb recorded at baseline. The remaining 1763 (76% of the total trial population) served as the study sample, providing complete data for analysis.
Laboratory Measures and Calculation of Glomerular Filtration Rate
Hemoglobin and serum creatinine (SCr) were obtained from the reported values from the study sites recorded within one year prior to trial enrollment and performed by the local laboratory at each center. Detailed methods for local measurement of Hb and SCr were no available. Anemia was defined as a baseline Hb <13 g/dl and <12 g/dl in men and women (World Health Organization definition), respectively.14 Estimated glomerular filtration rates (eGFR ml/min/1.73 m2) were calculated using the four-variable Levey Modification of Diet in Renal Disease formula (186.3*[SCr- 1.154]*[age-.203]); calculated values were multiplied by 0.742 for women and by 1.21 for African Americans.15
Statistical Analysis
Statistical analyses were performed by the data coordinating center (Duke Clinical Research Institute, Durham, NC) using SAS software version 9.2 (SAS Institute Inc., Cary, NC). Baseline characteristics were reported as medians with 25th and 75th percentiles, or counts and proportions as appropriate. The primary composite end point was all-cause mortality or hospitalization. Pre-specified secondary end points were all-cause mortality, cardiovascular mortality or cardiovascular hospitalization, and cardiovascular mortality or heart failure hospitalization. Cumulative event rates were calculated using the Kaplan-Meier method. Time zero for censoring was the time of randomization. Relative risks were expressed as hazard ratios (HR) with 95% confidence intervals and were calculated using the Cox proportional hazards model. Two modeling approaches were taken. Model 1 was created a priori to predict each of the four main clinical endpoints using the entire 2331 subjects recruited into HF-ACTION with the goal of creating an adjustment model. For this method, included variables were identified via a bootstrapped backwards selection algorithm of a Cox proportional hazards model with the C-index used to choose the best model for each outcome (see appendix). This approach evaluated 61 candidate variables (including Hb) and was done independent and before the current analysis. This analysis did not identify Hb as a strong predictor of outcomes. After reviewing Model 1 results and the included covariates it was decided to create a model (Model 2) which focused on evaluating specifically whether anemia was a predictor of outcomes and included only the 1763 subjects with actual, non-imputed Hb values. Model 2 used variables found to be differentially associated with Hb (Table 1) and known to be clinical confounders for HF including: age, African American versus other race, female gender, diabetes mellitus, ischemic etiology of HF, LVEF, and eGFR. Other variables in the HF-ACTION database excluded from Model 2 were those believed to result from anemia or related to other included variables (exercise performance variables, quality of life, and symptom scores), variables known to be confounded by indication (e.g. beta-blocker and diuretic dosage, use of nitrates), biologic representations of the same construct (e.g. SCr and blood urea nitrogen where renal filtration function represented by eGFR) and those with no biologic plausibility (randomization allocation, site of randomization). The univariate relationship of the continuous variables in this set (age, LVEF, eGFR) with each outcome was checked for linearity of the log HR, and piecewise linear splines were used as transformations when appropriate in Model 2. A two-tailed p-value < 0.05 was considered statistically significant.
Table 1.
Baseline characteristics by quintile of Hb
| Total (n=1763) | Quintile 1 of Hb (n = 373) |
Quintile 2 of Hb (n = 355) |
Quintile 3 of Hb (n = 339) |
Quintile 4 of Hb (n = 357) |
Quintile 5 of Hb (n = 339) |
||
|---|---|---|---|---|---|---|---|
| Hb (g/dl) | Median (Q1, Q3) | 13.5 (12.3, 14.6) | 11.4 (10.7, 11.8) | 12.7 (12.4, 12.9) | 13.5 (13.4, 13.7) | 14.4 (14.2, 14.6) | 15.6 (15.2, 16.1) |
| Age | Median (Q1, Q3) | 59 (51, 68) | 60 (52, 70) | 61 (54, 70) | 60 (51, 69) | 58 (50, 66) | 57 (60, 65) |
| Sex | Female n, % | 480 (27%) | 160 (43%) | 132 (37%) | 95 (28%) | 69 (19%) | 24 (7%) |
| Race | Black or African American n, % | 571 (33%) | 175 (48%) | 138 (39%) | 98 (30%) | 89 (25%) | 71 (21%) |
| White n, % | 1069 (62%) | 178 (48%) | 195 (55%) | 213 (65%) | 249 (71%) | 234 (71%) | |
| Other n, % | 93 (5%) | 15 (4%) | 21 (6%) | 18 (5%) | 13 (4%) | 26 (8%) | |
| Missing n,% | 30 (1.7%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Hypertension | n, % | 1068 (61%) | 257 (69%) | 206 (58%) | 196 (58%) | 201 (56%) | 208 (62%) |
| Diabetes Mellitus | n, % | 569 (32%) | 148 (40%) | 121 (34%) | 108 (32%) | 102 (29%) | 90 (27%) |
| COPD | n, % | 198 (11%) | 37 (10%) | 43 (12%) | 39 (12%) | 43 (12%) | 36 (11%) |
| Prior MI | n, % | 746 (42%) | 159 (43%) | 158 (45%) | 132 (39%) | 156 (44%) | 141 (42%) |
| PVD | n, % | 132 (7.5%) | 32 (8.6%) | 27 (7.6%) | 28 (8.3%) | 24 (6.7%) | 21 (6.2%) |
| Prior CABG | n, % | 458 (26%) | 110 (29%) | 104 (29%) | 78 (23%) | 85 (24%) | 81 (24%) |
| BMI (kg/m2) | Median (Q1, Q3) | 30 (26, 35) | 29 (25, 35) | 30 (26, 35) | 29 (25, 34) | 30 (26, 35) | 30 (27, 36) |
| Resting HR (bpm) | Median (Q1, Q3) | 70 (63, 77) | 70 (64, 78) | 69 (61, 76) | 70 (63, 76) | 70 (62, 76) | 71 (64, 78) |
| Systolic BP (mmHg) | Median (Q1, Q3) | 110 (100, 126) | 110 (100, 128) | 110 (100, 124) | 110 (100, 126) | 110 (100, 124) | 112 (104, 124) |
| Diastolic BP (mmHg) | Median (Q1, Q3) | 70 (60, 78) | 68 (60, 76) | 70 (60, 78) | 70 (60, 80) | 70 (60, 78) | 71 (64, 80) |
| NYHA class | II n, % | 1119 (63%) | 196 (53%) | 225 (63%) | 223 (66%) | 243 (68%) | 232 (68%) |
| III/IV n, % | 644 (37%) | 177 (47%) | 130 (37%) | 116 (34%) | 114 (32%) | 107 (32%) | |
| Angina class | No angina n, % | 1455 (83%) | 308 (83%) | 287 (81%) | 279 (82%) | 297 (83%) | 284 (84%) |
| I n, % | 166 (9%) | 29 (8%) | 38 (11%) | 36 (11%) | 30 (8%) | 33 (10%) | |
| II–IV n,% | 140 (8%) | 35 (9%) | 29 (8%) | 24 (7%) | 30 (8%) | 22 (6%) | |
| HF Etiology | Ischemic n, % | 923 (52%) | 206 (55%) | 207 (58%) | 168 (50%) | 177 (50%) | 165 (49%) |
| LVEF | Median (Q1, Q3) | 25 (20, 30) | 25 (20, 30) | 25 (20, 30) | 24 (20, 30) | 24 (20, 30) | 24 (21, 30) |
| ACEI and/or ARB | n, % | 1665 (94%) | 349 (94%) | 336 (95%) | 317 (94%) | 336 (94%) | 327 (96%) |
| Beta blocker | n, % | 1666 (94%) | 348 (93%) | 337 (95%) | 326 (96%) | 332 (93%) | 323 (95%) |
| Loop diuretics | n, % | 1396 (79%) | 333 (89%) | 274 (77%) | 266 (78%) | 275 (77%) | 248 (73%) |
| Nitrates | n, % | 446 (25%) | 113 (30%) | 81 (23%) | 86 (25%) | 89 (25%) | 77 (23%) |
| Calcium channel blockers | n, % | 123 (7%) | 32 (9%) | 27 (8%) | 22 (6%) | 19 (5%) | 23 (7%) |
| Spironolactone/Eplerenone | n, % | 798 (45%) | 172 (46%) | 146 (41%) | 155 (46%) | 166 (46%) | 159 (47%) |
| Digoxin | n, % | 798 (45%) | 166 (44%) | 159 (45%) | 153 (45%) | 177 (50%) | 143 (42%) |
| SCr (mg/dl) | Median (Q1, Q3) | 1.2 (1.0, 1.5) | 1.3 (1.0,1.7) | 1.2 (0.9,1.5) | 1.2 (1.0,1.5) | 1.2 (1.0,1.4) | 1.2 (1.0,1.4) |
| eGFR | Median (Q1, Q3) | 67 (51, 81) | 59 (44, 76) | 67 (50, 82) | 66 (53, 80) | 68 (55, 82) | 71 (56, 83) |
| BUN | Median (Q1, Q3) | 20 (15, 28) | 23 (16, 35) | 20 (15, 29) | 20 (16, 27) | 20 (15, 25) | 20 (15, 26) |
Quintile 1 of Hb is defined as Hb ≤12.1 g/dl, quintile 2 by 12.1 g/dl < Hb ≤13.1 g/dl, quintile 3 by 13.1 g/dl < Hb ≤13.9 g/dl, quintile 4 by 13.9 g/dl < Hb ≤14.9 g/dl, and quintile 5 by Hb > 14.9 g/dl; ACEI=angiotensin converting enzyme inhibitor, ARB = angiotensin II receptor blocker, COPD=chronic obstructive pulmonary disease, Hb=hemoglobin, MI=myocardial infarction, PVD=peripheral vascular disease, CABG=coronary artery bypass graft surgery, BMI=body mass index, HR=heart rate, BP=blood pressure, NYHA=New York Heart Association functional class, HF=heart failure, eGFR=estimated glomerular filtration rate, BUN=blood urea nitrogen
Results
Baseline Characteristics
Selected demographics for the study sample (n=1763) were as follows: median age 59 years (range 19–90); 73% male; 62% Caucasian, 33% African American and 5% other races (Table 1). The overall prevalence of anemia was 30.1% and 26.9% in men and women, respectively. The median Hb was 15.6 g/dl for Q5 compared to 11.4 g/dl for Q1. At baseline, we found that in patients with HFREF, reduced Hb was more frequent among the expected conditions including older age, female gender, African Americans diabetes, hypertension, and mildly reduced renal filtration function. In the lowest quintile of Hb, the median eGFR was 59 with an interquartile range of 44–76 ml/kg/1.73 m2. Thus, nearly all subjects were above critical levels of renal dysfunction. The most striking contrast was a prevalence of diabetes corresponding to 40% in Q1 compared to 27% in Hb Q5.
Adverse Events
The median follow-up times for those with and without anemia were 30 and 31 months, respectively, p=0.594. Table 2 displays selected adverse events reported by site investigators over the course of the trial according to the presence of anemia. These adverse events were not adjudicated by the endpoints committee nor adjusted for confounding variables. With the exception of transient ischemic attack, adverse events were more frequent for those with anemia. The fraction of subjects with at least one selected adverse cardiovascular event was 49% and 37%, respectively, for those with and without anemia.
Table 2.
Selected adverse outcomes according to presence or absence of anemia, N (%)
| All | Anemia | No Anemia | |
|---|---|---|---|
| N | 1763 | 515 | 1248 |
| Median Follow-up (median, 25 and 75 percentiles in months) | 30 (19–42) | 30 (18–43) | 31 (20–42) |
| Prespecified Adverse Cardiovascular Events | |||
| Worsening Heart Failure | 518 (29) | 200 (39) | 318 (25) |
| Unstable angina | 138 (7.8) | 51 (9.9) | 87 (7.0) |
| Serious arrhythmia | 261 (15) | 82 (16) | 179 (14) |
| Stroke | 52 (2.9) | 22 (4.3) | 30 (2.4) |
| Transient ischemic attack | 30 (1.7) | 7 (1.4) | 23 (1.8) |
| Any of the above events | 711 (40) | 253 (49) | 458 (37) |
Prespecified Primary and Secondary Outcomes in the Trial
Kaplan-Meier curves for the prespecified primary and secondary endpoints are shown in Figures 2–4. Individual trial outcomes and unadjusted/adjusted HRs for subjects with and without anemia are shown in Table 3. Over the median follow-up of 30 months, all-cause mortality or all-cause hospitalization occurred in 78% vs 64%, and all-cause mortality alone occurred in 23% vs 15% among patients with and without anemia, respectively, p<0.001 for both. Cardiovascular mortality or cardiovascular hospitalization and cardiovascular mortality or heart failure hospitalization occurred in 67% vs 54%, and 44% vs 29%, respectively, p<0.001 in those with and without anemia. HF hospitalizations occurred in 36% vs 22%, and urgent outpatient visits for HF exacerbations in 67% vs 55%, in the anemia versus no anemia groups respectively, p<0.001 for both. Kaplan-Meier curves demonstrating the time to first event are shown in Figure 1 for all-cause mortality or all-cause hospitalization as a composite. The results from univariate and multivariate adjustment Models 1 and 2 are shown in Table 3. For Model 1, the adjusted hazards ratio for anemia and the primary outcome of all cause mortality or all-cause hospitalization was 1.15 (CI 1.01–1.32), p=0.042. The adjusted hazards for anemia and the prespecified secondary outcomes were 0.99 (95% CI 0.77–1.27), p=0.919 for all-cause death; 1.22 (95% CI 0.97–1.30), p=0.120 for cardiovascular death or cardiovascular hospitalization; and 1.20 (95% CI 0.99–1.44), p=0.060 for cardiovascular death and heart failure hospitalization. For the modified model designed to evaluate anemia as a predictor of outcomes, the adjusted hazards ratio for anemia and the primary outcome of all cause mortality or all-cause hospitalization was 1.25 (95% CI 1.10–1.42), p<0.001. The adjusted hazards for anemia and the prespecified secondary outcomes were 1.11 (95% CI 0.87–1.42), p=0.385 for all-cause death; 1.16 (95% CI 1.01–1.33), p=0.035 for cardiovascular death or cardiovascular hospitalization; and 1.27 (95% CI 1.06–1.51), p=0.008 for cardiovascular death and heart failure hospitalization. All of these analyses demonstrated attenuation of the measure of association with adjustment, and this attenuation was greater for the global model (Model 1) than when adjusting for specific confounders of the anemia and outcomes association using Model 2 (i.e. age, African American versus other race, female gender, diabetes mellitus, ischemic etiology of HF, LVEF, and eGFR).
Figure 2.
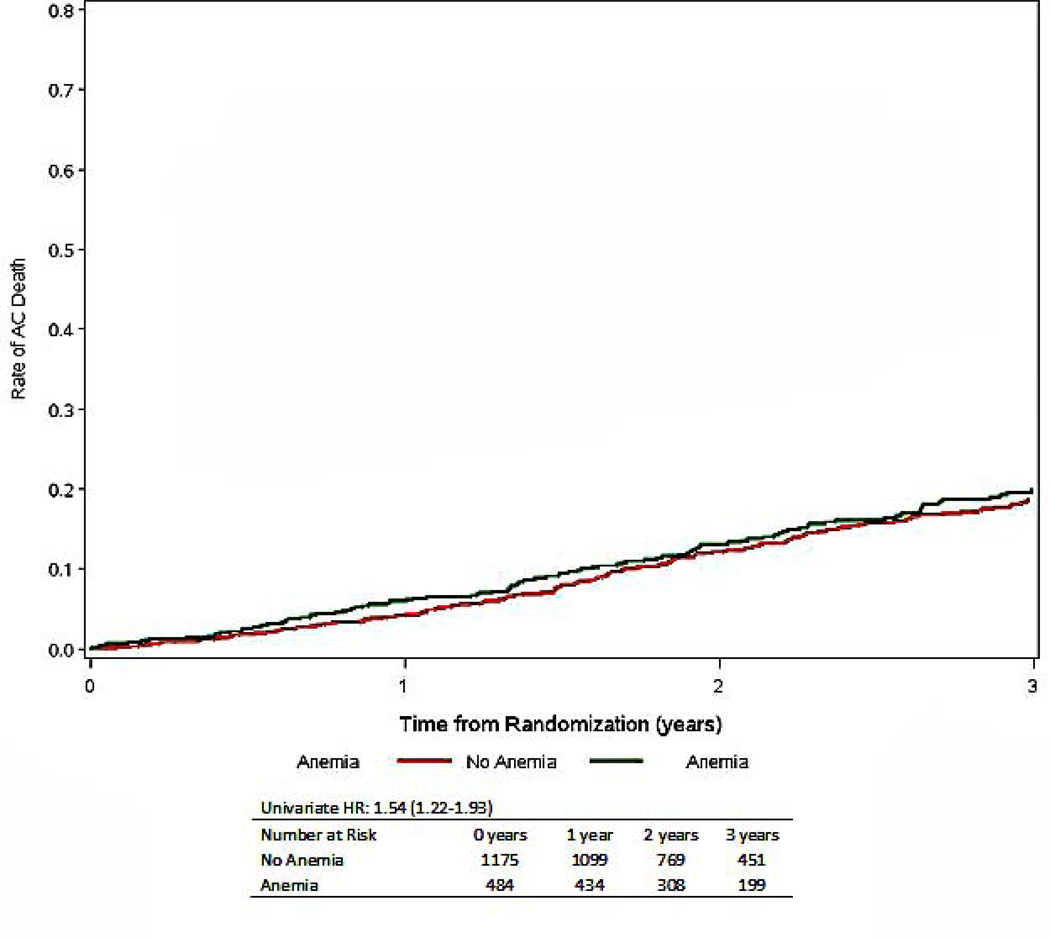
Time to all-cause mortality stratified by the presence or absence of anemia, numbers at risk include only those with nonmissing covariates (total is less than 1763)
Figure 4.
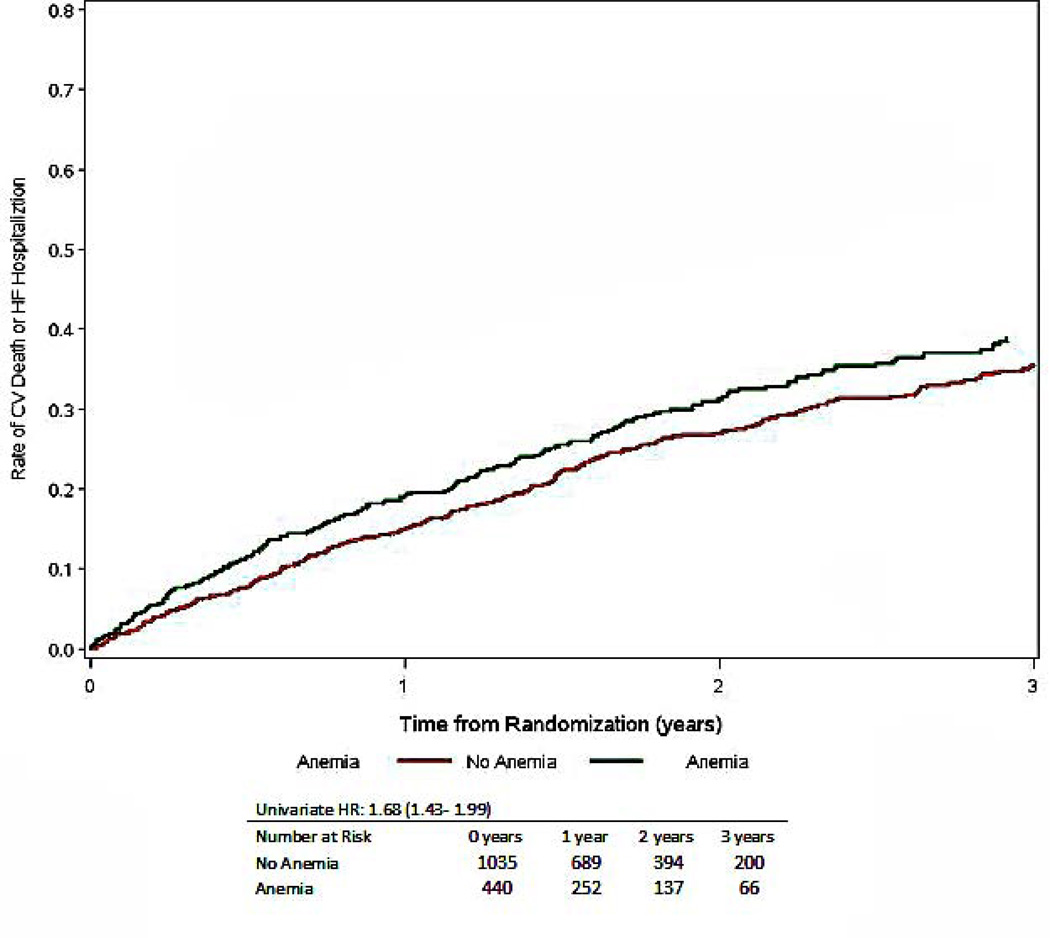
Time to CV mortality or HF hospitalization, stratified by the presence or absence of anemia, numbers at risk include only those with nonmissing covariates (total is less than 1763)
Table 3.
Trial outcomes according to the presence or absence of anemia with adjusted hazard ratios being derived from adjustment models 1 and 2 described in the methods section, CI=confidence interval, HR=hazard ratio
| Univariate (Unadjusted) | Model 1 Adjusted | Model 2 Adjusted | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All | Anemia | No Anemia | HR (95% CI) | P- value |
HR (95% CI) | P- Value |
HR (95% CI) | P-value | |
| N | 1763 | 515 | 1248 | - | - | ||||
| Mos. Follow-up med (IQR) | 30 (19–42) | 30 (18–43) | 31 (20–42) | - | 0.594 | ||||
| Trial Outcomes | |||||||||
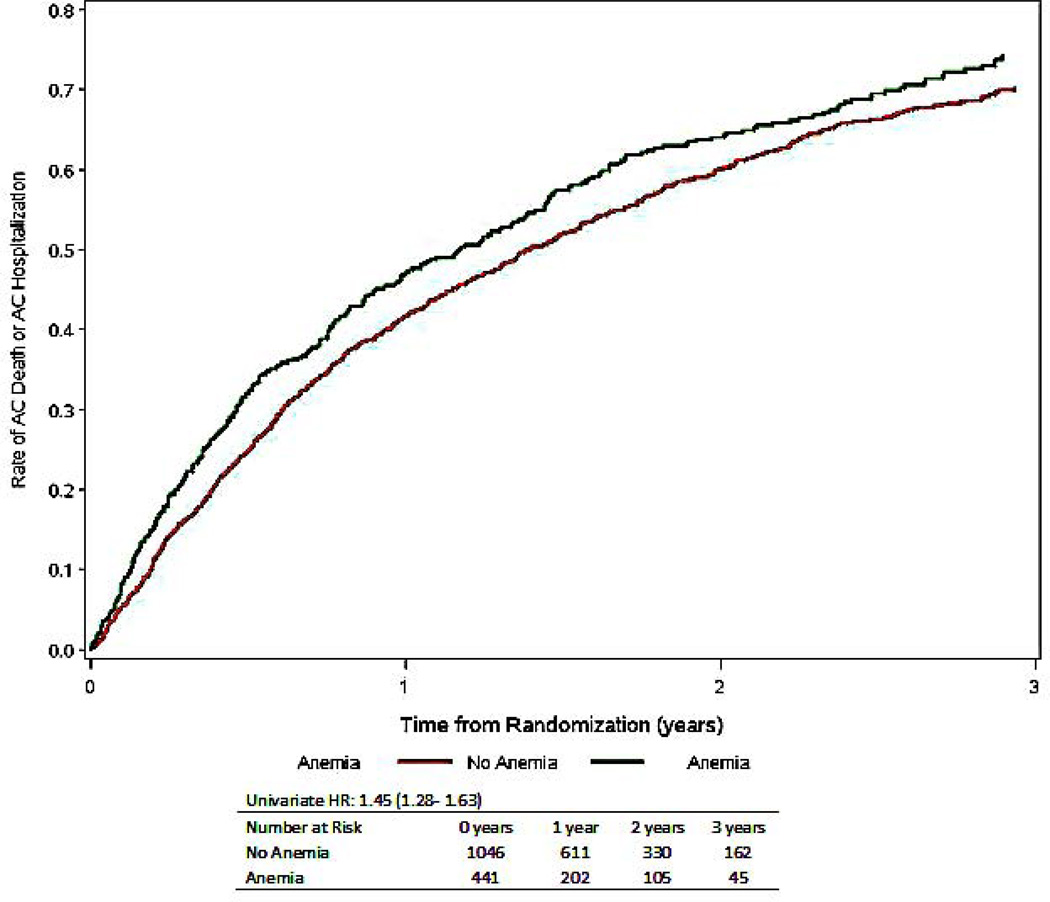
| All-Cause Mortality or All-Cause Hospitalization | 1203 (68) | 401 (78) | 802 (64) | 1.45 (1.28–1.63) | <0.001 | 1.15 (1.01–1.32) | 0.042 | 1.25 (1.10–1.42) | <0.001 |
| All-Cause Mortality | 308 (17) | 118 (23) | 190 (15) | 1.54 (1.22–1.93) | <0.001 | 0.99 (0.77–1.27) | 0.919 | 1.11 (0.87–1.42) | 0.385 |
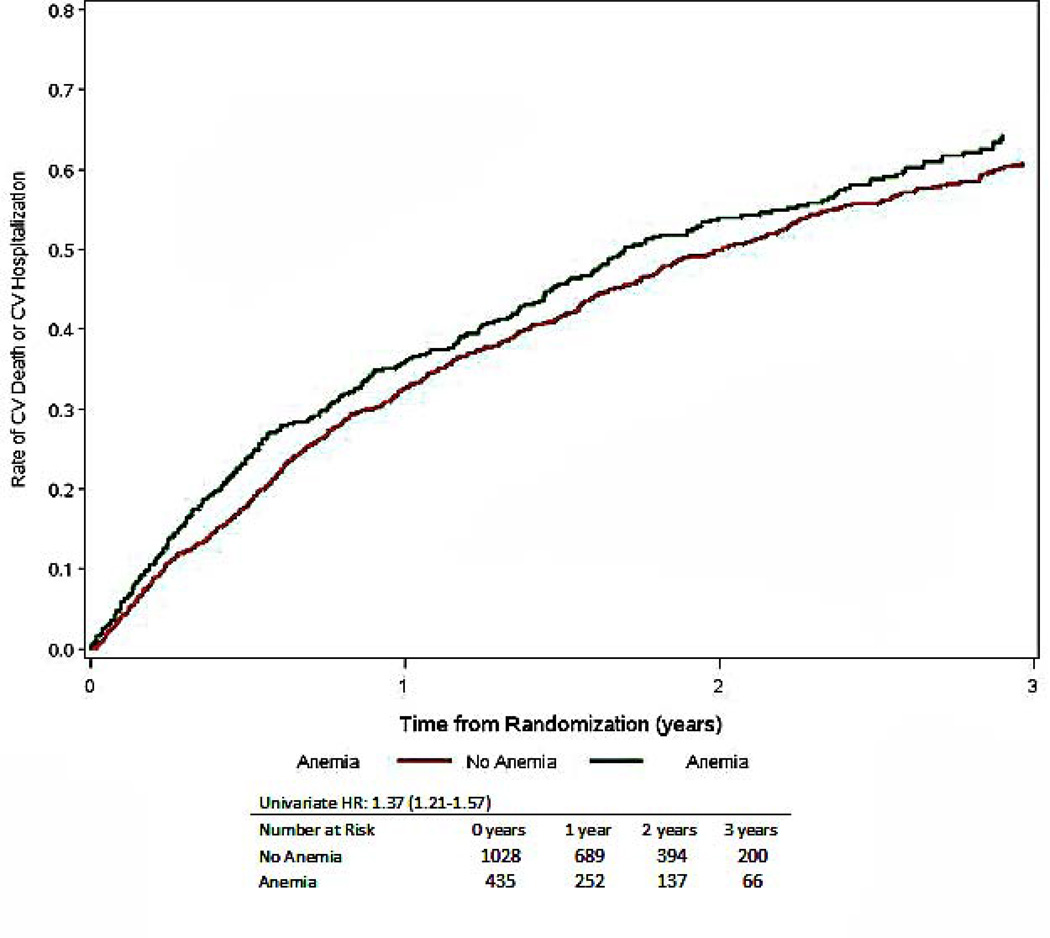
| Cardiovascular Mortality or Cardiovascular Hospitalization | 1021 (58) | 343 (67) | 678 (54) | 1.37 (1.21–1.57) | <0.001 | 1.22 (0.97– 1.30) | 0.120 | 1.16 (1.01–1.33) | 0.035 |
| Cardiovascular Mortality or Heart Failure Hospitalization | 590 (33) | 227 (44) | 363 (29) | 1.68 (1.43–1.99) | <0.001 | 1.20 (0.99–1.44) | 0.060 | 1.27 (1.06–1.51) | 0.008 |
| Cardiovascular Mortality | 261 (15) | 97(19) | 164 (13) | 1.46 (1.14–1.88) | 0.003 | 0.93 (0.71–1.23) | 0.619 | 1.09 (0.83–1.43) | 0.527 |
| All-Cause Hospitalization | 1150 (65) | 387 (75) | 763 (61) | 1.46 (1.29–1.65) | <0.001 | 1.16 (1.01–1.33) | 0.040 | 1.27 (1.12–1.45) | <0.001 |
| Cardiovascular Hospitalization | 950 (54) | 320 (62) | 630 (50) | 1.37 (1.20–1.57) | <0.001 | 1.12 (0.96–1.30) | 0.138 | 1.17 (1.02–1.35) | 0.03 |
| Heart Failure Hospitalization | 464 (26) | 186 (36) | 278 (22) | 1.79 (1.49–2.15) | <0.001 | 1.25 (1.02–1.54) | 0.032 | 1.33 (1.09–1.61) | 0.005 |
| Emergency department visit, or urgent clinic visit for heart failure exacerbation | 1033 (59) | 346 (67) | 687 (55) | 1.40 (1.25–1.57) | <0.001 | 1.21 (1.04–1.40) | 0.012 | 1.20 (1.05–1.38) | 0.008 |
Figure 1.
Time to all-cause mortality or all-cause hospitalization stratified by the presence or absence of anemia, numbers at risk include only those with nonmissing covariates (total is less than 1763)
Discussion
The current analysis provides a unique opportunity to evaluate the relationship of anemia to outcomes in stable HF patients with preserved eGFR. In general, all the unadjusted hazards for trial endpoints were elevated for those with anemia. There were no indications of competing risks given the similar follow-up times for those with and without anemia (median 30 months for each, Table 2). For both the original predictive model developed for the primary endpoint and the modified model, anemia was associated with the primary endpoint of time to all-cause mortality or hospitalization. Although the association with other trial outcomes were non-significant in the global model, these associations were evident for the primary and secondary outcomes when the model was modified to be more specific to an evaluation of anemia. These data are consistent with the notion that anemia, even in those with only mildly reduced or normal renal function, is an elevated risk state for patients with HFREF and represents a complicated clustering of modulators that are related to cardiac mortality or hospitalization.29 Anemia may be both a contributor and a result of HF as a chronic disease state. Reduced Hb is associated with impaired oxygen delivery, salt and water retention, and chronic volume overload which may exacerbate HF symptoms.28 It has been reported in clinical trials and observational studies that patients with the lowest Hb values have higher physician assigned NYHA class and worse HF symptoms.28 Additionally, anemia has been consistently associated with higher rates of hospitalization for heart failure and other causes as well as all-cause mortality in those with HF.19 20 Anemia can be considered a manifestation of the chronic cardiorenal syndrome, where either the HF worsens kidney function (Type 2) or vice versa (Type 4).21 In cardiorenal syndromes, anemia may play a role in reducing oxygen delivery, thus impairing myocardial contractility and systolic performance.22 In addition, increased LV mass is commonly associated with anemia and progressive renal failure and may be related to the impairment of cellular transport of electrolytes and oxygen which mechanical and metabolic functions.23 Chronic kidney disease and HF have been associated with increased levels of hepcidin-25, which inhibits the ferroportin transporter and blocks iron transport from the gut and at many somatic levels.24 In addition, reduced renal mass is associated with relative deficiency of erythropoietin, which is partially responsible for the predictable decline in erythroid mass as renal function declines. Moreover, deficient levels of erythropoietin may directly influence left ventricular remodeling and HF. 25 Lastly, anemia is associated with increased markers of inflammation and oxidative stress.26 27 Collectively, these pathologic changes may be responsible, for the higher morbidity and mortality associated with anemia in HFREF that we reported in HF-ACTION and has been observed in other studies.28
Our study adds to the literature in demonstrating that anemia, even at higher levels of eGFR at baseline is independently associated with an array of adverse outcomes including cardiac and all-cause hospitalization, emergency department visit, or urgent clinic visit for heart failure exacerbation, and death due to heart failure or other cardiac causes.
We recognize that both renal filtration function and older age are important confounders in the relationship between anemia and poor outcomes.29 30 Herzog and colleagues found among Medicare recipients not on dialysis, that the annual mortality rates were: 4% for those with no HF, CKD, 8% for anemia or CKD, 13% for HF, and 23% for all three conditions in the same patient population.31 The annual mortality rate in HF ACTION was < 10%, suggesting selection bias for the trial significantly reduced generalizability and may have reduced the relative impact of anemia on survival. Thus, both in community populations and clinical trials, senescent decline in both bone marrow and renal function may play a role in cardiac adverse events precipitating hospitalization, and in some circumstances, contributing to death.32 33
Our findings suggested that investigator reported adverse events related to the heart were more frequent in those with anemia. Finally, the triad of older age, anemia, and reduced renal function may identify a particularly frail population that is more prone to medication adverse events, complications with invasive procedures, and a greater susceptibility to event-driven mortality over the natural history of HF.34 35
Limitations
Our study has all the limitations of a post-hoc analysis from a large randomized trial. Selection bias for entry into the trial of exercise training yielded a younger and more robust group of HF patients able to exercise which may not represent those commonly seen in clinical practice. We did not have baseline hemoglobin or creatinine values on all the subjects in the trial; thus, this is a subgroup that has both measures recorded on the case report form up to one year prior to randomization. We did not have a background history of prior anemia or its treatments including iron or erythrocyte stimulating agents. Because subjects were restricted to having systolic HF, we cannot generalize our findings to those with more modestly reduced LVEF (36–45%) or to those with preserved systolic function. Hemoglobin and SCr values were obtained from clinical records and not measured in a core laboratory. Thus, differences in assay types and standardization probably contributed to variation in eGFR. Furthermore, we did not measure arterial blood gases, serum iron indices, folate, vitamin B12, or erythropoietin precluding the etiology of anemia. Lastly, we did not have measures of iron transport, inflammation, and oxidative stress or other biomarkers to elucidate the pathophysiologic mechanisms relating chronic failure of the heart, bone marrow, and kidneys.36 The current analysis provides results from two models, which both have limitations. Model 1 was derived a priori from the complete cohort. Hb values were missing in 24% of the cases and were imputed in order to be included in the preconstructed global model (Model 1). Derived variables integrating information such as eGFR or anemia as defined as a disease state in this paper were not considered at the time Model 1 was created. Model 1 also did not allow clinical refinement for multicollinearity and did not exclude potential symptomatic outcomes of anemia as predictors. Additionally, since Model 1 had a large number of predictor variables relative to the number of outcomes, it was subject to over-fitting and over-adjustment. While Model 2 addressed some of the shortcomings of Model 1, it excluded known predictors of clinical outcomes in heart failure featured in other HF-ACTION papers, including peak VO2, quality of life, medication treatment, and detailed echocardiographic measures. Model 2 was created based upon univariate findings and after Model 1 results were known. Both models have the limitation of unknown or unmeasured confounders that may account for the association between anemia and the clinical outcomes reported.
Conclusion
Anemia is associated with increased rates of death, hospitalization, and HF exacerbation in outpatients with chronic HFREF and with both preserved and decreased renal filtration function. After adjusting for other important covariates, anemia is independently associated with an excess hazard for all-cause mortality and all-cause hospitalization. Anemia is also associated with combinations of cardiovascular death and cardiovascular/heart failure hospitalizations as composite endpoints.
Supplementary Material
Figure 3.
Time to CV mortality or CV hospitalization, stratified by the presence or absence of anemia, numbers at risk include only those with nonmissing covariates (total is less than 1763)
Acknowledgments
Funding/Support: National Institutes of Health, National Heart Lung and Blood Institute, for the conduct of the HF-ACTION Trial, Amgen, Inc., for partial support of statistical analyses and manuscript preparation and finalization.
Footnotes
Conflict of Interest: Dr Kilpatrick is an employee and stockholder of Amgen, Inc., who provided funding for this analysis.
Contributor Information
Peter A. McCullough, St. John Providence Health System, Warren, MI, St. John Hospital and Medical Center, Detroit, St. John Macomb Oakland Center, Madison Heights, Providence Hospitals and Medical Centers, Southfield and Novi, MI.
Denise Barnard, University of California, San Diego School of Medicine; UCSD Health Systems, San Diego, CA.
Robert Clare, Duke Clinical Research Institute, Durham, NC.
Stephen J. Ellis, Duke Clinical Research Institute, Durham, NC.
Jerome L. Fleg, National Heart Lung and Blood Institute, Bethesda, MD.
Gregg C. Fonarow, University of California at Los Angeles School of Medicine, Los Angeles, CA.
Barry A. Franklin, Oakland University William Beaumont School of Medicine, Cardiac Rehabilitation, William Beaumont Hospital, Royal Oak, MI.
Ryan D. Kilpatrick, Amgen, Inc., Thousand Oaks, CA.
Dalane W. Kitzman, Advanced Cardiac Care and Heart Transplant Program, Wake Forest Baptist Health, Winston-Salem, NC.
Christopher M. O’Connor, Duke Clinical Research Institute, Durham, NC.
Ileana L. Piña, Albert Einstein College of Medicine, Division of Cardiology, Heart Failure/Transplant Program, Montefiore Medical Center, New York, NY.
Udho Thadani, University of Oklahoma Health Sciences Center and Veterans Administration Medical Center, Oklahoma City, OK.
Vinay Thohan, Advanced Cardiac Care and Heart Transplant Program, Wake Forest Baptist Health, Winston-Salem, NC.
David J. Whellan, Thomas Jefferson University School of Medicine Philadelphia, PA.
References
- 1.McCullough PA, Philbin EF, Spertus JA, Kaatz S, Sandberg KR, Weaver WD. Confirmation of a Heart Failure Epidemic: Findings from the Resource Utilization Among Congestive Heart Failure (R.E.A.C.H.) Study. J Am Coll Cardiol. 2002;39:60–69. doi: 10.1016/s0735-1097(01)01700-4. [DOI] [PubMed] [Google Scholar]
- 2.Szczech LA, Harmon W, Hostetter TH, Klotman PE, Powe NR, Sedor JR, Smedberg P, Himmelfarb J. World Kidney Day 2009: problems and challenges in the emerging epidemic of kidney disease. J Am Soc Nephrol. 2009 Mar;20(3):453–455. doi: 10.1681/ASN.2009010041. [DOI] [PubMed] [Google Scholar]
- 3.Whaley-Connell AT, Sowers JR, Stevens LA, McFarlane SI, Shlipak MG, Norris KC, Chen SC, Qiu Y, Wang C, Li S, Vassalotti JA, Collins AJ Kidney Early Evaluation Program Investigators. CKD in the United States: Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES) 1999–2004. Am J Kidney Dis. 2008 Apr;51(4) Suppl 2:S13–S20. doi: 10.1053/j.ajkd.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Whaley-Connell AT, Sowers JR, McFarlane SI, Norris KC, Chen SC, Li S, Qiu Y, Wang C, Stevens LA, Vassalotti JA, Collins AJ Kidney Early Evaluation Program Investigators. Diabetes mellitus in CKD: Kidney Early Evaluation Program (KEEP) and National Health and Nutrition and Examination Survey (NHANES) 1999–2004. Am J Kidney Dis. 2008 Apr;51(4) Suppl 2:S21–S29. doi: 10.1053/j.ajkd.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Artham SM, Lavie CJ, Patel HM, Ventura HO. Impact of obesity on the risk of heart failure and its prognosis. J Cardiometab Syndr. 2008 Summer;3(3):155–161. doi: 10.1111/j.1559-4572.2008.00001.x. [DOI] [PubMed] [Google Scholar]
- 6.Zalesin KC, Franklin BA, Miller WM, Peterson ED, McCullough PA. Impact of obesity on cardiovascular disease. Endocrinol Metab Clin North Am. 2008 Sep;37(3):663–684. doi: 10.1016/j.ecl.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 7.McFarlane SI, Chen SC, Whaley-Connell AT, Sowers JR, Vassalotti JA, Salifu MO, Li S, Wang C, Bakris G, McCullough PA, Collins AJ, Norris KC Kidney Early Evaluation Program Investigators. Prevalence and associations of anemia of CKD: Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES) 1999–2004. Am J Kidney Dis. 2008 Apr;51(4) Suppl 2:S46–S55. doi: 10.1053/j.ajkd.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 8.Wu AH, Omland T, Wold Knudsen C, McCord J, Nowak RM, Hollander JE, Duc P, Storrow AB, Abraham WT, Clopton P, Maisel AS, McCullough PA Breathing Not Properly Multinational Study Investigations. Relationship of B-type natriuretic peptide and anemia in patients with and without heart failure: a substudy from the Breathing Not Properly (BNP) Multinational Study. Am J Hematol. 2005 Nov;80(3):174–180. doi: 10.1002/ajh.20456. [DOI] [PubMed] [Google Scholar]
- 9.Smith GL, Lichtman JH, Bracken MB, Shlipak MG, Phillips CO, DiCapua P, Krumholz HM. Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol. 2006 May 16;47(10):1987–1996. doi: 10.1016/j.jacc.2005.11.084. [DOI] [PubMed] [Google Scholar]
- 10.McCullough PA, Lepor NE. Piecing together the evidence on anemia: the link between chronic kidney disease and cardiovascular disease. Rev Cardiovasc Med. 2005;6(Suppl 3):S4–S12. [PubMed] [Google Scholar]
- 11.Anand I, McMurray JJ, Whitmore J, Warren M, Pham A, McCamish MA, Burton PB. Anemia and its relationship to clinical outcome in heart failure. Circulation. 2004 Jul 13;110(2):149–154. doi: 10.1161/01.CIR.0000134279.79571.73. [DOI] [PubMed] [Google Scholar]
- 12.Whellan DJ, O'Connor CM, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Houston-Miller N, Fleg JL, Schulman KA, Piña IL HF-ACTION Trial Investigators. Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): design and rationale. Am Heart J. 2007 Feb;153(2):201–211. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 13.O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Piña IL HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009 Apr 8;301(14):1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Geneva: World Health Organization; 2008. Worldwide prevalence of anaemia 1993–2005. ISBN 9789241596657. [Google Scholar]
- 15.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999 Mar 16;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 16.O'Connor CM, Whellan DJ, Wojdyla D, Leifer E, Clare RM, Ellis SJ, Fine LJ, Fleg JL, Zannad F, Keteyian SJ, Kitzman DW, Kraus WE, Rendall D, Piña IL, Cooper LS, Fiuzat M, Lee KL. Factors related to morbidity and mortality in patients with chronic heart failure with systolic dysfunction: the HF-ACTION predictive risk score model. Circ Heart Fail. 2012 Jan 1;5(1):63–71. doi: 10.1161/CIRCHEARTFAILURE.111.963462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enders CK. Missing Data Analysis. New York, NY: Guilford; 2010. [Google Scholar]
- 18.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009 Jul;20(4):488–495. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Haehling S, van Veldhuisen DJ, Roughton M, Babalis D, de Boer RA, Coats AJ, Manzano L, Flather M, Anker SD. Anaemia among patients with heart failure and preserved or reduced ejection fraction: results from the SENIORS study. Eur J Heart Fail. 2011 Jun;13(6):656–663. doi: 10.1093/eurjhf/hfr044. [DOI] [PubMed] [Google Scholar]
- 20.Hamaguchi S, Tsuchihashi-Makaya M, Kinugawa S, Yokota T, Takeshita A, Yokoshiki H, Tsutsui H JCARECARD Investigators. Anemia is an independent predictor of long-term adverse outcomes in patients hospitalized with heart failure in Japan. A report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARECARD) Circ J. 2009 Oct;73(10):1901–1908. doi: 10.1253/circj.cj-09-0184. [DOI] [PubMed] [Google Scholar]
- 21.Ronco C, McCullough P, Anker SD, Anand I, Aspromonte N, Bagshaw SM, Bellomo R, Berl T, Bobek I, Cruz DN, Daliento L, Davenport A, Haapio M, Hillege H, House AA, Katz N, Maisel A, Mankad S, Zanco P, Mebazaa A, Palazzuoli A, Ronco F, Shaw A, Sheinfeld G, Soni S, Vescovo G, Zamperetti N, Ponikowski P for the Acute Dialysis Quality Initiative (ADQI) consensus group. Cardio-renal syndromes: report from the consensus conference of the Acute Dialysis Quality Initiative. Eur Heart J. 2009 Dec 25; doi: 10.1093/eurheartj/ehp507. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weisensee D, Schnaars Y, Schoeppe W, Bereiter-Hahn J, Löw-Friedrich I. Potential uremic toxins modulate energy metabolism of cardiac myocytes in vitro. Exp Nephrol. 1997 May-Jun;5(3):194–200. [PubMed] [Google Scholar]
- 23.Yerkey MW, Kernis SJ, Franklin BA, Sandberg KR, McCullough PA. Renal dysfunction and acceleration of coronary disease. Heart. 2004 Aug;90(8):961–966. doi: 10.1136/hrt.2003.015503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato A. Increased hepcidin-25 and erythropoietin responsiveness in patients with cardio-renal anemia syndrome. Future Cardiol. 2010 Nov;6(6):769–771. doi: 10.2217/fca.10.97. [DOI] [PubMed] [Google Scholar]
- 25.Smith K, Semple D, Bhandari S, Seymour AM. Cellular basis of uraemic cardiomyopathy: a role for erythropoietin? Eur J Heart Fail. 2009 Aug;11(8):732–738. doi: 10.1093/eurjhf/hfp093. [DOI] [PubMed] [Google Scholar]
- 26.van der Zee S, Baber U, Elmariah S, Winston J, Fuster V. Cardiovascular risk factors in patients with chronic kidney disease. Nat Rev Cardiol. 2009 Jul 21; doi: 10.1038/nrcardio.2009.121. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Kovesdy CP, Kalantar-Zadeh K. Review article: Biomarkers of clinical outcomes in advanced chronic kidney disease. Nephrology (Carlton) 2009 Jun;14(4):408–415. doi: 10.1111/j.1440-1797.2009.01119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCullough PA, Lepor NE. The deadly triangle of anemia, renal insufficiency, and cardiovascular disease: implications for prognosis and treatment. Rev Cardiovasc Med. 2005 Winter;6(1):1–10. [PubMed] [Google Scholar]
- 29.McCullough PA, Franklin BA, Leifer E, Fonarow GC. Impact of reduced kidney function on cardiopulmonary fitness in patients with systolic heart failure. Am J Nephrol. 2010;32(3):226–233. doi: 10.1159/000317544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hawkins S, Wiswell R. Rate and mechanism of maximal oxygen consumption decline with aging: implications for exercise training. Sports Med. 2003;33(12):877–888. doi: 10.2165/00007256-200333120-00002. [DOI] [PubMed] [Google Scholar]
- 31.Herzog CA, Muster HA, Li S, Collins AJ. Impact of congestive heart failure, chronic kidney disease, and anemia on survival in the Medicare population. J Card Fail. 2004 Dec;10(6):467–472. doi: 10.1016/j.cardfail.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Anderson S, Halter JB, Hazzard WR, Himmelfarb J, Horne FM, Kaysen GA, Kusek JW, Nayfield SG, Schmader K, Tian Y, Ashworth JR, Clayton CP, Parker RP, Tarver ED, Woolard NF, High KP workshop participants. Prediction, progression, and outcomes of chronic kidney disease in older adults. J Am Soc Nephrol. 2009 Jun;20(6):1199–1209. doi: 10.1681/ASN.2008080860. [DOI] [PubMed] [Google Scholar]
- 33.Shlipak MG, Katz R, Kestenbaum B, Fried LF, Siscovick D, Sarnak MJ. Clinical and subclinical cardiovascular disease and kidney function decline in the elderly. Atherosclerosis. 2009 May;204(1):298–303. doi: 10.1016/j.atherosclerosis.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franklin BA. Survival of the fittest: evidence for high-risk and cardioprotective fitness levels. Curr Sports Med Rep. 2002 Oct;1(5):257–259. doi: 10.1249/00149619-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 35.McCullough PA. Why is chronic kidney disease the"spoiler" for cardiovascular outcomes? J Am Coll Cardiol. 2003 Mar 5;41(5):725–728. doi: 10.1016/s0735-1097(02)02955-8. [DOI] [PubMed] [Google Scholar]
- 36.Stenvinkel P, Carrero JJ, Axelsson J, Lindholm B, Heimbürger O, Massy Z. Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: how do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol. 2008 Mar;3(2):505–521. doi: 10.2215/CJN.03670807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.