Abstract
Objectives
While age-related brain changes are becoming better understood, midlife patterns of change are still in need of characterization, and longitudinal studies are lacking. The aim of this study was to determine if baseline fractional anisotropy (FA), obtained from diffusion tensor imaging (DTI) predicts volume change over a four-year interval.
Experimental design
Forty-four cognitively healthy middle-age adults underwent baseline DTI and longitudinal T1-weighted magnetic resonance imaging. Tensor Based Morphometry methods were used to evaluate volume change over time. FA values were extracted from regions of interest that included the cingulum, entorhinal white matter, and the genu and splenium of the corpus callosum. Baseline FA was used as a predictor variable, while gray and white matter atrophy rates as indexed by Tensor Based Morphometry were the dependent variables.
Principal observations
Over a four-year period, participants showed significant contraction of white matter, especially in frontal, temporal, and cerebellar regions (p<0.05, corrected for multiple comparisons). Baseline FA in entorhinal white matter, genu, and splenium, was associated with longitudinal rates of atrophy in regions that included the superior longitudinal fasciculus, anterior corona radiata, temporal stem, and white matter of the inferior temporal gyrus (p<0.001, uncorrected for multiple comparisons).
Conclusions
Brain change with aging is characterized by extensive shrinkage of white matter. Baseline white matter microstructure as indexed by DTI was associated with some of the observed regional volume loss. The findings suggest that both white matter volume loss and microstructural alterations should be considered more prominently in models of aging and neurodegenerative diseases.
Keywords: aging, fractional anisotropy, microstructure, atrophy, longitudinal, diffusion tensor imaging, tensor-based morphometry
Introduction
Normal aging is accompanied by a progressive cognitive decline and neural degeneration; however, the mechanisms for these changes are not fully understood. Histological studies have established that there is a decrease in the number and dendritic extent of cortical neurons (Coleman and Flood, 1987) and shrinkage of neurons (Terry et al., 1987), with consequent cerebral atrophy. While gray matter loss is evident in aging, several studies suggest that the age-related structural deterioration of white matter (Tang et al., 1997) is central in the brain aging process (O’Sullivan et al., 2001; Pfefferbaum et al., 2005) and may be involved in disruption of neural networks underlying normal cognitive function (Grady, 2008; Greenwood, 2007). Moreover, human white matter development is thought to be heterochronic and regionally heterogeneous; specifically, axons from the prefrontal and other association areas continue to myelinate temporally longer than, for example, sensory or motor areas (Bartzokis et al., 2004; Benes, 2004). Fundamental questions still remain, however, about the temporal relationship between white and gray matter changes in normal aging. Recently, it has been proposed that white matter alterations may precede gray matter changes (Bartzokis, 2004; Bartzokis et al., 2004).
Longitudinal in vivo brain imaging of white and gray matter may help define the temporal relationship of brain tissue change. Diffusion tensor imaging (DTI) in particular has allowed investigators to examine white matter in a way that was previously not possible (Basser, 1995; Basser and Pierpaoli, 1996b). Derived from DTI, fractional anisotropy (FA) (Giorgio et al., 2010) is a quantitative index of the directionality of water diffusion reflecting the integrity of the brain tissue (Basser and Pierpaoli, 1996a). Alterations in the microstructure environment, such as demyelination of axons and loss of axonal structure, reduce directional water diffusion and thus reduce FA (Englund, 1998). Several DTI studies of healthy aging have shown widespread age-related reductions in FA and elevations in diffusivity in white matter (Ardekani et al., 2007; Benedetti et al., 2006; Charlton et al., 2008; Grieve et al., 2007); however, across studies there is regional variability, suggesting that the effect of aging on white matter is still in need of clarification. Decreases in FA have been consistently reported in large cerebral white matter regions such as the centrum semiovale, corona radiata, frontal and parietal pericallosal areas, and periventricular regions, whereas less consistent findings have been detected in the splenium of the corpus callosum, parietal white matter, and limbs of the internal capsule (Hugenschmidt et al., 2008; Madden et al., 2004; Pfefferbaum et al., 2005; Salat et al., 2005a).
Although it is well established that white matter integrity as indexed by FA, decreases with age, the relationship between these microstructural changes and volume loss remains to be fully elucidated. In a review article evaluating voxel-based morphometry (VBM) and DTI studies of prefrontal white matter (Salat et al., 2005b), a positive correlation between FA and volume was observed only in participants over age 40. Whereas another study (Fjell et al., 2008) found moderately correlated regional white matter volume and FA in a similar cohort, the Salat et al. result is consistent with previous research (Bartzokis et al., 2001; Courchesne et al., 2000; Raz et al., 2004), showing that anterior white matter myelination peaks during middle age and subsequently declines. The authors, Salat et al., suggest that FA may be a microstructural marker of volumetric measures and thus, reduced FA may reflect decreased white matter volume. In contrast, a previous study (Benedetti et al., 2006) used whole-brain histograms and found no correlation between mean diffusivity and volume, as measured through magnetization transfer MRI. However, this inconclusive result may be due to averaging whole-brain FA and volume, a method that may overlook regional variability. Using DTI and VBM, Hugenschmidt and colleagues (Hugenschmidt et al., 2008) showed that regions exhibiting decreased FA in middle age were the same areas that exhibit white matter volume loss in older age, suggesting that microstructural FA changes may precede and predict white matter atrophy, although proving temporal ordering is difficult. .
Testing the extent to which microstructural alterations precede volume loss requires a within subject longitudinal approach and was a primary focus of this study. Using imaging data acquired in a sample of healthy middle-aged adults, the aim of this study was to understand if microstructural alterations, as indexed by FA, were related to gray and white matter volume change over time. Baseline white matter health was assessed in regions of interest (ROIs) within multiple white matter tracts where age-related declines in tissue integrity have been found previously. The selected white matter regions included the cingulum adjacent to hippocampus, entorhinal white matter, and the cingulum subjacent to the posterior cingulate, all association fibers observed to be susceptible to age-related deterioration in comparison to projection fibers (Stadlbauer et al., 2008). The ROIs also included the genu and splenium of the corpus callosum, both of which have been reported to have age-related FA decreases (Bhagat and Beaulieu, 2004; Head et al., 2004; Ota et al., 2006; Pfefferbaum et al., 2005; Pfefferbaum et al., 2000; Sullivan et al., 2006). FA was chosen based on its reliable relationship with age related white matter alterations (Pfefferbaum et al., 2000; Salat et al., 2005a; Westlye et al., 2010). The relationship between FA and white matter alteration has been observed to be stronger in adults over age 40 (Salat et al., 2005a), similar in age range to the present sample. Thus, we expected that the FA signal from the pre-selected white matter tracts would predict volume loss in related white matter regions and gray matter structures, including frontal, parietal, basal temporal, and parahippocampal regions.
Methods
Participants
Forty-four cognitively healthy participants underwent two magnetic resonance imaging (MRI) sessions (baseline and follow-up) as part of previous functional MRI studies of memory and aging. All participants were from the Wisconsin Registry for Alzheimer’s Prevention (WRAP) (Sager et al., 2005), which is a registry of healthy middle-aged adults who have at least one parent with late onset Alzheimer’s disease (AD) or no parental family history of AD. The sample included participants with parental family history and genetic risk for AD, specifically, positive Apolipoprotein E ε4 (APOE4) status. All participants underwent a baseline MRI and a follow-up MRI approximately 4 years later. In addition to MRI, participants received a neuropsychological assessment. Demographics and cognitive performance scores are shown in Table 1.
Table 1.
Demographic features and cognitive performance.
| Total N | 44 |
| Female: N (%) | 27 (61%) |
| Parental family history AD: N (%) | 25 (56%) |
| APOE4 positive: N (%) | 20 (45%) |
| Baseline Age: years, SD (range) | 56.3 ± 6.9 (42–75) |
| Baseline Education: years, SD (range) | 15.9 ± 2.4 (12–20) |
| Time from baseline scan to follow-up: years, SD (range) | 3.48 ± 0.88 (2.17–4.92) |
| MMSE: Mean, SD | 29.6 ± .66 |
| WRAT-III reading: Mean, SD | 52.2 ± 3.5 |
| BVMT-R total: Mean, SD | 24.9 ± 7.1 |
| BVMT-R delayed recall: Mean, SD | 9.7 ± 2.0 |
| RAVLT delayed recall: Mean, SD | 10.7 ± 2.9 |
| Digit Span: Mean, SD | 18.0 ± 3.4 |
| TMT A: Mean, SD | 28.4 ± 8.5 |
| TMT B: Mean, SD | 58.7 ± 20.9 |
| BNT: Mean, SD | 56.0 ± 7.8 |
All neuropsychological scores reported above are raw scores. AD: Alzheimer’s disease; APOE4: Apolipoprotein E, ε4; BNT: Boston Naming Test (Kaplan, et al. 2001); BVMT-R: Brief Visuospatial Memory Test-Revised (Benedict 1997); Digit Span (from WAIS-III) (Wechsler 1987); MMSE: Mini Mental State Examination (Folstein, et al. 1975); RAVLT: Rey Auditory Verbal Learning Test (Rey 1964); TMTA/B: Trail Making Test A and B (Reitan RM 1993); WRAT-III: Wide Range Achievement Test-III reading subtest (Jastak 1993).
Inclusion criteria for all subjects consisted of the following: normal cognitive function determined by neuropsychological evaluation, no current diagnosis of major psychiatric disease or other major medical conditions (e.g., diabetes, myocardial infarction, or recent history of cancer), no history of head trauma, and no contraindications for a MRI scan. Study procedures were approved by the University of Wisconsin Health Sciences Institutional Review Board and were in accordance with U.S. federal regulations. All participants provided written informed consent.
MRI acquisition
Participants were imaged on a General Electric 3.0 Tesla SIGNA (Waukesha, WI) MRI system with a quadrature birdcage head coil at baseline and after four years. At baseline, cardiac-gated diffusion-weighted echo planar magnetic resonance images were acquired using twelve optimum non-collinear encoding directions (obtained by minimum energy numerical optimization) with a diffusion weighting of 1114 s/mm2 and a non-DWT2-weighted reference image. The effective TR was 10–13 heartbeats (~10–15 s) dependent upon the subject’s heart rate. Other imaging parameters were TE=78.2 ms, 3 averages (NEX: magnitude averaging), and an image acquisition matrix of 120×120 over a field of view of 240×240mm2. Three averages were acquired and the cerebrum was covered using 39 contiguous 3-mm thick axial slices. The acquired voxel size of 2 × 2 × 3 mm was interpolated to 0.9375 mm isotropic dimensions (256×256 in plane image matrix). The total acquisition time was between 6.5 and 8 min dependent upon the heart rate. High order shimming was performed prior to the DTI acquisition to optimize the homogeneity of the magnetic field across the brain and to minimize EPI distortions.
3D T1-weighted volumes were obtained at baseline and follow-up using an inversion recovery prepared fast gradient echo pulse sequence. The whole brain was imaged in the axial plane with the following parameters: TI = 600 ms; TR = 9 ms; TE = 1.8 ms; NEX = 1; flip angle = 20°; acquisition matrix = 256 × 192 × 124, interpolated to 256 × 256 × 124; FOV = 240 mm; slice thickness = 1.2 mm (124 slices), receiver bandwidth = ± 16 kHz; acquisition time ~ 7.5 min.
Diffusion Tensor Imaging (DTI) and Tract-Based Spatial Statistics (TBSS) preprocessing
Diffusion-weighted DICOM images acquired at baseline were converted into NIFTI format using AFNI (http://afni.nimh.nih.gov/). FA maps were generated via the FMRIB Software Library (FSL) (http://www.fmrib.ox.ac.uk/fsl/fdt/index.html (Behrens et al., 2003) using the following procedures: (1) image distortions in the DTI data caused by eddy currents were corrected; (2) estimation of diffusion tensors was achieved using DTIFIT; (3) three-dimensional maps of FA images were computed from the tensors from step (2). The FA maps were then aligned using registration methods based on the Tract-Based Spatial Statistics (TBSS: http://www.fmrib.ox.ac.uk/fsl/tbss/index.html) processing scheme. TBSS methods were employed because the method is known to provide accurate registration of FA maps, the method allowed us to confidently position ROIs for extraction of FA values, and this method of registration reduces the inclusion of CSF voxels in the final extracted FA estimates. TBSS performs alignment of all FA data by projecting the original FA maps onto a mean FA skeleton. The main steps of the procedure we employed were as follows: a) FA images were eroded slightly and the end slices were zeroed to remove outliers from the diffusion tensor fitting; b) A non linear registration was estimated to align the FA images to a 1×1×1mm standard space. The target image was affine transformed to Montreal Neurological Institute (MNI) space and each subject’s FA image had its nonlinear transform to the target and an affine transform to MNI space applied, resulting in a transformation of the original FA image into MNI space. c) The mean of all FA images was created and the image was skeletonized. d) The mean FA skeleton was then thresholded to produce a binary skeleton mask that defined the set of voxels used in all subsequent processing. e) A “distance map” was then created from the skeleton mask. This was used in the projection of the subjects’ FA maps onto the skeleton. f) All of the subjects’ aligned FA data were projected onto the mean FA skeleton using warping methods that are based on free-form deformations and B-Splines (Rueckert et al., 1999). The process is achieved by filling the skeleton with FA values from the nearest relevant tract center. This was performed for each skeleton voxel, by searching perpendicular to the local skeleton structure for the maximum value in the subject’s FA image. g) After projection onto the mean FA skeleton, the skeletonized data in standard space were used for the ROI analyses.
Regions of Interest
Each ROI was drawn on a common space skeleton mask in FSLview (http://www.fmrib.ox.ac.uk/fsl/fslview/index.html) and was then applied to the normalized individual maps. ROIs drawn on the template were individually checked to ensure correct placement on the single-subject normalized FA maps. Individual FA values for each ROI were extracted by acquiring the mean value across the tract labels of interest. ROIs were drawn bilaterally and included the cingulum subjacent to the posterior cingulate, cingulum adjacent to hippocampus, entorhinal white matter, and the genu and splenium of the corpus callosum (Figure 1). Corticospinal tract was included as a control ROI based on literature suggesting these tracts, along with primary sensory and motor cortices are preserved during aging relative to association cortices.
Figure 1.
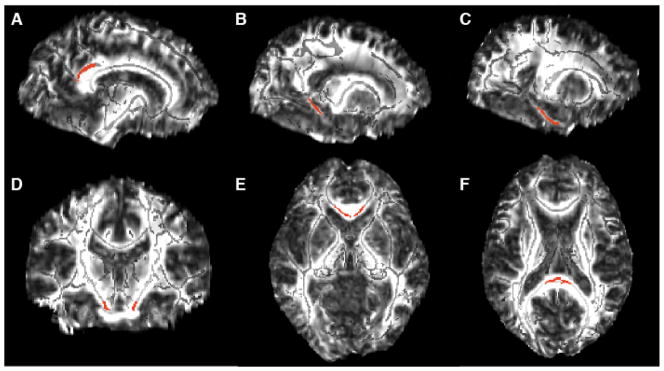
White matter ROIs (shown in red) overlayed on the FA template image (skeletonized FA underlayed in light gray). The bilateral ROIs included: A. cingulum bundle subjacent to posterior cingulate (119 voxels, MNI coordinates ±11 −45 28), B. cingulum adjacent to hippocampus (60 voxels, MNI coordinates ± 20 −42 −2), C. entorhinal white matter (96 voxels, MNI coordinates ±24 −26 −19), D. corticospinal tract (31 voxels, MNI coordinates ±10 −20 −24), E. splenium (49 voxels, MNI coordinates ±1 −35 14) and F. genu (48 voxels, MNI coordinates ±4 23 −1) of the corpus callosum. A–C are shown in sagittal view, D is shown in coronal view, and E–F are shown in an axial view.
Tensor-Based Morphometry
In order to produce estimates of volume change from baseline to follow-up, we employed Tensor-Based Morphometry (TBM) methods implemented in the SPM5 software package (http://www.fil.ion.ucl.ac.uk/spm/software/spm5/). First, bias correction with eight iterations, a FWHM of Gaussian smoothing set at a 60mm cutoff, and a medium level of regularization was applied to both the baseline and follow-up scans to correct for intensity non-uniformity. TBM procedures followed those described by Kipps and colleagues (Kipps et al., 2005). Briefly, a high-dimensional deformation field was used to warp the corrected late image to match the baseline scan within subject (Ashburner and Friston, 2000). The amount of volume change was indexed by the determinant of the gradient of deformation at a single-voxel level (Jacobian determinants). The Jacobian image represented a measure of the brain specific volume change between the first and the second scan. The maps were converted to annual rate of change maps using the formula: Annual Rate = ((Jacobian determinant)^(1/Interscan duration)−1), where “Interscan duration” was the number of years between baseline and follow-up scans. In order to warp the final TBM maps to template space, normalization parameters were estimated by matching the brain-volume images from the baseline scan with the MNI brain-volume template, these were then applied to the Jacobian image (Ashburner and Friston, 1999). Finally, the normalized TBM maps were smoothed using an 8 mm isotropic Gaussian kernel.
Statistical analyses
In order to test the extent to which participants showed tissue change over a four year period, tissue maps were thresholded to either 1) values above zero to reflect tissue atrophy, or 2) values below zero to reflect contraction. One-way t-tests were used to determine significant regions of change. In order to test the extent to which FA obtained at baseline predicted volume change, individual FA estimates from each ROI were entered into a multiple regression analysis, where the independent predictor variable was baseline FA, and the dependent variable (volume change) was the participant’s TBM map thresholded above zero (reflecting tissue atrophy). For all models, the covariates were baseline age and gender. Results were considered significant at p < 0.001 (uncorrected).
Results
Participants with parental family history of AD or positive APOE4 status did not differ with respect to demographic characteristics or neuropsychological test performance compared to those with no AD risk factors.
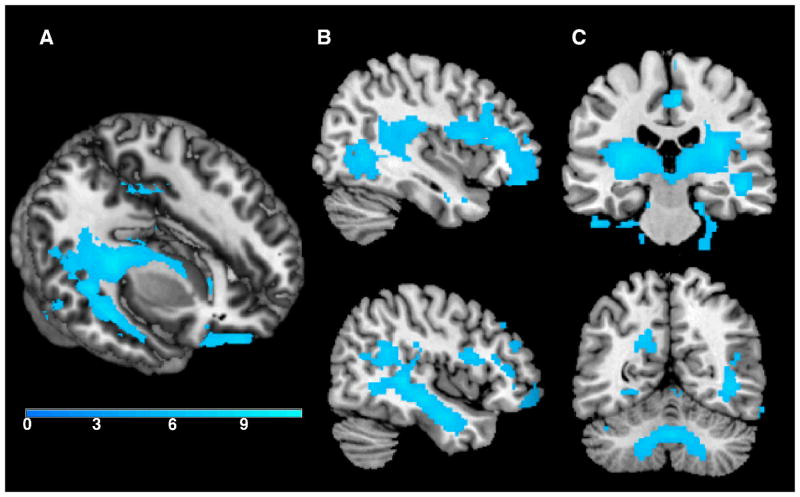
There was a significant change in volume over four years. As detailed in Table 2 and shown in Figure 2, significant volume contraction was observed predominantly in frontal, temporal, and cerebellar regions. These results survived family wise error correction (FWE) p <0.05. In contrast, a voxel-wise analysis revealed no areas of significant expansion (p <0.001, uncorrected).
Table 2.
Regions of tissue contraction over four years (p <0.05, FWE corrected).
| Location | Cluster size | MNI coordinates of peak voxel | Peak-level T-statistic | ||
|---|---|---|---|---|---|
| x | y | z | |||
| R Medial Orbital Gyrus | 12841 | 16 | 32 | −28 | 11.10 |
| R Cingulate Gyrus | 1263 | 6 | −22 | 48 | 9.22 |
| R Superior Frontal Gyrus WM | 498 | 8 | −4 | 66 | 8.56 |
| L Posterior Orbital Gyrus WM | 502 | −32 | 38 | −20 | 8.37 |
| L Posterior Corona Radiata | 492 | −18 | −48 | 30 | 8.20 |
| R Cerebellar Hemisphere | 318 | 20 | −26 | −22 | 8.06 |
| L Superior Frontal Gyrus WM | 346 | −10 | 4 | 62 | 7.84 |
| CSF space ventral to L Inferior Temporal Gyrus | 299 | −50 | −30 | −32 | 7.81 |
| R Cuneus | 99 | 4 | −78 | 40 | 7.11 |
| R Precentral Gyrus | 69 | 6 | −30 | 72 | 7.01 |
| CSF space ventral to R Fusiform Gyrus | 35 | 18 | 4 | −46 | 6.77 |
| L Fusiform Gyrus WM | 55 | −32 | −42 | −16 | 6.77 |
| L Lyngual Gyrus WM | 179 | −16 | −68 | −8 | 6.58 |
| L Cerebellar Hemisphere | 94 | −26 | −72 | −26 | 6.45 |
| R Precentral Gyrus WM | 32 | 14 | −18 | 64 | 6.38 |
| R Middle Temporal Gyrus | 67 | 62 | −48 | −4 | 6.19 |
| R Fusiform Gyrus | 21 | 60 | −60 | −20 | 6.17 |
| R Supramarginal Gyrus | 76 | 62 | −40 | 38 | 6.16 |
| CSF space anterior to L Cerebellar Hemisphere | 58 | −20 | −30 | −46 | 6.16 |
| R Middle Frontal Gyrus | 71 | 46 | 38 | 24 | 6.07 |
| R Middle Frontal Gyrus | 34 | 28 | 62 | 20 | 5.93 |
L = Left, R = Right, WM= white matter. Cluster size is expressed in number of voxels.
Figure 2.
Regions of tissue contraction over four years (p <0.05, FWE corrected). As shown in the 3D render (A) and sagittal cross-section (B) there was significant contraction in temporal stem white matter over four years. Additionally, as shown in the coronal sections (C) there was significant contraction in large portions of bilateral sub-cortical white matter and the cerebellum. The color bar represents T values.
With regard to FA, there was no significant effect of AD risk factors. The mean FA values extracted from bilateral ROIs were as follows: .70 (± .06) from the corticospinal tract, 0.71 (± .03) from the genu, 0.54 (± .06) from the entorhinal white matter, 0.53 (± .03) from the cingulum subjacent to the posterior cingulate bundle, 0.51 (± .04) from the cingulum adjacent to hippocampus, and 0.87 (± 0.03) from the splenium. No significant differences were found between the left and right hemisphere per each of the investigated ROIs and thus left and right were averaged for the remaining analyses.
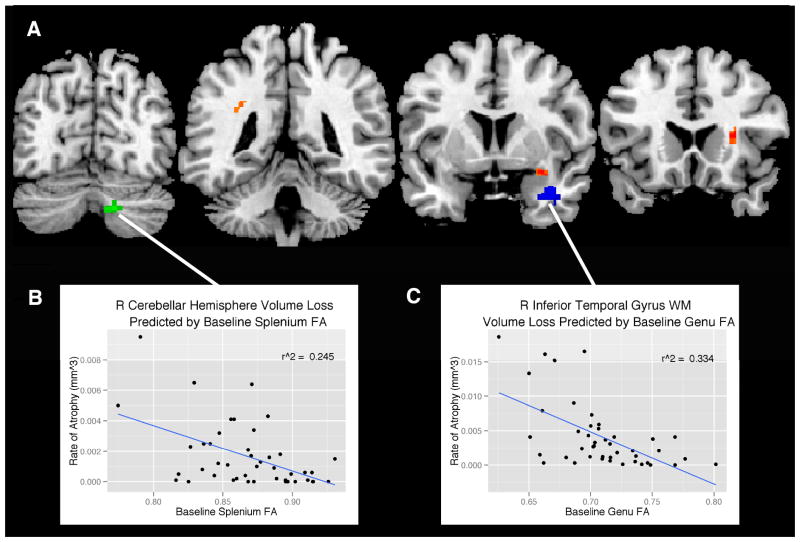
As detailed in Table 3, baseline FA in entorhinal white matter, and genu and splenium of the corpus callosum predicted atrophy (p <0.001, uncorrected) as indexed by the TBM maps (Figure 3). With the exception of the right cerebellar hemisphere volume loss predicted by splenium FA, the majority of tissue contraction was primarily in white matter regions, specifically within the superior longitudinal fasciculus, anterior corona radiata, and the temporal stem, all regions predicted by baseline entorhinal FA. In addition, atrophy in the white matter of the inferior temporal gyrus was predicted by baseline genu FA. Baseline FA in the corticospinal tract, cingulum adjacent to hippocampus, and cingulum subjacent to the posterior cingulate did not predict any regions of volume loss.
Table 3.
Regions of tissue contraction (p <0.001, uncorrected) predicted by baseline FA.
| Baseline ROI where FA was extracted | Cluster size | Location | MNI coordinates of peak voxel | Peak-level T-statistic | r2 | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Entorhinal | 36 | R Anterior Corona Radiata | 28 | 18 | 12 | 4.0 | 0.25 |
| Entorhinal | 22 | R Temporal Stem | 32 | 4 | −14 | 3.9 | 0.21 |
| Entorhinal | 27 | L Superior Longitudinal Fasciculus | −30 | −46 | 30 | 3.66 | 0.21 |
| Genu | 91 | R Inferior Temporal Gyrus WM | 36 | 4 | −28 | 4.59 | 0.33 |
| Splenium | 42 | R Cerebellar Hemisphere | 16 | −72 | −36 | 4.6 | 0.25 |
L = Left, R = Right, WM= white matter. Cluster size is expressed in number of voxels.
Figure 3.
Regions (A) where baseline FA from the splenium (green), entorhinal white matter (orange), and genu (blue) predict volume loss from baseline to follow up (p <0.001, uncorrected). The statistical map is overlaid on coronal sections of the “CH2” template available in MRIcron (Rorden, 2007). The correlation between (B) baseline splenium FA and cerebellar hemisphere volume loss was r2 = 0.25, p < 0.001 and (C) baseline genu FA and inferior temporal gyrus WM volume loss was r2 = 0.33, p < 0.001. Data points represent individual participants.
Discussion
The results of this study indicate that over a four year period, middle-aged adults show significant shrinkage of white matter. Further, midlife measures of FA—a putative marker of white matter integrity—predict longitudinal rates of white matter atrophy. While T1-weighted imaging in this study and other studies appears to be sensitive to gross volume loss, techniques such as DTI are sensitive to microstructural alterations, and the results suggest that they may in fact be predictive of subsequent volume loss.
One of the largest areas of atrophy was observed in the white matter of the inferior temporal gyrus, as predicted by genu FA. Additionally, entorhinal FA was associated with atrophy in temporal stem. This is in accord with prior findings, with both volume (Raz et al., 2004) and anisotropy (Head et al., 2004; Salat et al., 2005a) in the temporal lobes showing moderate decline with age. This decline is second to the frontal cortices and is followed by smaller decreases in the parietal and occipital lobes, suggesting an anterior to posterior gradient. A volumetric cross-sectional study in men showed a quadratic relationship between age and white matter volume in the temporal lobes, with white matter volume increasing to the age of 47 years and declining subsequently (Bartzokis et al., 2001). The mean age of the current cohort was 56 years at baseline, suggesting that the majority of the cohort had crossed over the peak of myelination in this brain region, and was on a downward trajectory over the subsequent four years. The non-linear nature of white matter development over the life-span contrasts with the linear decline of gray matter volume throughout most of adulthood, and this is important to take into consideration in studies of midlife white matter change.
Another significant region of atrophy, predicted by splenium FA, was located in the right cerebellar hemisphere, which is consistent with several findings of age-related decreases in total cerebellar volume, cerebellar white matter, and other cerebellar structures (Jernigan et al., 2001; Liu et al., 2003; Luft et al., 1999; Raz et al., 2001; Sullivan et al., 2000; Walhovd et al., 2005). Moreover, longitudinally, the cerebellum shows pronounced longitudinal shrinkage with advancing age (Raz et al., 2010; Raz et al., 2005), possibly beginning to decline during the fifth decade of life, reflecting an exponential fit (Luft, 1999).
We observed a region of volume loss in the superior longitudinal fasciculus (SLF), predicted by baseline entorhinal FA. This tract is a heavily myelinated white matter bundle that connects the anterior and posterior regions of the cerebrum, sending projections to the temporal lobes (Wakana et al., 2004). Findings of longitudinal changes in the SLF converge with a longitudinal DTI study showing reduced FA in the SLF in the healthy elderly subjects (Teipel et al.), as well as in older individuals with mild cognitive impairment (Cho et al., 2008). Age-related decreases in FA of the SLF has been shown to be associated with poorer performance in a number of cognitive tasks involved in set-shifting (Perry et al., 2009), episodic memory (Lockhart et al.), executive function (Sasson et al.), and word finding (Stamatakis et al.). In addition, late-life depressed individuals exhibited greater white matter hyperintensity burden in this region (Sheline et al., 2008). Although atrophy reflected by the TBM maps was not correlated with neuropsychological performance (likely due to limited variability in this cognitively healthy sample), this volume loss may predict cognitive changes as the cohort ages, and will be tested at future follow-ups. In addition to the SLF, entorhinal FA was associated with atrophy in anterior corona radiata. These results replicate findings in a multimodal imaging study in younger individuals where significant quadratic relationships between white matter volume and age were observed in the superior corona radiata bilaterally and in the left SLF (Giorgio et al., 2010). Overall, baseline entorhinal FA was associated with the majority of regions of longitudinal atrophy observed in this study. While specific pathways in humans have not been well-characterized, work in non-human primates suggest that the entorhinal region is widely connected with association cortices (Insausti et al., 1987), linking the hippocampus to the association areas of the frontal, parietal, temporal, and occipital lobes (Van Hoesen and Pandya, 1975).
Interestingly, baseline corticospinal FA, the control ROI, did not predict any areas of atrophy. The results are in accord with observations that primary motor and sensory cortices are relatively spared during aging. A recent study (Jang, 2011) has shown corticospinal tract FA decreases with age, whereby participants who are 50 and older show lower FA compared to participants in the third decade of life. The results of our study suggest that changes in microstructural parameters of the corticospinal tract may occur in the absence of volume loss and may not predict downstream volume change, at least during a 4-year interval in middle-aged years. Moreover, baseline FA within the cingulum adjacent to hippocampus and cingulum subjacent to the posterior cingulate did not predict any volume loss. These negative findings may suggest that the microstructural integrity of the cingulum does not decline as rapidly during middle age.
The largest areas of atrophy were primarily in frontal and temporal white matter, findings which complement a cross-sectional study from Hugenschmidt, et al. 2008, where FA had significant relationships with several areas of white matter volume loss, including temporal and parietal regions of the corona radiata, the length of the corpus callosum, and centrum semiovale (Hugenschmidt et al., 2008). In addition, our results are consistent with the convergence of research showing cerebral white matter to have an anterior-posterior gradient of decline (Buckner, 2004; Head et al., 2004; Raz, 2000). This decline in prefrontal white matter follows an inverted-U trajectory, with a linear increase in young adulthood, a plateau in middle age and significant contraction starting in the fifth decade of life (Bartzokis et al., 2001; Courchesne et al., 2000; Raz et al., 2004), the average age of our participants. This rate of decline increases with age (Raz et al., 2005), which is in line with other age-related acceleration in other indices of white matter integrity, such as MRI relaxation times (Bartzokis, 2004; Bartzokis et al., 2003) and ratio of small to large myelinated axons (Tang et al., 1997). These studies suggest that certain brain regions that are late to mature and which contain a high ratio of thinly myelinated fibers (e.g. prefrontal cortex) may be more susceptible to age-related atrophy.
Several of the white matter changes found in aging are likely to affect measurements of water diffusion anisotropy. Histopathological studies have shown that aging is associated with white matter deterioration that include myelin pallor (Kemper, 1994), loss of myelinated fibers (Marner et al., 2003; Meier-Ruge et al., 1992; Pakkenberg and Gundersen, 1997), and in non-human primates, malformation of myelin sheaths (Peters and Sethares, 2002). Further, non-human primate studies also show that age is associated with decreases in synapses, dendritic spines, and myelin sheath degradation in the upper layers of neocortex (Peters, 2002a; Peters, 2002b). These histological studies reveal localized splitting of myelin lamellae causing spherical cytoplasmic cavities or ‘balloons’ within the myelin sheath, and continued myelin production constructing double myelin sheaths, where fluid may build up between layers. Furthermore, as suggested by Bartzokis et al (Bartzokis, 2004) this later myelination is more vulnerable, and age-related declines in membrane cholesterol—a hydrophobic molecule—make myelin more water permissive. All of these changes are candidates for influencing measurements of diffusion anisotropy, in addition to being candidates for predicting later volume loss. Additional histopathological studies will be needed to determine how closely microstructural changes link to overt volume loss; however, only brain imaging studies—while limited in their ability to directly measure pathology—are currently the sole approach to mapping out in vivo changes longitudinally.
Our findings must be interpreted in light of several limitations. Since our sample incorporates individuals with varying risk for Alzheimer’s disease, we cannot rule out the possibility that our findings are Alzheimer’s risk specific. However, participants carrying risk factors for Alzheimer’s did not differ with regards to demographics, neuropsychological scores, or baseline FA measures. While our results showing white matter contraction over fours years survived correction for multiple comparisons, our models showing the predictive value of FA are reported at an uncorrected threshold and thus, we can not rule out the possibility of Type 1 error. Despite this, the exploratory analyses converge on white matter volume loss and thus are less likely to be due to chance. We should also note that while we used DTI measures as the predictor variables in our study design, we can not definitively conclude that microstructural alterations precede volume loss. The temporal ordering of microstructural and volumetric changes over the lifespan is still in need of further characterization. Finally, although other DTI indices, such as axial or radial diffusion, do inform about axonal morphology and myelin characteristics, respectively, we decided to only use FA due to its consistency in the normal aging literature and reflection of several factors, such as changes in axon density, myelination, axonal membrane integrity, fiber orientation, and other alterations.
To our knowledge, this is the first study demonstrating that white matter alterations collected at baseline are associated with future longitudinal white matter volume loss in cognitively normal adults. Following these individuals as they enter the “golden years” will help in further fleshing out the time course of structural brain changes and also determine whether any of the individual variability in atrophy in middle age is due to preclinical diagnosis of age-related neurodegenerative disease. Although longitudinal studies on age-related neurodegenerative diseases including AD are still needed to evaluate patterns of degeneration in pathological processes, this study suggests that DTI may be useful for characterizing the distribution and time course of alterations that occur in the brain with normative aging, improving models of disease progression, and will likely be important for early diagnosis and for monitoring the efficacy of treatments.
Acknowledgments
This work was supported by the National Institutes of Health (AG027161, AG021155, R01AG037639, P50 AG033514) and by a Merit Review Grant (I01CX000165) from the Department of Veterans Affairs. The project was also facilitated by the facilities and resources at the Geriatric Research, Education, and Clinical Center (GRECC) of the William S. Middleton Memorial Veterans Hospital, Madison, WI, GRECC MS# 2013-05. We would like to acknowledge the support of researchers and staff at the Waisman Center, University of Wisconsin–Madison, where imaging data were collected. We thank Aparna Sodhi for her helpful technical assistance on the manuscript. Finally, we thank our dedicated participants for their valuable time.
Footnotes
None of the authors have a conflict of interest to declare.
References
- Ardekani S, Kumar A, Bartzokis G, Sinha U. Exploratory voxel-based analysis of diffusion indices and hemispheric asymmetry in normal aging. Magnetic resonance imaging. 2007;25(2):154–67. doi: 10.1016/j.mri.2006.09.045. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7(4):254–66. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11(6 Pt 1):805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer’s disease. Neurobiology of Aging. 2004;25(1):5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. author reply 49–62. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men: a magnetic resonance imaging study. Archives of General Psychiatry. 2001;58(5):461–5. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Cummings JL, Sultzer D, Henderson VW, Nuechterlein KH, Mintz J. White matter structural integrity in healthy aging adults and patients with Alzheimer disease: a magnetic resonance imaging study. Archives of Neurology. 2003;60(3):393–8. doi: 10.1001/archneur.60.3.393. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Sultzer D, Lu PH, Nuechterlein KH, Mintz J, Cummings JL. Heterogeneous age-related breakdown of white matter structural integrity: implications for cortical “disconnection” in aging and Alzheimer’s disease. Neurobiology of Aging. 2004;25(7):843–51. doi: 10.1016/j.neurobiolaging.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR in Biomedicine. 1995;8(7–8):333–44. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996a;111(3):209–19. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of Magnetic Resonance Series B. 1996b;111(3):209–19. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50(5):1077–88. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Benedetti B, Charil A, Rovaris M, Judica E, Valsasina P, Sormani MP, Filippi M. Influence of aging on brain gray and white matter changes assessed by conventional, MT, and DT MRI. Neurology. 2006;66(4):535–9. doi: 10.1212/01.wnl.0000198510.73363.c6. [DOI] [PubMed] [Google Scholar]
- Benedict R. Brief Visuospatial Memory Test-Revised. Lutz, FL: Psychological Assessment Resources Inc; 1997. [Google Scholar]
- Benes FM. A disturbance of late myelination as a trigger for Alzheimer’s disease. Neurobiology of Aging. 2004;25(1):41–3. doi: 10.1016/j.neurobiolaging.2003.06.003. [DOI] [PubMed] [Google Scholar]
- Bhagat YA, Beaulieu C. Diffusion anisotropy in subcortical white matter and cortical gray matter: changes with aging and the role of CSF-suppression. Journal of magnetic resonance imaging: JMRI. 2004;20(2):216–27. doi: 10.1002/jmri.20102. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44(1):195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Landau S, Schiavone F, Barrick TR, Clark CA, Markus HS, Morris RG. A structural equation modeling investigation of age-related variance in executive function and DTI measured white matter damage. Neurobiology of Aging. 2008;29(10):1547–55. doi: 10.1016/j.neurobiolaging.2007.03.017. [DOI] [PubMed] [Google Scholar]
- Cho H, Yang DW, Shon YM, Kim BS, Kim YI, Choi YB, Lee KS, Shim YS, Yoon B, Kim W, et al. Abnormal integrity of corticocortical tracts in mild cognitive impairment: a diffusion tensor imaging study. Journal of Korean Medical Science. 2008;23(3):477–83. doi: 10.3346/jkms.2008.23.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman PD, Flood DG. Neuron numbers and dendritic extent in normal aging and Alzheimer’s disease. Neurobiology of Aging. 1987;8(6):521–45. doi: 10.1016/0197-4580(87)90127-8. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216(3):672–82. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Englund E. Neuropathology of white matter changes in Alzheimer’s disease and vascular dementia. Dement Geriatr Cogn Disord. 1998;9(Suppl 1):6–12. doi: 10.1159/000051183. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Greve DN, Fischl B, Benner T, van der Kouwe AJ, Salat D, Bjornerud A, Due-Tonnessen P, Walhovd KB. The relationship between diffusion tensor imaging and volumetry as measures of white matter properties. Neuroimage. 2008;42(4):1654–68. doi: 10.1016/j.neuroimage.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Giorgio A, Santelli L, Tomassini V, Bosnell R, Smith S, De Stefano N, Johansen-Berg H. Age-related changes in grey and white matter structure throughout adulthood. Neuroimage. 2010;51(3):943–51. doi: 10.1016/j.neuroimage.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL. Cognitive neuroscience of aging. Annals of the New York Academy of Sciences. 2008;1124:127–44. doi: 10.1196/annals.1440.009. [DOI] [PubMed] [Google Scholar]
- Greenwood PM. Functional plasticity in cognitive aging: review and hypothesis. Neuropsychology. 2007;21(6):657–73. doi: 10.1037/0894-4105.21.6.657. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. Cognitive aging, executive function, and fractional anisotropy: a diffusion tensor MR imaging study. AJNR Am J Neuroradiol. 2007;28(2):226–35. [PMC free article] [PubMed] [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, McAvoy M, Morris JC, Snyder AZ. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: evidence from diffusion tensor imaging. Cerebral Cortex. 2004;14(4):410–23. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- Hugenschmidt CE, Peiffer AM, Kraft RA, Casanova R, Deibler AR, Burdette JH, Maldjian JA, Laurienti PJ. Relating imaging indices of white matter integrity and volume in healthy older adults. Cereb Cortex. 2008;18(2):433–42. doi: 10.1093/cercor/bhm080. [DOI] [PubMed] [Google Scholar]
- Insausti R, Amaral DG, Cowan WM. The entorhinal cortex of the monkey: II. Cortical afferents. Journal of Comparative Neurology. 1987;264(3):356–95. doi: 10.1002/cne.902640306. [DOI] [PubMed] [Google Scholar]
- Jang SH, Cho SH, Lee MY, Kwon YH, Chang MC. Age-related changes of the corticospinal tract in the human brain A diffusion tensor imaging study. Neural Regeneration Research. 2011;6(4):283–287. [Google Scholar]
- Jastak A. Wide Range Achievement Test. 3. Wilmington: Wide Range Inc; 1993. [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiology of Aging. 2001;22(4):581–94. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. 2. Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- Kemper TL. Neuroanatomical and neuropathological changes during aging and dementia. In: Albert MLKJ, editor. Clinical Neurology of Aging. New York: Oxford University Press; 1994. pp. 3–67. [Google Scholar]
- Kipps CM, Duggins AJ, Mahant N, Gomes L, Ashburner J, McCusker EA. Progression of structural neuropathology in preclinical Huntington’s disease: a tensor based morphometry study. J Neurol Neurosurg Psychiatry. 2005;76(5):650–5. doi: 10.1136/jnnp.2004.047993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RS, Lemieux L, Bell GS, Sisodiya SM, Shorvon SD, Sander JW, Duncan JS. A longitudinal study of brain morphometrics using quantitative magnetic resonance imaging and difference image analysis. Neuroimage. 2003;20(1):22–33. doi: 10.1016/s1053-8119(03)00219-2. [DOI] [PubMed] [Google Scholar]
- Lockhart SN, Mayda AB, Roach AE, Fletcher E, Carmichael O, Maillard P, Schwarz CG, Yonelinas AP, Ranganath C, Decarli C. Episodic memory function is associated with multiple measures of white matter integrity in cognitive aging. Front Hum Neurosci. 6:56. doi: 10.3389/fnhum.2012.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft AR, Skalej M, Schulz JB, Welte D, Kolb R, Burk K, Klockgether T, Voight K. Patterns of age-related shrinkage in cerebellum and brainstem observed in vivo using three-dimensional MRI volumetry. Cerebral Cortex. 1999;9(7):712–21. doi: 10.1093/cercor/9.7.712. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM. Diffusion tensor imaging of adult age differences in cerebral white matter: relation to response time. Neuroimage. 2004;21(3):1174–81. doi: 10.1016/j.neuroimage.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. Journal of Comparative Neurology. 2003;462(2):144–52. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- Meier-Ruge W, Ulrich J, Bruhlmann M, Meier E. Age-related white matter atrophy in the human brain. Annals of the New York Academy of Sciences. 1992;673:260–9. doi: 10.1111/j.1749-6632.1992.tb27462.x. [DOI] [PubMed] [Google Scholar]
- O’Sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology. 2001;57(4):632–8. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- Ota M, Obata T, Akine Y, Ito H, Ikehira H, Asada T, Suhara T. Age-related degeneration of corpus callosum measured with diffusion tensor imaging. Neuroimage. 2006;31(4):1445–52. doi: 10.1016/j.neuroimage.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJ. Neocortical neuron number in humans: effect of sex and age. Journal of Comparative Neurology. 1997;384(2):312–20. [PubMed] [Google Scholar]
- Perry ME, McDonald CR, Hagler DJ, Jr, Gharapetian L, Kuperman JM, Koyama AK, Dale AM, McEvoy LK. White matter tracts associated with set-shifting in healthy aging. Neuropsychologia. 2009;47(13):2835–42. doi: 10.1016/j.neuropsychologia.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A. The effects of normal aging on myelin and nerve fibers: a review. Journal of Neurocytology. 2002a;31(8–9):581–93. doi: 10.1023/a:1025731309829. [DOI] [PubMed] [Google Scholar]
- Peters A. Structural changes that occur during normal aging of primate cerebral hemispheres. Neuroscience and Biobehavioral Reviews. 2002b;26(7):733–41. doi: 10.1016/s0149-7634(02)00060-x. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C. Aging and the myelinated fibers in prefrontal cortex and corpus callosum of the monkey. Journal of Comparative Neurology. 2002;442(3):277–91. doi: 10.1002/cne.10099. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Frontal circuitry degradation marks healthy adult aging: Evidence from diffusion tensor imaging. Neuroimage. 2005;26(3):891–9. doi: 10.1016/j.neuroimage.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magnetic Resonance in Medicine. 2000;44(2):259–68. doi: 10.1002/1522-2594(200008)44:2<259::aid-mrm13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Raz J. Aging of the brain and its impact on cognitive performance: integration of structural and functional findings. In: Craik F, Salthouse TA, editors. Handbook of aging and cognition: Erlbaum. Vol. 2. 2000. pp. 1–90. [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U. Trajectories of brain aging in middle-aged and older adults: regional and individual differences. Neuroimage. 2010;51(2):501–11. doi: 10.1016/j.neuroimage.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Williamson A, Acker JD. Age and sex differences in the cerebellum and the ventral pons: a prospective MR study of healthy adults. AJNR American Journal of Neuroradiology. 2001;22(6):1161–7. [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cerebral Cortex. 2005;15(11):1676–89. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD. Differential aging of the medial temporal lobe: a study of a five-year change. Neurology. 2004;62(3):433–8. doi: 10.1212/01.wnl.0000106466.09835.46. [DOI] [PubMed] [Google Scholar]
- Reitan RMWD. The Halstead-Reitan Neuropsychological Test Battery: Theory and clinical interpretation. Tucson: Neuropsychology Press; 1993. [Google Scholar]
- Rey A. L’examen clinique en psychologie. Paris: Presses Universitaires de France; 1964. [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. 1999;18(8):712–21. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- Sager MA, Hermann B, La Rue A. Middle-aged children of persons with Alzheimer’s disease: APOE genotypes and cognitive function in the Wisconsin Registry for Alzheimer’s Prevention. J Geriatr Psychiatry Neurol. 2005;18(4):245–9. doi: 10.1177/0891988705281882. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJ, Hevelone ND, Zaleta AK, Rosen BR, Fischl B, Corkin S, Rosas HD, et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging. 2005a;26(8):1215–27. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Hevelone ND, Fischl B, Corkin S, Rosas HD, Dale AM. Age-related changes in prefrontal white matter measured by diffusion tensor imaging. Annals of the New York Academy of Sciences. 2005b;1064:37–49. doi: 10.1196/annals.1340.009. [DOI] [PubMed] [Google Scholar]
- Sasson E, Doniger GM, Pasternak O, Tarrasch R, Assaf Y. Structural correlates of cognitive domains in normal aging with diffusion tensor imaging. Brain Struct Funct. 217(2):503–15. doi: 10.1007/s00429-011-0344-7. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Vaishnavi SN, Mintun MA, Barch DM, Epstein AA, Wilkins CH, Snyder AZ, Couture L, Schechtman K, et al. Regional white matter hyperintensity burden in automated segmentation distinguishes late-life depressed subjects from comparison subjects matched for vascular risk factors. American Journal of Psychiatry. 2008;165(4):524–32. doi: 10.1176/appi.ajp.2007.07010175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadlbauer A, Salomonowitz E, Strunk G, Hammen T, Ganslandt O. Age-related degradation in the central nervous system: assessment with diffusion-tensor imaging and quantitative fiber tracking. Radiology. 2008;247(1):179–88. doi: 10.1148/radiol.2471070707. [DOI] [PubMed] [Google Scholar]
- Stamatakis EA, Shafto MA, Williams G, Tam P, Tyler LK. White matter changes and word finding failures with increasing age. PLoS One. 6(1):e14496. doi: 10.1371/journal.pone.0014496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Pfefferbaum A. Selective age-related degradation of anterior callosal fiber bundles quantified in vivo with fiber tracking. Cerebral Cortex. 2006;16(7):1030–9. doi: 10.1093/cercor/bhj045. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A. Cerebellar volume decline in normal aging, alcoholism, and Korsakoff’s syndrome: relation to ataxia. Neuropsychology. 2000;14(3):341–52. doi: 10.1037//0894-4105.14.3.341. [DOI] [PubMed] [Google Scholar]
- Tang Y, Nyengaard JR, Pakkenberg B, Gundersen HJ. Age-induced white matter changes in the human brain: a stereological investigation. Neurobiology of Aging. 1997;18(6):609–15. doi: 10.1016/s0197-4580(97)00155-3. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Meindl T, Wagner M, Stieltjes B, Reuter S, Hauenstein KH, Filippi M, Ernemann U, Reiser MF, Hampel H. Longitudinal changes in fiber tract integrity in healthy aging and mild cognitive impairment: a DTI follow-up study. J Alzheimers Dis. 22(2):507–22. doi: 10.3233/JAD-2010-100234. [DOI] [PubMed] [Google Scholar]
- Terry RD, DeTeresa R, Hansen LA. Neocortical cell counts in normal human adult aging. Ann Neurol. 1987;21(6):530–9. doi: 10.1002/ana.410210603. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Pandya DN. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. III. Efferent connections. Brain Research. 1975;95(1):39–59. doi: 10.1016/0006-8993(75)90206-1. [DOI] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230(1):77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Quinn BT, Salat D, Makris N, Fischl B. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiology of Aging. 2005;26(9):1261–70. doi: 10.1016/j.neurobiolaging.2005.05.020. discussion 1275–8. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Memory Scale-Revised. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, Bjornerud A, Due-Tonnessen P, Engvig A, Grydeland H, Tamnes CK, Ostby Y, Fjell AM. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cerebral Cortex. 2010;20(9):2055–68. doi: 10.1093/cercor/bhp280. [DOI] [PubMed] [Google Scholar]