Abstract
Vitamin D and vitamin D metabolites such as 25-hydroxyvitamin D and 1α, 25-dihydroxyvitamin D (1α, 25(OH)2D3) circulate in the serum of fish. The receptor for 1α, 25(OH)2D3 (VDR) has previously been cloned from fish intestine, and ligand binding assays have demonstrated the presence of the VDR in the gills, intestine and liver of fish. Using immunohistochemical methods with specific antibodies against the VDR, we now report that the VDR is widely expressed in tissues of the adult male and female zebrafish, Danio rerio, specifically in epithelial cells of gills, tubular cells of the kidney, and in absorptive cells in the intestine. Additionally, the VDR is expressed in the skin, the olfactory organ, in the retina, brain, and spinal cord. Sertoli cells of the testis, oocytes, acinar cells of the pancreas, hepatocytes and bile duct epithelial cells express substantial amounts of the receptor. Osteoblast-like cells and chondrocytes also express the VDR. Pre-immune serum and antiserum pre-adsorbed with Danio VDR protein fails to detect the VDR in the same tissues. The VDR is also present in the developing eye, brain, and otic vesicle of 48 h and 96h post-fertilization zebrafish embryos. Parenteral administration of 1α, 25(OH)2D3 increases concentrations of the VDR in intestinal epithelial cells but not in epithelial cells of the gills. Lithocholic acid, however, does not alter concentrations of the VDR following parenteral administration. The data suggest that the VDR is widely distributed in tissues of the zebrafish, Danio rerio, and is likely to play important roles in epithelial transport, bone and endocrine function. Furthermore, concentrations of the receptor appear to be regulated by its ligand, 1α, 25-dihydroxyvitamin D, but not by lithocholic acid. Zebrafish may serve as a useful model in which to assess the function of the VDR in diverse tissues.
Keywords: Zebrafish; Vitamin D Receptor; 1α,25-dihydroxyvitamin D; calcium
INTRODUCTION
The vitamin D endocrine system plays a vital role in mineral homeostasis, endocrine, immune, and neural function (1–5). The active metabolite of vitamin D3, 1α, 25(OH)2D3, is formed from vitamin D3 as a result of a multistep process involving hydroxylations at C-25 and C-1 that occur in the liver and kidney, respectively (1, 2). 1α, 25(OH)2D3 binds to its receptor, the vitamin D receptor (VDR), with high affinity and subsequently regulates the expression of several genes (4). The crucial role of the VDR in mediating the function of 1α, 25(OH)2D3 is demonstrated by the occurrence of disordered calcium and phosphorous homeostasis in humans and animal models which have mutations of the VDR (6–9).
Previous immunohistochemical studies from our laboratory have demonstrated that the VDR is present in several epithelial tissues of terrestrial mammals (10–16). However, little is known of the distribution of the VDR in fish. Fish are a source of vitamin D3 (17–19) and have detectable concentrations of 25-hydroxyvitamin D3 and 1α, 25(OH)2D3 in serum (20–23). Two species of the VDR have been cloned from the intestine of the flounder and pufferfish (24, 25), and the VDR is present in the lamprey, a fish lacking any skeletal elements, suggesting that the VDR may play a role in processes other than bone mineralization such as xenobiotic metabolism (26, 27). Published literature suggests that Danio rerio, however, expresses a single VDR although sequencing of expressed RNAs suggests some heterogeneity in the sequence (26, 28–30). 1α, 25(OH)2D3 alters mineral transport in fish gills, and vitamin D deficiency is associated with altered growth and mineralization of the fish skeleton (31–33). Indeed, changes in 1α, 25(OH)2D3 and VDR concentrations have been noted in salmon undergoing smoltification and migrating from freshwater (low calcium concentrations) to seawater (high calcium concentrations) suggesting that synthesis of the sterol and its receptor undergo alterations depending upon ambient calcium concentrations (32). Furthermore, the administration of vitamin D3 or 1α, 25-dihydroxyvitamin D3 is associated with increased mineralization in developing fish embryos (34). There have been no reports, however, that have examined the organ-specific and cellular distribution of the VDR in tissues of fish using specific antibodies.
In order to establish the zebrafish, Danio rerio, as a model organism in which to study the multiple effects of 1α, 25(OH)2D3, we examined the distribution of the VDR in adult male and female fish and in developing fish embryos. Danio rerio is an important model organism in which disruption of gene expression often results in a phenotype that can be readily observed. Additionally, the effects of exposure to toxins and other xenobiotics can be readily observed in this organism, and the effects of disruptions of key proteins in the adaptation to exposure to xenobiotics can be assessed (35). We now demonstrate that the VDR is present in multiple epithelial tissues such as the gill, kidney and intestine, which are important in the movement of calcium and other ions. In addition, we demonstrate that the VDR is present in bone and endocrine tissues such as the pancreas, testis and ovary suggesting that 1α, 25(OH)2D3 may be important in the appropriate function of these organs. The VDR is detected in the brain, retina and olfactory organs suggesting that it may be important in their function. We also show that the VDR is present in the developing fish embryo. In adult fish, the parenteral administration of 1α, 25(OH)2D3 increases VDR expression in the intestine but not in gills. Lithocholic acid does not increase VDR concentrations in the intestine or gills when administered parenterally in fish.
METHODS AND MATERIALS
Expression methods for Danio rerio VDR 33-453
Reverse transcription and polymerase chain reaction (RT-PCR) was carried out using a Titanium One Step RT PCR kit (Clontech, Mountain View, CA) to obtain a full length cDNA clone for Danio rerio VDR (GenBank Accession NM_130919) using total RNA prepared from Danio rerio. Appropriate primers contained BamHI and EcoRI restriction sites to allow for subsequent cloning into an expression plasmid. PCR products for 33-453 Danio rerio Q47E P54R VDR (which corresponds, in length, to residues in full length (FL) human VDR) were treated with BamHI and EcoRI and then ligated into BamHI/EcoRI treated pGEX-6P-1 (GE Amersham Biosciences, Piscataway, NJ). E. coli BL21 host cells (Novagen/EMD) were transformed with the VDR-pGEX-6P-1 chimeric plasmid for subsequent protein expression. The DNA sequence was verified by sequencing both strands of the plasmid constructs. The 33-453 VDR of Danio rerio was expressed using bacterial expression methods for the VDR (36–40). Briefly, the Danio rerio VDR construct was expressed as a GST (glutathione S-transferase) fusion protein. E. coli BL21 cells were transformed with the expression plasmid were plated on antibiotic plates and single colonies were grown in 100 ml starter cultures in 2X YT medium with appropriate antibiotic (ampicillin 100 μg/ml). Ten liters of 2X YT medium with appropriate antibiotic were inoculated with starter cultures and cells were grown to an OD600 of ~1 at 37 °C. The temperature was decreased to 20 °C for 30 min, and isopropylthiogalactoside (IPTG) was added to a concentration of 0.1 mM. Cells were allowed to grow for ~5 hours and bacterial pellets were harvested by centrifugation. Bacteria were lysed in PBS, 5 mM EDTA, 10 mM β-mercaptoethanol (β-ME), pH 7.4 (lysis buffer) containing 4 mM phenylmethylsulfonylfluoride (PMSF) using 0.1 mm glass beads and an ice-jacketed bead beater (Biospec Products, Inc., Bartlesville, OK). Bacterial lysates, from bacteria expressing the GST-VDR were centrifuged at 20,000 × g and the clarified supernatants were applied to glutathione-Sepharose using a batch procedure at 4 °C for ~1.5 hr. (Clontech; Miltenyi Biotec Inc., Auburn, CA). The matrix was washed extensively with lysis buffer at 4 °C followed by washes with PBS, 10 mM β-ME. The glutathione resin (7.5 ml) to which GST-VDR was bound was washed with 150 ml of 50 mM Tris, 1 mM DTT, 1 mM EDTA, 150 mM NaCl, pH 7.0. The resin with GST-VDR bound to it was treated with 60 units PreScission Protease (GE Amersham Biosciences) at 4 °C. Danio rerio 33-453 VDR was collected after cleavage was complete (as determined by SDS-PAGE) by addition of PreScission Protease buffer and collection of the column effluent. Danio rerio 33-453 VDR was further purified by dilution of the protein with 6 volumes 50 mM Tris, 2 mM DTT pH 7.0 (Buffer A) which was then filtered through a 0.2 μm nylon filter. The protein was applied to a Tricorn MonoQ 50/5 column (GE Amersham Biosciences). Highly purified Danio rerio 33-453 VDR was eluted by a gradient of increasing amounts of buffer B (buffer A containing IM NaCl). The pure protein eluted at about 32% buffer B. Protein purity was assessed by SDS-polyacrylamide gel electrophoresis using Coomassie blue and silver stained Phastgels (GE Amersham Biosciences, and by western analyses using rabbit anti-human VDR (# 2-152) and anti-rabbit horseradish peroxidase conjugated IgG secondary antibody (Dako, Carpinteria, CA). Bound secondary antibody was detected by use of a chemiluminescence substrate (Roche Diagnostics, Indianapolis, IN).
Fixation of Zebrafish for Immunohistochemistry
Adult male and female zebrafish (approximately 5 months of age) and raised under standard conditions in fish water (Instant Ocean, Spectrum Inc., Atlanta, GA; final conductivity 450 μS/cm2, pH 7.5; final calcium concentration 0.25mM) were obtained from Dr. Xiaolei Xu (Department of Biochemistry and Molecular Biology, Mayo Clinic). The fish were fed brine shrimp 4 times per day. The fish were euthanized with 1% Tricaine in fish water. A slit was cut in the ventral portion of the abdomen using a sharp scalpel. The fish were placed in Dietrich’s solution (30% ethanol, 10% formalin and 2% acetic acid, pH 2 .7) for decalcification and fixation at room temperature for 96 hours. The fixed and decalcified fish were embedded in paraffin and serially sectioned to obtain 4 μm sections.
Forty eight and ninety-six hour post-fertilization zebrafish embryos were fixed as previously described (10, 14).
Immunohistochemistry using VDR antibody
Immunohistochemistry for the VDR was performed using methods described earlier (10, 12–16). VDR antibodies were prepared as described earlier (10, 12–16). 4 μm thick serial sections from adult fish were placed on silanized slides. The slides were deparaffinized in xylene, rehydrated in a series of ethanol solutions, and rinsed in water. Endogenous peroxidase activity was blocked using 0.6% H202 in methanol. After a water rinse, sections were placed in 10 mM citric acid, pH 6.0, and heated in a microwave two times for 2 min in a 750-W oven set on high. After cooling, sections were treated with 5% normal goat serum in phosphate-buffered saline, pH 7.4 containing 0.05% Tween 20 for 15 min and incubated for 60 min with rabbit-anti-VDR, at a dilution of 1:500 or 1:1000, at room temperature. After rinsing, sections were treated with biotinylated goat anti-rabbit immunoglobulin G (1:200) (Dako), followed by peroxidase-labeled streptavidin (1:500) (Dako) for 30 min at room temperature. Sections were developed by adding 0.1 M sodium acetate, pH 5.2, containing aminoethyl carbazole and H2O2 for 15 min. Sections were counterstained with hematoxylin and placed on a coverslip with aqueous mounting media. Negative controls for nonspecific staining were done on tissue sections using pre-immune rabbit serum diluted 1: 500 in place of primary antibodies. To assure specificity slides were also stained using primary antibodies that were pre-adsorbed with Danio VDR antigen or VDR buffer (as control) prepared as described above.
Immunofluorescence fixation and labeling
Adult zebrafish for immunofluorescence were obtained from Jennifer Liang (Case Western Reserve University). Fish were fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) in PBS overnight at 4 °C followed by incubation in 30% sucrose in PBS overnight at 4 °C. Fish were embedded in Tissue Tek (Electron Microscopy Sciences) and cut in 20 μm sections on a Leica cryotome. Frozen sections for fluorescence labeling were rehydrated in PBS and blocked in goat blocking buffer (5% normal goat serum, 1% glycerol, 0.1% each fish skin gelatin and BSA in PBS, pH 7.2). Sections were incubated with rabbit anti-VDR (1/1000) 2 h at room temperature followed by Cy5 conjugated goat anti-rabbit IgG (1/800) (Invitrogen Corp., Carlsbad, CA) for 1 h at room temperature.
Regulation of the VDR by 1α, 25(OH)2D3 and lithocholic acid
Adult male fish were administered intramuscularly 25 ng of 1α, 25(OH)2D3 (Dr. Milan Uskokovic, Roche, Nutley, NJ) in 5 μL propylene glycol or 25 ng of lithocholic acid (Sigma Chemicals, St. Louis, MO) in 5 μL propylene glycol or 5 μL of propylene glycol alone. Twenty two hours later the fish were euthanized in tricaine as described above and sections of fish containing the intestine and gills were isolated by a transverse cut behind the gill operculum, and homogenized in buffer containing 50 mM Tris, 25 mM sodium chloride, 6 mM EDTA, 10 mM DTT, pH 7.4, containing a protease-inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). The homogenates were centrifuged at 16,000 X at 4°C for one hour. The supernatant protein was quantitated in each sample using Bio-Rad protein reagent (Bio-Rad, Hercules, CA) and 60 μg of protein homogenate were separated by SDS-PAGE. The separated proteins were transferred to PVDF membranes and exposed to anti-VDR antibody (1:500) in TBS (50 mM Tris, 150 mM sodium chloride, pH 7.5) containing 0.5% blocking agent (Roche) for one hour. The membranes were washed 3 times in TBS, 0.1% Tween-20, and exposed to secondary antibody (goat-anti-rabbit HRP-IgG, Dako Corp, Carpinteria, CA, 1: 2000, in TBS containing 0.5% blocking agent) for one hour. The membrane was then washed 3 times in TBS, 0.1% Tween-20. The membrane was exposed to BM chemiluminescent reagent (Roche Diagnostics) for one minute. Signal was detected by exposing the membranes to x-ray film (Kodak, Biomax MR). The optical density of bands at Mr 52,000 was quantitated using a Kodak ds-1D Digital System Version 3.0.0 (Kodak, New Haven, CT).
RESULTS
A low-power view of the sagittally-sectioned, posterior portion, of an adult male zebrafish demonstrates the presence of the VDR in the intestine (I), the liver (L), the testis (T), and the kidney (K) (Figure 1A). Studies performed with the pre-immune serum show absent staining in these organs (Figure 1B). A low-power view of the sagittally-sectioned, mid-portion, of an adult male zebrafish demonstrates immunostaining for the VDR in the gills (G), the epithelium of the oropharynx, and the brain (B) (Figure 1C). A low-power view of a transverse section of the mid-portion of an adult male zebrafish shows staining for the VDR in the gills (G), the epithelium of the oropharynx (O), and the spinal cord (SC) (Figure 1D). A low-power view of the sagittally-sectioned, anterior portion, of an adult zebrafish demonstrates the presence of the VDR in layers of the eye (E) and the olfactory organ (OO) (Figure 1E). Figure 1F shows the absence of immunostaining using pre-immune serum.
Figure 1.
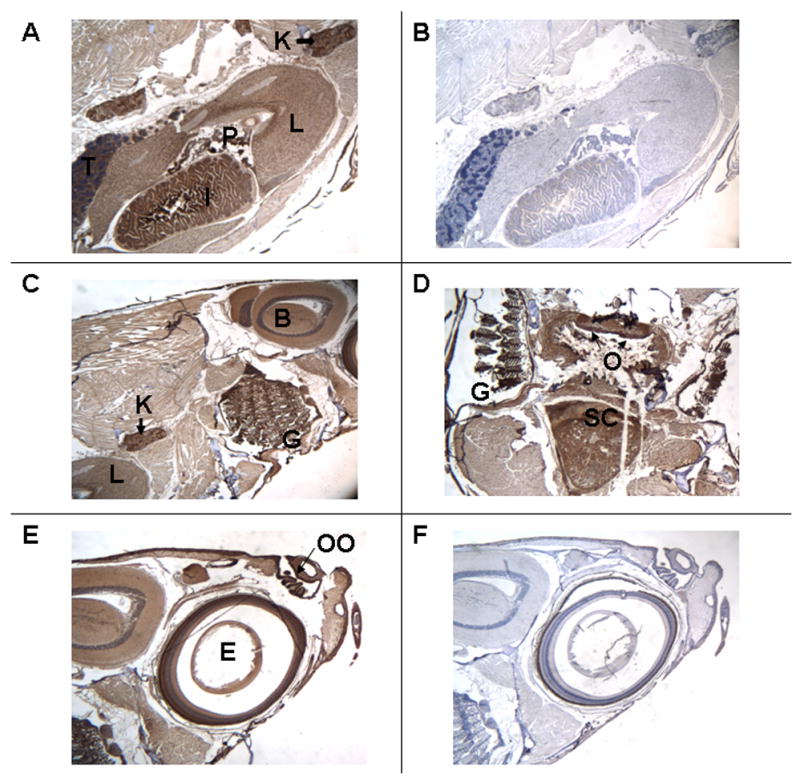
Section of adult male zebrafish. Immunostaining with anti-VDR antibody or pre-immune serum. 50 X original magnification. Panel A. Sagittal section. Immunostaining with anti-VDR antibody of the intestine (I), Liver (L), pancreas (P), kidney (K and arrow), and testis (T). Panel B. Sagittal section. Pre-immune serum. Note absence of staining in the intestine, liver, pancreas, kidney and testis. Panel C. Sagittal section. Immunostaining with anti-VDR antibody of the gills (G), kidney (K), liver (L) and brain (B). Panel D. Transverse section. Immunostaining with anti-VDR antibody of gills (G), epithelial lining of oropharynx (O), and spinal cord (SC). Panel E. Sagittal section. Immunostaining with anti-VDR antibody of olfactory organ (OO) and eye (E). Panel F. Sagittal section. Pre-immune serum. Note absence of staining of olfactory organ (OO) and eye (E). The dark brown color represents the VDR.
In order to assure specificity of the antibody, in anti-VDR immune serum was pre-adsorbed with excess Danio rerio recombinant VDR. No immunostaining was observed using pre-adsorbed VDR antibody in the intestine (Figure 2A), kidney or liver (Figure 2B), gill membranes or oropharynx (Figure 2C), testis (Figure 2D) or in the various layers of the eye (Figure 2E).
Figure 2.
Section of adult male zebrafish. Immunostaining with anti-VDR antibody pre-adsorbed with recombinant Danio rerio full-length VDR. 50 X original magnification. Panel A. Intestine. Panel B. Liver and kidney. Panel C. Gills. Panel D. Testis. Panel E. Eye. Note absence of immunostaining in all tissues.
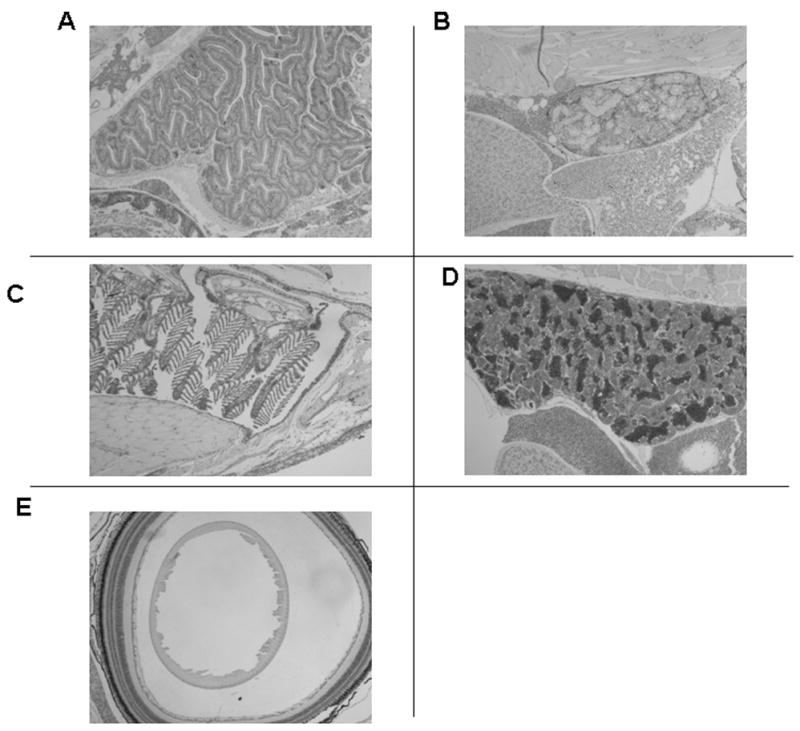
In the kidney immunostaining is visible in the renal tubules (T) and in the glomerular epithelium (G) (Figure 3A). Immunostaining is present throughout the tubular cell and is not exclusively localized to the nucleus. There is no staining visible in the kidney when pre-immune serum is used (Figure 3B). In Figure 3C, immunostaining for the VDR is clearly visible in epithelial cells of gill lamellae (L). Additionally, immunostaining for the VDR is visible in chondrocyte cells of the bony portion of the filaments of the gill (CC in F; see discussion below regarding distribution in bone). Pre-immune serum shows the appropriate negative results for VDR immunostaining (Figure 3D). In figure 3E is shown immunostaining for the VDR with immune serum in the epithelial cells of the olfactory organ (OOE, arrows). Results with pre-immune serum are appropriately negative (Figure 3F). There is intense staining for the VDR in epithelial cells of the intestine (EC; Figure 3G). The bodies of goblet cells which contain mucin-like material immunostain with VDR-antibodies. The mucinous material does not immunostain. Results with the pre-immune serum are negative (Figure 3H). Of note, as demonstrated in Figure 2, pre-adsorbed antiserum reveals no immunostaining in various organs.
Figure 3.
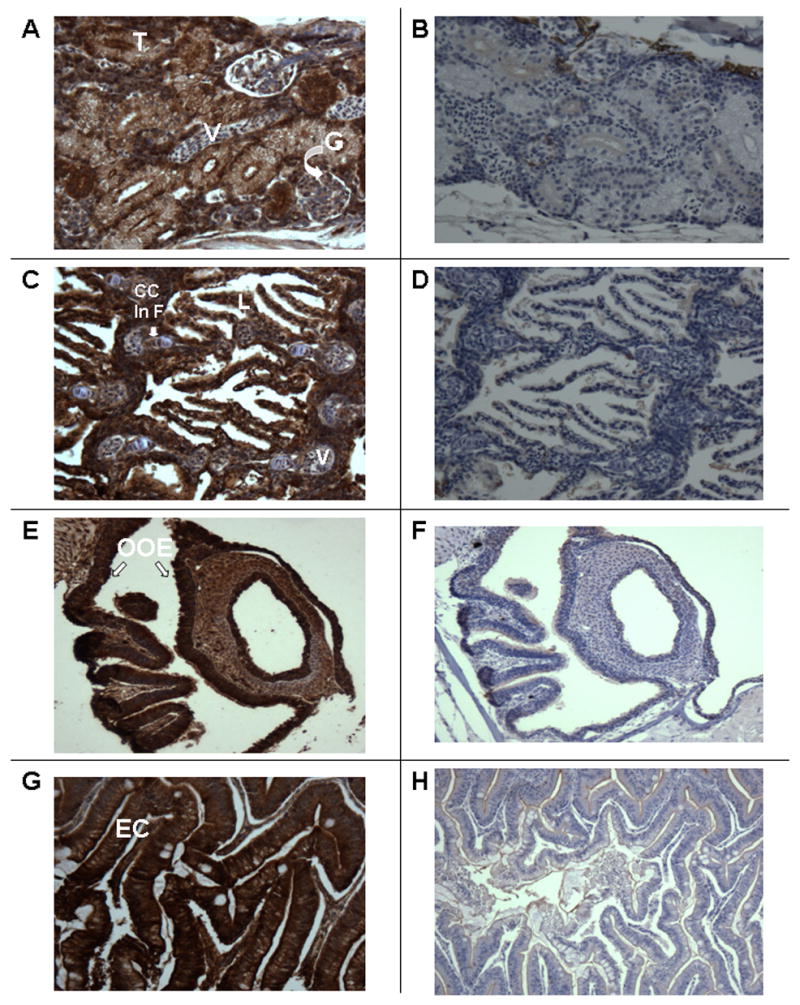
Section of adult male zebrafish. Immunostaining with anti-VDR antibody or pre-immune serum. 200 X original magnification. Panel A. Sagittal section. Immunostaining with anti-VDR antibody of kidney. Note staining of glomerular epithelium (G) and tubular epithelium (T). Note absence of staining of vessels (V). Panel B. Sagittal section. Pre-immune serum. Note absence of staining of tubules glomeruli and blood vessels of the kidney. Panel C. Sagittal section. Immunostaining with anti-VDR antibody of gills. Note staining of epithelial cells in the gill lamellae (L). Also notes staining of chondrocytes in gill filaments (CC in F). Panel D. Sagittal section. Pre-immune serum. Note absence of staining of gill structures. Panel E. Sagittal section. Immunostaining with anti-VDR antibody. Note immunostaining of olfactory organ epithelium (OOE). Panel F. Sagittal section. Pre-immune serum. Note absence of staining of olfactory organ epithelium. Panel G. Sagittal section. Immunostaining with anti-VDR antibody. Note immunostaining of epithelial cells of the intestine (EC). Unstained cells are goblet cells. Panel H. Sagittal section. Pre-immune serum. Note absence of staining of epithelial cells of the intestine.
Besides epithelial cells, the VDR is also detected in acinar cells of the pancreas (AC; Figure 4A). Interestingly, VDR immunostaining is faint or absent in islet cells of the pancreas (I; Figure 4A). In bone, osteoblasts stain intensely for the VDR (Ob; Figure 4B). VDR immunostaining is also present in hepatocytes and cholangiocytes (epithelial cells lining the biliary ductules) of the liver (Hc and Cc; Figure 4C). Pre-immune serum shows no immunostaining for the VDR in the liver (Figure 4D). Similar results were obtained with pre-adsorbed serum. In the heart, no immunostaining for the VDR is noted in myocytes (compare Figure 4E with Figure 4F). Staining in the chambers of the heart is probably due to peroxidase activity exhibited by red cells and not blocked sufficiently by peroxide pretreatment. In Figure 4G, VDR immunostaining in Sertoli cells in the testis is shown. Pre-immune serum shows no VDR immunostaining in testicular cells (Figure 4H).
Figure 4.
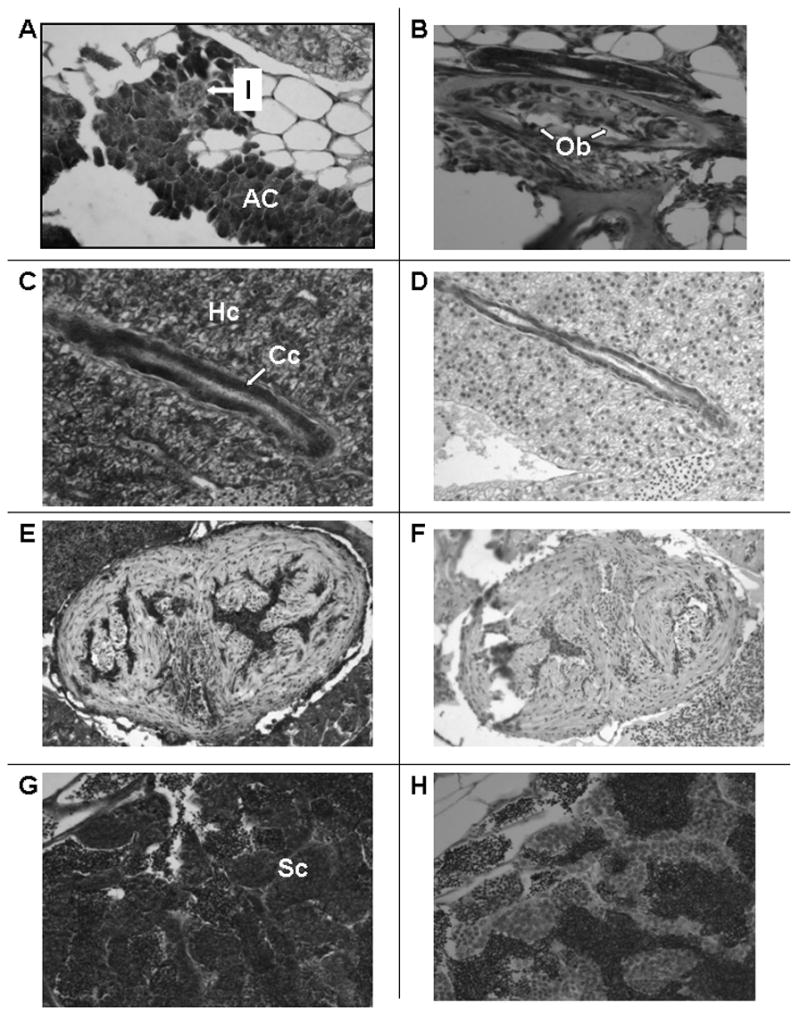
Section of adult male zebrafish. Immunostaining with anti-VDR antibody or pre-immune serum. 200 X original magnification. Panel A. Sagittal section. Immunostaining with anti-VDR antibody of pancreas. Note staining of acinar cells of the pancreas (AC) and absence of staining of islets (I). Panel B. Sagittal section. Immunostaining with anti-VDR antibody of bone. Note staining of osteoblasts lining decalcified bone (Ob). Panel C. Sagittal section. Immunostaining with anti-VDR antibody of liver. Note immunostaining of hepatocytes (Hc) and cholangiocytes (Cc). Panel D. Sagittal section. Pre-immune serum. Note absence of staining of hepatocytes and cholangiocytes. Panel E. Sagittal section. Immunostaining with anti-VDR antibody. Note faint immunostaining of cardiac myocytes. The immuno-peroxidase staining within the ventricular cavity represents staining of erythrocytes whose endogenous peroxidase activity has not been completely suppressed. Panel F. Sagittal section. Pre-immune serum. Note absence of staining of cardiac myocytes. Panel G. Sagittal section. Immunostaining with anti-VDR antibody. Note immunostaining of Sertoli cells (Sc) of the testis. Panel H. Sagittal section. Pre-immune serum. Note absence of staining of Sertoli cells of the testis.
In adult female fish all tissues except reproductive tissues, immunostained for the VDR in a manner identical to that seen in adult male fish. In the female ovary, VDR immunostaining was noted in developing and mature ovarian follicles (Figure 5A). No signal was noted with pre-immune serum (Figure 5B). An example of positive immunostaining in non-reproductive, adult female zebrafish tissue (gills) is seen in Figure 5C. No VDR signal was noted when pre-immune serum was used to immunostain gills (Figure 5D).
Figure 5.
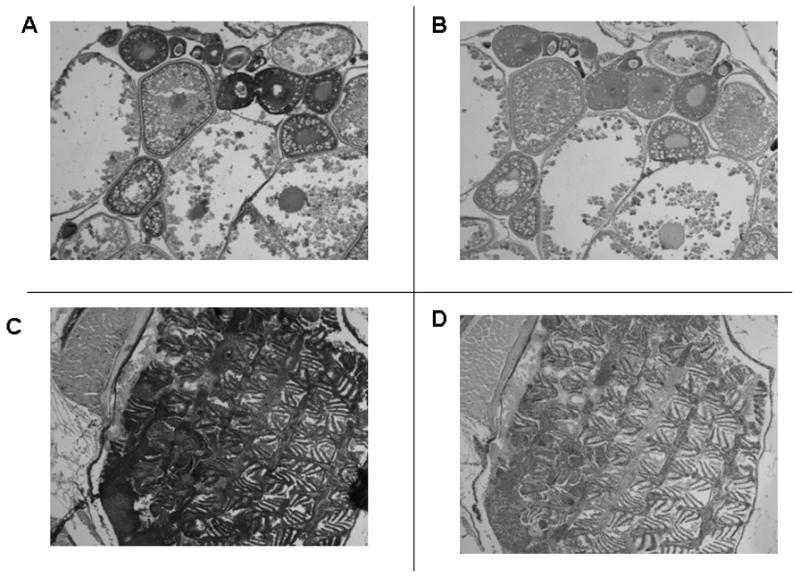
Immunostaining of tissues from adult female zebrafish with anti-VDR antibody or pre-immune serum. 50 X original magnification. Panel A. Developing oocytes immunostained with anti-VDR antibody. Panel B. Immunostained with pre-immune serum. Panel C. The gills immunostained with anti-VDR antibody. Panel D. The gills immunostained with pre-immune serum.
VDR immunostaining is also evident in the ganglion cell layer of the retina (a), the inner plexiform layer (b), the inner nuclear layer (c), the outer plexiform layer (d), the outer nuclear layer (e) and in the photo receptor layer (f) (Figure 6A). No immunostaining is discerned in the retinal pigmented layer. In contrast, no staining is observed with pre-immune serum (Figure 6B). VDR immunostaining is observed in cells of the brain and spinal cord (Figures 6C, 6E, and 6F). No immunostaining is noted with pre-immune serum (Figure 6D).
Figure 6.
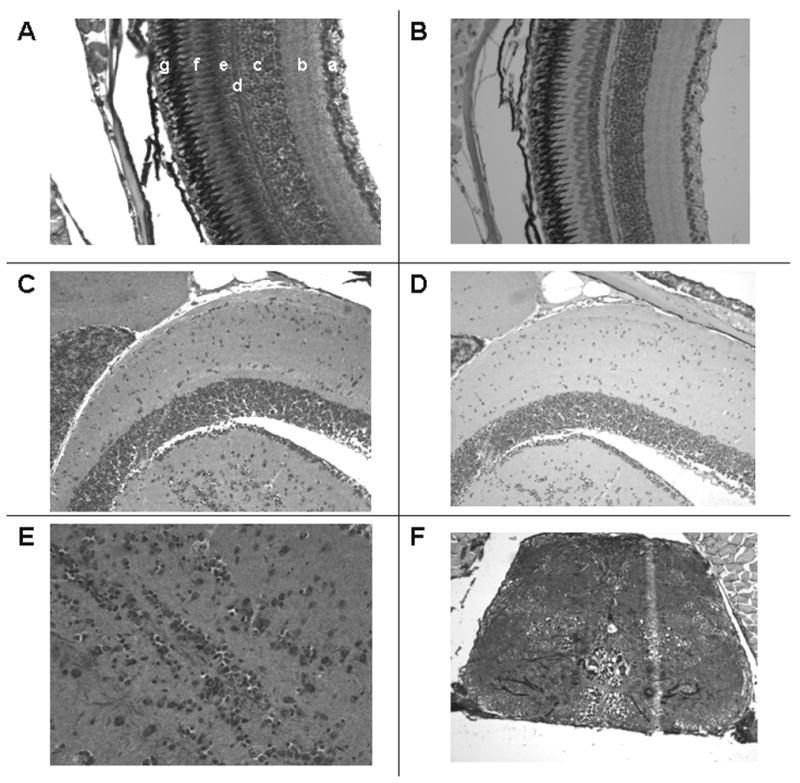
Immunostaining of the eye and neural tissues with anti-VDR antibody or pre-immune serum. 200 X original magnification. Panel A. Section of the retina immunostained with anti-VDR antibody. a = ganglion cell layer; b = inner plexiform layer; c = inner nuclear layer; d = outer plexiform layer; e = outer nuclear layer; f = rods; g = pigmented layer. Panel B. Section of retina using pre-immune serum. Note absence of staining in all layers. Panel C. Section of brain immunostained with anti-VDR antibody. Panel D. Section of brain immunostained with pre-immune serum. Panel E. Section of brain immunostained with anti-VDR antibody (400 X). Panel F. Section of spinal cord staining with anti-VDR antibody.
We used an alternative technique to demonstrate VDR immunostaining in bone, retina, neural tissue and gills. Fluorescent labeling is seen in the spinal cord (SC) and cancellous bone of the vertebral body (boxed area) (Figure 7A). The location and number of the cells labeled in the vertebral body suggests these cells are osteoblasts (Figure 7B). The absence of deep labeling in the cortical bone is likely due to lack of penetration of antibodies deep within the dense cortical bone. VDR fluorescent-immunostaining is also evident in the ganglion cell layer of the retina (a), the inner plexiform layer (b), the inner nuclear layer (c), the outer plexiform layer (d), the outer nuclear layer (e) and in the photo receptor layer (f) (Figure 7C). Immunofluorescence studies show intense labeling of VDR at the dorsal surface of the gills (Fig 7D).
Figure 7.
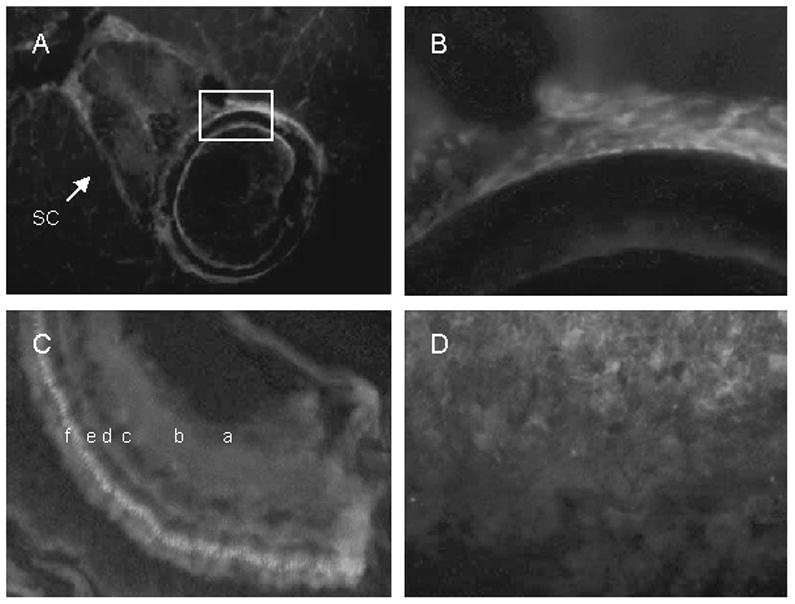
Cryosections of adult zebrafish labeled with VDR antibody visualized with Cy5 (red). Nuclei were labeled with DAPI (blue). A. Vertebra showing labeling in spinal cord (sc), vertebral body (boxed area), and surrounding muscle. B. Higher magnification of the vertebral body (boxed region). C, retina showing ganglion cell layer (a), inner plexiform layer (b), inner nuclear layer (c), outer plexiform layer (d), outer nuclear layer (e), and photo receptor layer (f). D, gills.
We examined the distribution of the VDR in 48 h and 96h post-fertilization zebrafish embryos. In the 48 h post-fertilization embryo, the VDR is seen in the developing brain (diencephalon) and in cells of the neural retina (Figure 8A, coronal section and Figure 8B, sagittal section)). The VDR is also seen the developing mandible (Figure 8A and Figure 8B). No immunostaining is observed in similar cells when pre-immune serum is used (Figure 8C). Figure 9A demonstrates results obtained with immune serum in 96 h post-fertilization embryos. VDR immunostaining is observed in the eye (E), portions of the brain (B), and the otic vesicle (O). Pre-immune serum failed to demonstrate the presence of the VDR (Figure 9B). A higher magnification view of the developing eye is seen in Figure 9C. Cells of the lens (L) do not immunostain. Ganglion cells appear to immunostain (G). Rods (R) do not immunostain. Cells lining the otic vesicle (OE) immunostain for the VDR (Figure 8D).
Figure 8.
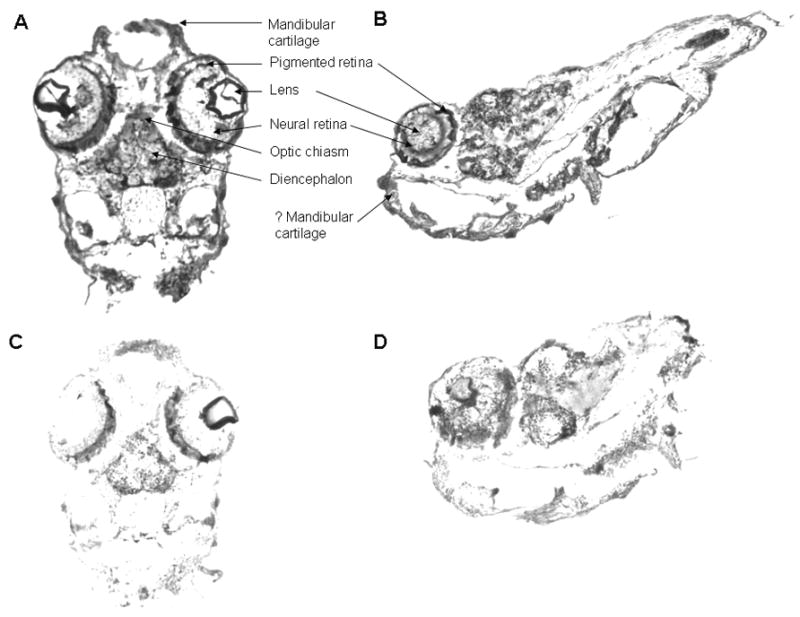
Immunostaining of 48 hour post-fertilization zebrafish embryos with anti-VDR antibody or pre-immune serum. 100 X original magnification. Panel A. Coronal section. 48 hour post-fertilization embryo immunostained with anti-VDR antibody. Note staining (brown color) of cells within the eye (neural retina), the brain (diencephalon) and the developing mandible. 100 X original magnification. Panel B. Sagittal section. 48 hour post-fertilization embryo immunostained with immune serum. Note staining of cells of the neural retina, brain and developing mandible. Panel C. Coronal section. 48 hour post-fertilization embryo immunostained with pre-immune serum. Note absence of staining. 100 X original magnification. Panel D. Sagittal section. 48 hour post-fertilization embryo immunostained with pre-immune serum. Note absence of staining in cells of the neural retina, brain and developing mandible
Figure 9.
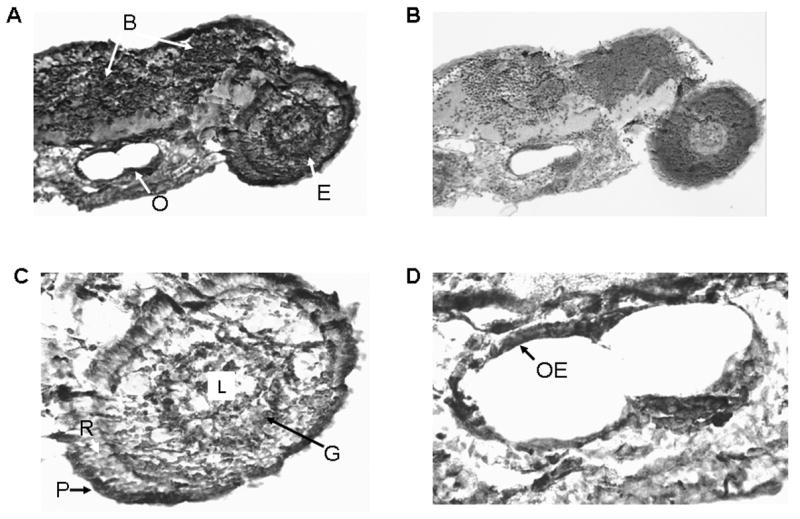
Immunostaining of 96 hour post-fertilization zebrafish embryo with anti-VDR antibody or pre-immune serum. 100 X original magnification. Panel A. 96 hour post-fertilization embryo immunostained with anti-VDR antibody. Note staining (brown color) of cells within the eye (E), the brain (B) and the otic vesicle (O). 100 X original magnification. Panel B. 96 hour post-fertilization embryo immunostained with pre-immune serum. Note absence of staining for the VDR. 100 X original magnification. Panel C. Section of the eye immunostained with anti-VDR antibody; 200 X original magnification. L = lens; G = ganglion cells; R = photoreceptor cells; P = pigmented epithelial cells. Panel D. Otic vesicle immunostained with anti-VDR antibody. 200 X. original magnification.
We investigated whether the VDR in zebrafish is regulated by its ligands, 1α, 25(OH)2D3 and lithocholic acid. We administered 25 ng of either 1α, 25(OH)2D3, lithocholic acid or vehicle intramuscularly into male zebrafish. We observed an up regulation of the VDR in intestinal tissues (Figure 10, p<0.05) following the administration of 1α, 25(OH)2D3 but not with lithocholic acid.
Figure 10.
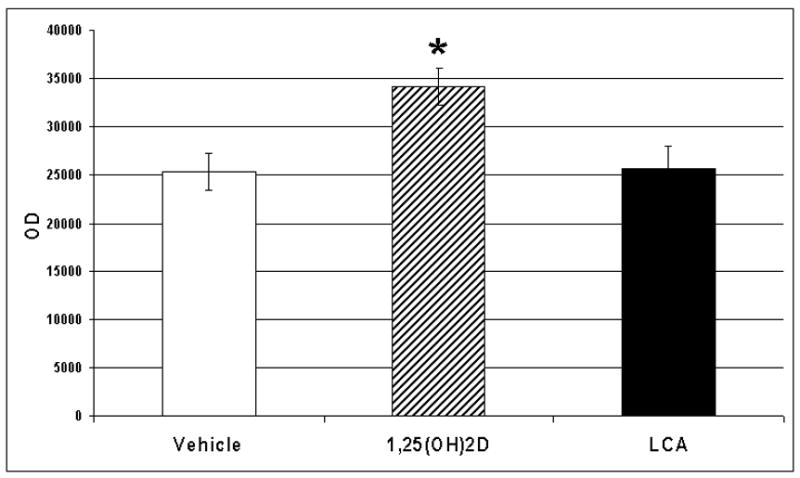
Relative concentrations of VDR in the intestine following treatment of adult male zebrafish with vehicle, 1α, 25(OH)2D3 or lithocholic acid (LCA). * p<0.05. Adult zebrafish were administered 25 ng of 1α, 25(OH)2D3 in 5 μl of propylene glycol, 25 ng of lithocholic acid in 5 μl of propylene glycol, or vehicle (5 μl of propylene glycol) parenterally. Twenty two hours later the fish were killed and tissues harvested as described. Equal amounts of protein (60 μg) were separated by SDS-PAGE using 10% acrylamide gels. Following electrophoresis, proteins were transferred to PVDF membranes and the VDR was detected by Western blotting methods as described.
DISCUSSION
We demonstrate, widespread expression of the VDR in epithelial solute transporting tissues of the zebrafish, Danio rerio Immunostaining for the VDR is found in tissues such as the intestine, liver, and gills where specific 1α, 25(OH)2D3 ligand-binding had been previously detected by Sundell et al. (23). Our findings show that specific cells in these organs immunostain for the VDR. For example, in the intestine, both absorptive cells and goblet cells appear to stain for the VDR. In the gills, absorptive epithelium present on gill lamellae expresses the VDR. Interestingly, chondrocytes present in gill filaments also stain for the VDR. We show that the VDR is present both in tubular epithelial cells, as well as, in epithelial cells of the glomerulus. This is consistent with findings in the rat kidney reported by us earlier (14). Sundell et al. did not detect the presence of the VDR in the kidney (23). The reason for this discrepancy is uncertain but may be due to the relative insensitivity of ligand-bind assays compared to immunostaining methods used by us in this report. It is very likely that the VDR plays an important role in the regulation of calcium and phosphorous transport in epithelial cells of the zebrafish intestine, kidney, and gills. Indeed, Qiu et al. have shown that 1α, 25(OH)2D3 increases the uptake of zinc in the gills, and it has been demonstrated that vitamin D deficiency alters mineral metabolism and bone development in fish, suggesting an important role for the VDR in calcium transport in the intestine (33). In the liver, both hepatocytes and cholangiocytes which have widely differing biochemical functions express the VDR. Cholangiocytes (biliary duct epithelial lining cells) also transport solutes and water and the presence of the VDR in these cells suggests that it may function to control solute transport in these cells in a manner similar to that observed in transporting epithelia of the intestine, kidney and gills. The effects of 1α, 25(OH)2D3 on the transport of minerals and other solutes in transporting epithelia of Danio warrants further investigation.
The VDR is also expressed in endocrine cells such as the acinar cells of the pancreas. Interestingly, in contrast to what is found in mammals and birds (10, 41–43), immunostaining in pancreatic islets of the zebrafish is minimal. These data suggest that the VDR does not play a role in modulating insulin secretion in Danio but plays a role in altering pancreatic secretions through its actions on acinar cells. Alternatively, low levels of VDR may be present in islet cells but are not detected by our antibody. The VDR is also expressed in Sertoli cells in the testis and in developing oocytes in ovarian tissue suggesting role for the VDR and vitamin D-endocrine system in reproductive function in male Danio. Other than the distribution of the VDR in either testis or ovary, VDR distribution is identical in male and female zebrafish.
The bone is a major target of 1α, 25(OH)2D3, independent of the effect of the hormone on concentrations of calcium and phosphorus in the extracellular fluid. Both osteoblast and osteoclast function is affected by the hormone. We demonstrate the presence of VDR in both chondrocytes and osteoblasts. The precise biochemical events modulated by 1α, 25(OH)2D3 in these cells in Danio remains to be determined.
Previous reports have demonstrated the presence of the VDR in cells of the nervous system (44–47). We show that in the zebrafish, VDR expression is observed in cells of the brain, the spinal cord and in epithelial cells of the olfactory organ. Of interest, the VDR is expressed in the adult zebrafish eye in ganglion cells, inner nuclear layer cells and rods in the retina. The exact role of the VDR in the different parts of the nervous system of the fish remains to be elucidated. The zebrafish may be well suited to study the effects of the VDR on nervous system development.
In order to determine whether the VDR is expressed in the developing zebrafish embryo, we sectioned 48 and 96 hour post-fertilization zebrafish embryos and immunostained them with VDR antibodies. We observed VDR immunostaining in the developing brain, in epithelial cells lining the otic vesicle and in the ganglion cells of eye. We did not examine earlier stages of development (<48 h post-fertilization), as immunohistochemistry at earlier time points is technically difficult. Other methods e.g. in situ hybridization may yield information regarding VDR distribution and expression in very early developing zebrafish embryos.
To gain insights into the regulation of the VDR we administered either 1α, 25(OH)2D3 or lithocholic acid parenterally to determine whether these ligands modulate VDR concentrations in various tissues. We observed that 1α, 25(OH)2D3 increased VDR expression in the intestine but not in the gill. Lithocholic acid was without effect.
In conclusion, the VDR is widely expressed in epithelial endocrine and neural tissues of the male and female adult zebrafish, Danio rerio. It is also expressed in developing embryos of the zebrafish as early as 48 h post-fertilization. The VDR concentrations in the intestine are modulated by 1α, 25(OH)2D3. These data suggest an important role of the VDR in fish biology. The zebrafish is a novel and readily manipulated model system in which to further explore the biology of the VDR.
Acknowledgments
Supported by NIH grants DK 65830, DK 76829 and DK 73369 (to RK) and AHA grant D630137N (to CRS).
Footnotes
Publisher's Disclaimer: All authored papers and editorial news and comments, opinions, findings, conclusions or recommendations in JBMR WebFirst papers are those of the author(s) and do not necessarily reflect the views of the JBMR and its publisher, nor does their publication imply any endorsement. No responsibility is assumed, and responsibility is hereby disclaimed, by the American Society for Bone and Mineral Research and the Journal of Bone and Mineral Research for any injury and/or damage to persons or property as a matter of products liability, negligence or otherwise, or from any use or operation of methods, products, instructions or ideas presented in these articles. Independent verification of diagnosis and drug dosages should be made. Discussions, views and recommendations as to medical procedures, choice of drugs and drug dosages are the responsibility of the authors. JBMR WebFirst papers have been peer-reviewed, however the articles have not gone through the copyediting process. Papers will not appear in JBMR style and format until the final print and online version is available. The WebFirst publication date is the official date of publication for each paper. There will be minor changes made to the WebFirst paper in the copyediting process, however no scientific content will be changed. The final paper published in the print Journal and on JBMR Online will not change in scientific content, only in presentation and to adhere to JBMR style.
Conflict of Interest: The authors state that they have no conflicts of interest.
Digital Object Identifier (DOI): 10.1359/JBMR.080403
References
- 1.DeLuca HF, Schnoes HK. Vitamin D: recent advances. Annu Rev Biochem. 1983;52:411–439. doi: 10.1146/annurev.bi.52.070183.002211. [DOI] [PubMed] [Google Scholar]
- 2.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 3.Norman AW. Minireview: vitamin D receptor: new assignments for an already busy receptor. Endocrinology. 2006;147:5542–5548. doi: 10.1210/en.2006-0946. [DOI] [PubMed] [Google Scholar]
- 4.Jurutka PW, Whitfield GK, Hsieh JC, Thompson PD, Haussler CA, Haussler MR. Molecular nature of the vitamin D receptor and its role in regulation of gene expression. Rev Endocr Metab Disord. 2001;2:203–216. doi: 10.1023/a:1010062929140. [DOI] [PubMed] [Google Scholar]
- 5.Johnson JA, Kumar R. Vitamin D and renal calcium transport. Curr Opin Nephrol Hypertens. 1994;3:424–429. doi: 10.1097/00041552-199407000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Hughes MR, Malloy PJ, O’Malley BW, Pike JW, Feldman D. Genetic defects of the 1,25-dihydroxyvitamin D3 receptor. J Recept Res. 1991;11:699–716. doi: 10.3109/10799899109066437. [DOI] [PubMed] [Google Scholar]
- 7.Kato S, Takeyama K, Kitanaka S, Murayama A, Sekine K, Yoshizawa T. In vivo function of VDR in gene expression-VDR knock-out mice. J Steroid Biochem Mol Biol. 1999;69:247–251. doi: 10.1016/s0960-0760(99)00042-4. [DOI] [PubMed] [Google Scholar]
- 8.Kato S. The function of vitamin D receptor in vitamin D action. J Biochem (Tokyo) 2000;127:717–722. doi: 10.1093/oxfordjournals.jbchem.a022662. [DOI] [PubMed] [Google Scholar]
- 9.Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, Demay MB. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci U S A. 1997;94:9831–9835. doi: 10.1073/pnas.94.18.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson JA, Grande JP, Roche PC, Kumar R. Immunohistochemical localization of the 1,25(OH)2D3 receptor and calbindin D28k in human and rat pancreas. Am J Physiol. 1994;267:E356–360. doi: 10.1152/ajpendo.1994.267.3.E356. [DOI] [PubMed] [Google Scholar]
- 11.Johnson JA, Kumar R. Renal and intestinal calcium transport: roles of vitamin D and vitamin D-dependent calcium binding proteins. Semin Nephrol. 1994;14:119–128. [PubMed] [Google Scholar]
- 12.Johnson JA, Grande JP, Roche PC, Campbell RJ, Kumar R. Immunolocalization of the calcitriol receptor, calbindin-D28k and the plasma membrane calcium pump in the human eye. Curr Eye Res. 1995;14:101–108. doi: 10.3109/02713689508999921. [DOI] [PubMed] [Google Scholar]
- 13.Johnson JA, Grande JP, Roche PC, Campbell RJ, Kumar R. Immunolocalization of calcitriol receptor, plasma membrane calcium pump and calbindin-D28k in the cornea and ciliary body of the rat eye. Ophthalmic Res. 1995;27:42–47. doi: 10.1159/000267566. [DOI] [PubMed] [Google Scholar]
- 14.Johnson JA, Grande JP, Roche PC, Sweeney WE, Jr, Avner ED, Kumar R. 1 alpha, 25-dihydroxyvitamin D3 receptor ontogenesis in fetal renal development. Am J Physiol. 1995;269:F419–428. doi: 10.1152/ajprenal.1995.269.3.F419. [DOI] [PubMed] [Google Scholar]
- 15.Johnson JA, Grande JP, Roche PC, Kumar R. Immunohistochemical detection and distribution of the 1,25-dihydroxyvitamin D3 receptor in rat reproductive tissues. Histochem Cell Biol. 1996;105:7–15. doi: 10.1007/BF01450873. [DOI] [PubMed] [Google Scholar]
- 16.Johnson JA, Grande JP, Windebank AJ, Kumar R. 1,25-Dihydroxyvitamin D(3) receptors in developing dorsal root ganglia of fetal rats. Brain Res Dev Brain Res. 1996;92:120–124. doi: 10.1016/0165-3806(95)00204-9. [DOI] [PubMed] [Google Scholar]
- 17.Bills C, Massengale O, Hickman K, Le B, Gray E. A new vitamin D in cod liver oil. J Biol Chem. 1938;126:241–244. [Google Scholar]
- 18.Steenbock H, Kletzien SWF, Halpin JG. The reaction of the chicken to irradiated ergosterol and irradiated yeast as contrasted with the natural vitamin of fish liver oils. Journal of Biological Chemistry. 1932;97:249–264. [Google Scholar]
- 19.Bills C. The resistance of the anti-rachitic substance in cod liver oil to reagents. J Biol Chem. 1925;64:1–9. [Google Scholar]
- 20.Lu Z, Chen TC, Zhang A, Persons KS, Kohn N, Berkowitz R, Martinello S, Holick MF. An evaluation of the vitamin D3 content in fish: Is the vitamin D content adequate to satisfy the dietary requirement for vitamin D? J Steroid Biochem Mol Biol. 2007;103:642–644. doi: 10.1016/j.jsbmb.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holick MF. J Nutr Sci Vitaminol (Tokyo) 1992. Evolutionary biology and pathology of vitamin D. Spec No:79–83. [DOI] [PubMed] [Google Scholar]
- 22.Takeuchi A, Okano T, Sayamoto M, Sawamura S, Kobayashi T, Motosugi M, Yamakawa T. Tissue distribution of 7-dehydrocholesterol, vitamin D3 and 25-hydroxyvitamin D3 in several species of fishes. J Nutr Sci Vitaminol (Tokyo) 1986;32:13–22. doi: 10.3177/jnsv.32.13. [DOI] [PubMed] [Google Scholar]
- 23.Sundell K, Bishop JE, Bjornsson BT, Norman AW. 1,25-Dihydroxyvitamin D3 in the Atlantic cod: plasma levels, a plasma binding component, and organ distribution of a high affinity receptor. Endocrinology. 1992;131:2279–2286. doi: 10.1210/endo.131.5.1330497. [DOI] [PubMed] [Google Scholar]
- 24.Maglich JM, Caravella JA, Lambert MH, Willson TM, Moore JT, Ramamurthy L. The first completed genome sequence from a teleost fish (Fugu rubripes) adds significant diversity to the nuclear receptor superfamily. Nucleic Acids Res. 2003;31:4051–4058. doi: 10.1093/nar/gkg444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki T, Suzuki N, Srivastava AS, Kurokawa T. Identification of cDNAs encoding two subtypes of vitamin D receptor in flounder, Paralichthys olivaceus. Biochem Biophys Res Commun. 2000;270:40–45. doi: 10.1006/bbrc.2000.2378. [DOI] [PubMed] [Google Scholar]
- 26.Reschly EJ, Krasowski MD. Evolution and function of the NR1I nuclear hormone receptor subfamily (VDR, PXR, and CAR) with respect to metabolism of xenobiotics and endogenous compounds. Curr Drug Metab. 2006;7:349–365. doi: 10.2174/138920006776873526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitfield GK, Dang HT, Schluter SF, Bernstein RM, Bunag T, Manzon LA, Hsieh G, Dominguez CE, Youson JH, Haussler MR, et al. Cloning of a functional vitamin D receptor from the lamprey (Petromyzon marinus), an ancient vertebrate lacking a calcified skeleton and teeth. Endocrinology. 2003;144:2704–2716. doi: 10.1210/en.2002-221101. [DOI] [PubMed] [Google Scholar]
- 28.Ciesielski F, Rochel N, Moras D. Adaptability of the Vitamin D nuclear receptor to the synthetic ligand Gemini: remodelling the LBP with one side chain rotation. J Steroid Biochem Mol Biol. 2007;103:235–242. doi: 10.1016/j.jsbmb.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Krasowski MD, Yasuda K, Hagey LR, Schuetz EG. Evolutionary selection across the nuclear hormone receptor superfamily with a focus on the NR1I subfamily (vitamin D, pregnane X, and constitutive androstane receptors) Nucl Recept. 2005;3:2. doi: 10.1186/1478-1336-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ciesielski F, Rochel N, Mitschler A, Kouzmenko A, Moras D. Structural investigation of the ligand binding domain of the zebrafish VDR in complexes with 1alpha,25(OH)2D3 and Gemini: purification, crystallization and preliminary X-ray diffraction analysis. J Steroid Biochem Mol Biol. 2004;89–90:55–59. doi: 10.1016/j.jsbmb.2004.03.109. [DOI] [PubMed] [Google Scholar]
- 31.Qiu A, Glover CN, Hogstrand C. Regulation of branchial zinc uptake by 1alpha,25-(OH)(2)D(3) in rainbow trout and associated changes in expression of ZIP1 and ECaC. Aquat Toxicol. 2007;84:142–152. doi: 10.1016/j.aquatox.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 32.Lock EJ, Ornsrud R, Aksnes L, Spanings FA, Waagbo R, Flik G. The vitamin D receptor and its ligand 1alpha,25-dihydroxyvitamin D3 in Atlantic salmon (Salmo salar) J Endocrinol. 2007;193:459–471. doi: 10.1677/JOE-06-0198. [DOI] [PubMed] [Google Scholar]
- 33.Abbink W, Hang XM, Guerreiro PM, Spanings FA, Ross HA, Canario AV, Flik G. Parathyroid hormone-related protein and calcium regulation in vitamin D-deficient sea bream (Sparus auratus) J Endocrinol. 2007;193:473–480. doi: 10.1677/JOE-06-0164. [DOI] [PubMed] [Google Scholar]
- 34.Fleming A, Sato M, Goldsmith P. High-throughput in vivo screening for bone anabolic compounds with zebrafish. J Biomol Screen. 2005;10:823–831. doi: 10.1177/1087057105279952. [DOI] [PubMed] [Google Scholar]
- 35.Lefebvre KA, Trainer VL, Scholz NL. Morphological abnormalities and sensorimotor deficits in larval fish exposed to dissolved saxitoxin. Aquat Toxicol. 2004;66:159–170. doi: 10.1016/j.aquatox.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Craig TA, Kumar R. Synthesis and purification of soluble ligand binding domain of the human vitamin D3 receptor. Biochem Biophys Res Commun. 1996;218:902–907. doi: 10.1006/bbrc.1996.0160. [DOI] [PubMed] [Google Scholar]
- 37.McArdle CA. Functional interaction between gonadotropin-releasing hormone and PACAP in gonadotropes and alpha T3-1 cells. Ann N Y Acad Sci. 1996;805:112–120. doi: 10.1111/j.1749-6632.1996.tb17477.x. discussion 120-111. [DOI] [PubMed] [Google Scholar]
- 38.Veenstra TD, Benson LM, Craig TA, Tomlinson AJ, Kumar R, Naylor S. Metal mediated sterol receptor-DNA complex association and dissociation determined by electrospray ionization mass spectrometry. Nat Biotechnol. 1998;16:262–266. doi: 10.1038/nbt0398-262. [DOI] [PubMed] [Google Scholar]
- 39.Veenstra TD, Johnson KL, Tomlinson AJ, Craig TA, Kumar R, Naylor S. Zinc-induced conformational changes in the DNA-binding domain of the vitamin D receptor determined by electrospray ionization mass spectrometry. J Am Soc Mass Spectrom. 1998;9:8–14. doi: 10.1016/S1044-0305(97)00229-8. [DOI] [PubMed] [Google Scholar]
- 40.Craig TA, Lutz WH, Kumar R. Association of prokaryotic and eukaryotic chaperone proteins with the human 1alpha,25-dihydroxyvitamin D(3) receptor. Biochem Biophys Res Commun. 1999;260:446–452. doi: 10.1006/bbrc.1999.0931. [DOI] [PubMed] [Google Scholar]
- 41.Norman AW, Frankel JB, Heldt AM, Grodsky GM. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science. 1980;209:823–825. doi: 10.1126/science.6250216. [DOI] [PubMed] [Google Scholar]
- 42.Kadowaki S, Norman AW. Dietary vitamin D is essential for normal insulin secretion from the perfused rat pancreas. J Clin Invest. 1984;73:759–766. doi: 10.1172/JCI111269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kadowaki S, Norman AW. Pancreatic vitamin D-dependent calcium binding protein: biochemical properties and response to vitamin D. Arch Biochem Biophys. 1984;233:228–236. doi: 10.1016/0003-9861(84)90621-0. [DOI] [PubMed] [Google Scholar]
- 44.Veenstra TD, Fahnestock M, Kumar R. An AP-1 site in the nerve growth factor promoter is essential for 1, 25-dihydroxyvitamin D3-mediated nerve growth factor expression in osteoblasts. Biochemistry. 1998;37:5988–5994. doi: 10.1021/bi972965+. [DOI] [PubMed] [Google Scholar]
- 45.Veenstra TD, Londowski JM, Windebank AJ, Brimijoin S, Kumar R. Effects of 1,25-dihydroxyvitamin D3 on growth of mouse neuroblastoma cells. Brain Res Dev Brain Res. 1997;99:53–60. doi: 10.1016/s0165-3806(96)00196-4. [DOI] [PubMed] [Google Scholar]
- 46.Veenstra TD, Prufer K, Koenigsberger C, Brimijoin SW, Grande JP, Kumar R. 1,25-Dihydroxyvitamin D3 receptors in the central nervous system of the rat embryo. Brain Res. 1998;804:193–205. doi: 10.1016/s0006-8993(98)00565-4. [DOI] [PubMed] [Google Scholar]
- 47.Prufer K, Veenstra TD, Jirikowski GF, Kumar R. Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the rat brain and spinal cord. J Chem Neuroanat. 1999;16:135–145. doi: 10.1016/s0891-0618(99)00002-2. [DOI] [PubMed] [Google Scholar]


