Abstract
The purpose was to systematically investigate inter-limb interactions in chronic hemiparetic stroke. Fourteen post-stroke hemiparetic subjects (>1yr) performed maximum voluntary contraction (MVC) elbow flexion tasks without visual feedback with one (unilateral) and two limbs simultaneously (bilateral). At submaximal levels, subjects produced force to a visual target reflecting 20, 40, 60, and 80% of corresponding MVC in unilateral tasks, and of summated unilateral MVCs in bilateral tasks. Elbow flexion force and biceps surface EMG were measured bilaterally. Proportionally increased EMG activity on the contralateral limb (motor overflow) was observed during unilateral tasks of the non-impaired limb, but not of the impaired limb. During bilateral tasks at submaximal levels, the impaired limb produced less force (i.e., force deficit) as compared to expected forces based upon its unilateral MVC. Force deficit on the impaired limb was compensated by greater force production on the non-impaired limb such that the visual target was reached. However, force contribution to the total force progressively decreased from the non-impaired side, when the level of sub-maximal contractions increased. During bilateral MVC tasks, there was no force deficit on the impaired limb, but force deficit was observed on the non-impaired limb. A net result of a small bilateral deficit in force with parallel changes in EMG was observed. These novel findings of activation-level dependent interactions and asymmetrical contralateral motor overflow provide new insights that, among other compensatory mechanisms, ipsilateral corticospinal projections from the non-lesioned hemisphere play an important role in inter-limb interactions in chronic stroke, in addition to unbalanced interhemispheric inhibition.
Keywords: bilateral deficit, force deficit, stroke, voluntary contraction, hemiplegia, weakness, interlimb
Introduction
During a simultaneous bilateral maximum voluntary contraction (MVC) task, a healthy individual produces less total peak force than the sum of maximum forces created during unilateral MVC tasks. This phenomenon of bilateral deficit (BD) is commonly observed in a variety of upper and lower extremity tasks in healthy individuals (Grant et al. 1994; Howard and Enoka 1991; Jakobi and Cafarelli 1998; Latash et al. 2002; Li et al. 2001; 2000a; b; Li et al. 2003). Similarly, bilateral deficit is observed in stroke survivors in bilateral MVC tasks (DeJong and Lang 2012; Lewek et al. 2010; Li et al. 2003; McQuade et al. 2008). When force of each limb is compared, both limbs produce less force in a bilateral MVC task than in individual unilateral tasks (i.e., force deficit) in healthy subjects (DeJong and Lang 2012; Li et al. 2003). On the contrary, force deficit has been observed only on the non-impaired limb, but not on the impaired limb in hemiparetic stroke subjects (DeJong and Lang 2012; McQuade et al. 2008). Interestingly, DeJong and Lang have reported that the impaired side even produces higher maximal grip force, i.e., facilitation, in bilateral MVC tasks (DeJong and Lang 2012). These studies indicate that asymmetrical muscle strength and altered inter-limb interactions coexist in hemiplegic stroke survivors in bilateral MVC tasks.
Strength asymmetry also influences inter-limb interactions at the submaximal levels. In bilateral submaximal tasks where a visual target represents a percentage of summated individual MVCs, subjects are explicitly instructed to produce a total force with both sides simultaneously to match the target. Without specific instructions on force production on each side, healthy subjects have relatively equal force contribution to the total force from both sides (DeJong and Lang 2012), while hemiparetic stroke subjects produce less force on the impaired side (target of 30%MVC in (DeJong and Lang 2012); 5%, 25%, and 50%MVC in (Lodha et al. 2012)) and the percent of total force for each side remains consistent from low to moderate levels of force production (Lodha et al. 2012). Even explicitly instructed to produce equal amount of forces simultaneously on the impaired and non-impaired sides but without visual feedback (low to moderately high levels: 25%, 50%, and 65%MVC), subjects produce significantly less force on the impaired limb. However, the ratio of forces (non-impaired/impaired) at the submaximal levels is related to the ratio of MVCs of each side (Bertrand et al. 2004). In other words, forces on the impaired and non-impaired sides are proportional to individual MVCs, i.e., no force deficit at the tested submaximal levels.
These reports collectively demonstrate differences in force production of both impaired and non-impaired sides between submaximal (low to moderate) and maximal bilateral tasks in chronic hemiparetic stroke. The underlying mechanisms remain unknown, though these previous studies suggest inter-limb interactions between the impaired and non-impaired limbs are activation level dependent. To systematically examine inter-limb interactions, we measured voluntary elbow flexion forces of the impaired and non-impaired limbs during bilateral voluntary elbow flexion at submaximal and maximal levels (20% to 100% MVC). We hypothesized that there are activation-level-dependent dynamic inter-limb interactions in chronic stroke; specifically there is force deficit on the impaired limb during bilateral tasks at submaximal levels, but no or minimal force deficit on the impaired side at the maximal level. We also recorded surface EMGs from bilateral biceps during unilateral and bilateral elbow flexion tasks to examine whether inter-limb force interactions are accompanied by parallel EMG changes. Part of the results has been presented in a conference (Durand-Sanchez et al. 2012).
Methods
Subjects
Fourteen chronic stroke subjects (mean 63.9 ± 14.9 years of age) were recruited (See Table 1 for subject characteristics). Inclusion criteria were subjects who had: 1) hemiplegia secondary to a single ischemic or hemorrhagic stroke; 2) at least 12 months post-stroke; 3) residual voluntary elbow flexion force on the impaired side; 4) full passive range of motion in the impaired shoulder and elbow joints; 5) intact cognitive ability for the purpose of giving informed consent and understanding instructions related to the experiment. Subjects were excluded if they had: 1) a history of multiple strokes or bilateral involvement; 2) presence of contracture that would limit a full range of motion of the elbow joint on the impaired side; 3) neglect and/or cognitive deficit. All subjects gave an informed consent prior to participation. This study was approved by the Committee for the Protection of Human Subjects at the local institutes.
Table 1.
Subject characteristics
| Age (years of age) | 63.9 ± 14.9 |
| Gender | Female=7, Male=7 |
| Impaired side | Left=7, Right=7 |
| Post-Stroke (month) | 74.9 ± 41.0 |
| Modified Ashworth Scale (MAS) | 0 = 6 |
| 1 = 4 | |
| 1+ = 3 | |
| 2 = 0 | |
| 3 = 1 |
Procedure
Apparatus
Subjects were seated on a height-adjustable chair. Both upper limbs were symmetrically positioned as follows: the shoulders were slightly flexed and abducted to approximately 45°, the elbows flexed to approximately 90°, and the forearms/wrists were in a neutral position. A load cell (208C02; PCB Piezotronics, Depew, NY) was placed perpendicular to the distal end of each forearm to measure the isometric elbow flexion force, as described in a recent study (Chang et al. in press). Extra stabilization straps were applied to the distal forearm on the impaired side. A harness with shoulder straps held the trunk against a firm back support to prevent compensatory axial motion or shoulder protraction and retraction force generation across the elbow joint during the tasks. Bipolar EMG bar (22×33 mm Ag/AgCl) electrodes were placed over the biceps muscle bellies bilaterally. A reference electrode was placed at the lateral humeral epicondyle on the left side. The EMG electrodes were connected to an EMG amplifier (modified Bagnoli 8, Delsys, Boston, MA). Force and EMG signals were digitized at 5000 Hz (PCI-6229, National Instruments, Austin, TX) using a personal computer with custom LabVIEW software (National Instruments) and saved for off-line analysis.
Tasks
Subjects first performed 3 sets of elbow flexion MVC tasks without visual feedback in a randomized order: 1) unilateral MVC tasks on the impaired side, 2) unilateral MVC tasks on the non-impaired side, and 3) bilateral MVC tasks. The highest force value of 3 unilateral MVC trials was chosen as 100% MVC for that limb. These unilateral MVCs were used to determine force targets of 20%, 40%, 60% and 80% of MVC for unilateral submaximal tasks. As in previous studies (DeJong and Lang 2012; Lodha et al. 2012), the sum of unilateral MVCs was used to establish corresponding force targets for bilateral tasks at submaximal levels.
After at least 3 practice trials, subjects were asked to perform unilateral and bilateral tasks at submaximal levels in a randomized order. Each trial lasted 20s. Auditory cues signaled the start of contraction at the 5th second and the stop at the 15th second of a trial. A thick red horizontal line indicating the target force was displayed on the computer screen. A real-time visual display of the force amplitude (the total force if in bilateral tasks) ran from left to right across the screen. Subjects were verbally encouraged to match the target red line with the real force. Three trials were performed in a randomized order for each condition (×3) and each target force (×4), with at least 1 minute rest in between trials and conditions.
Data Analysis
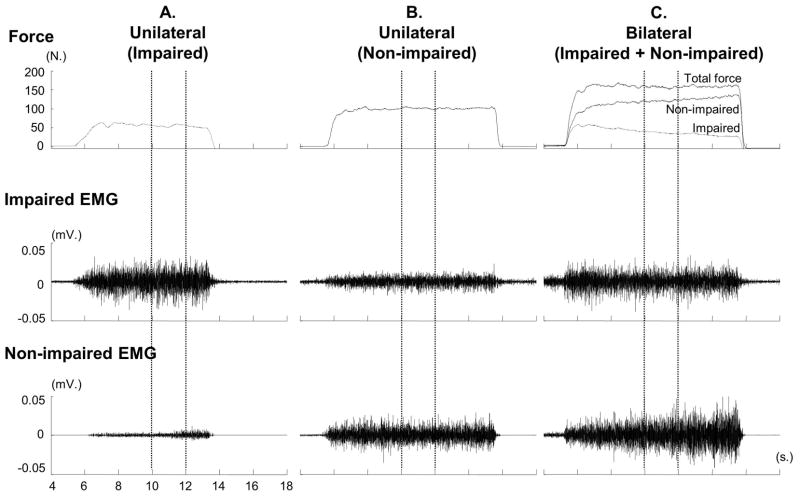
Force and EMG signals were analyzed offline using a custom MATLAB program. Force signals were filtered with a low-pass Butterworth filter (10-Hz cutoff). EMG signals were filtered with a band-pass 4th order Butterworth filter (20 to 300 Hz), rectified, and smoothed with a 4th order Butterworth filter with a cutoff frequency at 20Hz. For maximal MVC trials, peak force value was identified (McQuade et al. 2008) and root-mean-square (RMS) EMG (peak EMG) was averaged from a 200-ms window centered on the peak force. For each 20-s submaximal (20%, 40%, 60% and 80% of MVC) trial, a period of 3-s (10th – 12th s of the trial) of EMG and force signals was used to standardize the analysis because stroke subjects may have difficulty maintaining steady force for a long period of time (Lodha et al. 2010) (see also Figure 1). Average amplitudes of force and RMS EMG in the 3-s segment were calculated for each limb.
Figure 1.
Representative trials with raw force and EMG signals from 4th to 18th -s during unilateral and bilateral tasks at 60% MVC in a subject. The 2-s force and EMG signals (between two vertical dotted lines) were analyzed.
We compared the sum of unilateral MVCs (Fsum) and the total force (Ftot) during a bilateral MVC task to calculate bilateral deficit (BD) using the following equation: BDForce = ((Fsum − Ftot)/Fsum) × 100%. Similarly, BD for EMG was calculated BDEMG= ((EMGsum − EMGtot)/EMGsum) × 100%. If BD > 0, it represents bilateral deficit; and BD < 0 represents bilateral facilitation during a bilateral MVC task.
We further compared forces of each limb between unilateral (Funi) and bilateral (Fbi) tasks at the same level (maximal and submaximal) to calculate force deficit (FD) of a limb during bilateral tasks. The following equation was used: FD= (Funi−Fbi)/Funi×100%. If FD >0, it represents force deficit of a limb during a bilateral task, if FD < 0, it represents force facilitation of a limb during a bilateral task. Force contribution (FC) of each limb to the total force (Ftot) at different target levels may vary. We calculated force contribution of the impaired limb (FCi) as follows: FCi = (Fbi/Ftot) ×100%. FC of the non-impaired limb was 1−FCi.
As shown in Figure 1, we observed EMG activities on the contralateral resting biceps brachii muscle during unilateral tasks, i.e., overflow. The amount of overflow EMG activity recorded in the resting limb, compared to the amount of EMG activity generated in the active limb was measured for each force level. An overflow percentage was calculated using the normalized EMG (nEMG) in the contralateral resting limb to the normalized EMG in the contracting limb following equation: (nEMGrest/nEMGcontract) ×100%.
Statistical Analysis
Descriptive statistics and repeated-measures ANOVAs were performed. We performed separate analysis of forces for tasks at maximal and submaximal force levels. Paired t-tests were used to compare the sum of forces and the total force for MVC tasks. ANOVA tests were conducted to examine the force variables (sum of forces and total force) among submaximal force levels. Factors included CONDITION (2 levels, unilateral vs. bilateral) and FORCE LEVEL (4 levels, 20%, 40%, 60%, 80% of MVC). Similarly, individual limb forces during unilateral and bilateral tasks were analyzed using paired t-tests for MVC tasks and two-way ANOVAs for submaximal tasks. Factors included CONDITION and FORCE LEVEL. Further ANOVA tests were performed to examine FD between limbs and among submaximal tasks. Factors included SIDE (2 levels, Impaired vs. Non-impaired) and FORCE LEVEL. To examine the activation-level dependent effect of force contribution, a one-way ANOVA with factor ACTIVATION (5 levels, 20%, 40%, 60%, 80% and 100% of MVC) was performed on force contribution. Post hoc Tukey’s HSD tests were performed when there was a significant effect in ANOVA tests. The alpha level required for all statistical significance was set at .05. Data are reported as means ± standard deviation within the text and displayed as means ± standard errors in the figures.
Results
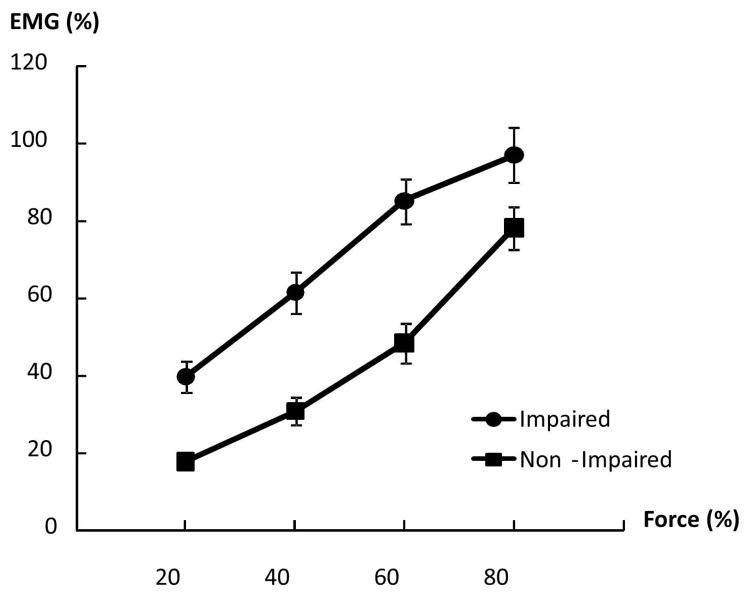
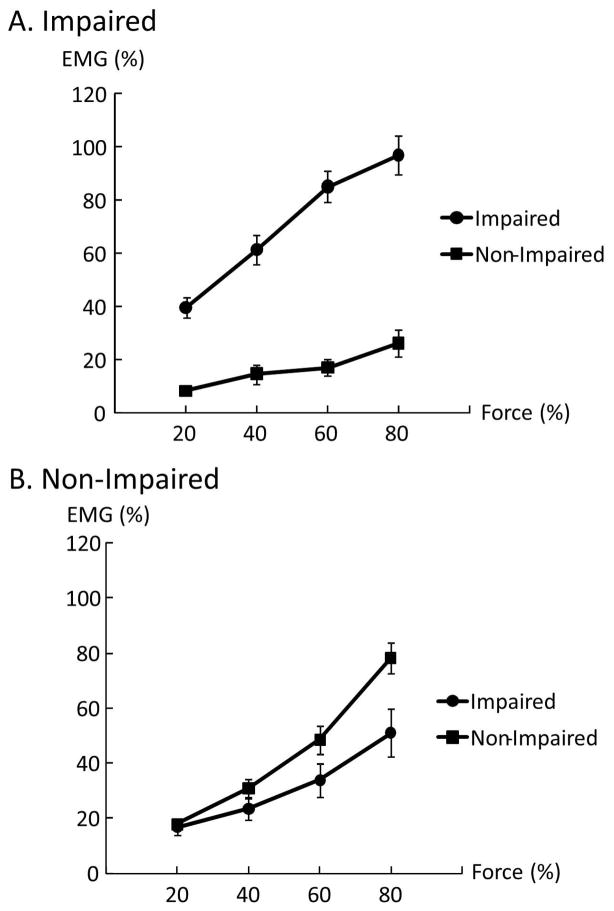
Forces of each limb during unilateral and bilateral tasks are summarized in Table 2. Representative raw force and EMG signals from one subject during unilateral tasks and a bilateral task at 60% MVC are shown in Figure 1. On average, as we expected, EMG–force curves revealed a positive linear relationship between normalized EMG and force in both limbs. The EMG-force curve on the impaired limb was shifted upward but remained parallel to the curve on the non-impaired limb (Fig 2). As shown in Figure 1A & 1B, motor overflow was greater during unilateral activation of the non-impaired limb than the impaired side (Fig 3). During unilateral activation of the impaired limb, the overflow ratios were: 23.2 ± 21.6% at 20% MVC, 28.9 ± 31.8% at 40% MVC, 20.4 ±13.4% at 60% MVC, and 26.4 ± 17.3% at 80% MVC. During unilateral activation of the non-impaired limb, the overflow ratios were: 101.1±84.7% at 20% MVC, 83.8±65.7% at 40% MVC, 70.9±43.2% at 60% MVC and 63.6±35.0% at 80% MVC. Two-way SIDE × FORCE LEVEL ANOVA tests on the overflow ratio revealed a significant effect of SIDE (F[1,13]=49.35, p<0.0001), but no main effects of FORCE LEVEL (F[3,39]=1.14, p=0.3439) or significant interactions between SIDE and FORCE LEVEL (F[3,39]=1.17, p=0.3333).
Table 2.
Mean and standard error (SE) of the mean force (in Newtons) and RMS EMG (in μ.v.) in unilateral and bilateral tasks
| Force levels (%MVC) | Unilateral Task | Bilateral Task | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Impaired | Non-Impaired | Impaired | Non-Impaired | Total | ||||||
| Force | RMS EMG | Force | RMS EMG | Force | RMS EMG | Force | RMS EMG | Force | RMS EMG | |
| 20% | 13.0 (2.0) | 26.2 (5.6) | 21.7 (1.8) | 19.8 (3.2) | 10.4 (2.0) | 21.4 (5.0) | 26.8 (2.5) | 23.1 (4.1) | 37.2 (3.3) | 44.5 (7.9) |
| 40% | 26.0 (4.1) | 41.9 (8.9) | 43.3 (3.5) | 33.7 (6.0) | 22.7 (4.1) | 40.8 (9.2) | 48.6 (5.1) | 41.5 (7.9) | 71.3 (6.7) | 82.3 (14.2) |
| 60% | 39.0 (6.1) | 61.0 (14.3) | 65.0 (5.3) | 53.7 (9.7) | 33.3 (6.0) | 58.5 (14.2) | 67.2 (7.7) | 64.3 (12.5) | 100.5 (10.0) | 122.8 (20.6) |
| 80% | 52.0 (8.2) | 75.5 (21.5) | 86.6 (7.1) | 89.9 (14.4) | 44.6 (8.1) | 75.4 (21.6) | 82.3 (7.5) | 95.6 (17.9) | 126.9 (11.7) | 170.9 (34.7) |
| 100% | 65.0 (10.2) | 81.6 (21.0) | 108.3 (8.8) | 147.3 (23.0) | 63.0 (10.6) | 75.0 (22.6) | 96.4 (10.3) | 111.9 (22.2) | 159.3 (14.9) | 186.9 (39.3) |
Figure 2.
Linear EMG-force relations in impaired and non-impaired limbs. The EMG-force relation in the impaired limb was upward shifted but paralleled to the EMG-force relation in the non-impaired limb.
Figure 3.
Overflow EMG activities of the resting biceps during voluntary activation of the contralateral impaired limb (A) and non-impaired limb (B).
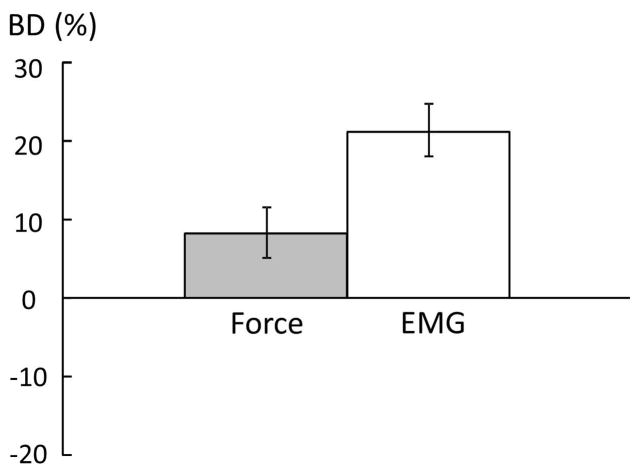
When the total force of a bilateral task was analyzed, there was bilateral deficit (BD) in force and EMG during bilateral MVC tasks. Figure 4 shows the average BD in force and EMG. Paired t-tests with raw force and EMG values revealed the total force during bilateral tasks was lower than the sum of forces during unilateral tasks (Ftot=159.3±55.9 N., Fsum=172.6±55.3 N., t(13) =2.89, p=0.0127). Similarly, EMGtot was lower than EMGsum (0.187±0.147 m.v. vs. 0.229 ± 0.144 m.v., t(13)=5.15, p=0.002) at 100% MVC. BDForce and BDEMG were 8.2±11.8% and 18.2±13.0%, respectively. During bilateral submaximal tasks, as expected, the total force matched the visual target, i.e., no bilateral deficit. Two-way CONDITION × FORCE LEVEL ANOVA tests in raw force and EMG revealed a main effect of FORCE LEVEL (F[3,39] >49.65, p<0.0001), but no significant effects of CONDITION or interactions between CONDITION and FORCE LEVEL were found.
Figure 4.
Average bilateral deficit (BD) in force and EMG during bilateral MVC tasks. Positive value indicates bilateral deficit, while negative value indicates bilateral facilitation.
When forces of individual limbs were analyzed, results showed an activation level-dependent pattern. During bilateral MVC tasks, the non-impaired limb produced significantly less force (96.4 ± 38.4 N vs. 108.3± 33.1 N; t(13) =2.75, p=0.0167), while the impaired limb produced a similar force (63.0 ± 39.7 N vs. 65.0± 38.2 N), as compared to corresponding unilateral MVC tasks (see Table 2). At submaximal levels, the impaired limb produced less force in the bilateral tasks than in corresponding unilateral tasks across all force levels (i.e. force deficit). Two-way CONDITION × FORCE LEVEL ANOVA tests for the impaired limb revealed main effects of CONDITION (F[1, 13]=6.52, p=0.0241) and FORCE LEVEL (F[3,39]=71.34, p<0.0001), but no significant interactions between CONDITION and FORCE LEVEL. However, the same analysis for the non-impaired limb showed no significant effects of CONDITION or interactions between CONDITION and FORCE LEVEL. The results showed no force deficit in the non-impaired limb between the bilateral and unilateral tasks at submaximal levels.
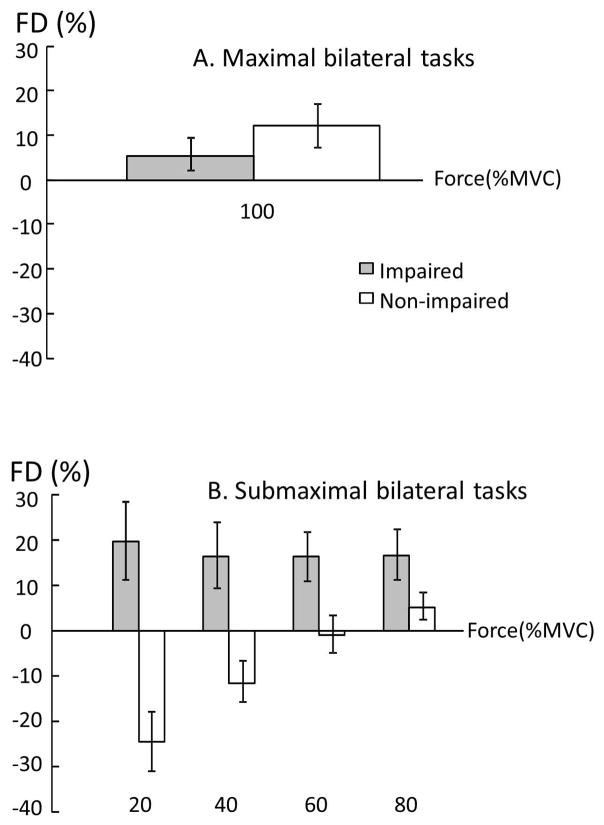
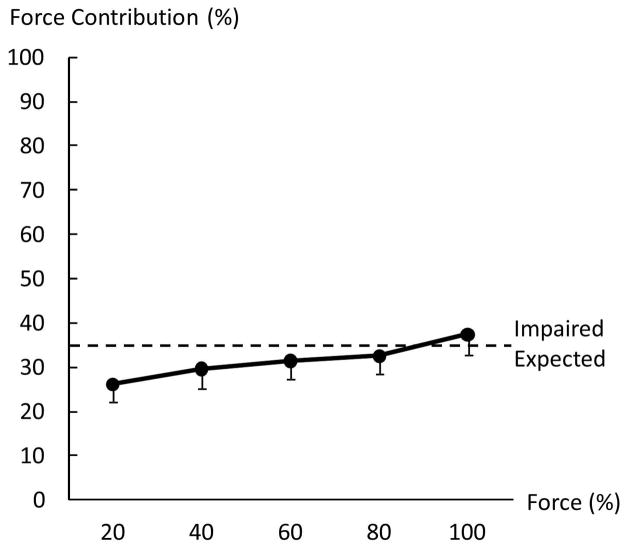
When force deficit (FD) was compared between the impaired and non-impaired limb, two-way ANOVA tests revealed a main effect of SIDE (F[1,13]=36.8, p<0.0001) and significant interactions between SIDE and FORCE LEVEL (F[3,39]=3.30, p=0.0303). In the impaired limb, FDi was similar among 4 submaximal MVCs (20%:19.6 ± 32.3%, 40%: 16.5 ± 27.6 %, 60%:16.2 ± 20.3%, 80%:16.6 ± 20.9%). In the non-impaired limb, FDni was negative (i.e. facilitation) at 20% MVC (−24.6 ± 24.4%), and progressed to be positive at 80% MVC (5.2± 11.4%, p=0.0008). FD was higher in the impaired limb than in the non-impaired limb at 20% (p<0.0001), 40% (p=0.0015) and 60% (p=0.0432) MVC but not at 80% MVC (Figure 5B). In other words, even the target force was matched by the total force during submaximal bilateral tasks, the non-impaired limb progressively produced less force contribution when the target level increased. Force contribution (FC) of the impaired side also reflected this trend (Fig 6). A one-way ANOVA showed the main effect of ACTIVATION (F[4,52]=6.8816; p=0.00016), indicating progressive increases of force contribution from the impaired limb. According to post-hoc tests, FC at 100%MVC was significantly higher than at 20% and 40%MVC; FC at 80%MVC was significantly higher than at 20%MVC (p>0.0001).
Figure 5.
Average force deficit index (FD) at maximal (A) and submaximal (B) levels.
Figure 6.
Force contribution (FC) of the impaired limb to the total force during bilateral tasks. FC of the impaired side progressively increased when the level of voluntary activation increased. Expected: the visual target was preset proportional to the sum of unilateral MVCs during bilateral submaximal tasks. Each limb was expected to produce a force proportional to its own MVC across all submaximal levels.
Discussion
In this study, stroke subjects performed voluntary elbow flexion at maximal and submaximal levels with the impaired and non-impaired limbs unilaterally and bilaterally. Our results confirmed previous findings, including that 1) there is bilateral deficit during bilateral MVC tasks in stroke subjects; 2) bilateral deficit is primarily due to force deficit on the non-impaired side (DeJong and Lang 2012; McQuade et al. 2008); 3) bilateral deficit is accompanied by parallel changes in EMG (Howard and Enoka 1991; Post et al. 2007); 4) there are similar EMG-force relations on both sides (Chang et al. in press). Our novel findings from systematical examination of forces of individual limbs in unilateral and bilateral tasks expanded the previous findings: 1) activation level dependent inter-limb interactions during bilateral tasks were resulted from progressively decreased force contribution from the non-impaired side; 2) motor overflow to the contralateral resting limb was proportional to unilateral activation of the non-impaired side, but not the impaired side. These novel findings provide some new insights on inter-limb interactions in chronic hemiparetic stroke.
During bilateral submaximal tasks, subjects were instructed to produce a total force to match a visual target. The target was preset proportional to the sum of unilateral MVCs. As such, force contribution of each limb to the total force was the same if each limb produced a force proportional to its unilateral MVC across the different submaximal levels (i.e., expected, thus no force deficit). In the present study, force contribution from each limb changed progressively as a result of inter-limb interactions and was related to the level of voluntary activation. As revealed in Figure 5, the non-impaired limb produced more than expected force (i.e., negative force deficit) to compensate for force deficit on the impaired limb at low levels, in order to match the target force. When the target force increased, force contribution of the non-impaired limb progressively decreased while force deficit on the impaired side remained relatively the same (Figure 5). This trend continued until the non-impaired limb failed to reach the required activation during bilateral MVC tasks, i.e., force deficit, but there was no force deficit on the impaired limb. As such, force contribution of the impaired limb progressively increased as the level of voluntary activation increased (Figure 6). The present results of activation level dependent inter-limb interactions were different from previous studies that showed constant ratio between forces of the non-impaired and impaired limbs (Bertrand et al. 2004; Lodha et al. 2012). The difference may be ascribed to the fact that only low to moderate high levels were tested in the past (<65%MVC).
Interhemispheric inhibition (IHI) has been viewed previously as the predominant mechanism underlying the bilateral deficit phenomenon in healthy subjects (Archontides and Fazey 1993). In IHI, a high level of cortical activation in one hemisphere causes inhibition of the homologous cortical area in the contralateral hemisphere. The IHI effect is equally distributed to each limb in healthy individuals from low to high levels of bilateral activation (Archontides and Fazey 1993; Owings and Grabiner 1998; Soteropoulos and Perez 2011). Recent studies in chronic stroke subjects, however, have shown that IHI from the lesioned to non-lesioned hemisphere decreases (Liepert et al. 2000), while IHI from the non-lesioned to lesioned side remains intact or can be exaggerated (Butefisch et al. 2008; Perez and Cohen 2009). In this study, perhaps unbalanced IHI inhibition from the non-lesioned to the lesioned hemisphere resulted in our findings of less than expected force production on the impaired limb, and more than expected force production on the non-impaired limb at submaximal levels.
At the level of maximal levels, however, our findings are reversed that the impaired limb produced the predicted amount of force and a force deficit on the non-impaired side was observed. This argues against IHI as the predominant mechanism for the present findings at the maximal level. Post-stroke compensatory mechanisms may be involved in bilateral tasks (DeJong and Lang 2012), in addition to the interhemispheric inhibition (IHI) mechanism. These compensatory mechanisms may include 1) activation of secondary motor areas of the lesioned hemisphere (Whitall et al. 2011); 2) unmasking of uncrossed ipsilateral corticospinal tracts from the non-lesioned hemisphere (Khodiguian et al. 2003; Lewek et al. 2010); 3) increased descending drive from the reticulospinal system after unilateral brain lesions (Benecke et al. 1991; Dewald et al. 1995) that are likely bilateral projections (Baker 2011; Buford and Davidson 2004).
Motor overflow to the impaired side of stroke survivors during voluntary contraction of the non-impaired side has been previously reported (Hwang et al. 2005). In our study, we compared motor overflow to the impaired side and to the non-impaired side during contralateral elbow flexion. We observed asymmetry in motor overflow between impaired and non-impaired elbow flexion in stroke subjects. Our novel findings of asymmetrical contralateral motor overflow and progressively decreased force contribution on the non-impaired side provide some evidence that unmasking ipsilateral corticospinal projections, among possible compensatory mechanisms, play an important role in inter-limb interactions in chronic stroke, in addition to IHI. Firstly, motor overflow was observed on the resting impaired side proportional to unilateral activation of the non-impaired side, but not vice versa (Figure 3). This asymmetry suggests that motor overflow is not likely from the reticulospinal projections. Secondly, the result of progressively decreased force contribution of the non-impaired side to the total force at the submximal levels was possibly due to the fact that part of descending activation from the non-lesioned ipsilateral hemisphere is channeled to the impaired side, i.e., sharing of the same activation source. This possibility was further corroborated by the results of force deficit on the non-impaired side, i.e., ceiling effect (Latash et al. 2002; Li et al. 2001), but no deficit on the impaired side for the bilateral MVC tasks. These results would be not possible if primarily mediated by activation of secondary motor areas of the lesioned hemisphere, though not ruled out in the present study. Lastly, previous fMRI studies indicate that the non-lesioned hemisphere is involved in movement of the impaired and non-impaired sides (Feydy et al. 2002; Kim et al. 2006; Marshall et al. 2000).
There are limitations in the present study. This study provides evidence of contralateral motor overflow in chronic stroke. Testing of age- and gender-matched control subjects could provide results contrasting to this pattern of pathological findings in stroke. Only 3 seconds of data during a 10-s contraction were analyzed in a 20-s trial. This was chosen to standardize the analysis where patients had stable force output in the middle to late contraction. Although slow in initiation and development of force, stroke subjects usually maintained force output well. A longer duration of data for analysis could provide more robust results.
Concluding remarks
During simultaneous force production with both impaired and non-impaired limbs in chronic stroke subjects, our results confirmed previous findings of the bilateral deficit phenomenon. Our novel findings indicated 1) activation level dependent inter-limb interactions during bilateral tasks that were resulted from progressively decreased force contribution from the non-impaired side, and 2) motor overflow to the contralateral resting limb during unilateral activation of the non-impaired side. These findings suggest the important role of unmasking ipsilateral projections from the non-lesioned hemisphere in the inter-limb interactions in addition to inter-hemispheric inhibition. Recently, bilateral training has been used to facilitate motor recovery of the impaired limb in chronic stroke. In most bilateral training protocols, the impaired side moves simultaneously with the non-impaired limb in reaching or other functional tasks at submaximal levels (cf reviews (Cauraugh et al. 2010; Coupar et al. 2010; van Delden et al. 2012; Waller and Whitall 2008)). Our results suggest that the impaired limb could benefit more from bilateral training when high activation of both limbs is required. At high activation levels, it is more likely to recruit alternative compensatory mechanisms to facilitate recovery.
Acknowledgments
This study was supported in part by NIH grants (NIH/NINDS R01NS060774; NIH/NICHD/NCMRR R24 HD050821-08 under subcontract with Rehabilitation Institute of Chicago).
References
- Archontides C, Fazey JA. Inter-limb interactions and constraints in the expression of maximum force; A review, some implications and suggested underlying mechanisms. J Sports Sci. 1993;11:145–158. doi: 10.1080/02640419308729978. [DOI] [PubMed] [Google Scholar]
- Baker SN. The primate reticulospinal tract, hand function and functional recovery. J Physiol. 2011;589:5603–5612. doi: 10.1113/jphysiol.2011.215160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benecke R, Meyer BU, Freund HJ. Reorganisation of descending motor pathways in patients after hemispherectomy and severe hemispheric lesions demonstrated by magnetic brain stimulation. Exp Brain Res. 1991;83:419–426. doi: 10.1007/BF00231167. [DOI] [PubMed] [Google Scholar]
- Bertrand AM, Mercier C, Shun PLW, Bourbonnais D, Desrosiers J. Effects of Weakness on Symmetrical Bilateral Grip Force Exertion in Subjects with Hemiparesis. J Neurophysiol. 2004;91:1579– 1585. doi: 10.1152/jn.00597.2003. [DOI] [PubMed] [Google Scholar]
- Buford JA, Davidson AG. Movement-related and preparatory activity in the reticulospinal system of the monkey. Exp Brain Res. 2004;159:284–300. doi: 10.1007/s00221-004-1956-4. [DOI] [PubMed] [Google Scholar]
- Butefisch CM, Wessling M, Netz J, Seitz RJ, Homberg V. Relationship between interhemispheric inhibition and motor cortex excitability in subacute stroke patients. Neurorehabil Neural Repair. 2008;22:4–21. doi: 10.1177/1545968307301769. [DOI] [PubMed] [Google Scholar]
- Cauraugh JH, Lodha N, Naik SK, Summers JJ. Bilateral movement training and stroke motor recovery progress: A structured review and meta-analysis. Hum Mov Sci. 2010;29:853–870. doi: 10.1016/j.humov.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S-H, Francisco GE, Zhou P, Rymer WZ, Li S. Weakness, spasticity, force variability and spontaneous motor unit discharges of resting spastic-paretic biceps brachii in chronic stroke. Muscle & Nerve. doi: 10.1002/mus.23699. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupar F, Pollock A, van Wijck F, Morris J, Langhorne P. Simultaneous bilateral training for improving arm function after stroke. Cochrane Database Syst Rev. 2010:CD006432. doi: 10.1002/14651858.CD006432.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJong SL, Lang CE. The bilateral movement condition facilitates maximal but not submaximal paretic-limb grip force in people with post-stroke hemiparesis. Clin Neurophysiol. 2012;123:1616–1623. doi: 10.1016/j.clinph.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118 (Pt 2):495–510. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- Durand-Sanchez A, Chang SH, Ditommaso C, Li S. Does the non-impaired limb help the impaired limb during bilateral motor tasks in hemiparetic stroke patients? Am J Phys Med Rehabil. 2012;91:286–287. [Google Scholar]
- Feydy A, Carlier R, Roby-Brami A, Bussel B, Cazalis F, Pierot L, Burnod Y, Maier MA. Longitudinal study of motor recovery after stroke: recruitment and focusing of brain activation. Stroke. 2002;33:1610–1617. doi: 10.1161/01.str.0000017100.68294.52. [DOI] [PubMed] [Google Scholar]
- Grant AK, Habes JD, Putz-Anderson V. Psychophysical and EMG correlates of force exertion in manual work. Intl J Indus Ergon. 1994;13:31–39. [Google Scholar]
- Howard JD, Enoka RM. Maximum bilateral contractions are modified by neurally mediated interlimb effects. J Appl Physiol. 1991;70:306–316. doi: 10.1152/jappl.1991.70.1.306. [DOI] [PubMed] [Google Scholar]
- Hwang IS, Tung LC, Yang JF, Chen YC, Yeh CY, Wang CH. Electromyographic analyses of global synkinesis in the paretic upper limb after stroke. Phys Ther. 2005;85:755–765. [PubMed] [Google Scholar]
- Jakobi JM, Cafarelli E. Neuromuscular drive and force production are not altered during bilateral contractions. J Appl Physiol. 1998;84(1):200–206. doi: 10.1152/jappl.1998.84.1.200. [DOI] [PubMed] [Google Scholar]
- Khodiguian N, Cornwell A, Lares E, DiCaprio PA, Hawkins SA. Expression of the bilateral deficit during reflexively evoked contractions. J Appl Physiol. 2003;94:171–178. doi: 10.1152/japplphysiol.00703.2002. [DOI] [PubMed] [Google Scholar]
- Kim YH, You SH, Kwon YH, Hallett M, Kim JH, Jang SH. Longitudinal fMRI study for locomotor recovery in patients with stroke. Neurology. 2006;67:330–333. doi: 10.1212/01.wnl.0000225178.85833.0d. [DOI] [PubMed] [Google Scholar]
- Latash ML, Li S, Danion F, Zatsiorsky VM. Central mechanisms of finger interaction during one- and two-hand force production at distal and proximal phalanges. Brain Res. 2002;924:198–208. doi: 10.1016/s0006-8993(01)03234-6. [DOI] [PubMed] [Google Scholar]
- Lewek MD, Breslin R, Hlad L, Lanton A, St John J. Non-paretic quadriceps activity influences paretic quadriceps activity post-stroke. Clin Neurophysiol. 2010;121:1962–1967. doi: 10.1016/j.clinph.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Li S, Danion F, Latash ML, Li ZM, Zatsiorsky VM. Bilateral deficit and symmetry in finger force production during two- hand multifinger tasks. Exp Brain Res. 2001;141:530–540. doi: 10.1007/s002210100893. [DOI] [PubMed] [Google Scholar]
- Li S, Danion F, Latash ML, Li ZM, Zatsiorsky VM. Characteristics of finger force production during one and two-hand tasks. Hum Mov Sci. 2000a;19:897–924. [Google Scholar]
- Li S, Danion F, Latash ML, Li ZM, Zatsiorsky VM. Finger coordination in multi-finger force production tasks involving fingers of the right hand and/or fingers of the left hand. J Appl Biomech. 2000b;16:379–391. [Google Scholar]
- Li S, Latash ML, Yue GH, Siemionow V, Sahgal V. The effects of stroke and age on finger interaction in multi-finger force production tasks. Clin Neurophysiol. 2003;114:1646–1655. doi: 10.1016/s1388-2457(03)00164-0. [DOI] [PubMed] [Google Scholar]
- Liepert J, Storch P, Fritsch A, Weiller C. Motor cortex disinhibition in acute stroke. Clin Neurophysiol. 2000;111:671–676. doi: 10.1016/s1388-2457(99)00312-0. [DOI] [PubMed] [Google Scholar]
- Lodha N, Coombes SA, Cauraugh JH. Bimanual isometric force control: Asymmetry and coordination evidence post stroke. Clin Neurophysiol. 2012;123:787–795. doi: 10.1016/j.clinph.2011.08.014. [DOI] [PubMed] [Google Scholar]
- Lodha N, Naik SK, Coombes SA, Cauraugh JH. Force control and degree of motor impairments in chronic stroke. Clin Neurophysiol. 2010;121:1952–1961. doi: 10.1016/j.clinph.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Marshall RS, Perera GM, Lazar RM, Krakauer JW, Constantine RC, DeLaPaz RL. Evolution of Cortical Activation During Recovery From Corticospinal Tract Infarction. Stroke. 2000;31:656–661. doi: 10.1161/01.str.31.3.656. [DOI] [PubMed] [Google Scholar]
- McQuade K, Harris-Love ML, Whitall J. Maximal voluntary isometric elbow flexion force during unilateral versus bilateral contractions in individuals with chronic stroke. J Appl Biomech. 2008;24:69–74. doi: 10.1123/jab.24.1.69. [DOI] [PubMed] [Google Scholar]
- Owings TM, Grabiner MD. Normally aging older adults demonstrate the bilateral deficit during ramp and hold contractions. J Gerontol A Biol Sci Med Sci. 1998;53:B425–429. doi: 10.1093/gerona/53a.6.b425. [DOI] [PubMed] [Google Scholar]
- Perez MA, Cohen LG. The corticospinal system and transcranial magnetic stimulation in stroke. Top Stroke Rehabil. 2009;16:254–269. doi: 10.1310/tsr1604-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post M, van Duinen H, Steens A, Renken R, Kuipers B, Maurits N, Zijdewind I. Reduced cortical activity during maximal bilateral contractions of the index finger. NeuroImage. 2007;35:16. doi: 10.1016/j.neuroimage.2006.11.050. [DOI] [PubMed] [Google Scholar]
- Soteropoulos DS, Perez MA. Physiological changes underlying bilateral isometric arm voluntary contractions in healthy humans. J Neurophysiol. 2011;105:1594–1602. doi: 10.1152/jn.00678.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Delden AE, Peper CE, Beek PJ, Kwakkel G. Unilateral versus bilateral upper limb exercise therapy after stroke: a systematic review. J Rehabil Med. 2012;44:106–117. doi: 10.2340/16501977-0928. [DOI] [PubMed] [Google Scholar]
- Waller SM, Whitall J. Bilateral arm training: Why and who benefits? NeuroRehabilitation. 2008;23:29–41. [PMC free article] [PubMed] [Google Scholar]
- Whitall J, Waller SM, Sorkin JD, Forrester LW, Macko RF, Hanley DF, Goldberg AP, Luft A. Bilateral and unilateral arm training improve motor function through differing neuroplastic mechanisms: a single-blinded randomized controlled trial. Neurorehabil Neural Repair. 2011;25:118–129. doi: 10.1177/1545968310380685. [DOI] [PMC free article] [PubMed] [Google Scholar]