Abstract
Screening compounds for in vivo activity can be used as a first step to identify candidates that may be developed into pharmacological agents1,2. We developed a novel nanoinjection/electrophysiology assay that allows the detection of bioactive modulatory effects of compounds on the function of a neuronal circuit that mediates the escape response in Drosophila melanogaster3,4. Our in vivo assay, which uses the Drosophila Giant Fiber System (GFS, Figure 1) allows screening of different types of compounds, such as small molecules or peptides, and requires only minimal quantities to elicit an effect. In addition, the Drosophila GFS offers a large variety of potential molecular targets on neurons or muscles. The Giant Fibers (GFs) synapse electrically (Gap Junctions) as well as chemically (cholinergic) onto a Peripheral Synapsing Interneuron (PSI) and the Tergo Trochanteral Muscle neuron (TTMn)5. The PSI to DLMn (Dorsal Longitudinal Muscle neuron) connection is dependent on Dα7 nicotinic acetylcholine receptors (nAChRs)6. Finally, the neuromuscular junctions (NMJ) of the TTMn and the DLMn with the jump (TTM) and flight muscles (DLM) are glutamatergic7-12. Here, we demonstrate how to inject nanoliter quantities of a compound, while obtaining electrophysiological intracellular recordings from the Giant Fiber System13 and how to monitor the effects of the compound on the function of this circuit. We show specificity of the assay with methyllycaconitine citrate (MLA), a nAChR antagonist, which disrupts the PSI to DLMn connection but not the GF to TTMn connection or the function of the NMJ at the jump or flight muscles.
Before beginning this video it is critical that you carefully watch and become familiar with the JoVE video titled “Electrophysiological Recordings from the Giant Fiber Pathway of D. melanogaster” from Augustin et al7, as the video presented here is intended as an expansion to this existing technique. Here we use the electrophysiological recordings method and focus in detail only on the addition of the paired nanoinjections and monitoring technique.
Keywords: Drosophila melanogaster, Giant Fiber Circuit, screening, in vivo, nanoinjection, electrophysiology, modulatory compounds
Protocol Text
STEPS
1. Electrophysiology rig set-up
-
1.1
The required equipment for the electrophysiology rig set-up is described in detail by Augustin et al. in this Journal14. Please refer to this article for a detailed explanation of the electrophysiological apparatuses needed.
-
1.2
Modify the previously described electrophysiology rig set-up14 by adding a sixth micromanipulator, which holds the nanoinjector. For easy access to the head of the animal it should be placed between the two stimulating electrode micromanipulators as shown in Figure 2.
-
1.3
Before starting the experiment, ensure that you have a comfortable range on all axes of rotation with all micromanipulators, and that all electrodes as well as the injection micropipette (see step 2) can reach the animal.
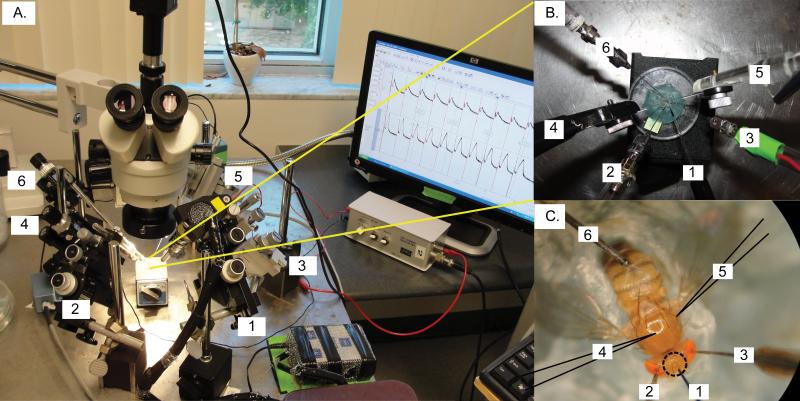
Figure 2. Micromanipulators set-up.
(A)A modified set-up of a previously published protocol14 is used to fit the injection micromanipulator for paired GFS recordings with simultaneous nanoinjections. The mounted fly preparation is oriented with the head of the fly towards the experimenter. The injection micromanipulator (#1) is placed in front of the experimenter between the two manipulators for the tungsten stimulating electrodes (2# and #3). The two micromanipulators for the glass recording electrodes (#4 and #5) are placed on the left and right side, respectively. The micromanipulator for the tungsten ground electrode (#6) is placed furthest in the back either on the left side (shown here) or on the right side.
(B) A close up view from the top of the arrangements of the various electrodes and injection micropipette.
(C) A properly mounted D. melanogaster impaled with electrodes and injection micropipette ready for injection. Notice that the animal's body is mounted with its thorax horizontally and its wings spread out. The wax is securely wrapped around its body preventing the animal from moving. Additionally, the ground electrode (#6, in the abdomen), the glass recording electrodes (#4 and #5, in the thorax, highlighted by dark outlines), and the stimulating electrodes (#2 and #3, one in each eye) are impaled in place, as previously described14. The injection micropipette (#1) is properly aligned with the center of the three ocelli (circled). Insertion of the injection micropipette should be placed in this area.
2. Nanoinjection set-up
-
2.1
The nanoinjection set-up requires a Nanoliter2000 (World Precision Instruments, Sarasota, FL, U.S.A.) or similar type of injector that allows controlled injections in nanoliter quantities.
-
2.2
Prepare injection needle using the glass micropipettes supplied with the injector by pulling them to a resistance of 80-100 MΩ with an electrode puller.
-
2.3
For smooth injections it is required to bevel the micropipettes to an 11-17 μm opening at a 45 degree angle (Figure 3).
-
2.4
Slowly backfill the injection micropipette with synthetic oil using a Hamilton syringe as instructed by the Nanoliter2000 manual, ensuring that no air bubbles are present.
-
2.5
Carefully secure the micropipette on the nanoinjector and prepare it to load the compound by emptying excess oil as instructed by the Nanoliter2000 manual.
-
2.6
Place the injector on the micromanipulator and load the compound as instructed in the Nanoliter2000 manual. Make sure that the tip of the micropipette does not break during this procedure.
-
2.7
Set the desired amount of nanoliters to be injected in the injector's control box as instructed by the Nanoliter2000 manual. Please note that the total amount injected should not exceed 100 nl. We found that larger quantities of saline control solutions may affect the function of the GFS circuit.
-
2.8
It is critical to have the injector unplugged from the control box during recording acquisition with the exception of the injection itself, since the power supply of the nanoinjector interferes with the recordings, which is visible as background noise (Figure 4). However, do not disconnect the power supply to the control box as it will re-set it.
Figure 3. Beveled injection micropipette.
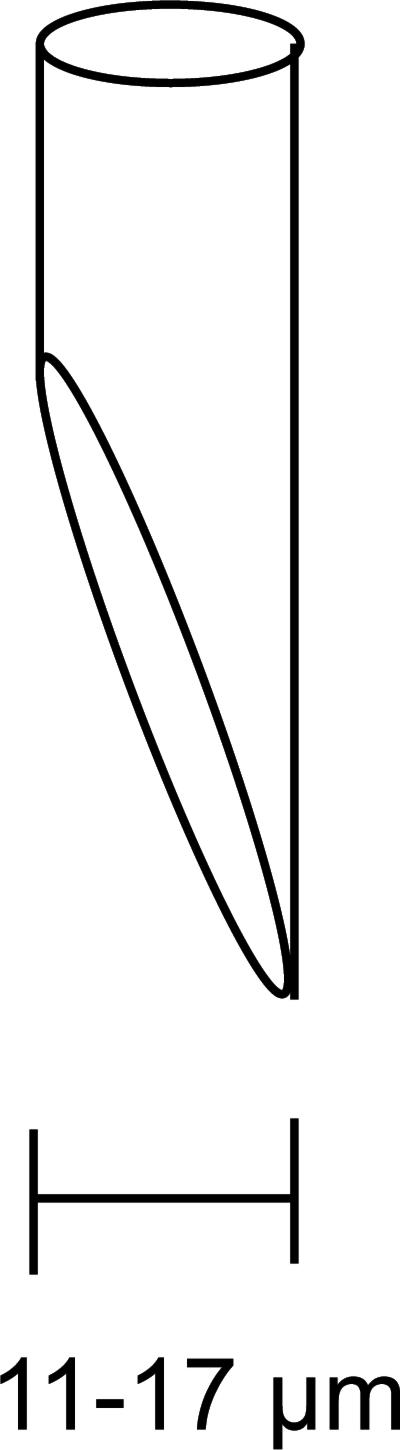
A diagram of a properly beveled micropipette is shown here. The electrode opening should be beveled at a 45 degree angle and have an opening between 11 to 17 μm. A proper beveled injection micropipette is crucial for a smooth injection with minimal damage to the fly.
Figure 4. Overall scheme of the nanoinjection/electrophysiology protocol.
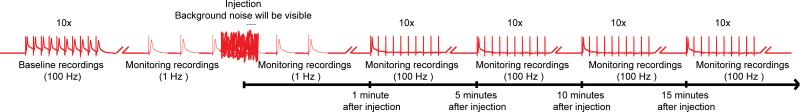
A representative diagram of the overall scheme for the nanoinjection/electrophysiology protocol. Start by obtaining a baseline recording by stimulating the Giant Fibers (GFs) at 100 Hz with 10 trains of 10 stimuli each (only one train shown here). Before injection, begin the 1 Hz stimulations one second apart. During injection time (while injector is plugged in to the control box), you will observe significant background noise; however, do not discontinue the recordings. After injection (and injector is unplugged from control box), continue the 1 Hz stimulation for about 1 more minute. Finally, proceed to stress the GFs with 10 trains of 10 stimuli at 100 Hz and continue to test the function of the GF pathways with this paradigm every 5 minutes up to 15 minutes. Note: recordings were manipulated to create the overall scheme and do not represent a specific result obtained. Not to scale, not all traces are shown.
3. Drosophila melanogaster preparation
-
3.1
Anesthetize 2 to 6 day old flies with CO2 or on ice as previously described14,15.
-
3.2
Once immobile, use a pair of tweezers to transfer the animal to a small plate with soft dental wax by picking it up from its legs. Please note that a male fruit fly weighs about 1.0 mg and a female fruit fly weighs about 1.2 mg, thus compound-to-body weight ratio is different in male versus female flies. Therefore, it is recommended to use only one gender for the experiments.
-
3.3
As previously described14,15, carefully mount the fly dorsal side up and ensure that the thorax and head are immobilized with soft dental wax placed around the body. Carefully spread the wings out so that they lay perpendicular to the thorax (Figure 2, C). The fly should be mounted with as little damage as possible.
4. Paired nanoinjection/electrophysiology
-
4.1
Place the mounted fly on the electrophysiology rig with its head towards the stimulating electrodes.
-
4.2
Impale the animal with the corresponding stimulating, ground and recording electrodes as previously described14,15 (Figure 2, C). Unless otherwise desired, place one recording electrode into the DLM and the other into the TTM muscle. The DLM muscle is located in the thorax between the anterior Dorso-Central hairs and the midline of the fly. The TTM muscle is located near the wing attachments, between the posterior and anterior Supra-Alar hairs of the fly7.
-
4.3
Align the injection micropipette containing the compound with the center of the three ocelli situated on the medial posterior portion of the head but do not inject yet (Figure 2, C).
-
4.4
Before compound injection obtain a baseline recording of the GF to TTM and GF to DLM pathways of the Giant Fiber System (GFS, Figure 1) via brain stimulation. To do so, activate the Giant Fibers (GFs) with 10 trains of 10 stimuli (40-50 mV) each given for a duration of 0.03 ms at 100 Hz with a 1 second delay between the trains14,15 (Figure 4). Wild type flies should be able to follow one-to-one at this rate of stimulation for both DLM and TTM pathways. Discard the fly if the GF to DLM and GF to TTM pathways do not follow the 100 Hz stimulation at a 1:1 ratio.
-
4.5
Switch to continuous stimulation of the GF with single pulses at 1 Hz (Figure 4).
-
4.6
Quickly plug the injector into the control box. Even though background noise will interfere with the 1 Hz stimulation recordings, do not discontinue it.
-
4.7
Carefully insert the injection micropipette into the head capsule of the fly just below the cuticule, and inject the desired amount of compound into the hemolymph of the fly while maintaining the 1 Hz stimulation (Figure 4). Due to the open circulatory system of the fly, the entire nervous system will be exposed to the compound within seconds. Though a particular injection site is not crictial to deliver compounds into the hemolymph, we find the region of the ocelli, which are localized medial at the most dorsal side of the head capsule, to be a convenient site that allows for an easy injection that leads to rapid and even distribution of the compound.
-
4.8
Immediately remove injection micropipette from site of injection and unplug the injector from the control box while continuing the 1 Hz GF stimulation up to 1 min after injection (Figure 4).
-
4.9
In order to reveal more subtle effects of compounds on the GFS, stress the Giant Fibers (GFs) with 10 trains of 10 stimuli at 100 Hz with a 1 second delay between the trains. Continue to test the function of the GF pathways with this paradigm every 5 minutes up to 15 minutes (Figure 4). However, shorter intervals or longer monitoring periods are also possible.
-
4.10
In order to test whether the compound has an effect at the neuromuscular junctions (NMJs) of the GFS, and possibly narrow down the effects of the compound, proceed to activate the motor neurons directly by thoracic stimulation. For this, remove the stimulating electrodes from the eyes and replace them on the anterior sides of the thorax in order to stimulate the motor neurons with 10 trains of 10 stimuli at 100 Hz.
Figure 1. The Giant Fiber System.
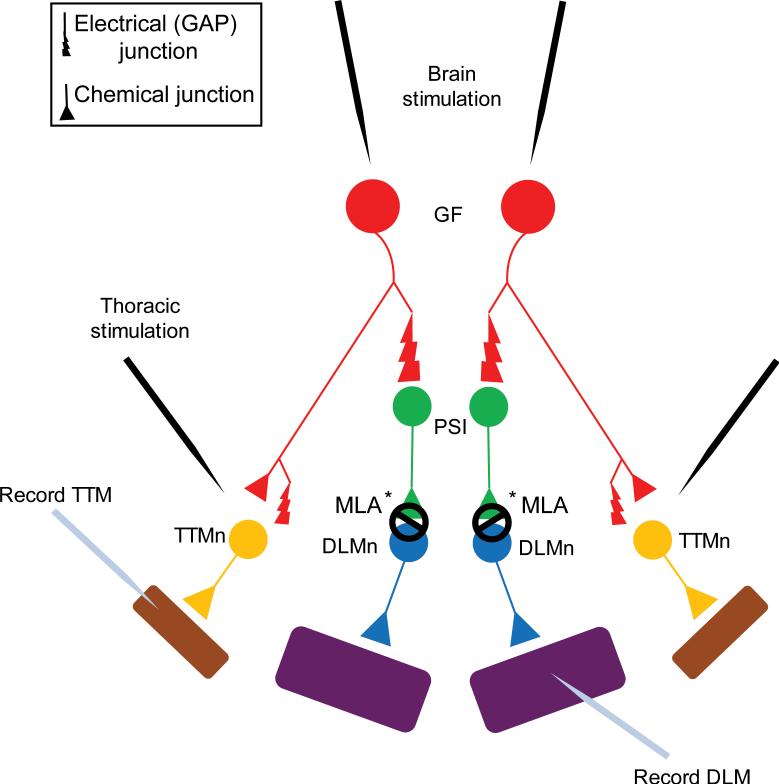
Diagram of the Giant Fiber System (GFS). The Giant Fibers (GFs, shown in red) synapse electrically (Gap Junctions) as well as chemically (cholinergic) onto a Peripheral Synapsing Interneuron (PSI, shown in green) and the Tergo Trochanteral Muscle neuron (TTMn, shown in yellow)5. The PSI to DLMn (Dorsal Longitudinal Muscle neuron, shown in blue) connection is dependent on Dα7 nAChR subtype6. Finally the neuromuscular junction (NMJ) of the TTMn and the DLMn onto the jump (TTM, shown in purple) and flight muscles (DLM, shown in purple) is glutamatergic.
Note: The GF to PSI connection is both electrical and chemical. However, in shakB mutants (which lack gap junctions), no response can be recorded from the DLM upon stimulation of the GFs in the brain, demonstrating that the chemical component in the absence of electrical connections is not sufficient to evoke an action potential in the PSI5,16-18. Because the GF to PSI connectionis gap junction dependent, this figure shows only the GAP junction at the synapse for simplicity reasons.
Note: The electrophysiology traces shown in the video do not correspond to the effects of pure dye injection.
Representative Results
Effect of an antagonist on the PSI to DLM synapse of the Giant Fiber System
Methyllycaconitine citrate (MLA) is a nAChR antagonist that is specific for α7 nAChR subtypes. The PSI to DLMn synapse in the GF-DLM pathway is dependent on the Dα7 nAChR subtype for proper function, while genetic removal of Dα7 nAChR subtype has no effect on the GF-TTM pathway5,6. In order to demonstrate the specificity and sensitivity of our assay we injected MLA at different concentrations (0, 0.02, 0.04, 0.08, 0.12 ng/mg, 46 nl injected) into the head of the animal (n=10 per compound treatment; n=15 for saline treatment). Only male flies (of the wild type genotype wild 10E) were used, and the effect of the compound was monitored for a total of 15 minutes after injection.
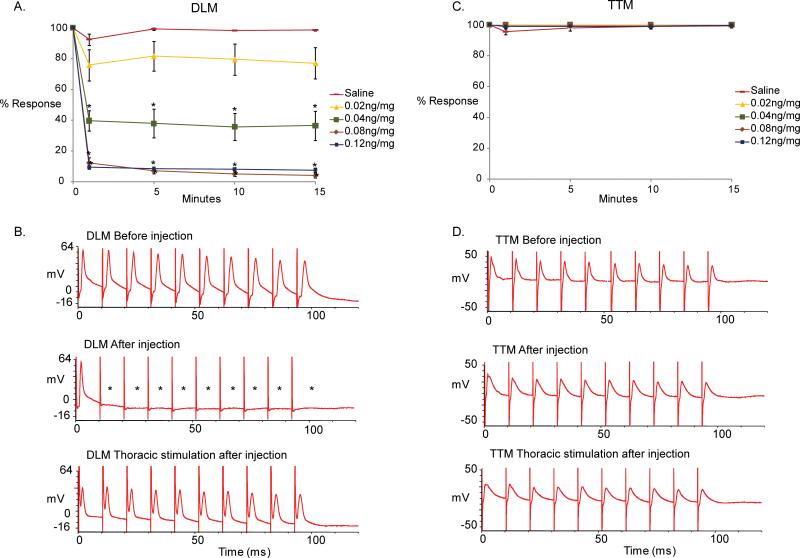
Figure 5 depicts the difference between baseline recordings obtained before injection and those obtained after injection in response to MLA and saline control solution. We found that injection of MLA resulted in the inability of the GF-DLM pathway to follow one-to-one at 100 Hz by stimulations of the GFs in the brain while the GF-TTM pathway remained unaffected. (Figure 5, Top and middle trace, t-test performed between saline controls [0 ng/mg] and the different concentrations of MLA at each time point unless the data is non-parametric [normality and equal variances tested], otherwise we use a Mann-Whitney Rank Sum Test. *p<0.001). However, a one-to-one response of the DLM was observed when the motor neurons were stimulated directly (Figure 5, bottom trace), demonstrating that the NMJ function of the DLM and TTM is not affected by MLA. MLA appeared to reach its maximum effect 1 minute after injection for 0.04, 0.08 and 0.12 ng/mg of MLA injected, as no further significant changes were noted during the following 15 minutes of testing period. Moreover, the compound reached a maximum effect at 0.08 ng/mg since stronger responses were not observed with the higher dosage of 0.12 ng/mg
Figure 5. The effects of MLA in the GFS.
(A)A graphical depiction of the effects of the α7 nAChR antagonist Methyllycaconitine citrate (MLA) on the GF-DLM pathway of the fruit fly at different concentrations (0, 0.02, 0.04, 0.08, 0.12 ng/mg. n=10 per compound treatment; n=15 for saline treatment). Only one minute after injection, a significant and immediate effect was seen with 0.04 ng/mg of MLA. A significant effect was also seen with 0.8ng/mg and 0.12ng/mg of MLA at 100Hz stimulation of the GFs. No significant difference was noted between saline controls and 0.02 ng/mg of MLA. Additionally, no change in effect was seen after 1 minute post injection during the time tested (15 minutes). A t-test was performed between saline controls (0 ng/mg) and the different concentrations of MLA at each time point. Levels are reported as mean +/− SEM, *p < 0.001.
(B) Sample traces of the DLM responses at 100 Hz stimulation. Top trace shows the responses of the muscle before MLA injection upon GF stimulation in the brain. Notice that the muscle is able to respond to each stimulus one-to-one at 100 Hz. Middle trace displays the responses of the DLM after MLA injection (0.12 ng/mg). Notice that the muscle is not able to respond to each stimulus one-to-one at 100 Hz. (asterisks). Bottom trace shows the responses of the DLM of the same preparation (0.12 ng/mg) upon direct stimulation of the motor neurons in the thorax. Because DLM responds one-to-one at 100 Hz with thoracic stimulation, the failure of responses with brain stimulations can be attributed to the cholinergic PSI-DLMn connection.
(C) A graphical depiction of the effects with different MLA concentrations (0, 0.02, 0.04, 0.08, 0.12 ng/mg) on the GF-TTM pathway. No significant effects were seen between saline (0 ng/mg) and compound injections at any time point. A t-test was performed between saline controls (0 ng/mg) and the different concentrations of MLA at each time point, *p < 0.001.
(D)Sample traces of the TTM responses at 100 Hz stimulation. Top trace shows the responses of the muscle before MLA injection upon GF activation with stimulation in the brain. Notice that the muscle is able to respond to all stimuli at 100 Hz. Middle trace shows the responses of the DLM after MLA injection (0.12 ng/mg). Responses from the TTM muscle to stimulation of the GF in the brain remain one-to-one. Bottom trace shows the responses of the TTM of the same preparation to 100 Hz stimulation of the motor neurons in the thorax (0.12 ng/mg).
Discussion
The nanoinjection/electrophysiology bioassay presented here allows for a rapid screening of compounds in the nervous system of the fruit fly. This is a novel in vivo technique that requires small quantities of a compound to elicit an effect on a variety of molecular targets in a well-characterized neuronal circuit. This method can be used to test the bioactivity of different compounds, from unknown toxins to commercially available pharmacological agents.
Here we demonstrated the function of our assay using MLA, which had an effect on the Giant Fiber System (GFS) of the fruit fly (Figure 5). We found that it selectively disrupted the GF to DLM pathway but not the GF to TTM pathway. Activating the motor neurons directly via thoracic stimulation demonstrated that the defect in the GF to DLM pathway was not due to a dysfunction at the neuromuscular junction (NMJ) but it was consistent with the antagonistic effect of MLA at the Dα7 nAChR subtypes present at the PSI-DLMn synapse6 (Figure 1). Although the GF to TTMn connection was shown to be cholinergic, it is unknown whether Dα7 nAChR subunits are present at this synapse. Furthermore, the genetic absence of the choline acetyltransferase (Cha) gene or the Dα7 nAChR subtype (Dα7) gene does not disrupt the function of the GF-TTMn connection because of the concurrent presence of an electrical junction5,6,17,19,20, which makes the pathway unlikely to be affected by MLA.
After compound injection, the solution should immediately immerse the entire nervous system of the animal due to its open circulatory system21. If properly injected, the compound usually reaches the thorax and abdomen within seconds, but a homogenous dispersion can take up to a minute. However, if the compound is not injected properly into the hemolymph (i.e. injecting the micropipette too deep going into the brain tissue) then slower dispersion throughout the animal is observed. While dye may be used to practice a proper injection technique as shown in the video, it is not recommended to co-inject the blue food coloring with a compound to be tested as it may alter the properties of the compound and thus its bioactivity. Additionally, since most solutions used as solvent are clear in color (saline, DMSO, etc), it is difficult to see whether or not the compound was ejected from the injection needle. Therefore, when dissolving a specific compound it is important to ensure that it goes completely into solution; otherwise undissolved particles will rapidly clog the injection tip, preventing from any ejection of fluid. Furthermore, although compound dispersion may be immediate throughout the hemolymph, reaching the targets in the central nervous system, as well as reaching its maximum dosage, may take longer based on the compound's chemical properties, such as size and polarity, and its ability to permeate the fly's blood brain barrier.22 Thus, it is important to monitor potential effects of unknown compounds several minutes after injection because different compounds can have variation in times of onset effects, which may increase over time in some cases. Strong and immediate effects of the compound that completely block the function of neurons can already be seen with the triggered responses at 1 Hz, while stimulation of the GFS at higher frequencies (100 Hz) is used to detect more subtle effects due to lower dosage or potency of a compound. If no effects are observed after compound injection it can be due to either small drug dosage or the fact that the compound's specific molecular target is not present in the GFS.
Moreover, when using the bioassay presented here as a screening tool for novel compounds (such as conotoxins) it is important to note that the assay is restricted to the molecular targets found in the GFS of the fly. Although the assay itself does not permit locating the actual molecular targets of the compound injected, it does allow for the narrowing down of potential targets within the GFS. Additional tests, such as patch clamp on neurons or muscles or genetic interaction studies with Drosophila melanogaster mutants, can be done to determine the specific target of these compounds. Finally, the presented recording protocol was designed to detect antagonistic effects on the function of the GFS. However, the recording protocol may be easily adjusted to monitor for agonistic effects by passively monitoring for responses induced by the compound rather than testing if the GFS is not able to respond reliably when the circuit is stimulated by the experimenter.
Table of specific reagents and equipment:
| Name of the reagent | Company | Catalogue number | Comments (optional) |
|---|---|---|---|
| Recording glass electrodes: borosilicate glass capillaries | World Precision Instruments | 1B100F-4 | 1.0mm OD, 0.58mm ID |
| Stimulator | Grass Instruments | Model S48 | |
| Amplifier | Getting Instruments Inc. | Model 5A | |
| Data acquisition Software: Digidata | Molecular Devices® | Model 1440A | |
| Data collection software: pCLAMP | Molecular Devices® | Version 10 | |
| Stereomicroscope with fiber optic microscope ring illuminator | AmScope | SM-4T Model HL250-AR | |
| Dissecting scope for mounting | AmScope | SM-2TZ | |
| Kite Manual Micromanipulator & Tilting Base | World Precision Instruments | Model # M3301 Kite: Model # KITE-M3-L |
|
| Drosophila melanogaster Wild 10E genotype (wild type strain) | Bloomington Stock center | Stock # 3892 | |
| Vertical pipette puller | David Kopf Instruments | Model 700c | |
| Injection glass micropipettes: Borosilicate glass capillaries | World Precision Instruments | Catalogue # 4878 | 1.14mm OD, 0.5mm ID |
| Silicon oil | Fisher | Catalogue # S159-500 | |
| Beveler | Sutter Instrument Co. | K.T. Brown Type Model # BV-10 |
|
| Nanoliter2000 | World Precision Instruments | Catalogue # B203XVY | |
| Blue food coloring | McCormick® | N/A | Ingredients: Water, Propylene Glycol, FD&C Blue 1, and 0.1% Propylparaben (preservative). |
| Methyllycaconitine citrate (MLA) | Tocris Bioscience | Catalogue # 1029 | |
| Plastic wax sticks | Hygenic Corporation (Akron Ohio USA) |
Acknowledgments
We would like to acknowledge the members of the Mari lab and the Godenschwege lab, in particular Aline Yonezawa, for comments and help with this protocol. This work was funded by the National Institute for Neurological Disorders and Stroke grant R21NS06637 to F.M. and T.A.G.; A.B. was funded by the National Science Foundation award number 082925, URM: Integrative Biology for future researchers.
Footnotes
Disclosures:
I have nothing to disclose.
Contributor Information
Monica Mejia, Department of Biological Sciences. Florida Atlantic University. 777 Glades Road, Boca Raton, Fl 33431, USA. mmejia8@fau.edu.
Mari D. Heghinian, Department of Chemistry & Biochemistry. Florida Atlantic University. 777 Glades Road, Boca Raton, Fl 33431, USA. mheghini@fau.edu
Alexandra Busch, Department of Biological Sciences. Florida Atlantic University. 777 Glades Road, Boca Raton, Fl 33431, USA. abusch@fau.edu.
Frank Marí, Department of Chemistry & Biochemistry. Florida Atlantic University. 777 Glades Road, Boca Raton, Fl 33431, USA. mari@fau.edu.
Tanja A. Godenschwege, Department of Biological Sciences. Florida Atlantic University. 777 Glades Road, Boca Raton, Fl 33431, USA.
References
- 1.Koehn FE, Carter GT. The evolving role of natural products in drug discovery. Nat Rev Drug Discov. 2005;4:206–220. doi: 10.1038/nrd1657. [DOI] [PubMed] [Google Scholar]
- 2.Miljanich GP. Ziconotide: neuronal calcium channel blocker for treating severe chronic pain. Curr Med Chem. 2004;11:3029–3040. doi: 10.2174/0929867043363884. [DOI] [PubMed] [Google Scholar]
- 3.Layer RT, Wagstaff JD, White HS. Conantokins: peptide antagonists of NMDA receptors. Curr Med Chem. 2004;11:3073–3084. doi: 10.2174/0929867043363901. [DOI] [PubMed] [Google Scholar]
- 4.Lewis RJ. Conotoxins as selective inhibitors of neuronal ion channels, receptors and transporters. IUBMB Life. 2004;56:89–93. doi: 10.1080/15216540410001668055. [DOI] [PubMed] [Google Scholar]
- 5.Allen MJ, Godenschwege TA, Tanouye MA, Phelan P. Making an escape: development and function of the Drosophila giant fibre system. Semin Cell Dev Biol. 2006;17:31–41. doi: 10.1016/j.semcdb.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Fayyazuddin A, Zaheer MA, Hiesinger PR, Bellen HJ. The nicotinic acetylcholine receptor Dalpha7 is required for an escape behavior in Drosophila. PLoS biology. 2006;4:e63. doi: 10.1371/journal.pbio.0040063. doi:10.1371/journal.pbio.0040063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jan LY, Jan YN. L-glutamate as an excitatory transmitter at the Drosophila larval neuromuscular junction. The Journal of physiology. 1976;262:215–236. doi: 10.1113/jphysiol.1976.sp011593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Usherwood PN, Machili P, Leaf G. L-Glutamate at insect excitatory nerve-muscle synapses. Nature. 1968;219:1169–1172. doi: 10.1038/2191169a0. [DOI] [PubMed] [Google Scholar]
- 9.Marrus SB, Portman SL, Allen MJ, Moffat KG, DiAntonio A. Differential localization of glutamate receptor subunits at the Drosophila neuromuscular junction. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:1406–1415. doi: 10.1523/JNEUROSCI.1575-03.2004. doi:10.1523/JNEUROSCI.1575-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen SA, Fetter RD, Noordermeer JN, Goodman CS, DiAntonio A. Genetic analysis of glutamate receptors in Drosophila reveals a retrograde signal regulating presynaptic transmitter release. Neuron. 1997;19:1237–1248. doi: 10.1016/s0896-6273(00)80415-8. [DOI] [PubMed] [Google Scholar]
- 11.Qin G, et al. Four different subunits are essential for expressing the synaptic glutamate receptor at neuromuscular junctions of Drosophila. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:3209–3218. doi: 10.1523/JNEUROSCI.4194-04.2005. doi:10.1523/JNEUROSCI.4194-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuster CM, et al. Molecular cloning of an invertebrate glutamate receptor subunit expressed in Drosophila muscle. Science. 1991;254:112–114. doi: 10.1126/science.1681587. [DOI] [PubMed] [Google Scholar]
- 13.Tanouye MA, Wyman RJ. Motor outputs of giant nerve fiber in Drosophila. Journal of neurophysiology. 1980;44:405–421. doi: 10.1152/jn.1980.44.2.405. [DOI] [PubMed] [Google Scholar]
- 14.Augustin H, Allen MJ, Partridge L. Electrophysiological Recordings from the Giant Fiber Pathway of <em>D. melanogaster</em>. J Vis Exp. 2011 doi: 10.3791/2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen MJ, Godenschwege T. In: Drosophila Neurobiology. Zhang B, Freeman MR, Waddell S, editors. Cold Spring Harbor Laboratory Press; 2010. pp. 215–224. [Google Scholar]
- 16.Blagburn JM, Alexopoulos H, Davies JA, Bacon JP. Null mutation in shaking-B eliminates electrical, but not chemical, synapses in the Drosophila giant fiber system: a structural study. J Comp Neurol. 1999;404:449–458. [PubMed] [Google Scholar]
- 17.Thomas JB, Wyman RJ. Mutations altering synaptic connectivity between identified neurons in Drosophila. J Neurosci. 1984;4:530–538. doi: 10.1523/JNEUROSCI.04-02-00530.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baird DH, Schalet AP, Wyman RJ. The Passover locus in Drosophila melanogaster: complex complementation and different effects on the giant fiber neural pathway. Genetics. 1990;126:1045–1059. doi: 10.1093/genetics/126.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorczyca M, Hall JC. Identification of a cholinergic synapse in the giant fiber pathway of Drosophila using conditional mutations of acetylcholine synthesis. J Neurogenet. 1984;1:289–313. doi: 10.3109/01677068409107093. [DOI] [PubMed] [Google Scholar]
- 20.Allen MJ, Murphey RK. The chemical component of the mixed GF-TTMn synapse in Drosophila melanogaster uses acetylcholine as its neurotransmitter. The European journal of neuroscience. 2007;26:439–445. doi: 10.1111/j.1460-9568.2007.05686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mejia M, et al. A novel approach for in vivo screening of toxins using the Drosophila Giant Fiber circuit. Toxicon. 2010;56:1398–1407. doi: 10.1016/j.toxicon.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stork T, et al. Organization and function of the blood-brain barrier in Drosophila. J Neurosci. 2008;28:587–597. doi: 10.1523/JNEUROSCI.4367-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]