Abstract
Severe respiratory viral infections are associated with spread to the alveoli of the lungs. There are multiple murine models of severe respiratory viral infections that have been used to identify viral and host factors that contribute to disease severity. Primary cultures of murine alveolar epithelial cells provide a robust in vitro model to perform mechanistic studies that can be correlated with in vivo studies to identify cell type-specific factors that contribute to pathology within the alveoli of the lung during viral infection. In this study, we established an in vitro model to compare the responses of type I (ATI) and type II (ATII) alveolar epithelial cells to infection by respiratory viruses used in murine models: mouse-adapted severe acute respiratory syndrome-associated coronavirus (SARS-CoV, v2163), murine coronavirus MHV-1, and influenza A (H1N1) virus, strain PR8. Murine alveolar cells cultured to maintain an ATII cell phenotype, determined by expression of LBP180, were susceptible to infection by all three viruses. In contrast, ATII cells that were cultured to trans-differentiate into an ATI-like cell phenotype were susceptible to MHV-1 and PR8, but not mouse-adapted SARS-CoV. Epithelial cells produce cytokines in response to viral infections, thereby activating immune responses. Thus, virus-induced cytokine expression was quantified in ATI and ATII cells. Both cell types had increased expression of IL-1β mRNA upon viral infection, though at different levels. While MHV-1 and PR8 induced expression of a number of shared cytokines in ATI cells, there were several cytokines whose expression was induced uniquely by MHV-1 infection. In summary, ATI and ATII cells exhibited differential susceptibilities and cytokine responses to infection by respiratory viruses. This in vitro model will be critical for future studies to determine the roles of these specialized cell types in the pathogenesis of respiratory viral infection.
Keywords: alveolar epithelial cells, influenza A virus, murine coronavirus, mouse-adapted SARS-coronavirus, type I pneumocyte, type II pneumocyte, cytokines
1. Introduction
Respiratory viral pathogens from several different families are a major source of morbidity and mortality worldwide. While infection of the upper respiratory tract is common and results in subclinical or mild disease, infection of the lungs can result in severe, potentially lethal diseases, including viral pneumonia, acute respiratory distress syndrome (ARDS), and severe acute respiratory syndrome (SARS). These severe diseases can result from infection by currently circulating viruses, including influenza viruses and respiratory syncytial virus (RSV), or by new viruses that emerge in the human population from animal reservoirs, such as new strains of influenza A virus (IAV) and SARS-associated coronavirus (SARS-CoV). Murine models have been invaluable in the identification of virus and host determinants of disease pathogenesis during respiratory viral infections. While these models provide a complex view of host/pathogen interactions, there is a critical need to have physiologically relevant in vitro models that can be used to delineate cell type-specific mechanisms that contribute to disease pathogenesis in the lung. The goal of this study was to develop such an in vitro model, from which data can be correlated to well-established in vivo models of respiratory viral pathogenesis.
The alveolar epithelium is a critical target for severe respiratory virus infections. The extensive surface area of the alveolar epithelium is composed of two morphologically and functionally distinct cell types. Type I alveolar (ATI) cells, which cover 95% of the surface area of the epithelium, are large thin cells that function in gas and ion exchange and fluid transport (Williams, 2003). The type II alveolar (ATII) cells produce pulmonary surfactant that is required to prevent alveolar collapse and proteins that participate in innate defense of the lung (Mason, 2006). As the dividing cells of the alveolar epithelium, ATII cells serve as progenitors to repair damaged epithelium. Infection of ATI or ATII alveolar epithelial cells of the distal lung has been detected in fatal cases of avian (H5N1) and 2009 pandemic (pH1N1) IAV, RSV, and SARS-CoV (Johnson et al., 2007; Nicholls et al., 2006; Shieh et al., 2010; Shieh et al., 2005; Uiprasertkul et al., 2007). Infection of alveolar epithelial cells is also associated with severe disease in murine models of respiratory viral infections, including mouse-adapted IAV and SARS-CoV (Blazejewska et al., 2011; Hrincius et al., 2012; Roberts et al., 2007). Viral infection of these physiologically critical cell types causes direct damage to the alveolar epithelium and also immune-mediated pathology, both of which will impair respiration and/or lead to lung collapse due to impaired surfactant production. Alveolar epithelial cells produce inflammatory cytokines and chemokines in response to viral infection and thereby may elicit responses that contribute to both viral clearance and immune-mediated pathology.
Primary cultures of differentiated alveolar epithelial cells are a valuable model to study virus-host interactions in physiologically relevant cell types in vitro. ATII cells can be isolated from murine lungs and cultured to maintain an ATII cell phenotype or trans-differentiate into cells with an ATI-like phenotype in vitro (Corti et al., 1996; DeMaio et al., 2009; Rice et al., 2002). The goals of this study were to culture primary murine ATII cells to maintain an ATII cell phenotype or trans-differentiate into an ATI cell phenotype, then compare the susceptibility of ATI and ATII cultures to infection by respiratory viruses that cause severe disease in mice: Influenza A virus (PR8; Family Orthomyxoviridae), Murine coronavirus (MHV-1; Family Coronaviridae), and mouse-adapted SARS-CoV (v2163; Family Coronaviridae). We further evaluated expression of inflammatory cytokines by ATI and ATII cultures in response to infection by these viruses. Based on their susceptibility and response to infection by respiratory viral pathogens, these cultures will be valuable in future studies to characterize the differential responses of ATI and ATII cells to viral infection and to identify the pathological mechanisms associated with viral infection in these biologically relevant cell types.
2. Materials and Methods
2.1 Cell lines and viruses
Vero E6 (ATCC: CRL-1586) and Madin-Darby canine kidney (MDCK; ATCC: CCL-34) cells were cultured in MEM (Invitrogen, Carlsbad, CA) containing 10% FBS (Atlanta Biologicals, Norcross, GA) and 1% antibiotic-antimycotic (Invitrogen). 17Cl-1, a spontaneously transformed clone of BALB/c 3T3 cells (provided by Dr. Kathryn Holmes, University of Colorado Denver School of Medicine), were cultured in DMEM (Invitrogen) with 10% FBS and 1% antibiotic-antimycotic (Sturman and Takemoto, 1972). MHV-1 and IAV (A/Puerto Rico/8/1934 (H1N1)) were obtained from the American Type Culture Collection and BEI Resources, respectively. MHV-1 was propagated in 17Cl-1 cells, purified by sucrose gradient centrifugation, and titrated by plaque assay on 17Cl-1 cells, as previously described (Frana et al., 1985; Sturman et al., 1980). PR8 was propagated and titrated by plaque assay in MDCK cells in media containing 1% BSA and TPCK-trypsin (1 ug/ml). Mouse-adapted SARS-CoV (v2163), provided by Dr. Ralph Baric (University of North Carolina), was passaged once in Vero E6 cells before use (Day et al., 2009). All experiments with v2163 were performed in a certified biosafety level 3 laboratory using protocols approved by the University of Idaho Biosafety Committee under guidelines provided in the Biosafety in Microbiological and Biomedical Laboratories, 5th Edition (Centers for Disease Control and Prevention and National Institutes of Health).
2.2 Primary cell isolation and culture
Animal protocols were approved by the University of Idaho Animal Care and Use Committee according to the National Research Council Guide for the Care and Use of Laboratory Animals. Female C57BL/6 mice (8 weeks; 16-21 g) were obtained from the Center for Reproductive Biology at Washington State University (Pullman, WA). ATII cells were isolated from mice using a previously published protocol (Corti et al., 1996). Briefly, epithelial cells were dissociated from lung tissues by incubation in dispase (BD Biosciences, San Jose, CA), followed by mechanical disruption of the alveoli in DMEM with 0.01% DNase (Sigma-Aldrich, St. Louis, MO). The cells were filtered and incubated with biotinylated monoclonal antibodies to CD16/32 and CD45 (Southern Biotechnology, Birmingham, AL), followed by magnetic selection with Dynabeads (Invitrogen) to remove hematopoietic cells. The cells were incubated three times for 40 min at 37°C on tissue culture treated dishes and non-adherent cells were plated as described below. Freshly isolated cells were analyzed by IFA for expression of ATII cell marker protein, LBP180, and ranged from 83-93% positive (Supplemental Fig. 1). To maintain an ATII cell phenotype, the ATII cells were cultured on millicell inserts (EMD Millipore Corp., Billerica, MA) coated with 70% rat tail collagen and 30% BD Matrigel (BD Biosciences), in DMEM/10% FBS supplemented with keratinocyte growth factor (KGF; 10 ng/ml; ProSpec, Rehovot, Israel) for 5 days. To promote trans-differentiation into an ATI cell phenotype, ATII cells were cultured on fibronectin (Sigma-Aldrich; 5 ug/ml) in DMEM/10% FBS for five days. The cultures were maintained at 37°C and 10% CO2.
2.3 Indirect immunofluorescence assay (IFA)
The expression of ATI and ATII phenotypic markers and viral proteins was analyzed by indirect immunofluorescence assay. Cells were fixed with 4% formaldehyde and permeabilized with 0.2% Triton X-100. Cellular proteins were detected with mouse monoclonal antibody to LBP180 (Abcam, Cambridge, MA) or Syrian hamster anti-T1α (provided by Dr. Maria Ramirez, Boston University School of Medicine) and secondary antibodies: goat anti-mouse-488 (Invitrogen) or rabbit anti-hamster-FITC (Abcam), respectively. v2163 infection was detected using monoclonal antibody NR-619, which recognizes the nucleocapsid protein of SARS-CoV (BEI Resources) and anti-mouse IgM-TRITC (Millipore). Surface expression of ACE2 and TMPRSS2 was evaluated on non-permeabilized cells using rabbit primary antibodies (Abcam) and anti-rabbit-488 (Invitrogen). MHV-1 infection was evaluated using a monoclonal antibody that recognizes the nucleocapsid protein of MHV-1 (provided by Dr. Julian Leibowitz, Texas A&M University), followed by goat anti-mouse-488 (Invitrogen). Goat antiserum NR-3148, which recognizes the hemagglutinin protein of PR8 (BEI Resources), and anti-goat-555 (Invitrogen) were used to detect PR8 infection. Cells were stained with DAPI to visualize nuclei and were photographed on a Nikon Eclipse Epifluorescent Microscope with a Hamamatsu digital camera and MetaMorph software (Molecular Devices).
2.4 Western blot analysis
Cells were lysed in RIPA buffer and equal amounts of cell protein, as determined by BCA Protein Assay (Thermo Fisher Scientific, Rockford, IL), were resolved by SDS-PAGE under reducing conditions and transferred to Immobilon PVDF membranes (Millipore). Membranes were incubated with primary antibody to T1α, followed by HRP-conjugated anti-hamster (Abcam), which was detected by chemiluminescence. Protein loading was evaluated using an antibody to β-actin (Abcam).
2.5 Cytokine gene expression analysis
Total cellular RNA was isolated using Trizol Reagent, according to the manufacturer’s recommendations (Invitrogen). Mouse Inflammatory Cytokines and Receptors RT2 Profiler PCR Arrays were used to quantify the expression of 84 inflammatory genes in IAV-, MHV-1-, and mock-inoculated ATI-like cells (SABiosciences/QIAGEN, Valencia, CA). Where indicated, lipopolysaccharide (LPS; Sigma Aldrich; 1 ug/ml) was used to induce cytokine expression. Expression of IL-1β was quantified in mock-inoculated and virus-infected ATI-like and ATII cells by qPCR using published primer sequences (Oh et al., 2011) and SYBR green in a StepOnePlus instrument (Applied Biosystems, Carlsbad, CA). Expression of β-actin was quantified to determine relative expression levels, using primers: 5′-AAGTCCCCTCACCCTCCCAAAAG and 5′-AAGCAATGCTGTCACCTTCCC. Relative expression was determined by the reciprocal of the ΔCt (1/Ct[IL-1β] − Ct[β-actin]). Means and standard errors from at least three experiments were used to test for statistical significance compared to expression in mock-treated cells using an unpaired t test and p<0.05 was determined to be significant.
3. Results
3.1. Phenotypes of primary alveolar epithelial cells
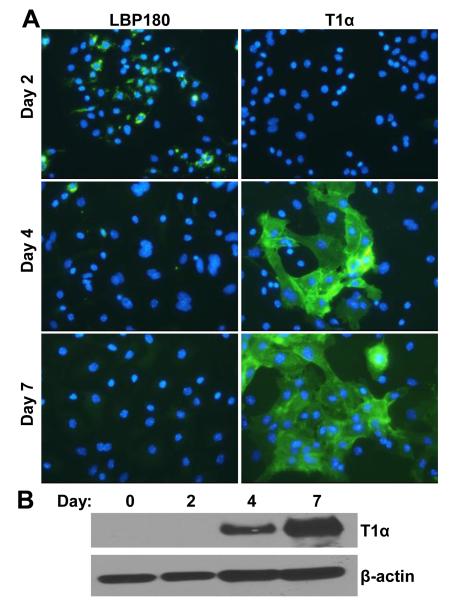
Both ATI and ATII cells are infected by IAV and SARS-CoV in vivo, in fatal human cases and mouse models (Korteweg and Gu, 2008; Nicholls et al., 2006; Roberts et al., 2007; Shieh et al., 2010; Shieh et al., 2005; Tate et al., 2011). In order to study viral infection of these highly specialized cell types in vitro, we evaluated culture conditions to maintain the phenotype of primary murine ATII cells, or to acquire an ATI cell phenotype. Expression of lamellar body protein 180 (LBP180/ABCA3) was evaluated to identify differentiated ATII cells (Mulugeta et al., 2002; Zen et al., 1998). T1α is a marker of differentiated ATI cells that is not expressed by ATII cells, and was used to monitor expression of an ATI-like phenotype (Dobbs et al., 1988; Ramirez et al., 2003). When cultured in vitro, ATII cells readily lose expression of ATII-specific proteins and gain expression of ATI-specific proteins, suggesting trans-differentiation to an ATI cell phenotype (DeMaio et al., 2009; Marsh et al., 2009; Mendez et al., 2006). Furthermore, the loss of ATII-specific markers coincides with changes in morphology and the disappearance of lamellar bodies and surfactant production (Paine et al., 1990; Zen et al., 1998). Freshly isolated ATII cells were analyzed for LBP180 expression by IFA and random fields were counted to estimate the percentage of positive cells. Approximately 90% of freshly isolated cells expressed the ATII-specific protein, LBP180 (Supplemental Fig. 1). The percentage of cells expressing LBP180 rapidly decreased during culture on fibronectin-coated coverslips, to approximately 62% positive by day 2 after isolation (Supplemental Fig. 1 and Fig. 1A). Further culture of murine ATII cells on fibronectin resulted in significantly reduced expression of LBP180 by day 4 after isolation and LBP180 was not detected on day 7 (Fig. 1A, left panels). Under these same culture conditions, the expression of T1α (ATI-specific) was increased by day 4 after isolation, which increased further by day 7 (Figs. 1A and B). Thus, concomitant with the loss of LBP180 expression, these primary cultures acquired expression of T1α, suggesting a transition from a predominantly ATII to ATI-like phenotype.
Fig. 1.
Trans-differentiation of murine ATII cells to an ATI-like cell phenotype. (A) ATII cells were cultured on fibronectin-coated coverslips for the indicated times and immunofluorescence assay was used to detect expression of phenotypic marker proteins of ATII cells, LBP180, or ATI cells, T1α. Nuclei were stained with DAPI, inset panels. (B) ATII cells were lysed on the day of isolation (day 0) or cultured on fibronectin and lysed on the indicated days. Cell lysates were analyzed by western blot analysis using antibody against T1α or β-actin, as a protein loading control. The images shown are representative of three replicate experiments.
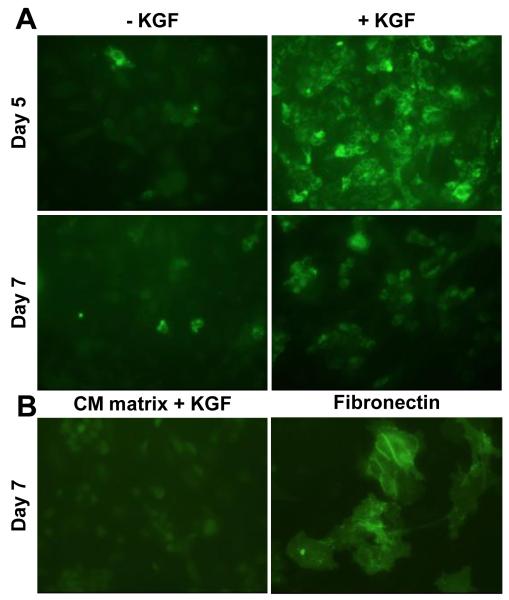
In order to promote expansion of the ATII cells while maintaining their phenotype, we plated the cells on 70% rat tail collagen and 30% Matrigel (CM matrix) and provided KGF in the growth medium. ATII cells cultured on CM matrix with KGF maintained expression of LBP180 through 7 days after isolation, with maximal expression on day 5 (Fig. 2A, right panels). Exclusion of KGF from the medium resulted in significantly fewer LBP180-expressing cells on days 5 and 7 after isolation (Fig. 2A, left panels). Furthermore, ATII cells cultured on CM matrix with KGF did not express an ATI cell-specific protein, T1α, in comparison to trans-differentiated ATI-like cells cultured on fibronectin (Fig. 2B). ATII cells cultured on matrices with an increased proportion of collagen to matrigel (80:20), had reduced expression of LBP180 (Data not shown). Thus, murine ATII cells maintained expression of LBP180 for short time periods (2 days) of culture on fibronectin alone and for longer time periods (5-7 days) when cultured on 70:30 CM matrix with KGF.
Fig. 2.
Maintenance of ATII cell phenotype in cultured murine cells. ATII cells were cultured on 70% collagen/30% matrigel (CM) matrix for 5 or 7 days with or without keratinocyte growth factor (KGF) in the medium. Immunofluorescence assay was used to detect the expression of (A) ATII marker protein, LBP180, or (B) ATI marker protein, T1α. Cells cultured on fibronectin were used as a positive control for T1α expression.
3.2 Mouse-adapted SARS-CoV, v2163, infects murine cultures with an ATII, but not ATI, cell phenotype
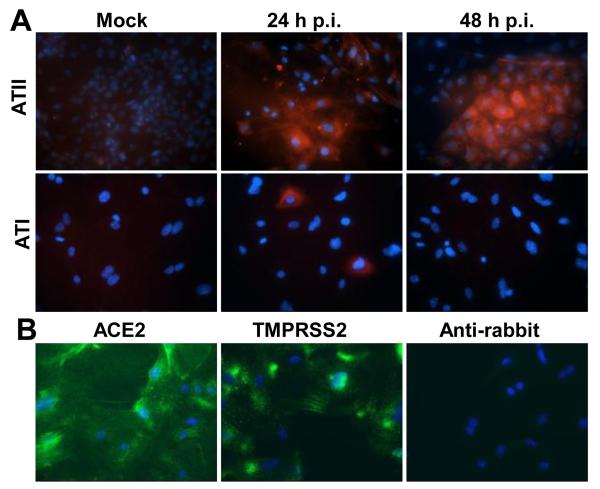
SARS-CoV RNA and antigens were detected in both ATI and ATII cells in lung tissues from fatal SARS cases (Nicholls et al., 2006). Human isolates of SARS-CoV do not cause lethal disease in mice, but acquire virulence upon serial passage in mice (Day et al., 2009; Roberts et al., 2007). These mouse-adapted isolates are used to study the mechanisms of SARS-CoV pathogenesis in vivo (Frieman et al., 2012). v2163 is an isolate of SARS-CoV that was passaged 25 times in the lungs of BALB/c mice and causes a highly lethal pulmonary infection in mice (Day et al., 2009). We tested murine pneumocytes with either an ATI or ATII cell phenotype for susceptibility to infection by v2163. Cultures with an ATI cell phenotype rarely showed cells with viral antigen 24 h post-inoculation (p.i.), and no viral antigen was detected by 48 h p.i. (Figure 3A, bottom panels). In contrast, viral antigen was detected at both 24 and 48 h p.i. in cells cultured with an ATII phenotype (Figure 3A, top panels). Thus, mouse-adapted SARS-CoV preferentially infected alveolar epithelial cells with an ATII cell phenotype in vitro.
Fig. 3.
Susceptibility of primary murine alveolar epithelial cells to infection by mouse-adapted SARS-CoV. (A) Murine cells were cultured as either an ATI or ATII cell phenotype for 5 days, then were inoculated with mouse-adapted SARS-CoV, isolate v2163 for 24 or 48 h. Infection was analyzed by immunofluorescence assay of viral nucleocapsid protein (red) and nuclei were stained with DAPI (blue). (B) Cells cultured for 5 days with an ATI cell phenotype were analyzed by immunofluorescence using rabbit antibodies against ACE2 or TMPRSS2 (green) and nuclei were stained with DAPI (blue). A fluorescently labeled antibody against rabbit IgG was used as a negative control.
In order to determine if the lack of SARS-CoV receptor expression by ATI-like cells was responsible for them being refractory to v2163 infection, we evaluated expression of murine ACE2 by IFA on non-permeabilized ATI-like cells. ATI-like cells that were evaluated for ACE2 expression on day 5 after isolation, the same day cells were inoculated with v2163, had robust expression of ACE2 on the cell surface (Fig. 3B). In addition, these cells were evaluated for expression of TMPRSS2, a serine protease that interacts with ACE2 and enhances infection of cells by SARS-CoV (Shulla et al., 2011). The ATI-like cells also had robust surface expression of TMPRSS2 on day 5 after isolation (Fig. 3B). Thus, expression of ACE2 and TMPRSS2 does not correspond with the susceptibility of murine alveolar epithelial cells to infection by v2163.
3.3. MHV-1 replicates in primary murine cultures with an ATI or ATII cell phenotype
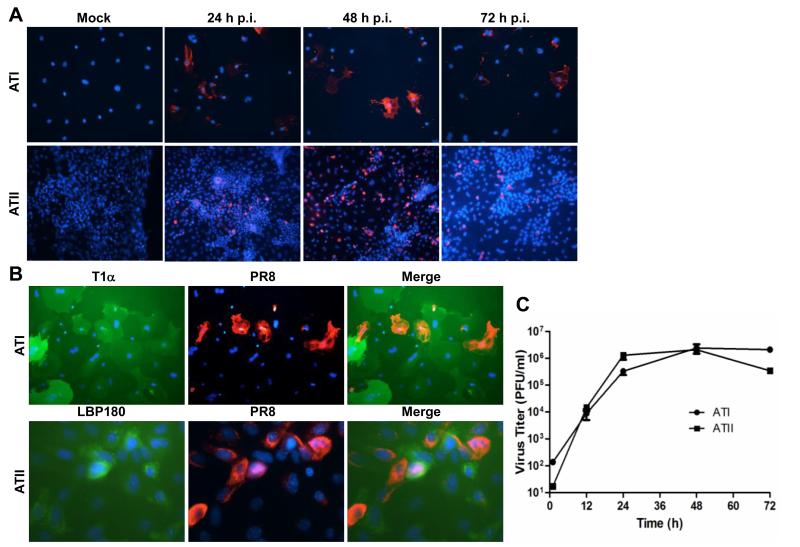
MHV-1 is a murine coronavirus that causes severe disease and pathology in the lungs of specific genetic lines of mice (De Albuquerque et al., 2006; Khanolkar et al., 2009). The pathology of MHV-1 infection in A/J mice resembles that seen in fatal SARS cases, thus MHV-1 is studied as a model of SARS pulmonary pathogenesis (De Albuquerque et al., 2006). ATI-like cells were inoculated with MHV-1 and monitored for cytopathic effects (CPE), the presence of viral antigen, and production of infectious virus over time. MHV-1 infection of ATI-like cells resulted in the formation of syncytia and rounding up of cells in the monolayer by 24 h p.i. (Fig. 4A, top panels, and 4B). Viral nucleocapsid protein antigen was detected in approximately 50% of the cells by 12 h p.i., and expanded to the majority of cells in the culture by 24 h p.i. (Fig. 4A, top panels). The presence of viral antigen corresponded to increased release of infectious virus through 24 h p.i. (Fig. 4C).
Fig. 4.
Susceptibility of primary murine alveolar epithelial cells to infection by murine coronavirus, MHV-1. (A) Murine cells were cultured as either an ATI or ATII cell phenotype for 5 days, then were inoculated with MHV-1 for 12 h or 24 h. Infection was analyzed by immunofluorescence assay of viral nucleocapsid protein (green) and nuclei were stained with DAPI (blue). (B) Cells were cultured on fibronectin (ATI phenotype) and inoculated with MHV-1 or mock-inoculated. Cells were photographed on phase contrast at 24 h p.i. (C) Infection of MHV-1-inoculated ATI and ATII cells was analyzed by plaque assay of supernatant medium at the indicated times. The mean virus titers and standard errors from four replicate experiments are shown.
ATII cultures were also tested for susceptibility to infection by MHV-1. In agreement with infection of ATI-like cells, viral antigen was detected in ATII cells at 12 and 24 h p.i., and the titer of infectious virus in the medium increased over a similar time course (Fig. 4C). In contrast to infection of ATI-like cells, MHV-1 did not induce syncytia formation or visible CPE in ATII cell cultures. Despite a lower proportion of viral antigen-expressing cells, cultures with an ATII phenotype produced higher titers of virus through 24 h p.i. (Fig. 4C). Syncytia formation and cell death seen in the ATI-like cells may limit the production of MHV-1 compared to infection in ATII cultures. These results show that MHV-1 established a productive infection in cultures with either ATI or ATII cell phenotypes, which differed in CPE and virus production.
3.4 PR8 replicates in primary murine cultures with an ATI or ATII cell phenotype
The PR8 strain of influenza A virus is frequently used as a model for viral pneumonia, and the spread of PR8 to the alveoli in infected mice corresponds with increased disease severity (Blazejewska et al., 2011; Hrincius et al., 2012). To determine whether primary murine cells with an ATI or ATII cell phenotype are susceptible to PR8 infection in vitro, primary cultures were inoculated with PR8 and infection was analyzed by IFA and plaque assay. Like murine coronavirus, MHV-1, PR8 infected primary cultures with both ATI and ATII cell phenotypes (Fig. 5). However, the kinetics of infection was slower, with the peak of viral antigen expressing cells present at 48 h p.i. Although CPE were not apparent, reduced viral antigen expression late in infection (72 h p.i.) suggests a decline in the number of susceptible cells in the culture. The viral growth curves for PR8 replication in ATI and ATII cells were indistinguishable (Fig. 5C).
Fig. 5.
Susceptibility of primary murine alveolar epithelial cells to infection by influenza A virus, PR8. (A) Murine cells were cultured as either an ATI or ATII cell phenotype for 5 days, then were inoculated with PR8. Infection was analyzed by immunofluorescence assay of viral hemagglutinin protein (red) and nuclei were stained with DAPI (blue) at the indicated times post infection (p.i.). (B) Co-localization of ATI (T1α) or ATII (LBP180) phenotypic proteins (green) and PR8 antigen (red) was analyzed by dual IFA 24 h p.i. Nuclei were stained with DAPI (blue). (C) Infection of PR8-inoculated ATI and ATII cells was analyzed by plaque assay of supernatant medium at the indicated times. The mean virus titers and standard errors from five replicates are shown.
In both ATI and ATII cultures, a lower proportion of cells were infected by PR8 compared to MHV-1. As our phenotypic analysis of these cultures demonstrated heterogeneity in expression of ATI and ATII marker proteins (Fig. 1 and 2), we next determined whether PR8 infects differentiated cells or other cells within these cultures. Co-localization of viral antigen and T1α (ATI-specific protein) were evaluated in ATI cultures 24 h after inoculation with PR8 by dual IFA. A majority of cells that contained PR8 antigen were also positive for T1α expression, which demonstrated that PR8 infects cells with an ATI cell phenotype (Fig. 5B, top panels). However, many T1α positive cells were not infected by PR8. Similarly, in cells cultured with an ATII phenotype, PR8 antigens were detected in cells expressing ATII phenotypic marker, LBP180 (Fig. 5B, bottom panels). However, PR8 infection was not exclusively found in LBP180-positive cells. A majority of ATII cells plated on fibronectin remained LBP180 positive by day 2 after isolation (Supplemental Fig. 1). Therefore, we inoculated ATII cells with PR8 one day after isolation and performed dual IFA for LBP180 and PR8 antigens 24 h p.i. (Supplemental Fig. 2). As with our ATII cells plated on CM matrix, some but not all LBP180 positive cells were also positive for PR8 antigens. Taken together, these experiments demonstrate that PR8 does infect alveolar cells expressing ATI or ATII specific proteins.
3.5 Cytokine gene expression in virus-infected alveolar epithelial cells
Alveolar epithelial cells express cytokines and chemokines in response to viral infection, thereby contributing to the influx of inflammatory cells into the lungs. We evaluated the response of ATI-like cells to infection by MHV-1 and PR8 using RT-PCR arrays that quantify RNA for 84 cytokines. Compared to mock-inoculated cells, PR8 and MHV-1 induced mRNA expression of a shared group of cytokines (IL-10, IL-16, IL-1β, IL-36α, TNF-α, LT-α, LT-β), chemokines (CCL2, 4, 7, 8, CXCL1, 10, 14), and receptors (CCR3, TNFRII) (Table 1). In addition to these shared cytokines, MHV-1 induced expression of several additional genes that were not induced by PR8 infection in ATI-like cells (Supplemental Table 1). In contrast, only three chemokine genes (CCL5, CCL9, and CXCL11) were differentially expressed by PR8-infected cells.
Table 1. Cytokine gene expression in ATI-like cells infected by IAV and MHV-1a.
| Fold Change vs. Mockb |
||
|---|---|---|
| Gene | IAV | MHV-1 |
| Bcl6 | 2.8 | 4.0 |
| Ccl2 | 2.2 | 3.2 |
| Ccl20 | 3.1 | 11.1 |
| Ccl4 | 2.1 | 2.0 |
| Ccl7 | 2.2 | 3.0 |
| Ccl8 | 2.6 | 2.3 |
| Ccr3 | 3.2 | 3.8 |
| Cxcl1 | 5.0 | 11.5 |
| Cxcl10 | 8.0 | 5.2 |
| Cxcl13 | 5.6 | 2.1 |
| Il10 | 2.4 | 10.7 |
| Il16 | 2.8 | 5.8 |
| Il1b | 2.1 | 10.3 |
| Il1f6 | 3.0 | 3.7 |
| Lta | 2.6 | 5.6 |
| Ltb | 2.3 | 25.2 |
| Tnf | 9.1 | 6.0 |
| Tnfrsf1b | 2.7 | 5.2 |
RNA was extracted from IAV or MHV-1 infected ATI-likecells 24 hpi and mRNA for inflammatory cytokines and receptors was quantified by qPCR Array (SABiosciences). Genes included are those with at least a two fold increase compared to mock-inoculated cells in at least two replicate experiments.
Fold change values are representative values from three (IAV) or two (MHV-1) replicate samples.
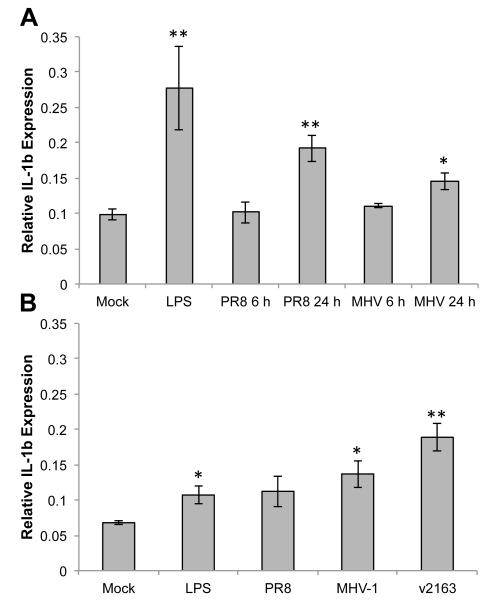
As a canonical pro-inflammatory cytokine that contributes to the local recruitment and activation of inflammatory cells in addition to systemic symptoms of viral infection, the production of IL-1β has important consequences for both viral clearance and disease severity. Expression of IL-1β mRNA was quantified by qRT-PCR in ATI-like cells infected by MHV-1 or PR8 at 6 and 24 h p.i. (Fig. 6A), and in ATII cells infected by PR8, MHV-1, or v2163 at 24 h p.i. (Fig. 6B). LPS was used as a positive control to induce IL-1β expression in both cell types. Expression of IL-1β was induced significantly by PR8 and MHV-1 infection of ATI-like cells by 24 h p.i. (Fig. 6A). MHV-1 infection stimulated a similar increase in IL-1β expression in both ATI-like and ATII cells (Fig. 6A and B), however PR8 infection in ATII cells stimulated a lower level of IL-1β expression, which did not reach statistical significance (Fig. 6B). Infection of ATII cells by v2163 also resulted in increased IL-1β expression (Fig. 6B). Interestingly, while both ATI-like and ATII cells expressed IL-1β in response to LPS, expression in ATI-like cells was induced to a higher level. These results show that primary murine alveolar epithelial cells respond differently to viral infection and LPS stimulation under culture conditions that generate an ATI-like or ATII phenotype.
Fig. 6.
Expression of IL-1β by primary murine alveolar epithelial cells in response to respiratory viral infection. Murine cells were cultured as an ATI (A) or ATII (B) cell phenotype, infected by the indicated viruses, and IL-1β mRNA was quantified by RT-qPCR. All ATII cell samples (B) were infected for 24 h. Mock-inoculated and LPS-treated cells were used as negative and positive controls, respectively. Expression of IL-1β was normalized to that of β-actin and the means and standard errors from 3-5 replicate experiments are shown. Statistically significant differences in expression compared to mock samples were determined by unpaired t test. *= p<0.05, **= p<0.005
4. Discussion
Due to its critical function in respiration, damage to the alveolar epithelium by viral infection results in severe, potentially fatal, disease. In order to design novel therapies that successfully limit this damage, it is imperative that we identify the mechanisms that contribute to lung pathology during infection of alveolar epithelial cells. The response of alveolar epithelial cells to viral infection has been studied in a variety of models, including continuous cell lines and primary cell cultures. Continuous cell lines, such as A549 that are used as a model of ATII cells, have key biochemical and structural differences compared to their in vivo counterparts (Mason and Williams, 1980; Swain et al., 2010). These differences limit the usefulness of continuous lines in identifying physiologically relevant mechanisms of viral pathogenesis. Primary alveolar epithelial cells isolated from human lung tissues have been used to study respiratory viral pathogenesis in differentiated cell types in vitro (Kosmider et al., 2012; Mossel et al., 2008; Wang et al., 2011; Wang et al., 2009). However, access to human lung tissues and genetic variability are factors that limit their usefulness for mechanistic studies. Primary rat alveolar epithelial cells cultured with distinct ATI and ATII phenotypes have been widely used as in vitro models for studies of lung physiology (Gonzalez et al., 2005; Qiao et al., 2003). Limitations of rat model systems include the very few genetic knock-out lines and established models of respiratory viral infections (Funk et al., 2009; Miura et al., 2007; Rzepka et al., 2012). Based on the availability of well-characterized genetic lines and established models of respiratory viral pathogenesis, it is highly desirable to establish murine ATI and ATII cell models to study virus-host interactions in the differentiated cell types of the alveolar epithelium. Other researchers have optimized the isolation and culture of differentiated ATII cells from mice and established culture conditions to maintain their ATII phenotype or trans-differentiate into cells with the morphological and functional characteristics of ATI cells (Corti et al., 1996; Herold et al., 2006; Rice et al., 2002). Murine ATI-like cells that were derived from isolated ATII cells have been used to study influenza virus infection in vitro (Corti et al., 1996; Herold et al., 2006; Rice et al., 2002). In the present study, primary murine alveolar cells were cultured with ATI or ATII cell phenotypes and their susceptibility and inflammatory response to infection by respiratory viruses were compared. This in vitro system will be invaluable in future studies to identify the mechanisms of pathogenesis during viral infection of the alveolar epithelium that can be directly compared to robust murine models of viral pathogenesis in vivo.
Serial passage of the Urbani strain of SARS-CoV in the lungs of mice resulted in enhanced virulence of the virus in mice and thus provides an animal model that replicates the disease pathogenesis observed in human patients (Day et al., 2009; Roberts et al., 2007). The v2163 strain, which is the result of 25 passages in the lungs of BALB/c mice, causes dose-dependent lethality in young (5-6 week old) mice (Day et al., 2009). During serial passage, v2163 acquired four mutations that result in amino acid changes in the spike glycoprotein. Two of these changes are in residues of the receptor binding motif that interacts with ACE2 and, as predicted by structural modeling, may enhance binding to murine ACE2 (Day et al., 2009; Frieman et al., 2012). Adaptation of the spike glycoprotein to bind more efficiently to ACE2 proteins of different species is thought to be critical for changes in the host range and tissue tropism of SARS-CoV (Becker et al., 2008; Frieman et al., 2012; Li, 2008; Sheahan et al., 2008). We observed that primary murine ATI-like cells were not susceptible to infection by v2163, so ACE2 expression by these cells was evaluated. ATI-like cells had robust surface expression of ACE2, suggesting that ACE2 binding is not the limiting factor in susceptibility to v2163 infection. Other groups have recently demonstrated the importance of a cellular protease, TMPRSS2, in enhancing ACE2-dependent and -independent infection by SARS-CoV (Glowacka et al., 2011; Matsuyama et al., 2010; Shulla et al., 2011). It is not known whether TMPRSS2 is important for infection by mouse-adapted strains of SARS-CoV. Regardless, we detected high levels of TMPRSS2 on the surface of murine ATI-like cells. The mechanism(s) that restricts replication of v2163 in murine ATI-like cells is not known, but our study suggests that expression of murine ACE2 and TMPRSS2 are not limiting factors. Like our study, Mossel et al. found human primary ATI-like cells to be refractory to SARS-CoV infection in vitro (Mossel et al., 2008). However, they observed that SARS-CoV infection of primary cells corresponded with the level of ACE2 expressed by the cells.
In addition to mouse-adapted SARS-CoV, we evaluated the susceptibility of murine ATI-like and ATII cells for infection by a murine coronavirus, MHV-1. MHV-1 infects the respiratory tract and causes disease with a wide range of severities, depending upon the genetic line of mice (De Albuquerque et al., 2006; Khanolkar et al., 2009). Upon intranasal inoculation with MHV-1, BALB/cJ mice exhibit some of the pathological characteristics of SARS in humans, including pulmonary congestion, alveolar and interstitial inflammation, and hyaline membranes. However, MHV-1 infection is not lethal in BALB/cJ mice, which show only mild clinical signs of disease and recover completely. In contrast, infection of A/J mice with MHV-1 results in severe lung pathology and death of infected mice. The lung pathology seen in MHV-1 infected A/J mice is similar to fatal SARS cases in humans, including severe edema, thickening of the alveolar epithelium, interstitial inflammation, fibrin deposits, and hyaline membranes. Although MHV-1 virions have been detected by electron microscopy in alveolar macrophages late during infection of A/J mice, infection of pneumocytes early during infection cannot be excluded. Furthermore, ATI cells express fibroleukin during MHV-1 infection of A/J mice, suggesting a role for these cells in fibrin deposition in the alveoli (De Albuquerque et al., 2006). Primary murine ATI-like cells provide an attractive model for studying the signaling pathways involved in MHV-1-induced fibroleukin expression and their potential role in fibrin deposition during viral infection in the lung. The mechanisms responsible for the differential pathogenesis seen during respiratory infection of various mouse strains by MHV-1 have not been determined. Future studies using primary alveolar epithelial cells from different genetic lines of mice will determine the potential contributions of these cell types in pathogenic outcomes of infection.
The pulmonary pathology in fatal cases of influenza viral pneumonia is characterized by diffuse alveolar damage, including the presence of hyaline membranes, inflammatory cells, and edema in the alveoli (Liem et al., 2008; Nakajima et al., 2012; Shieh et al., 2010). The mouse-adapted PR8 strain of influenza A virus has been widely studied as a model for influenza pneumonia because it induces similar pathology in specific inbred strains of mice (Blazejewska et al., 2011; Fukushi et al., 2011). The virulence of different PR8 variants in various murine genetic lines is correlated with increased viral replication and spread to the alveolar regions of the lung (Blazejewska et al., 2011). Infection of the alveoli by PR8 results in dramatic pathology associated with inflammation, leukocytic infiltration, and destruction of the epithelial surface (Blazejewska et al., 2011; Fukushi et al., 2011; Loosli et al., 1975). Epithelial damage is likely due to a combination of direct viral cytopathic effects and the inflammatory response. Inhibition of inflammatory mediators during influenza virus infection in mice lessens morbidity and mortality, suggesting that the inflammatory response is a critical determinant of severe disease outcomes (Herold et al., 2008; Le Goffic et al., 2006; Vlahos et al., 2011). Herold et al. further demonstrated that macrophages recruited to the lungs during PR8 infection in mice have a direct role in alveolar damage by inducing apoptosis of ATI cells (Herold et al., 2008). Murine ATII cells cultured to acquire an ATI cell phenotype in vitro have been used to study influenza virus infection in the alveolar epithelium and how infected ATI cells interact with monocytes to mediate disease pathology (Herold et al., 2008; Herold et al., 2006). Our study confirmed results of previous studies demonstrating susceptibility of and cytokine expression by murine alveolar epithelial cells in response to PR8 infection (Herold et al., 2006; Tate et al., 2011). We also established the susceptibility of primary murine cells with an ATII phenotype to PR8 infection. While infection of ATI and ATII cultures by PR8 was not as robust as MHV-1, a significant amount of infectious PR8 was released from these cultures. We further demonstrated that PR8 infection co-localized with ATI and ATII phenotypic markers, suggesting infection of these cell types. However, in ATII cell cultures, PR8 antigens were also detected in LBP180-negative cells. While the exact nature of these cells is not known, many possibilities exist. Our ATII cell isolations ranged from 83-93% LBP180-positive, which is similar to the purity reported by others (Corti et al., 1996; Messier et al., 2012). Thus, the LBP180-negative cells could be contaminating cell types from the isolation procedure, including fibroblasts, endothelial cells, or white blood cells. Furthermore, ATII cells can trans-differentiate into ATI-like cells or undergo epithelial to mesenchymal transition when cultured in vitro (Kim et al., 2006). The LBP180-negative cells in our cultures may be ATII cells that are no longer fully differentiated. However, our dual antigen labeling demonstrated that alveolar cells with either ATI or ATII phenotype can be infected by PR8. As ATI and ATII cells are both targeted in severe influenza virus infections, it is important to identify the responses of these unique cell types to infection. In addition to inflammation-induced damage, electron microscopy has demonstrated budding of PR8 virions from ATI and ATII cells early during infection with extensive damage to the epithelial surface over time (Loosli et al., 1975). Differentiated cultures of alveolar epithelial cells will be critical for identifying the mechanisms responsible for direct viral damage to the alveolar epithelium during infection.
The pathogenesis of respiratory viral infections is often correlated with an excessive inflammatory response in the lungs. Alveolar epithelial cells express proinflammatory cytokines and chemokines in response to viral infection and may be important in the initiation of these damaging inflammatory responses (Herold et al., 2006; Miura et al., 2007; Rzepka et al., 2012; Tate et al., 2011; Wang et al., 2011). In this study, we compared cytokine expression by murine ATI-like cells infected by MHV-1 or PR8, and IL-1β expression by ATI-like and ATII cells infected by MHV-1, PR8, or v2163. Infection of ATI-like cells by MHV-1 or PR8 induced similar cytokine profiles, which correspond to cytokine expression in the lungs of infected mice (Alberts et al., 2010; Leibowitz et al., 2010; Srivastava et al., 2009). Interestingly, MHV-1 also stimulated expression of several cytokines and chemokines that were not expressed upon PR8 infection. It is not known whether this difference has biological relevance during in vivo infection. The inflammatory response to MHV-1 has been characterized in A/J mice, which have severe disease outcomes to infection (De Albuquerque et al., 2006). Our primary cells were isolated from C57Bl/6 mice, which have mild infection in the respiratory tract by MHV-1 (De Albuquerque et al., 2006). The mechanisms that underlie the differences in pathogenesis of MHV-1 infection in different genetic lines of mice have not been clearly defined. In general, increased cytokine expression correlates with disease severity in both MHV-1 and PR8 infection models. Thus, it will be important to identify the mechanisms whereby alveolar epithelial cells detect viral infection and determine their role in the detrimental inflammatory response to viral infection in the lung. ATI and ATII cells are both targets for viral infection within the alveoli. In this study, we directly compared expression of IL-1β by murine ATI-like and ATII cells in response to viral infection. IL-1β expression was induced by LPS to a high level in ATI-like, but only moderately in ATII cells. Raoust et al. also observed cytokine expression by primary murine cells cultured with an ATI cell phenotype upon treatment with LPS (Raoust et al., 2009). A recent study demonstrated that murine ATII cells cultured similarly to those in our study express chemokines CCL2 and CXCL2 in response to LPS treatment, suggesting that these cells are capable of LPS-induced responses through TLR4 signaling (Bello-Irizarry et al., 2012). Infection by PR8 and MHV-1 induced IL-1β expression by 24 h p.i. in ATI-like cells. Viral infection induced expression of IL-1β in ATII cells, yet similar to LPS, expression was not as robust as in ATI cells. Thus, ATI-like and ATII cells responded differently to the same viral infections, and the levels of IL-1β expression was dependent both on the cell phenotype and the specific virus. Other studies have reported differential expression of cytokines by primary alveolar epithelial cells cultured with an ATI-like or ATII cell phenotype (Mir-Kasimov et al., 2012; Yu et al., 2011). Human primary alveolar epithelial cells cultured with an ATI-like or ATII phenotype express very similar levels of cytokine mRNA upon infection by influenza viruses (Yu et al., 2011). Despite similarities in mRNA levels, secretion of MCP-1 is dramatically increased upon influenza virus infection of ATI-like, but not ATII, cells (Yu et al., 2011). A similar finding between our study and others is the observation that despite having a similar susceptibility to viral infection, cultures with ATI and ATII phenotypes can have different responses to these infections. Determining the mechanisms whereby ATI and ATII cells detect viral infections and regulate cytokine expression in the lungs is a critical next step of these studies. Primary murine alveolar epithelial cells will be crucial in the identification of these mechanisms.
Supplementary Material
Highlights.
Alveolar epithelial cells are important targets in severe respiratory viral infection
Murine ATI and ATII cultures are an in vitro model for viral pathogenesis
ATI cells are infected by IAV and MHV-1, not mouse-adapted SARS-CoV
ATII cells are infected by IAV, MHV-1, and mouse-adapted SARS-CoV
ATI and ATII cells express cytokines upon infection by respiratory viruses
Acknowledgements
This study was supported by a Career Development Award from the Pacific Northwest Regional Center of Excellence (NIH/NIAID: U54 AI081680) and by grant P20 RR015587 from the National Center for Research Resources (NCRR/NIH). L.P.K. is supported by the Botswana International University of Science and Technology (BIUST). The sponsors had no role in study design, collection, analysis, and interpretation of the data, writing the report, and in the decision to publish the results of the study. The authors are grateful to Drs. Ralph Baric and Amy Sims, University of North Carolina, Dr. Kathryn Holmes, University of Colorado at Denver School of Medicine, Dr. Julian Leibowitz, Texas A&M University, and Drs. Maria Ramirez and Mary Williams, Boston University School of Medicine for viruses, cells, and antibodies that were used in this study. The following reagents were obtained through the NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH: Influenza A Virus, A/Puerto Rico/8/34 (H1N1), NR-3169; Polyclonal Anti-Influenza Virus H1 (H0) Hemagglutinin (HA), A/Puerto Rico/8/34 (H1H1), (Antiserum, Goat), NR-3148; and Monoclonal Anti-SARS-CoV N Protein (Similar to 42C), NR-619. Finally, the authors would like to thank Dr. Elizabeth Fortunato for careful reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberts R, Srivastava B, Wu H, Viegas N, Geffers R, Klawonn F, Novoselova N, do Valle TZ, Panthier JJ, Schughart K. Gene expression changes in the host response between resistant and susceptible inbred mouse strains after influenza A infection. Microbes and infection / Institut Pasteur. 2010;12(4):309–318. doi: 10.1016/j.micinf.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Becker MM, Graham RL, Donaldson EF, Rockx B, Sims AC, Sheahan T, Pickles RJ, Corti D, Johnston RE, Baric RS, Denison MR. Synthetic recombinant bat SARS-like coronavirus is infectious in cultured cells and in mice. Proc Natl Acad Sci U S A. 2008;105(50):19944–19949. doi: 10.1073/pnas.0808116105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello-Irizarry SN, Wang J, Olsen K, Gigliotti F, Wright TW. The Alveolar Epithelial Cell Chemokine Response to Pneumocystis Requires Adaptor Molecule MyD88 and Interleukin-1 Receptor but Not Toll-Like Receptor 2 or 4. Infection and immunity. 2012;80(11):3912–3920. doi: 10.1128/IAI.00708-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazejewska P, Koscinski L, Viegas N, Anhlan D, Ludwig S, Schughart K. Pathogenicity of different PR8 influenza A virus variants in mice is determined by both viral and host factors. Virology. 2011;412(1):36–45. doi: 10.1016/j.virol.2010.12.047. [DOI] [PubMed] [Google Scholar]
- Corti M, Brody AR, Harrison JH. Isolation and primary culture of murine alveolar type II cells. Am J Respir Cell Mol Biol. 1996;14(4):309–315. doi: 10.1165/ajrcmb.14.4.8600933. [DOI] [PubMed] [Google Scholar]
- Day CW, Baric R, Cai SX, Frieman M, Kumaki Y, Morrey JD, Smee DF, Barnard DL. A new mouse-adapted strain of SARS-CoV as a lethal model for evaluating antiviral agents in vitro and in vivo. Virology. 2009;395(2):210–222. doi: 10.1016/j.virol.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Albuquerque N, Baig E, Ma X, Zhang J, He W, Rowe A, Habal M, Liu M, Shalev I, Downey GP, Gorczynski R, Butany J, Leibowitz J, Weiss SR, McGilvray ID, Phillips MJ, Fish EN, Levy GA. Murine hepatitis virus strain 1 produces a clinically relevant model of severe acute respiratory syndrome in A/J mice. J Virol. 2006;80(21):10382–10394. doi: 10.1128/JVI.00747-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaio L, Tseng W, Balverde Z, Alvarez JR, Kim KJ, Kelley DG, Senior RM, Crandall ED, Borok Z. Characterization of mouse alveolar epithelial cell monolayers. American journal of physiology. Lung cellular and molecular physiology. 2009;296(6):L1051–1058. doi: 10.1152/ajplung.00021.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs LG, Williams MC, Gonzalez R. Monoclonal antibodies specific to apical surfaces of rat alveolar type I cells bind to surfaces of cultured, but not freshly isolated, type II cells. Biochim Biophys Acta. 1988;970(2):146–156. doi: 10.1016/0167-4889(88)90173-5. [DOI] [PubMed] [Google Scholar]
- Frana MF, Behnke JN, Sturman LS, Holmes KV. Proteolytic cleavage of the E2 glycoprotein of murine coronavirus: host-dependent differences in proteolytic cleavage and cell fusion. J Virol. 1985;56(3):912–920. doi: 10.1128/jvi.56.3.912-920.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieman M, Yount B, Agnihothram S, Page C, Donaldson E, Roberts A, Vogel L, Woodruff B, Scorpio D, Subbarao K, Baric RS. Molecular determinants of severe acute respiratory syndrome coronavirus pathogenesis and virulence in young and aged mouse models of human disease. J Virol. 2012;86(2):884–897. doi: 10.1128/JVI.05957-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushi M, Ito T, Oka T, Kitazawa T, Miyoshi-Akiyama T, Kirikae T, Yamashita M, Kudo K. Serial histopathological examination of the lungs of mice infected with influenza A virus PR8 strain. PLoS One. 2011;6(6):e21207. doi: 10.1371/journal.pone.0021207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CJ, Manzer R, Miura TA, Groshong SD, Ito Y, Travanty EA, Leete J, Holmes KV, Mason RJ. Rat respiratory coronavirus infection: replication in airway and alveolar epithelial cells and the innate immune response. J Gen Virol. 2009;90(Pt 12):2956–2964. doi: 10.1099/vir.0.014282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowacka I, Bertram S, Muller MA, Allen P, Soilleux E, Pfefferle S, Steffen I, Tsegaye TS, He Y, Gnirss K, Niemeyer D, Schneider H, Drosten C, Pohlmann S. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. 2011;85(9):4122–4134. doi: 10.1128/JVI.02232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Yang YH, Griffin C, Allen L, Tigue Z, Dobbs L. Freshly isolated rat alveolar type I cells, type II cells, and cultured type II cells have distinct molecular phenotypes. American journal of physiology. Lung cellular and molecular physiology. 2005;288(1):L179–189. doi: 10.1152/ajplung.00272.2004. [DOI] [PubMed] [Google Scholar]
- Herold S, Steinmueller M, von Wulffen W, Cakarova L, Pinto R, Pleschka S, Mack M, Kuziel WA, Corazza N, Brunner T, Seeger W, Lohmeyer J. Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand. J Exp Med. 2008;205(13):3065–3077. doi: 10.1084/jem.20080201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold S, von Wulffen W, Steinmueller M, Pleschka S, Kuziel WA, Mack M, Srivastava M, Seeger W, Maus UA, Lohmeyer J. Alveolar epithelial cells direct monocyte transepithelial migration upon influenza virus infection: impact of chemokines and adhesion molecules. J Immunol. 2006;177(3):1817–1824. doi: 10.4049/jimmunol.177.3.1817. [DOI] [PubMed] [Google Scholar]
- Hrincius ER, Hennecke AK, Gensler L, Nordhoff C, Anhlan D, Vogel P, McCullers JA, Ludwig S, Ehrhardt C. A single point mutation (Y89F) within the non-structural protein 1 of influenza A viruses limits epithelial cell tropism and virulence in mice. The American journal of pathology. 2012;180(6):2361–2374. doi: 10.1016/j.ajpath.2012.02.029. [DOI] [PubMed] [Google Scholar]
- Johnson JE, Gonzales RA, Olson SJ, Wright PF, Graham BS. The histopathology of fatal untreated human respiratory syncytial virus infection. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2007;20(1):108–119. doi: 10.1038/modpathol.3800725. [DOI] [PubMed] [Google Scholar]
- Khanolkar A, Hartwig SM, Haag BA, Meyerholz DK, Harty JT, Varga SM. Toll-like receptor 4 deficiency increases disease and mortality after mouse hepatitis virus type 1 infection of susceptible C3H mice. J Virol. 2009;83(17):8946–8956. doi: 10.1128/JVI.01857-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A. 2006;103(35):13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korteweg C, Gu J. Pathology, molecular biology, and pathogenesis of avian influenza A (H5N1) infection in humans. The American journal of pathology. 2008;172(5):1155–1170. doi: 10.2353/ajpath.2008.070791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmider B, Messier EM, Janssen WJ, Nahreini P, Wang J, Hartshorn KL, Mason RJ. Nrf2 protects human alveolar epithelial cells against injury induced by influenza A virus. Respir Res. 2012;13(1):43. doi: 10.1186/1465-9921-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goffic R, Balloy V, Lagranderie M, Alexopoulou L, Escriou N, Flavell R, Chignard M, Si-Tahar M. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2006;2(6):e53. doi: 10.1371/journal.ppat.0020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz JL, Srinivasa R, Williamson ST, Chua MM, Liu M, Wu S, Kang H, Ma XZ, Zhang J, Shalev I, Smith R, Phillips MJ, Levy GA, Weiss SR. Genetic determinants of mouse hepatitis virus strain 1 pneumovirulence. J Virol. 2010;84(18):9278–9291. doi: 10.1128/JVI.00330-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. Structural analysis of major species barriers between humans and palm civets for severe acute respiratory syndrome coronavirus infections. J Virol. 2008;82(14):6984–6991. doi: 10.1128/JVI.00442-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem NT, Nakajima N, Phat le P, Sato Y, Thach HN, Hung PV, San LT, Katano H, Kumasaka T, Oka T, Kawachi S, Matsushita T, Sata T, Kudo K, Suzuki K. H5N1-infected cells in lung with diffuse alveolar damage in exudative phase from a fatal case in Vietnam. Japanese journal of infectious diseases. 2008;61(2):157–160. [PubMed] [Google Scholar]
- Loosli CG, Stinson SF, Ryan DP, Hertweck MS, Hardy JD, Serebrin R. The destruction of type 2 pneumocytes by airborne influenza PR8-A virus; its effect on surfactant and lecithin content of the pneumonic lesions of mice. Chest. 1975;67(2 Suppl):7S–14S. doi: 10.1378/chest.67.2_supplement.7s. [DOI] [PubMed] [Google Scholar]
- Marsh LM, Cakarova L, Kwapiszewska G, von Wulffen W, Herold S, Seeger W, Lohmeyer J. Surface expression of CD74 by type II alveolar epithelial cells: a potential mechanism for macrophage migration inhibitory factor-induced epithelial repair. American journal of physiology. Lung cellular and molecular physiology. 2009;296(3):L442–452. doi: 10.1152/ajplung.00525.2007. [DOI] [PubMed] [Google Scholar]
- Mason RJ. Biology of alveolar type II cells. Respirology. 2006;11:S12–15. doi: 10.1111/j.1440-1843.2006.00800.x. Suppl. [DOI] [PubMed] [Google Scholar]
- Mason RJ, Williams MC. Phospholipid composition and ultrastructure of A549 cells and other cultured pulmonary epithelial cells of presumed type II cell origin. Biochim Biophys Acta. 1980;617(1):36–50. doi: 10.1016/0005-2760(80)90222-2. [DOI] [PubMed] [Google Scholar]
- Matsuyama S, Nagata N, Shirato K, Kawase M, Takeda M, Taguchi F. Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J Virol. 2010;84(24):12658–12664. doi: 10.1128/JVI.01542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MP, Morris SB, Wilcoxen S, Greeson E, Moore B, Paine R., 3rd Shedding of soluble ICAM-1 into the alveolar space in murine models of acute lung injury. American journal of physiology. Lung cellular and molecular physiology. 2006;290(5):L962–970. doi: 10.1152/ajplung.00352.2005. [DOI] [PubMed] [Google Scholar]
- Messier EM, Mason RJ, Kosmider B. Efficient and rapid isolation and purification of mouse alveolar type II epithelial cells. Experimental lung research. 2012;38(7):363–373. doi: 10.3109/01902148.2012.713077. [DOI] [PubMed] [Google Scholar]
- Mir-Kasimov M, Sturrock A, McManus M, Paine R., 3rd Effect of alveolar epithelial cell plasticity on the regulation of GM-CSF expression. American journal of physiology. Lung cellular and molecular physiology. 2012;302(6):L504–511. doi: 10.1152/ajplung.00303.2010. [DOI] [PubMed] [Google Scholar]
- Miura TA, Wang J, Holmes KV, Mason RJ. Rat coronaviruses infect rat alveolar type I epithelial cells and induce expression of CXC chemokines. Virology. 2007;369(2):288–298. doi: 10.1016/j.virol.2007.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossel EC, Wang J, Jeffers S, Edeen KE, Wang S, Cosgrove GP, Funk CJ, Manzer R, Miura TA, Pearson LD, Holmes KV, Mason RJ. SARS-CoV replicates in primary human alveolar type II cell cultures but not in type I-like cells. Virology. 2008;372(1):127–135. doi: 10.1016/j.virol.2007.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulugeta S, Gray JM, Notarfrancesco KL, Gonzales LW, Koval M, Feinstein SI, Ballard PL, Fisher AB, Shuman H. Identification of LBM180, a lamellar body limiting membrane protein of alveolar type II cells, as the ABC transporter protein ABCA3. J Biol Chem. 2002;277(25):22147–22155. doi: 10.1074/jbc.M201812200. [DOI] [PubMed] [Google Scholar]
- Nakajima N, Van Tin N, Sato Y, Thach HN, Katano H, Diep PH, Kumasaka T, Thuy NT, Hasegawa H, San LT, Kawachi S, Liem NT, Suzuki K, Sata T. Pathological study of archival lung tissues from five fatal cases of avian H5N1 influenza in Vietnam. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2012 doi: 10.1038/modpathol.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls JM, Butany J, Poon LL, Chan KH, Beh SL, Poutanen S, Peiris JS, Wong M. Time course and cellular localization of SARS-CoV nucleoprotein and RNA in lungs from fatal cases of SARS. PLoS Med. 2006;3(2):e27. doi: 10.1371/journal.pmed.0030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh HA, Park CS, Ahn HJ, Park YS, Kim HM. Effect of Perilla frutescens var. acuta Kudo and rosmarinic acid on allergic inflammatory reactions. Exp Biol Med (Maywood) 2011;236(1):99–106. doi: 10.1258/ebm.2010.010252. [DOI] [PubMed] [Google Scholar]
- Paine R, 3rd, Joyce-Brady M, Clement A, Brody JS. Serum accelerates the loss of type II cell differentiation in vitro. Am J Respir Cell Mol Biol. 1990;3(4):311–323. doi: 10.1165/ajrcmb/3.4.311. [DOI] [PubMed] [Google Scholar]
- Qiao R, Zhou B, Liebler JM, Li X, Crandall ED, Borok Z. Identification of three genes of known function expressed by alveolar epithelial type I cells. Am J Respir Cell Mol Biol. 2003;29(1):98–105. doi: 10.1165/rcmb.2002-0196OC. [DOI] [PubMed] [Google Scholar]
- Ramirez MI, Millien G, Hinds A, Cao Y, Seldin DC, Williams MC. T1alpha, a lung type I cell differentiation gene, is required for normal lung cell proliferation and alveolus formation at birth. Dev Biol. 2003;256(1):61–72. doi: 10.1016/s0012-1606(02)00098-2. [DOI] [PubMed] [Google Scholar]
- Raoust E.s., Balloy V, Garcia-Verdugo I, Touqui L, Ramphal R, Chignard M. Pseudomonas aeruginosa LPS or Flagellin Are Sufficient to Activate TLR-Dependent Signaling in Murine Alveolar Macrophages and Airway Epithelial Cells. PLoS ONE. 2009;4(10):e7259. doi: 10.1371/journal.pone.0007259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice WR, Conkright JJ, Na C-L, Ikegami M, Shannon JM, Weaver TE. Maintenance of the mouse type II cell phenotype in vitro. American journal of physiology. Lung cellular and molecular physiology. 2002;283(2):L256–264. doi: 10.1152/ajplung.00302.2001. [DOI] [PubMed] [Google Scholar]
- Roberts A, Deming D, Paddock CD, Cheng A, Yount B, Vogel L, Herman BD, Sheahan T, Heise M, Genrich GL, Zaki SR, Baric R, Subbarao K. A mouse-adapted SARS-coronavirus causes disease and mortality in BALB/c mice. PLoS Pathog. 2007;3(1):e5. doi: 10.1371/journal.ppat.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzepka JP, Haick AK, Miura TA. Virus-infected alveolar epithelial cells direct neutrophil chemotaxis and inhibit their apoptosis. Am J Respir Cell Mol Biol. 2012;46(6):833–841. doi: 10.1165/rcmb.2011-0230OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T, Rockx B, Donaldson E, Sims A, Pickles R, Corti D, Baric R. Mechanisms of zoonotic severe acute respiratory syndrome coronavirus host range expansion in human airway epithelium. J Virol. 2008;82(5):2274–2285. doi: 10.1128/JVI.02041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh W-J, Blau DM, Denison AM, DeLeon-Carnes M, Adem P, Bhatnagar J, Sumner J, Liu L, Patel M, Batten B, Greer P, Jones T, Smith C, Bartlett J, Montague J, White E, Rollin D, Gao R, Seales C, Jost H, Metcalfe M, Goldsmith CS, Humphrey C, Schmitz A, Drew C, Paddock C, Uyeki TM, Zaki SR. 2009 Pandemic Influenza A (H1N1): Pathology and Pathogenesis of 100 Fatal Cases in the United States. The American journal of pathology. 2010;177(1):166–175. doi: 10.2353/ajpath.2010.100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh WJ, Hsiao CH, Paddock CD, Guarner J, Goldsmith CS, Tatti K, Packard M, Mueller L, Wu MZ, Rollin P, Su IJ, Zaki SR. Immunohistochemical, in situ hybridization, and ultrastructural localization of SARS-associated coronavirus in lung of a fatal case of severe acute respiratory syndrome in Taiwan. Hum Pathol. 2005;36(3):303–309. doi: 10.1016/j.humpath.2004.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulla A, Heald-Sargent T, Subramanya G, Zhao J, Perlman S, Gallagher T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J Virol. 2011;85(2):873–882. doi: 10.1128/JVI.02062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava B, Blazejewska P, Hessmann M, Bruder D, Geffers R, Mauel S, Gruber AD, Schughart K. Host genetic background strongly influences the response to influenza a virus infections. PLoS One. 2009;4(3):e4857. doi: 10.1371/journal.pone.0004857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman LS, Holmes KV, Behnke J. Isolation of coronavirus envelope glycoproteins and interaction with the viral nucleocapsid. J Virol. 1980;33(1):449–462. doi: 10.1128/jvi.33.1.449-462.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman LS, Takemoto KK. Enhanced growth of a murine coronavirus in transformed mouse cells. Infection and immunity. 1972;6(4):501–507. doi: 10.1128/iai.6.4.501-507.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain RJ, Kemp SJ, Goldstraw P, Tetley TD, Stevens MM. Assessment of cell line models of primary human cells by Raman spectral phenotyping. Biophysical journal. 2010;98(8):1703–1711. doi: 10.1016/j.bpj.2009.12.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate MD, Schilter HC, Brooks AG, Reading PC. Responses of mouse airway epithelial cells and alveolar macrophages to virulent and avirulent strains of influenza A virus. Viral immunology. 2011;24(2):77–88. doi: 10.1089/vim.2010.0118. [DOI] [PubMed] [Google Scholar]
- Uiprasertkul M, Kitphati R, Puthavathana P, Kriwong R, Kongchanagul A, Ungchusak K, Angkasekwinai S, Chokephaibulkit K, Srisook K, Vanprapar N, Auewarakul P. Apoptosis and pathogenesis of avian influenza A (H5N1) virus in humans. Emerging infectious diseases. 2007;13(5):708–712. doi: 10.3201/eid1305.060572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahos R, Stambas J, Bozinovski S, Broughton BR, Drummond GR, Selemidis S. Inhibition of Nox2 oxidase activity ameliorates influenza A virus-induced lung inflammation. PLoS Pathog. 2011;7(2):e1001271. doi: 10.1371/journal.ppat.1001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Nikrad MP, Phang T, Gao B, Alford T, Ito Y, Edeen K, Travanty EA, Kosmider B, Hartshorn K, Mason RJ. Innate immune response to influenza A virus in differentiated human alveolar type II cells. Am J Respir Cell Mol Biol. 2011;45(3):582–591. doi: 10.1165/rcmb.2010-0108OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Oberley-Deegan R, Wang S, Nikrad M, Funk CJ, Hartshorn KL, Mason RJ. Differentiated human alveolar type II cells secrete antiviral IL-29 (IFN-lambda 1) in response to influenza A infection. J Immunol. 2009;182(3):1296–1304. doi: 10.4049/jimmunol.182.3.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MC. Alveolar type I cells: molecular phenotype and development. Annu Rev Physiol. 2003;65:669–695. doi: 10.1146/annurev.physiol.65.092101.142446. [DOI] [PubMed] [Google Scholar]
- Yu WC, Chan RW, Wang J, Travanty EA, Nicholls JM, Peiris JS, Mason RJ, Chan MC. Viral replication and innate host responses in primary human alveolar epithelial cells and alveolar macrophages infected with influenza H5N1 and H1N1 viruses. J Virol. 2011;85(14):6844–6855. doi: 10.1128/JVI.02200-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zen K, Notarfrancesco K, Oorschot V, Slot JW, Fisher AB, Shuman H. Generation and characterization of monoclonal antibodies to alveolar type II cell lamellar body membrane. The American journal of physiology. 1998;275(1 Pt 1):L172–183. doi: 10.1152/ajplung.1998.275.1.L172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.