Abstract
A salient visual stimulus can be rendered invisible by presenting it to one eye while flashing a mask to the other eye. This procedure, called continuous flash suppression (CFS), has been proposed as an ideal way of studying awareness as it can make a stimulus imperceptible for extended periods of time. Previous studies have reported robust suppression of cortical activity in higher visual areas during CFS, but the role of primary visual cortex (V1) is still controversial. In this study, we resolve this controversy on the role of V1 in CFS and also begin characterizing the computational processes underlying CFS. Early visual cortical activity was measured with functional magnetic resonance imaging while human subjects viewed stimuli composed of target and mask, presented to the same or different eyes. Functional MRI responses in early visual cortex were smaller when target and mask were in different eyes compared to the same eye, not only for the lowest contrast target rendered invisible by CFS, but also for higher contrast targets which were visible even when presented to the eye opposite the mask. We infer that CFS is based on modulating the gain of neural responses, akin to reducing target contrast.
Introduction
One way of studying the neural basis of visual awareness is by comparing activity evoked by a visible image with that evoked by a similar image that is rendered invisible. Changing the physical properties of a stimulus (e.g., lowering contrast) is a trivial way of rendering it unperceivable, but such changes would make the visible and invisible categories incomparable. Another idea for making a stimulus unperceivable while keeping its physical properties intact is to change the context instead of changing the stimulus itself. Continuous flash suppression (CFS) has been proposed to do just that (Tsuchiya and Koch, 2005). CFS is based on binocular flash suppression (Wolfe, 1984) and binocular rivalry (Wheatstone, 1838). By presenting a flashing mask to one eye, a low-contrast target presented to the other eye can be rendered invisible for longer than with similar methods (such as standard binocular rivalry). Because this compelling perceptual phenomenon is achieved without changing the target, CFS has been used to study visual awareness using behavioral measurements (Jiang et al., 2006; Yang et al., 2007), functional magnetic resonance imaging (fMRI), and electrophysiology (Sterzer et al., 2009; Kang et al., 2011). A similar phenomenon called generalized flash suppression (GFS) has been used in monkey electrophysiology and fMRI studies (Wilke et al., 2003; Maier et al., 2008).
Previous studies reported robust suppression of activity in higher visual areas during CFS (Fang and He, 2005; Jiang and He, 2006; Hesselmann and Malach, 2011), but the role of primary visual cortex (V1) is controversial. One study reported no evidence for differences (a null result) in human V1 fMRI responses between visible and invisible stimuli (Watanabe et al., 2011), but another study found a difference in monkey V1 fMRI responses (Maier et al., 2008). Possible explanations for this discrepancy are: 1) lack of statistical power underlying the null result, 2) different species, 3) different stimulus configurations, and 4) an attention confound, an attention confound, that the positive result in the monkey study “because attention was not thoroughly controlled” (Watanabe et al., 2011).
Here, we resolve this controversy on the role of V1 in CFS, and offer an explanation of the computational processes underlying CFS. We measured activity in human V1 using fMRI while subjects viewed CFS stimuli composed of a target and mask, presented to the same eye (visible) or to different eyes (invisible). Subjects performed a task that diverted attention away from the stimuli. Each subject participated in multiple fMRI scanning sessions to ensure sufficient statistical power. The amplitudes of fMRI responses in V1 were smaller when target and mask were in different eyes, compared to the same eye. Our results suggest that CFS is a form of perceptual masking, for which the presence of a mask suppresses activity evoked by the target (Legge and Foley, 1980; Carandini and Heeger, 2012), and that the suppression is stronger when mask and target are presented to different eyes. We propose that CFS impacts awareness by modulating the gain of neural responses to the target, at an early stage of visual processing, akin to reducing target contrast.
Materials and Methods
Subjects and experimental sessions
Data were acquired from four healthy subjects with normal or corrected-to-normal vision (2 females, age range, 21–42). One subject was an author (S4). Experiments were conducted with the written consent of each subject and in accordance with the safety guidelines of fMRI research, as approved by the University Committee on Activities Involving Human Subjects at New York University. Each subject participated in five MRI scanning sessions: one session to obtain a high-resolution anatomical volume, one session for standard retinotopic mapping (Engel et al., 1994; Larsson and Heeger, 2006; Wandell et al., 2007), and three sessions of the main experiment. Each subject participated in a behavioral pilot experiment to select the target stimulus contrasts, in the MRI scanner. In addition, subjects S2–S4 participated in a behavioral control experiment, including 2–3 sessions outside the MRI scanner.
Stimuli
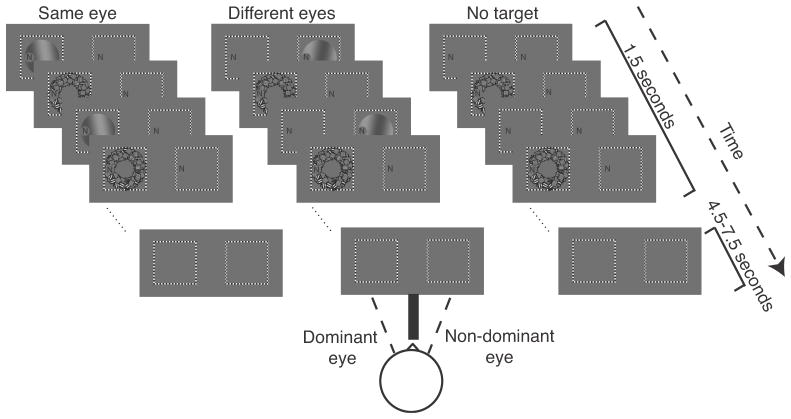
A mask was presented to one eye and a target to either the same eye as the mask (“same eye”) or to the other eye (“different eyes”). The target and mask configuration was nearly identical to that used in a previous fMRI study of CFS that reported no evidence for a difference in V1 activity between same eye (visible) and different eyes (invisible) (Watanabe et al., 2011), and was further optimized to maximize suppression (see Results and Figure 1). There were two differences between the stimuli in previous study and the current study. 1) We used a lower target spatial frequency and slower motion (0.2 cpd and 1 Hz vs. 1.5 cpd and 5 Hz) because with these parameters the target was suppressed more efficiently in our hands. 2) The stimulus aperture was smaller in the current experiment (2.9º vs 5º radius) because the display size was limited by our binocular setup. The mask was composed of 200 moving, square-wave grating patches (3, 7, 11 or 15 cpd; 1 Hz), placed at random positions and orientations within an annulus (inner radius 2°, outer radius 3°), centered 1.5° to the right of fixation (Figure 1). The locations and orientations of the component gratings were selected randomly and updated at 12 Hz. The target was a moving, sinusoidal grating (0.2 cpd; 1 Hz), slightly tilted from vertical (10°, either clockwise or counter clockwise), in a circular aperture (radius 2.9°), centered with the mask (Figure 1). The target had one of four contrast levels: no target (0 contrast), low contrast (visible for same eye but invisible for different eyes), medium contrast (2 times the low contrast, and visible for both same and different eyes) or high contrast (4 times the low contrast and visible). The low contrast was determined with a pretest to measure visibility threshold (see details below and Table 1). The target and mask were presented in alternating screen refreshes, creating a perception of target blended within the mask (in conditions when the target was visible). In addition, a stream of letters (size ~0.25°) was presented at fixation and refreshed at a rate of 5 Hz. Stimuli were generated using Matlab (MathWorks) and MGL (available at http://justingardner.net/mgl) on a Macintosh computer. Stimuli were displayed in the MRI scanner with an LCD projector (Eiki LC-XG250, 60Hz refresh rate) onto a back-projection screen in the bore of the magnet. Subjects were supine and viewed the projected stimuli through converging prisms and an angled mirror.
Figure 1.
Stimuli and experimental protocol. CFS stimuli were presented for 1.5 sec, followed by an intertrial interval (4.5–7.5 sec). Left, target and mask presented to the same eye. Middle, target and mask presented to different eyes. Right, mask only (no target). Stimuli were presented to the two eyes on a split screen. Binocular fusion was achieved with the use of converging prisms. The checkerboard-textured frames and the stream of letters were identical in the two eyes, assisting fusion. The resulting fused precept was of a centered single frame with a single letter stream to the left of the frame’s center. Subjects were instructed to fixate the letters. The letters in the figure are presented at a larger scale than the rest of the figure to enhance their visibility.
Table 1.
Behavioral performance in psychophysical pretests
| Subject | Target contrast (%) | Detection (d’): different eyes | Orientation discrimination (% correct): different eyes | Orientation discrimination (% correct): same eye |
|---|---|---|---|---|
| S1 | 12 | 0.12 (p=0.36) | 50 (p=0.56) | 66.67 (p=0.01) |
| S2 | 12 | −0.35 (p=0.93) | 42 (p=0.9) | 69.39 (p=0.004) |
| S3 | 15 | −0.18 (p=0.82) | 61.22 (p=0.08) | 78 (p<0.0001) |
| S4 | 20 | −0.13 (p=0.75) | 59.26 (p=0.24) | 85.18 (p<0.0001) |
| mean ± SEM | 14.75 ± 1.89 | −0.13 ± 0.1 | 53.12 ± 4.44 | 74.81 ± 4.21 |
Each row corresponds to a different subject. Bottom row, mean and SEM across subjects. Columns (left to right): target contrasts; close-to-zero d’ for target and mask presented to different eyes; close-to-chance orientation-discrimination performance for target and mask presented to different eyes; well-above-chance orientation-discrimination performance for target and mask presented to the same eye.
Binocular Fusion
Stimuli were presented to the two eyes on a split screen, in two checkerboard-textured frames 6° to the left and right of center (Figure 1). A septum (black, cardboard divider) ensured that the right image was visible exclusively to the right eye and the left image to the left eye. Binocular fusion was achieved with the aid of converging (prism) lenses, placed in an MRI-compatible glasses frame (Schurger, 2009). The checkerboard-textured frames and the stream of letters were identical in the two eyes, assisting fusion. The resulting fused precept was of a centered single frame with a single letter stream 2.5° to the left of the frame’s center. Subjects were instructed to fixate the letters.
Main experiment (fMRI)
Each session included at least ten 5 minute runs, each including 48–49 trials. The mask was presented in all trials to one eye, the eye for which suppression was found to be strongest (as determined for each subject individually, see below), and the experimental conditions differed in the presentation of the target. The target was either absent (“mask only”) or presented with either low, medium or high contrasts, in equal probabilities. The target was presented to the same eye as the mask (“same eye” condition) for half the trials, and to the other eye (“different eyes”) for the other half. This resulted in a total of 7 trial types: mask-only, low-same, low-different, medium-same, medium-different, high-same and high-different. These 7 trial types were presented in random order. For 3 out of the 4 subjects (S2–S4) there were approximately equal numbers of trials for each of the 7 trial types (189–344 per trial type with an average of 258). Subject S1 participated in an earlier version of the experiment which was identical to the final version in every respect except that the 7 stimulus conditions did not have equal probabilities of being presented on each trial. This subject performed fewer high-contrast trials (same eye: 157; different eyes: 159) and fewer medium-contrast trials (same eye: 36; different eyes: 35) than the rest of the subjects. Each trial was 1.5 s in duration. Each inter-trial interval (ITI) was chosen randomly, with equal probabilities, to be either 4.5, 6 or 7.5 seconds.
fMRI responses in visual cortex are strongly influenced by fluctuations in attention, blinks, and eye movements, which in turn can be influenced by task demands and perceptual decisions (Gandhi et al., 1999; Tse et al., 2010; Hupé et al., 2012). In addition, interocular conflict has been shown to attract attention (Paffen et al., 2012). To emphasize the stimulus-evoked visual responses, subjects performed a task during the main experiment that controlled and diverted the focus of attention away from the CFS targets (see control experiment below). Specifically, subjects performed a continuous detection task on the letter stream throughout each run. The task was to indicate, by means of a button press, whether the current letter was an ‘X’. To help ensure that blinks were not synchronized with target grating visibility, subjects were also instructed to blink only when pressing the button in response to the letter stream.
Pretest to determine threshold
When using CFS to study unawareness it is crucial to measure the visibility of the target. We determined each subject’s visibility threshold with a behavioral pretest, including catch trials with no target. This pretest was conducted prior to the main experiment, while subjects were lying in the scanner. However, subjects did not perform this task while acquiring fMRI measurements during the main experiment; instead, they performed a continuous detection task (see above).
The pretest consisted of two stages. The purpose of the first stage was to choose the highest contrast that would be invisible when mask and target were presented to different eyes (under CFS). The purpose of the second stage was to confirm that this contrast was visible when mask and target were presented to the same eye. The display was identical to the main experiment except that a fixation cross replaced the letter stream and the ITI was fixed at 0.5 seconds. Stage 1 started with a contrast that was robustly visible in all subjects (30%), gradually decreasing until the subject reported that he/she could no longer see the stimulus. Following this the subject performed a stimulus detection task for 200 trials, 50% mask-only trials and 50% with mask and target in different eyes, randomly interleaved. Subjects were instructed to press 1 of 2 buttons to indicate if the target was present. Subjects were informed that 50% of the trials include a target, encouraging them to adopt a balanced criterion. Detection performance was quantified by measuring d’ from the hit rate and false-alarm rate. If the resulting d’ was reliably greater than 0, then the procedure was repeated with another 100 trials at a lower contrast. This procedure was repeated until d’ was statistically indistinguishable from, or less than, 0. This test was performed twice, with the mask presented to the left and to the right eye. The eye which provided the higher contrast threshold was chosen as the “mask eye” for the main experiment. The purpose of the second stage was to measure orientation-discrimination performance with target and mask in the same eye or in different eyes, using the contrast level and the mask eye determined in stage 1. Targets were tilted 10° from vertical, either clockwise or counterclockwise. Same-eye and different-eyes trials, and clockwise and counterclockwise tilts, were presented in random order with equal probabilities. Subjects were instructed to press 1 of 2 buttons to indicate the target tilt (clockwise or counterclockwise from vertical). In both stages of the pretest, the color of the fixation cross provided feedback by changing to red when the subject’s response was incorrect or to green when it was correct.
Diverted attention control experiment
The purpose of this experiment was to measure the efficacy of the letter-detection task in diverting attention from the CFS stimuli. Three out of the 4 subjects participated (S1 was unavailable). This control experiment had two stages. For both stages, subjects viewed stimuli in a dark psychophysics lab room, on a chin rest, at a distance of 57 cm from a CRT monitor (HP p1230, 75 Hz refresh rate). The first stage was identical to the first pretest (see above), with the purpose of choosing a contrast that was suppressed to invisibility like the low contrast used in the main experiment. In the second stage, the experimental procedure was similar to that of the main experiment, but with the following differences. The letters were replaced by a fixation cross between trials. In each trial the grating target was either present (low contrast) or absent and the stream of letters either included one letter X or did not include any. In separate blocks, the mask and target were presented to the same or to different eyes. Subjects were instructed to perform two tasks sequentially: target detection (press 1 of 2 buttons to indicate if a target was present) and letter detection (press 1 of 2 buttons to indicate if an X was present). Button presses were performed during the ISI and triggered a brief change in the color of fixation that indicated that the button press was registered, but did not provide correct/incorrect feedback. The grating-detection task was primary in one session, i.e., subjects were instructed to attend and respond first to the grating-detection task. The letter-detection task was primary in another session, i.e., subjects were instructed to attend and respond first to the letter-detection task. The two sessions were performed on separate days to avoid confusion about which task was primary. For 2 out of the 3 subjects, the letter-discrimination task was primary in the first of these two sessions, and the target-detection task was primary in the second session, vice versa for the third subject.
MRI acquisition
MRI data were acquired on a Siemens 3T Allegra head-only scanner using a head coil (NM-011) for transmitting and an eight-channel phased-array surface coil (MMSC-071; both Nova Medical) for receiving. We used gradient-recalled echo-planar imaging to measure blood oxygen level dependent changes in image intensity (Ogawa et al., 1990). We acquired 23 slices oriented perpendicular to the calcarine sulcus and positioned with the most posterior slice at the occipital pole (1500 ms repetition time; 30 ms echo time; 72° flip angle; 2×2×2.5 mm voxel size; 104×80 voxel grid). A T1-weighted magnetization-prepared rapid gradient echo anatomical volume (MPRAGE) was acquired in each scanning session with the same slice prescriptions as the functional images (1530 ms repetition time; 3.8 ms echo time; 8° flip angle; 1×1×2.5 mm voxel size; 256X160 voxel grid). A high-resolution anatomical volume, acquired in a separate session, was the average of three MPRAGE scans that were aligned to one another and averaged (2500 ms repetition time; 3.93 ms echo time; 8° flip angle; 1×1×1 mm voxel size; 256×256 voxel grid). This high-resolution anatomical scan was used both for registration across scanning sessions and for gray-matter segmentation and cortical flattening.
fMRI data analysis
The fMRI data were analyzed using Matlab (MathWorks) and mrTools (available at http://www.cns.nyu.edu/heegerlab) on a Macintosh computer. The anatomical volume acquired in each scanning session was aligned to the high-resolution anatomical volume of the same subject’s brain, using a robust image-registration algorithm (Nestares and Heeger, 2000). Data from the first 8 frames of each functional run were discarded to minimize the effect of transient magnetic saturation and allow the hemodynamic response to reach steady state. Head movement within and across runs was compensated for using standard procedures (Nestares and Heeger, 2000). The time series from each voxel was divided by its mean to convert from arbitrary intensity units to percentage modulation and was high-pass filtered (cutoff: 0.01 Hz) to remove low-frequency noise and drift (Smith et al., 1999).
The fMRI data from the main experiment were analyzed in regions of interest (ROIs) in the cortical gray matter corresponding to the representations in V1, V2, and V3 of the inner circle of the mask’s annulus (see below). The preprocessed time series were averaged across gray matter voxels within each ROI.
We performed a deconvolution analysis (Dale, 1999) to estimate the fMRI response time course for each individual subject and each stimulus condition (Figure 4B). This procedure is equivalent to trial-triggered averaging with correction for overlap between temporally adjacent responses (Dale, 1999), based on the assumption that hemodynamic responses superimpose linearly over time (Boynton et al., 1996; Cardoso et al., 2012). The individual subject fMRI response time courses plotted in Figure 4B were each rescaled relative to the peak of that subject’s high contrast same eye curve. The response time courses were also averaged across all trials, separately for each ROI, normalized (sum=1), and used as each subject’s own typical hemodynamic response function (HRF) for that ROI to estimate response amplitudes.
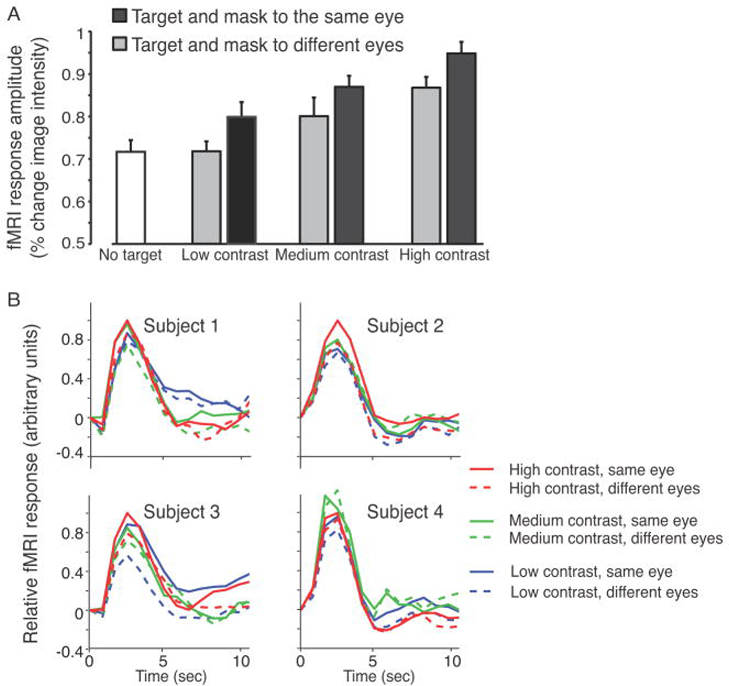
Figure 4.
fMRI responses from V1. (A) Response amplitudes, averaged across the 4 subjects. Error bars, 68% confidence interval computed with bootstrapping (see Materials and Methods). V1 responses were larger when the target and mask were presented to the same eye compared to different eyes, for all three contrast levels. (B) fMRI response time courses, from each of the four subjects. Solid curves, same eye. Dashed curves, different eyes. Red, high contrast. Green, middle contrast. Blue, low contrast.
Response amplitudes were estimated, separately for each trial type and separately for each subject, using linear regression. Each row of the regression matrix corresponded to a time point in the experiment and each column represented one of the 7 stimulus conditions. The expected neural activity (each column of the matrix) was convolved with the subject’s own HRF (as described above) to create a model of the expected fMRI responses (Boynton et al., 1996). We then estimated the response amplitudes (β values) by solving an equation of the form y = Ax, where the vector y was the measured fMRI time series during one run, the vector x was the response amplitudes, and A was the regression matrix described above. The response amplitudes were then averaged across subjects (Figure 4A).
Statistics
Non-parametric randomization tests were used to assess the statistical reliability of the differences between the responses to the different stimulus conditions. For each of the three contrasts, we obtained a null distribution for the difference in fMRI response amplitudes between same eye and different eyes. This distribution was created by repeating the regression analysis described above (including averaging across subjects) 1000 times, each time following random shuffling of trial tags (same vs. different eyes). The measured difference value was then compared to the null distribution; the proportion of the null distribution greater than the measured value was designated as the (one-tailed) p-value. The analysis accounted for the different number of trials per condition in subject S1. Performing the same analyses without including subject S1 supported the same conclusions.
A bootstrap procedure specified the error bars for the response amplitudes to each stimulus condition (Figure 4A). This was done by repeating the regression 1000 times, randomly resampling (with replacement) the data from each subject. We resampled entire runs from each scanning session (not individual trials), because the bootstrap procedure presumed that each resampling of the data was statistically independent. The analysis again accounted for the different number of trials per condition in subject S1.
A similar bootstrap procedure was used to compare suppression across visual areas. We defined a suppression index: (Rd − Rm)/(Rd + Rm), where Rd was the response amplitude when target and mask were presented to different eyes and Rm was the response amplitude to the mask only stimulus. We then calculated the distribution of the pairwise differences between suppression indices for each pair of visual areas (e.g., V1 and V2). This created a distribution of the differences in suppression indices. This was done by repeating the regression 1000 times, randomly resampling (with replacement) the data from each subject. The proportion of the resulting difference distribution that was greater than (or less than) 0 was designated as the (two-tailed) p-value.
A complementary procedure tested the statistical significance of differences in activity, for each subject individually. For each experimental session and contrast, the response amplitude evoked by the same eye was subtracted from that evoked by the corresponding different eyes stimulus condition. A Wilcoxon signed-rank test (Wilcoxon, 1945) was then used to determine if these differences were significantly larger than 0.
A randomization procedure assessed statistical significance of the the behavioral data (pretest and diverted attention control). The null distribution was created by repeatedly calculating accuracy rates 1000 times, each time following random shuffling of trial tags (same vs. different eyes). The proportion of the null distribution greater than the measured value was designated as the (one-tailed) p-value.
Defining retinotopic regions of interest
V1, V2 and V3 were identified in each hemisphere of each subject by measuring retinotopic maps. A traveling-wave retinotopic-mapping protocol was utilized for one of the subjects (Engel et al., 1997; Larsson and Heeger, 2006). A population-receptive-field-mapping protocol was used for the other three subjects (Dumoulin and Wandell, 2008). Visual cortical areas were identified by the retinotopic maps on flattened representations of the cortical surface. For each observer, cortical reconstruction and volumetric segmentation was performed with the Freesurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu/). The technical details of these procedures are described in prior publications (Larsson and Heeger, 2006). Visual area boundaries were drawn by hand on the flattened representations of each subject’s cortical surface, following published conventions (Wandell et al., 2007), and the corresponding gray matter coordinates were recorded.
Regions of interest (ROIs) were defined, individually for each subject, to include the subarea of V1, V2 and V3 responsive to the inner circle of the mask’s annulus (Figure 2). These ROIs included both dorsal and ventral subregions of V2 and V3. These subregions of each visual area were identified according to a localizer experiment acquired at the beginning of each scanning session of the main experiment. During these localizer runs, subjects held fixation while the display alternated every 9 s between a uniform gray field and a high-contrast checkerboard pattern within the inner circle of the mask annulus. This inner circle was the region of the display where the target was presented, but not overlapping with the mask. We identified voxels within V1, V2 and V3 gray matter for which the activity strongly correlated with the stimulus alternation (r > 0.5). These ROIs were thus defined based on measurements (from the retinotopic mapping and localizer experiments), that were statistically independent of those from the main experiment, i.e., measured in separate runs (Figure 2A).
Figure 2.
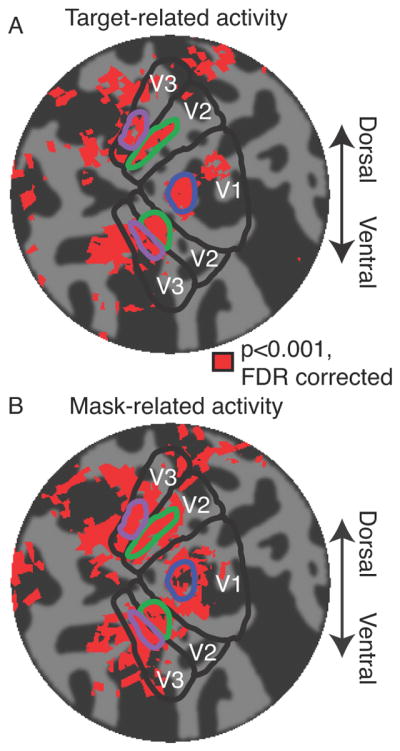
Regions of interest (ROIs), for a typical example subject, visualized on a flattened representation of the subject’s occipital lobe. (A) Target-related activity. Activity corresponding to retinotopic location of the target, averaged across 3 localizer runs. Dark gray corresponds to sulci. Light gray corresponds to gyri. Red patches correspond to statistically significant activity (see Materials and Methods). Blue outline, V1 target ROI. Green outlines, V2 target ROI. Purple outlines, V3 target ROI. (B) Mask-relate activity. Activity evoked by mask-only trials during the main experiment (same format as panel A).
Statistical parameter mapping
We performed complimentary statistical parameter mapping analysis, in addition to the main analysis (see above). The goal of this analysis was to search for post hoc differences in responses (same vs. different eyes) across all gray matter voxels in the acquired slices. Response amplitudes were recalculated as outlined above, but separately for each voxel. Statistics (p-values) were computed using two complementary procedures. First, we performed a Wilcoxon signed-rank test (Wilcoxon, 1945) as outlined above, but separately for each voxel, and individually for each subject. The resulting p-values were then corrected for multiple comparisons using the false discovery rate (FDR) procedure (Benjamini and Hochberg, 1995). Second, we performed a non-parametric randomization test (Raz et al., 2003; Gardner et al., 2008), individually for each subject. Same eye and different eyes trial tags were randomly shuffled and response amplitudes were recalculated for each voxel. Differences between response amplitudes computed with the randomized trial sequence were considered samples from null distributions, i.e., values that would be expected by chance according to the null hypothesis that there was no difference in mean activity when target and mask were presented to the same eye versus different eyes. The randomization was repeated 1000 times to produce null distributions for each voxel. The actual response difference (computed using the correct trial sequence) was compared to the null distribution, separately for each voxel; the percentage of the null distribution greater than or equal to the actual response difference was designated as the (one-tailed) p-value. The resulting p-values were then corrected for multiple comparisons using FDR (Benjamini and Hochberg, 1995).
Analogous randomization procedures were used to compute maps of activity evoked by the the target localizer (see Defining retinotopic regions of interest) and by mask (using the mask-only trials from the main experiment). To obtain the null distribution of target-related activity the trial tags of the localizer experiment were shuffled. To obtain the null distribution of mask-related activity, trial order and inter-trial intervals of the main experiment were shuffled. These maps were visualized on flattened representations of each subject’s cortical surface (Figure 2).
Results
Pretest to determine threshold
The low contrast was selected by the pretest, individually for each subject, as the highest contrast producing robust invisibility under CFS (see Materials and Methods). Detection was indistinguishable from chance for targets of that contrast (Table 1; p >=0.36 for each subject individually; non-parametric, one-tailed randomization test, see Materials and Methods). The poor psychophysical performance (close-to-zero d’ values) confirmed subjective reports by all 4 subjects that the target was invisible; the mean d’ value was 11 standard errors less than d’=1, which is typically considered psychophysical threshold. A second experiment (see Materials and Methods) confirmed that, with this contrast, orientation-discrimination accuracy was above-chance when target and mask were presented to the same eye (Table 1; p <=0.01 for each subject individually), but was indistinguishable from chance when presented to different eyes (Table 1). Together, these results demonstrate that the target was visible for same eye but invisible for different eyes.
Diverted attention
A control experiment confirmed that the continuous letter-detection task successfully diverted attention from the CFS targets (Figure 3). In this experiment, subjects were instructed to perform two tasks sequentially: target detection (press 1 of 2 buttons to indicate if a grating target was present) and letter detection (press 1 of 2 buttons to indicate if an X was present) (see Materials and Methods). Performance in both tasks depended on which of the two tasks was primary. For mask and target presented to the same eye, performance accuracy was higher in the grating-detection task when it was the primary task compared to when the letter-detection task was primary (p<0.0001, one-tailed, randomization test on the difference in performance). For target and mask presented to different eyes, performance in the grating-detection task was statistically indistinguishable from chance (by design, see above and Table 1), and consequently performance in the grating-detection task did not depend on which of the two tasks was primary (p=0.54, one-tailed randomization test on the difference in performance). Performance accuracy was higher in the letter-detection task when it was the primary task compared to when the grating-detection task was primary, regardless of whether that mask and target were presented to the same eye or different eyes (p<0.0001, one-tailed randomization test on the difference in performance).
Figure 3.
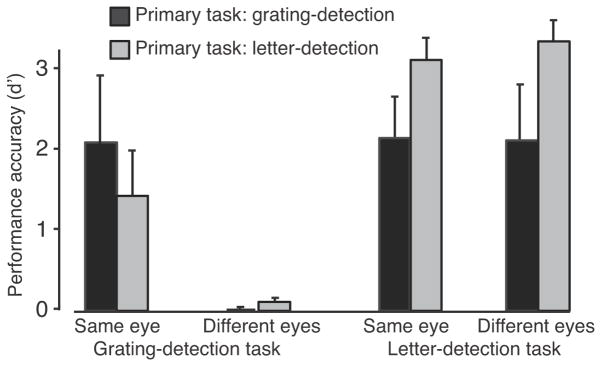
Performance accuracy (d’) for the diverted attention control experiment, averaged across the 4 subjects. Values are presented for the two tasks and the two stimulus conditions (same eye and different eyes). Black, primary task grating-detection. Gray, primary task was letter-detection.
Performance accuracy for the continuous letter detection task during the main experiment (in the scanner) was similar to performance accuracy when letter detection was primary during the diverted attention experiment. For the main experiment, a hit was defined as a correct button press recorded within the 1.2 seconds (6 letters) following target appearance. A false alarm was defined as an incorrect button press when no target was presented during the previous 1.2 seconds. Hit rates were high for each of the four subjects (78.9%, 77.01%, 81.8%, 88.64%), and false alarm rates were low (2.06%, 0%, 0.8%, 4.11%). Averaging the hit and false alarm rates across subjects yielded an average performance accuracy of d’=3.
V1 responses during CFS
V1 activity reflected both target contrast and target visibility (Figure 4). fMRI responses were measured in predefined subregions of each subject’s V1, corresponding retinotopically to the location of the target. The measured response amplitudes evoked by the targets, within these predefined ROIs, were smaller when mask and target were presented to different eyes compared to the same eye, for all 3 contrasts (low contrast: p=0.016, medium contrast: p=0.034, high contrast: p=0.004; non-parametric, one-tailed randomization test, see Materials and Methods). This result was evident for each subject individually; fMRI responses were smaller when mask and target were presented to different eyes compared to the same eye (p<0.05 for each subject individually, Wilcoxon signed-rank test, df=8, see Materials and Methods). In addition, the low-contrast, different eyes stimulus (which was invisible) evoked V1 activity that was statistically indistinguishable from the mask only (no-target) stimulus (p=0.48, one-tailed randomization test), and was smaller in amplitude than any of the stimuli for which the target was visible (p<0.05 for all 5 conditions, one-tailed randomization test).
CFS suppression at higher stages of visual processing
The results in V2 and V3 were similar to those in V1 (Figure 5). Response amplitudes in V2 were generally higher than in V1. This was probably due to mask-evoked activity that was included in V2 ROI and not in V1 ROI (Figure 2). V2 has larger receptive fields and a less precise retinotopic map than V1 and consequently its ROI was less accurately restricted to exclude most of the mask-evoked activity as V1 ROI.
Figure 5.
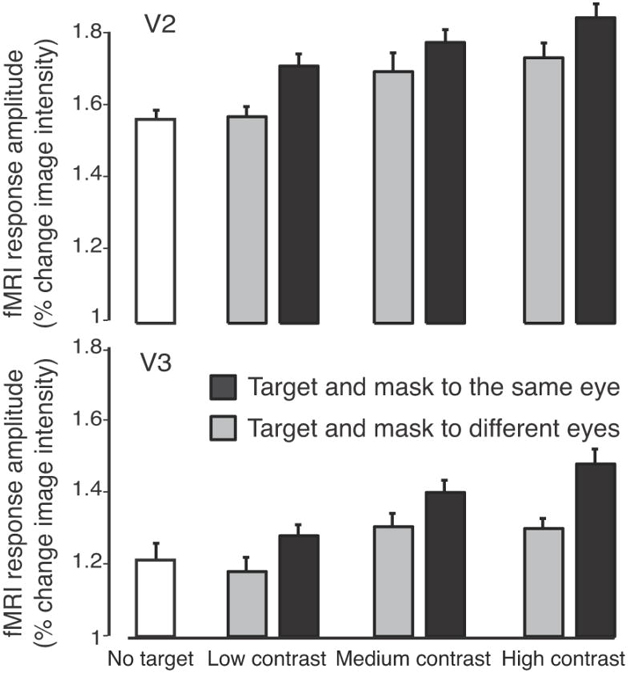
fMRI responses from V2 and V3 (same format as Figure 4A).
Response amplitudes for the targets were smaller when mask and target were presented to different eyes compared to the same eye, for all three contrasts (V2 ROI: low contrast: p=0.002, medium contrast: p=0.04, high contrast: p=0.009; V3 ROI: low contrast: p=0.001, medium contrast: p=0.03, high contrast: p=0.006). The low-contrast, different eye stimulus evoked activity in V2 and V3 which was statically indistinguishable from the mask only stimulus (V2: p=0.55; V3: p=0.17), and smaller in amplitude than for any of the visible targets (p<0.05 for all 5 conditions, one-tailed randomization test).
Our results are consistent with the hypothesis that the effect of CFS is entirely mediated by suppression in V1, and that subsequent stages of visual processing (such as V2 and V3) simply inherit the suppression from V1. A suppression index was used to compare visual areas (see Materials and Methods). There was no evidence for any differences in this suppression index across visual areas (two-tailed p>0.05 for all pairs of areas in all contrasts).
It might be that additional suppression is evident in later stages of processing beyond V3, the strength of suppression might depend on the stimulus conditions, and/or suppression in early visual areas might be modulated (via feedback) by suppression in higher visual areas. There is evidence that CFS is stronger for some visual features than others (Jiang et al., 2007; Yang et al., 2007; Zhou et al., 2010; Mudrik et al., 2011; Sterzer et al., 2011). Specifically, CFS has been found to be strongest for targets and masks with low spatial frequencies (Yang and Blake, 2012), suggesting that suppression is strongest for particular combinations of visual features in the target and/or mask. Some stimuli (like those used in the current study) are tailor-made to evoke cooperative and competitive interactions among subpopulations of neurons in V1, because V1 shows a functional organization for these stimulus features (orientation columns, ocular dominance columns, precise retinotopic map). Other stimuli, because of their spatial configuration or temporal properties, might evoke stronger suppression in later visual areas, as has been shown for binocular rivalry (Logothetis et al., 1996; Alais and Blake, 1999; Nguyen et al., 2001; Alais and Melcher, 2007) Additional measurements like those reported here could test for a correspondence between perceptual suppression due to CFS and its neural correlates, while systematically manipulating these various stimulus factors.
Statistical parameter maps
The main analysis described above was hypothesis-driven and based on predefined ROIs. In addition, we performed complimentary statistical parameter mapping analyses, to search for post hoc differences in responses (same vs. different eyes) across all gray matter voxels in the acquired slices, individually for each subject (see Materials and Methods). No significant differences in activity were found, except for a few scattered voxels. It is likely that there are widespread effects of CFS in activity in multiple cortical areas, which might be revealed using hypothesis-driven, ROI-based analyses like that we utilized in our main analysis. But the data at hand does not provide evidence for activity outside the hypothesized ROIs with this exploratory statistical parameter mapping analysis.
Discussion
CFS changes the gain of V1 responses
Responses in early visual cortical areas (V1, V2, V3) were smaller when the target and mask were presented to different eyes compared to same eye. This was the case for all three contrast levels, including the low contrast for which CFS rendered the target invisible and the medium-to-high contrasts for which the target was visible even when presented to the eye opposite the mask. These results are analogous to the changes in perception and neural activity that have been measured for a variety of other types of visual masking (Legge and Foley, 1980; Carandini and Heeger, 2012).
Visual masking is hypothesized to be a consequence of normalization (Heeger, 1992; Foley, 1994; Heeger, 1994; Petrov et al., 2005; Busse et al., 2009; Carandini and Heeger, 2012). The idea of the normalization model is that the response of a neuron to its preferred stimulus (i.e., the target) is suppressed by the pooled (summed) activity of a population of neurons (the normalization pool) that are responding to the surrounding context (i.e., the mask). Specifically, the excitatory drive from the target depends on the contrast energy of the target, and it is normalized (divided) by the activity of the normalization pool which depends on the contrast energy of the mask. The effect of the mask is, therefore, a change in contrast gain; superimposing the mask reduces responses evoked by the target in a manner equivalent to scaling its contrast (Carandini and Heeger, 2012). Such suppression has been reported even when target and mask were presented to opposite eyes (Walker et al., 1998; Truchard et al., 2000; Wilke et al., 2003; Moradi and Heeger, 2009). Indeed, inter-ocular suppression (from different eyes) in V1 can be explained by the normalization model (Moradi and Heeger, 2009). Different masks (e.g., same eye versus different eyes) can have different impacts on contrast gain; GFS (which is similar to CFS) has been reported to be most effective when the target and mask were presented to different eyes (Wilke et al., 2003), and monocular rivalry is weaker than binocular rivalry, even though they are are similarly affected by stimulus properties (O’Shea et al., 2009). For all of these variations, the normalization model predicts that any given mask is equivalent to reducing target contrast by a fixed scale factor.
The implication of this model is that CFS does not switch neural activity “on” and “off”, reflecting the dichotomy between “aware” and “unaware” states, but rather that it influences awareness by modulating the contrast gain of neural responses. A prerequisite for visual awareness is that the sensory/perceptual representation must be sufficiently stable and robust, i.e., that the stimulus-evoked responses are sufficiently large relative to the inherent variability in neural activity. Because CFS reduces the gain of the stimulus-evoked responses, the responses evoked by a low-contrast target do not satisfy this prerequisite. CFS has gained increasing popularity for studying visual awareness (Fang and He, 2005; Tsuchiya and Koch, 2005; Jiang et al., 2006; Bahrami et al., 2007; Yang et al., 2007; Jiang et al., 2009; Sterzer et al., 2009; Tsuchiya et al., 2009; Hesselmann and Malach, 2011; Kang et al., 2011; Mudrik et al., 2011; Watanabe et al., 2011), because it seems to be an elegant way to suppress a target without changing its basic visual properties. This is only partially true. CFS does not involve modifying the target itself, but does involve changing the context in which the target is presented. The mask is a more effective suppressor when target and mask are presented to different eyes compared to when they are presented to the same eye. This implies that the mask contributes more strongly to the normalization pool when target and mask are presented to different eyes compared to when they are presented to the same eye. This explanation of CFS is supported by the observation that CFS suppression is more effective when the target and the mask share similar spatiotemporal properties (Yang and Blake, 2012). CFS does not “shut off” visual processing but rather selectively attenuates the activity in a subpopulation of neurons, just like other types of visual masking (Zadbood et al., 2011). We conclude that CFS affects the gain of early visual responses, consistent with the extensive literature on visual masking.
Some studies have ostensibly shown that high level information is suppressed by conventional masking and not by CFS, supposedly contradicting the hypothesis that both methods are based on gain modulation in early visual cortex. These studies used a method termed “breaking CFS” (b-CFS), in which the contrast of the CFS-suppressed target image is gradually ramped up until the observer can report its spatial location (Jiang et al., 2007; Yang et al., 2007; Zhou et al., 2010; Mudrik et al., 2011; Stein et al., 2011). The b-CFS studies have been criticized, however, for not measuring or controlling response criteria, temporal uncertainties, and other factors impacting subjects’ strategies (Stein et al., 2011). When these factors were equated, no such differences were found between CFS and conventional masking, at least for the case of detecting upright and inverted faces (Stein et al., 2011). It remains to be seen if the same applies to other b-CFS findings.
Previous CFS imaging studies
Previous fMRI studies have reported suppression of visual cortical activity during CFS (Fang and He, 2005; Hesselmann and Malach, 2011). This suppression was reported in both early (Maier et al., 2008) and late (Jiang and He, 2006) visual cortical areas and both the dorsal and the ventral visual pathways (Fang and He, 2005; Hesselmann and Malach, 2011).
There is, however, one previous fMRI study that failed to find a significant difference in activity between visible and invisible targets (Watanabe et al., 2011). This study used fMRI with CFS to study the neural correlates of visual awareness and attention in human primary visual cortex (V1). Visual awareness was manipulated by presenting a target and mask either to the same eye, or to different eyes. In addition, attention was manipulated by instructing subjects to attend either to the region where the grating targets were presented, or to a target-irrelevant task at fixation. Activity evoked by visible (same eye) and invisible (different eyes) targets was indistinguishable in V1 (both when attended and when unattended), but attention to the spatial location of the target increased activity substantially in the retinotopically-corresponding region of V1. The effect of attention on V1 activity has been reported numerous times (Gandhi et al., 1999; Kastner et al., 1999; Martinez et al., 1999), including an interaction between attention and CFS in V1 (Bahrami et al., 2007), and hence the novel finding was the lack of a difference in activity in V1 (a null result) between visible and invisible targets. The authors wrote that “the results regarding awareness in our study challenge the currently established view that the BOLD signal in V1 correlates robustly with the contents of perception” and concluded that “the difference in the lower-level limit of BOLD activation between attention and awareness illustrates dissociated neural correlates of the two processes” (Watanabe et al., 2011).
Our current results overturn this negative finding. Using nearly identical stimulus conditions, we found that there was a difference in V1 activity for visible (low contrast, same eye) and invisible (low contrast, different eyes) targets. The most obvious difference between our experiment and theirs is that we averaged the measurements across ~3 times more trials per subject, to obtain the statistical power needed for distinguishing small differences in activity. We infer that the invisible and visible conditions corresponded, respectively, to V1 responses with low gain and slightly-higher gain, and the differences in V1 activity between these two conditions were small and hard to detect. However, we make no claim about the relation between V1 activity and awareness.
The experimental protocol adopted by the previous fMRI study (Watanabe et al., 2011) did not include target-absent catch trials, so it is unclear if the targets were really invisible. Subjects in that study were instructed to attend to the targets and indicate target visibility, but their button press responses could have been based on each subject’s ability to detect any difference between the two (same eye, different eyes) conditions, and hence is not sufficient proof that the targets were invisible. In addition, without catch trials it was impossible for them to determine if their measurements had sufficiently high signal-to-noise ratio. Only by including catch trials were we able to establish that the low-contrast targets were invisible when target and mask were presented to different eyes, and that responses to visible (low contrast, same eye) targets differed from the responses to catch trials (no target), bracketing the range of response amplitudes that might be expected for the invisible (low contrast, different eyes) targets.
Conclusion
We infer that CFS changes the gain of stimulus-evoked neural responses. According to this idea, a target that is presented with a mask in different eyes is perceptually equivalent to a target of lower contrast with a mask in the same eye. This conclusion offers a step toward characterizing the computational processes underlying CFS. It also offers a quantitative point of view for studying awareness, based on visual masking, for which the empirical methods and theoretical principles have become well-established over the past 40 years.
Acknowledgments
This study was supported by grants from NIH (R01-EY016752), the US-Israeli Binational Science Foundation (2007224) and the Weizmann-NYU Demonstration Fund in Neuroscience (D.J.H). Author S.Y.G was supported by the EU Marie Curie outgoing international fellowship and by the Weizmann Institute Advancing Women in Science award.
Contributor Information
Shlomit Yuval-Greenberg, Department of Psychology and Center for Neural Science, New York University.
David J. Heeger, Department of Psychology and Center for Neural Science, New York University
References
- Alais D, Blake R. Grouping visual features during binocular rivalry. Vision Research. 1999 doi: 10.1016/s0042-6989(99)00146-7. [DOI] [PubMed] [Google Scholar]
- Alais D, Melcher D. Strength and coherence of binocular rivalry depends on shared stimulus complexity. Vision Research. 2007 doi: 10.1016/j.visres.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Bahrami B, Lavie N, Rees G. Attentional load modulates responses of human primary visual cortex to invisible stimuli. Curr Biol. 2007;17:509–513. doi: 10.1016/j.cub.2007.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57:289–300. [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. The Journal of Neuroscience. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse L, Wade AR, Carandini M. Representation of Concurrent Stimuli by Population Activity in Visual Cortex. Neuron. 2009;64:931–942. doi: 10.1016/j.neuron.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandini M, Heeger DJ. Normalization as a canonical neural computation. Nat Rev Neurosci. 2012;13:51–62. doi: 10.1038/nrn3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso MMB, Sirotin YB, Lima B, Glushenkova E, Das A. The neuroimaging signal is a linear sum of neurally distinct stimulus- and task-related components. Nat Neurosci. 2012;15:1298–1306. doi: 10.1038/nn.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoulin SO, Wandell BA. Population receptive field estimates in human visual cortex. Neuroimage. 2008;39:647–660. doi: 10.1016/j.neuroimage.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SA, Glover GH, Wandell BA. Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cereb Cortex. 1997;7:181–192. doi: 10.1093/cercor/7.2.181. [DOI] [PubMed] [Google Scholar]
- Engel SA, Rumelhart DE, Wandell BA, Lee AT, Glover GH, Chichilnisky EJ, Shadlen MN. fMRI of human visual cortex. Nature. 1994;369:525. doi: 10.1038/369525a0. [DOI] [PubMed] [Google Scholar]
- Fang F, He S. Cortical responses to invisible objects in the human dorsal and ventral pathways. Nat Neurosci. 2005;8:1380–1385. doi: 10.1038/nn1537. [DOI] [PubMed] [Google Scholar]
- Foley JM. Human luminance pattern-vision mechanisms: masking experiments require a new model. J Opt Soc Am A Opt Image Sci Vis. 1994;11:1710–1719. doi: 10.1364/josaa.11.001710. [DOI] [PubMed] [Google Scholar]
- Gandhi SP, Heeger DJ, Boynton GM. Spatial attention affects brain activity in human primary visual cortex. P Natl Acad Sci Usa. 1999;96:3314–3319. doi: 10.1073/pnas.96.6.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JL, Merriam EP, Movshon JA, Heeger DJ. Maps of visual space in human occipital cortex are retinotopic, not spatiotopic. J Neurosci. 2008;28:3988–3999. doi: 10.1523/JNEUROSCI.5476-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeger DJ. Normalization of cell responses in cat striate cortex. Vis Neurosci. 1992;9:181–197. doi: 10.1017/s0952523800009640. [DOI] [PubMed] [Google Scholar]
- Heeger DJ. The representation of visual stimuli in primary visual cortex. Current Directions in Psychological Science 1994 [Google Scholar]
- Hesselmann G, Malach R. The Link between fMRI-BOLD Activation and Perceptual Awareness Is "Stream-Invariant" in the Human Visual System. Cerebral Cortex. 2011;21:2829–2837. doi: 10.1093/cercor/bhr085. [DOI] [PubMed] [Google Scholar]
- Hupé J-M, Bordier C, Dojat M. A BOLD signature of eyeblinks in the visual cortex. Neuroimage. 2012;61:149–161. doi: 10.1016/j.neuroimage.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Costello P, Fang F, Huang M, He S. A gender- and sexual orientation-dependent spatial attentional effect of invisible images. P Natl Acad Sci Usa. 2006;103:17048–17052. doi: 10.1073/pnas.0605678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Costello P, He S. Processing of invisible stimuli: advantage of upright faces and recognizable words in overcoming interocular suppression. Psychological Science. 2007;18:349–355. doi: 10.1111/j.1467-9280.2007.01902.x. [DOI] [PubMed] [Google Scholar]
- Jiang Y, He S. Cortical Responses to Invisible Faces: Dissociating Subsystems for Facial-Information Processing. Curr Biol. 2006;16:2023–2029. doi: 10.1016/j.cub.2006.08.084. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Shannon RW, Vizueta N, Bernat EM, Patrick CJ, He S. Dynamics of processing invisible faces in the brain: automatic neural encoding of facial expression information. Neuroimage. 2009;44:1171–1177. doi: 10.1016/j.neuroimage.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M-S, Blake R, Woodman GF. Semantic analysis does not occur in the absence of awareness induced by interocular suppression. Journal of Neuroscience. 2011;31:13535–13545. doi: 10.1523/JNEUROSCI.1691-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Larsson J, Heeger DJ. Two retinotopic visual areas in human lateral occipital cortex. Journal of Neuroscience. 2006;26:13128–13142. doi: 10.1523/JNEUROSCI.1657-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legge GE, Foley JM. Contrast masking in human vision. J Opt Soc Am. 1980;70:1458–1471. doi: 10.1364/josa.70.001458. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Leopold DA, Sheinberg DL. What is rivalling during binocular rivalry? 1996 doi: 10.1038/380621a0. [DOI] [PubMed] [Google Scholar]
- Maier A, Wilke M, Aura C, Zhu C, Ye FQ, Leopold DA. Divergence of fMRI and neural signals in V1 during perceptual suppression in the awake monkey. Nat Neurosci. 2008;11:1193–1200. doi: 10.1038/nn.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A, Anllo-Vento L, Sereno MI, Frank LR, Buxton RB, Dubowitz DJ, Wong EC, Hinrichs H, Heinze HJ, Hillyard SA. Involvement of striate and extrastriate visual cortical areas in spatial attention. Nat Neurosci. 1999;2:364–369. doi: 10.1038/7274. [DOI] [PubMed] [Google Scholar]
- Moradi F, Heeger DJ. Inter-ocular contrast normalization in human visual cortex. Journal of Vision. 2009;9:13.1–22. doi: 10.1167/9.3.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudrik L, Breska A, Lamy D, Deouell LY. Integration Without Awareness: Expanding the Limits of Unconscious Processing. Psychological Science. 2011;22:764–770. doi: 10.1177/0956797611408736. [DOI] [PubMed] [Google Scholar]
- Nestares O, Heeger DJ. Robust multiresolution alignment of MRI brain volumes. Magn Reson Med. 2000;43:705–715. doi: 10.1002/(sici)1522-2594(200005)43:5<705::aid-mrm13>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Nguyen VA, Freeman AW, Wenderoth P. The depth and selectivity of suppression in binocular rivalry. Perception & Psychophysics. 2001;63:348–360. doi: 10.3758/bf03194475. [DOI] [PubMed] [Google Scholar]
- O’Shea RP, Parker A, La Rooy DJ. Monocular rivalry exhibits three hallmarks of binocular rivalry: Evidence for common processes. Vision Research. 2009;49 doi: 10.1016/j.visres.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. P Natl Acad Sci Usa. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paffen C, Hessels RS, Van der Stigchel S. Interocular conflict attracts attention. Attention. 2012 doi: 10.3758/s13414-011-0256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov Y, Carandini M, McKee S. Two distinct mechanisms of suppression in human vision. Journal of Neuroscience. 2005;25:8704–8707. doi: 10.1523/JNEUROSCI.2871-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz J, Zheng H, Ombao H, Turetsky B. Statistical tests for fMRI based on experimental randomization. Neuroimage. 2003;19:226–232. doi: 10.1016/s1053-8119(03)00115-0. [DOI] [PubMed] [Google Scholar]
- Schurger A. A very inexpensive MRI-compatible method for dichoptic visual stimulation. J Neurosci Methods. 2009;177:199–202. doi: 10.1016/j.jneumeth.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Smith AM, Lewis BK, Ruttimann UE, Ye FQ, Sinnwell TM, Yang Y, Duyn JH, Frank JA. Investigation of low frequency drift in fMRI signal. Neuroimage. 1999;9:526–533. doi: 10.1006/nimg.1999.0435. [DOI] [PubMed] [Google Scholar]
- Stein T, Hebart MN, Sterzer P. Breaking Continuous Flash Suppression: A New Measure of Unconscious Processing during Interocular Suppression? Front Hum Neurosci. 2011;5:167. doi: 10.3389/fnhum.2011.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterzer P, Hilgenfeldt T, Freudenberg P, Bermpohl F, Adli M. Access of emotional information to visual awareness in patients with major depressive disorder. Psychol Med. 2011;41:1615–1624. doi: 10.1017/S0033291710002540. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Jalkanen L, Rees G. Electromagnetic responses to invisible face stimuli during binocular suppression. Neuroimage. 2009;46:803–808. doi: 10.1016/j.neuroimage.2009.02.046. [DOI] [PubMed] [Google Scholar]
- Truchard AM, Ohzawa I, Freeman RD. Contrast gain control in the visual cortex: monocular versus binocular mechanisms. Journal of Neuroscience. 2000;20:3017–3032. doi: 10.1523/JNEUROSCI.20-08-03017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse PU, Baumgartner FJ, Greenlee MW. Event-related functional MRI of cortical activity evoked by microsaccades, small visually-guided saccades, and eyeblinks in human visual cortex. Neuroimage. 2010;49:805–816. doi: 10.1016/j.neuroimage.2009.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya N, Koch C. Continuous flash suppression reduces negative afterimages. Nat Neurosci. 2005;8:1096–1101. doi: 10.1038/nn1500. [DOI] [PubMed] [Google Scholar]
- Tsuchiya N, Moradi F, Felsen C, Yamazaki M, Adolphs R. Intact rapid detection of fearful faces in the absence of the amygdala. Nat Neurosci. 2009;12:1224–1225. doi: 10.1038/nn.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker GA, Ohzawa I, Freeman RD. Binocular cross-orientation suppression in the cat's striate cortex. J Neurophysiol. 1998;79:227–239. doi: 10.1152/jn.1998.79.1.227. [DOI] [PubMed] [Google Scholar]
- Wandell BA, Dumoulin SO, Brewer AA. Visual field maps in human cortex. Neuron. 2007;56:366–383. doi: 10.1016/j.neuron.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Cheng K, Murayama Y, Ueno K, Asamizuya T, Tanaka K, Logothetis N. Attention But Not Awareness Modulates the BOLD Signal in the Human V1 During Binocular Suppression. Science. 2011;334:829–831. doi: 10.1126/science.1203161. [DOI] [PubMed] [Google Scholar]
- Wheatstone C. Contributions to the Physiology of Vision. Part the First. On Some Remarkable, and Hitherto Unobserved, Phenomena of Binocular Vision. Philosophical transactions of the Royal Society of London. 1838;128:371–394. [Google Scholar]
- Wilcoxon F. Individual Comparisons by Ranking Methods. Biometrics Bulletin. 1945;1:80–83. [Google Scholar]
- Wilke M, Logothetis NK, Leopold DA. Generalized flash suppression of salient visual targets. Neuron. 2003;39:1043–1052. doi: 10.1016/j.neuron.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Wolfe JM. Reversing ocular dominance and suppression in a single flash. Vision Research. 1984;24:471–478. doi: 10.1016/0042-6989(84)90044-0. [DOI] [PubMed] [Google Scholar]
- Yang E, Blake R. Deconstructing continuous flash suppression. Journal of Vision. 2012;12:8–8. doi: 10.1167/12.3.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang E, Zald DH, Blake R. Fearful expressions gain preferential access to awareness during continuous flash suppression. Emotion. 2007;7:882. doi: 10.1037/1528-3542.7.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadbood A, Lee S-H, Blake R. Stimulus fractionation by interocular suppression. Front Hum Neurosci. 2011;5:135. doi: 10.3389/fnhum.2011.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Zhang L, Liu J, Yang J, Qu Z. Specificity of face processing without awareness. Consciousness and Cognition. 2010;19:408–412. doi: 10.1016/j.concog.2009.12.009. [DOI] [PubMed] [Google Scholar]