Abstract
Regulated exocytosis mediates the release of hormones and transmitters. The last step of this process is represented by the merger between the vesicle and the plasma membranes, and the formation of a fusion pore. Once formed, the initially stable and narrow fusion pore may reversibly widen (transient exocytosis) or fully open (full-fusion exocytosis). Exocytosis is typically triggered by an elevation in cytosolic calcium activity. However, other second messengers, such as cyclic AMP (cAMP), have been reported to modulate secretion. The way in which cAMP influences the transitions between different fusion pore states remains unclear. Here, hormone release studies show that prolactin release from isolated rat lactotrophs stimulated by forskolin, an activator of adenylyl cyclases, and by membrane-permeable cAMP analog (dbcAMP), exhibit a biphasic concentration dependency. While at lower concentrations (2–10 μM forskolin and 2.5–5 mM dbcAMP) these agents stimulate prolactin release, an inhibition is measured at higher concentrations (50 μM forskolin and 10–15 mM dbcAMP). By using high-resolution capacitance (Cm) measurements, we recorded discrete increases in Cm, which represent elementary exocytic events. An elevation of cAMP leaves the frequency of full-fusion events unchanged, while increasing the frequency of transient events. These exhibited a wider fusion pore as measured by increased fusion pore conductance and a prolonged fusion pore dwell-time. The probability of observing rhythmic reopening of transient fusion pores was elevated by dbcAMP. In conclusion, cAMP-mediated stabilization of wide fusion pores prevents vesicles from proceeding to the full-fusion stage of exocytosis, which hinders vesicle content discharge at high cAMP concentrations.
Keywords: lactotrophs, exocytosis, cAMP, membrane capacitance, fusion pore, prolactin release
Introduction
Regulated exocytosis mediates the release of hormones and transmitters stored in vesicles (Jahn et al., 2003). This process ends with the merger of the vesicle membrane and the plasma membrane, leading to the formation of a stable and narrow fusion pore, through which secretions exit the cell (Spruce at al., 1990; Lollike et al., 1995; Vardjan et al., 2007). An increase in cytosolic calcium concentration ([Ca2+]i) leads to the fusion pore diameter increase, which eventually either fully opens (full-fusion exocytosis) or reversibly closes (transient exocytosis) (Vardjan et al., 2007; Jorgačevski et al., 2007). Fluctuations between fusion pore states with different diameters have been reported, lasting from milliseconds to minutes before full-fusion (Fernandez et al., 1984; Vardjan et al., 2007; Jorgačevski et al., 2010). These fluctuations can exhibit remarkable rhythmicity (Henkel et al., 2000; Stenovec et al., 2004; Vardjan et al., 2007), but their nature remains elusive.
Changes in [Ca2+]i are likely to play a role in regulating the transitions between stages of exocytosis (Alés et al., 1999; Jorgačevski et al., 2008). Additionally, elevations in cAMP affect exocytosis (Renström et al., 1997; Sikdar et al., 1998; Cochilla et al., 2000; Kostic et al., 2002; Sedej et al., 2005; Gonzalez-Iglesias et al., 2006), but it is less clear exactly which exocytic stages are modulated by cAMP. In lactotrophs, cAMP facilitates hormone release via several mechanisms (Gonzalez-Iglesias et al., 2006, 2008; Stojilkovic et al., 2010), also by affecting the exocytic machinery (Sikdar et al., 1990). Interestingly, cAMP may shift full-fusion to transient exocytosis, as shown in insulin-secreting cells (Hanna et al., 2009). In contrast, in melanotrophs, cAMP mediates preferential fusion of larger vesicles without increasing the frequency of events (Sikdar et al., 1998).
To investigate the nature of the transitions between stages vesicles undergo in regulated exocytosis, we studied peptidergic vesicles of rat pituitary lactotrophs, cells in which unitary exocytic events can be studied (Stenovec et al., 2004; Jorgačevski et al., 2008). We first asked how elevations in intracellular cAMP affect hormone release from the population of lactotrophs. The results revealed a biphasic effect of cAMP. At relatively low cAMP elevations, prolactin release was augmented, whereas a decreased release in prolactin was recorded at higher cAMP levels. Next, the cell-attached patch clamp was used to monitor discrete changes in membrane capacitance (Cm), which represent unitary exocytic events (Neher and Marty, 1982) and permit measurements of fusion pore conductance (Gp) and fusion pore dwell-time (Lollike and Lindau, 1999; Jorgačevski et al., 2008; Jorgačevski et al., 2011). Elevations in cAMP increased the frequency of transient, but not full-fusion events. Transient fusion pore openings exhibited increased Gp and prolonged fusion pore dwell-time. Moreover, cAMP increased the probability of rhythmic reopenings of transient fusion pores. Although cAMP increased the frequency of unitary exocytic events, cAMP-mediated stabilization of widely open transient fusion pores may hinder the discharge of vesicle contents.
Material and Methods
Cell cultures
Lactotrophs were isolated from adult male Wistar rats as described (Ben-Tabou et al., 1994). Briefly, cells were plated on glass coverslips coated with poly-L-lysine and maintained in the feeding medium (high-glucose Dulbecco’s modified Eagle’s medium supplemented with 10 percent newborn calf serum 1.5 μM bovine serum albumin and 2 mM L-glutamine) in an atmosphere of humidified air (95 percent) and CO2 (5 percent) at 37 °C. The feeding medium was replaced every other day. The animals were euthanized in accordance with the International Guiding Principles for Biomedical Research Involving Animals developed by the Council for International Organizations of Medical Sciences, the Directive on Conditions for Issue of License for Animal Experiments for Scientific Research Purposes (Official Gazette of the Republic of Slovenia 40/85 and 22/87), and by the NICHD Animal Care and Use Committee. The procedures using animals were approved by the Veterinary Administration of the Republic of Slovenia (approval no. 3440-29/2006). Experiments were carried out at room temperature one to four days after the isolation.
Prolactin-release and cAMP measurements
Prolactin (PRL) and cAMP release was monitored using cell column perifusion experiments. Briefly, 1.2 × 107 cells were incubated with pre-swollen cytodex-1 beads in 60 mm petri dishes for 18 h. The beads were then transferred to 0.5 ml chambers and perifused with Hanks’ M199 containing 25 mM HEPES, 0.1 percent BSA and penicillin (100 units/ml)/streptomycin (100 μg/ml) for 2.5 h at a flow rate of 0.8 ml/min and at 37 °C to establish stable basal secretion. Fractions were collected at 1 min intervals and later assayed for PRL and cAMP contents using radioimmunoassay. The primary antibody and standard for PRL assay were purchased from the National Pituitary Agency and Dr. AF Parlow (Harbor-UCLA Medical Center, Torrance, USA). Cyclic AMP was determined using specific antiserum provided by Albert Baukal (NICHD, Bethesda, USA). 125I-PRL and 125I-cAMP were purchased from PerkinElmer Life Sciences (Boston, USA).
Electrophysiology
Glass pipettes were fire-polished and heavily coated with Sylgard® (Midland, USA). The resistance of pipettes was 3–6 MΩ. Cell-attached capacitance measurements were performed with a dual-phase lock-in patch-clamp amplifier (SWAM IIC; Celica, Ljubljana, Slovenia) as described (Vardjan et al., 2007, Jorgačevski et al., 2010). A sine wave voltage (1591 Hz, 111 mV r.m.s.) was applied to the pipette, while holding the pipette potential at 0 mV. The phase of the dual-phase lock-in amplifier was adjusted and checked at regular intervals as described previously (Vardjan et al., 2007; Jorgačevski et al., 2010). We performed capacitance measurements under non-stimulated conditions and after stimulation with different cAMP-increasing agents: 1 mM IBMX (3-isobutyl-1-methylxanthine, a phosphodiesterase inhibitor to increase cytosolic cAMP concentration), 10 mM dbcAMP (N6,2′-O-dibutyryl adenosine-3′,5′-cyclic monophosphate, a membrane permeable cAMP analog) and 1 μM forskolin. dbcAMP and IBMX were added as a bolus of the stock solutions, which were prepared in extracellular solution. Stimulation with forskolin was performed by a 30 min pre-incubation of cells with forskolin.
Data Analysis
Electrophysiological recordings were analyzed in the custom-made software (CellAn, Celica, Slovenia) written for MATLAB (Math Works, Natick, USA). For transient fusion events, vesicle capacitance (Cv) and fusion pore conductance (Gp) were calculated from the imaginary (Im) and the real (Re) part of the admittance signals, as reported (Lollike and Lindau, 1999): Cv=[(Re2 + Im2)/Im]/ω, where ω is the angular frequency (ω=2πf, f is the sine-wave frequency, 1591 Hz) and Gp=(Re2 + Im2)/Re. Fusion-pore radius was estimated by using the equation Gp=(πr2)/(ρλ), where r denotes fusion pore radius, λ the estimated resistivity of the saline (100 Ωcm), and ρ the length of a gap junction channel (15 nm; Spruce et al., 1990). Vesicle diameter was calculated by using specific membrane capacitance (cm) of 8 fF/μm−2. A burst was considered to consist of no less than five transient events, with no more than 5 s between the ensuing events. Transient events in a burst were considered periodic when times between the ensuing events were normally distributed (Shapiro-Wilk normality test) and the coefficient of variation of the Gaussian curve fitted to the data was less than 0.2. All statistics were performed with Sigma Plot® (Systat Software; San Jose, USA). Results are presented as mean ± standard error of the mean (s.e.m.). Statistical significance was evaluated by using Student’s t-test for normally and Man-Whitney for non-normally distributed data. Considering p<0.05 (*), p<0.01 (**).
Solutions
The extracellular solution consisted of 10 mM HEPES/NaOH (pH 7.4), 10 mM D-glucose, 130 mM NaCl, 8 mM CaCl2, 1 mM MgCl2, and 5 mM KCl. Unless stated otherwise, all chemicals of highest purity available were purchased from Sigma-Aldrich (St. Louis, USA).
Results
The effect of cAMP-increasing agents on cAMP and PRL release in pituitary cells
cAMP is a secondary messenger capable of enhancing hormone release by promoting Ca2+ influx into cells or by directly modulating specific steps in the secretory pathway (reviewed in Seino and Shibasaki, 2005). Previously we have confirmed a linear relationship between intracellular and extracellular cyclic nucleotide concentrations in lactotrophs treated with cAMP-increasing agents (Gonzalez-Iglesias et al., 2006). Forskolin, an adenylyl cyclase activator, dose-dependently (added to the culture medium at 2, 10, and 50 μM; see Fig. 1a) increased the concentration of released cAMP in cultured cells, as reported (Gonzalez-Iglesias et al., 2006). To determine whether forskolin also affects the release of hormones from lactotrophs, we measured PRL release from perifused pituitary lactotrophs when forskolin was applied for a period of 40 min (Fig. 1b). At lower concentrations of forskolin (2 μM and 10 μM), PRL release was enhanced immediately after the addition of forskolin and reached a steady-state that was 1.6-fold higher than in controls. However, at higher concentration of forskolin (50 μM) PRL release increased only after a delay of 30 min (Fig. 1b). Because different concentrations of forskolin had different effects on PRL release from lactotrophs, we wanted to determine whether this effect was specific for forskolin. Next, we used dbcAMP, a membrane permeable cAMP analog. In Fig. 1c, the results show that dbcAMP increased PRL release by 1.5-fold at 2.5 and 5 mM. However, the addition of 10 mM dbcAMP decreased the rate of PRL release, which reached levels lower than those recorded in controls. At 15 mM dbcAMP, the rate of PRL release continued to decrease but reverted to partial recovery upon rinsing dbcAMP from the bath.
Figure 1. Elevation in cAMP cytosolic concentration increases prolactin (PRL) release from perifused pituitary lactotrophs.
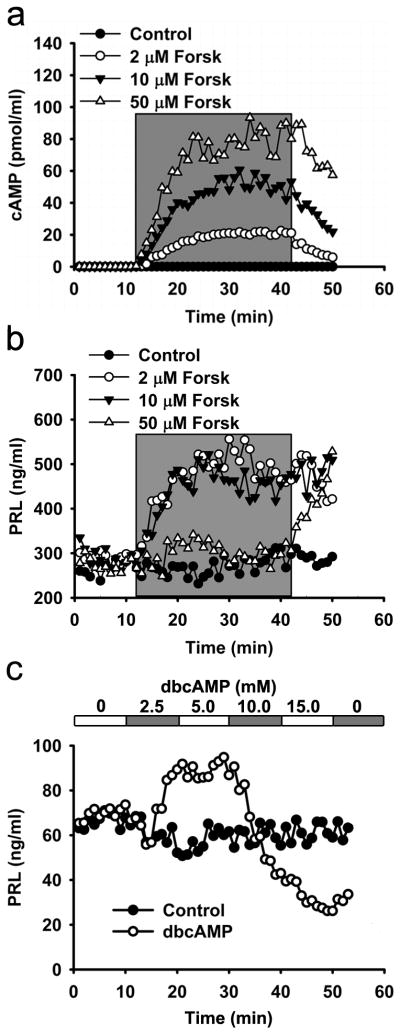
Cultured pituitary lactotrophs were perifused with extracellular solutions containing different concentrations of forskolin, an adenyl cyclase activator, and dbcAMP (N6,2′-O-dibutyryl adenosine-3′,5′-cyclic monophosphate). Samples were collected every minute and analyzed for either cAMP or PRL. (a) Dose-dependent effect of forskolin on cAMP release in perifused lactotrophs. The grey part denotes the period of forskolin application. (b) Dose-dependent effect of forskolin on PRL release from perifused lactotrophs. The grey part denotes the period of forskolin application. (c) The effect of different concentrations of dbcAMP on the PRL release from perifused lactotrophs. The top x-axis shows the concentrations of applied dbcAMP. Shown are representative experiments from four independent experiments.
These results indicate that cAMP potentiates PRL release at relatively low concentrations, while at higher cAMP concentrations it inhibits PRL release. Therefore, our next goal was to study how cAMP-increasing agents affect the exocytic machinery directly. To this end, we recorded unitary exocytic events by the high-resolution membrane capacitance technique (Neher and Marty, 1982; Zorec et al., 1991).
cAMP increases the frequency of transient exocytic events
Although single fusion events can be studied in single cells (Kreft and Zorec, 1997; Vardjan et al., 2007; Jorgačevski et al., 2008), few studies address the question of how cAMP modulates unitary exocytotic events (Sikdar et al., 1998; Hanna et al., 2009). Here, we studied the effects of cAMP on the occurrence and on the properties of the unitary exocytic events of PRL-containing vesicles. We used the cell-attached patch-clamp technique to monitor membrane capacitance (Cm), a parameter linearly related to the plasma membrane surface area in control conditions (extracellular solution) and in the presence of different cAMP-increasing agents. To elevate cAMP levels we applied: IBMX, a non-specific phosphodiesterase inhibitor (1 mM), and forskolin (1 μM), in two separate sets of experiments. Both of which have been shown to increase cAMP and PRL release from lactotrophs (Fig. 1b, Gonzalez-Iglesias et al., 2006). We performed an additional set of experiments where we applied 10 mM dbcAMP, which inhibits PRL release (Fig. 1c).
The recordings were made from 50 membrane patches, each on a different cell, with a total recording time of 13.9 hours (3.2 hours at control conditions and 10.7 hours at stimulated conditions) and at an average time of 1000 s per recording. We observed the presence of discrete irreversible upward and downward steps in Cm, likely indicating full-fusion exocytosis and endocytosis, respectively (Heuser and Reese, 1973; Neher and Marty, 1982; see Fig. 2a). We also recorded reversible steps in Cm that likely represent transient exocytosis (Alvarez de Toledo et al., 1993; Fesce et al., 1994; Jorgačevski et al., 2008; see Fig. 2b). Transient exocytic events, observed in the imaginary part of the admittance trace (Im), often exhibited a crosstalk, observed on the real part of the admittance trace (Re), indicating the presence of a narrow fusion pore (Lollike and Lindau, 1999; see Fig. 2b). For these events we calculated the vesicle capacitance (Cv) and fusion pore conductance (Gp), as described in Materials and Methods (see Fig. 2c; Lollike and Lindau, 1999). The average Cv amplitude of exocytic events recorded in control and in stimulated conditions was 0.88 ± 0.03 fF (range 0.2–4 fF). By assuming specific membrane capacitance of 8 fF/μm−2 and spherical geometry of vesicles, the amplitude corresponds to vesicle diameter of 176 ± 10 nm, which is in agreement with previously published results on PRL-containing vesicles (Smets et al., 1987; Angleson et al., 1999; Vardjan et al., 2007, Jorgačevski et al., 2008, 2011).
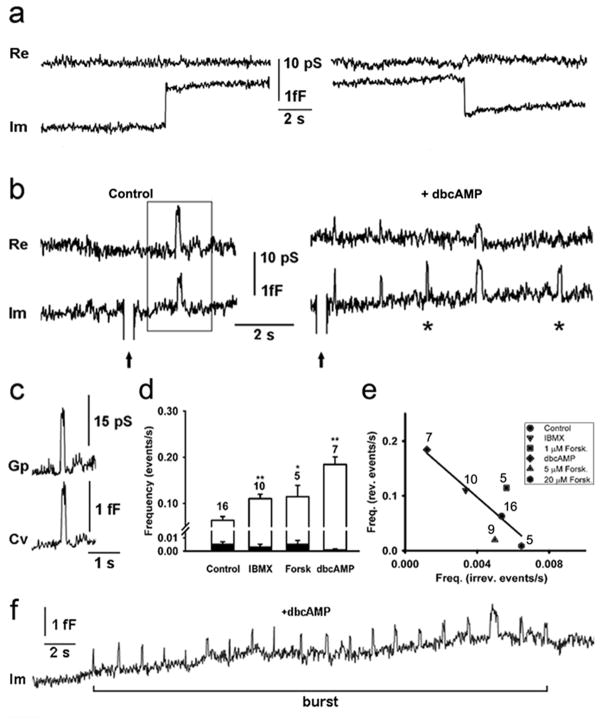
Figure 2. cAMP-increasing agents augment the frequency of reversible but not the irreversible discrete capacitance steps.
The irreversible events are represented by discrete upward or downward step in the imaginary admittance trace (Im), which is proportional to the membrane capacitance. Reversible events consist of an upward and a subsequent downward discrete step in Im, which follows within 5 s. (a) A representative example of irreversible upward (left) and downward steps (right) in Im trace and the corresponding real part of the admittance trace (Re, top). (b) Reversible events in Im before (Control, left) and after the stimulation by dbcAMP (10 mM, +dbcAMP, right). Note that some of the reversible events in Im trace exhibit projections to the Re trace, while the others were devoid of projections (asterisks). Arrows denote calibration pulses in Im, used to adjust the phase of the lock-in amplifier. (c) Projected reversible events were used to calculate fusion pore conductance (Gp) and vesicle capacitance (Cv), as described in the Materials and Methods section. Shown is a representative example framed in panel b. (d) The average frequency of irreversible upward events (full bars) in controls was 0.005 ± 0.002 s−1 (n=16). The addition of phosphodiesterase inhibitor (IBMX, 1 mM), an agent to activate adenylyl cyclase (forskolin 1 μM, Forsk), and a membrane permeable cAMP analog (dbcAMP, 10 mM) did not affect (p>0.05) the frequency of irreversible upward events, (0.003 ± 0.002 s−1, 0.006 ± 0.002 s−1 and 0.001 ± 0.001 s−1) respectively. The average frequency of reversible events (open bars) increased from 0.06 ± 0.01 s−1 in controls to 0.11 ± 0.01 s−1 (p<0.01; IBMX), 0.11 ± 0.02 s−1 (p<0.05; forskolin) and 0.18 ± 0.02 s−1 (p<0.01; dbcAMP). Values are means ± s.e.m. Numbers above error bars indicate the number of patches. (e) We observed negative relationships between the average frequency of irreversible upward events and the average frequency of reversible events for each condition, which was best fitted with the linear regression: y (the average frequency of reversible events)=(−21 ± 9) × x (the average frequency of irreversible up-ward events) + (0.20 ± 0.04) with the correlation coefficient, r=0.85. (f) Representative Im trace, showing a burst of reversible events. We defined a burst as a minimum of five reversible events with less than 5 s between ensuing reversible events.
The occurrence of irreversible upward events did not significantly change after the addition of any of the cAMP-increasing agents (Fig. 2d, black bars; see Table 1). However, both IBMX and forskolin applications increased the occurrence of transient exocytic events twofold, from 0.06 ± 0.01 s−1 to 0.11 ± 0.01 s−1 (p<0.01) and to 0.11 ± 0.02 s−1 (p<0.05), respectively (Fig. 2d, open bars). The stimulation with dbcAMP elicited an even greater increase in the frequency of transient exocytic events, to 0.18 ± 0.02 s−1 (Fig. 2d, open bars p<0.01). Observed changes were not an artifact of the vehicle (20 mM DMSO) because control experiments where we added the vehicle without cAMP increasing agents did not affect the frequency of transient exocytic events (data not shown).
Table 1.
Types of unitary exocytic events in controls and in the presence of dbcAMP.
| Type of exocytic events | Percentage of different exocytic events | ||
|---|---|---|---|
| Full-fusion events (No. of events/all events) | Transient events with wide fusion pores (No. of events/all events) | Transient events with narrow fusion pores (No. of events/all events) | |
| Control | 8.3% (n=25/300) | 66.6% (n=200/300) | 25.0% (n=75/300) |
| +dbcAMP | 0.6% (n=7/1152) | 98.5% (n=1136/1152) | 0.9% (n=9/1152) |
The inverse linear relation between the occurrences of reversible (transient) vs. irreversible (full-fusion) exocytic events indicates that cAMP-stimulation augments the former while inhibiting the latter events (Fig. 2e), which is consistent with the view that cAMP up-regulates the transient mode of exocytosis (Hanna et al., 2009). We also observed that the nature of appearance of transient exocytic events was different after stimulation. Prior to the stimulation transient exocytic events usually occurred as independent events, while after the addition of dbcAMP, they often appeared in bursts (Fig. 2f).
Fig. 3a shows the Cv amplitude distribution of transient exocytic events recorded in a representative patch of membrane. Note the unchanged Cv amplitude before and after dbcAMP application (Gaussian mean 1.09 ± 0.05 fF; n=11 and 1.08 ± 0.01 fF; n=64), indicating that dbcAMP-stimulation modulated the properties of a pre-existing fusion pore. To determine whether transient events in Cm represent the repetitive fusion pore opening of a single vesicle, we compared the Cv amplitudes of upward and downward steps in this recording. Regression lines for control (n=11 events, filled circles) and dbcAMP-stimulated conditions (n=64 events, open circles) had slopes close to 1 (1.0 ± 0.1 and 0.8 ± 0.1, respectively; see Fig. 3aii) and high correlation coefficients (r=0.95 and r=0.78, respectively; see Fig. 3aii). These results are consistent with the view that transient exocytic events represent a single vesicle interacting with the plasma membrane. To further test if this is valid for all patches, we compared the average upward and downward amplitudes in Cv in controls (n=16 cells) and after cAMP-stimulation (n=21 cells). Similarly as for the single patch, the results were best fitted with the regression line with the slope near one (0.97 ± 0.04 for control and 1.04 ± 0.02 after stimulation) and with even higher correlation coefficients (r=0.99 and 0.99, respectively; see Fig. 3b). We also investigated whether the stimulation by cAMP affects the distribution of Cv amplitudes of transient exocytic events. Fig. 3c shows that in controls, the distribution of Cv amplitudes consisted of two peaks (with mean amplitudes of 0.58 ± 0.01 fF and 1.44 ± 0.02 fF), indicating two populations of vesicles interacting with the plasma membrane. However, following stimulation by cAMP, the predominant vesicle population that interacted with the plasma membrane exhibited a lower Cv amplitude of 0.61 ± 0.01 fF. This population of vesicles represented 50 percent of all vesicles interacting with the plasma membrane in controls, whereas it increased to 93 percent following cAMP stimulation.
Figure 3. Reversible events mirror repetitive fusion of single vesicle.
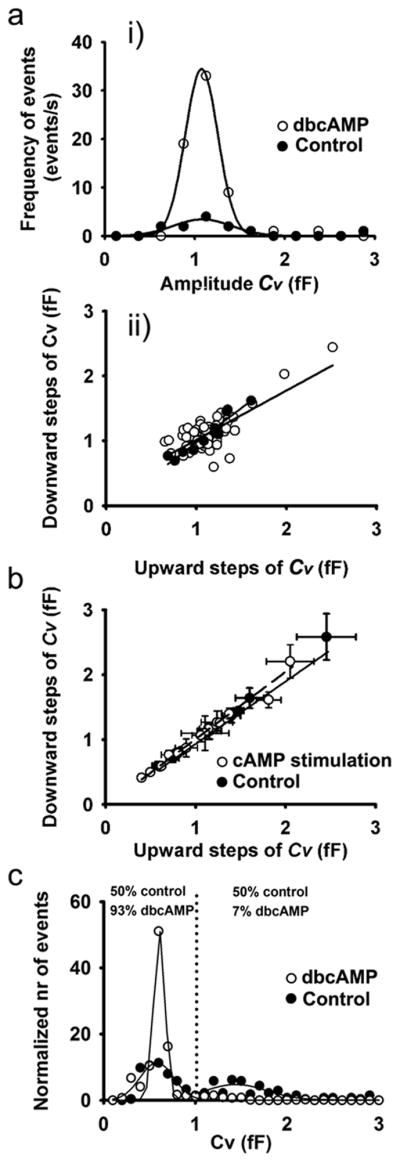
(a)(i) The distributions of vesicle membrane capacitance (Cv) amplitudes of reversible events from a representative patch, before and after the stimulation with dbcAMP, were similar. Lines show fitted Gaussian curves with means of 1.09 ± 0.05 fF (control, correlation coefficient r=0.92; n=11 events) and 1.08 ± 0.01 fF (dbcAMP, r=0.99; n=64 events), respectively. (ii) The relationship between amplitudes of the downward and the preceding upward Cv steps of reversible events: the regression line represents the best fit with parameters: y (Cv amplitude of the downward step)=(1.0 ± 0.1) × x (Cv amplitude of the upward step) + (−0.1 ± 0.1) (r=0.95, n=11 events) before stimulation and the regression line y (Cv amplitude of the downward step)=(0.8 ± 0.1) × x (Cv amplitude of the upward step) + (0.26 ± 0.10) (r=0.76, n=64 events) after the stimulation (open circles). The slopes of both regression lines were similar (p=0.2). (b) The relationship between the average Cv amplitudes of downward vs. upward discrete steps of reversible events in distinct membrane patches before and after the addition of cAMP-increasing agents. The solid line represents linear fit of the controls: y (the average Cv amplitudes of down-ward steps)=(0.97 ± 0.04) × x (the average Cv amplitudes of upward steps) + (−0.03 ± 0.15) (r=0.99, n=9 cells) and the dashed line represents linear fit to the data obtained after the addition of cAMP-increasing agents: y (the average Cv amplitudes of upward steps)=(1.04 ± 0.02) × x (the average Cv amplitudes of downward steps) + (−0.03 ± 0.05) (r= 0.99, n=16 cells). The slopes of both regression lines were similar (p=0.3). Values are means ± s.e.m. (c) Distribution of Cv amplitudes of all events in control conditions shows two population best fitted with Gaussian curves with means: 0.58 ± 0.01 fF (r=0.82) and 1.44 ± 0.02 fF (r=0.80), each corresponding to 50 percent of all events in control conditions. After dbcAMP, we only observed one population best fitted with Gaussian curve with mean 0.61 ± 0.01 fF (r=0.99) that represents 93 percent of all events after dbcAMP.
These results indicate that stimulation by cAMP primarily affects smaller peptidergic vesicles, which are engaged in transient mode of exocytosis.
cAMP affects the fusion pore diameter and dwell-time
In the transient mode of exocytosis, vesicle cargo discharge can be constrained by a narrow fusion pore and by the relatively short effective fusion pore dwell-time (Barg et al., 2002; Tsuboi and Rutter, 2003; Stenovec et al., 2004; Obermüller et al., 2005). Therefore, our next aim was to determine if cAMP-increasing agents alter the fusion pore geometry. Thus, we calculated fusion pore conductance (Gp; see Fig. 2c; Lollike and Lindau, 1999; see Materials and Methods), a parameter related to the fusion pore diameter (Breckenridge and Almers, 1987). However, Gp can only be calculated for transient fusion events exhibiting a significant crosstalk between the Im (proportional to the vesicle capacitance Cv) and the Re traces. In this case, a relatively narrow fusion pore acts as a resistor which produces a measurable projection to the Re part of the admittance signal (reflecting the conductance of the fusion pore; Lollike and Lindau, 1999).
The majority of transient fusion events, observed in controls and in dbcAMP-stimulated conditions exhibited wide fusion pores. Stimulation with dbcAMP increased the percentage of transient exocytic events with wide fusion pores from 67 percent to 99 percent (Table 1). Transient exocytic events with narrow fusion pores decreased from 25 percent in controls to approximately 1 percent after treatment with dbcAMP (Table 1). In addition, the percentage of full-fusion events decreased after stimulation with dbcAMP from 8 percent (control) to less than 1 percent (Table 1).
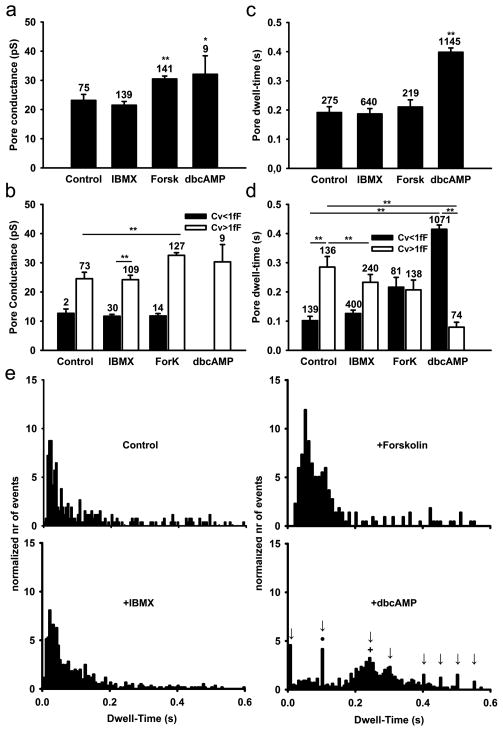
To learn about the influence of each of the cAMP-enhancing agents on Gp in detail, we analyzed the average Gp in each set of experiments, respectively (Fig. 4a). Following the treatment with IBMX, the average Gp was 21 ± 1 pS (n=139 events), similar to the average Gp in controls (23 ± 2 pS; n=75 events; see Fig. 4a). However, a significant increase in the average Gp was observed following the treatment by forskolin and dbcAMP; 30 ± 1 pS (n=141; p<0.01) and 32 ± 6 pS (n=9; p<0.05; see Fig 4a), respectively. Interestingly, if we analyzed the cAMP-mediated change in fusion pore conductance as a function of Cv, the results revealed that larger vesicles with Cv > 1 fF exhibited larger Gp than events with Cv < 1fF (Fig. 4b).
Figure 4. cAMP-increasing agents affect the fusion pore conductance and the pore dwell-time.
(a) The average fusion pore conductance (Gp), determined for reversible events with measurable crosstalk between Re and Im traces in controls was 23 ± 2 pS (n=75 events). The addition of IBMX, forskolin, and dbcAMP increased the average Gp to 21 ± 1 pS (n=139 events; P=0.3), 30 ± 1 pS (141 events; p<0.001), and 32 ± 6 pS (n=9 events; p<0.05), respectively. Values are means ± s.e.m. (b) The average fusion pore dwell-time of controls was 0.19 ± 0.02 s (n=275 events) and remained unchanged after the addition of IBMX and forskolin (0.19 ± 0.02 s; n=640 events and 0.21 ± 0.02 s; n=219 events, respectively). DbcAMP treatment increased the average fusion pore dwell-time to 0.40 ± 0.02 s (n=1145 events, p<0.001). Values are means ± s.e.m. (c) Fusion pore conductance displayed as a function of Cv > 1 fF (white columns) and Cv < 1 fF (black columns). (d) Changes in fusion pore dwell-time displayed as a function of Cv > 1 fF (white columns) and Cv < 1 fF (black columns) (e) The frequency distribution of fusion pore dwell-times in controls and after the addition of cAMP increasing agents. Arrows in the panel showing the distribution of fusion pore dwell-times after the addition of dbcAMP (+dbcAMP) point to the modal values of the dwell-times, which belong to the respective bursts. Two modal dwell-times, which are marked with the cross and the dot, denote two bursts, shown in panels a and b of Fig. 5.
The dwell-time of transient fusion pore openings was measured as the time between the upward and the ensuing downward step in Cm, as reported (Vardjan et al., 2007; Jorgačevski et al., 2008). Stimulation with IBMX or forskolin did not affect the average fusion pore dwell-time (0.19 ± 0.02 s in control vs. 0.19 ± 0.02 s with IBMX and 0.21 ± 0.02 s with forskolin). However, stimulation with dbcAMP doubled the average fusion pore dwell-time to 0.40 ± 0.02 s (Fig. 4c; p<0.01). The probability of the open fusion state, which we calculated as the sum of all fusion pore dwell-times divided by the total recording time, increased after dbcAMP stimulation threefold, from 0.02 (n=16 cells) to 0.07 (n=7 cells). In contrast, IBMX (n=10 cells) and forskolin (n=5 cells) stimulation did not significantly affect the probability of open fusion pore state (0.02 and 0.03, respectively). Moreover, the cAMP-mediated effects of fusion pore dwell-time depended on the Cv amplitude. In controls, fusion pore dwell-time was shorter for events with Cv < 1fF than with Cv > 1fF (Fig. 4d).
Frequency histograms of fusion pore dwell-time (Fig. 4e) show that in controls and after simulation with IBMX, distributions of fusion pore dwell-times are similar (modal peaks at ~0.03 s). However, after stimulation with forskolin, fusion pore dwell-time distribution shifted to higher values (note a second peak at ~0.07 s). After stimulation with dbcAMP, the distribution of fusion pore dwell-times was different. Instead of a single or double mode distribution, we observed several modes (Fig. 4e, bottom right). Each of the modal peaks (highlighted by arrows on Fig. 4e, lower right panel) denotes a particular burst of events. Multimodality was observed also for separated data of Cv < 1fF and Cv > 1fF (data not shown). The prolonged fusion pore dwell-time, observed after the stimulation with forskolin and dbcAMP, is consistent with previous reports (Ohara-Imaizumi et al., 2002; Gandhi and Stevens, 2003; Perrais et al., 2004; Thorn and Parker, 2005). Our results indicate that the stimulation with low concentration of IBMX and forskolin is not sufficient to affect fusion pore dwell-time. On the other hand, the mean dwell-time of ~250 ms after dbcAMP stimulation is similar to the reported value after depolarization with KCl (Vardjan et al., 2007).
The addition of dbcAMP elicits periodicity of transient fusion events within a burst
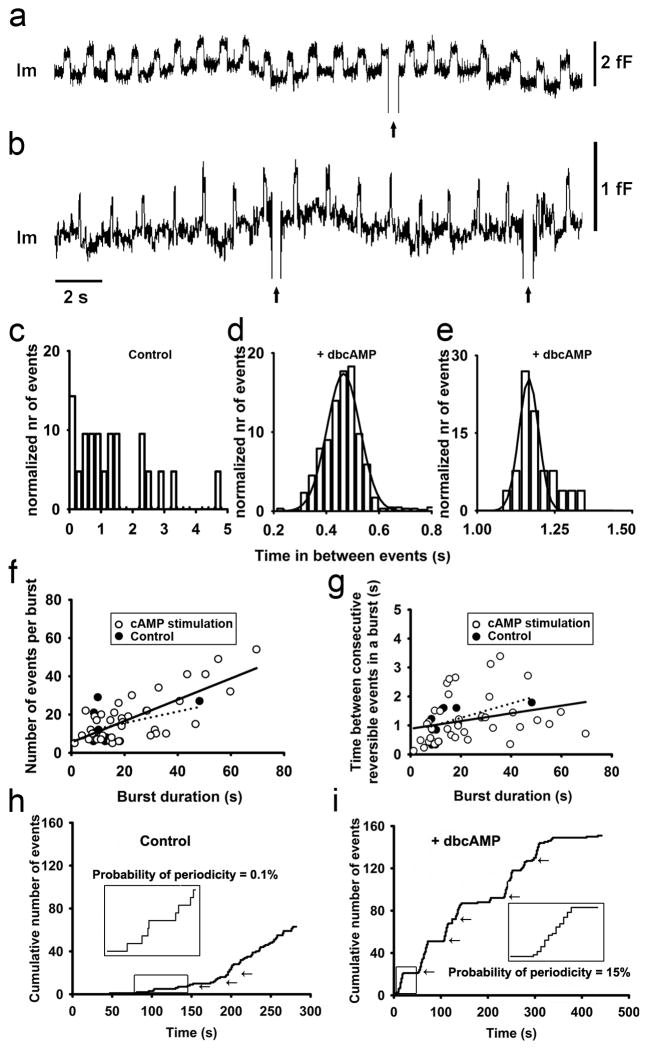
The increased intracellular level of cAMP elicited the periodicity of transient fusion events, which appeared in bursts (Fig. 2f and Fig. 5a, b). Within a burst, the transient fusion pore events represent repetitive reopening of the single vesicle fusion pore as indicated by practically identical Cv amplitudes (see also Fig. 2f). Moreover, fusion pore dwell-times of the repetitive reversible events in the same burst were relatively constant, contributing to the distinct modal peaks seen in Fig. 4e (lower right panel; see also Henkel et al., 2000 and Stenovec et al., 2004). To study periodicity of transient fusion events in more detail, we determined the burst parameters. We considered a burst to be composed of at list five transient events, with at most 5 s in between ensuing events. In controls, 42 percent of transient fusion events occurred in bursts (n=115 events in bursts). The percentage of events appearing in bursts increased to 53 percent (n=338 events in bursts) after IBMX stimulation and to 70 percent (n=153 events in bursts) after forskolin stimulation. After dbcAMP stimulation, almost all events appeared in bursts (~94 percent; n=1078 events in bursts). We then measured the time between ensuing events within bursts. The distribution of these, prior to stimulation, was complex (Fig. 5c). In contrast, stimulation with dbcAMP resulted in a bell-shaped distribution (Fig. 5d, e), indicating that the fusion pores reopened at predictable times within a given patch of membrane where the periodic behavior was recorded. To confirm the periodicity, we fitted the distributions with Gaussian curves (Fig. 5d, e) with means 0.465 ± 0.001 s and 1.166 ± 0.002 s. Similar values were reported previously (Stenovec et al., 2004).
Figure 5. The addition of dbcAMP results in rhythmic reopening of the same fusion pore.
(a, b) Two epochs of the representative Im traces showing rhythmic fusion pore activity in two different cells after the addition of dbcAMP. This activity was part of two bursts (see Fig. 1e for definition) with durations of 180 s and 41s s. (c, d, e) Representative histograms of times in between ensuing transient exocytic events within a burst in controls (c) and after the addition of dbcAMP (d, e). The histogram of controls shows a random distribution, while histograms after the addition of dbcAMP (which are partially shown in panels a, b) appear normally distributed and were fitted with Gaussian curves with the means of 0.465 ± 0.001 s (n=645 events) and 1.166 ± 0.002 s (n=26 events), respectively. (f) The number of reversible events in a burst depends on the burst duration. The control data points were fitted with the regression line (dotted line) with parameters: y (number of events per burst)=(0.30 ± 0.27) × x (burst duration in s) + (10 ± 6) (correlation coefficient r=0.41, n=8 bursts, p=0.3) and after stimulation with cAMP (solid line): y (number of events per burst)=(0.56 ± 0.08) × x (burst duration in s) + (6 ± 2) (correlation coefficient r=0.76, n=44 bursts, p<0.001). Note that the number of bursts of controls is much reduced. (g) The time between consecutive reversible events in a burst did not depend on the burst duration. Control data points were fitted with the regression line (dotted line) with parameters: y (time between consecutive reversible events in a burst in s)=(0.025 ± 0.015) × x (burst duration in s) + (0.8 ± 0.3) (correlation coefficient r=0.57, n=8 bursts, p=0.1) and after cAMP stimulation data points were fitted with the regression line (solid line) with parameters: y (time between consecutive reversible events in a burst in s)=(0.013 ± 0.007) × x (burst duration in s) + (0.9 ± 0.2) (correlation coefficient r=0.27, n=44 bursts, p=0.08). Note that the burst duration of controls was typically shorter than 20 s. (h) The cumulative number of reversible events as a function of time in a representative control cell. Arrows denote bursts of reversible events. The inset shows a magnified epoch (marked in fig. with rectangle) where the time between ensuing reversible events is random. (i) The cumulative number of reversible events as a function of time in a representative cell after the addition of dbcAMP. Arrows denote bursts of reversible events. Inset shows a magnified burst (marked in fig. with rectangle) with remarkably constant time between ensuing reversible events.
Then we analyzed the relationship between the number of transient fusion events within a burst and the duration of the burst (Fig. 5f). In bursts after cAMP stimulation we observed that the number of transient fusion events was correlated with the duration of the burst (r=0.76; n=44 bursts), with the average frequency of 0.56 ± 0.08 events/s in bursts. Fig. 5f shows that the majority of bursts that occurred after the addition of cAMP-increasing agents had increased number of events per burst, and prolonged burst duration, compared to controls. The average number of transient fusion events in a burst increased; from 14 ± 4 events in controls (n=115 events in 8 bursts) to 17 ± 3 events after addition of IBMX (n=338 events in 19 bursts), to 29 ± 20 events after addition of forskolin (n=153 events in 8 bursts) and to 27 ± 6 events after addition of dbcAMP (n=1078 events in 17 bursts). The average burst duration was similar in control and in IBMX-stimulated condition (~18 s), whereas after forskolin- and dbcAMP-stimulations, burst durations were prolonged (~24 s and ~33 s, respectively; data not shown). We also analyzed the relationship between the time between consecutive transient events within a burst and burst duration, which showed a poor correlation (r=0.29; n=50 bursts) and slope near 0 (0.01 ± 0.007; Fig. 5g). Note also that the majority of bursts recorded in the presence of elevated cAMP exhibit longer periods between consecutive transient events in addition to the prolonged burst durations, compared to controls (Fig. 5g). Therefore, the burst duration is related to the number but not the time in between consecutive transient fusion events. Panels h and i of Fig. 5 show cumulative numbers of transient events in two representative recordings. The appearance of bursts both in control and in dbcAMP-stimulated conditions are highlighted in insets, respectively. In controls, the time between consecutive events is rarely periodic with the probability of periodicity of 0.1 percent, calculated as the time in which reversible events were periodic, divided by the total time of recordings (see Materials and Methods). However, the inset of the dbcAMP-stimulated recording shows highly periodic transient exocytic events. The overall probability of periodicity after the addition of dbcAMP was 15 percent, much more than in spontaneous conditions, where it was 0,1 percent.
These results indicate that dbcAMP-stimulation not only increases the frequency of transient exocytic events, but also affects the nature of their appearance – transient exocytic events become periodic, with periodic fusion pore dwell-times and more frequently appear in bursts.
cAMP stabilizes transient, widely open fusion pores
The results shown in Table 1 demonstrate that dbcAMP decreases the fraction of transient exocytic events with relatively narrow (measurable) fusion pores. Moreover, the remaining transient exocytic events with measurable fusion pores exhibit increased diameters, compared to controls (Fig. 4a). Both results indicate that the addition of dbcAMP promote wider fusion pores. Previously we observed that fusion pore diameter is related to the diameter of vesicles (Jorgačevski et al., 2010). To check the possibility that the elevation of cAMP influences previously observed relationship, we plotted Cv and the respective Gp for each event in controls and after the addition of cAMP-increasing agents (Fig. 6a). The data points were best fitted with linear regressions with the following slopes: 2.3 ± 0.5 pS/fF (control) and 7.0 ± 0.5 pS/fF (cAMP-increasing agents) (p<0.001). These results show that a vesicle of a given Cv (diameter) may attain a threefold larger Gp (wider fusion pore) in the presence of a cAMP-increasing agent (Fig. 6a).
Figure 6. The presence of cAMP increasing agents affects the relationship between the fusion pore diameter and the vesicle diameter.
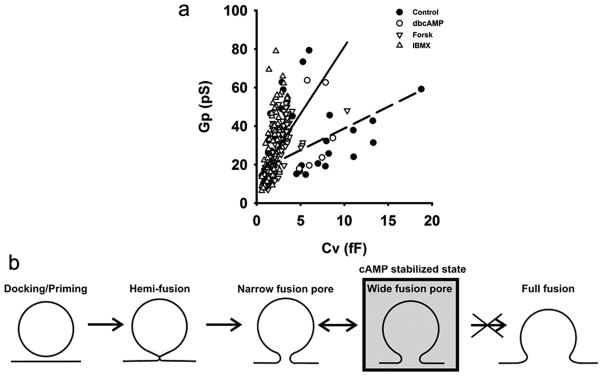
(a) Scatter plot diagram of fusion pore conductance vs. vesicle capacitance of respective reversible events in controls (full circles) and after the addition of cAMP increasing agents (empty symbols). Respective data points were best fitted with: y (Gp)=(2.3 ± 0.5) × x (Cv) + (16 ± 2) (control, dashed line, r=0.48) and y (Gp)=(7.0 ± 0.5) × x (Cv) + (11 ± 1) (cAMP-increasing agents, solid line, r=0.61). Both slopes are significantly different from each other (p<0.001). Significance was tested using one-way ANCOVA for two independent samples. (b) Model describing the effect of cAMP on exocytotic cycle of lactotroph vesicles.
Discussion
Regulated exocytosis consists of several distinct stages (Coorssen and Zorec, 2012). The key finding of this work is that cAMP stabilizes a stage, where the vesicle fusion pore transiently opens to relatively wide diameters, but has a reduced probability to enter into the full-fusion exocytic stage (Fig. 6b). Stabilization of a particular stage may represent a rate-limiting step for vesicle cargo release, implying that secretion can be regulated at the post-fusion state (Rahamimoff and Fernandez, 1997; Barg et al., 2002; Tsuboi and Rutter, 2003; Obermüller et al., 2005; Hanna et al., 2009; Thorn, 2009; Jorgačevski et al., 2011). This condition can be attained by regulating fusion pore gating (kinetics) and by regulating fusion pore diameter (Staal et al., 2004; Stenovec et al., 2004; Vardjan et al., 2007; Jorgačevski et al., 2008; Zhang and Jackson, 2008; Jorgačevski et al., 2010), as demonstrated in the present work for cAMP-modulation of regulated exocytosis.
Hormone-release studies have shown that cAMP-elevating agents stimulate PRL release from pituitary cells at relatively low forskolin and dbcAMP concentrations, whereas at higher concentrations an inhibition of PRL release was recorded (Fig. 1). The latter finding contrasts with previous studies, where IBMX increases the content of cAMP in cells and also the release of PRL in a dose-dependent manner (Gonzalez-Iglesias et al., 2006). In the present work, elevated forskolin (50 μM) and dbcAMP (>10 mM) concentrations are inhibitory for PRL release (Fig. 1). Among many possible mechanisms, the inhibition of PRL release at elevated levels of cAMP may be due to direct cAMP-mediated modulation of the exocytic machinery (Sikdar et al., 1990, 1998; Hanna et al., 2009).
cAMP increases the occurrence of transient exocytic events
As shown previously (Zorec et al., 1991; Jorgačevski et al., 2008, 2011), we recorded unitary exocytic events in lactotrophs (Figs. 2, 5). The amplitude of ~1 fF of these events is consistent with the view that they represent peptidergic vesicles interacting with the plasma membrane (Jorgačevski et al., 2011). Two kinds of discrete increases in Cm were present: irreversible, representing full-fusion, and reversible, representing the transient mode of exocytosis (Fig. 2). Interestingly, cAMP-increasing agents did not increase the frequency of the former, but only the frequency of the latter (Figs. 2, 3). The increased frequency of transient events is consistent with the increased PRL release recorded in these cells (Fig. 1). Furthermore, transient exocytic events exhibited wider fusion pore diameters and prolonged fusion pore dwell-times (Fig. 4), all facilitating the exit of vesicle cargo into the extracellular space. However, the relative reduction of the occurrence of discrete irreversible steps in Cm observed in the presence of cAMP-elevating agents (Fig. 2e), indicates that vesicle discharge may not attain full rates because it is hindered by the inability of transient exocytic events transiting into the full-fusion stage of regulated exocytosis (Fig. 6b). This condition is likely reflected in reduced rates of PRL release recorded at relatively high dbcAMP concentrations (Fig. 1). These results are in agreement with the decrease in cAMP-dependent full-fusion events in β-cells (Hanna et al., 2009). As in our experiments, where cAMP-increasing agents increase the frequency of transient exocytic events (Fig. 2d, e), a similar augmentation of the occurrence of transient exocytotic events was observed in PC12, chromaffin, and pancreatic β-cells (Wang et al., 2003; MacDonald et al., 2006; Hanna et al., 2009), but not in islet cells (Hatakeyama et al., 2006). Furthermore, Cochilla and co-workers (2000) have shown that the increase of intracellular cAMP does not affect the number of plasma membrane fusion sites. Thus, the enhanced, cAMP-stimulated PRL release observed (Fig. 1b; Gonzalez-Iglesias et al., 2006) is due to the increased secretion of prolactin per fusion site, possibly via more efficient vesicle discharge (i.e. increased frequency of reversible exocytic events and increased Gp; Figs. 2d and 4a). Most likely, the afore mentioned cAMP-stimulated PRL release cannot be attributed to the compound exocytosis (Vardjan et al., 2009), since the results on the vesicle sizes (estimated from Cv; Fig. 3) do not support this conclusion.
The fusion pore has long been considered an unstable intermediate leading to complete merger of the vesicle membrane with the plasma membrane (Heuser and Reese, 1973). However, recent evidence indicates that the fusion pore can exhibit remarkable stability and can fluctuate between several open and closed states (Vardjan et al., 2007; Jorgačevski et al., 2010). The results in this study show that cAMP may stabilize one of these intermediates, that is, transient fusion pore openings with relatively wide diameters and prolonged dwell-times. However, this intermediate unlikely proceeds to the full-fusion stage of exocytosis (Fig. 6b) and thus represents a hindrance for the fastest rates of PRL release. A similar stabilization, albeit with fusion pore narrowing, was observed in lactotrophs transfected with Munc 18-1 mutants (Jorgačevski et al., 2011) and in lactotrophs exposed to Al3+ (Calejo et al., 2012).
The increase of intracellular cAMP resulted in fusion of larger vesicles in melanotrophs (Sikdar et al., 1998). Our results show that the amplitudes of Cv of transient events are similar in control and cAMP conditions in a given patch of membrane (Figs. 2b, f and 3). However, when we plotted the Cv amplitudes of all recorded events, the amplitudes of Cv appeared to consist of two vesicle populations being engaged in regulated exocytosis. The recalculated average vesicle diameters were 145 nm and 225 nm (see Materials and Methods), corresponding well with the size of prolactin containing vesicles (Smets et al., 1987; Angleson et al., 1999). Stimulation with cAMP-increasing agents increased the occurrence of the smaller amplitude vesicles (Fig. 4c). Why cAMP-mediated effects are related to mainly the smaller sized vesicles interacting with the plasma membrane is unclear, but may relate to the observation that fusion pore diameter attained at equilibrium depends on vesicle diameter and on the intrinsic shape of membrane constituents in the region of the fusion pore (Jorgačevski et al., 2010). The latter could be affected by cAMP-dependent alteration of the local phospholipid environment (Su et al., 2012), while size dependent modulation of vesicles of different diameters was observed also in cells treated by Munc 18-1 mutations (Jorgačevski et al., 2011).
cAMP-increasing agents elevate the fusion pore conductance and invoke rhythmicity
The release of vesicle content through a transient fusion pore depends on the effective diameter and the lifetime of the fusion pore. Previous studies have shown that KCl and hyposmotic stimulation affects both parameters in rat lactotrophs (Vardjan et al., 2007; Jorgačevski et al., 2008). In the present work, 25 percent of transient events, measured in control conditions, exhibited fusion pores, with the diameters < 3 nm (a limitation of our experimental setup; see Debus and Lindau, 2000; Table 1) as reported and too narrow for the exit of PRL from the vesicle (Vardjan et al., 2007). Stimulation with cAMP-increasing agents decreased the percentage of narrow fusion pores and increased the average fusion pore diameter of measurable fusion pores (Table 1; Fig. 4). Only stimulation with IBMX failed to result in a statistically significant increase of the average fusion pore diameter. This failure could be due to the slower time course of the cAMP increase that follows this type of stimulation. The other possibility is that the level of cAMP concentration achieved with this stimulation is below the level of that achieved with forskolin and dbcAMP. In agreement with these two notions, stimulation with dbcAMP had the most robust effects on the kinetics and conductance of the fusion events (Figs. 4, 5, 6). MacDonald et al. (2006) failed to observe changes in Gp following the stimulation with 5 μM of forskolin, which could be due to the different concentration used or due to the different cell model.
Rhythmicity of transient fusion events
Our results show that the average fusion pore dwell-time significantly increased only following the stimulation with dbcAMP (Figs. 4, 6a). This result may reflect the fact that the intracellular cAMP concentration must reach a threshold value in order to increase the fusion pore dwell-time. The distribution of fusion pore dwell-times show that in controls the modal peak is similar to the one reported (Stenovec et al., 2004) and is not changed after the stimulation with IBMX (Fig. 4c). Stimulation with forskolin shifted the modal peak dwell-time to a higher value. On the other hand, stimulation with dbcAMP resulted in several modal values, which represent the distribution of fusion pore dwell-times within bursts (Figs. 2f, 5a, 5b) and are similar to the observations of stimulated fission pore open states (Henkel et al., 2000). Moreover, time in between ensuing exocytic events was regular and could be fitted with a Gaussian curve (Figs. 5d, e). Amperometric measurements in giant dopamine neurons of freshwater snail showed a similar phenomenon: a bursting of periodic exocytotic events (Chen et al., 1996). The physiological significance of this periodic exocytic activity is still unknown but may involve cationic fluxes through the fusion pore and the vesicle membrane, as previously modeled (Kabaso et al., 2012).
In conclusion, the regulation of cAMP-mediated PRL-release is much more complicated than initially believed. Even though the increase in cAMP to a certain level stimulates PRL- release, at much higher levels of cAMP, the PRL release is significantly reduced mainly because of a decrease in the full-fusion exocytic events. We attribute transient increase in PRL release to stabilized fusion pores, which open more frequently, have on average prolonged dwell-time and wider diameters, but are unable to transit into the full-fusion exocytic state.
Acknowledgments
This work was supported by the Ministry of Higher Education, Sciences, and Technology of the Republic of Slovenia (P3 310; J3 3654; J3 4051; J3 4146); the Portuguese Foundation of Science and Technology of the Portuguese Ministry of Sciences, Technology, and High Education (Bilateral Agreement between Portugal and Slovenia - Proj. 441.00 SLOVENIA, SFRH/BD/41217/2007 to A.I.C.); and an NICHD Intramural Grant.
Footnotes
Competing financial interest
The authors declare that they have no competing financial interests.
References
- Alés E, Tabares L, Poyato JM, Valero V, Lindau M, Alvarez de Toledo G. High calcium concentrations shift the mode of exocytosis to the kiss-and-run mechanism. Nat Cell Biol. 1999;1:40–44. doi: 10.1038/9012. [DOI] [PubMed] [Google Scholar]
- Alvarez de Toledo G, Fernández-Chacón R, Fernández J. Release of secretory products during transient vesicle fusion. Nature. 1993;363:554–558. doi: 10.1038/363554a0. [DOI] [PubMed] [Google Scholar]
- Angleson J, Cochilla A, Kilic G, Nussinovitch I, Betz W. Regulation of dense core release from neuroendocrine cells revealed by imaging single exocytotic events. Nat Neurosci. 1999;2:440–446. doi: 10.1038/8107. [DOI] [PubMed] [Google Scholar]
- Barg S, Olofsson C, Schriever-Abeln J, Wendt A, Gebre-Medhin S, Renstrom E, Rorsman P. Delay between fusion pore opening and peptide release from large-dense-core vesicles from neuroendocrine cells. Neuron. 2002;33:287–299. doi: 10.1016/s0896-6273(02)00563-9. [DOI] [PubMed] [Google Scholar]
- Ben-Tabou S, Keller E, Nussinovitch I. Mechanosensitivity of voltage-gated calcium currents in rat anterior pituitary cells. J Physiol. 1994;476:29–39. [PMC free article] [PubMed] [Google Scholar]
- Breckenridge LJ, Almers W. Currents through the fusion pore that forms during exocytosis of a secretory vesicle. Nature. 1987;328:814–817. doi: 10.1038/328814a0. [DOI] [PubMed] [Google Scholar]
- Calejo AI, Jorgačevski J, Silva VS, Stenovec M, Kreft M, Gonçalves PP, Zorec R. Aluminum-induced changes of fusion pore properties attenuate prolactin secretion in rat pituitary lactotrophs. Neuroscience. 2012;201:57–66. doi: 10.1016/j.neuroscience.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Chen G, Gutman DA, Zerby SE, Ewing AG. Electrochemical monitoring of bursting exocytotic events from the giant dopamine neuron of Planorbis corneus. Brain Res. 1996;733:119–124. doi: 10.1016/0006-8993(96)00754-8. [DOI] [PubMed] [Google Scholar]
- Cochilla AJ, Angleson JK, Betz WJ. Differential regulation of granule-to-granule and granule-to-plasma membrane fusion during secretion from rat pituitary lactotrophs. J Cell Biol. 2000;150:839–848. doi: 10.1083/jcb.150.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coorssen JR, Zorec R. Regulated exocytosis per partes. Cell Calcium. 2012;52:191–195. doi: 10.1016/j.ceca.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Debus K, Lindau M. Resolution of patch capacitance recordings and of fusion pore conductances in small vesicles. Biophys J. 2000;78:2983–2997. doi: 10.1016/S0006-3495(00)76837-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez J, Neher E, Gomperts B. Capacitance measurements reveal stepwise fusion events in degranulating mast cells. Nature. 1984;312:453–455. doi: 10.1038/312453a0. [DOI] [PubMed] [Google Scholar]
- Fesce R, Grohovaz F, Valtorta F, Meldolesi J. Neurotransmitter release: fusion or “kiss-and-run”? Trends Cell Biol. 1994;4:1–4. doi: 10.1016/0962-8924(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Gandhi S, Stevens C. Three modes of synaptic vesicular recycling revealed by single-vesicle imaging. Nature. 2003;423:607–613. doi: 10.1038/nature01677. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Iglesias AE, Jiang Y, Tomic M, Kretschmannova K, Andric SA, Zemkova H, Stojilkovic SS. Dependence of electrical activity and calcium influx-controlled prolactin release on adenylyl cyclase signalling pathway in pituitary lactotrophs. Mol Endocrinol. 2006;20:2231–2246. doi: 10.1210/me.2005-0363. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Iglesias AE, Murano T, Li S, Tomic M, Stojilkovic SS. Dopamine inhibits prolactin release in pituitary lactotrophs through pertussis toxin-sensitive and -insensitive signaling pathways. Endocrinology. 2008;149:1470–1479. doi: 10.1210/en.2007-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna ST, Pigeau GM, Galvanovskis J, Clark A, Rorsman P, MacDonald PE. Kiss-and-run exocytosis and fusion pores of secretory vesicles in human β-cells. Pflugers Arch - Eur J Physiol. 2009;457:1343–1350. doi: 10.1007/s00424-008-0588-0. [DOI] [PubMed] [Google Scholar]
- Hatakeyama H, Kishimoto T, Nemoto T, Kasai H, Takahashi N. Rapid glucose sensing by protein kinase A for insulin exocytosis in mouse pancreatic islets. J Physiol. 2006;570:271–282. doi: 10.1113/jphysiol.2005.096560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel AW, Meiri H, Horstmann H, Lindau M, Almers W. Rhythmic opening and closing of vesicles during constitutive exo- and endocytosis in chromaffin cells. EMBO J. 2000;19:84–93. doi: 10.1093/emboj/19.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J, Reese T. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973;57:315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Lang T, Südhof T. Membrane fusion. Cell. 2003;112:519–533. doi: 10.1016/s0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- Jorgačevski J, Stenovec M, Kreft M, Bajić A, Rituper B, Vardjan N, Stojilkovic S, Zorec R. Hypotonicity and peptide discharge from a single vesicle. Am J Physiol Cell Physiol. 2008;295:624–631. doi: 10.1152/ajpcell.00303.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgačevski J, Fošnarič M, Vardjan N, Stenovec M, Potokar M, Kreft M, Kralj-Iglič V, Iglič A, Zorec R. Fusion pore stability of peptidergic vesicles. Mol Membr Biol. 2010;27:65–80. doi: 10.3109/09687681003597104. [DOI] [PubMed] [Google Scholar]
- Jorgačevski J, Potokar M, Grilc S, Kreft M, Liu W, Barclay WF, Buckers J, Medda R, Hell SH, Parpura V, Burgoyne RD, Zorec R. Munc18-1 Tuning of Vesicle Merger and Fusion pore Properties. J Neurosci. 2011;31:9055–9066. doi: 10.1523/JNEUROSCI.0185-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabaso D, Calejo AI, Jorgačevski J, Kreft M, Zorec R, Iglič A. Fusion pore diameter regulation by cations modulating local membrane anisotropy. Scientific World Journal. 2012;2012:983138. doi: 10.1100/2012/983138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostic TS, Tomic M, Andric SA, Stojilkovic SS. Calcium-independent and cAMP-dependent modulation of soluble guanylyl cyclase activity by G protein-couple receptors in pitiitary cells. J Biol Chem. 2002;277:16412–16418. doi: 10.1074/jbc.M112439200. [DOI] [PubMed] [Google Scholar]
- Kreft M, Zorec R. Cell-attached measurements of attofarad capacitance steps in rat melanotrophs. Pflugers Arch. 1997;434:212–214. doi: 10.1007/s004240050387. [DOI] [PubMed] [Google Scholar]
- Lollike K, Borregaard N, Lindau M. The exocytotic fusion pore of small granules has a conductance similar to an ion channel. J Cell Biol. 1995;129:99–104. doi: 10.1083/jcb.129.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lollike K, Lindau M. Membrane capacitance techniques to monitor granule exocytosis in neutrophils. J Immunol Methods. 1999;232:111–120. doi: 10.1016/s0022-1759(99)00169-6. [DOI] [PubMed] [Google Scholar]
- MacDonald PE, Braun M, Galvanovskis J, Rorsman P. Release of small transmitters through kiss-and-run fusion pores in rat pancreatic β cells. Cell Metabolism. 2006;4:283–290. doi: 10.1016/j.cmet.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Neher E, Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci USA. 1982;79:6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermüller S, Lindqvist A, Karanauskaite J, Galvanovskis J, Rorsman P, Barg S. Selective nucleotide-release from dense-core granules in insulin-secreting cells. J Cell Sci. 2005;118:4271–4282. doi: 10.1242/jcs.02549. [DOI] [PubMed] [Google Scholar]
- Ohara-Imaizumi M, Nakamichi Y, Tanaka T, Katsuta H, Ishida H, Nagamatsu S. Monitoring of exocytosis and endocytosis of insulin secretory granules in the pancreatic beta-cell line MIN6 using pH-sensitive green fluorescent protein (pHluorin) and confocal laser microscopy. Biochem J. 2002;363:73–80. doi: 10.1042/0264-6021:3630073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrais D, Kleppe I, Taraska J, Almers W. Recapture after exocytosis causes differential retention of protein in granules of bovine chromaffin cells. J Physiol. 2004;560:413–428. doi: 10.1113/jphysiol.2004.064410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahamimoff R, Fernandez JM. Pre- and post-fusion regulation of transmitter release. Neuron. 1997;18:17–27. doi: 10.1016/s0896-6273(01)80043-x. [DOI] [PubMed] [Google Scholar]
- Renström E, Eliasson L, Rorsman P. Protein kinase A-dependent and -independent stimulation of exocytosis by cAMP in mouse pancreatic B-cells. J Physiol. 1997;502:105–118. doi: 10.1111/j.1469-7793.1997.105bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedej S, Rose T, Rupnik M. cAMP increases Ca2+-dependent exocytosis through both PKA and Epac2 in mouse melanotrophs from pituitary slices. J Physiol. 2005;567:799–813. doi: 10.1113/jphysiol.2005.090381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino S, Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocyosis. Physiol Rev. 2005;85:1303–1342. doi: 10.1152/physrev.00001.2005. [DOI] [PubMed] [Google Scholar]
- Su WM, Han GS, Casciano J, Carman GM. Protein Kinase A-mediated Phosphorylation of Pah1p Phosphatidate Phosphatase Functions in Conjunction with the Pho85p-Pho80p and Cdc28p-Cyclin B Kinases to Regulate Lipid Synthesis in Yeast. J Biol Chem. 2012;40:33364–33376. doi: 10.1074/jbc.M112.402339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikdar SK, Zorec R, Mason WT. cAMP directly facilitates Ca-induced exocytosis in bovine lactotrophs. FEBS Lett. 1990;273:150–154. doi: 10.1016/0014-5793(90)81072-v. [DOI] [PubMed] [Google Scholar]
- Sikdar SK, Kreft M, Zorec R. Modulation of unitary exocytotic event amplitude by cAMP in rat melanotrophs. J Physiol. 1998;511:851–859. doi: 10.1111/j.1469-7793.1998.851bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smets G, Velkeniers B, Finne E, Baldys A, Gepts W, Vanhaelst L. Postnatal development of growth hormone and prolactin cells in male and female rat pituitary. An immunocytochemical light and electron microscopic study. J Histochem Cytochem. 1987;35:335–341. doi: 10.1177/35.3.3819376. [DOI] [PubMed] [Google Scholar]
- Spruce A, Breckenridge L, Lee A, Almers W. Properties of the fusion pore that forms during exocytosis of a mast cell secretory vesicle. Neuron. 1990;4:643–654. doi: 10.1016/0896-6273(90)90192-i. [DOI] [PubMed] [Google Scholar]
- Staal R, Mosharov E, Sulzer D. Dopamine neurons release transmitter via a flickering fusion pore. Nat Neurosci. 2004;7:341–346. doi: 10.1038/nn1205. [DOI] [PubMed] [Google Scholar]
- Stenovec M, Kreft M, Poberaj I, Betz W, Zorec R. Slow spontaneous secretion from single large dense-core vesicles monitored in neuroendocrine cells. FASEB J. 2004;18:1270–1272. doi: 10.1096/fj.03-1397fje. [DOI] [PubMed] [Google Scholar]
- Stojilkovic SS, Tabak J, Bertram R. Ion channels and signaling in the pituitary gland. Endocr Rev. 2010;31:845–915. doi: 10.1210/er.2010-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su WM, Han GS, Casciano J, Carman GM. Protein Kinase A-mediated Phosphorylation of Pah1p Phosphatidate Phosphatase Functions in Conjunction with the Pho85p-Pho80p and Cdc28p-Cyclin B Kinases to Regulate Lipid Synthesis in Yeast. J Biol Chem. 2012;40:33364–33376. doi: 10.1074/jbc.M112.402339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn P. New insights into the control of secretion. Commun Integr Biol. 2009;2:315–317. doi: 10.4161/cib.2.4.8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn P, Parker I. Two phases of zymogen granule lifetime in mouse pancreas: Ghost granules linger after exocytosis of contents. J Physiol. 2005;563:433–442. doi: 10.1113/jphysiol.2004.077230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi T, Rutter G. Multiple forms of “kiss-and-run” exocytosis revealed by evanescent wave microscopy. Curr Biol. 2003;13:563–567. doi: 10.1016/s0960-9822(03)00176-3. [DOI] [PubMed] [Google Scholar]
- Vardjan N, Stenovec M, Jorgačevski J, Kreft M, Zorec R. Subnanometer fusion pores in spontaneous exocytosis of peptidergic vesicles. J Neurosci. 2007;27:4737–4746. doi: 10.1523/JNEUROSCI.0351-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardjan N, Jorgačevski J, Stenovec M, Kreft M, Zorec R. Compound exocytosis in pituitary cells. Ann N Y Acad Sci. 2009;1152:63–75. doi: 10.1111/j.1749-6632.2008.04008.x. [DOI] [PubMed] [Google Scholar]
- Wang CT, Lu JC, Bai J, Chang PY, Martin TFJ, Chapman ER, Jackson MB. Different domains of synaptotagmin control the choice between kiss-and-run and full fusion. Nature. 2003;424:943–947. doi: 10.1038/nature01857. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Jackson MB. Temperature dependence of fusion kinetics and fusion pores in Ca2+-triggered exocytosis from PC12 cells. J Gen Physiol. 2008;131:117–124. doi: 10.1085/jgp.200709891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorec R, Sikdar SK, Mason WT. Increased Cytosolic Calcium Stimulates Exocytosis in Bovine Lactotrophs. J Gen Physiol. 1991;97:473–497. doi: 10.1085/jgp.97.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]